Abstract
Background & Aims
Non-steroidal anti-inflammatory drugs (NSAIDs) are effective cancer chemopreventive agents. However, chronic administration of NSAIDs is associated with significant side effects, mainly gastrointestinal. Given these limitations, we synthesized phospho-sulindac (P-S; OXT-328), a novel sulindac derivative.
Methods
Here, we evaluated the safety and efficacy of P-S in preclinical models, including its mechanism of action using human colon cancer cell (HCCC) lines and animal tumor models.
Results
a) Compared to sulindac, P-S is much more potent in inhibiting the growth of cultured HCCC and more efficacious in preventing the growth of HT-29 xenografts in nude mice. P-S also prevents the growth of intestinal tumors in Apc/Min mice, b) in combination with difluoromethylornithine (DFMO), P-S reduced tumor multiplicity in Apc/Min mice by 90%; and c) P-S is much safer than sulindac as evidenced by its in vitro toxicological evaluation and animal toxicity studies. Mechanistically, P-S increases the intracellular levels of reactive oxygen and nitrogen species, which are key early mediators of its chemopreventive effect. Moreover, P-S induces spermidine/spermine N1-acetyltransferase enzymatic activity, and together with DFMO it reduces polyamine levels in vitro and in vivo.
Conclusions
P-S displays considerable safety and efficacy, two pharmacological properties that are essential for a potential cancer chemopreventive agent, and thus merits further evaluation.
Keywords: Colon cancer, Phospho-sulindac, Sulindac, Polyamines, Reactive oxygen species
INTRODUCTION
Colon cancer, one of the most frequent human malignancies in the western world, is a preventable disease. In 2009, the National Cancer Institute estimated that in the United States there were about 150,000 new cases and 50,000 deaths from colorectal cancer. Thus, the development of safe and effective chemopreventive agents represents a pressing need.
The non-steroidal anti-inflammatory drug (NSAID) sulindac, alone or in combination with difluoromethylornithine (DFMO), has been shown to be effective in the prevention of colon cancer.1–3 A recent study by Meyskens et al, reaffirmed the feasibility of this approach. In humans, when compared to controls, sulindac plus DFMO reduced the recurrence of one or more adenomas by 70% and of more advanced adenomas by 92%.3 However, a limiting factor in the long term use of sulindac is its toxicity, primarily gastrointestinal and renal, that can affect up to 20% of patients.4 These considerations prompted us to synthesize phospho-sulindac (P-S; OXT-328; Fig. 1A), a novel derivative of sulindac, and evaluate its safety and efficacy against colon cancer. We anticipated enhanced safety of P-S, because it is modified at its −COOH moiety, which is considered critical for its toxicity.5
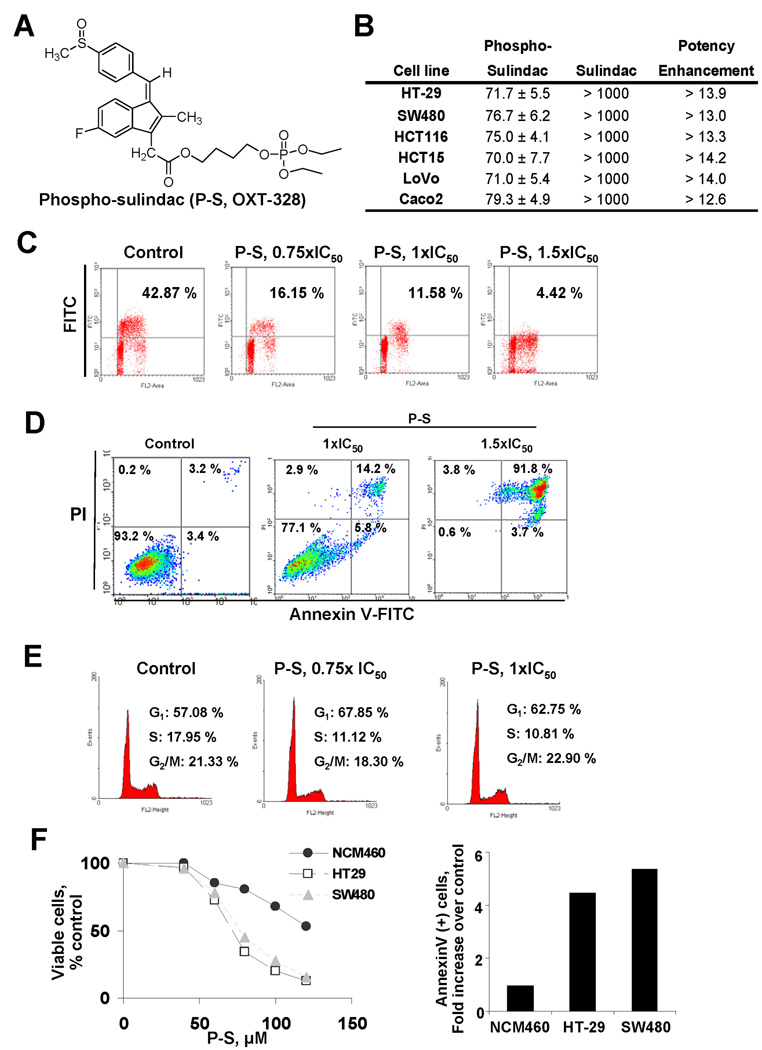
Figure 1. Phospho-sulindac inhibits cell proliferation, and induces cell cycle arrest and cell death by apoptosis in colon cancer cells.
(A) Chemical structure of phospho sulindac (P-S; OXT-328). (B) IC50 values for colon cancer cells treated with P-S or sulindac for 24 h (mean ± SEM). (C) Histograms of BrdU incorporation in HT-29 cells treated for 24 h without (control) or with 0.75×, 1× or 1.5× IC50 P-S. (D) The percentage of SW480 apoptotic cells, determined by flow cytometry using the dual staining (Annexin V and PI), are indicated in each quadrant. (E) Cell cycle progression in HT-29 cells. Representative profiles of the distribution of cells in G1, G2/M and S phases are shown. (F) Differential cytotoxic effect of P-S in colon cancer cells compared to the normal human colon cell line NCM460. Left panel: NCM460, HT-29 and SW480 cells were incubated without or with variable concentrations (0–120 µM) of phospho-sulindac for 24 h. Cell growth was expressed as % control. Right panel: Cell death by apoptosis was determined by flow cytometry in NCM460, HT-29 and SW480 cells incubated without or with 110 µM of P-S for 24 h. Results are expressed as fold-increase compared to the percentages of apoptotic cells in the NCM460 cells.
Here, we report the enhanced potency of P-S over sulindac in inhibiting the growth of human colon cancer cell lines, its efficacy in animal tumor models, its apparent safety based on toxicological evaluation and animal testing, and provide a mechanism for its chemopreventive effect.
