Abstract
Background & Aims
Infection with the gastric mucosal pathogen H. pylori is the strongest identified risk factor for distal gastric cancer. These bacteria colonize a significant part of the world’s population. We investigated the molecular mechanisms of p53 regulation in H. pylori-infected cells.
Methods
Mongolian gerbils were challenged with H. pylori and their gastric tissues were analyzed by immunohistochemistry and immunoblotting with p53 antibodies. Gastric epithelial cells were co-cultured with H. pylori and the regulation of p53 was assessed by real-time PCR, immunoblotting, immunofluorescence, and cell survival assays. shRNA and dominant-negative mutants were used to inhibit activities of HDM2 and AKT.
Results
We found that in addition to previously reported up-regulation of p53, H. pylori can also negatively regulate p53 by increasing ubiquitination and proteasomal degradation via activation of the serine/threonine kinase AKT, which phosphorylates and activates the ubiquitin ligase HDM2. These effects were mediated by the bacterial virulence factor, CagA; ectopic expression of CagA in gastric epithelial cells increased phosphorylation of HDM2 along with the ubiquitination and proteasomal degradation of p53. The decrease in p53 levels increased survival of gastric epithelial cells that had sustained DNA damage.
Conclusion
H. pylori is able to inhibit the tumor suppressor p53. H. pylori activates AKT, resulting in phosphorylation and activation of HDM2 and subsequent degradation of p53 in gastric epithelial cells. H. pylori-induced dysregulation of p53 is a potential mechanism by which the microorganism increases the risk of gastric cancer in infected individuals.
Keywords: p73, p53 protein family, apoptosis, stomach cancer
Introduction
H. pylori is a Gram-negative pathogen that colonizes the stomachs of approximately half of the world’s population and is the strongest identified risk factor for the development of distal gastric cancer.
Although H. pylori is one of the most common bacterial infections globally, not all individuals develop gastric neoplasms. It is likely that a complex interplay between bacterial virulence and host factors is paramount in determining the progression to gastric cancer.1 Virulence factors allow H. pylori to induce an intense inflammatory response leading to gastric tissue damage that may result in premalignant pathological lesions and subsequently gastric cancer. The most distinguishing virulence constituent of H. pylori is the cag pathogenicity island (cag PAI), a 40kb region of DNA that encodes a type IV secretion system. A product of the cag PAI, CagA, is delivered by this secretion system into epithelial cells after bacterial attachment.
Recent studies have confirmed that CagA functions as a bacterial oncoprotein. It has been reported that CagA promotes anchor-independent growth of gastric epithelial cells in soft agar.2 Transgenic mice expressing CagA develop gastric epithelial neoplasms.3 Epidemiological studies have shown that the presence of CagA significantly increases the risk of gastric atrophy and gastric cancer. However, CagA is one of several bacterial factors known to be involved in H. pylori pathogenesis.
Although many oncogenic pathways induced by H. pylori have been characterized, less is known about tumor suppressors that may potentially counteract its tumorigenic function. p53 is a key tumor suppressor that is inactivated by mutations in approximately 40% of gastric tumors. The cag PAI contributes to p53 inactivation, as individuals infected with H. pylori cag+ strains have a higher likelihood of harboring p53 mutations.4 p53 can also be inhibited by non-mutational mechanisms. A number of oncoviruses specifically inactivate p53 as a part of their replication cycle.5 The Epstein-Barr virus, which has been implicated in the development of H. pylori-negative gastric tumors, expresses an immediate-early BZLF1 protein that inhibits p53 transcription activity.6 Thus, inactivation of p53 is a critical step and a common feature of gastric cancer development.
Currently, the regulation of p53 in H. pylori-infected cells is not well understood. Several immunohistochemical studies have found increased p53 levels in H. pylori infected patients.7–9 Up-regulation and activation of p53 have also been shown in vitro.10, 11 However, other studies reported either no significant difference in p53 expression and activity or decreased levels after exposure to H. pylori.12–15 No increased p53 expression has been reported in dyspeptic patients.12 In this study, only one out of 54 patients infected with H. pylori showed p53-positive immunohistochemical staining. The variability of clinical specimens with respect to pathological conditions, differences in H. pylori strains, and duration of infection as well as complex regulations of p53 expression are most likely contributed to different outcomes of the p53 analyses.
Here, we investigated mechanisms that regulate p53 in H. pylori-infected gastric epithelial cells.
Materials and Methods
Cell culture and H. pylori strains
Human gastric cancer cell lines SNU1, AGS, and Kato III were maintained in F-12 medium. HFE-145,16 a human gastric epithelial cell line, was cultured in DMEM/F-12 medium. p53-null osteosarcoma cell line, SaOs2, was grown in RPMI-1640 medium. All media were supplied by Invitrogen (Carlsbad, CA) and supplemented with 10% fetal bovine serum (FBS). The cagA+ H. pylori clinical strain J166 and rodent-adapted strain 7.13 were grown in Brucella broth with 5% FBS for 18 hours, harvested by centrifugation, and added to gastric cells at a bacteria-to-cell ratio of 100:1 or as indicated. Isogenic cagA− and cagE− mutants were constructed within strains J166 and 7.13 by insertional mutagenesis using aphA and selected with kanamycin.13 Heat-inactivated H. pylori were generated by heating the bacteria to 80°C for 10 minutes.
