Abstract
The peptide melanin-concentrating hormone (MCH), produced mainly by cells in the lateral hypothalamus (LH), perifornical area (PF) and zona incerta (ZI), is suggested to have a role in the consumption of rewarding substances, such as ethanol, sucrose and palatable food. However, there is limited information on the specific brain sites where MCH acts to stimulate intake of these rewarding substances and on the feedback effects that their consumption has on the expression of endogenous MCH. The current study investigated MCH in relation to ethanol consumption, in Sprague-Dawley rats. In Experiment 1, chronic consumption of ethanol (from 0.70 to 2.7 g/kg/day) dose-dependently reduced MCH gene expression in the LH. In Experiments 2–4, the opposite effect was observed with acute oral ethanol, which stimulated MCH expression specifically in the LH but not the ZI. In Experiment 5, the effect of MCH injection in brain-cannulated rats on ethanol consumption was examined. Compared to saline, MCH injected in the paraventricular nucleus (PVN) and nucleus accumbens (NAc) selectively stimulated ethanol consumption without affecting food or water intake. In contrast, it reduced ethanol intake when administered into the LH, while having no effect in the ZI. These results demonstrate that voluntary, chronic consumption of ethanol leads to local negative feedback control of MCH expression in the LH. However, with a brief exposure, ethanol stimulates MCH-expressing neurons in this region, which through projections to the feeding-related PVN and reward-related NAc can promote further drinking behavior.
Keywords: Melanin-concentrating hormone, Lateral hypothalamus, Perifornical hypothalamus, Zona incerta, Paraventricular nucleus of the hypothalamus, Nucleus accumbens, Ethanol
1. Introduction
Recent reports from this and other laboratories support the involvement of hypothalamic orexigenic peptides in controlling consummatory behavior [1, 2]. These include galanin (GAL), enkephalin (ENK) and hypocretin/orexin (OX), each of which stimulates the consumption of ethanol, as well as food and palatable diets [3–8]. Evidence now supports the idea that these hypothalamic peptides project to and interact with extra-hypothalamic reward circuits to promote consummatory behavior, playing a role in food and drug reinforcement in addition to energy balance [9].
The lateral hypothalamus (LH) has long been recognized as a brain region important for controlling both energy intake and reward-related behaviors [10, 11]. In addition to OX, there exists in this area a distinct cell population that expresses the peptide, melanin-concentrating hormone (MCH) [12–14]. This peptide has a prominent role in energy balance, mediating various aspects of consummatory behavior [15–19]. MCH is a cyclic nonadecapeptide, which is produced almost exclusively in neurons of the LH, the perifornical hypothalamus (PF) immediately surrounding the fornix, and the zona incerta (ZI), that project fibers throughout the brain [13, 20–23]. Although two main MCH receptors have been identified in humans [24–26], only one subtype has been cloned in rodents, the MCH R1 [25]. This receptor has a potent, inhibitory effect on neuronal activity through activation of the inhibitory G-protein (Gi) coupled receptor [27–29], and it is shown in non-neuronal cell lines to increase intracellular calcium release and activity of phospholipase C by activation of the Gq coupled receptor [30, 31]. In addition to the hypothalamus, there is dense expression of MCH R1 in the nucleus accumbens (NAc) and other limbic brain regions, suggesting a role for this peptide in motivation and reward-related behaviors, including feeding [28, 32].
The first studies of MCH focused on its role in feeding behavior. Investigations in rodents have shown the expression of this peptide to be increased by fasting while reduced by food intake [16, 17] and the administration of MCH in the cerebral ventricles to rapidly increase food intake [17, 19, 33]. Moreover, mutant mice carrying a targeted deletion of the MCH gene are hypophagic and lean [16], while mice over-expressing the peptide develop obesity and insulin resistance [34]. When injected in the hypothalamus, MCH is found to have a region-specific effect, stimulating feeding when administered in the medial paraventricular, arcuate and dorsomedial nuclei but having no effect in the LH [35]. In addition to the hypothalamus, MCH injected into the NAc, a region associated with the hedonic aspects of feeding, is also shown to enhance feeding behavior [18, 36]. More recently, a role for MCH in the consumption of other rewarding substances has been investigated. Ventricular administration of MCH stimulates the consumption of sucrose [37–39] and a high-fat diet [19, 40], and it enhances the rewarding effects of cocaine [41]. Ventricular injection of MCH also increases the consumption of and lever pressing for ethanol [38, 42], although mutant mice with a deletion of MCH R1 unexpectedly exhibit a similar increase in ethanol intake compared to wild-type mice [43]. All together, these studies indicate that MCH may function to promote consummatory behavior, not only of dietary nutrients but also of drugs of abuse such as ethanol, and to act in a region-specific manner.
This investigation further explored the role of MCH in the consumption of ethanol. The first issue to address was whether ethanol itself feeds back to control the expression of endogenous MCH in different areas of the LH and ZI. Our previous work with other hypothalamic peptides, which stimulate ethanol intake when injected into the PVN, has shown the expression of GAL and ENK in the PVN and OX in the perifornical LH to be stimulated by ethanol administration, suggesting that they function within a positive feedback circuit to promote ethanol consumption [3, 4, 44, 45]. The question to be addressed here is whether the MCH system in the LH or ZI functions in a similar manner. The second issue is whether MCH, shown to stimulate ethanol consumption when injected into the cerebral ventricles, can also affect the drinking of ethanol when administered into hypothalamic nuclei and NAc shell, regions known to be involved in consummatory behavior and motivation, respectively. These experiments, which involve both measurements and injections of MCH, should increase our understanding of the function of MCH in controlling the drinking of ethanol.
2. Methods and Materials
2.1 Animals
Adult, male Sprague-Dawley rats (Charles River Breeding Labs, Kingston, NY) were housed individually, on a 12-h reversed light/dark cycle in a fully accredited American Association for the Accreditation of Laboratory Animal Care facility, according to institutionally approved protocols as specified in the NIH Guide to the Use and Care of Animals and also with the approval of the Rockefeller University Animal Care Committee. The rats in each set of water- and ethanol-drinking groups were approximately matched for body weight, with an overall range of 300–350 g at the start of the experiment. All animals were allowed 1 week to acclimate to their individual housing conditions, during which time they were given ad libitum access to standard rodent chow (LabDiet Rodent Chow 5001, St. Louis, MO; 12% fat, 60% carbohydrate, and 28% protein) and water.
2.2 Test Procedures
The five experiments described below were designed to determine the feedback relationship between ethanol and endogenous MCH expression in the LH and ZI regions and the effects on ethanol consumption produced by MCH injections in the PVN, PF, LH, ZI or NAc.
