Abstract
The tachykinin NK3 receptor (NK3R) is a G protein coupled receptor that is activated, internalized, and trafficked to the nuclei of magnocellular neurons in the paraventricular nucleus of the hypothalamus (PVN) in response to acute hyperosmolarity. The lack of information on the nuclear import pathway raises concerns about the physiological role of nuclear NK3R. NK3R contains a nuclear localizing sequence (NLS) and this raises the possibility that importins are involved in transport of NK3R through the nuclear pore complex. The following experiments utilized: 1) co-immunoprecipitation to determine if NK3R is associated with importin β-1 following activation in response to acute hyperosmolarity in-vivo, and 2) immuno-neutralization of importin β-1 in-vitro to determine if nuclear transport of NK3R was blocked. Rats were given an intravenous injection of hypertonic saline (2 M) and 10 min after the infusion, the PVN was removed and homogenized. Importin β-1 co-immunoprecipitated with the NK3R following treatment with 2 M NaCl, but not following isotonic saline treatment. Immuno-neutralization of importin β-1 decreased the transport of NK3R into the nuclei in a time dependent fashion. The results indicate that in response to acute hyperosmotic challenge, NK3R associates with importin β-1 which enables the nuclear transport of NK3R. This is the first in-vivo study linking importin β-1 and the nuclear transport of a G protein coupled receptor, the NK3R, in brain.
Keywords: GPCR, epigenetics, vasopressin, nuclear trafficking, nuclear pore, brain
1.1
The Neurokinin 3 receptor (NK3R) is a member of the tachykinin receptor family of G protein coupled receptors (GPCRs) and shows preferential affinity for the tachykinin peptide, neurokinin B (NKB) (Chawala et al., 1997; Colin et al., 2002). NK3Rs are dispersed in the brain and are highly expressed on vasopressin (VP) magnocellular neurons of the paraventricular (PVN) and supraoptic nuclei (SON) of the hypothalamus. The NK3R expressed on magnocellular PVN and SON neurons are activated and internalized to the cytoplasm in response to hypovolemia and hyperosmolarity (Howe et al., 2004; Haley and Flynn, 2008; Haley and Flynn, 2006), and central administration of a selective NK3R antagonist blocks the receptor internalization and the release of VP in response to these two challenges (Haley and Flynn, 2006; Howe et al., 2004). Furthermore, unilateral intra-PVN injection of the NK3R antagonist significantly reduces the systemic release of VP and the induction of c-Fos in the injected PVN in response to hypotension (Haley and Flynn, 2008).
1.2
Both hypotension and acute hyperosmolarity cause a ligand-mediated internalization of the NK3R to the cytoplasm of magnocellular neurons. This is consistent with the traditional view that GPCR signaling is isolated to the immediate activation of ion channels and messenger cascades on the plasma membrane that can then alter cell responses and ultimately gene expression. However, several studies now show that membrane receptors, including GPCRs, are translocated to the cell nucleus (Gobeil et al., 2006). Indeed, in response to hyperosmolarity and hypotension, NK3Rs are transported to the nuclei of magnocellular neurons in the PVN and SON (Haley and Flynn, 2006; Howe et al., 2004; Jensen et al., 2008). The initial confocal studies were supported by cell fractionation experiments that showed the presence of the NK3R in nuclei following activation by hyperosmolarity. Furthermore, transmission electron microscopy showed that the NK3R was in the nucleoplasm and not on the nuclear membrane (Jensen et al., 2008).
1.3
The pathway by which NK3R is transported to the nucleus has not been described. The nuclear trafficking of a number of proteins, including steroid receptors and transcription factors is enabled by a nuclear localization sequence (NLS). The NLS is an amino acid sequence that is recognized and bound by importins and the importin family allows for the movement of proteins into the nucleus. The nuclear transport of NLS containing proteins by importins involves three key steps. First, the NLS is recognized and bound by one of the importin α’s. Second, importin β associates with the importin α that is bound to the target protein. Third, importin β associates with proteins in the nuclear pore complex that allow for the movement of the target protein into the nucleus. The NK3R contains a NLS (a.a 348–351) on the cytoplasmic tail of the receptor (Lee et al., 2004) and hence this receptor may transport into the nucleus via the importin pathway, particularly through an association with importin β-1 (imp β-1) as this association with importin β-1 is necessary for movement into the nucleus. An association of a GPCR and importins in brain tissue has never been shown. We tested this hypothesis by first showing that imp β-1 immunoprecipitated with NK3R in PVN tissue isolated from rats administered 2 M NaCl, but not in control rats. Second, the functional significance of importins in the nuclear transport of NK3R was tested by incubating immortalized neuronal hypothalamic cells expressing both NK3R and imp β-1 in imp β-1 antibody (immuno-neutralization) and then measuring by Western blot the presence of nuclear NK3R.
Experimental Procedures
2.1.1 Animal Care and housing
All animal experiments were carried out in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals. All experiments were approved by the University of Wyoming Animal Care and Use Committee. Male Charles River rats (Charles River Laboratories, Wilmington, MA, USA, 300–350 g) were housed individually in hanging stainless steel cages and maintained in a controlled environment (25° C, 12 h light/ 12 h dark) with food and water ad libitum.
2.1.2 Cell Culture
CLU209 cells were purchased from Cedarlane (Ontario, Canada). CLU209 are a rat immortalized neuronal hypothalamic cell line collected on embryonic day 18. Cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM, Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum (FBS), 25mM glucose, and 1% penicillin/streptomycin for the first 3 passes. After 3 passes the cells were no longer grown in penicillin/streptomycin. CLU209 cells were incubated at 37°C with 5% CO2 and were grown as a monolayer in a T-75 flask. When the cells were 90% confluent they were split at a 1:8 ratio by trypsinization to get single cells in suspension. Cells were generally passed every 4 days.
2.2.1 RNA extraction
The CLU209 cell line are hypothalamic cells that express vasopressin and other markers similar to magnocellular neurons in the PVN but there was no data showing that the CLU209 cells express the NK3R or imp β-1. To confirm NK3R and imp β-1 mRNA expression in the CLU209 cells, reverse transcription PCR (RT-PCR) was performed using mRNA from the CLU209 cell flasks (n=2) in tandem with RNA extracted from the rat PVN (n=1). Total RNA was extracted from the flask of cells or from the PVN tissue block using Trizol reagent (Invitrogen, Carlsbad, CA) following the manufactures protocols. Briefly, the PVN block was homogenized in 1 ml of Trizol or for the cell culture the media was removed from the cells and 2 ml of Trizol was added to the flask. The cells were vortexed for 5 min to lyse the cells. Trizol cell lysate (1 ml) or PVN lysate was moved to a 2 ml Eppendorf tube and 0.2 ml of chloroform was added. The sample was mixed and left at room temperature for 5 min. The sample was then centrifuged 12,000 × g for 15 min. The aqueous phase was moved to a fresh tube and 0.5 ml of isopropyl alcohol was added. The sample was incubated at room temperature for 10 min and then centrifuged 12,000 × g for 10 min. The supernatant was removed and the RNA pellet was washed in 70% ethanol and then centrifuged 12,000 × g for 5 min. The supernatant was removed and the pellet was air dried to remove any ethanol left in the tube. The RNA pellet was then dissolved in 50 µl of RNAse free dH2O. The RNA was assayed for concentration using the Nanodrop spectrophotometer (Thermo Scientific Nanodrop Products, Wilmington, DE).