Antibodies
Antibodies to the following proteins were used: p53(DO-1), p53(DO-7), p21(Ab-1), HDM2(Ab-1), and p73(Ab-3) from Calbiochem; anti-CagA from Austral Biologicals (San Ramon, CA); pAKT(Ser473), pHDM2(Ser166) and AKT from Cell Signaling; anti-GFP from Clontech; p53(CM-1) and p53(NCL-p53-505) from Novocastra (UK); anti-ubiquitin from Santa Cruz; AKT(pT308) from Epitomics (Burlingame, CA), and MDM2 (154–167) from Spring Bioscience (Pleasanton, CA).
Gerbil infection and immunohistochemistry
All animal experiments and procedures were approved by the Institutional Animal Care Committee at Vanderbilt University. Four to eight week-old pathogen-free Mongolian gerbils purchased from Harlan Labs were orogastrically challenged with either sterile Brucella broth or rodent-adapted H. pylori strain 7.13 or its isogenic cagA− mutant. The animals were euthanized at indicated time points. At necropsy, linear strips extending from the squamocolumnar junction through the proximal duodenum were fixed in 10% neutral-buffered formalin, paraffin embedded, and stained with hematoxylin and eosin or with p53 (DO-1) antibody (1:200). The percentage of epithelial cells positive for p53 was determined by a pathologist in a blind manner.
Cell Cycle Analysis
Cell cycle analysis was carried out as previously described.17 CagA expression was induced by adding 2 μg/mL doxycycline to AGS cells that express CagA in a tetracycline-inducible manner. Cells were then treated with hydrogen peroxide for 36 hours, stained with propidium iodide, and analyzed by flow cytometry.
Clonogenic survival assay
CagA expression was induced with 2 μg/mL doxycycline in AGS cells that inducibly express CagA. An equal number of control(uninduced) and CagA-expressing cells were treated with 50 μM H2O2 for 4 hours in triplicate and replated onto Petri dishes. After 12–14 days of incubation the plates were gently washed and stained with crystal violet. Viable colonies containing more than 50 cells were counted.
Analysis of CagA-induced degradation of p53
p53-null cells, Kato III or SaOs2, were co-transfected with p53 and either empty vector, CagA or HDM2 using Lipofectamine 2000 at a 1:20 ratio. To normalize for transfection efficiency, cells were also co-transfected with GFP. Cell extracts were prepared 48 hours after transfection and analyzed for p53 expression using Western blotting with the p53 antibody (DO-1; 1:2000).
Statistical Analysis
Statistical analysis was performed using the Student t-test and Mann-Whitney tests, depending on the data set. Results were expressed as mean values. Results were considered significant if p< .05.
Additional information on primers, vectors, chemicals, immunofluorescence, and ubiquitination assays are described in the supplementary materials.
Results
Protein levels of p53 are dynamically changed in H. pylori-infected cells
Although p53 expression has been assessed in gastric tissues from H. pylori-infected patients, the dynamics of p53 alterations have not been defined in detail. In addition, there are no data on p53 alterations during the initial phases of infection. To examine how H. pylori affects p53 in vivo, we used Mongolian gerbils, as gastric pathology induced by H. pylori in this system more closely recapitulates human infection than murine models.18 As a prelude to our analyses, we characterized gerbil p53 using a panel of rodent and human p53 antibodies. Among the antibodies that recognize gerbil p53, antibody DO-1 was selected since it can be used for both immunohistochemistry and Western blotting and recognizes an epitope (amino acids 11–25) of human p53 that is phylogenetically conserved between these species (Supplementary figure 1).
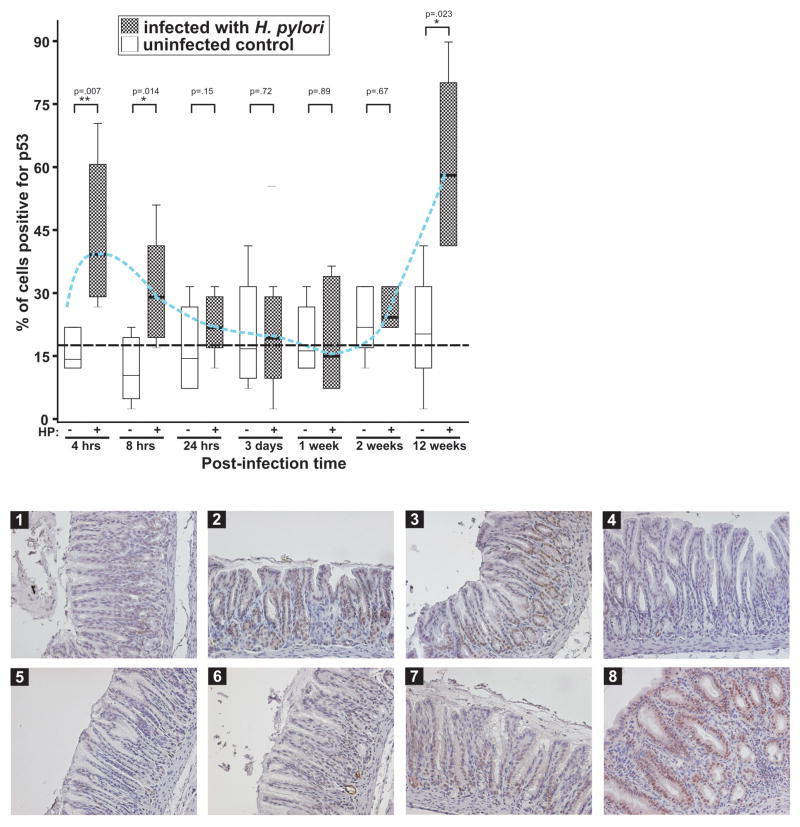
Mongolian gerbils (n=48) were infected with rodent adapted H. pylori strain 7.13, and disease outcome was followed. A control group of animals (n=48) received sterile broth alone. At indicated time intervals, gastric tissues were collected and examined for expression of p53 using immunohistochemistry. Levels of p53 in all uninfected animals were either low or undetectable. In contrast, the dynamics of p53 alterations in infected gerbils followed a pattern consisting of two peaks (Figure 1). Strong up-regulation of p53 was observed acutely in the antrum at 4–8 hours after infection and was strictly confined to the nuclei of epithelial cells. However, by 24 hours, the levels of p53 rapidly decreased despite the presence of bacteria. At three days, one and two weeks post-challenge, the levels were equal to or lower than in the control group of animals. The second peak of p53 up-regulation occurred later (12 weeks) and was accompanied by intense inflammation (Figure 1).