In Experiment 1, the effect of chronic ethanol consumption at different concentrations on MCH expression in the LH was examined. Two sets of rats were given ad libitum access to lab chow, water and, in some cases, ethanol over a 28-day period. The water-drinking control rats (n=5/experiment) were maintained on chow and water, and the ethanol-drinking experimental groups (n=10–15/experiment) were additionally given access to ethanol (95% ethanol, David Sherman Corp., St. Louis, MO) diluted with water. The ethanol and water were presented in the home cage in plastic 8 oz bottles, with a steel ball as a tip valve to prevent spillage (PETCO Animal Supplies, Inc, San Diego, CA). In order to increase the amount of daily ethanol consumed, access to the ethanol-containing cylinder over the 28 days was provided for 12 h rather than 24 h each day, as described in our previous publications [46, 47]. The relative position of the water- and ethanol-containing bottles was alternated each day to prevent place preference. In the first set of animals (n=15), ethanol was provided as a 1% solution for the first four days and then kept at 2% for the remainder of the experiment. In the second set of animals (n=20), the concentration of ethanol was increased stepwise, every 4 days, from 1% to 2%, 4%, 7% and then 9% v/v, with the rats maintained for an additional 8 days on 9% ethanol. Animals consuming the 2% ethanol solution were further subdivided based on low and high daily ethanol consumption, while those in the 9% group were subdivided into a low, middle and high group. At the 2% concentration, the low drinkers consumed an average of 0.25 g/kg/day while the high drinkers consumed 0.75 g/kg/day, and at 9% ethanol, the low drinkers consumed 0.70 g/kg/day, the middle group consumed 1.5 g/kg/day, and the high drinkers consumed 2.7 g/kg/day. Body weight and daily food intake were measured every 4 days and showed no significant differences between the groups. Following the 28 days of ethanol exposure, in the absence of ethanol during the 12-h off period, the animals showed no physical or affective disturbances, motor abnormalities, convulsions or autonomic disturbances, indicating that they were not dependent. On the final day of ethanol drinking, chow was provided for 4 h after dark onset, to allow the animals to be satiated. The food was then removed to minimize its effect on ethanol consumption or absorption, and the rats were given water alone (control group) or ethanol plus water and allowed to drink ad libitum for the next 2 h. With this intermittent access schedule, the rats consumed a large percentage (25–30%) of their daily ethanol intake during the first few hours of exposure. After being allowed to drink for 2 h, the water- and ethanol-drinking rats were sacrificed by rapid decapitation, and their brains were removed and examined for peptide gene expression using quantitative real-time polymerase chain reaction (qRT-PCR).
In Experiment 2, four additional groups of rats (n=5/group) were used to determine the effects of acute ethanol administration on MCH expression in the LH. Oral gavage was used to administer the ethanol solution (30% v/v) in order to minimize the effects of stress on peptide expression. On the day before the experiment, the rats were separated into three groups of equal body weights and then given two days of mock gavages prior to the test day, to acclimate them to this procedure. Animals received a single gavage of water (n=5), 0.25 g/kg ethanol (n=5), 0.75 g/kg ethanol (n=5), or 2.5 g/kg ethanol (n=5). Animals were sacrificed 2 h after the gavage procedure by rapid decapitation, and their brains were processed for qRT-PCR according to experimental procedures described below.
In Experiment 3, a new set of animals was used to confirm with a different technique the results obtained using qRT-PCR in Experiment 2. Briefly, the animals (n=5/group) received an acute gavage of water or of 0.75 g/kg or 2.5 g/kg solution of ethanol (30% v/v) as described in Experiment 2. All animals were sacrificed 2 h later, and their brains were processed for analysis using in situ hybridization (ISH) with a radiolabeled probe, as described below. This experiment measured MCH mRNA expression in the entire LH and ZI regions together, since anatomical distinctions are not visible using this method.
In Experiment 4, three additional groups of animals (n=5/group) were examined for a more precise anatomical analysis of the MCH-expressing cells, not only in the LH but also in the ZI. The three groups received a single gavage of water or the 0.75 g/kg or 2.5 g/kg solution of ethanol (30% v/v), and they were sacrificed 2 h later. Their brains were examined using ISH with a digoxigenin-labeled probe (DIG), which allowed us to measure the density of MCH-expressing cells in the LH and ZI regions separately.
In Experiment 5, five groups of animals (n=8–10/group) were surgically implanted with cannulas aimed at 5 different brain sites, as described below, and were allowed to recover for one week. Following recovery, these rats were trained to consume a 6% ethanol solution, with the concentration gradient increased from 2% to 4% to 6% every 4 days, and they were maintained on the 6% solution for an additional 4 days before the beginning of testing. With water and ethanol solution available, animals were injected (0.5 µl) with either saline vehicle or MCH (0.6 nmol) into five brain regions, the PVN, LH, PF, ZI or NAc. Following injections, measurements of chow, water and ethanol intake were recorded at 1 h, 2 h, 3 h and 4 h after injection. The effects produced by each drug injection were compared to vehicle injections on counterbalanced consecutive days.
2.3 Brain Dissections
Immediately after sacrifice, the brains for Experiment 1 and 2 were removed for peptide measurements using qRT-PCR. Brains were placed in a matrix with the ventral surface facing up, and three 1.0 mm coronal sections were made, with the middle optic chiasm as the anterior boundary [48]. For microdissection, the sections were placed on a glass slide, and the LH (Bregma − 2.8 to − 3.6 mm) was removed under a microscope, using the fornix and third ventricle as landmarks. The LH was taken from the area surrounding the fornix, within a range of 0.2 mm ventral, 0.3 mm dorsal and 1mm lateral to the fornix. These dissections were stored in RNAlater (Sigma-Aldrich Co., St. Louis, MO) until processed.
2.4 Quantitative Real-Time PCR Analysis
In Experiments 1 and 2, qRT-PCR was used to measure MCH mRNA levels in the LH. For all groups in Experiments 1 and 2, total RNA from individual microdissected hypothalamic samples was extracted with the RNAEasy Mini Kit (Qiagen, CA) using 1.0 mm zirconia/silica beads (Biospec Products, OK), with the exception of the 2% ethanol drinking group in Experiment 1 that had their hypothalamic samples pooled and RNA extracted using Trizol reagent, according to our previously published methods [45]. For all groups, the cDNA and minus RT were synthesized using an oligo-dT primer with or without SuperScript II reverse transcriptase. The qRT-PCR experiments were conducted with Applied Biosystems (ABI) system. With Applied Biosystems Primer Express V1.5a software, primers were designed to have a melting temperature of 58–60°C and to produce an amplicon of 50–160 base pairs. The last five bases on the 3’ end contained no more than 2 G and/or C bases, to reduce the possibility of nonspecific product formation.
