Abstract
Cardiac glycosides, which inhibit the plasma membrane Na+ pump, are one of the four categories of drug recommended for routine use to treat heart failure, yet their therapeutic window is limited by toxic effects. Elevated cytoplasmic Na+ ([Na+]i) compromises mitochondrial energetics and redox balance by blunting mitochondrial Ca2+ ([Ca2+]m) accumulation, and this impairment can be prevented by enhancing [Ca2+]m. Here, we investigate whether this effect underlies the toxicity and arrhythmogenic effects of cardiac glycosides and if these effects can be prevented by suppressing mitochondrial Ca2+ efflux, via inhibition of the mitochondrial Na+/Ca2+ exchanger (mNCE). In isolated cardiomyocytes, ouabain elevated [Na+]i in a dose-dependent way, blunted [Ca2+]m accumulation, decreased the NADH/NAD+ redox potential, and increased reactive oxygen species (ROS). Concomitant treatment with the mNCE inhibitor CGP-37157 ameliorated these effects. CGP-37157 also attenuated ouabain-induced cellular Ca2+ overload and prevented delayed afterdepolarizations (DADs). In isolated perfused hearts, ouabain’s positive effects on contractility and respiration were markedly potentiated by CGP-37157, as were those mediated by β-adrenergic stimulation. Furthermore, CGP-37157 inhibited the arrhythmogenic effects of ouabain in both isolated perfused heart and in vivo. The findings reveal the mechanism behind cardiac glycoside toxicity and show that improving mitochondrial Ca2+ retention by mNCE inhibition can mitigate these effects, particularly with respect to the suppression of Ca2+-triggered arrhythmias, while enhancing positive inotropic actions. These results suggest a novel strategy for the treatment of heart failure.
Keywords: Cardiac glycosides, energy metabolism, Na+/Ca2+ exchanger, ion transport, heart failure, arrhythmias
Introduction
Cardiac glycosides have been widely used in the treatment of heart failure (HF) for more than 200 years, and they are one of the 4 categories of drug that are recommended for routine use to treat HF by The American College of Cardiology/American Heart Association Joint Guidelines[1]. Treatment with digitalis glycosides can improve HF symptoms, increase cardiac output, enhance quality of life, and decrease clinical decompensation and hospitalization, however, the digitalis toxicity-related death in HF patients especially in the subgroup with high serum digoxin concentration undermines the beneficial effect of digitalis treatment on total mortality rates (reviewed in [2]).
The primary action of cardiac glycosides is their ability to inhibit Na+/K+-ATPase (NKA), which elicits multiple effects on cardiac physiology and pathology (reviewed in[3]). Inhibition of NKA on the sarcolemma of cardiac myocytes has a positive inotropic effect, mediated by an elevation of intracellular Na+ ([Na+]i). Elevated [Na+]i increases sarcoplasmic reticulum (SR) Ca2+ load by affecting the activity of the sodium/calcium exchanger (NCX) on the sarcolemma, as a consequence of a reduction of the driving force for Ca2+ extrusion and/or an increase in Ca2+ influx via the NCX. The resulting increase of SR Ca2+ load is responsible not only for the inotropic effect, but also for the arrhythmogenic effects of glycosides; the major adverse effect of digitalis drugs. Besides its inotropic effect, clinical trials have shown that digitalis glycosides could also reduce plasma norepinephrine levels[4–6], serum aldosterone[4, 6, 7], and plasma renin activity [4, 7] in patients with HF. The beneficial effects of glycosides on HF are probably attributable to both their inotropic and neurohormonal effects. The adverse effects of glycoside have also been well documented, which include cardiac arrhythmias, gastrointestinal symptoms, and central nervous system abnormalities[1].
Our recent studies have led us to speculate that glycosides might impair mitochondrial energetics in cardiac myocytes due to elevated [Na+]i [8, 9]. Mitochondrial Ca2+ ([Ca2+]m) homeostasis plays a central role in energy supply and demand matching. Increased cardiac work leads to an increase in [Ca2+]m accumulation[8], which is critical for maintaining NADH/NAD+ redox potential[8, 9] by activating several enzymes in the tricarboxylic acid cycle[10]. Elevated [Na+]i blunts [Ca2+]m accumulation by activating the mitochondrial Na+/Ca2+ exchanger (mNCE), the major [Ca2+]m efflux pathway, and therefore it mediates net oxidation of NADH during increased work. Our recent studies have demonstrated this adverse effect of elevated [Na+]i on mitochondrial energetics in normal cardiac myocytes, with [Na+]i elevated artificially using the patch clamp technique, and in myocytes isolated from failing hearts, with a chronic pathological elevation of [Na+]i[8, 9]. Our previous studies also showed the potential therapeutic effect of CGP-37157, an inhibitor of mNCE. CGP-37157 enhances [Ca2+]m accumulation and restores mitochondrial NADH production in cells with elevated [Na+]i[9]. Mitochondria have several functions beyond ATP production, including acting as an intracellular Ca2+ buffering system, and we have shown that inhibition of [Ca2+]m efflux by CGP-37157 increases mitochondrial Ca2+ retention capacity and consequently decreases cytosolic Ca2+ ([Ca2+]c) cycling[8]: this effect might also attenuate glycoside-induced SR Ca2+ overload and arrhythmias.
In the present study, we investigated whether ouabain has adverse effects on mitochondrial energetics, redox status, and ROS balance, as well as whether CGP-37157 can prevent ouabain-induced mitochondrial dysfunction. Moreover, we also studied the role of mitochondrial dysfunction in ouabain toxicity by assessing the effects of CGP-37157 on ouabain-induced irregular [Ca2+]c cycling in isolated myocytes and on ouabain-induced arrhythmias in isolated perfused hearts. Finally, we show that CGP-37157 treatment has a beneficial effect to decrease in vivo arrhythmias in ouabain-treated guinea pigs.
Materials and Methods
Animal
250–300g Hartley guinea pigs were obtained from Hill Top and housed in an animal facility at the Johns Hopkins University. This study conforms to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85-23, revised 1996) and was approved by the Johns Hopkins Animal Care and Use Committee.
Cell isolation
Guinea pig ventricular myocytes were isolated by enzymatic digestion as described previously[11]. Cells were suspended in Dulbecco’s Modification of Eagle’s Medium (DMEM) supplemented with 5% fetal bovine serum, 1% penicillin/streptomycin, and 15 mmol/L HEPES, pH 7.4, and stored in a 5% CO2 incubator at 37°C.
Fluorescence recording
Cytosolic Ca2+ ([Ca2+]c), mitochondrial Ca2+ ([Ca2+]m), and [Na+]i were measured with the fluorescent indicators indo-1, rhod-2, and SBFI, respectively (Invitrogen), and mitochondrial NADH was measured as the cellular autofluorescence with 360nm excitation/450nm emission. Indo-1 was loaded into cell with the membrane permeable form, indo-1 AM. To minimize indo-1 loading into the mitochondrial compartment, cells were briefly incubated with indo-1 AM at room temperature for 20 min. Rhod-2 and SBFI experiments were carried out as previously described[8, 9]. Cytoplasmic rhod-2 was eliminated by using rhod-2-free patch pipettes[8, 9]. The ROS-sensitive fluorescent probe 5-(–6)-chloromethyl-2’,7’-dichlorohydrofluorescein diacetate (CM-H2DCFDA) was used to monitor the extent of oxidative stress, as previously described [12]. This probe is primarily localized in the mitochondrial matrix[12] and is oxidized by hydrogen peroxide to yield fluorescent CM-DCF, thus indirectly reporting mitochondrial superoxide production.
