Abstract
Inflammation and immunity have been implicated in a wide variety of diseases and disorders ranging from Alzheimer’s disease to cardiovascular disease to hemorrhagic shock. In this review, we will briefly consider the evidence for the neural concomitants of immunomodulation. First, we will briefly review the anatomy and physiology of neural-immune communication. Evidence for the somatotopic organization of the vagus nerve and for pain processes suggests that such an organization may be relevant for the investigation of the neural concomitants of immunity. Then we will provide an overview of what is known from both animal and human studies including neuroimaging and clinical studies. Finally, we will discuss some of the challenges and opportunities in this exciting area of investigation.
Keywords: Neural-immune, Vagus, Heart rate variability, Immune, Innervation, Brain
1. The anatomy and physiology of neural-immune communication
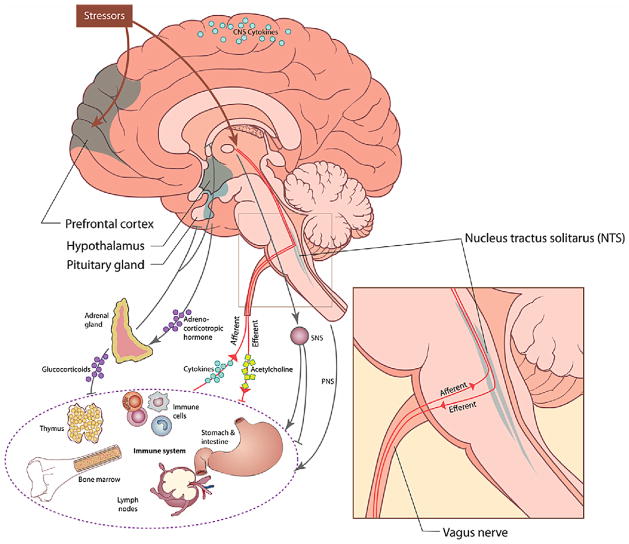
Although some controversy still exists surrounding specific aspects of neural-immune communications, the wealth of evidence provides strong support for numerous interactions among the central nervous system (CNS), peripheral nervous system (both sympathetic and parasympathetic branches), the endocrine system, and the immune system (Felton, 2000; Sternberg, 2006; Watkins and Maier, 1999; see Fig. 1). Neurohormonal pathways involving circulating hormones such as cortisol (in humans) and catecholamines, including norepinephrine, have been described in some detail (Sternberg, 1997, 2006). In addition to, and perhaps at least as important as the humoral pathway, are neural pathways, including the peripheral nervous system (reviewed in Sternberg (2006)), the sympathetic nervous system (SNS: reviewed in Nance and Sanders (2007)), and the parasympathetic nervous system. The latter is the focus of this review (see also Tracey (2002, 2007), and van der Zanden et al. (2009)).
Fig. 1.
While release of neurohormones into the circulation provides a mechanism for regulation of immunity at a systemic level, through neurohormonal binding to receptors in immune cells, neural pathway regulation of immunity confers an added dimension to immune regulation – that of anatomical location (Sternberg, 2006; Tracey, 2002). Thus, neural pathways regulate immunity at a local and regional level. This level of anatomical organization is important because immune organs are specialized to regulate different aspects of immune function (e.g., thymus and lymph nodes regulate cellular immunity; bone marrow and spleen regulate humoral immunity; skin and mucosa contain the first line defense cells of innate immunity). Specific regions within immune organs are further specialized to regulate different immune cells at different stages of development (Felton, 2000). Thus within the thymus and lymph nodes there are regions where developing and maturing T lymphocytes are exposed to antigen and either die through apoptosis or go on to mature to become specific immune activated cells, each with specialized function, for example capable of killing viruses or cancer cells. Within the spleen and bone marrow, B lymphocytes mature to the stage where they can produce specific antibodies.
Superimposed on this anatomical structure of functional specificity of immune cells and organs, is the structure and specificity of the neural pathways that innervate these organs. The sympathetic nervous system regulates immunity at a regional level, through innervation of immune organs including the spleen, thymus and lymph nodes (Nance and Sanders, 2007; Sternberg, 2006). Sympathetic influences can be both pro- and anti-inflammatory (Elenkov et al., 2000; Thayer and Fischer, 2009). The SNS plays a role in redistribution of immune-cell populations acutely, and also can be immunosuppressive, affecting immune-cell function during conditions of massive norepinephrine (NE) release within these organs, such as occurs during stress (reviewed in Kennedy et al. (2005), Nance and Sanders (2007)). Clinically relevant effects of NE have been found as both human and animal studies suggest that NE is involved in wound healing (Yang et al., 2002; Gosain et al., 2006). The peripheral nervous system regulates immunity at sites of inflammation, wherever in the body this might occur. Neuropeptides released from peripheral nerves tend to be pro-inflammatory and are largely responsible for the characteristic features of “calor, rubor and dolor” (heat, redness and pain) at inflammatory sites (Sternberg, 2006).