The SYBR Green PCR core reagents kit (ABI, CA) was used with cyclophilin (cyc) as an endogenous control. PCR was performed in MicroAmp Optic 96-well Reaction Plates (ABI) on an ABI PRISM 7900 Sequence Detection system, with the condition of 2 min at 50°C, 10 min at 95°C, then 40 cycles of 15 sec at 95°C and 1 min at 60°C. Each study consisted of 4 independent runs of PCR in triplicate, and each run included a standard curve, non-template control, and negative RT control. The levels of target gene expression were quantified relative to the level of cyc by standard curve method, based on threshold with Ct value of 18–25 for the different genes. For our initial MCH expression experiments, we used cyc, β- actin and GAPDH as controls. Since cyc gave the most reliable data with no region or treatment specific changes in quantity, we continued to use cyc to normalize our data for OX expression. The primers, designed with ABI Primer Express V.1.5a software based on published sequences, were: 1) cyc: 5’- GTGTTCTTCGACATCACGGCT -3’ (forward) and 5’- CTGTCTTTGGAACTTTGTCTGCA - 3’ (reverse); and 2) MCH: 5’- ATCGGTTGTTGCTCCTTCTCTG -3’ (forward) and 5’- TCT GCT TGG AGC CTG TGT TCT T - 3’ (reverse). The concentrations of primers were 100 to 200 nM, and all reagents, unless indicated, were from Invitrogen (Carlsbad, CA). The specificities of RT-PCR products were confirmed by both a single dissociation curve of the product and a single band with a corresponding molecular weight revealed by an agarose gel electrophoresis. In addition to the non-template control and a negative RT control, the specificity of the quantitative PCR was verified with an anatomical negative control by using the corpus callossum in the same brain. No signals above threshold of all targeted genes were detected by qRT-PCR in all of the controls.
2.5 Radiolabeled In Situ Hybridization Histochemistry
In Experiment 3, the mRNA levels of MCH were measured by radiolabeled ISH histochemistry in animals treated with water or an acute gavage of either 0.75 g/kg or 2.5 g/kg ethanol (n=5/group). The animals were sacrificed by rapid decapitation, and the brains were immediately removed and fixed in 4% paraformaldehyde in phosphate buffer (PB) (0.1M pH 7.2) for 48–72 h, cryoprotected in 25% sucrose for 48–72 h, and then frozen and stored at −80°C. The antisense and sense MCH RNA probes were donated by Dr. Nicholas A. Tritos and labeled with 35S-UTP (Perkin Elmer, Waltham, MA) as described [49]. Free-floating 30 µm coronal sections were processed as follows: 10 min in 0.001% proteinase K, 5 min in 4% paraformaldehyde, and 10 min each in 0.2 N HCl and acetylation solution, with 10 min wash in PB between each step. After washing, the sections were hybridized with 35S-labeled probe (103 cpm/ ml) at 55°C for 18 h. Following hybridization, the sections were washed in 4 × SSC, and nonspecifically bound probe was removed by RNase (Sigma, St. Louis, MO) treatment for 30 min at 37°C. Then, sections were run through a series of stringency washes with 0.1 M dithiothreicitol (Sigma, St. Louis, MO) in 2 × SSC and 1 × SSC and 0.1 × SSC at 55°C. Finally, sections were mounted, air-dried and exposed to Kodak BioMax MR film for 8–18 h at −80°C, developed and microscopically analyzed. The sense probe control was performed in the same tissue, and no signal was found.
Gene expression level was determined with a computer-assisted microdensitometry of autoradiographic images on the MCID image analysis system (Image Research, Inc., St. Catherines, Canada) as described [50, 51]. Microscale 14C standards (Amersham Biosciences, Piscataway, NJ) were exposed on the same Kodak film with the sections and digitized. Gray level/optical density calibrations were performed by using a calibrated film strip ladder (Imaging Research, St. Catherines, ON, Canada) for optical density. Optical density was plotted as a function of microscale calibration values. It was determined that all subsequent optical density values of digitized autoradiographic images fell within the linear range of the function. The values obtained represent the average of measurements taken from 10–12 sections per animal from Bregma −2.8 to −3.1. In each section, the optical density was recorded for the LH and ZI together, as it was difficult to delineate these two structures using radiolabeled probes. The background optical density from a same size area in the thalamus was subtracted from these measurements and the mean value of density for the 0.75 g/kg ethanol and 2.5 g/kg ethanol groups in each experiment was reported as percentage of the water group.
2.6 Digoxigenin-Labeled In Situ Hybridization Histochemistry
As previously described [44], DIG antisense RNA probes and 30-lm free-floating cryostat sections were used for ISH histochemistry. AP-conjugated sheep anti-digoxigenin Fab fragments (1:1000, Roche, Nutley, NJ) and NBT / BCIP (Roche, Nutley, NJ) were used to visualize the signal. Gene expression level was measured by semi-quantification with Image-Pro Plus software, version 4.5 (Media Cybernetics, Inc., Silver Spring, MD, USA) and was expressed as cells /mm2, reflecting density of mRNA containing cells. In all analyses, the cell number was counted only on one plane in each section, and only those cells containing a nucleus in the plane (>10 lm2) were counted, thereby excluding fractions of cells. This analysis with the DIG-labeled probe allowed for more anatomically precise measurements to be made, thus permitting us to analyze separately the sub-populations of MCH neurons in the LH and ZI. The values obtained represent an average of measurements taken from 10–12 sections per animal from Bregma −2.8 to −3.1. All MCH cells from 0.2 to 0.8 mm lateral to the fornix were considered to be in the LH, while all MCH cells between 0.8 and 1.4 mm dorsal to the fornix were considered in the ZI. The average cell density in each region for the different groups was compared and statistically analyzed, with the analyses being performed by an observer who was blind to the identity of the rats.
2.7 Surgery
Subjects were anesthetized with a combination of ketamine (80 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.), supplemented with ketamine when necessary. Guide shafts (21-gauge stainless steel, 10 mm in length) were implanted perpendicularly according to the atlas of Paxinos and Watson [52], in the PVN (A-P −1.8, L 0.4, V 3.8), LH (A-P −2.9, L 2.2, V 4.5), PF (A-P −2.9, L 1.6, V 4.5), ZI (A-P −2.9, L 1.6, V 3.1), and NAc shell (A-P +1.8, L 0.8, V 4.2), with reference to bregma, the midsaggital sinus, and the level of skull surface. The injectors protruded 4.5 mm beyond the guide shafts for injection. Injections were made unilaterally for the PVN, as it lies along the midline, but bilaterally for the other regions. Other than the time of injection, stainless steel stylets were left in the guide shafts to prevent occlusion.
2.8 Microinjection Procedures
All solutions were delivered through concentric microinjectors made of 26-gauge stainless steel outside with fused-silica tubing inside (74 µm ID, 154 µm OD, Polymicro Technologies, Phoenix AZ). The silica injector tip protruded beyond the implanted guide shaft to reach into the region of interest (V 8.0 for PVN, V 9.0 for LH, V 9.0 for PF, V 7.6 for ZI, V 8.7 for NAc). The dose was chosen based on our preliminary tests and the literature on feeding behavior [18, 35, 53]. The MCH (0.6 nmol; Sigma-Aldrich Co., St. Louis, MO) was dissolved in preservative-free 0.9% NaCl solution (Hospira Inc, Lake Forest, IL) and prepared fresh immediately prior to microinjection.