Protocol for experiments in isolated myocytes
Isolated ventricular myocytes were loaded into a heated field-stimulation chamber (Warner Instruments, Hamden CT) at 37°C on the stage of a fluorescence microscope (Nikon Eclipse TE300). The myocytes were first superfused with control Tyrode’s solution containing (in mmol/L): NaCl 130, KCl 5, MgCl2 1, Na-HEPES 10, CaCl2 2, glucose 10, pH 7.4. After 1min recording in the absence of electrical stimulation in control buffer, 1 µM ouabain and 100nM isoproterenol with or without 1 µM CGP-37157 were added to buffer. After another 5 min of recording, myocytes were field-stimulated at 1Hz for 3 min followed by 2min recording after returning to the resting state. To record [Ca2+]m and action potentials, myocytes were whole-cell patch-clamped at 37°C. Tyrode’s solution was used as external solution and the internal solution contained (in mmol/L): NaCl 10, KCl 19, K-glutamate 125, MgCl2 0.5, HEPES 10, MgATP 5, pH7.25.
Protocol for isolated perfused heart experiments
Guinea pig hearts were quickly excised under anesthesia and mounted on a modified Langendorff apparatus attached to a Powerlab system (AD Instruments). Hearts were perfused with gassed (95/5% O2/CO2) control buffer containing (in mM) 118 NaCl, 24 NaHCO3, 1.2 KH2PO4, 4.75 KCl, 1.2 MgSO4, 2.0 CaCl2, and 10 glucose. A buffer-filled latex balloon (Harvard apparatus) was inserted through the mitral valve into the left ventricle. Hearts were suspended in a buffer-filled heating chamber maintained at 37°C and two electrodes were placed into the bath for volume-conducted electrocardiogram recordings. Heart rate, left ventricular developed pressure (LVDP), maximal rates of contraction and relaxation (±dP/dt), oxygen consumption, and electrocardiograms (ECG) were recorded on a computer. After a 10min equilibration period, hearts were subjected to the following protocol: 10min baseline recording while the heart was perfused with control buffer, followed by application of 0.25µM ouabain with or without 1µM CGP-37157 and another 10min recording, then 25nM isoproterenol was added to the buffer and recorded for 60 min.. The measurements of hemodynammic parameters were determined by taking the average during the last 2 minutes before ouabain treatment (baseline) and for 2 minutes during the maximal effect on LVDP of either ouabain or isoproterenol.
O2 consumption
Cardiac O2 concentration was measured with an O2 probe attached to a PowerLab system (AD Instruments). The probe was calibrated with 95% O2-saturated buffer as 100% and 100% N2-saturated buffer as 0%. The molar concentration of O2 consumption was calculated from percentage concentration with the following equation:
Where CF is coronary flow (ml/min); 0.02373 is absorption coefficient of O2 in H2O at 37°C; 22.414 is the volume (L) of 1 mol gas at STP; 760 is 1 atm in mm Hg; p equals 47.067, which is vapor pressure of H2O at 37°C; ΔO2% is the difference in O2 percentage concentration between inflow buffer collected from cannula before heart was mounted and outflow buffer collected from pulmonary artery; dwt is dry heart weight.
Assessment of cardiac arrhythmias
Arrhythmias were characterized in accordance with the Lambeth Conventions[13] and scores were tabulated for the 10min period of ouabain treatment using modified Score A as described by Curtis and Walker[14]. Briefly, each heart was given a score based on the following criteria: 0: <50 ventricular premature beats; 1: 50–499 ventricular premature beats; 2: >500 ventricular premature beats and/or 1 episode of spontaneously reverting ventricular tachycardia or ventricular fibrillation; 3: more than one episode of spontaneously reverting ventricular tachycardia or fibrillation (<2 min); 4: >2 min of ventricular tachycardia or fibrillation.
In vivo study of arrhythmia
Surface ECG was recorded from normal guinea pigs and drugs were introduced with intraperitoneal injection. After 20 min baseline recording, 0.02mg/kg CGP or vehicle was administrated followed by 0.05mg/kg ouabain administration. After 15min, second dose of ouabain was introduced, which was followed by 0.25mg/kg iso administration after another 15min. Arrhythmia was evaluated with the incidence of ventricular premature beat during a 30-min period after isoproterenol administration.
Statistical analysis
Data are expressed as mean ± SEM. Effects of ouabain and CGP-37157 on the cytosolic Ca2+ transient and on the arrhythmia scores were analyzed with unpaired t-tests. Effects of ouabain and isoproterenol on hemodynamics were analyzed with paired t-tests. Differences in the incidence of VF were analyzed with Fisher’s Exact Test. O2 consumption was analyzed with one way ANOVA.
Results
Effects of ouabain on [Na+]i
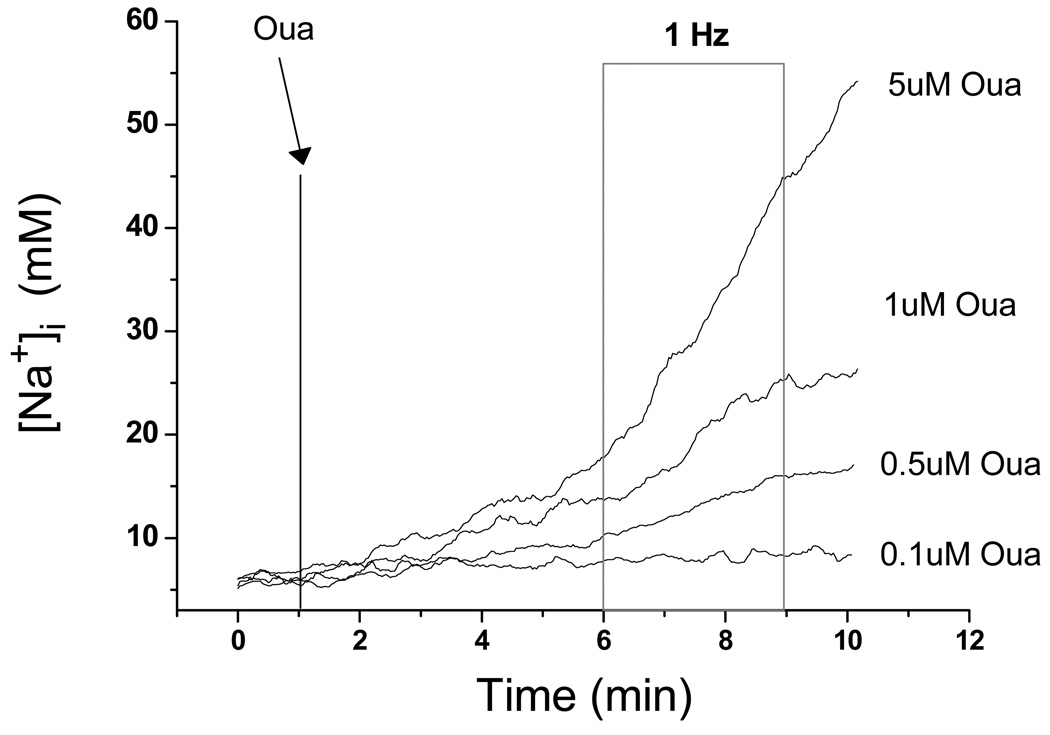
Application of ouabain to isolated myocytes at rest or with 1Hz stimulation elevated [Na+]i monotonically in a dose-dependent way (Fig. 1). 1Hz stimulation increased the rate of [Na+]i accumulation slightly (Fig. 1). To optimize the conditions of our study so that [Na+]i could be elevated efficiently, but with acceptable toxicity during the protocol, 1µM ouabain was used in the following isolated myocyte experiments.
Figure 1.
Representative recordings of [Na+]i. Isolated cardiac myocytes were loaded with SBFI-AM. After 1 min recording, cells were treated with 100nM isoproterenol and ouabain at different concentrations as indicated for 5 min, and then were field-stimulated at 1 Hz for 3min (gray box). After stimulation, cells were recorded at rest for 1 min.