Figure 1. p53 levels are dynamically altered following H. pylori infection in vivo.
Upper panel: gastric tissues harvested from gerbils infected with H. pylori strain 7.13 at the indicated time were immunostained for p53 and quantitated using a blind protocol. Results are expressed as the percentage of p53 positive cells per sample. Mean values ( ) for infected and uninfected animals are shown. The blue dashed line connects mean values for p53 expression in infected gerbils. The black dashed line depicts the average levels of p53 in the uninfected control. Lower panel: representative staining for p53 (x20) is shown for uninfected control (1) and infected animals for 4 hrs(2), 8 hrs(3), 24 hrs(4), 3 days(5), 1 week(6), 2 weeks(7), and 12 weeks(8).
) for infected and uninfected animals are shown. The blue dashed line connects mean values for p53 expression in infected gerbils. The black dashed line depicts the average levels of p53 in the uninfected control. Lower panel: representative staining for p53 (x20) is shown for uninfected control (1) and infected animals for 4 hrs(2), 8 hrs(3), 24 hrs(4), 3 days(5), 1 week(6), 2 weeks(7), and 12 weeks(8).
Since H. pylori altered the levels of p53 acutely, we next examined whether H. pylori affects p53 protein levels in vitro. SNU1 gastric epithelial cells that express functional wild-type p53 were co-cultured with H. pylori strains 7.13 or J166 for 2–72 hours and p53 was analyzed by Western blotting (Figure 2A and data not shown). Similar to the dynamics of p53 in vivo, endogenous levels of p53 rapidly increased and then decreased following a co-culture with both H. pylori strains. Notably, p53 was decreased to levels that were lower than in uninfected control cells. Heat inactivation of H. pylori failed to induce changes in p53 levels, suggesting that live bacteria are needed for the regulation of p53 (Figure 2A, lower panel).
Figure 2. Dysregulation of p53 in gastric epithelial cells co-cultured with H. pylori.
(A) Protein lysates were prepared from control SNU1 cells (−) or those co-cultured with H. pylori (+) strain J166 for the indicated time and analyzed for expression of p53 by Western blotting (upper panel). The bottom panel shows cells co-cultured with heat-inactivated bacteria. (B) The same as (A) except AGS cells were used. Bottom panel: expression of TAp73β in AGS cells co-cultured with H. pylori. (C) SNU1 cells co-cultured with H. pylori strain J166 or treated with 10 nM camptothecin (CPT) for the indicated time were analyzed for p53 by Western blotting. Expression of p53 protein was quantitated by densitometry and normalized to actin expression. Data depicted as mean ± SEM (n=3). (D) AGS cells treated with 50 nM camptothecin for 6 hours. Camptothecin was removed and cells were then either supplemented with fresh media (upper panel) or co-cultured with H. pylori strain J166 (bottom panel) for 72 hours.
The initial increase in p53 followed by the rapid down-regulation was also observed in another gastric epithelial cell line, AGS (Figure 2B). The reduction in p53 was specific for this molecule as another member of the p53 family, p73, was not decreased but rather increased by co-culture of AGS or SNU1 cells with H. pylori (Figure 2B, bottom panel and data not shown).
We then compared changes in p53 that were induced by H. pylori with other known p53 inducers. Camptothecin was selected, as it is commonly used for induction of p53.17, 19 This natural alkaloid induces double-strand breaks in DNA leading to the activation of p53. We first determined the minimal concentration of camptothecin (10 nM) that induces p53 to the same levels as H. pylori and then compared the dynamics of p53 alterations for both p53 inducers. While p53 up-regulation was similar for H. pylori and camptothecin, co-culture of SNU1 cells with H. pylori led to the rapid decrease of p53, which was not observed in camptothecin-treated cells (Figure 2C). To confirm that H. pylori actively facilitates the decrease of p53, p53 was induced by camptothecin for 6 hours. The drug was then removed by washing with fresh media and cells were co-cultured with H. pylori strain J166. The co-culture of AGS cells with H. pylori led to rapid down-regulation of p53 compared to uninfected cells, which retained high levels of p53 (Figure 2D, compare lower and upper panels).
Thus, alterations in the levels of p53 can be characterized as an initial increase that is followed by rapid decrease induced by H. pylori.