MATERIALS AND METHODS
Reagents - Cell culture
P-S was synthesized as reported.6 Sulindac, A23187 and N-acetyl-L-cysteine (NAC) were from Sigma. Human colon cancer cell lines and the normal human colon cell line NCM460 were grown as monolayer as suggested by ATTC (Manassas, VA). Cell growth was determined using the MTT assay (Promega, Madison, WI) following the manufacturer’s instructions.7 Cells were treated with P-S ± DFMO for up to 48 h. Cell proliferation was assayed by 5-bromo-2′-deoxyuridine (BrdU) incorporation; apoptosis and necrosis by staining with Annexin V-FITC and propidium iodide (PI) and analyzing the fluorescence intensities by FACScaliber (BD Bioscience); and cell cycle by flow cytometry, all as described.7
Redox assays
Reactive oxygen and nitrogen species (RONS) levels were determined using the general RONS probe DCFDA.6 HT-29 cells treated with the test compound(s) for 2 h, were loaded with DCFDA and fluorescence intensity was analyzed by FACScaliber.7 Mitochondrial superoxide was determined by fluorescence microscopy. Cells seeded overnight in glass bottom culture dishes (MatTek, Ashland, MA) were treated with the test compound(s) for 0 – 60 min and assayed by fluorescence microscopy.6 Glutathione (oxidized and reduced) was determined by the GSH reductase-coupled 5,5'-dithiobis(2-nitrobenzoic acid) assay.8 The Thioredoxin redox status assay was performed as described.6
Electrophoretic mobility shift assay (EMSA)
Nuclear fractions were isolated from 2×106 cells (treated and controls) and subjected to EMSA.7 Oligonucleotides containing the consensus sequence for NF-κB or OCT-1 (control) were end-labeled with [γ-32P]-ATP using T4 polynucleotide kinase.
Cyclooxygenase (COX) activity and PGE2 levels
COX activity and PGE2 levels were determined by immunoassays following the manufacturer’s instructions (Cayman Chemical).
Polyamines and spermidine/spermine-N1-acetyltransferase (SAT1) activity
Cells treated with test compound(s) for 24 h, were assayed for polyamine levels by HPLC (internal standard: 1,7-diaminoheptane).9 For the SAT1 activity, cells (3×106), treated with test compound(s) were harvested by scraping, disrupted by sonication in buffer [10 mmol/L Tris-HCl (pH 7.5), 2.5 mmol/L DTT, 1 mmol/L EDTA] and pelleted. SAT1 activity was based on the amount of labeled N1-acetylspermidine synthesized from [14C]acetyl-CoA and unlabelled spermidine.10
siRNA gene knock-down
Cells were transfected with 200 ng of control or sat1 siRNA in Lipofectamine 2000 (Invitrogen) and 48 h later were replated and treated with test compound(s).
Animal studies
All studies were approved by our Institutional Animal Care and Use Committee.
Acute toxicity
Six week-old female C57BL/6J+/+ mice (n=8/group), were treated for 5 days by oral gavage with equimolar amounts of P-S (317 mg/kg/d) or sulindac (200 mg/kg/d) or vehicle. Body weight was recorded every other day. All mice were necropsied.
Survival study
Six week-old female nude mice (n=6/group), were treated for 21 days by oral gavage with vehicle, P-S (100 mg/kg/d), or sulindac 66 mg/kg/d (equimolar) or 100 mg/kg/d (equi-dose). Body weight was recorded every other day. On day 21, surviving animals were euthanized, serum was collected, and their liver, kidneys and pancreas were removed, preserved in formalin and evaluated histologically.
Gastrointestinal toxicity
The gastrointestinal toxicity of P-S was determined in rats following a standard protocol.11 Six week-old Sprague Dawley rats (n=3/group) were treated for 4 days by gavage with vehicle, indomethacin 4.75 mg/kg/d (positive control) or equimolar amounts of P-S (317 mg/kg/d) or sulindac (200 mg/kg/d). On day 5, animals were injected with 1% Evans blue solution, sacrificed, and the number and size of small intestinal ulcerations were recorded. The toxicity scoring ranges between 0 (intact small intestine with no ulcerations or mucosal damage) and 5 (animal dies prior to study’s end).11
Efficacy in nude mouse xenografts
Six week-old female NCr nude mice (Taconic; n=6/group) were treated by gavage with sulindac (33 mg/kg/d), P-S (50 or 100 mg/kg/d) or vehicle (corn oil). Five days later, mice were inoculated s.c with 2×106 HT-29 colon cancer cells on each flank. Tumor size was determined with a digital microcalipter (tumor volume = [length × width × (length + width/2) × 0.56]). Eighteen days after cell inoculation, animals were sacrificed and tumors were removed and weighed.
Efficacy in APCMin/+ mice
1) Treatment protocol study: Eleven week-old male C57BL/6J APCMin/+ (Jackson Laboratories; n=10/group) were treated by gavage with P-S 100 mg/kg/d or vehicle (corn oil) for 28 days. At sacrifice, their small intestine and colon segments were removed, opened longitudinally and tumors were counted under a magnifying lens. 2) Prevention protocol study: Six week-old C57BL/6J APCMin/+ mice (n=9/group) received daily: group 1, vehicle (corn oil) by gavage; group 2, P-S 100 mg/kg by gavage; group 3, DFMO 2% in drinking water; and group 4, P-S and DFMO as above. Seven weeks later, all animals were euthanized; their small intestine and colon were removed and processed as above. Tissue samples were preserved in formalin and studied by immunohistochemistry following standard protocols8 or snap-frozen for biochemical analyses.
Statistical Analysis
Results, from at least three independent experiments and expressed as mean±SEM were analyzed by one-factor analysis of variance followed by Tukey test for multiple comparisons. P<0.05 was statistically significant.
RESULTS
P-S inhibits the growth of cultured colon cancer cells through a strong cytokinetic effect
To study the effect of P-S on cell growth, we determined the 24-h IC50 values of P-S and sulindac in various human colon cancer cells (Fig. 1B). The IC50 values of P-S varied little among these cell lines (70–79.3 µM), whereas those of sulindac were consistently >1,000 µM indicating a potency enhancement of >14.2-fold.
We then elucidated the underlying cytokinetic effect of P-S in HT-29 and SW480 cells. P-S markedly reduced concentration-dependent cell proliferation (Fig. 1C). For instance, at its IC50 P-S reduced HT-29 cell proliferation from 43% in controls to 12% and at 1.5×IC50 to 4%, accounting to 73% and 91% reduction, respectively. P-S induced concentration-dependent apoptosis in HT-29 and SW480 cells. Both early and late apoptosis were present but the latter predominated. At 24 h, in SW480 cells, the annexin V(+) cells increased from 5.6% in control to 20% at P-S 1×IC50 and to 95.5% at 1.5×IC50 (Fig. 1D). Finally, P-S blocked the G1→ S transition. The percentage of cells in G1 phase was significantly higher in cells treated with 0.75×IC50 P-S than in controls (80% vs. 57%, and 68% vs. 57% for HT-29 and SW480 cells, respectively; Fig. 1E).
We next compared the effect of P-S on colon cancer cells against that on the normal human colon epithelial cell line NCM460. After 24 h-treatment with 100 µM P-S, only 20.4% and 27.7% of HT29 and SW480 cells, respectively, remained viable. However, under the same experimental conditions, 67.7% of NCM460 cells were viable (Fig. 1F). In addition, incubation of HT-29 and SW480 cells with 110 µM P-S generated a 4.5- and 5-fold increase in annexin V(+) cells. In contrast, only a 1-fold increase in annexin V(+) cells was observed for NCM460 cells (Fig. 1F). This indicates that P-S decreases cell growth and induces apoptosis preferentially in colon cancer cells compared to a normal epithelial colon cell line.
Cell signaling effects of P-S
We examined the effect of P-S on the redox status of colon cancer cell line, an effect potentially important for its mechanism of action,6, 8 its effect on polyamines, known to be critical to the anticancer effect of sulindac,2 and a series of redox-sensitive signaling pathways.