Of particular relevance for this review, the parasympathetic nervous system has been shown to play a crucial role in immunomodulation. Both afferent and efferent parasympathetic activity is thought to play a role in immunomodulation (Sternberg, 2006; Tracey, 2007; van der Zanden et al., 2009). By the nature of its “wandering” route through the body the vagus nerve may be uniquely structured to provide an effective early warning system for the detection of pathogens as well as a source of negative feedback to the immune system after the pathogens have been cleared (Berthoud and Neuhuber, 2000). The vast majority of vagal fibers (upwards of 80%) are sensory in nature and thus provide an effective coverage of the body for the detection of invaders (Berthoud and Neuhuber, 2000). The anatomy of the afferent vagus has been described in detail by Berthoud and Neuhuber (2000) and in many species including humans has been shown to have connections to the heart, lungs, esophagus, and liver among other organs. Moreover, the afferent vagus has interleukin (IL)-1 receptors expressed by paraganglia cells situated in parasympathetic ganglia (Sternberg, 2006; Watkins and Maier, 1999). The presence of cytokines such as IL-1 in the periphery is relayed via the vagus nerve to CNS structures, one of the most important being the nucleus of the solitary tract (NTS). At the NTS the afferent and efferent aspects of the parasympathetic nervous system meet. Therefore the NTS is a major relay station for neural-immune communication (Sternberg, 2006). On the afferent side, vagal afferents terminate in the NTS in a somatotopic manner resulting in functional divisions of the NTS (Maier et al., 1998). This somatotopic organization may allow for a high degree of localization and specificity of immune-to-brain communication. This is important as the anatomical location of the pathogens conveys information necessary to mount a location specific and thereby more effective response. Moreover, the NTS has direct and indirect connections to a wide range of neural structures thus giving the vagus nerve the capacity to influence a broad array of processes (Groves and Brown, 2005). On the efferent side, the NTS provides input to the dorsal motor nucleus of the vagus (DMV) and the nucleus ambiguous (NA). These are the sources of the efferent signals which innervate many of the organs associated with the immune system including the heart, liver, and gastrointestinal system. Acetylcholine release from the vagus nerve modulates immune responses at least in part via alpha 7 nicotinic receptors that inhibit NF kappa B and thus cytokine synthesis and release (Rosas-Ballina and Tracey, 2009). It should be noted that the source of the regulatory acetylcholine is not unambiguous and it has been suggested that it may be immune-cell derived instead of being released from nerve endings (Kawashima and Fujii, 2004). Taken together these parasympathetic pathways form what has been termed “the cholinergic anti-inflammatory pathway” (Tracey, 2007, 2009). This mechanism of immunomodulation is particularly relevant to this review as several neuroimaging studies in humans have identified CNS structures associated with both immunomodulation and (cardio)vagal modulation.
One point of controversy as noted above is the source of the regulatory acetylcholine and the innervation of the spleen in particular. Early studies by Dale suggested that the spleen was directly innervated by the vagus nerve (Dale and Dudley, 1929). However, subsequent studies called those findings into question (reviewed in Nance and Sanders (2007) but see Migini et al. (2005)). The current literature remains equivocal with some researchers providing evidence for direct innervation of the spleen (Buijs et al., 2008; Chen et al., 1996) whereas other researchers provide evidence for indirect or no innervation of the spleen (Bellinger et al., 1993; Nance and Burns, 1989; Rosas-Ballina et al., 2008). Buijs et al. (2008) directly examined the autonomic innervation of the spleen by injecting the trans-synaptic retrograde tracer Pseudorabies virus (PRV) into the spleen and observing the presence of PRV positive neurons in various target areas in the central nervous system. PRV positive neurons were found in sympathetic motor neurons in the interomedio lateral (IML) column and in the parasympathetic neurons of the dorsal motor nucleus of the vagus (DMV) after 2 days. Importantly, these researchers performed additional experiments using selective denervation and longer survival times. After selective sympathetic denervation and 2 day survival time, PRV positive neurons could no longer be found in the IML whereas PRV positive neurons continued to be found in the DMV. After selective parasympathetic denervation and 2 day survival time, PRV positive neurons could no longer be found in the DMV whereas PRV positive neurons continued to be found in the IML. After longer survival times a larger number of brain stem and higher brain areas, including the bed nucleus of the stria terminalis and the central nucleus of the amygdala, were associated with the direct parasympathetic innervation of the spleen compared to the sympathetic innervation. Thus these findings suggest that both the sympathetic and the parasympathetic nervous systems innervate the spleen directly.