2.2.2 RT-PCR
RT-PCR was performed using the extracted RNA with the Quantitect reverse transcription kit (Qiagen, Valencia, CA). RNA (1 µg) was incubated with genomic DNA wipeout buffer for 2 min at 42° C in a Minicycler (Bio-Rad, Hercules, CA) to remove genomic DNA contamination. The RT-PCR was then run following kit protocols to make the cDNA. Validated primer sets targeting NK3R (103 bp amplicon), VP (173 bp amplicon) and imp β-1 (91 bp amplicon) were purchased from Geneglobe (Qiagen, Valencia, CA). PCR was run using 0.5 µg of the cDNA with the Core Taq polymerase kit (Qiagen, Valencia, CA) and the Geneglobe primer sets. PCR product was electrophoresed at 110 V for 45 minutes on a 1% agarose gel. DNA bands were detected with ethidium bromide staining. Gels were imaged using the Gel Doc XRS imaging system with Quantity one software (Bio-Rad, Hercules, CA).
2.3.1 Western blot and co-immunoprecipitation
Antibodies
Two different antibodies for the NK3R were used for Western blot and co-immunoprecipitation. The first, the K7 anti-NK3R antibody is directed against COOH-terminus, amino acids 434–452, of the receptor and was raised in rabbit (kindly provided by Dr. James Krause; (Mileusnic et al., 1999). The specificity of the K7 antibody for NK3R and NK3R alone was shown by Jensen and associates (Jensen et al., 2008) by unilateral PVN injections of siRNA targeting the NK3R. The siRNA temporarily knocked down the expression of the NK3R. As the receptor was knocked down in-vivo, the corresponding band on Western blot was also knocked down. As the effects of the siRNA diminished the NK3R was expressed and was once again detected on Western blot. The K7 antibody was diluted at 1:4000 for Western blot application. The Thermo Scientific goat anti-rabbit HRP secondary was used at a dilution of 1:500,000. The second anti-NK3R antibody, sheep anti-NK3R, is a polyclonal antibody that was developed against a synthetic peptide in house in sheep and targets amino acids 180–192 on the 2nd extracellular loop of the NK3R. The sheep anti-NK3R was used at a dilution of 2 µg/ml in Western blot labeling. The secondary antibody was a donkey anti-sheep from Abcam (Cambridge, MA) and was used at a dilution of 1:20,000. The K7 and the sheep anti-NK3R antibodies detect a 126 kD band on Western blot. The 126 kD band is just over twice the size of the proposed molecular weight for the NK3R (56 kD). This increase in weight is due to the formation of a stable dimer by the NK3R. The specificity of the sheep anti-NK3R antibody for the NK3R was demonstrated by: (1) preabsoption of the antibody with the immunizing peptide and (2) the K7 and sheep anti-NK3R antibodies, although targeting different regions of the NK3R, both label the same band on Western blots, and (3) the sheep anti-NK3R antibody immunoprecipitated the same band that was detected by the K7 antibody. These data along with the previous knockdown experiments using the K7 antibody demonstrate that both the K7 and sheep anti-NK3R meet established criterion for antibody specificity (Burry, 2000; Holmseth et al., 2006; Larsson, 1981; Rhodes and Trimmer, 2006; Saper and Sawchenko, 2003; Saper, 2005). There was no cross reaction of either antibody with imp β-1.
2.3.2
Imp β-1 antibodies were purchased from Bethyl Laboratories (KPNB1; Montgomery, TX) and from Santa Cruz Biotechnology (karyopherin β-1; Santa Cruz, CA). KPNB1 from Bethyl was raised in rabbit and targets the COOH-terminus of the importin β-1 protein. KPNB1 was used at a concentration of 0.4 µg/ml for Western blot. The karyopherin β-1 antibody from Santa Cruz was also raised in rabbit but targets the NH2-terminus of the importin β-1. Karyopherin β-1 was used at a concentration of 4 µg/ml for Western blot. Both antibodies could be pre-absorbed by their blocking peptides. Both antibodies detected the same band (~97 kD) when the Bethyl KPNB1 was used to immunoprecipitate imp β-1. This suggests that they exhibit specific labeling for the imp β-1 protein (Holmseth et al., 2006; Rhodes and Trimmer, 2006). Neither antibody cross reacted with the NK3R.
2.3.3
The rats (n=2) were deeply anesthetized with a lethal dose of ketamine (333 mg/kg) and decapitated. The brains were removed and the PVN was blocked based on visual land marks (Jensen et al., 2008). The tissue blocks were homogenized in RIPA buffer (Thermo Scientific, Rockford, IL) supplemented with Halt Protease and Phosphatase Inhibitors (Thermo Scientific, Rockford, IL). For the CLU209 cells, the media was removed from the flask (n=2) and the cells are scraped from the flask into 1 ml of ice cold PBS. The cells were then pelleted by centrifugation (1,000 × g for 10 min). The PBS was then removed and 200 µl of RIPA buffer supplemented with Halt Protease and Phosphatase Inhibitors was added. The cells were then homogenized with a Dounce Homogenizer and the protein concentration of the samples was determined by photo absorbance. Protein (40 µg) was heated to 99° C for 10 min in Laemmli’s buffer with 2- β mercaptoethanol to further denature the proteins. The protein was then run on a 4–15% SDS polyacrylamide gel and transferred to Immun-Blot 0.2 µm PVDF membrane (Bio-Rad, Hercules, CA) for blotting. Subsequently, the membrane was washed in TBST (Tris buffered saline, 0.05% Tween 20) and blocked for 1 h in blocking buffer (TBST, 5% Blotto [Bio-Rad, Hercules, CA]). The membrane was then incubated with primary antibody targeting either NK3R or imp β-1 in blocking buffer for 1 h at room temperature on a rocker. The membrane was washed 3 × for 5 min in TBST and then incubated with secondary antibody conjugated to HRP. The protein band was visualized using either the SuperSignal West Femto kit or SuperSignal West Pico kit (Thermo Scientific, Rockford, IL) depending on the target protein. The Gel Doc XRS digital imaging system (Bio-Rad, Hercules, CA) was used to capture images of the membranes. Antigen signal was quantified by pixel density using arbitrary pixel units with the Quantity One software (Bio-Rad, Hercules, CA). When applicable membrane labeling was removed by incubation with 15 ml of Restore Plus Western Blot Stripping Buffer (Thermo Scientific, Rockford, IL) for 10 min. The membranes were then washed and re-probed for different proteins.
2.4.1 Conjugation of Antibodies to beads
For immunoprecipitation of the NK3R, the sheep anti-NK3R antibody was conjugated to MagnaBind Carboxyl Derivatized Beads (Thermo Scientific, Rockford, IL). The conjugation of antibodies to the MagnaBind Carboxyl Derivatized Beads was accomplished by following the protocols provided by the manufacturer. Briefly, MagnaBind Carboxyl Derivatized Bead slurry (200 µl) was washed 3 times in 1 ml of PBS. Conjugation buffer (200 µl, 0.1 M MES, 0.9% NaCl, pH 7.4) containing 200 µg of the sheep anti-NK3R antibody was added to the beads. The cross linker 1-Ehtyl-3-(3-dimethylaminopropyl) carbodiimide HCl (EDC, 2 mg) was dissolved in 200 µl of conjugation buffer and then added to the bead antibody mixture. The mixture was placed on a rotating mixer and mixed at room temperature for 30 min. Beads were then washed 3 × in 1 ml of PBS and stored in 200 µl of PBS at 4° C until used. Immunoprecipitation targeting imp β-1 was performed with anti-imp β-1 antibodies in conjunction with Protein G coated agarose beads. Additional modifications to the co-immunoprecipitation procedure with the imp β-1 antibody are stated below (2.6.2).
2.5.1 Intravenous catheter surgery
Male Charles River rats (300–350 g; n=10) were anesthetized by administering an intraperitoneal injection of ketamine hydrochloride (70 mg/kg). Rats were fitted with femoral intravenous (i.v.) catheters made from polyethylene (PE-50) tubing (Haley and Flynn, 2006). Briefly, the femoral vein was exposed by blunt excision, punctured, and a 3 cm length of PE-50 tubing was inserted into the femoral vein and held in place by sutures tied around the vein. The catheter exited through a small puncture between the scapulae, was filled with heparinized saline (50 U/ml), and heat sealed. Rats were allowed to recover overnight.