P-S induces oxidative stress in colon cancer cells
To explore the effect of P-S on cellular RONS, we loaded SW480 cells treated with 0.5× or 2×IC50 P-S for 1 h with DCFDA, a general RONS probe. The level of RONS in response to P-S at 0.5× and 1×IC50 for cell growth, increased 1.8 and 2.8 fold over control (Fig. 2A), whereas P-S at 1× and 1.5×IC50 gave intermediate results (not shown). In HT-29 cells, P-S 1×IC50 increased mitochondria superoxide levels in a time-dependent manner (Fig. 2B). We then evaluated GSH, a major antioxidant system in mammalian cells, levels in response to P-S. Treatment of HT-29 cells with P-S 1×IC50 for 4 h deceased GSH levels by 50% (Fig. 2C). The GSH synthase inhibitor BSO had a similar effect (41% reduction), while pretreatment for 1 h with NAC, largely abrogated P-S’s effect on GSH. Furthermore, P-S induced the oxidized form of Trx-1, a key antioxidant enzyme, most notably between 15 and 60 min (Fig. 2E).
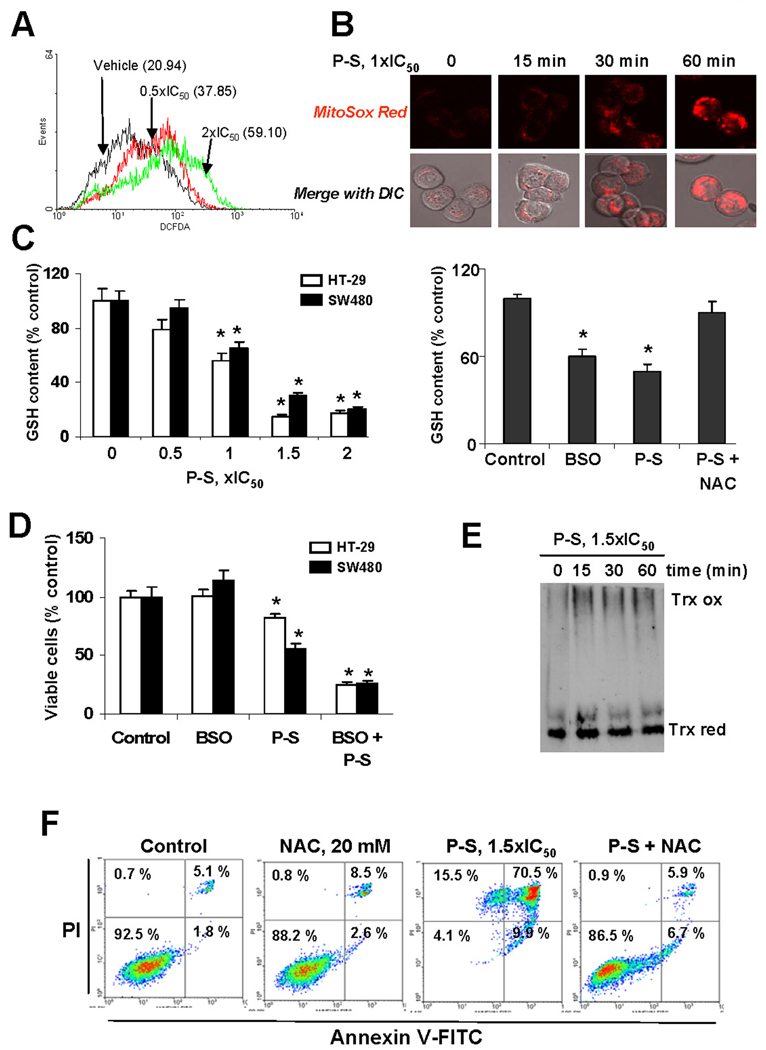
Figure 2. Phospho-sulindac induces RONS in colon cancer cells: Effect on cell growth and apoptosis.
(A) DCFDA fluorescence was measured by flow cytometry in SW480 cells treated with 0.5×IC50 or 2×IC50 P-S for 1 h. (B) P-S induces a time-dependent increase in mitochondrial superoxide levels. HT-29 cells incubated with 1×IC50 P-S for different time periods. After adding the selective MitoSOX probe, cells were subjected to confocal microscopy (×40). (C) P-S reduces GSH content. Left panel: GSH levels were determined in HT-29 cells incubated with various concentrations of P-S (left panel) or with 1×IC50 P-S, BSO, or with NAC and P-S for 4 h (right panel). Values are shown as means ± SEM of 3 independent experiments; *p<0.05 vs. control. (D) HT-29 and SW480 cells were treated with 100 µM BSO followed by treatment with P-S for 24 h. Cell growth was expressed as % control. (E) P-S affects the thioredoxin system. The levels of oxidized (Trx ox) and reduced (Trx red) Trx-1 were detected by native immunoblot in whole-cell lysates from SW480 cells treated with P-S 1.5×IC50 for different time periods. (F) NAC prevents cell death induced by P-S. HT-29 cells were either untreated or pretreated with NAC for 1 h followed by P-S for 24 h. Cell death by apoptosis was determined by flow cytometry.
We next examined whether P-S may stimulate apoptosis by inducing a state of oxidative stress. Pre-treatment with NAC for 1 h, prior to P-S, reduced SW480 cell death by 84% (Fig. 2F). Moreover, GSH depletion induced by BSO enhanced the cell growth-induced inhibition by P-S (Fig 2D). While P-S inhibited the growth of HT-29 and SW480 cells (IC50 >80 µM under this experimental protocol), pretreatment with 100 µM BSO for 24 h reduced the IC50 to 25 and 26 µM for HT-29 and SW480 cells, respectively (Fig. 2D). Overall, these findings indicate that RONS decide the fate of colon cancer cells in response to P-S.
P-S reduces the level of polyamines by inducing SAT1 activity in colon cancer cells
Sulindac is known to reduce polyamine levels in colon cancer cells. Thus, we evaluated whether P-S could have a similar effect. Treatment of HT-29 or SW480 cells with 1×IC50 P-S for 24 h markedly diminished the levels of spermidine and spermine, without affecting putrescine levels (Fig. 3A).
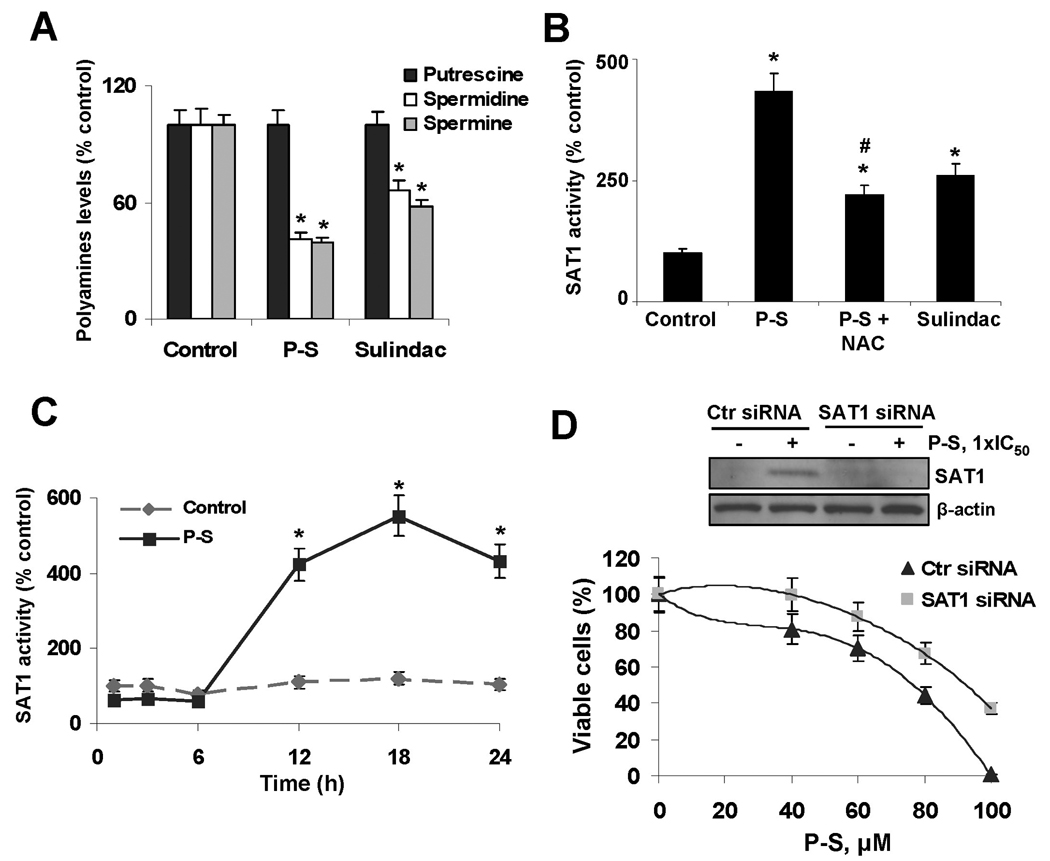
Figure 3. Phospho-sulindac reduces polyamines levels by inducing SAT1 in colon cancer cells.