H. pylori-induced down-regulation of p53 is mediated by AKT in an HDM2-dependent manner
To define in more detail the molecular regulation of p53 by H. pylori, we determined changes of p53 mRNA in AGS cells after co-culture with H. pylori. Our analysis revealed that mRNA levels of p53 did not change significantly (Figure 3A, left panel). Similar effects were seen in gastric epithelial cells, HFE-145, and the gerbil gastric mucosa (Supplementary figures 2A and 3A) indicating that p53 protein is regulated by post-translational mechanisms. Indeed, as shown in Figure 3A (right panel), the protein stability of endogenous p53 protein was decreased by H. pylori in AGS cells in which protein synthesis is blocked by cycloheximide.
Figure 3. p53 is down-regulated by H. pylori in an HDM2-dependent manner.
(A) Left panel: The bar graph represents quantitative real-time RT-PCR analysis of the p53 transcript in AGS cells co-cultured with H. pylori strain J166 for the indicated time. Data were normalized to HPRT1 mRNA. Expression of p53 mRNA in uninfected cells was arbitrarily set at 1. Right panel: Co-culture of AGS cells with H. pylori strain J166 decreases the protein stability of p53 after inhibition of protein synthesis with cycloheximide. Expression of p53 protein was quantitated by densitometry and normalized to actin. Data depicted as mean ± SEM (n=3). (B) Levels of p53 in AGS cells treated with proteasomal inhibitor MG-132 and co-cultured with H. pylori for 24 hours. (C) Treatment of AGS cells with Nutlin3 (10 μM), which interferes with p53-HDM2 interaction, suppresses H. pylori-induced inhibition of p53. (D) Inhibition of HDM2 by siRNA alleviates the down-regulation of p53 induced by H. pylori strain J166 in AGS cells. Right panel: HDM2 siRNA inhibited expression of the HDM2 protein.
As p53 can be regulated by ubiquitination and proteasome degradation, we next pre-treated AGS cells with 20 μM proteasome inhibitor MG-132 for 1 hour and co-cultured these cells with H. pylori for 24 hours. In untreated control cells p53 was down-regulated by H. pylori, whereas no effect was found in MG-132-treated cells (Figure 3B). Given that MG-132 did not adversely affect H. pylori viability at the used concentration,20 this result suggests that the decrease in p53 induced by H. pylori depends on proteasomal activity.
HDM2 is a main E3 ubiquitin ligase that mediates p53 degradation, therefore we next used Nutlin3 that inhibits the p53-HDM2 interactions. The specific inhibition of p53 degradation with Nutlin3 suppressed the down-regulation of p53 induced by H. pylori, indicating that HDM2 mediates this process (Figure 3C). Indeed, using RNA interference we found that inhibition of HDM2 in AGS cells with HDM2 siRNA alleviates the suppression of p53 by H. pylori (Figure 3D).
HDM2 activity is regulated by serine-threonine kinase AKT that phosphorylates HDM2.21 It has recently been reported that AKT is phosphorylated and activated in gastric epithelial cells infected with H. pylori.22–24 Consistent with these observations, an increased phosphorylation of AKT at Thr308 and Ser473 following H. pylori infection was observed in our studies (Supplementary Figure 4). Moreover, we found that H. pylori induces phosphorylation of HDM2 at Ser166, which is phosphorylated by AKT and leads to the activation of HDM221 in AGS or SNU1 cells co-cultured with H. pylori (Figure 4A). Notably, an increased MDM2 phosphorylation was also found in the gerbil gastric mucosa after H. pylori infection (Figure 4B). Increased HDM2 phosphorylation was not accompanied by an increase in total protein levels of HDM2.
Figure 4. AKT protein kinase regulates degradation of p53 in H. pylori-infected cells.
(A) Protein lysates were prepared from control AGS and SNU1 cells (−) or those co-cultured with H. pylori strain J166 (+) for the indicated time, and were analyzed by Western blotting with pHDM2 antibody that recognizes phosphorylation at position Ser166. (B) Analysis of MDM2 protein phosphorylation in gerbil gastric tissues after infection with H. pylori strain 7.13 using a pMDM2(Ser166) and MDM2 (154–169) antibodies. (C) Inhibition of AKT by siRNA suppressed H. pylori-mediated degradation of p53 and HDM2 phosphorylation at Ser166 in AGS cells.
To directly assess the role of AKT, we next inhibited AKT using siRNA and co-cultured these AGS cells with H. pylori, which resulted in a decrease of HDM2 phosphorylation as well as an increase in p53 levels in cells infected with H. pylori (Figure 4C).
Collectively, our data show that H. pylori increases phosphorylation and activation of HDM2 that induce proteasomal degradation of p53. This process in turn is mediated by the activation of AKT.
Role of H. pylori virulence constituents in regulation of p53
We next defined the specific H. pylori virulence factors that may regulate p53 protein levels. Since genes within the cag PAI have been shown to significantly affect the interaction with host epithelial cells, isogenic cagA− and cagE-null mutants were generated in H. pylori strains J166 and 7.13 and their ability to modulate p53 protein was examined. AGS cells were co-cultured with wild-type or isogenic H. pylori mutants and p53 protein levels were analyzed by Western blotting (Figure 5A). We found that deletion of cagA inhibits degradation of p53 induced by H. pylori. Notably, the cagE− mutant that does not form the type IV secretion system was also deficient in p53 regulation. Similarly, the ability of H. pylori to affect p53 was significantly compromised by loss of either cagA or cagE in SNU1 cells (data not shown). These results indicate that CagA, a component of the cag PAI, is involved in negative regulation of p53.
Figure 5. CagA regulates p53 levels in H. pylori-infected cells.