To minimize stress from the injections, the animals were handled extensively throughout their ethanol training prior to the initiation of the tests. Injections were counterbalanced so that each animal received vehicle or MCH in opposite order on two consecutive days, and they were given early in the dark cycle (2 hours after dark onset), immediately prior to daily ethanol access. All injections were made using a syringe pump, which infused 0.5 µl during 47 sec at a flow rate of 0.6 µl/min, and the microinjector was kept in place for another 47 sec, to allow diffusion before removal. Ethanol, food and water intake were measured every hour for four hours after injections and ethanol access. Each group received only a single set of tests (MCH versus saline).
2.9 Histology
Injection sites were verified by injecting 0.25 µl methylene blue dye (Sigma, St. Louis, MO). Brains were kept in formalin for a minimum of 1 week prior to slicing, then cut in 40 µm sections on a freezing microtome and slide-mounted for microscopic verification. Behavioral data from animals with injector tips in the region of interest were included in the analysis, while those with probes 0.5 mm or farther from the target regions (1–2/group) were discarded from the analysis.
2.10 Data Analysis
The peptide expression values in the figures are expressed as mean ± SEM. Statistical analyses of these data were performed using a one-way analysis of variance (ANOVA), followed by post-hoc tests (Holm-sidak) for multiple comparisons. For the injection data, values are expressed as mean ± SEM and compared between MCH and vehicle. Ethanol, food and water intake data were each analyzed separately using 2-way repeated measures ANOVA, with two levels of treatment (MCH and saline vehicle) and four levels of time (1–4 h) as the within-subject independent variables. Follow-up pairwise comparisons were made using two-tailed t-tests, where p<0.05 was considered significant. Since we knew a priori that time could be a confounding factor in the injection studies, follow-up statistical tests were used in one case despite a negative main result, to determine significance at specific time points. These probability values given in the text or legends to the figures and tables reflect the results of these tests.
3. Results
3.1 Experiment 1: Effect of Chronic Ethanol Intake on MCH Expression in the LH as Measured by qRT-PCR
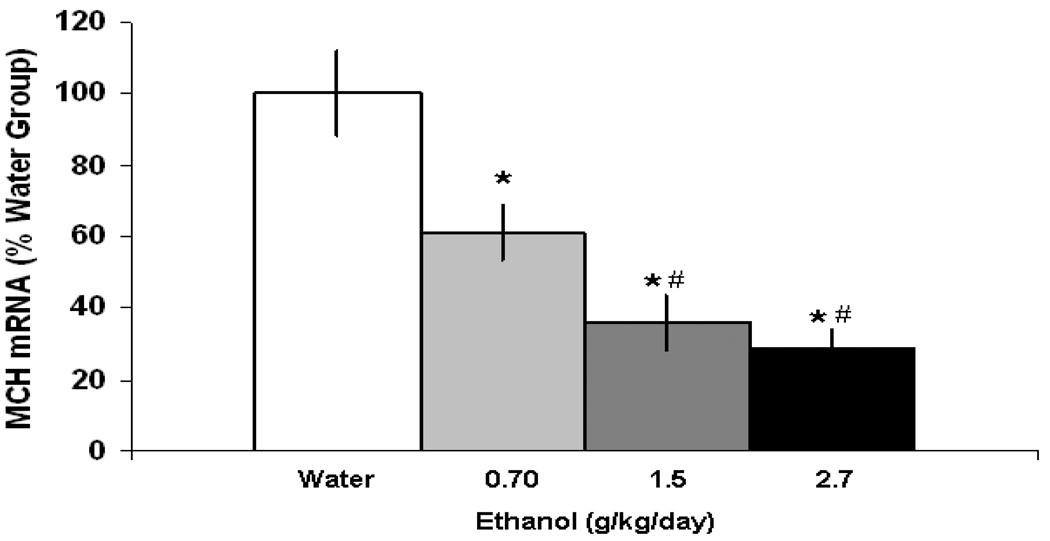
The first experiment tested the effects of chronic ethanol consumption compared to water on MCH expression in the LH of Sprague-Dawley rats. Animals were maintained for 28 days on either water (n=5/experiment), 2% ethanol (n=10) or 9% (n=15) ethanol, and with a range of intake across animals, subgroups could be formed with rats that consumed an average of 0.25 g/kg/day or 0.75 g/kg/day on 2% ethanol or an average of 0.70 g/kg/day, 1.5 g/kg/day or 2.7 g/kg/day on 9% ethanol. Chronic consumption of 9% ethanol solution compared to water produced a dose-dependent reduction in MCH mRNA expression in the LH [F(3,19) = 25.9, p<0.001] (Fig. 1). Compared to the water drinking rats, animals consuming 1.5 g/kg or 2.7 g/kg of ethanol showed a 64–70% reduction in MCH gene expression (p<0.001), which was stronger than the 39% reduction measured in the animals drinking 0.70 g/kg/day ethanol (p<0.001). A small but significant reduction (−45%) in MCH expression was obtained with chronic consumption of 2% ethanol in animals consuming 0.75 g/kg/day ethanol (p<0.001), with no significant change observed in animals consuming 0.25 g/kg/day ethanol compared to water (data not shown). These results demonstrate that voluntary, chronic intake of ethanol dose-dependently reduces MCH gene expression in the LH.
Figure 1.
Effects of chronic ethanol intake on the expression of MCH in the LH, as measured by qRT-PCR. In the 9% ethanol drinking animals, the data (mean ± SEM) revealed a significant reduction in expression of MCH in the 0.70 g/kg/day, 1.5 g/kg/day and 2.7 g/kg/day ethanol groups (* p<0.001 compared to water, # p<0.001 compared to 0.70 g/kg/day).