(A) Polyamine levels in SW480 cells treated with P-S 80 µM or sulindac 800 µM for 24 h. *p<0.05 vs. control. (B) P-S induces SAT1 enzymatic activity. SW480 cells were incubated with P-S, or NAC and P-S or sulindac for 24 h. *p<0.05 vs. control; #p<0.05 vs. P-S. (C) Time dependency on the effect of P-S on SAT1 activity. *p<0.05 vs. control. (D) Silencing SAT1 attenuates P-S-induced cell growth reduction. SW480 cells were transfected with either control or SAT1 siRNA. After transfection, cells were treated with various concentrations of P-S for 24 h and cell growth was evaluated; *p<0.05 vs. control, (bottom panel). After transfection a subset of cells were treated with 1×IC50 P-S for 24 h. Immunoblots to verify SAT1 silencing were performed on whole cell extracts obtained from these cells (top panel).
We examined the effect of P-S on SAT1 activity, an enzyme known to be induced by sulindac.12 While incubation for 24 h with 85 µM P-S increased SAT1 activity by 3- and 4.4-fold (P<0.05 versus control), 800 µM sulindac increased it by 1.5 and 2.5-fold (P<0.05 versus control) in HT-29 and SW480 cells, respectively (Fig. 3B). Since RONS can modulate SAT1 activity,10 we tested whether NAC could prevent the induction of SAT1 activity by P-S. Indeed, NAC 20 mM prevented, in part, the effect of P-S on SAT1, suggesting a redox-dependent effect (Fig. 3B). Of note, the effect of P-S on SAT1 was time-dependent. While no difference in SAT1 activity was observed during the first 6 h of treatment with P-S, after 12 h this activity was increased 4-fold compared to controls and continued to a maximum of 5.5-fold increase after 18 h (Fig. 3C).
To evaluate the role of SAT1 in P-S-induced cell growth inhibition, we silenced SAT1. Compared to controls, knocking-down sat1 rendered SW480 cells resistance to the growth inhibitory effect of P-S. While cells transfected with nonspecific siRNA required 75 µM P-S to decrease their number of viable cells by half, cells transfected with siRNA against sat1 required 93 µM P-S (24% more) for the same effect (Fig. 3D).
P-S modulates redox-sensitive signaling molecules
NF-κB
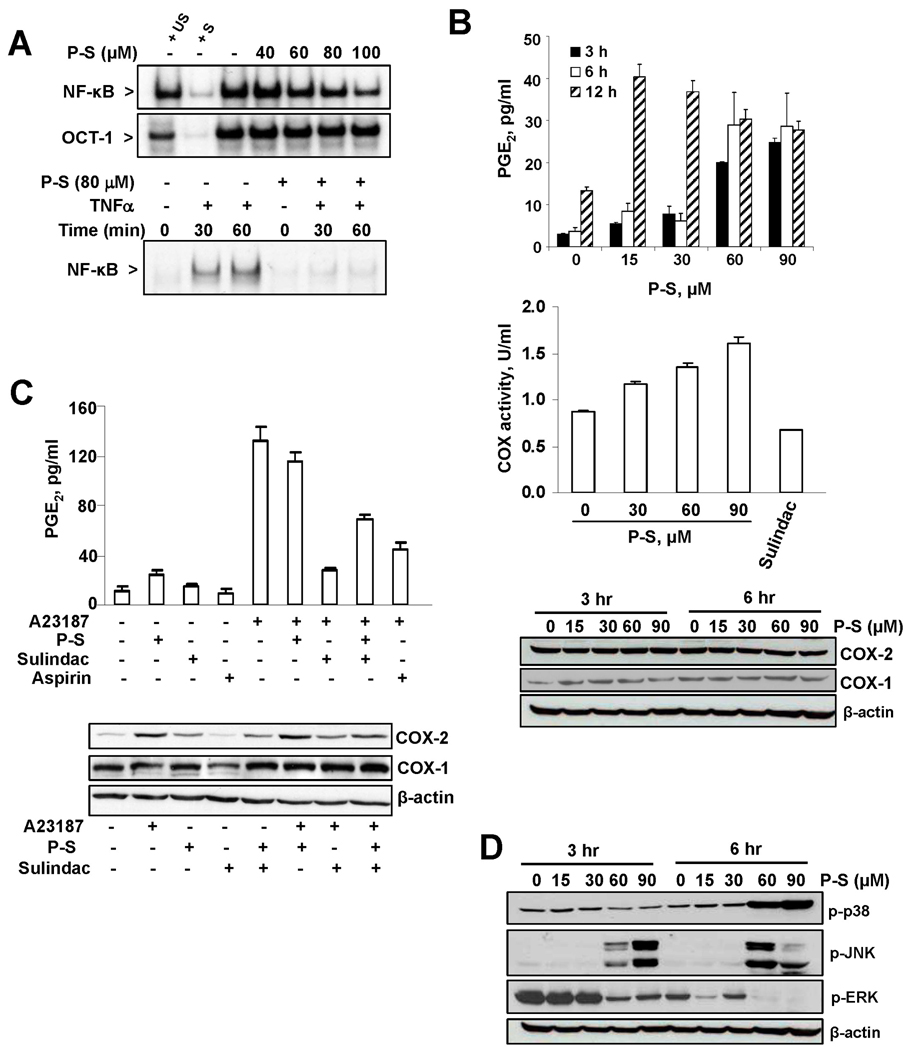
A redox-sensitive dimer, NF-κB modulates cell growth and inflammation, especially in cancer.13 Treatment of HT-29 cells with P-S suppressed NF-κB activation in a concentration-dependent manner (Fig. 4A). Moreover, while TNF-α rapidly activated NF-κB, a 4 h pre-incubation with P-S abrogated this effect.
Figure 4. Phospho-sulindac modulates redox-sensitive signaling pathways in colon cancer cells.
(A) P-S inhibits constitutive and TNF-α-induced NF-κB activation. Upper panel: EMSA for NF-κB and OCT-1 of nuclear fractions isolated from HT-29 cells after 4 h-treatment without or with 40–100 µM P-S. To determine the specificity of each transcription factor-DNA complex, the control nuclear fraction (−) was incubated in the presence of 100-fold molar excess of unlabeled oligonucleotide containing the consensus sequence for either the specific (+ S) or an unspecific (+ US) transcription factor. Lower panel: EMSA for NF-κB of nuclear fractions isolated, after 4 h of preincubation without or with P-S and further 0, 30 or 60 min incubation without (−) or with 10 ng/ml TNFα. n.s.: non specific. (B) P-S increased PGE2 levels, measured by ELISA, in a time- and concentration-dependent manner; and upregulated COX-2 activity, measured by ELISA after a 6-h incubation with P-S or 1.2 mM sulindac. COX-2 and COX-1 expression, by immunoblot in total fractions isolated from HT-29 cells treated with P-S, were unaffected. (C) P-S did not prevent the increase in PGE2 levels induced by A23187. HT-29 cells were pre-treated with P-S 1×IC50, sulindac 1.2 mM or aspirin 0.4 mM for 30 min followed by A23187 5 µM for 3 h. PGE2 levels, measured by ELISA, COX-2 and COX-1 expression by Immunoblots, are shown. (D) P-S modulates MAPKs. Levels of phosphorylated p38, JNK and ERK (p-p38, p-JNK and p-ERK, respectively) were measured by immunoblot in total fractions isolated from HT-29 cells treated with P-S. β-actin levels are shown as loading controls.
