Abstract
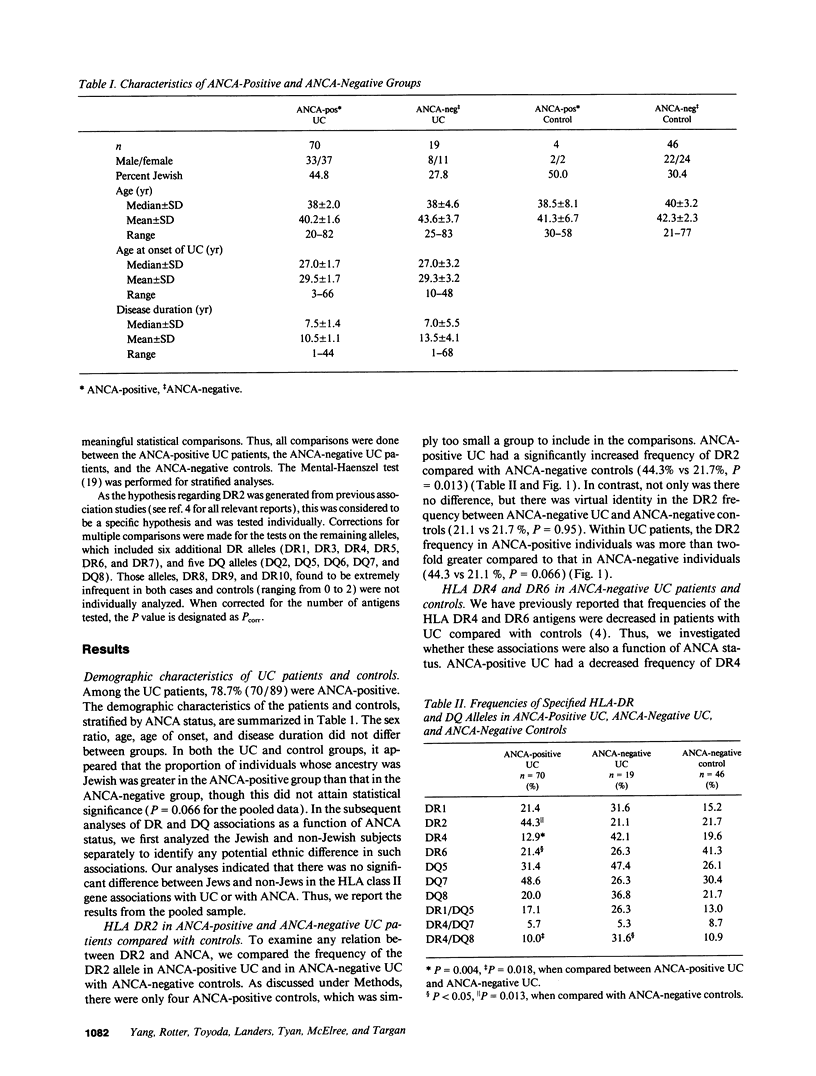
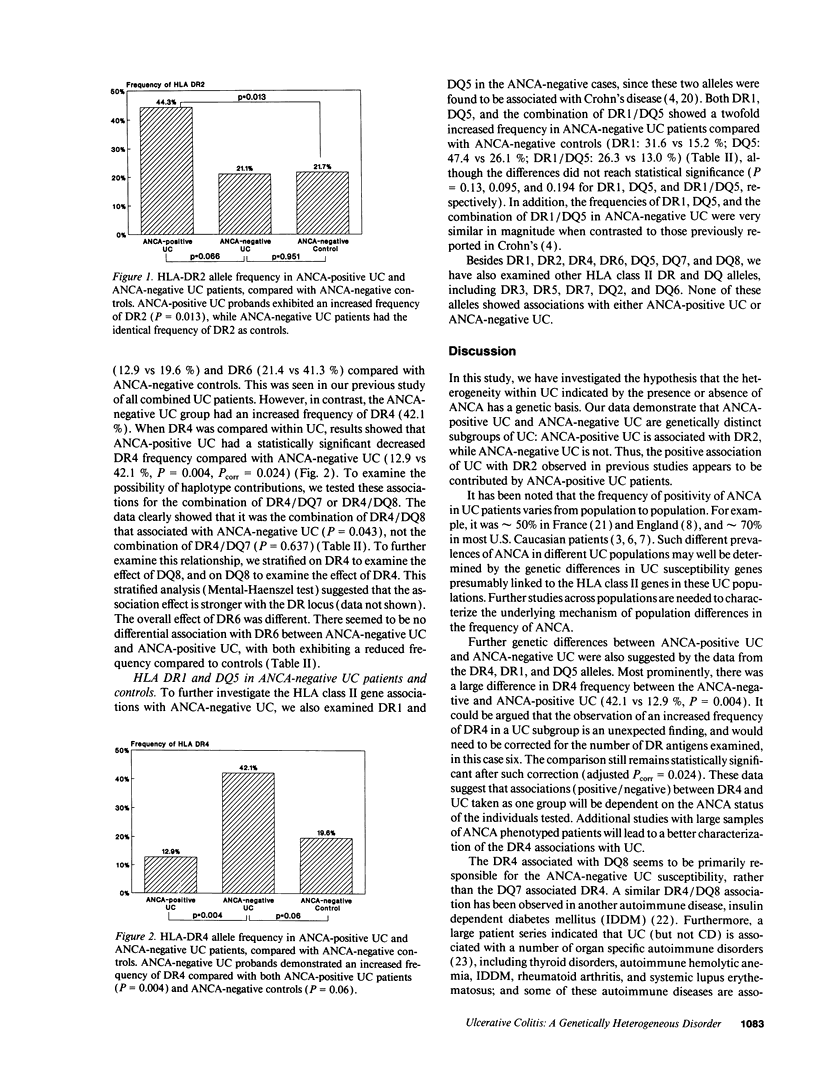
Newly described distinct associations of HLA class II genes with ulcerative colitis (UC) (DR2) and Crohn's disease (CD) (DR1/DQ5) provide strong evidence for genetic heterogeneity of susceptibility between these two forms of inflammatory bowel disease. A familial distribution of antineutrophil cytoplasmic antibodies (ANCAs, a subclinical marker of UC) in UC families has further implied the existence of heterogeneity within UC. To test the hypothesis that the heterogeneity within UC indicated by ANCAs has a genetic basis that resides within the HLA region, we studied 89 UC cases and an ethnically matched control group (n = 50). Serological and molecular typing techniques were applied to define HLA class II genes (DR, DQ). ANCAs were detected using an enzyme-linked immunosorbent assay, and positive values were confirmed by indirect immunofluorescence. We observed that ANCA-positive UC patients (n = 70) had a significantly increased frequency of DR2 compared with ANCA-negative controls (n = 46) (44% vs 22%, P = 0.01). In contrast, the frequency of DR2 in ANCA-negative UC cases (21%) was virtually identical to that in controls (22%, P = 0.9). Furthermore, the ANCA-negative UC patients had an increase in the DR4 allele compared with ANCA-positive UC (P = 0.004). Thus, with the combination of a subclinical marker (ANCAs) and molecular genetic markers, genetic heterogeneity has been demonstrated within UC: ANCA-positive UC associated with DR2, and ANCA-negative UC likely associated with DR4.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cambridge G., Rampton D. S., Stevens T. R., McCarthy D. A., Kamm M., Leaker B. Anti-neutrophil antibodies in inflammatory bowel disease: prevalence and diagnostic role. Gut. 1992 May;33(5):668–674. doi: 10.1136/gut.33.5.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerr R. H., Targan S. R., Landers C. J., Sutherland L. R., Shanahan F. Anti-neutrophil cytoplasmic antibodies in ulcerative colitis. Comparison with other colitides/diarrheal illnesses. Gastroenterology. 