Effects on [Ca2+]m and mitochondrial NADH during increased work
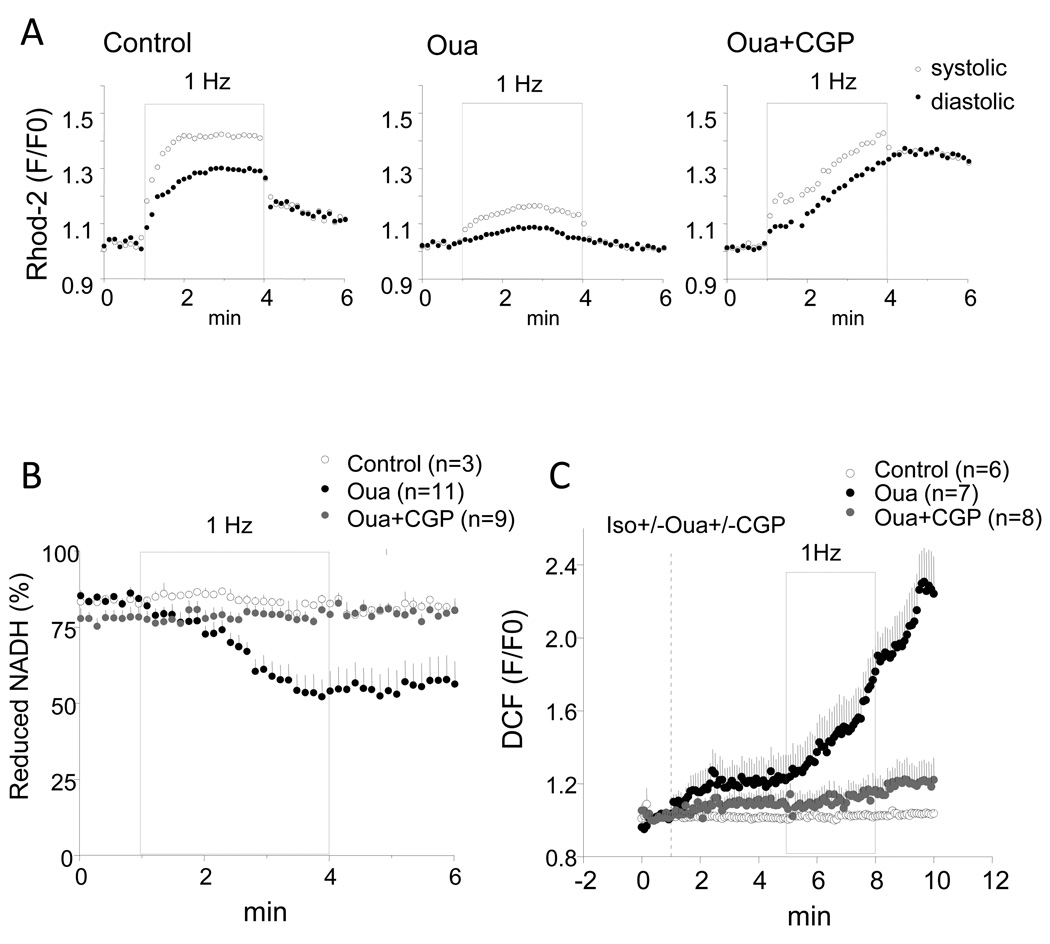
In the presence of 100nM isoproterenol, 1Hz stimulation induced [Ca2+]m accumulation in control cell (without ouabain treatment) with a 30% increase of diastolic rhod-2 signal at the end of stimulation compared to baseline level before stimulation (Fig.2A left), whereas, 1 µM ouabain treatment blunted [Ca2+]m accumulation and the increase of rhod-2 signal during stimulation was less than 10% (Fig.2A middle). The effect of ouabain on [Ca2+]m accumulation was reversed by 1 µM CGP-37157 (Fig.2A left). The decreased [Ca2+]m accumulation by ouabain was associated with net mitochondrial NADH oxidation (Fig. 2B). In ouabain-treated myocytes, the NADH level decreased significantly during 1 Hz stimulation from 81.7±1.8% before stimulation to 52.3±5.7% by the end of stimulation, whereas, in control cells or ouabain-treated cells with administration of CGP-37157, NADH level was well maintained during stimulation (before stimulation: 84.1±0.3% and 78.3±3.2%; at the end of stimulation: 84.1±1.6% and 80.6±2.5% in control cells and ouabain-treated cells in the presence of CGP, respectively.) (Fig.2B).
Figure 2.
Effects of ouabain and CGP-37157 on mitochondrial Ca2+ accumulation, NADH production, and oxidative stress. In the presence of 100nM isoproterenol, control cells and ouabain-treated cells with or without CGP-37157 were stimulated at 1Hz for 3 min (grey box) followed by 2-min recovery at resting state. A. Representative recording of rhod-2 signals in control cell (left), cell treated with 1 µM ouabain (middle), and cell treated with ouabain plus CGP-37157 (right). B. Recording of NADH autofluorescence. C. Recording of DCF fluorescence.
The oxidation of the NADH pool during the increase in work in the presence of ouabain did not reverse after cessation of electrical stimulation. In keeping with our hypothesis that the lack of NADH recovery under high [Na+]i conditions might be a consequence of oxidative damage[15], ouabain-treated myocytes displayed higher rates of oxidation of CM-DCF; an effect further exacerbated by electrical stimulation (Fig. 2C). Co-application of CGP-37157 abolished the increase in oxidative stress induced by ouabain.