On the other hand Rosas-Ballina et al. (2008) propose that the vagus nerve may not innervate the spleen directly but modulates splenic nerve function via nicotinic acetylcholine receptors on the celiac-superior mesenteric ganglion. This mechanism is thought to involve the stimulation of adrenegeric receptors on spleen immune cells.
One reason for the differing findings in the literature may have to due with differences in the methods for assessing the innervation of the spleen. For example, Buijs et al. (2008) used PRV and selective denervation of the sympathetic and parasympathetic inputs to the spleen whereas Rosas-Ballina et al. (2008) used staining for antibodies against acetyltransferase and vesicular acetylcholine transporter to infer that the spleen was not directly innervated by the vagus. However, as noted by Buijs et al. (2008, p. e3152) the absence of staining for antibodies against acetyltransferase and vesicular acetylcholine transporter does not necessarily indicate the absence of direct vagal innervation (cf., the liver). Differences in methodology may not completely explain the conflicting reports in the literature (see below for additional factors). For example, Cano et al. (2001) using PRV staining methods also failed to find evidence for direct vagal innervation of the spleen. Thus whereas the exact structural relationship between the vagus and the spleen remains a topic of debate, it appears that functionally the vagus is able to modulate immune activity of the spleen.
Another factor that may contribute to this controversy may be the evolving understanding of neurotransmission in general and of the nature of the autonomic neuroeffector junction in particular (Burnstock, 2009). A full exposition is not possible here and the interested reader is referred to Burnstock (2009) for a thorough review but several aspects are of particular importance and will be briefly described.
First, unlike the skeletal neuromuscular junction and the neuronal synapse, the autonomic neuroeffector junction is comprised of continuously moving varicosities whose relationship with the neuroeffector cell membranes changes over time. Thus even close junctions may be only temporary (Burnstock, 2009, p. 3). This may make it difficult to find evidence for “direct” innervation of various organs by the autonomic nervous system. As noted by Burnstock (2008, 2009, p. 5) this may be particularly relevant for neural-immune communication. Importantly, the classical idea that Sir Henry Dale (1934) put forward that each neuron had only one neurotransmitter associated with it, (i.e., the post-ganglionic sympathetic neurons used norepinephrine exclusively whereas the post-ganglionic parasympathetic neurons used acetylcholine exclusively) has been shown to be incorrect. Thus neurons may release a number of substances that may serve to modulate each other and thus produce a complex range of effects. This process has been called co-transmission (Burnstock, 2009).
Relevant to the current discussion, Straub et al. (2000) provided evidence for the complexity of sympathetic modulation of immune function at the spleen. Specifically it was demonstrated that neuropeptide Y (NPY), which is co-released from sympathetic nerves with NE, has both inhibitory effects via alpha-adrenergic receptors at low concentrations of NE and stimulatory effects via beta-adrenergic receptors at higher concentrations of NE as indexed by IL-6 secretions of spleen slices.
Similarly, Hoover et al. (2009) provided evidence of co-transmission of acetylcholine and nitric oxide (NO) at cardiac cholinergic nerves. Importantly, there is also some evidence for the existence of co-transmission of acetylcholine and NE among a subset of cholinergic neurons including some cardiac neurons (Hoover et al., 2009; Weihe et al., 2005). To the best of our knowledge there is no evidence for these dual phenotype cholinergic/adrenergic neurons in the innervation of the spleen. Co-transmission is an as yet incompletely understood process and has been shown to differ among species. Therefore findings based in one animal may not generalize to another animal including humans. Thus as new techniques to assess and characterize the autonomic neuroeffector junction become available our understanding of the innervation of the immune system may continue to evolve.
2. Neural concomitants of immune function
The evidence for anatomic specialization of immune regulation in both efferent and afferent directions suggests that different regions of the immune response may indeed be reflected centrally in an anatomical representation within the brain, which reflects the anatomical organization of the immune system. Such organization could, if proven to exist, potentially confer differential and specialized control of immune responses through efferent neural routes.