(A) AGS cells were cultured in the presence of the wild-type H. pylori strain 7.13 or isogenic cagA− or cagE− null mutants, and protein levels of p53 were assessed by Western blotting. (B) Left panel: gastric tissues harvested from gerbils infected with H. pylori strain 7.13 or isogenic cagA-null mutant at indicated time points were immunostained for p53 and quantitated using a blind protocol. Results are expressed as the percentage of p53 positive cells per sample. Mean values ( ) for cagA− and cagA+ infected animals are shown. A dashed line depicts the average levels of p53 in the uninfected control animals. Right panel: representative immunohistochemical staining for p53 (x20) is shown for uninfected animals (1) and those infected with wild-type (2) or cagA− (3) isogenic H. pylori strains for 6 hours (at the peak of p53 increase). Levels of p53 were also analyzed by Western blotting with p53-specific antibody at 6 hours. (C) Analysis of HDM2 phosphorylation in AGS cells co-cultured with H. pylori strain J166 or its isogenic cagA− or cagE− derivatives. (D) Analysis of p53 ubiquitination in AGS cells co-cultured with the indicated isogenic H. pylori strains for 24 hours. Proteasomal degradation was inhibited with MG-132.
) for cagA− and cagA+ infected animals are shown. A dashed line depicts the average levels of p53 in the uninfected control animals. Right panel: representative immunohistochemical staining for p53 (x20) is shown for uninfected animals (1) and those infected with wild-type (2) or cagA− (3) isogenic H. pylori strains for 6 hours (at the peak of p53 increase). Levels of p53 were also analyzed by Western blotting with p53-specific antibody at 6 hours. (C) Analysis of HDM2 phosphorylation in AGS cells co-cultured with H. pylori strain J166 or its isogenic cagA− or cagE− derivatives. (D) Analysis of p53 ubiquitination in AGS cells co-cultured with the indicated isogenic H. pylori strains for 24 hours. Proteasomal degradation was inhibited with MG-132.
To explore the effect of CagA on p53 within the gastric niche, two groups of gerbils were infected (n=8, per group) with isogenic wild-type or cagA− H. pylori 7.13 strains. At indicated times, gastric tissues were collected and analyzed for p53 expression by immunohistochemistry and Western blotting. Infection with both strains resulted in a rapid increase in p53 protein levels in the gerbils 4–6 hours after infection, when compared to uninfected control animals inoculated with broth alone. However, p53 increase at 6 hours was significantly higher in animals infected with the cagA− mutant than wild-type progenitor strain (Figure 5B). We also determined levels of p53 at a later time point, 12 weeks after infection. We found that in contrast to the earlier period of infection, gerbils challenged with isogenic wild-type H. pylori had significantly higher levels of inflammation and p53 when compared to animals infected with the isogenic cagA− mutant. The latter were characterized by weak p53 staining and mild inflammation.
Role of CagA in regulation of p53
To directly examine the role of CagA on p53 regulation, we next assessed the effect of this protein on phosphorylation of HDM2 and ubiquitination of p53. AGS cells were co-cultured with wild-type, cagA or cagE isogenic H. pylori mutants for 24 hours and HDM2 phosphorylation was analyzed by Western blotting. We found that deletion of cagA or cagE inhibit phosphorylation of HDM2 (Figure 5C). We next co-cultured AGS cells with wild-type, cagA or cagE isogenic H. pylori mutants, and p53 protein degradation was blocked by proteasomal inhibitor MG-132 (Figure 5D and Supplementary Figure 5), as described previously.25 Consistent with our results above, inactivation of CagA led to a decrease of ubiquitination of p53.
Next, we co-transfected p53 and CagA expression vectors into Kato III gastric epithelial cells, which lack the endogenous p53 gene expression. As a positive control, HDM2 and p53 were also co-expressed. As expected, ectopic HDM2 expression facilitated the degradation of p53 (Figure 6A, left panel). Similarly, expression of cagA led to a decrease of p53 in these cells although transfection with HDM2 was more potent in the induction of p53 degradation; most likely because CagA-induced degradation of p53 is mediated by low levels of endogenous HDM2. The decrease in p53 levels induced by CagA also led to the down-regulation of p21/Waf1, a well-known p53 transcription target (Figure 6A), and was accompanied by increased phosphorylation of endogenous HDM2 at Ser166 (Figure 6A and Supplementary Figure 6). We confirmed these data by co-transfection of CagA and p53 into another p53-null cell line, SaOs2, which has been previously used for studies of HDM2 mediated degradation of p53.25 Similar to Kato III cells, CagA transfection led to increased degradation of p53 in SaOs2 cells (Figure 6A, right panel). Our data suggest that this process is independent of phosphorylation of CagA (Supplementary Figure 7).
Figure 6. CagA induces degradation of p53.
(A) Left panel: p53-null Kato III cells were co-transfected with the indicated plasmids and GFP for 48 hours and then analyzed for p53 expression. Gel loading was normalized to GFP expression. HDM2 and p53 co-transfection was used as an additional positive control. Right panel: the same as the left panel but another p53-null osteosarcoma cell line, SaOs2, was used. (B) AGS cells were transfected with CagA-IRES-GFP (CagA) or empty IRES-GFP (Control) vectors. Twenty-four hours post-transfection cells were analyzed by immunofluorescence for p53 (red) in GFP-expressing cells (green). Nuclear p53 protein disappeared in 58% of CagA-expressing cells whereas only 13.5% of control GFP-positive cells were negative for p53. (C) Left panel: AGS cells that express CagA under control of tetracycline-inducible promoter were treated with hydrogen peroxide or left untreated and then analyzed for p53. Right panel: Control AGS cells (−DOX) or ones expressing CagA (+DOX) were treated with indicated concentrations of H2O2 for 24 hours. Cell death was assessed by flow cytometry after propidium iodide staining. The proportion of cells in subG1 is shown. CagA significantly increased survival of cells treated with H2O2. **, p< 0.01 vs. uninduced cells (n=3). (D) CagA increased long-term survival of AGS cells treated with H2O2. Analysis was conducted as described in the Materials and Methods section. The bar graph shows the number of colonies. **, p< 0.01 vs. uninduced cells (n=3).