Inhibition of mNCE mitigates ouabain-induced [Ca2+]c dysfunction and DAD-triggered action potentials
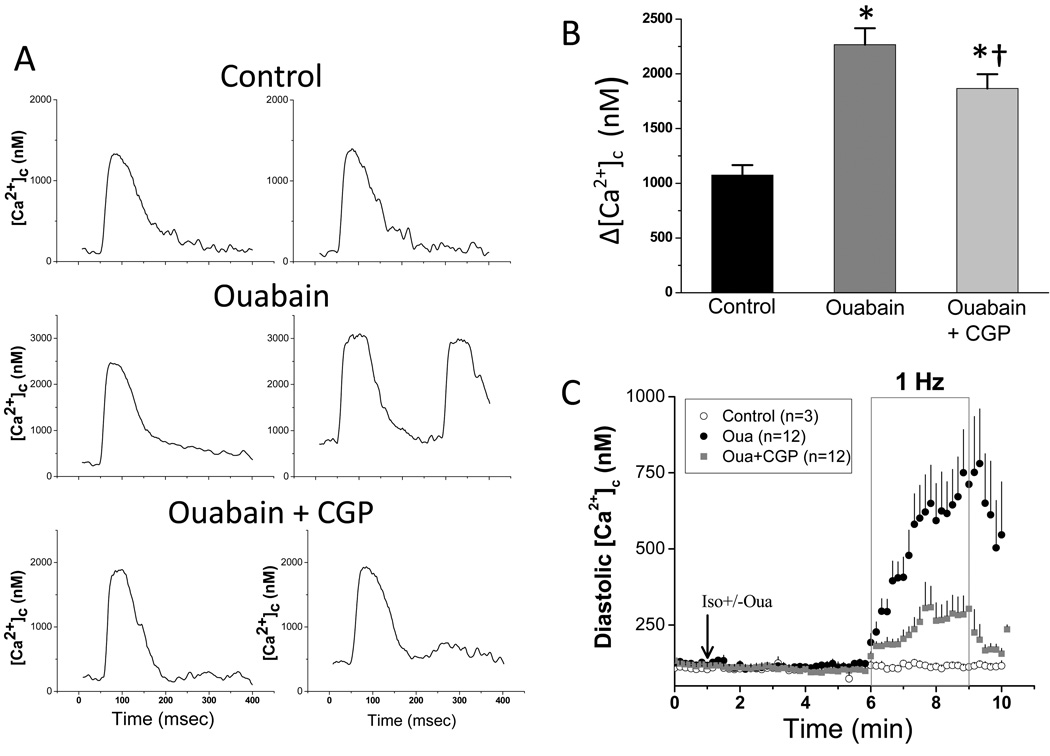
To investigate the effects of ouabain and CGP-37157 on [Ca2+]c cycling, [Ca2+]c was monitored with indo-1. Representative [Ca2+]c recordings at the onset (Fig. 3A; left panels) and at the end (Fig. 3A; right panels) of 1Hz stimulation in the presence of 100nM isoproterenol reveal that ouabain increases the probability of cytosolic Ca2+ overload and extrasystolic Ca2+ release (Fig. 3A; OUA). In the absence of CGP-37157, ouabain treatment increased the amplitude of [Ca2+]c transient (Δ[Ca2+]c) by 112% compared to that of control, whereas the addition of CGP-37157 attenuated the effect of ouabain on Δ[Ca2+]c, with a 74% increase compared to control (Fig. 3B). The attenuation of the Δ[Ca2+]c increase by CGP-37157 suggests that mitochondria play a role in EC coupling as a Ca2+ sink. Although administration of ouabain did not affect diastolic [Ca2+]c level significantly in myocytes in the resting state, diastolic [Ca2+]c was dramatically increased when the cells were paced at 1 Hz (Fig. 3C). After 3 min of stimulation, diastolic [Ca2+]c in cells treated with ouabain was 750±142 nM in contrast to 123±9 nM before stimulation. CGP-37157 reduced the accumulation of diastolic [Ca2+]c (370±60 nM at the end of stimulation versus 99±14 nM before stimulation). In control cells, 1 Hz stimulation did not increase diastolic [Ca2+]c (117±12 nM before stimulation and 111±7 nM at the end of stimulation). As a result of increased SR Ca2+ loading and elevated diastolic [Ca2+]c, the majority of cells treated with ouabain developed spontaneous Ca2+ oscillations, extrasystolic contractions, and eventual hypercontracture during prolonged stimulation, whereas CGP-37157 significantly reduced the incidence of discordant contractions (data not shown).
Figure 3.
Effects of ouabain and CGP-37157 on [Ca2+]c cycling. Isolated cardiac myocytes were loaded with indo-1 AM. After 5 min treatment of 100nM isoproterenol with or without ouabain and CGP-37157, cells were field stimulated for 3 min. A) Representative [Ca2+]c traces at the beginning (left panels) and end of stimulation (right panels). B) The average Δ[Ca2+]c recorded at the midpoint of stimulation was is shown for control cells (n=3), ouabain treated cells (n=12), and ouabain plus CGP-37157 treated cells (n=12). * p<0.01 while compared to control; † p<0.05 while compared to ouabain treated group. C) The average of diastolic [Ca2+]c recorded in control (isoproterenol only), ouabain-treated, and ouabain plus CGP-37157 -treated cells.
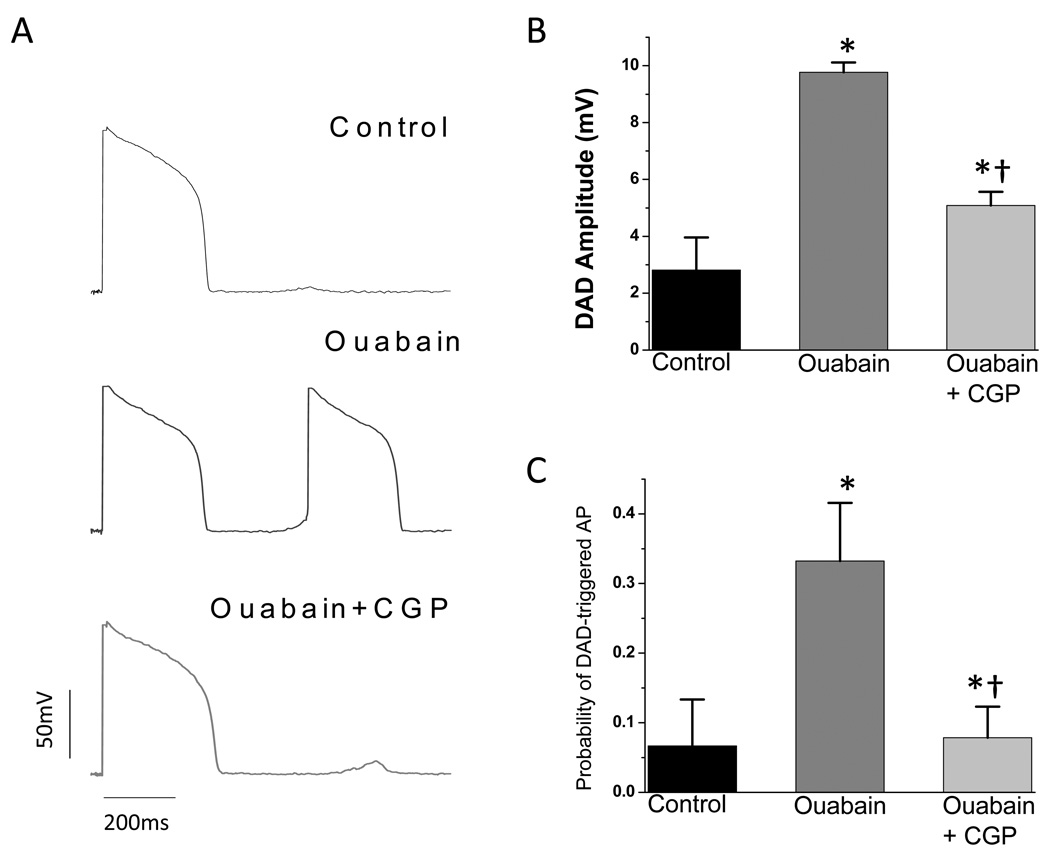
In current-clamped myocytes, electrically-stimulated action potentials (APs) were recorded at 1Hz and the probability of observing one or more delayed afterdepolarization-(DAD) triggered action potentials was determined during the last minuate of stimulation. Ouabain treatment caused a significant increase in the incidence of DAD-triggered APs (Fig. 4A and 4C). In myocytes treated with ouabain plus CGP-37157, DADs were still observed occasionally, but they were of smaller amplitude than in ouabain alone (Fig. 4B), and the incidence of DAD-triggered APs was not significantly different from controls (Fig. 4C).
Figure 4.
Effects of ouabain and CGP-37157 on DADs. In the presence of 100nM isoproterenol, current-clamped cells were treated with 1µM ouabain with or without 1µM CGP-37157 for 5 min, and action potentials (APs) were recorded at 1Hz for 3min. A) Representative AP traces. B) Amplitude of DADs. C) Probability of DAD-triggered AP activation. * p<0.01 compared to control; † p<0.01 compared to ouabain treated group.