1991 Jun;100(6):1590–1596. doi: 10.1016/0016-5085(91)90657-7. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Larhammar D., Schenning L., Gustafsson K., Wiman K., Claesson L., Rask L., Peterson P. A. Complete amino acid sequence of an HLA-DR antigen-like beta chain as predicted from the nucleotide sequence: similarities with immunoglobulins and HLA-A, -B, and -C antigens. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3687–3691. doi: 10.1073/pnas.79.12.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizak G. E., Grumet F. C. A new micromethod for the in vitro detection of antiplatelet antibodies: C-FDA thrombocytotoxicity. Hum Immunol. 1980 Jul;1(1):87–96. doi: 10.1016/0198-8859(80)90012-9. [DOI] [PubMed] [Google Scholar]
- Long E. O., Wake C. T., Gorski J., Mach B. Complete sequence of an HLA-dR beta chain deduced from a cDNA clone and identification of multiple non-allelic DR beta chain genes. EMBO J. 1983;2(3):389–394. doi: 10.1002/j.1460-2075.1983.tb01435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepom G. T., Erlich H. MHC class-II molecules and autoimmunity. Annu Rev Immunol. 1991;9:493–525. doi: 10.1146/annurev.iy.09.040191.002425. [DOI] [PubMed] [Google Scholar]
- Rang E. H., Brooke B. N., Hermon-Taylor J. Association of ulcerative colitis with multiple sclerosis. Lancet. 1982 Sep 4;2(8297):555–555. doi: 10.1016/s0140-6736(82)90629-8. [DOI] [PubMed] [Google Scholar]
- Redford A., Magalong D., Onohara-Toyoda M., Tyan D., Riley W. J., Maclaren N. K., Rotter J. I., Toyoda H. Restriction fragment length polymorphism (RFLP) heterogeneity of HLA-DQ beta genes associated with DNA fragment identical to the DR1-beta DNA structure. Dis Markers. 1991 Sep-Oct;9(5):257–263. [PubMed] [Google Scholar]
- Roth M. P., Petersen G. M., McElree C., Vadheim C. M., Panish J. F., Rotter J. I. Familial empiric risk estimates of inflammatory bowel disease in Ashkenazi Jews. Gastroenterology. 1989 Apr;96(4):1016–1020. doi: 10.1016/0016-5085(89)91618-1. [DOI] [PubMed] [Google Scholar]