To investigate the effect of CagA on endogenous p53, CagA was subcloned into a MSCV-IRES-GFP vector that bi-cistronically expresses CagA and GFP. AGS cells were then transfected with this vector and endogenous p53 levels were traced using indirect immunofluorescence (Figure 6B). We found that cells expressing CagA lack or have significantly lower levels of p53 than control cells that express GFP alone. Thus, CagA facilitates degradation of endogenous p53.
To assess the biological effect of CagA in our system of interest, we next constructed AGS cells that express CagA under the control of tetracycline-inducible promoter. Induction of CagA with doxycycline led to a decrease of endogenous p53 in these cells (Figure 6C). To analyze whether the down-regulation of p53 induced CagA affected DNA damage response, CagA was induced by doxycycline for 4 hours and cells were then treated with hydrogen peroxide, since this compound induces cell death through a p53-dependent pathway and is produced during H. pylori infection.26 Survival of treated cells was assessed using flow cytometry after propidium iodide labeling. We found that AGS cells that express CagA were significantly more resistant to the cytotoxic effects of hydrogen peroxide compared to control cells, which do not express CagA (Figure 6C). To determine whether CagA affects the long-term survival of cells, we carried out a clonogenic survival assay (Figure 6D). While control cells underwent significant cell death, cells in which CagA was induced by doxycycline survived and produced multiple colonies. CagA also protected AGS cells against another DNA-damaging agent, etoposide (data not shown). Thus, CagA increases the short- and long-term survival of gastric epithelial cells that have sustained DNA damage.
Discussion
In this study, we investigated the regulation of the p53 protein in H. pylori-infected gastric epithelial cells and found that this microorganism actively alters levels of p53. Previously, it has been reported that H. pylori infection increases the levels of the p53 protein in the gastric mucosa.7–9 Consistent with these reports, we observed up-regulation of p53 in Mongolian gerbils following H. pylori infection. However, our studies also provided evidence that regulation of p53 is more complex. We found that p53 was increased in a bimodal fashion in vivo. Acute accumulation of p53 occurred at 4–6 hours after infection and was followed by rapid decrease. A second peak of p53 was observed later upon gastritis development. Between the two peaks, levels of p53 remained relatively low for several weeks despite the presence of live bacteria. The complex p53 dynamics may have practical implications for studies of the p53 protein in the H. pylori-infected mucosa; depending on the time of analysis, the snapshot results of p53 might vary.
Transient up- and down-regulation of p53 was also found in vitro. We investigated the mechanisms of p53 down-regulation and found that H. pylori can accelerate ubiquitination and proteasomal degradation of p53. Notably, H. pylori specifically targets p53 as another member of the p53 family, p73, is not negatively affected by these bacteria. The down-regulation of p53 is mediated by a cellular HDM2 protein as suppression of its activity with Nutlin3 or siRNA inhibits H. pylori-induced degradation. Interestingly, the protein levels of HDM2 are not increased following H. pylori infection, but rather the levels of its phosphorylation by AKT kinase. Previously, it has been reported that AKT is activated in H. pylori-infected cells.22, 23 Our analysis was consistent with these findings. However, we also found that AKT mediates phosphorylation of HDM2 at serine 166, leading to the activation of HDM2 in H. pylori-infected cells (Figure 4). The increased phosphorylation of HDM2 was also found in H. pylori-infected gerbils. This phosphorylation event coincided with a decrease of p53 in the gastric mucosa.
Another important finding directly related to the mechanism of p53 regulation by H. pylori is the role of the cag PAI. CagA is a bacterial protein that is delivered by the type IV secretion system into epithelial cells after bacterial attachment. This subsequently activates multiple intracellular signaling cascades, eventuating in cellular morphological changes and alteration in the apoptotic response.27 To investigate the role of CagA in the regulation of p53, we used isogenic cagA− and cagE− H. pylori mutants. The loss of cagA inhibited the ability of H. pylori to down-regulate p53. Our findings also demonstrated that ectopic expression of CagA induces phosphorylation of HDM2 and decreases the levels of the p53 protein as well as increases survival of DNA damaged cells. We found that CagA-expressing cells have increased short- and long-term the survival rates after treatment with hydrogen peroxide and other DNA-damaging agents. These finding are consistent with a previous report showing that ectopic expression of CagA inhibits p53-dependent apoptosis induced by hydroxyurea in pre-B cells.28
However, the tumorigenic effect of H. pylori cannot be solely explained by the negative effects of CagA. For instance, H. felis, which lacks the cag PAI, is still able to induce severe inflammation and gastric cancer in rodent models. In addition, multiple mechanisms may lead to p53 inactivation. Approximately 40% of gastric tumors carry mutations in the p53 gene. Recently, it has been reported that H. pylori directly contributes to p53 mutagenesis by inducing AID expression.29 Down-regulation of the p53 protein by H. pylori may also play a role in the tumorigenic processes. Analogous to oncogenic viruses, which inhibit and/or degrade wild-type p53 protein as a part their infection cycle,5 down-regulation of p53 by H. pylori may lead to accumulation of oncogenic changes in normal cells.