Effects on cardiac function and oxygen consumption
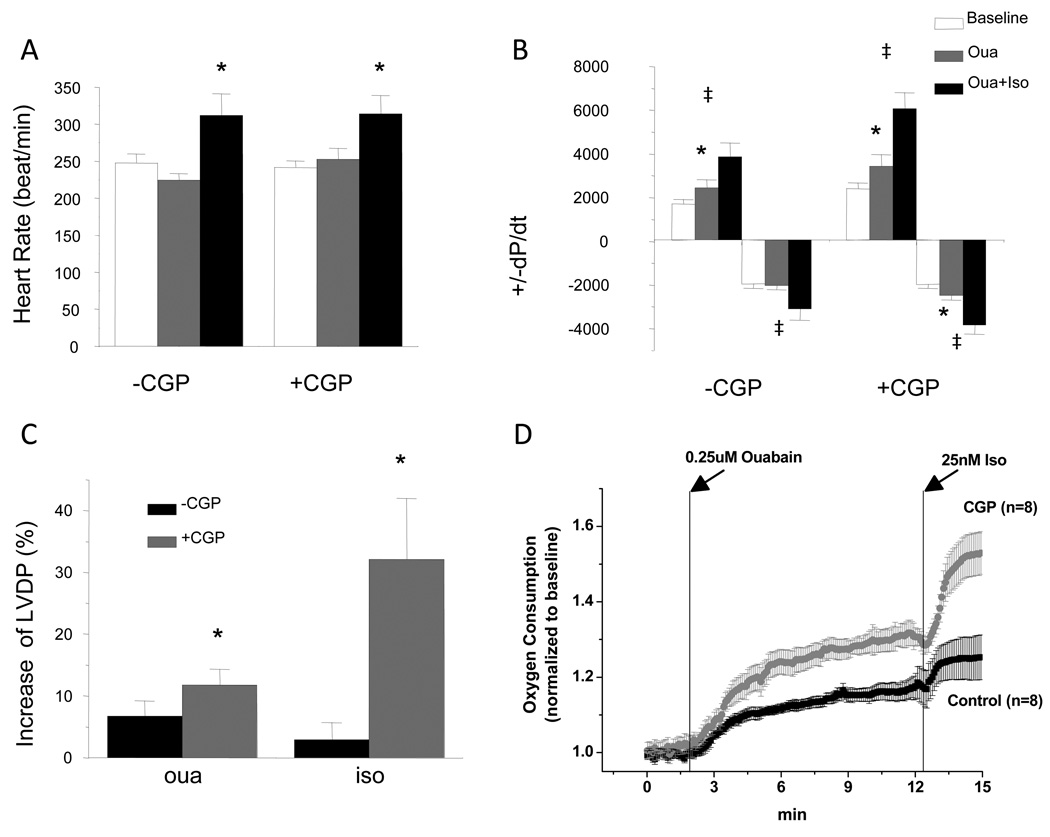
To investigate whether the impact of ouabain and CGP-37157 on NADH production had any effects on cardiac energetics and function, whole-heart studies were performed and cardiac oxygen consumption and hemodynamics were recorded. Heart rate was not affected by application of ouabain (Fig.5A). Consistent with its positive inotropic effects, application of ouabain led to a 7% (p<0.05) increase of LVDP compared to baseline (Fig.5C). Rates of contraction and relaxation were also improved with a 44% and 4% increase of +dP/dt and −dP/dt, respectively (Fig.5B). Application of ouabain with CGP-37157 mediated a 12% increase of LVDP a 42% increase of +dP/dt, and a much larger increase in −dP/dt (~25%) (Fig.5B). Compared to the level with ouabain treatment, administration of 25 nM isoproterenol further enhanced chronotropy and inotropy. Heart rate was increased similarly in the groups treated with isoproterenol in the absence (28% increase) or presence of CGP-37157 (23% increase) (Fig.5A). Remarkably, in the group without treatment of CGP-37157, LVDP was only increased by 2.5% (p=0.45) after administration of isoproterenol, whereas +dP/dt and −dP/dt were increased by 57% and 52%, respectively (Fig.5B and C). In the group treated with CGP-37157, LVDP was increased by 32%, and +dP/dt and −dP/dt were increased by 77% and 55%, respectively (Fig.5B). Associated with the increased cardiac function, cardiac oxygen consumption increased upon administration of ouabain and isoproterenol. After 10 min treatment of ouabain, whole heart oxygen consumption increased by 18% and isoproterenol further increased oxygen consumption to a level 25% above baseline (Fig. 5D). In keeping with the improved hemodynamic responses mentioned above, CGP-37157 also potentiated the increases in VO2 induced by ouabain and isoproterenol: ouabain increased VO2 by 32% while isoproterenol increased VO2 by 53% compared to baseline (Fig. 5D).
Figure 5.
Effects of ouabain and isoproterenol on cardiac function and oxygen consumption in isolated perfused hearts. A-C, Heart rates (A), ±dP/dt (B), and LVDP (C) measured at baseline and steady states after ouabain and isoproterenol application in groups with and without treatment of CGP-37157. * p<0.05 compared to baseline level; ‡ p<0.01 compared to that after ouabain application; † p<0.05 compared to that after isoproterenol application in the group without treatment of CGP-37157. D. Increases of LVDP by ouabain and isoproterenol. LVDPs measured at steady state after ouabain or isoproterenol application were compared to those before their application. * p<0.001. E. Measurements of oxygen consumption are displayed before and after application of ouabain and isoproterenol with or without CGP-37157, showing their effects on oxygen consumption.
Effects on arrhythmias
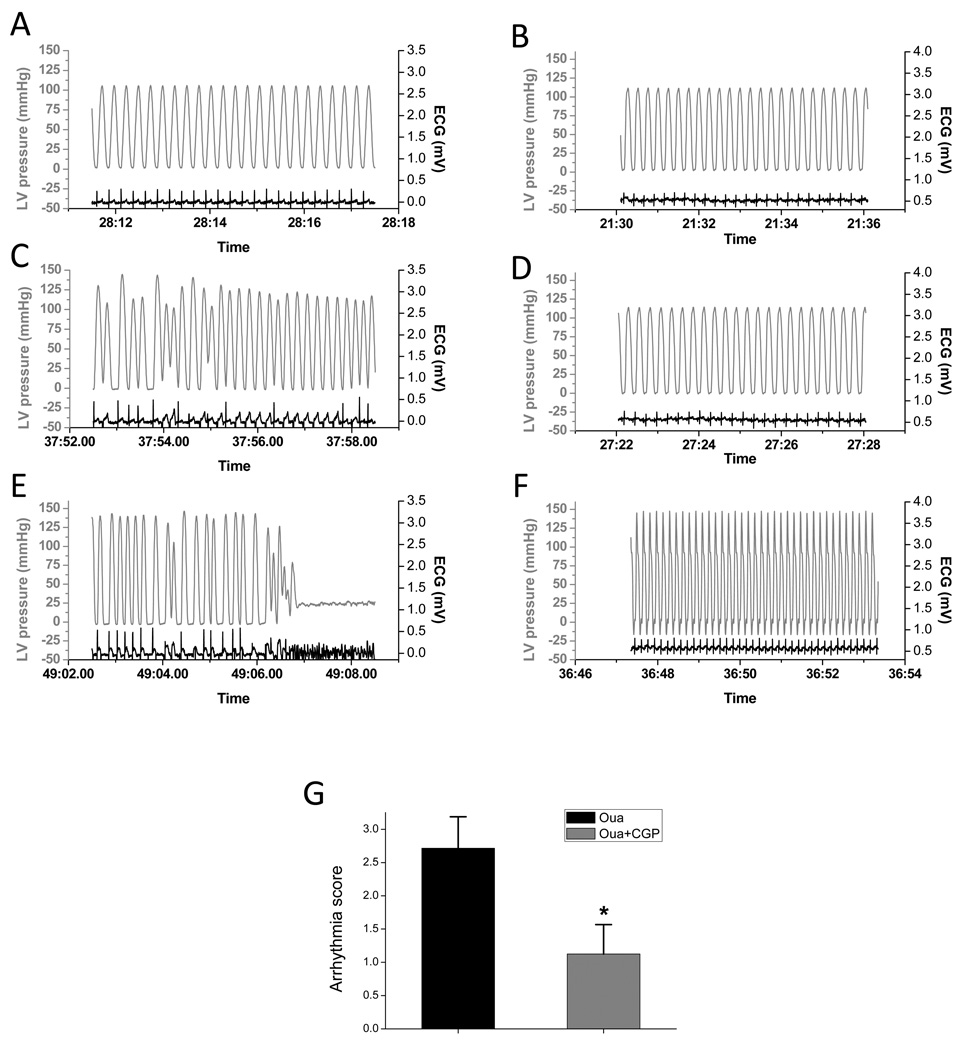
Study of isolated perfused heart indicated that ouabain treatment (0.25 µM) had a proarrhythmic effect, with an arrhythmia score of 2.7±0.5 during the 10-min treatment (Fig. 6). This effect became exacerbated after administration of isoproterenol - ventricular fibrillation occurred in 7 out of 8 hearts. However, treatment with CGP-37157 significantly attenuated the pro-arrhythmic effects of ouabain. The arrhythmia score during 10-min ouabain treatment was reduced to 1.1±0.4 (Fig. 6G) and the incidence of VF was also significantly decreased (2 out of 7 hearts, p<0.05).