2.6.1 Co-immunoprecipitation
Rats were removed from their cages and the i.v. catheters were connected to syringes mounted on a syringe pump (Model 200, KD scientific, Holliston, MA). The rats were then injected with 2 ml of 2 M NaCl or isotonic NaCl over 10 minutes (0.2 ml/min; n=3/group). Rats were then sacrificed by an overdose of sodium nembutal (120 mg/kg) and the brains extracted. The PVN region was excised and placed in a Dounce homogenizer with 200 µl of Co-IP lysis buffer (50 mM Tris HCl pH 7.2, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100) supplemented with Halt Protease and Phosphatase Inhibitors (Thermo Scientific, Rockford, IL). Samples were homogenized in a Dounce homogenizer by 25 strokes of both loose and tight pestles. The samples were then transferred to 1.7 ml Eppendorf tubes and centrifuged at 5,000 × g at 4° C for 5 min. The supernatant was placed in new 1.7 ml Eppendorf tubes and 800 µl of Co-IP lysis buffer and 50 µl of MagnaBind magnetic beads coupled to the sheep anti-NK3R antibodies were added to each sample. Samples were then mixed on a rotating mixer at 4° C for 18 h and then washed 4 × 1 ml of Co-IP lysis buffer. Samples were eluted from the antibody by heating the samples to 99° C for 10 min in 40 µl of Laemmli’s buffer supplemented with 0.1 M dithiothreitol (DTT). Samples were then centrifuged at 14,000 × g for 30 sec to pellet the beads. The supernatant was transferred to a fresh tube. The samples were then processed via Western Blot (see above, section 2.3.3) to evaluate the presence of both the NK3R and the imp β-1 proteins. Quantification of pixel densities was determined by the Quantity one software. Density of the imp β-1 in the different experimental groups was normalized to the levels of NK3R as it was the targeted protein in the immunoprecipitation.
2.6.2
The reverse co-immunoprecipitation was done with slight modifications. Rats (n=2/group) were treated the same as stated earlier but magnetic protein G coated beads were used instead of direct antibody-bead conjugation. The homogenate from control and 2 M NaCl challenged rats was pre-cleared with 50 µl of protein G coated beads washed 2 × in 500 µl of Co-IP lysis buffer. The beads were removed and the samples were then incubated for 18 h on a rotating mixer with 5 µg of the imp β-1 antibody at 4° C. Washed Protein G coated beads (50 µl) were then added to the sample and incubated in a rotating mixer for 1 h at 4° C. Beads were pelleted and the supernatant was removed. The beads were then washed 4 × in 1 ml of Co-IP lysis buffer. The antibody protein complexes were then stripped from the beads in the same manner stated above (2.6.1).
2.7.1 Importin β immuno-neutralization
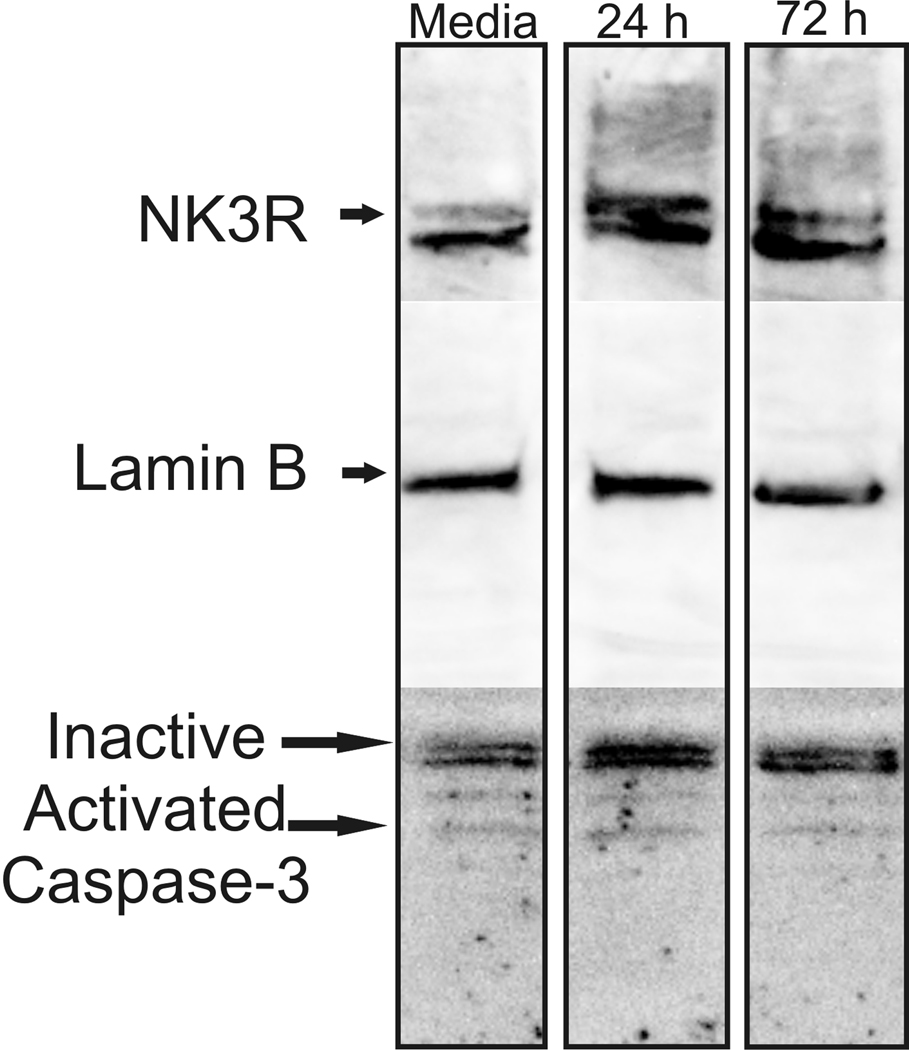
CLU209 were used when they reached 85–90% confluency. Plated cells were incubated for either 24 h or 72 h in media or media plus 40 µg/ml anti imp β-1 antibody (Bethyl Laboratories, n= 3 flasks per group). The experiment was repeated three times, 2 replications looked at the effects of the importin antibody on the nuclear transport of the NK3R, and one replication was used to identify possible cytotoxic effects of incubating the CLU209 cells with the importin antibody. At the end of the assigned time, the cells were harvested and the nuclei were extracted for Western blot analysis of NK3R trafficking. Each flask contained approximately 4.2 × 106 cells when harvested. One flask from each group was used to confirm that the incubation of the cells with the imp β-1 antibody was not altering the expression of NK3R or cytotoxic. Whole cellular protein was extracted by homogenization of the cells in RIPA buffer with a Dounce homogenizer and protein (50 µg per sample) was processed using Western blot. The membrane was probed with the sheep anti-NK3R antibody, Lamin B (Invitrogen, Camarillo CA., 1 µg/ml), and caspase-3 antibodies (Novus Biologicals, Littleton CO, 1:1000). Lamin B is a nuclear membrane protein and was used to normalize the expression of nuclear protein across samples. Membranes were then probed for Caspase-3, a member of group II effector caspases that are important in apoptotic signaling (Lavrik et al., 2005), to determine if immuno-neutralization of imp β-1 had a deleterious effect on the cells. The caspase-3 antibody recognized 3 different bands corresponding to different activation states of the caspases-3 protein. The 32 kD band is the inactive version of caspase-3, and 2 smaller bands (10 kD and 14 kD) are the activated pro-apoptotic markers.