Eicosanoids
COX, considered important in cancer, is inhibited by sulindac. We evaluated the effect of P-S on the COX/PGE2 cascade. In HT-29 cells, P-S left unaltered the expression of COX-2 and COX-1, but increased COX activity, leading to significantly increased PGE2 production (Fig. 4B). The COX-2 inhibitors sulindac and aspirin abrogated the increase in PGE2 levels by the calcium ionophore A23187, but P-S failed to prevent it (Fig. 4C), suggesting that it lacks an anti-COX effect. P-S plus sulindac suppressed COX-2 expression and PGE2 levels, although less than sulindac alone.
MAPKs
MAPKs are important mediators of intracellular signaling, whereas p38 and JNK, two of the major MAPKs, are redox-dependent.14 In HT-29 cells, P-S upregulated phosphorylated p38 (p-p38) and JNK (p-JNK). P-S 15 or 30 µM failed to induce formation of p-JNK, but at 60 and 90 µM it greatly increased p-JNK formation at 3 and 6 h (Fig. 4D). In the case of p38, 60 and 90 µM of P-S markedly stimulated its phosphorylation, which became evident at 6 h. Finally, P-S clearly decreased ERK1/2 phosphorylation, which is important in cell survival.15
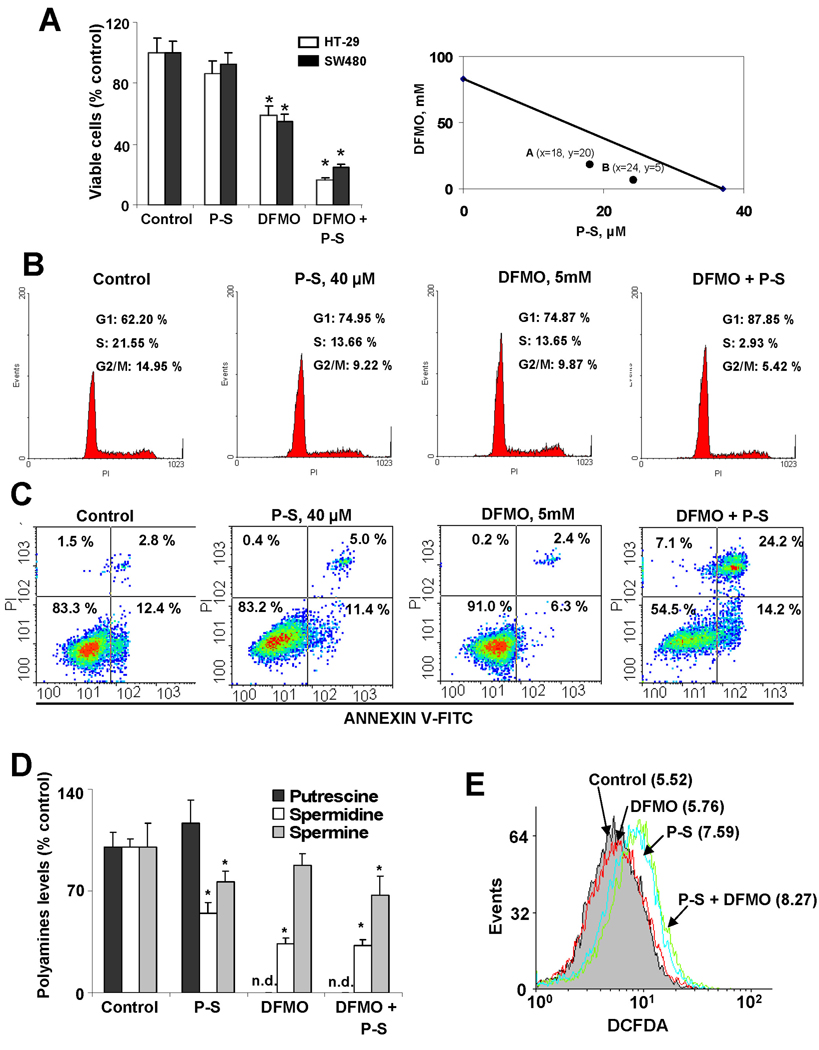
DFMO synergizes with P-S to inhibit colon cancer cell growth
By analogy with sulindac, which synergizes with DFMO to prevent colon cancer,3 we expected synergy between DFMO and P-S. At 48 h, DFMO 5 mM and P-S 40 µM each alone modestly inhibited cell growth, but their combination was more effective than the sum of the two. The reductions in cell number were: a) in HT-29 cells: DFMO 14%, P-S 41%, both 84%; and b) in SW480 cells: DFMO 8%, P-S 45%, both 75%. The isobologram confirms as clear-cut the pharmacological synergy between the two agents (Fig. 5A).
Figure 5. DFMO enhances phospho-sulindac-induced inhibition of colon cancer cell growth.
(A) Left panel: Cell viability was determined in HT-29 and SW-480 cells after 48 h of incubation with 5 mM DFMO, 40 µM P-S or both. Results are expressed as % control; *p<0.02 vs. control. Right panel: In this isobologram the additivity line connects the IC50 value of each compound used alone. A and B represent two different dose pairs of each compound (their respective concentrations are shown in parentheses). The location of both A and B below the additivity line signifies synergy. (B) Cell cycle progression in HT-29 cells incubated for 48 h as indicated. Representative profiles of the distribution of cells in G1, G2/M and S phases are shown. (C) The percentages of apoptotic cells determined by flow cytometry using the dual staining (Annexin V and PI) are indicated in each quadrant. Representative images are shown. (D) Polyamines levels in SW480 cells treated with P-S 1×IC50, DFMO 5 mM or a combination of both for 24 h; *p<0.05 vs. control; n.d: non detected. (E) DCFDA fluorescence was measured by flow cytometry in SW480 cells treated with P-S 1×IC50, DFMO 5mM or both for 1 h.
DFMO and P-S also synergize to inhibit cell cycle phase transitions. For example, the proportion of HT-29 cells in S phase was 13.6% for either alone and was reduced to 2.9% for both (Fig. 5B). A similar synergistic effect was observed in the induction of apoptosis. After 48 h of incubation with DFMO and P-S, the percentage of apoptotic cells was 38.4%, compared to 8.7% and 16.5% for DFMO and P-S alone, respectively (Fig. 5C). Of note, the concentrations of both compounds were below their IC50s for cell growth.
To elucidate the mechanism of the combination, we measured polyamines levels. As expected, P-S decreased spermidine and spermine, while DFMO completely abrogated putrescine levels. Combination of P-S and DFMO decreased the levels of the three polyamines measured (Fig. 5D). We also evaluated if DFMO could enhance the increase in RONS levels induced by P-S. Although P-S increased RONS levels after 1 h-incubation, co-treatment with DFMO did not additionally increase RONS levels in SW480 cells (Fig. 5E).
P-S shows no genotoxicity and no gastrotoxicity in rats and mice
We evaluated the safety of P-S by examining its genotoxicity by the Ames test and its gastrointestinal and other toxicity in mice and rats and compared it to sulindac.