Figure 6.
Effects of ouabain and CGP-37157 on arrhythmia in isolated perfused heart. A-F. Representative tracings of LV pressure and ECG waveforms. A and B) Recording at the end of baseline recording; C and D) recording at the end of 10 min ouabain application; E and F) recording after isoproterenol administration. A, C, and E were recorded in a heart without CGP-37157 treatment. B, D, and F were recorded in a CGP-37157 treated heart. G. Comparison of arrhythmia scores. Arrhythmia scores were tabulated for the 10 min ouabain treatment period for hearts treated with (n=8) or without (n=8) CGP-37157. * p<0.01.
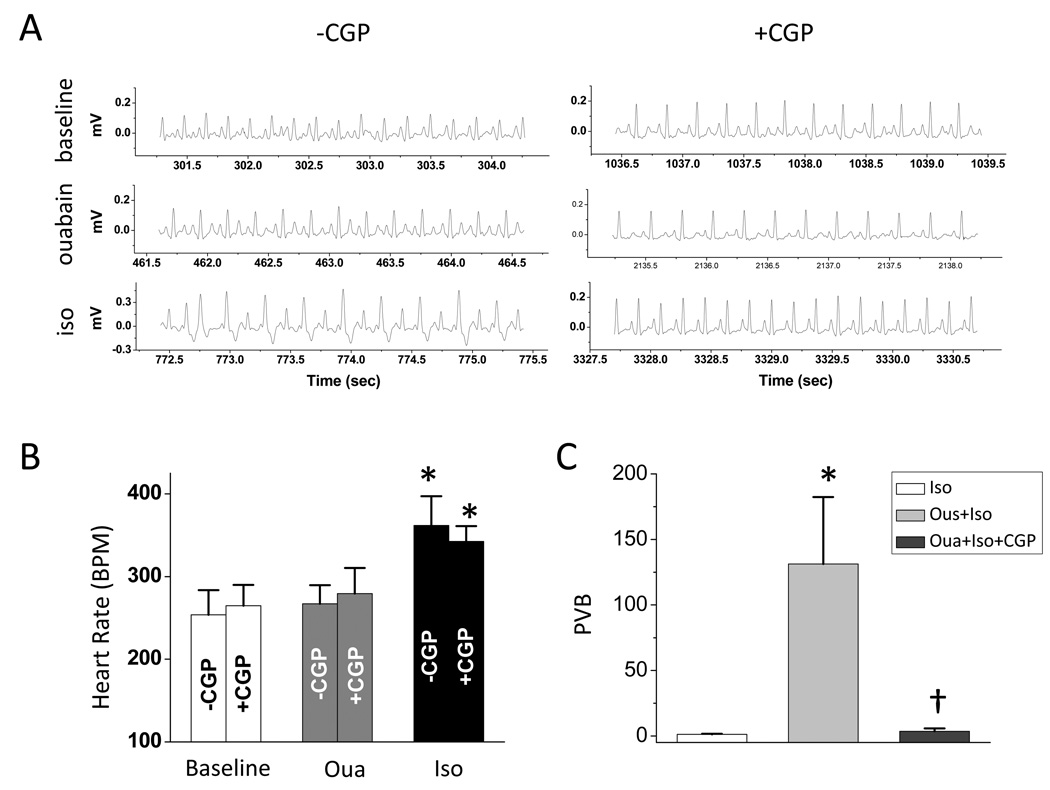
In our in vivo study, intraperitoneal administration of 2 doses of ouabain (0.05 mg/kg) increased the susceptibility of animal to isoproterenol-induced arrhythmia. Administration of 0.25 mg/kg isoproterenol following ouabain treatment induced arrhythmia (Fig. 7A), whereas isoproterenol (0.25 mg/kg) itself is insufficient to induce arrhythmia (Fig. 7C). Arrhythmias induced by ouabain and isoproterenol was inhibited by the pretreatment of CGP-37157 (Fig. A and C).
Figure 7.
in vivo study of effect of CGP-37157 on ouabain-induced arrhythmia: A. representative traces of surface ECG recorded at different time points: baseline (upper), after ouabain administration (middle), and after isoproterenol administration (lower). B. heart rate at baseline, after ouabain administration, and after isoproterenol administration with (n=5) or without (n=5) pretreatment of CGP-37157. C. incidence of PVB after the administration of isoproterenol. In the group of iso, animals (n=4) were only administrated with 0.25mg/kg iso without pretreatment of ouabain.
Discussion
This study is the first to describe that ouabain has adverse effects on mitochondrial function and that the consequent mitochondrial dysfunction contributes to the toxicity of ouabain. As we hypothesized, ouabain treatment impairs NADH production in isolated myocytes as a consequence of cytosolic Na+ loading, and this effect was prevented by CGP-37157. In isolated myocytes, CGP-37157 was shown to i) enhance mitochondrial Ca2+ uptake in the presence of ouabain, ii) decrease cytosolic diastolic Ca2+ overload, blunting spontaneous Ca2+ release, and iii) decrease the incidence of DAD-triggered action potentials. At the whole heart level, CGP-37157 prevented the ouabain-induced impairment of mitochondrial energetics, which limits the positive inotropic effects of both ouabain and isoproterenol. Moreover, improved contractility with CGP-37157 treatment correlated with increased rates of mitochondrial respiration. Consistently, CGP-37157 attenuated ouabain-induced arrhythmias and significantly decreased the incidence of VF.
The effects of ouabain on cardiac energetics
In previous studies, we have shown that elevated [Na+]i impairs mitochondrial NADH production by blunting [Ca2+]m accumulation during increased work, and that partial inhibition of mNCE by CGP-37157 restores [Ca2+]m accumulation and NADH production[8, 9]. These findings motivated us to investigate whether the known toxicity of digitalis therapy might involve impairment of cardiac energetics, and if inhibition of mNCE could abrogate this negative effect. In the present study, we examined the effects of ouabain and CGP-37157 on NADH production in isolated myocytes, and O2 consumption and cardiac performance in whole heart. In isolated cardiac myocytes, the elevation of [Na+]i induced by NKA inhibition with ouabain significantly oxidized the mitochondrial NADH pool, which was well maintained in the presence of CGP-37157. In intact perfused hearts, CGP-37157 enhanced the positive inotropic effects of both ouabain (+dP/dt was 41% higher) and isoproterenol (+dP/dt was 57% higher), and increased the maximal VO2 response. The additive effect of CGP-37157 indicates that ouabain’s positive effects on Ca2+ cycling and contractility are limited by a mismatch of energy supply and demand. Impairment of mitochondrial NADH balance led to Ca2+ dysregulation and the triggering of spontaneous SR Ca2+ release and DAD-triggered APs in cardiomyocytes. These effects were shown to be mediated by elevated [Na+]i and depressed [Ca2+]m accumulation because CGP-37157 restored the [Ca2+]m response, maintained the NAD+/NADH redox potential during increased work, and enhanced the whole heart respiration rate.