2.8.1 Nuclear Isolation
CLU209 cells were incubated with the KPNB1 antibody for the designated time and then were scraped from the flask into 1 ml phosphate buffered saline (PBS) using a cell scraper. Cells were pelleted by centrifugation at 1000 × g for 15 min at 4°C. Supernatant was discarded and 1 ml of homogenization buffer (0.32 M Sucrose, 3 mM CaCl2, 2 mM MgCl2, 0.1 mM EDTA, 0.1% Triton X-100, 1 mM DTT, 10 mM Tris-HCl, pH 8.0) supplemented with Halt protease inhibitor was added to re-suspend the pellet. The sample was transferred to a 1 ml Dounce Homogenizer and homogenized using 20 strokes of both loose and tight pestles. Next, the sample was placed in a pre-cooled microfuge tube and spun at 1000 × g for 15 min at 4°C. The supernatant was carefully removed and discarded, 1 ml of homogenization buffer was added and the sample was vortexed until pellet was fully suspended. The sample was centrifuged again at 1000 × g for 15 min at 4°C and the supernatant was removed and the pellet was re-suspended in 700 µl of homogenization buffer and 700 µl of sucrose buffer (1.8 M sucrose, 5 mM MgCl2, 0.1 mM EDTA, 1 mM DTT, 10 mM Tris-HCl, pH 8.0) and vortexed. The sample was overlaid on 1 ml of sucrose buffer in high speed centrifuge tubes. The overlaid samples were immediately spun at 30,000 × g for 1 h at 4°C using a swinging bucket rotor. The supernatant was discarded and the nuclei were re-suspended in 200 µl homogenizer buffer, transferred to a pre-cooled microfuge tube already containing 550 µl of buffer. Centrifuge sample at 1000 × g for 15 min at 4°C. Re-suspension and centrifugation was repeated 2 times. The supernatant was discarded and the nuclear pellets were dissolved in 50 µl of 0.1 M DTT in Laemmli’s buffer and heated at 99°C for 10 min. The nuclear extract was processed using Western blot to determine the presence of target proteins. These nuclear samples were labeled with additional antibodies to determine the purity of the nuclear sample. To check for cytoplasmic contamination of the nuclear extract, the membrane was incubated with anti-calcium calmodulin kinase 1 (CaMK1; Abcam, Cambridge, MA) at 2µg/ml. The secondary antibody (goat anti-rabbit) was used at a 1:5000 dilution. The nuclear marker Lamin B (Invitrogen, Camarillo CA., 1µg/ml) was used as a control protein for the nuclear samples. The secondary goat anti-mouse was used at 1:20,000 dilution. Pixel density of the Lamin B band was used to normalize the amount of nuclear protein present across the samples.
Results
3.1
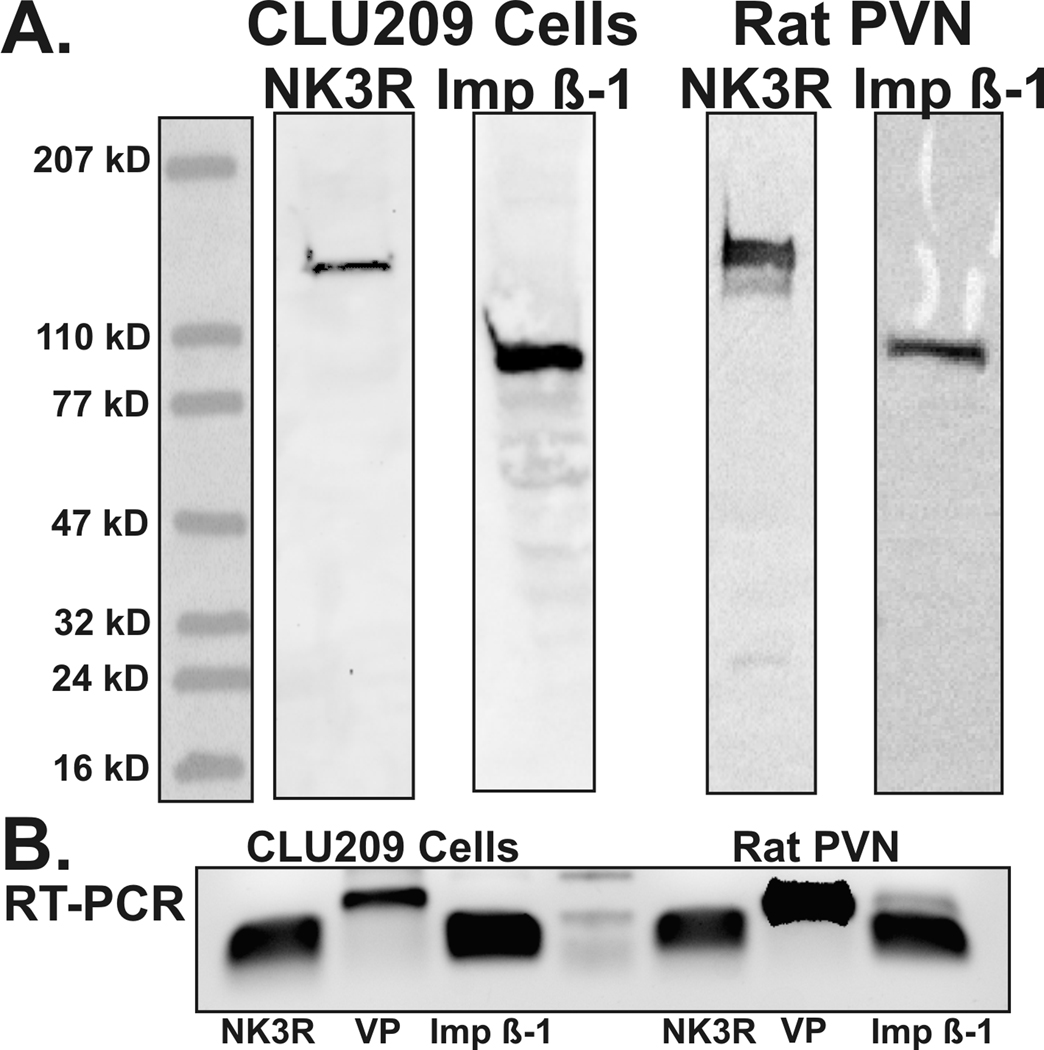
Western blots for imp β-1 using the Bethyl and the Santa Cruz antibodies detected one, 97 kD band, from PVN homogenate and from the CLU209 cell line (Figure 1). The 97 kD band is the suggested size of imp β-1. RT-PCR also showed that the mRNA for imp β-1 is readily expressed in the PVN of rat and in the CLU209 cells (Figure 1). It is important to note that the importin antibody only recognized a single band and that it did not recognize the larger 126 kD band that was detected by the NK3R antibodies. Conversely the NK3R antibodies recognized a 126 kD band and did not recognize any band that was detected by the imp β-1 antibody. Hence, the antibodies used in immunoprecipitation and Western blot experiments do not overlap in their recognition of their respective target sequences.
Figure 1.
Western blots from CLU209 cells and rat PVN (A) showing the labeling of the neurokinin 3 receptor (NK3R, sheep anti-NK3R in CLU209, K7 anti-NK3R in rat) and importin β-1 (Imp β-1, KPNB1 labeling for both samples). RT-PCR from the CLU209 and rat PVN (B) also demonstrate that mRNA for NK3R and imp β-1 are expressed in these samples along with vasopressin (VP). The center marker shows the 300 bp, 150 bp and 50 bp bands respectively.