Both animal and human studies have implicated CNS structures in immunomodulation (Goehler et al., 2000; Ohira et al., 2006). Ascending from the NTS the vagus reaches the parabrachial nucleus, the thalamus, the paraventricular nucleus, the central nucleus of the amygdala, the insula cortex, and in animals the infralimbic cortex including the homologus sites in humans of the anterior cingulate cortex (ACC) and the medial prefrontal cortex (MPFC), (Ter Horst and Postema, 1997; Thayer and Lane, 2009). In humans a growing number of neuroimaging studies have investigated the neural concomitants of immune function and have generally confirmed the importance of the insula, the ACC, and the MPFC (Ohira et al., 2006; Rosenkranz et al., 2005). Ohira et al. (2006) using positron emission tomography (PET) reported that increasing natural killer (NK) cell counts were associated with increasing activity in the orbitofrontal cortex (OFC) and left insula cortex whereas decreases in CD4+T lymphocyte numbers were associated with decreases in activity in the medial orbitofrontal cortex and the right insula. Rosenkranz et al. (2005) using fMRI showed associations between tumor necrosis factor (TNF)-alpha measured in the periphery in response to immunological challenge and activity in the anterior cingulate cortex (ACC) as well as between eosinophils and activity in the insula. Similarly, Eisenberger et al. (2009) found that in females but not males endotoxin induced increases in IL-6 were associated with increased blood flow as indexed by fMRI in the dorsal ACC and the right anterior insula. O’Connor et al. (2009) using salivary measures of IL-1 beta and TNF receptor II in bereaved women found positive associations between the pro-inflammatory cytokines and activity in the subgenual ACC and the OFC. In a replication and extension of their earlier work Ohira et al. (2009) found NK cell counts during either a controllable or an uncontrollable stochastic mental stress task to be positively associated with a number of brain regions both inside and outside the prefrontal cortex including the ACC, the OFC, the right dorsolateral prefrontal cortex (DLPFC) and the medial prefrontal cortex (MPFC) among others. Interestingly these researchers also measured HF-HRV and found it to be positively associated with activity in the MPFC and the adjacent ACC as well as the DLPFC. This latter study suggests that there may be overlap between the brain regions associated with immunomodulation and those associated with cardiovagal modulation. Taken together these human imaging studies of the neural concomitants of immunomodulation suggest that specific brain regions may be associated with regulation of specific immune functions.
Relatedly, neuroimaging studies of cardiovagal function have identified a similar set of structuresassociated with autonomic modulation (e.g., Critchley et al., 2003; Gianaros et al., 2004; Lane et al., 2009). For example, Lane et al. (2009) found positive associations between vagally mediated heart rate variability (HRV) and activity in the ACC and insula among other structures. A recent meta-analysis of studies that measured HRV and cerebral blood flow suggested a number of areas including the amygdala and the various regions of the ACC that were positively associated with HRV (Thayer et al., in press-a). As suggested by the Ohira et al. (2009) study the neural structures associated with immunomodulation may bear similarity to those associated with cardiovagal modulation. For example, one region in the MPFC that Ohira et al. (2009) found to correlate positively with NK cell counts overlaps with a region in the meta-analysis associated with HF-HRV. Importantly, the regions that have been associated with various immune functions as well as those identified as being associated with HRV are part of a medial prefrontal-brainstem network that Lane and Wager (2009) have suggested as being critically involved in the regulation of the autonomic nervous system, the endocrine system and the immune system as well as the related functions of pain, emotion, and behavioral regulation. Thus, evidence from both animal and human studies provide support for the idea that forebrain structures may be involved in immunomodulation at least partially via the neural concomitants of the cholinergic anti-inflammatory pathway. However, systematic studies are needed in which specific immune processes from specific locations are mapped onto specific brain regions. Furthermore, whereas there is evidence that the NTS has a somatotopic organization there is as yet no evidence that this extends into the forebrain regions that have been linked to immune processes.
Neuroimaging studies are not without their ambiguities. Many of the neural functions associated with immunomodulation may be inhibitory in nature. It is an underappreciated fact of neuroimaging that the tight link between observed signal and the underlying metabolic activity is only monotonic for glutamate and excitatory neural activity (Magistretti and Pellerin, 1999). On the other hand it has been shown that the association between the observed signal and the underlying metabolic activity for inhibitory processes is certainly not monotonic (Aron, 2007, p. 220; Chatton et al., 2003; Thayer, 2006). Given that inhibitory interneurons may be critical to the modulation of large scale excitatory neural networks, this lack of ability to clearly observe inhibitory activity represents a significant challenge to the study of the inhibitory cholinergic anti-inflammatory pathway and may impact the ability to study neural-immune communication more generally. Other paradigms from neuroscience as well as appropriately designed animal studies may be a necessary complement to human neuroimaging studies in the search for the neural concomitants of immunomodulation.