3.2 Experiment 2: Effect of Acute Ethanol on MCH Expression in the LH as Measured by qRT-PCR
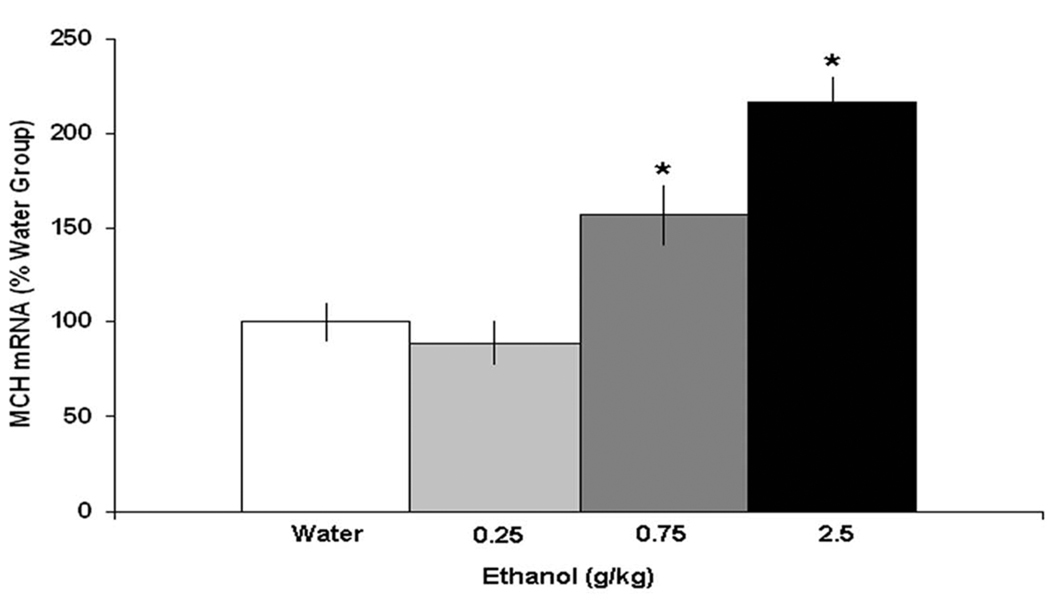
The second experiment examined the effects of acute ethanol administration on MCH expression in the LH. Sprague-Dawley rats (N=20) were administered a single gavage of either water or ethanol, at doses of 0.25, 0.75 or 2.5 g/kg, and gene expression was examined 2 h later. In contrast to chronic ethanol intake, acute oral administration of ethanol compared to the water control group was found to stimulate MCH expression in the LH [F(3,19) = 36.5, p<0.001] (Fig. 2). While the lowest 0.25 g/kg dose produced little change, a significant increase (+57%) in MCH gene expression was observed following 0.75 g/kg of ethanol (p< 0.001), and a larger increase (+116%) was seen with the 2.5 g/kg dose (p< 0.001). Thus, in contrast to the effects of chronic ethanol consumption, these results demonstrate that acute oral administration of ethanol can dose-dependently stimulate MCH peptide expression in the LH.
Figure 2.
Effects of acute ethanol gavage on the expression of MCH in the LH, as measured by qRT-PCR. The data (mean ± SEM) showed a significant increase in MCH expression with the 0.75 g/kg and 2.5 g/kg dose of ethanol compared to water gavage (*p<0.001 compared to water and 0.25 g/kg).
3.3 Experiment 3: Effect of Acute Ethanol on MCH Expression in the LH Using Radiolabeled ISH
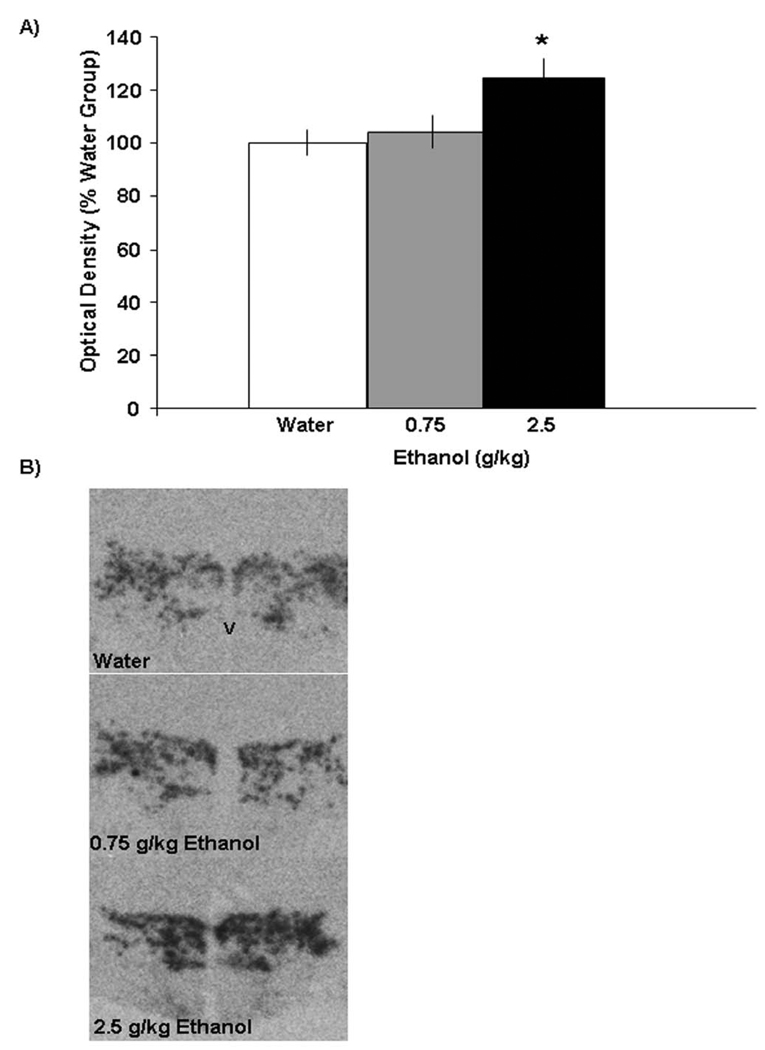
To further substantiate these findings, MCH gene expression in Experiment 3 was measured using radiolabeled ISH in rats (N=15) given oral administration of ethanol. Examination of the brains showed that the distribution of the radioactive probe was contained within the lateral and dorsal regions of the posterior hypothalamus, predominantly in the LH and nearby ZI region. The results obtained here with ISH confirmed those of Experiment 2 observed using qRT-PCR. As shown in Fig. 3, the 2.5 g/kg dose of ethanol compared to water produced a significant, 23% increase in mRNA expression of MCH in the LH/ZI region [F(2,14) = 4.61, p<0.05], with the lower 0.75 g/kg dose yielding no effect. The precise areas affected by ethanol are illustrated in the photomicrographs of Fig. 4. This evidence substantiates the finding that acute ethanol at 2.5 g/kg stimulates MCH gene expression, with the effect at the lower dose of 0.75 g/kg revealed only with qRT-PCR analysis.
Figure 3.