3.2
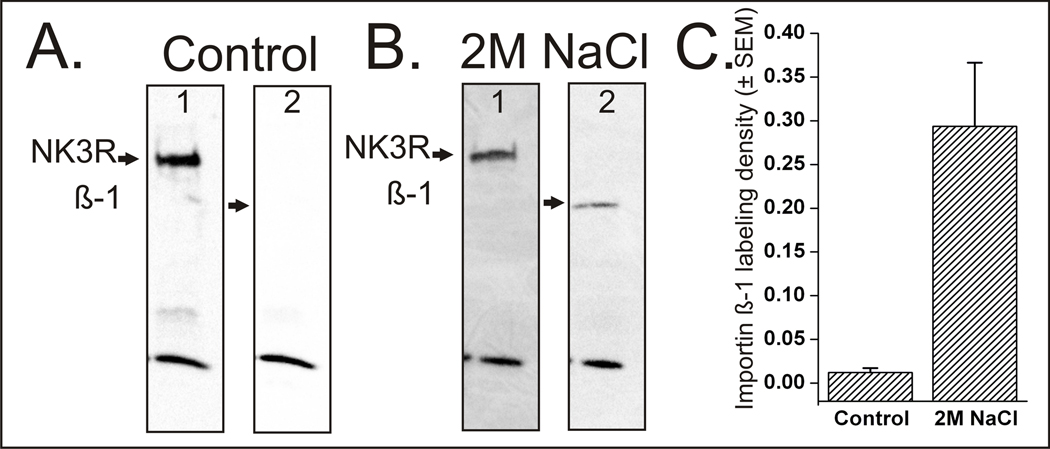
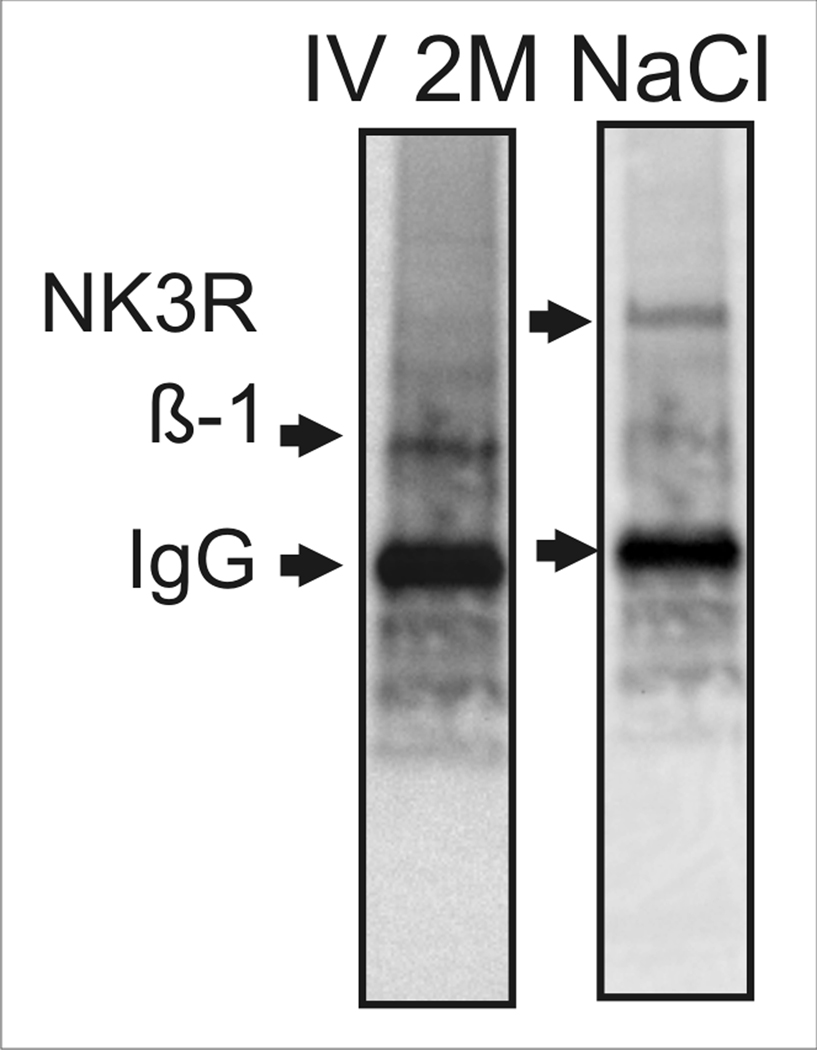
Following acute hyperosmotic challenge, the imp β-1 was co-immunoprecipitated with the NK3R. Imp β-1 was not co-immunoprecipitated with NK3R in samples from control rats as seen in Figure 2. Quantification of pixel densities of the Western blot images show that the labeling density for imp β-1 was 24 times denser in rats administered 2 M NaCl than compared to importin labeling density of control rats when samples were normalized to the levels of detectable NK3R (p<0.01, t-test). This increase in the labeling density for imp β-1 following the activation and internalization of NK3R shows that once NK3Rs are internalized, it then binds to imp β-1 in the cytoplasm. When the receptor is not activated there is no receptor internalization and as a result there is no interaction between NK3R and imp β-1. To further confirm the association of the NK3R with imp β-1, and rule out the possibility of interaction between imp β-1 and the NK3R antibodies, the reverse co-immunoprecipitation was performed (Figure 3). As expected, the reverse co-immunoprecipitation using the antibody targeting imp β-1 was able to immunoprecipitate the NK3R in 2 M NaCl challenged rats.
Figure 2.
Western blots following co-immunoprecipitation from control (A) or 2M NaCl challenged (B) rat. The control rat (A) shows that when the neurokinin 3 receptor (NK3R) was not activated or internalized there was no association with importin β-1 (β-1). Conversely when the NK3R was activated by a 2M NaCl challenge (B) it associated with importin β-1. Western blots also show a band at ~15kD that was present only in the co-immunoprecipitation blots. This band was present even in the absence of primary antibodies and is believed to be part of the magna bind beads that fractured during the elution process. Quantification of labeling density (C) shows that there is 24 times more importin β-1 labeling in the 2M NaCl challenged rats compared to the control rats.
Figure 3.
Western blot showing the immunoprecipitation of importin β-1 (β-1) in the first lane. The Western blot also shows that the neurokinin 3 receptor (NK3R, K7 anti-NK3R antibody) was co-immunoprecipitated with importin β-1 following 2M NaCl challenge.