3. Relationship between immune function and the autonomic nervous system: evidence from human clinical studies
The vast majority of the data for neural-immune interactions has come from animal studies. However, there is a growing body of literature from human clinical studies which suggests that autonomic modulation of immune responses may have important clinically relevant effects. Haensel et al. (2008) recently reviewed the literature on the relationship between HRV and inflammatory markers in adults. They identified thirteen studies involving healthy adults and patients with various disorders including cardiovascular, kidney, and renal disease. They concluded that the majority of studies found an inverse association between measures of HRV and inflammatory markers and that the range of observed correlations was between −0.020 and −0.40 (p. 1305).
One of the most important conclusions reached by Haensel et al. (2008) was that sympathetic nervous system function must be considered in interpreting the results of studies of the association between HRV and inflammation. Since their review numerous other reports have appeared. However, to our knowledge only one study has examined the association between HRV and inflammatory markers while also taking into account sympathetic nervous system function. Thayer and Fischer (2009) examined the relationships among vagal function as indexed by 24 h HRV, sympathetic activity as indexed by overnight urinary norepinephrine (NE), and inflammation as indexed by C-reactive protein (CRP) and white blood cell counts (WBC) in a sample of 611 apparently healthy adults. In univariate models (zero order correlations) they found that NE was positively related to WBC, and both root mean squared successive differences (RMSSD) and the percentage of interbeat interval differences >50 ms (pNN50) were inversely associated with CRP and WBC. Importantly, in multivariate models that controlled for a number of factors including sympathetic activity as measured by NE, RMSSD and pNN50 remained significantly inversely related to CRP and WBC. Thus, even when controlled for the effects of sympathetic nervous system activity, which could be pro- or anti-inflammatory (see above), measures of vagally mediated HRV remained inversely associated with markers of inflammation. These results help to clarify some of the previous studies in which associations between mixed measures of HRV such as the standard deviation of all interbeat intervals (SDNN) were found. It should be noted that various studies found associations between low frequency (LF) spectral power and inflammatory markers. LF power is an index that reflects both sympathetic and parasympathetic influences under certain conditions (Thayer et al., 2008). However, this index correlates very highly with measures of vagally mediated HRV and in the supine position reflects almost exclusively parasympathetic influences (Moak et al., 2007; Thayer et al., in press-b).
Experimental studies in humans also provide evidence for a role of vagal function in the regulation of immune responses. Weber et al. (in press) examined the influence of individual differences in vagally mediated HRV on the cardiovascular, endocrine, and immune systems during reactivity to and recovery from an acute mental stressor. In this study of 44 healthy young men it was found that those with low HRV had delayed recovery of diastolic blood pressure, cortisol, and TNF-alpha up to an hour after the stressor had ended. These results add to the human observational studies and suggest that vagal function is important in the regulation of both acute as well as chronic inflammation in apparently healthy humans.
4. Challenges and opportunities
Tracey (2007) has suggested that future research on the connections between the brain and the immune system may reveal an immunological homunculus. Analogous to the classical maps of the brain that somatotopically relate specific neural structures to specific action in the periphery, an immunological homunculus may reveal that there may be specific brain regions associated with the modulation of specific immune functions. Whereas the evidence for the somatotopic organization of immune functions is sparse as of yet, it has been demonstrated for the related phenomenon of pain (Henderson et al., 2007; Weiss et al., 2008). Henderson et al. (2007) using high-resolution functional magnetic resonance imaging (fMRI) showed that pain from different locations as well as from different tissues was represented somatotopically in the insula cortex. Weiss et al. (2008) using event-related fMRI showed that different afferent vagal nerve fibers (i.e., A δ versus C) were represented differentially in the cortex of humans. Advances in neuroimaging and other related technologies such as transcranial magnetic stimulation (TMS) may allow for the mapping of the neural concomitants of a range of immune functions such that for example, one brain region might be associated with the control of cytokine responses in the liver whereas another brain region may be associated with NK cell distribution. However, to take advantage of the opportunities afforded by these advances in technology several challenges have to be met. For example, the coordination of the timing of the various immune responses, which can span from seconds to hours, with the timing of various neuro-imaging paradigms such as PET or fMRI is an important hurdle to overcome if the association of specific brain regions with specific immune functions is to be physiologically meaningful.