We have previously demonstrated that if [Ca2+]m accumulation during an increase in work does not reach a critical threshold level, there is a linear correlation between [Ca2+]m and NADH level. In other words, more oxidation of the NADH pool occurs when the mitochondrial Ca2+ signal is insufficient to stimulate NADH production. This limitation can be overcome through several interventions that enhance mitochondrial Ca2+ uptake, such as decreasing cytoplasmic Na+, increasing cytosolic inorganic phosphate concentration, β-adrenergic stimulation, increasing extracellular Ca2+, or partially inhibiting mNCE[8]. In the whole heart, isoproterenol can facilitate [Ca2+]m uptake, both by increasing heart rate and by enhancing SR Ca2+ cycling; however, here we show that when [Na+]i is high as a result of ouabain treatment, even the β-adrenergic response is limited. This limitation can be overcome by improving [Ca2+]m accumulation with CGP-37157, as evidenced by an increased positive inotropic effect and enhanced VO2 max.
The role of mitochondria in ouabain-induced arrhythmias
Cardiac arrhythmias are one of the major adverse effects of cardiac glycosides in clinical use. The mechanisms underlying the arrhythmogenic effect are incompletely understood, but are thought to involve Ca2+-triggered events, occurring as a consequence of NKA inhibition and SR Ca2+ overload[16, 17]. As SR Ca2+ load rises, the open probability of RyR receptor increases and uncontrolled SR Ca2+ release may be initiated, promoting arrhythmogenic electrical signals including early- or delayed-afterdepolarizations (EADs or DADs). Discordant Ca2+ release was observed in the presence of ouabain, along with an excessive increase in diastolic Ca2+ (Fig. 3). Both of these effects were attenuated by CGP-37157, supporting the idea that mitochondria fulfill multiple roles in the integrated physiology of the cell, including buffering [Ca2+]c during inotropic stimuli, producing ATP to support normal Ca2+ cycling, and modulating the redox status of the cardiomyocyte. Enhancement of these mitochondrial properties by CGP-37157 suggests mNCE as a potential target for anti-arrhythmic therapy. Although the subject of some debate[18], there is evidence that mitochondria sequester [Ca2+]c during EC coupling on a beat-to-beat basis[8, 19–22]. The Ca2+ transient is increased when the mitochondrial Ca2+ uniporter is blocked[8, 21], and decreased when mNCE is inhibited[8]. In addition to the direct effect of mitochondrial Ca2+ dynamics on intracellular Ca2+ fluxes, mitochondria also appear to influence the propagation of Ca2+ waves. Boitier et al[23] demonstrated that when mitochondrial Ca2+ uptake was prevented by mitochondrial depolarization, the rate of propagation of Ca2+ wave was significantly increased in astrocytes. Similarly, Seguchi et al[21] inhibited mitochondrial Ca2+ uptake with Ru360 and converted non-propagating Ca2+ release into a propagating release in isolated ventricular myocytes. Therefore, CGP-37157 might have an anti-arrhythmic effect because it attenuates SR Ca2+ overload and inhibits uncontrolled propagation of local Ca2+ release events. Alternatively, the suppression of DAD-triggered APs and arrhythmias by CGP-37157 may be related to the reduced level of oxidative stress when mitochondrial function is preserved (Fig. 2C). In whole hearts, this beneficial effect of CGP-37157 was manifested as a decreased incidence of ouabain-induced arrhythmias, and, importantly, we also demonstrated that the progression to ventricular fibrillation by isoproterenol was suppressed by CGP-37157.
Potential clinical relevance
Cardiac glycosides have played an important role in the treatment of HF for over two hundred years. However the use of cardiac glycosides in HF has declined in the past decade[24]. One potential reason for this decline could be the lack of mortality benefit of digoxin as suggested by the Digoxin Investigation Group (DIG) trial[25]. In the DIG trial, it appeared that digoxin therapy did not affect overall mortality in HF patients although, like other studies[26, 27], it improved HF symptoms and reduced hospitalization rates due to worsening HF[25]. The improvement of cardiac function without benefit to mortality could be partially explained by an increase of cardiac sudden death in patients treated with digoxin. By considering serum digoxin concentration (SDC) as a factor, a recent post hoc analysis of the DIG trail demonstrated that digoxin therapy reduced mortality of all HF patients with a SDC of 0.5–0.9 ng/ml, whereas, at higher SDC (≥1.0ng/ml), digoxin therapy reduced hospitalizations with no effect on mortality[28]. This suggests that, as SDC increases, the beneficial effects of digoxin on mortality could be offset by increased incidence of arrhythmias and sudden death. The beneficial effects of CGP-37157 on arrhythmias and VF displayed in current study support a therapeutic strategy to improve cardiac glycoside treatment of patients in HF with combination therapy designed to inhibit both the NKA and the mNCE. In this regard, we previously showed that elevated [Na+]i in myocytes isolated from failing hearts impaired mitochondrial energetics during increased work[9]. Although digoxin therapy has been shown to increase cardiac contractility, exercise time, and O2 consumption of HF patients in clinical trials (reviewed in ref [2]), the adverse effect of elevated [Na+]i already present in HF could be intensified with digoxin treatment.
Our current study suggests that enhancement of [Ca2+]m by inhibition of mNCE could further improve cardiac function by digoxin therapy. Even apart from digoxin therapy, various endogenous digitalis-like compounds have been identified in human and animals, and their concentrations vary in different disease conditions (for review, see ref [29]). In 1991, Hamlyn et al [30] identified endogenous ouabain in human plasma, which is structurally, biologically, and immunologically indistinguishable from plant-derived ouabain. The plasma level of endogenous ouabain was then evaluated in HF patients and the result indicated a significant increase in endogenous ouabain in HF patients compared to normal controls[31]. Using an animal model, Fedorova et al [32] found that plasma levels of endogenous ouabain did not change in the stage of hypertrophy, but were substantially increased (by 3-fold) in the transition to heart failure. The adverse effects of cardiac glycosides on cardiac energetics and arrhythmias therefore might also exist in HF patients who are not receiving digoxin.
In summary, the current study demonstrates that ouabain-induced elevation of [Na+]i has an adverse effect on mitochondrial energetics, and CGP-37157 abrogates the negative effect on energetics and attenuates ouabain-induced arrhythmias. The results suggest a potential therapeutic strategy to improve digoxin’s positive effects while reducing the risk of digoxin toxicity.