Our findings also suggest that the level of p53 in H. pylori-infected cells reflects a balance between the p53 degradation induced by the bacteria and intrinsic cellular protection mechanisms, which up-regulate the p53 protein in response to cellular stresses (Figure 7). At the molecular level, it is manifested by negative and positive regulation of HDM2-dependent degradation of p53. Interactions between these processes may explain dynamic changes in the p53 levels during H. pylori infection. We think that the initial p53 increase is potentially related to an intracellular fail-safe mechanism that protects epithelial cells against aberrant activation of oncogenes induced by H. pylori and regulated by ARF protein.11 For example, well-known oncogenic inducers of p53 such as Ras, cMyc, and β-catenin are abnormally activated during H. pylori infection. The activation of β-catenin has recently been found to occur as early as four hours after infection of gerbils with H. pylori.30 DNA damage that has been shown to be induced by H. pylori may also lead to activation of p53.26 Between the two peaks of p53 up-regulation found in vivo, the steady-state level of p53 remains relatively low. This may play an important role in the adaptation of the bacteria. During this period of infection, inhibition of p53-dependent apoptosis may prevent excessive cell death, which triggers premature activation of immune response. Additional studies are needed to directly address these questions. Interestingly, the second peak of p53 is associated with a high level of inflammation. During this phase of infection, the p53 increase may be driven by DNA damage known to be associated with inflammatory processes and may lead to enhanced apoptosis in mucosal cells. This is consistent with the observations that eradication of H. pylori decreases the p53 levels.31
Figure 7.
Regulation of p53 by H. pylori.
In summary, this is the first demonstration that pathogenic bacteria can inhibit the tumor suppressor p53. The dysregulation of p53 by H. pylori may explain the increased risk of gastric cancer in individuals infected with this pathogenic microorganism.
Supplementary Material
Acknowledgments
This work was supported by the National Cancer Institute grants NIH CA108956, NIH CA129655, UL1 RR024975, and 1RO1 CA138833.
Abbreviations
- cagA
cytotoxin-associated antigen A
- cagE
cytotoxin-associated antigen E
Footnotes
The authors have no conflict of interest to disclose.
Role of authors:
Jinxiong Wei, Toni A. Nagy, Anna Vilgelm, Elena Zaika, Seth R. Ogden, Judith Romero-Gallo, Maria B. Piazuelo - acquisition of data and technical support;
Pelayo Correa, Mary K. Washington, Wael El-Rifai, Richard M. Peek - critical discussion of experimental data, material and technical support;
Alexander Zaika - study supervision.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Atherton JC, Blaser MJ. Coadaptation of Helicobacter pylori and humans: ancient history, modern implications. J Clin Invest. 2009;119:2475–87. doi: 10.1172/JCI38605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu Y, Zhong X, Zheng S, et al. Transformed immortalized gastric epithelial cells by virulence factor CagA of Helicobacter pylori through Erk mitogen-activated protein kinase pathway. Oncogene. 2005;24:3886–95. doi: 10.1038/sj.onc.1208551. [DOI] [PubMed] [Google Scholar]
- 3.Ohnishi N, Yuasa H, Tanaka S, et al. Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proc Natl Acad Sci U S A. 2008;105:1003–8. doi: 10.1073/pnas.0711183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shibata A, Parsonnet J, Longacre TA, et al. CagA status of Helicobacter pylori infection and p53 gene mutations in gastric adenocarcinoma. Carcinogenesis. 2002;23:419–24. doi: 10.1093/carcin/23.3.419. [DOI] [PubMed] [Google Scholar]
- 5.Levine AJ. The common mechanisms of transformation by the small DNA tumor viruses: The inactivation of tumor suppressor gene products: p53. Virology. 2009;384:285–93. doi: 10.1016/j.virol.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 6.Mauser A, Saito S, Appella E, et al. The Epstein-Barr virus immediate-early protein BZLF1 regulates p53 function through multiple mechanisms. J Virol. 2002;76:12503–12. doi: 10.1128/JVI.76.24.12503-12512.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gobbo Cesar AC, de Freitas Calmon M, Cury PM, et al. Genetic alterations in benign lesions: chronic gastritis and gastric ulcer. World J Gastroenterol. 2006;12:625–9. doi: 10.3748/wjg.v12.i4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozturk Y, Ozer E, Lebe B, et al. Immunohistochemical evaluation of p53 expression and proliferative activity in children with Helicobacter pylori associated gastritis. J Pediatr Gastroenterol Nutr. 2005;40:467–70. doi: 10.1097/01.mpg.0000148832.22130.d7. [DOI] [PubMed] [Google Scholar]
- 9.Unger Z, Molnar B, Pronai L, et al. Mutant p53 expression and apoptotic activity of Helicobacter pylori positive and negative gastritis in correlation with the presence of intestinal metaplasia. Eur J Gastroenterol Hepatol. 2003;15:389–93. doi: 10.1097/00042737-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed A, Smoot D, Littleton G, et al. Helicobacter pylori inhibits gastric cell cycle progression. Microbes Infect. 2000;2:1159–69. doi: 10.1016/s1286-4579(00)01270-3. [DOI] [PubMed] [Google Scholar]