- Sadovnick A. D., Paty D. W., Yannakoulias G. Concurrence of multiple sclerosis and inflammatory bowel disease. N Engl J Med. 1989 Sep 14;321(11):762–763. [PubMed] [Google Scholar]
- Saxon A., Shanahan F., Landers C., Ganz T., Targan S. A distinct subset of antineutrophil cytoplasmic antibodies is associated with inflammatory bowel disease. J Allergy Clin Immunol. 1990 Aug;86(2):202–210. doi: 10.1016/s0091-6749(05)80067-3. [DOI] [PubMed] [Google Scholar]
- Seibold F., Weber P., Klein R., Berg P. A., Wiedmann K. H. Clinical significance of antibodies against neutrophils in patients with inflammatory bowel disease and primary sclerosing cholangitis. Gut. 1992 May;33(5):657–662. doi: 10.1136/gut.33.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan F., Duerr R. H., Rotter J. I., Yang H., Sutherland L. R., McElree C., Landers C. J., Targan S. R. Neutrophil autoantibodies in ulcerative colitis: familial aggregation and genetic heterogeneity. Gastroenterology. 1992 Aug;103(2):456–461. doi: 10.1016/0016-5085(92)90834-l. [DOI] [PubMed] [Google Scholar]
- Snook J. A., de Silva H. J., Jewell D. P. The association of autoimmune disorders with inflammatory bowel disease. Q J Med. 1989 Sep;72(269):835–840. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Toyoda H. Long synthetic oligonucleotide probes for gene analysis. Methods Enzymol. 1992;216:108–115. doi: 10.1016/0076-6879(92)16013-a. [DOI] [PubMed] [Google Scholar]
- Toyoda H., Wang S. J., Yang H. Y., Redford A., Magalong D., Tyan D., McElree C. K., Pressman S. R., Shanahan F., Targan S. R. Distinct associations of HLA class II genes with inflammatory bowel disease. Gastroenterology. 1993 Mar;104(3):741–748. doi: 10.1016/0016-5085(93)91009-7. [DOI] [PubMed] [Google Scholar]
- van der Woude F. J., Rasmussen N., Lobatto S., Wiik A., Permin H., van Es L. A., van der Giessen M., van der Hem G. K., The T. H. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener's granulomatosis. Lancet. 1985 Feb 23;1(8426):425–429. doi: 10.1016/s0140-6736(85)91147-x. [DOI] [PubMed] [Google Scholar]