Genotoxicity
The mutagenic potential of P-S was evaluated by measuring its ability to induce reverse mutations at selected loci of two strains of Salmonella typhimurium in the presence and absence of S9 activation (performed by BioReliance, Rockville MD). All these studies were negative for genotoxicity.
Acute toxicity
Mice were treated for 5 days by oral gavage with equimolar amounts of P-S (317 mg/kg/d) or sulindac (200 mg/kg/day) or vehicle. Mice surviving to the end of the study were euthanized. P-S- and vehicle-treated mice a) maintained their weight (P-S = 16.3±1.2 → 15.7±1.2; vehicle = 16.1±1.0 g → 15.7±1.2, mean±SD); b) showed no evidence of gastrointestinal or other toxicity; c) all were alive and healthy at the conclusion of the study; and d) inspection of the heart, lungs, spleen, kidneys and liver showed no abnormalities. In contrast, sulindac-treated mice a) lost 20% of their weight (16.3±1.2 g → 13.0±0.5 g; mean±SD); b) showed significant mortality: 75% vs. 0% for P-S and vehicle (5 of the 8 mice died:1 on day 2; 2 on day 3; 2 on day 4; and 1 on day 5), and c) necropsies revealed upper gastrointestinal toxicity with macroscopically evident gastric ulcers in 3, gastric bleeding in 1, and perforation in 1. The stomachs of sulindac-treated animals were larger than those of the other two groups and in some the liver appeared hyperemic.
Gastrointestinal toxicity
Rats were treated for 4 days by oral gavage with equimolar amounts of P-S (317 mg/kg/d) or sulindac (200 mg/kg/day), indomethacin (4.75 mg/kg/d) or vehicle. As expected,11 treatment with indomethacin produced predominantly medium and large ulcerations (>4 mm) in the small intestine generating to a clinical score of 3.8. Vehicle- and P-S-treated rats showed no gastrointestinal toxicity, with no signs of ulcerations or mucosal damage (Fig. 6A). In contrast to P-S and similar to indomethacin, medium and large ulcerations in the small intestine were observed in the sulindac-treated rats.
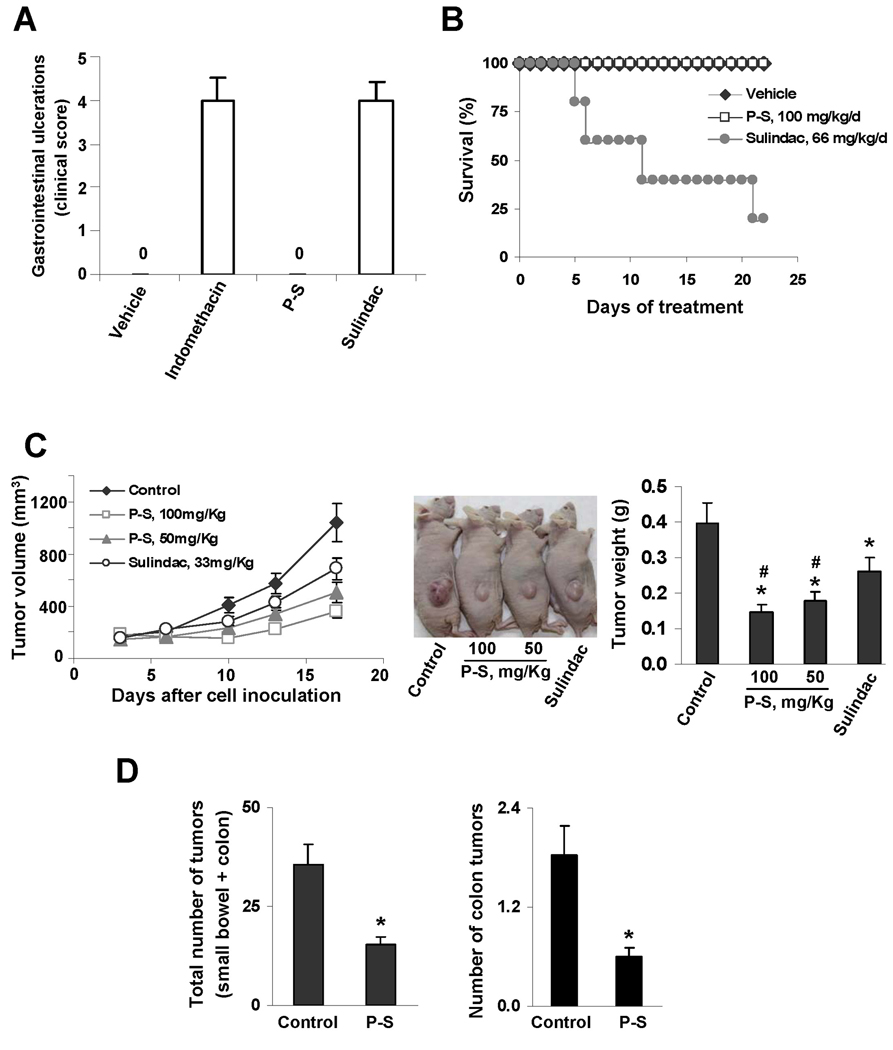
Figure 6. Phospho-sulindac is a safe and effective antitumor agent against colon cancer in vivo.
(A) Acute gastrointestinal toxicity of sulindac and P-S. Rats were treated with P-S or sulindac or indomethacin or vehicle, as in Methods. At day 5, the number and sizes of small intestine ulcerations were counted and scored according to the protocol.11 P-S-treated rats showed no gastrointestinal toxicity. (B) Survival curve for mice treated daily for three weeks with equimolar doses of P-S or sulindac. (C) Effect of P-S and sulindac on colon cancer xenografts in nude mice. Left panel: Tumor volume growth over time for control, P-S 50 mg/kg/d, P-S 100 mg/kg/d, and sulindac 33 mg/kg/d treated mice. Center panel: The difference in tumor size among the groups is apparent. Right panel: Mass of the dissected tumors. All values: mean±SEM, *p<0.05 vs. vehicle-treated mice; #p<0.05 vs. sulindac-treated mice. (D) Effect of P-S on tumor multiplicity in ApcMin/+ mice. The total number of tumors per animal in both small and large bowel (left panel) as well as those only in colon (right panel) was greatly reduced following 4 wks of treatment with P-S; *p<0.02 vs. control mice.
Survival of mice and organ toxicity
Mice were treated for 3 weeks by oral gavage with vehicle, P-S (100 mg/kg/d), or sulindac 66 mg/kg/d (equimolar) or 100 mg/kg/d (equi-dose). All vehicle- and P-S-treated mice were alive and healthy by the end of the study. However, 80% of the sulindac 66 mg/kg/d-treated mice died by 3 weeks (Fig. 6B), and all sulindac 100 mg/kg/d-treated mice died after seven days of treatment (Supplemental Fig. 1). Liver function tests (AST, ALT, alkaline phosphatase, GGTP and total bilirubin) were similar between P-S- and vehicle-treated mice. Finally, histologically the liver, pancreas and kidneys of mice treated with P-S showed no signs of toxicity (Supplemental Fig. 2).
P-S alone and in combination with DFMO inhibits colon carcinogenesis in vivo
We evaluated the chemotherapeutic potential of P-S and sulindac in a subcutaneous xenograft model of HT-29 cells. At sacrifice, while P-S (50 or 100 mg/kg/d by gavage) reduced tumor growth by 51 and 65%, respectively, compared to controls; sulindac (33 mg/kg/d, equimolar to P-S 50 mg/kg/d) reduced tumor growth by 34%. At both doses P-S reduced tumor growth significantly more than sulindac (p<0.05; Fig. 6C). In ApcMin/+ mice (predisposed to develop tumors in the small intestine and colon), P-S 100 mg/kg/d by gavage for 4 weeks decreased the number of tumors in the small intestine by 57.2% compared to controls (p<0.002); in the colon such reduction was 61.8 % (p<0.02; Fig. 6D). In both studies P-S was well tolerated with no weight loss during treatment.