A) Effects of acute ethanol gavage on the expression of MCH in the LH region, as measured by radiolabeled ISH. The data (mean ± SEM), presented as % of water control showed a significant increase in expression of MCH at the higher dose of ethanol (2.5 g/kg) as compared with the water group (*p<0.05). B) Photomicrographs illustrating the stimulatory effect of 2.5 g/kg ethanol on MCH mRNA expression, as measured using radiolabeled ISH. V-ventricle
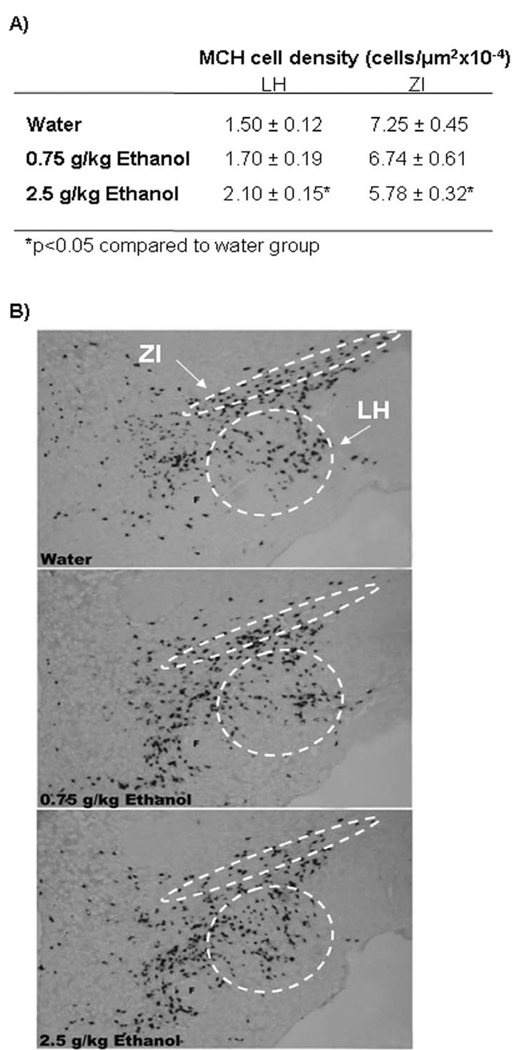
Figure 4.
A) Effects of acute ethanol on MCH cell density as measured by digoxigenin-labeled ISH. The data (mean ± SEM) showed a significant increase in expression of MCH in the LH and a reduction in expression of MCH in the ZI at the higher dose of ethanol (2.5 g/kg) as compared with the water group (*p<0.05). B) Photomicrographs illustrating the stimulatory effect of 2.5 g/kg ethanol on MCH-expressing neurons in the LH and inhibitory effect on neurons in the ZI region as measured by digoxigenin-labeled ISH. F- fornix
3.4 Experiment 4: Effect of Acute Ethanol on MCH Expression in LH and ZI Using DIG-Labeled ISH
In order to better visualize and anatomically differentiate MCH cells in the different areas of the ventral LH and dorsal ZI, DIG-labeled ISH was performed in an additional set of animals (N=15) given acute gavage of ethanol, as performed in Experiments 2 and 3. When compared to the water group, the 2.5 g/kg oral dose of ethanol produced a site-specific change in the density of MCH-expressing neurons (Table 1), as illustrated in the photomicrographs of Fig. 5. In the LH, acute oral ethanol significantly increased the density of the MCH neurons [F(2,14) = 4.50, p<0.05], consistent with the results of Experiments 2 and 3. Unexpectedly, acute ethanol produced the opposite effect in the ZI, where a marked reduction in the density of MCH neurons was observed at the higher, 2.5 g/kg dose [F(2,14) = 9.50, p<0.01]. These results demonstrate that the stimulatory effect of acute ethanol on MCH is specific to the LH neurons, with the ZI neurons showing a very different response.
Table 1.
Effects of MCH (0.6 nmol) injection in different brain regions on ethanol (g/kg), food(g), and water (ml) consumption during the first four hours after injection
| Region | Treatment | Nutrient | 1 h | 2 h | 3 h | 4 h |
|---|---|---|---|---|---|---|
| PVN | Saline | Ethanol | 0.22 ± 0.05 | 0.35 ± 0.08 | 0.65 ± 0.10 | 0.82 ± 0.13 |
| Food | 1.47 ± 0.67 | 3.74 ± 1.77 | 5.86 ± 1.67 | 7.89 ± 1.23 | ||
| Water | 2.23 ± 0.55 | 3.94 ± 0.97 | 5.59 ± 2.11 | 5.61 ± 2.12 | ||
| MCH | Ethanol | 0.36 ± 0.05 | 0.74 ± 0.08* | 1.10 ± 0.09* | 1.42 ± 0.17 | |
| Food | 1.36 ± 0.31 | 4.56 ± 0.47 | 5.49 ± 1.00 | 6.06 ± 0.95 | ||
| Water | 1.77 ± 0.54 | 4.23 ± 2.02 | 5.43 ± 1.97 | 6.25 ± 2.23 | ||
| LH | Saline | Ethanol | 0.63 ± 0.19 | 0.83 ± 0.18 | 1.15 ± 0.23 | 1.45 ± 0.27 |
| Food | 1.68 ± 0.66 | 3.74 ± 1.44 | 5.86 ± 1.67 | 7.87 ± 1.32 | ||
| Water | 3.03 ± 1.03 | 3.93 ± 0.98 | 5.63 ± 0.92 | 6.9 ± 0.96 | ||
| MCH | Ethanol | 0.25 ± 0.04* | 0.31 ± 0.08* | 0.61 ± 0.03* | 0.91 ± 0.15 | |
| Food | 0.97 ± 0.46 | 4.24 ± 1.28 | 6.34 ± 0.47 | 6.50 ± 0.40 | ||
| Water | 1.37 ± 0.21 | 2.83 ± 0.63 | 4.97 ± 0.91 | 6.56 ± 1.28 | ||
| PF | Saline | Ethanol | 0.47 ± 0.10 | 0.77 ± 0.17 | 1.13 ± 0.24 | 1.56 ± 0.31 |
| Food | 5.61 ± 0.66 | 7.78 ± 0.40 | 9.92 ± 0.32 | 13.33 ± 0.68 | ||
| Water | 2.54 ± 0.48 | 5.22 ± 0.81 | 8.76 ± 1.41 | 10.84 ± 1.80 | ||
| MCH | Ethanol | 0.25 ± 0.07* | 0.52 ± 0.07 | 0.98 ± 0.14 | 1.30 ± 0.19 | |
| Food | 3.81 ± 0.57 | 7.00 ± 0.43 | 9.46 ± 0.32 | 11.87 ± 0.61 | ||
| Water | 2.72 ± 0.59 | 5.39 ± 0.64 | 8.26 ± 1.10 | 11.00 ± 1.40 | ||
| ZI | Saline | Ethanol | 0.44 ± 0.10 | 0.53 ± 0.15 | 0.84 ± 0.14 | 1.17 ± 0.18 |
| Food | 2.33 ± 0.70 | 3.85 ± 0.93 | 6.38 ± 1.12 | 9.35 ± 1.08 | ||
| Water | 2.86 ± 0.74 | 4.35 ± 1.02 | 6.66 ± 1.37 | 7.87 ± 1.62 | ||
| MCH | Ethanol | 0.40 ± 0.09 | 0.62 ± 0.08 | 0.81 ± 0.10 | 0.99 ± 0.11 | |
| Food | 3.34 ± 0.67 | 5.35 ± 0.74 | 6.44 ± 0.81 | 7.60 ± 1.00 | ||
| Water | 2.45 ± 0.40 | 4.88 ± 0.73 | 5.87 ± 0.82 | 7.49 ± 0.94 | ||
| NAc | Saline | Ethanol | 0.14 ± 0.04 | 0.32 ± 0.09 | 0.57 ± 0.12 | 0.76 ± 0.15 |
| Food | 2.48 ± 0.67 | 4.48 ± 0.80 | 6.62 ± 0.85 | 8.90 ± 1.06 | ||
| Water | 2.09 ± 0.52 | 3.93 ± 0.80 | 5.80 ± 1.19 | 7.56 ± 1.77 | ||
| MCH | Ethanol | 0.35 ± 0.07* | 0.60 ± 0.11* | 0.82 ± 0.13* | 0.99 ± 0.16* | |
| Food | 2.16 ± 0.50 | 4.83 ± 0.60 | 6.47 ± 0.72 | 8.48 ± 0.91 | ||
| Water | 1.73 ± 0.22 | 3.60 ± 0.62 | 5.20 ± 0.94 | 7.17 ± 1.31 | ||
Data are presented as mean ± SEM,
p < 0.05 for comparisons between MCH and saline.