3.3
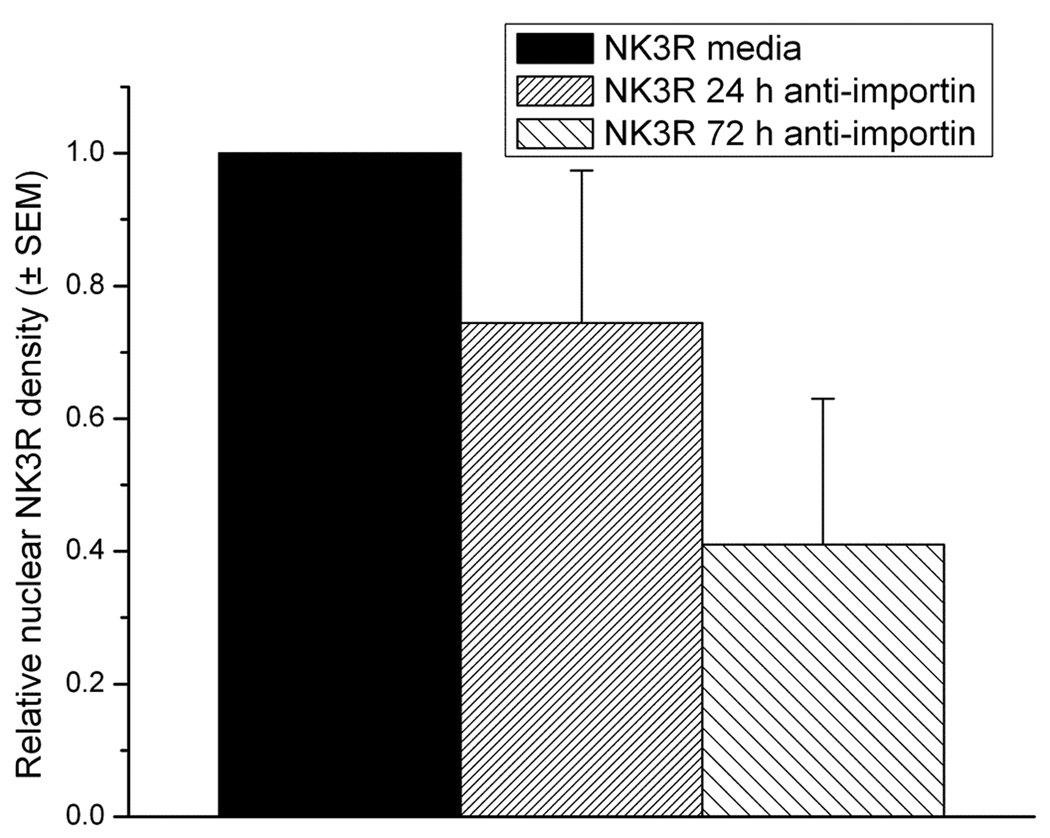
Western blots of proteins from the CLU209 cell line show that these cells express both the NK3R and importin. The NK3R antibody recognized a band (~126 kD) in the CLU209 cells while the imp β-1 antibody detected a 97 kD band (Figure 1). RT-PCR also confirmed that the cells express NK3R, imp β-1, as well as VP mRNA (Figure 1). Also, Western blot of nuclei isolated from CLU209 cells showed that in the absence of an agonist, NK3R was detected in the nucleoplasm. As such, NK3R are constitutively trafficked into the nucleus of these cells. The incubation of the cells with the anti-imp β-1 antibody reduced the nuclear transport of NK3R in a time dependent manner. A 24 h incubation with the imp β-1 antibody resulted in an average of a 26% decrease (replication 1= −10%; replication 2= −43%) while a 72 h incubation resulted in an average decrease of 59% (replication 1= −42%, replication 2= −73%) (Figure 4). The decrease in nuclear NK3R in cells incubated in importin antibody was not secondary to an overall reduction in NK3R or cell damage. Whole cell Western blot showed that the levels of NK3R remained constant across the experimental groups and caspase-3 staining showed no significant increase in the levels of apoptosis between the different cell experimental groups (Figure 5).
Figure 4.
Quantification of the labeling density of nuclear neurokinin 3 receptor (NK3R) in the CLU209 cells following importin β-1 immuno-neutralization. Lamin B was used as an internal loading control. The decrease in the nuclear presence of the NK3R was dependent on the time of incubation with the importin β-1 antibody.
Figure 5.
Western blots from CLU209 cell homogenate following incubation with the anti importin β-1 antibody. These data show that there was no decrease in the expression levels of neurokinin 3 receptor (NK3R, sheep anti-NK3R antibody) or lamin B following incubation with the importin β-1 antibody. These data also show that there was no activation of caspases-3 or increase in cell death due to incubation with the antibody.
Discussion
4.1
Confocal microscopy, Western blot of isolated nuclei, and immuno electron microscopy show the presence of NK3R protein in nuclei of PVN neurons following a hyperosmotic challenge (Jensen et al., 2008; Haley and Flynn, 2006). The objective of this study was to answer a fundamental question: what is the mechanism by which the NK3R is moved across the nuclear membrane following a hyperosmotic challenge in-vivo. As indicated earlier, for larger proteins to be transported into the nucleus through the nuclear pore complex (NPC) the protein must first associate with carrier proteins, like the importins. Importins bind to the NLS on the target protein and the association of the NLS with the importins is required for transport of larger proteins into the nucleus. The NLS is critical because mutation of the NLS blocks transport of the target protein into the nucleus (Kalderon et al., 1984; Savory et al., 1999) and co-immunoprecipitation studies show that NLS-containing proteins, including the glucocorticoid receptor and parathyroid hormone receptor associate with importins during nuclear transport (Liang et al., 2008; Tao et al., 2006; Pickard et al., 2006). Once the importin binds the NLS, the protein complex then moves to the NPC where imp β-1 docks with the NPC and proteins associated with imp β-1 are shuttled into the nucleus (Christophe et al., 2000; Johnson et al., 2004). The importin pathway mediates the nuclear transport of steroid receptors, transcription factors, and other NLS-containing proteins (Georget et al., 1997; Simental et al., 1991).
4.2
Nuclear transport of steroid receptors and transcription factors occurs throughout the brain and although the importin pathway has been linked to the nuclear transport of various receptors in-vitro (Tanaka et al., 2005; Otis et al., 2006; Pickard et al., 2006), there is very little information about the expression of importins in brain. A recent study was the first to demonstrate that imp β-1 mRNA was expressed in brain and furthermore that imp β-1 and other members of the importin family were expressed in the PVN (Hosokawa et al., 2008). Our present PCR results are consistent with this report in showing that the PVN expresses imp β-1 mRNA. We further show for the first time by Western blot that imp β-1 protein is present in the PVN. The presence of importin message and proteins identifies that the necessary machinery for the transport of proteins through the NPC is present in the PVN.
4.3
The importins that are present in the PVN and our present results indicate that they play a functional role in trafficking NK3R into the nucleus. In response to hyperosmolarity, NK3R were trafficked to the nuclei of PVN neurons. Furthermore, following NK3R receptor activation and internalization, NK3R associates with imp β-1. The association of NK3R and imp β-1 only occurred following the physiological challenge and was absent under basal, unstimulated conditions. The finding that imp β-1 was not pulled down with the NK3R in the control animals demonstrates that the receptor must be first activated and internalized for it to interact with imp β-1. The latter observation is consistent with previous results indicating that NK3R was not detected in the nuclei of PVN or SON neurons in control rats (Jensen et al., 2008; Howe et al., 2004; Haley and Flynn, 2006).
4.4
To further test the role of imp β-1 in the nuclear transport of NK3R, in-vitro immuno-neutralization of imp β-1 was performed in the CLU209 cell line. We first established that the cell line expresses the requisite proteins and furthermore mimic the nuclear presence of NK3R seen in PVN neurons, in-vivo. Western blot of CLU209 cell homogenate revealed that imp β-1 and NK3R are expressed by this cell line. The NK3R was also detected in the purified nuclear sample from the CLU209 cells. Furthermore, in the intact brain, NK3R are heavily expressed by vasopressinergic neurons (Ding et al., 1999; Haley and Flynn, 2006) and we show that the hypothalamic CLU209 cells express VP. Hence, CLU209 cells show protein expression pattern like that of magnocellular PVN neurons.
4.5
The concentration of imp β-1 is directly correlated with the import efficiency into the nucleus (Yang and Musser, 2006) and we reasoned that incubation of the CLU209 cells in imp β-1 antibody would functionally decrease the availability of imp β-1. Although the mechanism of antibody entry in to living cells is not fully understood it has been shown that antibodies are able to penetrate the plasma membrane and localize to various compartments within cells (Weisbart et al., 2000; Zack et al., 1996; Zack and Weisbart, 1998). Furthermore, once the antibodies have penetrated the cell they are still able to associate with their prospective targets (Zack et al., 1996). If the anti-imp β-1 antibodies were not able to enter the cell then they would not have access to imp β-1, as it is an intracellular protein, and there would be no effect on the nuclear trafficking of NK3R. NK3R was readily detected in the nuclear extract of cells raised in media but as imp β-1 is bound up by the anti imp β-1 antibodies the levels of nuclear NK3R decreased. As the amount of time that the cells were exposed to the importin antibody increased, the levels of detectable NK3R in the nucleus decreased. The importin antibody-mediated decrease in nuclear NK3R was not due to a reduction in total NK3R protein or damage to the cells. We interpret the findings to show that imp β-1 must be available to bind NK3R for transport into the nucleus. The functional effect that immune-neutralization of imp β-1 has on nuclear NK3R transport and the finding that NK3R associates with imp β-1 in brain are strong evidence that the importin pathway is the mechanism by which NK3R is translocated into the cell nucleus.