Supplementary Material
Acknowledgements
This work was supported by NIH grants P01 HL081427 and R01 HL101235 (BO’R).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None declared
References
- 1.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112(12):e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 2.Rahimtoola SH. Digitalis therapy for patients in clinical heart failure. Circulation. 2004;109(24):2942–2946. doi: 10.1161/01.CIR.0000132477.32438.03. [DOI] [PubMed] [Google Scholar]
- 3.Gheorghiade M, Adams KF, Jr., Colucci WS. Digoxin in the management of cardiovascular disorders. Circulation. 2004;109(24):2959–2964. doi: 10.1161/01.CIR.0000132482.95686.87. [DOI] [PubMed] [Google Scholar]
- 4.Ribner HS, Plucinski DA, Hsieh AM, Bresnahan D, Molteni A, Askenazi J, et al. Acute effects of digoxin on total systemic vascular resistance in congestive heart failure due to dilated cardiomyopathy: a hemodynamic-hormonal study. The American journal of cardiology. 1985;56(13):896–904. doi: 10.1016/0002-9149(85)90778-7. [DOI] [PubMed] [Google Scholar]
- 5.van Veldhuisen DJ, Man in 't Veld AJ, Dunselman PH, Lok DJ, Dohmen HJ, Poortermans JC, et al. Double-blind placebo-controlled study of ibopamine and digoxin in patients with mild to moderate heart failure: results of the Dutch Ibopamine Multicenter Trial (DIMT) Journal of the American College of Cardiology. 1993;22(6):1564–1573. doi: 10.1016/0735-1097(93)90579-p. [DOI] [PubMed] [Google Scholar]
- 6.Alicandri C, Fariello R, Boni E, Zaninelli A, Castellano M, Beschi M, et al. Captopril versus digoxin in mild-moderate chronic heart failure: a crossover study. Journal of cardiovascular pharmacology. 1987;9 Suppl 2:S61–S67. doi: 10.1097/00005344-198700002-00013. [DOI] [PubMed] [Google Scholar]
- 7.Covit AB, Schaer GL, Sealey JE, Laragh JH, Cody RJ. Suppression of the renin-angiotensin system by intravenous digoxin in chronic congestive heart failure. The American journal of medicine. 1983;75(3):445–447. doi: 10.1016/0002-9343(83)90346-7. [DOI] [PubMed] [Google Scholar]
- 8.Maack C, Cortassa S, Aon MA, Ganesan AN, Liu T, O'Rourke B. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circulation research. 2006;99(2):172–182. doi: 10.1161/01.RES.0000232546.92777.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu T, O'Rourke B. Enhancing mitochondrial Ca2+ uptake in myocytes from failing hearts restores energy supply and demand matching. Circulation research. 2008;103(3):279–288. doi: 10.1161/CIRCRESAHA.108.175919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiological reviews. 1990;70(2):391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- 11.O'Rourke B, Ramza BM, Marban E. Oscillations of membrane current and excitability driven by metabolic oscillations in heart cells. Science New York, NY. 1994;265(5174):962–966. doi: 10.1126/science.8052856. [DOI] [PubMed] [Google Scholar]
- 12.Aon MA, Cortassa S, Marban E, O'Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem. 2003;278(45):44735–44744. doi: 10.1074/jbc.M302673200. [DOI] [PubMed] [Google Scholar]
- 13.Walker MJ, Curtis MJ, Hearse DJ, Campbell RW, Janse MJ, Yellon DM, et al. The Lambeth Conventions: guidelines for the study of arrhythmias in ischaemia infarction, and reperfusion. Cardiovascular research. 1988;22(7):447–455. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- 14.Curtis MJ, Walker MJ. Quantification of arrhythmias using scoring systems: an examination of seven scores in an in vivo model of regional myocardial ischaemia. Cardiovascular research. 1988;22(9):656–665. doi: 10.1093/cvr/22.9.656. [DOI] [PubMed] [Google Scholar]
- 15.O'Rourke B, Maack C. The role of Na dysregulation in cardiac disease and how it impacts electrophysiology. Drug discovery today. 2007;4(4):207–217. doi: 10.1016/j.ddmod.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trafford AW, O'Neill SC, Eisner DA. Factors affecting the propagation of locally activated systolic Ca transients in rat ventricular myocytes. Pflugers Arch. 1993;425(1–2):181–183. doi: 10.1007/BF00374521. [DOI] [PubMed] [Google Scholar]
- 17.Lukyanenko V, Subramanian S, Gyorke I, Wiesner TF, Gyorke S. The role of luminal Ca2+ in the generation of Ca2+ waves in rat ventricular myocytes. J Physiol. 1999;518(Pt 1):173–186. doi: 10.1111/j.1469-7793.1999.0173r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Rourke B, Blatter LA. Mitochondrial Ca(2+) uptake: Tortoise or hare? Journal of molecular and cellular cardiology. 2008 doi: 10.1016/j.yjmcc.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bell CJ, Bright NA, Rutter GA, Griffiths EJ. ATP regulation in adult rat cardiomyocytes: time-resolved decoding of rapid mitochondrial calcium spiking imaged with targeted photoproteins. J Biol Chem. 2006;281(38):28058–28067. doi: 10.1074/jbc.M604540200. [DOI] [PubMed] [Google Scholar]
- 20.Robert V, Gurlini P, Tosello V, Nagai T, Miyawaki A, Di Lisa F, et al. Beat-to-beat oscillations of mitochondrial [Ca2+] in cardiac cells. Embo J. 2001;20(17):4998–5007. doi: 10.1093/emboj/20.17.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seguchi H, Ritter M, Shizukuishi M, Ishida H, Chokoh G, Nakazawa H, et al. Propagation of Ca2+ release in cardiac myocytes: role of mitochondria. Cell Calcium. 2005;38(1):1–9. doi: 10.1016/j.ceca.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Trollinger DR, Cascio WE, Lemasters JJ. Mitochondrial calcium transients in adult rabbit cardiac myocytes: inhibition by ruthenium red and artifacts caused by lysosomal loading of Ca(2+)-indicating fluorophores. Biophys J. 2000;79(1):39–50. doi: 10.1016/S0006-3495(00)76272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boitier E, Rea R, Duchen MR. Mitochondria exert a negative feedback on the propagation of intracellular Ca2+ waves in rat cortical astrocytes. J Cell Biol. 1999;145(4):795–808. doi: 10.1083/jcb.145.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams KF, Jr., Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149(2):209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 25.The effect of digoxin on mortality and morbidity in patients with heart failure. The Digitalis Investigation Group. N Engl J Med. 1997;336(8):525–533. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 26.Packer M, Gheorghiade M, Young JB, Costantini PJ, Adams KF, Cody RJ, et al. Withdrawal of digoxin from patients with chronic heart failure treated with angiotensin-converting-enzyme inhibitors. RADIANCE Study. N Engl J Med. 1993;329(1):1–7. doi: 10.1056/NEJM199307013290101. [DOI] [PubMed] [Google Scholar]
- 27.Uretsky BF, Young JB, Shahidi FE, Yellen LG, Harrison MC, Jolly MK. Randomized study assessing the effect of digoxin withdrawal in patients with mild to moderate chronic congestive heart failure: results of the PROVED trial. PROVED Investigative Group. J Am Coll Cardiol. 1993;22(4):955–962. doi: 10.1016/0735-1097(93)90403-n. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed A, Rich MW, Love TE, Lloyd-Jones DM, Aban IB, Colucci WS, et al. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur Heart J. 2006;27(2):178–186. doi: 10.1093/eurheartj/ehi687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bagrov AY, Shapiro JI. Endogenous digitalis: pathophysiologic roles and therapeutic applications. Nat Clin Pract Nephrol. 2008;4(7):378–392. doi: 10.1038/ncpneph0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamlyn JM, Blaustein MP, Bova S, DuCharme DW, Harris DW, Mandel F, et al. Identification and characterization of a ouabain-like compound from human plasma. Proc Natl Acad Sci U S A. 1991;88(14):6259–6263. doi: 10.1073/pnas.88.14.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gottlieb SS, Rogowski AC, Weinberg M, Krichten CM, Hamilton BP, Hamlyn JM. Elevated concentrations of endogenous ouabain in patients with congestive heart failure. Circulation. 1992;86(2):420–425. doi: 10.1161/01.cir.86.2.420. [DOI] [PubMed] [Google Scholar]
- 32.Fedorova OV, Talan MI, Agalakova NI, Lakatta EG, Bagrov AY. Coordinated shifts in Na/K-ATPase isoforms and their endogenous ligands during cardiac hypertrophy and failure in NaCl-sensitive hypertension. J Hypertens. 2004;22(2):389–397. doi: 10.1097/00004872-200402000-00025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.