Figure 5.
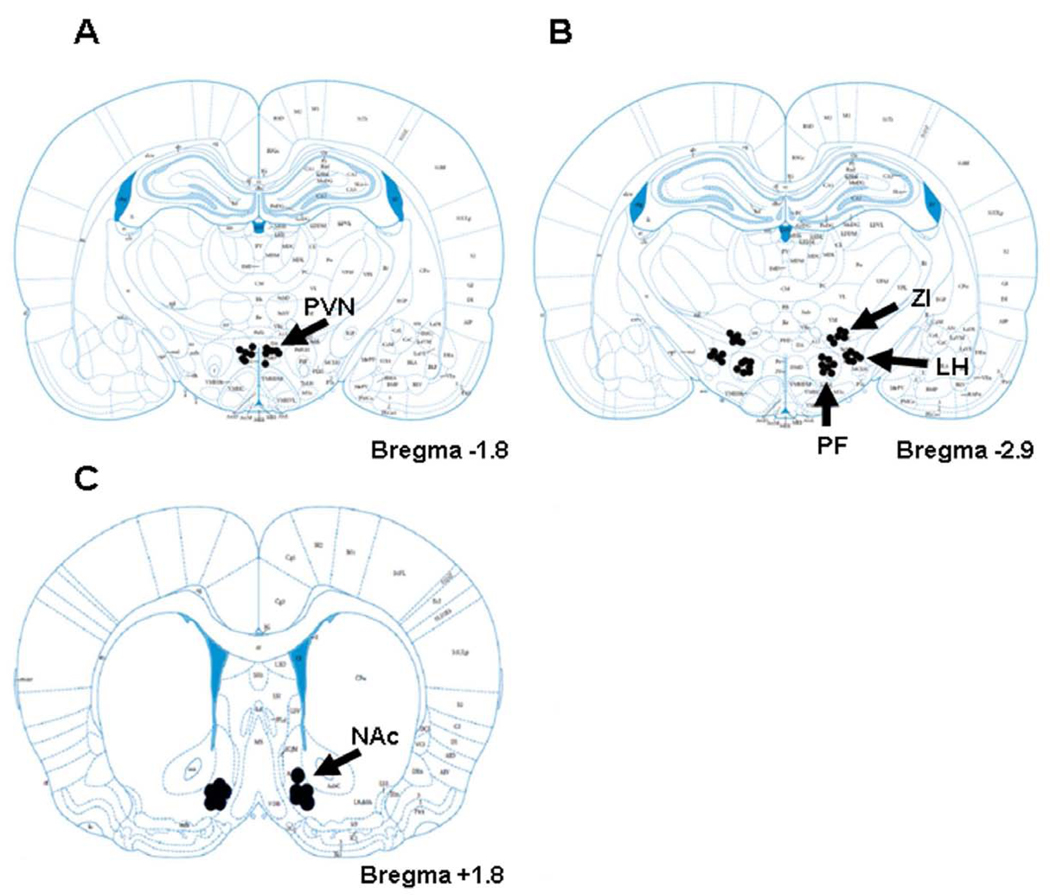
Black dots show the injection sites in the A) PVN, B) LH, PF and ZI, C) NAc for all animals used in the experiments. Sections are labeled according to the distance from Bregma along the rostral-caudal axis. Adapted from The Rat Brain, compact 3rd edition, G. Paxinos and C. Watson, Copyright 1997, with permission from Elsevier. PVN- paraventricular nucleus of the hypothalamus; LH- lateral hypothalamus; PF- perifornical area; ZI- zona incerta; NAc- nucleus accumbens
3.5 Experiment 5: Effect of MCH Injections into Different Brain Areas on Ethanol Consumption
This experiment tested whether the injection of MCH (0.6 nmol) compared to saline vehicle into different hypothalamic and mesolimbic sites can stimulate ethanol intake, similar to the effect produced by ventricular injections [38]. When injected into the PVN, MCH compared to vehicle resulted in a significant and specific increase in 6% ethanol consumption [F(1,6) = 7.66, p<0.05], while having no effect on food [F(1,6) = 0.40, n.s] or water [F(1,6) = 0.02, n.s] intake. This increase in ethanol consumption, as revealed by pairwise comparisons, occurred at 2 h (p<0.05), 3 h (p<0.05), and with a strong trend at 4 h (p=0.06) (Table 2), with injection sites seen in the ventral, medial parvocellular, and lateral magnocellular parts of the nucleus (Fig. 6A). In contrast to the PVN, injection of MCH into the LH region selectively decreased ethanol intake [F(1,6) = 6.62, p<0.05], without affecting food [F(1,6) = 0.05, n.s] or water [F(1,6) = 1.87, n.s] consumption (Table 2). In these sites lateral to the fornix (Fig. 6B), MCH reduced ethanol intake by 36% at 1 h (p<0.05), 50% at 2h (p<0.05), and 32% at 3h (p<0.05), with no difference at 4 h (Table 2). When injected into the PF, sites immediately surrounding the fornix (Fig. 6B), no main effects of MCH were observed on ethanol [F(1,8) = 2.25, n.s], food [F(1,8) = 3.00, n.s] or water [F(1,8) = 0.00, n.s] intake, although follow-up analyses at specific times revealed a significant reduction (−65%) in ethanol consumption at 1 h (p<0.05) (Table 2). In the more dorsal region of the ZI (Fig. 6B), MCH produced no change in ethanol [F(1,6) = 0.09, n.s], food [F(1,6) = 0.04, n.s] or water [F(1,6) =0.05, n.s] intake. In contrast to these effects in the LH but similar to that in the PVN, MCH injections into the NAc had a significant, stimulatory effect on ethanol intake [F(1,6) = 12.05, p<0.05], but not on food [F(1,6) = 1.30, ns] or water [F(1,6) = 0.40, n.s] intake. This increase in ethanol consumption, seen with cannula placements in the medial shell region of the NAc (Fig. 6C), occurred at 1 h, 2 h, 3 h, and 4 h (p<0.05) (Table 2). These results with different injection sites focus attention on the terminal sites of the PVN and NAc, where MCH specifically promotes the consumption of ethanol.