4.6
Our present findings showing that the NLS-containing NK3R transports into the nucleus via the importin pathway may help identify a shared mechanism for the transport of other cell surface GPCRs into the nucleus. Reports show that parathyroid hormone receptor (PTH1R) expresses an NLS and is trafficked into the nucleus by the importin pathway (Pickard et al., 2007). Several other GPCRs, such as the apelin, angiotensin I, bradykinin receptor, express putative NLSs and have been described in the cell nucleus (Lee et al., 2004; Bhattacharya et al., 1998). The mechanism of transport of these receptors into the nucleus has not been identified. Because two other GPCR’s that express an NLS translocate into the nucleus via importins (NK3R and PTH1R). The importin pathway may similarly transport any NLS containing GPCR through the nuclear pore complex.
4.7
The present results answer a fundamental question of how the NK3R protein moves into the nucleus. A remaining, and equally significant question is the function of NK3R and of any of the GPCRs reported to be transported into the cell nucleus. One idea is that once translocated into the nucleus the receptor affects gene transcription. Indeed, the nuclear transport of the AT1R correlated with the expression of norepinephrine transporter and tyrosine hydroxylase mRNA in-vitro (Lu et al., 1998). In the case of the NK3R, membrane activation results in the release of VP (Haley and Flynn, 2008; Haley and Flynn, 2006; Haley and Flynn, 2007),while the nuclear trafficking could then be a mechanism to affect gene expression, such as c-fos (Kawasaki et al., 2009). This idea is supported by immuno-electron microscopy showing that NK3R is present in the chromatin rich regions of nuclei isolated from the PVN of rats treated with an acute hyperosmotic challenge (Jensen et al., 2008). Furthermore, double immuno-electron microscopy and co-immunoprecipitation showed that within the nucleus, NK3R associates with histone H4, and specifically acetylated histone H4 following 2 M NaCl treatment (Xu submitted). NK3R lacks a traditional DNA binding domain but rather appears to interact with chromatin via p300/CBP-associated factor (PCAF), a histone acetyltransferase (Xu submitted). In this manner the nuclear transport of NK3R could be directly associated with chromatin structure and gene expression.
4.8
The nuclear transport of NK3Rs open up new possibilities in the signaling cascade for this and other GPCRs that is very distinct from the traditional role of GPCRs. Traditionally, the role of membrane bound GPCRs in cell signaling were thought to be isolated to the plasma membrane. GPCRs were activated by ligand binding and in turn activated a second messenger cascade within the cell. We now understand that GPCR signaling is more complex and has various levels of control at different locations within the cell (Luttrell, 2005). Also, many of the factors needed for GPCR signaling at the plasma membrane have been identified in the nucleus and on the inner nuclear membrane (Boivin et al., 2008; Boivin et al., 2006). Here we show for the first time in-vivo that the importin pathway helps regulate the nuclear trafficking of a GPCR. The presence of nuclear GPCR signaling cofactors and receptors would allow for a distinct signaling cascade separate for that found on the plasma membrane.
Acknowledgments
This research was supported by National Institutes of Health grants R01-NS57823 and P20-RR15640 awarded to F.W.F.
Abbreviations
- GPCR
G-protein coupled receptor
- imp β-1
Importin β-1
- NKB
Neurokinin B
- NK3R
Neurokinin 3 receptor
- NLS
Nuclear localization sequence
- PVN
Paraventricular nucleus
- SON
Supraoptic nucleus
- VP
Vasopressin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dane D. Jensen, Email: djensen@uwyo.edu.
Francis W. Flynn, Email: Flynn@uwyo.edu.
Reference List
- Bhattacharya M, Peri KG, Almazan G, Ribeiro-da-Silva A, Shichi H, Durocher Y, Abramovitz M, Hou X, Varma DR, Chemtob S. Nuclear localization of prostaglandin E2 receptors. Proc Natl Acad Sci U S A. 1998;95:15792–15797. doi: 10.1073/pnas.95.26.15792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin B, Lavoie C, Vaniotis G, Baragli A, Villeneuve LR, Ethier N, Trieu P, Allen BG, Hebert TE. Functional beta-adrenergic receptor signalling on nuclear membranes in adult rat and mouse ventricular cardiomyocytes. Cardiovasc Res. 2006;71:69–78. doi: 10.1016/j.cardiores.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Boivin B, Vaniotis G, Allen BG, Hebert TE. G protein-coupled receptors in and on the cell nucleus: a new signaling paradigm? J Recept Signal Transduct Res. 2008;28:15–28. doi: 10.1080/10799890801941889. [DOI] [PubMed] [Google Scholar]
- Burry RW. Specificity controls for immunocytochemical methods. J Histochem Cytochem. 2000;48:163–166. doi: 10.1177/002215540004800201. [DOI] [PubMed] [Google Scholar]
- Chawala M, Gutierrez G, Young W, III, McMullen N, Rance N. Localization of neurons expressing Substance P and Neurokinin B gene transcripts in the human hypothalamus and basal forebrain. The Journal of Comparative Neurology. 1997;384:429–442. doi: 10.1002/(sici)1096-9861(19970804)384:3<429::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Christophe D, Christophe-Hobertus C, Pichon B. Nuclear targeting of proteins: how many different signals? Cellular Signalling. 2000;12:337–341. doi: 10.1016/s0898-6568(00)00077-2. [DOI] [PubMed] [Google Scholar]
- Colin I, Blondeau C, Baude A. Neurokinin release in the rat nucleus of the solitary tract via NMDA and AMPA receptors. Neuroscience. 2002;115:1023–1033. doi: 10.1016/s0306-4522(02)00541-9. [DOI] [PubMed] [Google Scholar]
- Ding Y-Q, Lu B-Z, Guan Z-L, Wang D-S, Xu J-Q, Li J-H. Neurokinin B receptor (NK3)-containing neurons in the paraventricular and supraoptic nuclei of the rat hypothalamus synthesize vasopressin and express Fos following intravenous injection of hypertonic saline. Neuroscience. 1999;91:1077–1085. doi: 10.1016/s0306-4522(98)00643-5. [DOI] [PubMed] [Google Scholar]
- Georget V, Lobaccaro JM, Terouanne B, Mangeat P, Nicolas JC, Sultan C. Trafficking of the androgen receptor in living cells with fused green fluorescent protein-androgen receptor. Mol Cell Endocrinol. 1997;129:17–26. doi: 10.1016/s0303-7207(97)04034-3. [DOI] [PubMed] [Google Scholar]
- Gobeil F, Fortier A, Zhu T, Bossolasco M, Leduc M, Grandbois M, Heveker N, Bkaily G, Chemtob S, Barbaz D. G-protein-coupled receptors signalling at the cell nucleus: an emerging paradigm. Can J Physiol Pharmacol. 2006;84:287–297. doi: 10.1139/y05-127. [DOI] [PubMed] [Google Scholar]
- Haley GE, Flynn FW. Agonist and hypertonic saline-induced trafficking of the NK3-receptors on vasopressin neurons within the paraventricular nucleus of the hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1242–R1250. doi: 10.1152/ajpregu.00773.2005. [DOI] [PubMed] [Google Scholar]