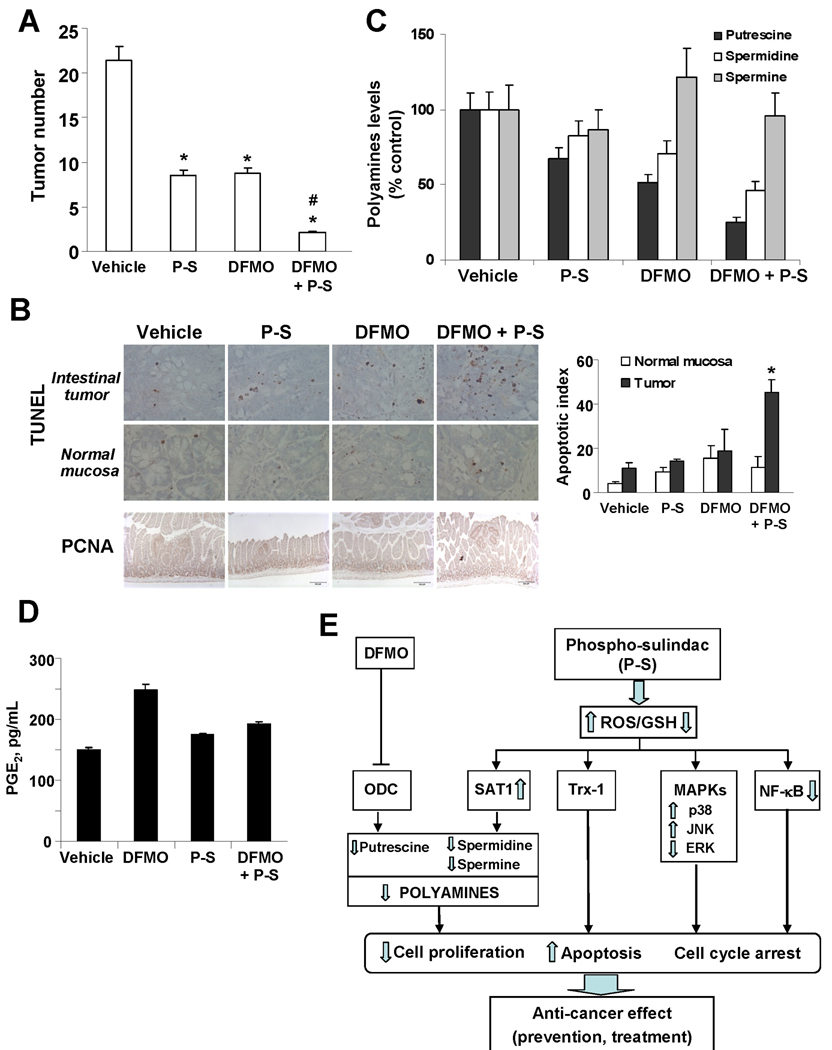
In another study, P-S (100 mg/kg by gavage) and DFMO (2% in drinking water) were administered alone and combined to ApcMin/+ mice between 6 and 13 weeks of age. DFMO and P-S alone reduced the number of all intestinal tumors by 50.8% and 52.1%, respectively, but their combination reduced it by 90.2% (p < 0.0001; Fig. 7A). P-S plus DFMO combination achieved this effect by inhibiting cell proliferation and inducing apoptosis (Fig. 7B). Interestingly, compared to the vehicle-treated group, P-S plus DFMO selectively induced apoptosis in the intestinal tumors but not in the normal mucosa (Fig. 7B). Mechanistically, this combination reduced total intestinal polyamine levels (Fig. 7C) but did not alter PGE2 tissue levels among the various groups (Fig. 7D).
Figure 7. Phospho-sulindac in combination with DFMO inhibits tumor multiplicity in ApcMin/+ mice.
(A) The number of tumors per animal was greatly reduced following 9 weeks of treatment with P-S, DFMO or both compared to controls, *p<0.01 vs. controls; #p<0.01 vs. P-S or DFMO-treated groups. (B) Combination between DFMO and P-S inhibited proliferation and selectively increased apoptosis in intestinal tumors. Intestinal tissue sections from ApcMin/+ mice treated without (vehicle) or with P-S, DFMO or both, were stained for PCNA expression as a proliferation marker (×10) or by the TUNEL method as an apoptosis marker (40×). Right panel: A total of 10 fields from tumor and normal mucosa for each group were examined and counted (n=5/group). Results are expressed as the Apoptotic index (TUNEL+ cells ± SEM per 40× field); *p<0.01 vs. control. (C) Polyamine content in small intestine of ApcMin/+ mice treated with P-S, DFMO or both. (D) Intestinal PGE2 levels of ApcMin/+ mice treated with P-S, DFMO or both, measured by ELISA, remain unchanged among the various groups. (E) Scheme illustrating the target pathways by which P-S alone, or in combination with DFMO, leads to colon cancer prevention.
DISCUSSION
The successful pharmacological agent against cancer must be effective and lack significant side effects. Here we demonstrate that P-S seems to meet both these requirements, markedly inhibiting intestinal tumors by itself and to a greater extent in combination with DFMO and appearing much safer than sulindac in preclinical studies.
P-S is a potent inhibitor of colon cancer cell lines (>14-fold more potent than sulindac) and exerts a profound inhibitory effect in preclinical models of colon cancer. While sulindac reduced the volume of colon xenografts by 34%, P-S at an equimolar dose reduced it by 51%, and by 65% at a higher dose. Furthermore, P-S reduced the number of all intestinal tumors in ApcMin/+ mice by 57.2%. Importantly, in contrast to sulindac, which stimulates tumor formation in the colon of ApcMin/+ mice,16 P-S reduced the number of colon tumors by 61.8%. This inhibitory effect of P-S appears to be caused by a triple cell kinetic effect: inhibition of proliferation, induction of apoptosis and necrosis, and block at the G1/S cell cycle transition; the proapoptotic effect being the dominant. Of note, a normal human colon epithelial cell line was resistant to P-S, indicating selectivity between normal and transformed colon cells. DFMO synergized with P-S In vivo, reducing the number of tumors in ApcMin/+ mice by 90.2%; their cytokinetic effect was synergistic, and apoptosis was induced selectively in the intestinal tumors and not in the normal mucosa. These results indicate that P-S ± DFMO are effective and act selectively against intestinal tumors.
Our work unraveled key parts of the mechanism of action of P-S (Fig. 7E). A major event is the induction of oxidative stress, followed by the activation of signaling cascades, involving polyamines, the thioredoxin system, MAPKs and NF-κB. The centrality of the oxidative stress (elevated RONS, suppressed GSH and increased oxidized Trx-1 levels) was underscored by manipulating the system: the antioxidant NAC greatly attenuated the apoptotic effect of P-S and decreased the activity of SAT1, the enzyme that acetylates and exports polyamines from the cells; BSO, which depletes intracellular GSH, enhanced its growth inhibitory effect; and as previously shown, knocking-down the expression of trx-1 rescued cells from the proapoptotic effect of P-S.6 These effects, combined with the inhibition of ODC by DFMO, culminates in the profound cytokinetic effect manifested as dramatic colon cancer prevention.