It also appears from both animal and human studies that there may be significant gender differences in neuroimmunomodulation. For example, Butts et al. (2008) reported that the effect of progesterone on bone marrow derived dendritic cells was more pronounced in females compared to male rodents as indexed by pro-inflammatory cytokine secretion. In humans, Thayer and Fischer (2009) found that the inverse association between HRV and CRP was 4.4 times greater in females than in males. Both of these studies suggested that these gender differences may have important implications for understanding the disparities in autoimmune disorders between males and females. Thus future research is needed to further clarify the nature of these gender differences in neuroimmunomodulation and their implications for health disparities.
In conclusion, converging evidence provides clear support for neuroimmunomodulation. The identification of the cholinergic anti-inflammatory pathway and the explication of its basic anatomy and physiology may provide the initial groundwork for the future development of an immunological homunculus much as is being revealed for pain and nocioception. However, this is not the only pathway that may be illuminated by neuroimaging studies of immune processes and research is needed that investigates all aspects of neuroimmunomodulation. Several important challenges, as outlined above, need to be met before such work will bear fruit.
References
- Aron AR. The neural basis of inhibition in cognitive control. Neuroscientist. 2007;13:214–228. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- Bellinger DL, Lorton D, Hamill RW, Felton SY, Felton DL. Acetylcholinesterase staining and choline acetyltransferase activity in the young adult rat spleen: lack of evidence for cholinergic innervation. Brain Behav Immun. 1993;7:191–204. doi: 10.1006/brbi.1993.1021. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- Buijs van der Vliet J, Garidou ML, Huitinga I, Escobar C. Spleen Vagal Denervation Inhibits the Production of Antibodies to Circulating Antigens. PLoS ONE. 3(9):e3152. doi: 10.1371/journal.pone.0003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Autonomic neurotransmission: 60 years since Henry Dale. Ann Rev Pharmacol Toxicol. 2009;49:1–30. doi: 10.1146/annurev.pharmtox.052808.102215. [DOI] [PubMed] [Google Scholar]
- Butts CL, Bowers E, Horn JC, Shukair SA, Belyavskaya E, Tonelli E, Sternberg EM. Inhibitory effects of progesterone differ in dendritic cells from female and male rodents. Gend Med. 2008;5:434–447. doi: 10.1016/j.genm.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano G, Sved AF, Rinaman L, Rabin BS, Card P. Characterization of the central nervous system innervation of the rat soleen using viral transneural tracing. J Comp Neurol. 2001;439:1–18. doi: 10.1002/cne.1331. [DOI] [PubMed] [Google Scholar]
- Chatton JY, Pellerin L, Magistretti PJ. GABA uptake into astrocytes is not associated with significant metabolic cost: implications for brain imaging of inhibitory transmission. Proc Natl Acad Sci. 2003;100:12456–12461. doi: 10.1073/pnas.2132096100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XH, Itoh M, Sun W, Miki T, Takeuchi Y. Localization of sympathetic and parasympathetic neurons innervating pancreas and spleen in the cat. J Auton Nerv Syst. 1996;59:12–16. doi: 10.1016/0165-1838(95)00136-0. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O’Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, Dolan RJ. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- Dale HH. Chemical transmission of the effects of nerve impulses. Brit J Med. 1934;1:835–841. doi: 10.1136/bmj.1.3827.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale HH, Dudley HW. The presence of histamine and acetylcholine in the spleen of the ox and horse. J Physiol (London) 1929;68:97–113. doi: 10.1113/jphysiol.1929.sp002598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Rameson LT, Mashal MN, Irwin MR. An fMRI study of cytokine-induced depressed mood and social pain: the role of sex differences. NeuroImage. 2009;47:881–890. doi: 10.1016/j.neuroimage.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve–an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- Felton DL. Neural influence on immune responses: underlying suppositions and basic principles of neural-immune signaling. Prog Brain Res. 2000;122:381–389. doi: 10.1016/s0079-6123(08)62152-4. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Van Der Veen FM, Jennings JR. Regional cerebral blood flow correlates with heart period and high-frequency heart period variability during working-memory tasks: implications for the cortical and subcortical regulation of cardiac autonomic activity. Psychophysiol. 2004;41:521–530. doi: 10.1111/1469-8986.2004.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RPA, Hansen MK, Anderson K, Maier SF, Watkins LR. Vagal immune-to-brain communication: a visceral chemosensory pathway. Auton Neurosci. 2000;85:49–59. doi: 10.1016/S1566-0702(00)00219-8. [DOI] [PubMed] [Google Scholar]