- 11.Ashktorab H, Ahmed A, Littleton G, et al. p53 and p14 increase sensitivity of gastric cells to H. pylori-induced apoptosis. Dig Dis Sci. 2003;48:1284–91. doi: 10.1023/a:1024198807619. [DOI] [PubMed] [Google Scholar]
- 12.Berloco P, Russo F, Cariola F, et al. Low presence of p53 abnormalities in H pylori-infected gastric mucosa and in gastric adenocarcinoma. J Gastroenterol. 2003;38:28–36. doi: 10.1007/s005350300003. [DOI] [PubMed] [Google Scholar]
- 13.Peek RM, Jr, Blaser MJ, Mays DJ, et al. Helicobacter pylori strain-specific genotypes and modulation of the gastric epithelial cell cycle. Cancer Res. 1999;59:6124–31. [PubMed] [Google Scholar]
- 14.Shirin H, Sordillo EM, Oh SH, et al. Helicobacter pylori inhibits the G1 to S transition in AGS gastric epithelial cells. Cancer Res. 1999;59:2277–81. [PubMed] [Google Scholar]
- 15.Suzuki H, Miyazawa M, Kai A, et al. No difference in the level of gastric mucosal cell apoptosis and proliferation in Helicobacter pylori-colonized p53 heterozygous knockout mice. Aliment Pharmacol Ther. 2002;16 (Suppl 2):158–66. doi: 10.1046/j.1365-2036.16.s2.18.x. [DOI] [PubMed] [Google Scholar]
- 16.Marlink KL, Bacon KD, Sheppard BC, et al. Effects of Helicobacter pylori on intracellular Ca2+ signaling in normal human gastric mucous epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2003;285:G163–76. doi: 10.1152/ajpgi.00257.2002. [DOI] [PubMed] [Google Scholar]
- 17.Wei J, O’Brien D, Vilgelm A, et al. A. Interaction of Helicobacter pylori with gastric epithelial cells is mediated by the p53 protein family. Gastroenterology. 2008;134:1412–23. doi: 10.1053/j.gastro.2008.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kodama M, Murakami K, Nishizono A, et al. Animal models for the study of Helicobacter-induced gastric carcinoma. J Infect Chemother. 2004;10:316–25. doi: 10.1007/s10156-004-0353-z. [DOI] [PubMed] [Google Scholar]
- 19.Vilgelm A, Wei JX, Piazuelo MB, et al. DeltaNp73alpha regulates MDR1 expression by inhibiting p53 function. Oncogene. 2008;27:2170–6. doi: 10.1038/sj.onc.1210862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eguchi H, Herschenhous N, Kuzushita N, et al. Helicobacter pylori increases proteasome-mediated degradation of p27(kip1) in gastric epithelial cells. Cancer Res. 2003;63:4739–46. [PubMed] [Google Scholar]
- 21.Zhou BP, Liao Y, Xia W, et al. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat Cell Biol. 2001;3:973–82. doi: 10.1038/ncb1101-973. [DOI] [PubMed] [Google Scholar]
- 22.Tabassam FH, Graham DY, Yamaoka Y. Helicobacter pylori activate epidermal growth factor receptor- and phosphatidylinositol 3-OH kinase-dependent Akt and glycogen synthase kinase 3beta phosphorylation. Cell Microbiol. 2009;11:70–82. doi: 10.1111/j.1462-5822.2008.01237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takeshima E, Tomimori K, Kawakami H, et al. NF-kappaB activation by Helicobacter pylori requires Akt-mediated phosphorylation of p65. BMC Microbiol. 2009;9:36. doi: 10.1186/1471-2180-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Suzuki M, Mimuro H, Kiga K, et al. Helicobacter pylori CagA phosphorylation-independent function in epithelial proliferation and inflammation. Cell Host Microbe. 2009;5:23–34. doi: 10.1016/j.chom.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Zaika A, Marchenko N, Moll UM. Cytoplasmically “sequestered” wild type p53 protein is resistant to Mdm2-mediated degradation. J Biol Chem. 1999;274:27474–80. doi: 10.1074/jbc.274.39.27474. [DOI] [PubMed] [Google Scholar]
- 26.Xu H, Chaturvedi R, Cheng Y, et al. Spermine oxidation induced by Helicobacter pylori results in apoptosis and DNA damage: implications for gastric carcinogenesis. Cancer Res. 2004;64:8521–5. doi: 10.1158/0008-5472.CAN-04-3511. [DOI] [PubMed] [Google Scholar]
- 27.Handa O, Naito Y, Yoshikawa T. CagA protein of Helicobacter pylori: A hijacker of gastric epithelial cell signaling. Biochem Pharmacol. 2006 doi: 10.1016/j.bcp.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 28.Umehara S, Higashi H, Ohnishi N, et al. Effects of Helicobacter pylori CagA protein on the growth and survival of B lymphocytes, the origin of MALT lymphoma. Oncogene. 2003;22:8337–42. doi: 10.1038/sj.onc.1207028. [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto Y, Marusawa H, Kinoshita K, et al. Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat Med. 2007;13:470–6. doi: 10.1038/nm1566. [DOI] [PubMed] [Google Scholar]
- 30.Franco AT, Israel DA, Washington MK, et al. Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc Natl Acad Sci U S A. 2005;102:10646–51. doi: 10.1073/pnas.0504927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kodama M, Fujioka T, Murakami K, et al. Eradication of Helicobacter pylori reduced the immunohistochemical detection of p53 and MDM2 in gastric mucosa. J Gastroenterol Hepatol. 2005;20:941–6. doi: 10.1111/j.1440-1746.2005.03880.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.