The inhibition of NF-κB activation by P-S and changes in three major MAPKs’ branches (activation of p38 and JNK and suppression of ERK1/2) are consistent with the growth inhibitory effect of P-S and likely mediate part of it. On the other hand, the expression of COX-2 and COX-1 were not affected by P-S; however, COX activity and PGE2 levels increased in vitro and remained unchanged in vivo. In contrast to sulindac, P-S did not inhibit COX-2 expression or PGE2 levels in response to the calcium ionophore A23287. Although sulindac inhibits COX, it is apparent that modifying it at its carboxylic group to generate P-S abrogates its ability to inhibit COX. This is in agreement with a recent study that confirms the crucial role of sulindac’s carboxylic moiety for COX-2 binding.5 Indeed, sulindac’s complex mode of action includes COX-dependent and -independent effects.
Increased polyamine levels have long been associated with colon carcinogenesis and are considered targets of both DFMO and NSAIDs, including sulindac.12 Sulindac stimulates polyamine acetylation and export by increasing SAT1 activity.12 Similar to sulindac, P-S per se reduced the levels of spermidine and spermine by inducing SAT1 activity. Indeed, SAT1 is responsible, in part, for the cell growth inhibitory effect of P-S; knocking-down sat1 expression rescued cell growth inhibition by P-S. The synergy of P-S and DFMO in suppressing polyamine levels is apparent both in vitro and in vivo.
The safety of an agent is of extreme importance. Although NSAIDs, including sulindac, are established as prototypical colon chemopreventive agents,17 their extended use in chemoprevention is limited because of significant toxicity, mostly gastrointestinal.4 Unlike sulindac, the novel P-S is a safer chemopreventive agent based on the lack of genotoxicity, and the absence of gastrointestinal toxicity in rats and mice.
In summary, our data indicate that the novel drug P-S is a safe and effective drug in preclinical models, two essential pharmacological properties for any candidate chemopreventive agent. Therefore, P-S merits further evaluation as a potentially chemopreventive agent against colon cancer.
Supplementary Material
Acknowledgments
Grant support: R01-CA139453; N01-CN-43302 WA#7
Abbreviations
- BSO
buthionine sulfoximine
- COX
cyclooxygenase
- DFMO
difluoromethylornithine
- EMSA
electrophoretic mobility shift assay
- NAC
N-acetyl-L-cysteine
- PI
propidium iodide
- P-S
phospho-sulindac
- RONS
reactive oxygen and nitrogen species
- SAT1
spermidine/spermine-N1-acetyltransferase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors have nothing to disclose except for BR, who has an equity position in Medicon, Inc.
Participation of each author in the study:
GGM: Study concept and design, acquisition of data, analysis and interpretation of data, drafting the manuscript.
YS, LH, GX and NO: acquisition of data, analysis and interpretation of data
RCG and FJ: OXT-328 synthesis, analysis and interpretation of data.
LK and DK: Participation in study design, data analysis and critical revision of the manuscript for important intellectual content.
BR: Study concept and design, analysis and interpretation of data, drafting the manuscript, study supervision.
REFERENCES
- 1.Beazer-Barclay Y, Levy DB, Moser AR, Dove WF, Hamilton SR, Vogelstein B, Kinzler KW. Sulindac suppresses tumorigenesis in the Min mouse. Carcinogenesis. 1996;17:1757–1760. doi: 10.1093/carcin/17.8.1757. [DOI] [PubMed] [Google Scholar]
- 2.Gerner EW, Meyskens FL., Jr Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer. 2004;4:781–792. doi: 10.1038/nrc1454. [DOI] [PubMed] [Google Scholar]
- 3.Meyskens FL, Jr, McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, Kelloff G, Lawson MJ, Kidao J, McCracken J, Albers CG, Ahnen DJ, Turgeon DK, Goldschmid S, Lance P, Hagedorn CH, Gillen DL, Gerner EW. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila Pa) 2008;1:32–38. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts J, II, Morrow J. Analgesic-antipyretic and antiinflammatory agents and drugs employed in the treatment gout. In: Hardman JG, Limbird LE, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York: McGraw-Hill; 2001. pp. 687–731. [Google Scholar]
- 5.Piazza GA, Keeton AB, Tinsley HN, Gary BD, Whitt JD, Mathew B, Thaiparambil J, Coward L, Gorman G, Li Y, Sani B, Hobrath JV, Maxuitenko YY, Reynolds RC. A novel sulindac derivative that does not inhibit cyclooxygenases but potently inhibits colon tumor cell growth and induces apoptosis with antitumor activity. Cancer Prev Res (Phila Pa) 2009;2:572–580. doi: 10.1158/1940-6207.CAPR-09-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun Y, Rigas B. The thioredoxin system mediates redox-induced cell death in human colon cancer cells: implications for the mechanism of action of anticancer agents. Cancer Res. 2008;68:8269–8277. doi: 10.1158/0008-5472.CAN-08-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao W, Mackenzie GG, Murray OT, Zhang Z, Rigas B. Phosphoaspirin (MDC-43), a novel benzyl ester of aspirin, inhibits the growth of human cancer cell lines more potently than aspirin: a redox-dependent effect. Carcinogenesis. 2009;30:512–519. doi: 10.1093/carcin/bgp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao J, Liu X, Rigas B. Nitric oxide-donating aspirin induces apoptosis in human colon cancer cells through induction of oxidative stress. Proc Natl Acad Sci U S A. 2005;102:17207–17212. doi: 10.1073/pnas.0506893102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seiler N, Knodgen B. High-performance liquid chromatographic procedure for the simultaneous determination of the natural polyamines and their monoacetyl derivatives. J Chromatogr. 1980;221:227–235. doi: 10.1016/s0378-4347(00)84307-8. [DOI] [PubMed] [Google Scholar]
- 10.Chopra S, Wallace HM. Induction of spermidine/spermine N1-acetyltransferase in human cancer cells in response to increased production of reactive oxygen species. Biochem Pharmacol. 1998;55:1119–1123. doi: 10.1016/s0006-2952(97)00601-1. [DOI] [PubMed] [Google Scholar]
- 11.Whiteley PE, Dalrymple SA. Models of inflammation: Measuring gastrointestinal ulcerations in the rat. In: Enna SJ, editor. Current Protocols in Pharmacology. Volume 3. New York: John Wiley & Sons, Inc.; 1998. [DOI] [PubMed] [Google Scholar]
- 12.Babbar N, Ignatenko NA, Casero RA, Jr, Gerner EW. Cyclooxygenase-independent induction of apoptosis by sulindac sulfone is mediated by polyamines in colon cancer. J Biol Chem. 2003;278:47762–47775. doi: 10.1074/jbc.M307265200. [DOI] [PubMed] [Google Scholar]
- 13.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 14.Benhar M, Engelberg D, Levitzki A. ROS, stress-activated kinases and stress signaling in cancer. EMBO Rep. 2002;3:420–425. doi: 10.1093/embo-reports/kvf094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balmanno K, Cook SJ. Tumour cell survival signalling by the ERK1/2 pathway. Cell Death Differ. 2009;16:368–377. doi: 10.1038/cdd.2008.148. [DOI] [PubMed] [Google Scholar]
- 16.Yang K, Fan K, Kurihara N, Shinozaki H, Rigas B, Augenlicht L, Kopelovich L, Edelmann W, Kucherlapati R, Lipkin M. Regional response leading to tumorigenesis after sulindac in small and large intestine of mice with Apc mutations. Carcinogenesis. 2003;24:605–611. doi: 10.1093/carcin/24.3.605. [DOI] [PubMed] [Google Scholar]
- 17.Baron JA. What now for aspirin and cancer prevention? J Natl Cancer Inst. 2004;96:4–5. doi: 10.1093/jnci/djh027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.