- Haley GE, Flynn FW. Tachykinin NK3 receptor contribution to systemic release of vasopressin and oxytocin in response to osmotic and hypotensive challenge. Am J Physiol Regul Integr Comp Physiol. 2007;293:R931–R937. doi: 10.1152/ajpregu.00196.2007. [DOI] [PubMed] [Google Scholar]
- Haley GE, Flynn FW. Blockade of NK3R signaling in the PVN decreases vasopressin and oxytocin release and c-Fos expression in the magnocellular neurons in response to hypotension. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1158–R1167. doi: 10.1152/ajpregu.90402.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmseth S, Lehre KP, Danbolt NC. Specificity controls for immunocytochemistry. Anat Embryol (Berl) 2006;211:257–266. doi: 10.1007/s00429-005-0077-6. [DOI] [PubMed] [Google Scholar]
- Hosokawa K, Nishi M, Sakamoto H, Tanaka Y, Kawata M. Regional distribution of importin subtype mRNA expression in the nervous system: study of early postnatal and adult mouse. Neuroscience. 2008;157:864–877. doi: 10.1016/j.neuroscience.2008.09.045. [DOI] [PubMed] [Google Scholar]
- Howe HE, Somponpun SJ, Sladek CD. Role of Neurokinin 3 Receptors in Supraoptic Vasopressin and Oxytocin Neurons. J Neurosci. 2004;24:10103–10110. doi: 10.1523/JNEUROSCI.3164-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen D, Zhang Z, Flynn FW. Trafficking of tachykinin neurokinin 3 receptor to nuclei of neurons in the paraventricular nucleus of the hypothalamus following osmotic challenge. Neuroscience. 2008;155:308–316. doi: 10.1016/j.neuroscience.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson HM, Subramaniam PS, Olsnes S, Jans DA. Trafficking and signaling pathways of nuclear localizing protein ligands and their receptors. Bioessays. 2004;26:993–1004. doi: 10.1002/bies.20086. [DOI] [PubMed] [Google Scholar]
- Kalderon D, Roberts BL, Richardson WD, Smith AE. A short amino acid sequence able to specify nuclear location. Cell. 1984;39:499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Kawasaki M, Ponzio TA, Yue C, Fields RL, Gainer H. Neurotransmitter regulation of c-fos and vasopressin gene expression in the rat supraoptic nucleus. Exp Neurol. 2009;219:212–222. doi: 10.1016/j.expneurol.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson LI. Peptide immunocytochemistry. Prog Histochem Cytochem. 1981;13:1–85. [PubMed] [Google Scholar]
- Lavrik IN, Golks A, Krammer PH. Caspases: pharmacological manipulation of cell death. J Clin Invest. 2005;115:2665–2672. doi: 10.1172/JCI26252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DK, Lanca AJ, Cheng R, Nguyen T, Ji XD, Gobeil F, Jr, Chemtob S, George SR, O'Dowd BF. Agonist-independent nuclear localization of the Apelin, angiotensin AT1, and bradykinin B2 receptors. J Biol Chem. 2004;279:7901–7908. doi: 10.1074/jbc.M306377200. [DOI] [PubMed] [Google Scholar]
- Liang J, Ke G, You W, Peng Z, Lan J, Kalesse M, Tartakoff AM, Kaplan F, Tao T. Interaction between importin 13 and myopodin suggests a nuclear import pathway for myopodin. Mol Cell Biochem. 2008;307:93–100. doi: 10.1007/s11010-007-9588-1. [DOI] [PubMed] [Google Scholar]
- Lu D, Yang H, Shaw G, Raizada MK. Angiotensin II-Induced Nuclear Targeting of the Angiotensin Type 1 (AT1) Receptor in Brain Neurons. Endocrinology. 1998;139:365–375. doi: 10.1210/endo.139.1.5679. [DOI] [PubMed] [Google Scholar]
- Luttrell LM. Composition and function of g protein-coupled receptor signalsomes controlling mitogen-activated protein kinase activity. J Mol Neurosci. 2005;26:253–264. doi: 10.1385/JMN:26:2-3:253. [DOI] [PubMed] [Google Scholar]
- Mileusnic D, Lee JM, Magnuson DJ, Hejna MJ, Krause JE, Lorens JB, Lorens SA. Neurokinin-3 receptor distribution in rat and human brain: an immunohistochemical study. Neuroscience. 1999;89:1269–1290. doi: 10.1016/s0306-4522(98)00349-2. [DOI] [PubMed] [Google Scholar]
- Otis KO, Thompson KR, Martin KC. Importin-mediated nuclear transport in neurons. Curr Opin Neurobiol. 2006;16:329–335. doi: 10.1016/j.conb.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Pickard BW, Hodsman AB, Fraher LJ, Watson PH. Type 1 Parathyroid Hormone Receptor (PTH1R) Nuclear Trafficking: Association of PTH1R with Importin {alpha}1 and {beta} Endocrinology. 2006;147:3326–3332. doi: 10.1210/en.2005-1408. [DOI] [PubMed] [Google Scholar]
- Pickard BW, Hodsman AB, Fraher LJ, Watson PH. Type 1 Parathyroid Hormone Receptor (PTH1R) Nuclear Trafficking: Regulation of PTH1R Nuclear-Cytoplasmic Shuttling by Importin-{alpha}/{beta} and Chromosomal Region Maintenance 1/Exportin 1. Endocrinology. 2007;148:2282–2289. doi: 10.1210/en.2007-0157. [DOI] [PubMed] [Google Scholar]
- Rhodes KJ, Trimmer JS. Antibodies as valuable neuroscience research tools versus reagents of mass distraction. J Neurosci. 2006;26:8017–8020. doi: 10.1523/JNEUROSCI.2728-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB. An open letter to our readers on the use of antibodies. J Comp Neurol. 2005;493:477–478. doi: 10.1002/cne.20839. [DOI] [PubMed] [Google Scholar]
- Saper CB, Sawchenko PE. Magic peptides, magic antibodies: guidelines for appropriate controls for immunohistochemistry. J Comp Neurol. 2003;465:161–163. doi: 10.1002/cne.10858. [DOI] [PubMed] [Google Scholar]
- Savory JG, Hsu B, Laquian IR, Giffin W, Reich T, Hache RJ, Lefebvre YA. Discrimination between NL1- and NL2-mediated nuclear localization of the glucocorticoid receptor. Mol Cell Biol. 1999;19:1025–1037. doi: 10.1128/mcb.19.2.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simental JA, Sar M, Lane MV, French FS, Wilson EM. Transcriptional activation and nuclear targeting signals of the human androgen receptor. J Biol Chem. 1991;266:510–518. [PubMed] [Google Scholar]
- Tanaka M, Nishi M, Morimoto M, Sugimoto T, Kawata M. Imaging analysis of mineralocorticoid receptor and importins in single living cells by using GFP color variants. Cell Tissue Res. 2005;320:447–453. doi: 10.1007/s00441-004-0984-5. [DOI] [PubMed] [Google Scholar]
- Tao T, Lan J, Lukacs GL, Hache RJG, Kaplan F. Importin 13 Regulates Nuclear Import of the Glucocorticoid Receptor in Airway Epithelial Cells. Am J Respir Cell Mol Biol. 2006;35:668–680. doi: 10.1165/rcmb.2006-0073OC. [DOI] [PubMed] [Google Scholar]
- Weisbart RH, Baldwin R, Huh B, Zack DJ, Nishimura R. Novel protein transfection of primary rat cortical neurons using an antibody that penetrates living cells. J Immunol. 2000;164:6020–6026. doi: 10.4049/jimmunol.164.11.6020. [DOI] [PubMed] [Google Scholar]
- Yang W, Musser SM. Nuclear import time and transport efficiency depend on importin beta concentration. J Cell Biol. 2006;174:951–961. doi: 10.1083/jcb.200605053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zack DJ, Stempniak M, Wong AL, Taylor C, Weisbart RH. Mechanisms of cellular penetration and nuclear localization of an anti-double strand DNA autoantibody. J Immunol. 1996;157:2082–2088. [PubMed] [Google Scholar]
- Zack DJ, Weisbart RH. Cell and Nuclear Penetration by Autoantibodies. In: Paul S, editor. Pathogenic Autoimmune Reactions. Totowa, NJ: Humana Press; 1998. pp. 305–319. [Google Scholar]