- Gosain A, Jones SB, Shankar R, Gamelli RL, DiPietro LA. Norepinephrine modulates the inflammatory and proliferative phases of wound healing. J Trauma. 2006;60:736–744. doi: 10.1097/01.ta.0000196802.91829.cc. [DOI] [PubMed] [Google Scholar]
- Groves DA, Brown VJ. Vagal nerve stimulation: a review of its applications and potential mechanisms that mediate its clinical effects. Neurosci Biobehav Rev. 2005;29:493–500. doi: 10.1016/j.neubiorev.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Haensel A, Mills PJ, Nelesen RA, Ziegler MG, Dimsdale JE. The relationship between heart rate variability and inflammatory markers in cardiovascular diseases. Psychoneuroendocrinology. 2008;33:1305–1312. doi: 10.1016/j.psyneuen.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LA, Gandevia SC, Macefield VG. Somatotopic organization of the processing of muscle and cutaneous pain in the left and right insula cortex: a single-trial fMRI study. Pain. 2007;128:20–30. doi: 10.1016/j.pain.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Hoover DB, Isaacs ER, Jacques F, Hoard JL, Page P, Armour JA. Localization of multiple neurotransmitters in surgically derived specimens of human atrial ganglia. Neuroscience. 2009;164:1170–1179. doi: 10.1016/j.neuroscience.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Kawashima K, Fujii T. Expression of non-neuronal acetylcholine in lymphocytes and its contribution to the regulation of immune function. Front Biosci. 2004;9:2063–2085. doi: 10.2741/1390. [DOI] [PubMed] [Google Scholar]
- Kennedy SL, Nickerson M, Campisi J, Johnson JD, Smith TP, Sharkey C, Fleshner MJ. Splenic norepinephrine depletion following acute stress suppresses in vivo antibody response. J Neuroimmunol. 2005;165 (1–2):150–160. doi: 10.1016/j.jneuroim.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Lane RD, McRae K, Reiman EM, Chen K, Ahern GL, Thayer JF. Neural correlates of heart rate variability during emotion. NeuroImage. 2009;44:213–222. doi: 10.1016/j.neuroimage.2008.07.056. [DOI] [PubMed] [Google Scholar]
- Lane RD, Wager TD. The new field of brain–body medicine: what we have learned and where are we headed? NeuroImage. 2009;47:1135–1140. doi: 10.1016/j.neuroimage.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and relevance to functional brain imaging. PhilosTrans R Soc Lond B Biol Sci. 1999;29:1155–1163. doi: 10.1098/rstb.1999.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Goehler LE, Fleshner M, Watkins LR. The role of the vagus nerve in cytokine-to-brain communication. Ann NY Acad Sci. 1998;840:289–300. doi: 10.1111/j.1749-6632.1998.tb09569.x. [DOI] [PubMed] [Google Scholar]
- Migini F, Streccioni V, Amanta F. Autonomic innervation of immune organs and neuroimmune modulation. Auton Autacoid Pharmacol. 2005;23:1–25. doi: 10.1046/j.1474-8673.2003.00280.x. [DOI] [PubMed] [Google Scholar]
- Moak JP, Goldstein DS, Eldadah BA, Saleem A, Holmes C, et al. Supine low frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Heart Rhythm. 2007;4:1523–1529. doi: 10.1016/j.hrthm.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance DM, Burns J. Innervation of the spleen in the rat: evidence for the absence of afferent innervation. Brain Behav Immun. 1989;1989:281–290. doi: 10.1016/0889-1591(89)90028-7. [DOI] [PubMed] [Google Scholar]
- Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007) Brain Behav Immun. 2007;21 (6):736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor MF, Irwin MR, Wellisch DK. When grief heats up: pro-inflammatory cytokines predict brain activation. NeuroImage. 2009;47:891–896. doi: 10.1016/j.neuroimage.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohira H, et al. Imaging brain and immune association accompanying cognitive appraisal of an acute stressor. NeuroImage. 2006;39:500–514. doi: 10.1016/j.neuroimage.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Ohira H, et al. Regulation of natural killer cell distribution by prefrontal cortex during stochastic learning. NeuroImage. 2009;47:897–907. doi: 10.1016/j.neuroimage.2009.04.088. [DOI] [PubMed] [Google Scholar]
- Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, Chavan S, Tracey KJ. Spenic nerve is required for cholinergic anti-inflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci USA. 2008;105:11008–11013. doi: 10.1073/pnas.0803237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M, Tracey KJ. Cholinergic control of inflammation. J Int Med. 2009;265:663–679. doi: 10.1111/j.1365-2796.2009.02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz MA, Busse WW, Johnston T, Swenson CA, Crisafi GM, Jackson MM, Bosch JA, Sheridan JF, Davidson RJ. Neural circuitry underlying the interaction between emotion and asthma symptom exacerbation. Proc Natl Acad Sci USA. 2005;102:13319–13324. doi: 10.1073/pnas.0504365102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg EM. Neural-immune interactions in health and disease. J Clin Invest. 1997;100:2641–2647. doi: 10.1172/JCI119807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific response to pathogens. Nat Rev Immunol. 2006;6:318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RH, Shaller T, Miller LE, von Horsten S, Jessop DS, Falk W, Scholmerich J. Neuropeptide Y cotransmission with norepinephrine in the sympathetic nerve-macrophage interplay. J Neurochem. 2000;75:2464–2471. doi: 10.1046/j.1471-4159.2000.0752464.x. [DOI] [PubMed] [Google Scholar]
- Ter Horst GJ, Postema F. Forebrain parasympathetic control of heart activity: retrograde transneuronal viral labeling in rats. Am J Physiol. 1997;273:H2926–H2930. doi: 10.1152/ajpheart.1997.273.6.H2926. [DOI] [PubMed] [Google Scholar]
- Thayer JF. On the importance of inhibition: central and peripheral manifestations of nonlinear inhibitory processes in neural systems. Dose–Response. 2006;4:2–21. doi: 10.2203/dose-response.004.01.002.Thayer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Ahs F, Fredrickson M, Sollers JJ, III, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Psychophysiology. doi: 10.1016/j.neubiorev.2011.11.009. in press. (abstract) [DOI] [PubMed] [Google Scholar]
- Thayer JF, Fischer JE. Heart rate variability, overnight urinary norepinephrine, and C-reactive protein: evidence for the cholinergic anti-inflammatory pathway in healthy human adults. J Int Med. 2009;265:439–447. doi: 10.1111/j.1365-2796.2008.02023.x. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Johnsen BH. Non-invasive assessment of autonomic influences on the heart: impedance cardiography and heart rate variability. In: Luecken LJ, Gallo LC, editors. Handbook of Physiological Research Methods in Health Psychology. Sage Publications; Newbury Park, CA: 2008. pp. 183–209. [Google Scholar]
- Thayer JF, Hansen AL, Johnsen BH. The non-invasive assessment of autonomic influences on the heart using impedance cardiography and heart rate variability. In: Steptoe A, editor. Handbook of Behavioral Medicine: Methods and Applications. Springer; New York: in press. [Google Scholar]
- Thayer JF, Lane RD. Claude Bernard and the heart–brain connection: further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev. 2009;33:81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. Physiology and immunology of the cholinergic anti-inflammatory pathway. J Clin Invest. 2007;117:289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9:418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Zanden EP, Boeckxstaens GE, De Jonge WJ. The vagus nerve as a modulator of intestinal inflammation. Neurogastroenterol Motil. 2009;21:6–17. doi: 10.1111/j.1365-2982.2008.01252.x. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. Implications of immune-to-brain communication for sickness and pain. Proc Natl Acad Sci USA. 1999;96:7710–7713. doi: 10.1073/pnas.96.14.7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber CS, Thayer JF, Rudat M, Wirtz PH, Zimmerman-Viehoff F, Thomas A, Perschel FH, Arck PC, Deter HC. Low vagal tone is associated with impaired post stress recovery of cardiovascular, endocrine, and immune markers. Eur J Appl Physiol. doi: 10.1007/s00421-009-1341-x. in press. [DOI] [PubMed] [Google Scholar]
- Weihe E, Schutz B, Hartschuh W, Anlauf M, Schafer CH, Eiden LE. Coexpression of cholinergic and noradrenergic phenotypes in human and nonhuman autonomic nervous system. J Comp Neurol. 2005;492:370–379. doi: 10.1002/cne.20745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss T, Straube T, Boettcher J, Hecht H, Spohn D, Miltner WHR. Brain activation upon selective stimulation of cutaneous C- and A δ-fibers. NeuroImage. 2008;41:1372–1381. doi: 10.1016/j.neuroimage.2008.03.047. [DOI] [PubMed] [Google Scholar]
- Yang EV, Bane CM, MacCallum RC, Kiecolt-Glaser JK, Malarkey WB, Glaser R. Stress-related modulation of matrix metalloproteinase expression. J Neuroimmunol. 2002;133:144–150. doi: 10.1016/s0165-5728(02)00270-9. [DOI] [PubMed] [Google Scholar]