4. Discussion
The present study demonstrated that, while chronic consumption of ethanol caused a dose-dependent reduction in MCH expression, acute oral administration of ethanol led to a stimulation of MCH-expressing cells. This latter effect was region-specific, apparent in the LH, but not in the ZI. The effect of MCH injections on ethanol consumption was also region-specific, with a stimulatory effect observed in two terminal areas, the PVN and NAc, and a suppressive effect seen in the cell body area of the LH, but not the ZI. These results focus attention on the MCH system of the LH in having a stimulatory effect on ethanol intake. In this area, acute ethanol exposure stimulates MCH-expressing neurons, which through projections outside the LH act positively to promote further ethanol intake but through local projections provide a negative feedback signal that limits excess consumption.
Chronic Ethanol Consumption Reduces MCH Gene Expression
The measured reduction in MCH gene expression with chronic ethanol consumption may be related to negative feedback signaling to the LH. Our results showed a dose-dependent decrease in MCH gene expression, with the most prominent effect observed in rats consuming between 1 and 2.5 g/kg/day of ethanol. A similar reduction in peptide expression with chronic ethanol has also been reported with OX in this same region [54]. Since OX can activate MCH neurons in the LH [55], these two effects may be closely related, with the reduced expression of OX contributing to the decrease in MCH. Another explanation for the reduction in MCH expression may involve negative feedback signals produced by ethanol exposure, such as a local release of γ-aminobutyric acid (GABA). Systemic ethanol administration increases GABA levels in the hypothalamus [56, 57], and the MCH as well as OX neurons express both GABA-A [58, 59] and GABA-B [60] receptors. The involvement of GABA in the inhibitory effect of chronic ethanol intake is further supported by evidence that local blockade of GABA signaling in the LH stimulates the immediate early gene c-Fos specifically in the MCH- and OX-producing cells [61]. This evidence supports the idea that GABA normally reduces the activity of neurons expressing MCH and OX in the LH and that chronic ethanol acts through this inhibitory neurochemical signaling to reduce their endogenous expression.
Acute Ethanol Stimulates MCH Gene Expression in the LH Region
In contrast to this inhibitory effect of chronic ethanol on MCH, acute oral administration of ethanol stimulated MCH expression in the LH region. Whereas there are several reports showing MCH expression to be increased in animals that overconsume and gain weight on a palatable, fat-rich diet [17, 62, 63], there is only one describing a strong trend toward a positive correlation between MCH expression and ethanol consumption [64]. Although the oral ethanol gavage procedure may have produced some stress in the animals, this stimulatory effect on MCH is more likely to be due to the ethanol itself than the stress, as MCH expression is found to be reduced by stressful conditions in rats and rainbow trout [65, 66]. The stimulatory effect of acute ethanol on MCH expression is similar to that seen in nearby OX-expressing cells in the LH region [54], and it may be a consequence of the increase in OX peptide, which is shown to stimulate MCH in the LH [55]. This increase in MCH expression may reflect a role for this peptide in stimulating ethanol consumption and mediating the reward properties of ethanol. These MCH-expressing neurons in the LH are known to project to multiple brain sites involved in reward functions, including the nearby hypothalamic nuclei, such as the PVN [67], and more distant mesolimbic regions, such as the NAc [28, 32]. The MCH R1 is abundantly expressed in these distal sites and is believed to mediate the stimulatory effect of MCH on consummatory and reward-related behaviors [28, 32, 68].
Acute Ethanol Reduces MCH Gene Expression in the ZI Region
The present results clearly differentiate the MCH neurons in the LH from those in the ZI, with the latter after acute ethanol exposure exhibiting a reduction in MCH expression. Although there is no evidence suggesting a direct role for the ZI in controlling ethanol consumption, this brain region has been closely associated with locomotor activity [69, 70], which in turn is positively related to ethanol intake [5, 71]. The ZI contains a very heterogeneous collection of cells, many of which are glutamatergic in nature and extend to regions of the basal ganglia to promote locomotor activity [72]. With evidence that MCH is generally neuronally inhibitory [31, 73], our results showing acute ethanol to reduce MCH-expressing neurons in the ZI suggests that local neural activity may be disinhibited, leading to an increase in locomotor activity and possibly intake of ethanol. This is supported by evidence showing that acute ethanol can enhance activity levels in animals [74, 75]. Thus, MCH neurons within the ZI, having distinct projection sites from the LH [76], may function to increase ethanol consumption by enhancing locomotor activity rather than reward-related behaviors.
MCH Enhances Ethanol Consumption in the PVN
With evidence suggesting that MCH projections to the PVN may enhance feeding behavior [35, 53], we were encouraged to examine the effects of MCH injections into this region on ethanol consumption. The original report relating MCH to ethanol intake showed a stimulatory effect with ventricular injections of this peptide [38, 42]. The present study is the first to demonstrate that this effect can occur with injections into specific brain sites and that MCH acts in a site-specific manner. Our results with hypothalamic injections in rats trained to drink ethanol focus attention on the PVN as one of the primary sites of action. When injected into the PVN, MCH preferentially increased ethanol intake in the second through fourth hours of testing, similar to the time course previously observed with feeding behavior [35], but it had no effect on food or water consumption. Using ethanol-naïve rats, previous studies have reported an increase in food intake with MCH injection in the PVN [35, 53]. The lack of this effect in ethanol-drinking rats suggests that ethanol may mask the feeding-stimulatory effects of MCH and supplant chow as the preferred substance. These stimulatory effects of PVN MCH injection on consummatory behavior may involve the activation of other peptide systems, such as galanin and enkephalin, which like MCH are found to promote both feeding and ethanol intake [45, 77]. With evidence that MCH reduces GABAergic activity [78], this activation of the local peptide systems may occur through a disinhibition of the large GABAergic cell population in the PVN [79], similar to that described in studies of MCH’s effect on corticotrophin-releasing factor in the PVN [80]. Since projections from the PVN extend directly to the mesolimbic reward circuit [81] and increase firing of dopamine neurons in the ventral tegmental area [82], MCH may also stimulate drinking behavior by acting through this system, which is intimately involved in reinforcing behavior [83–85]. Together, these studies suggest that MCH may act in the PVN to promote ethanol consumption and that this effect may occur, in part, through the activation of other local and projection peptide systems known to stimulate consummatory and reward-related behavior.
MCH Reduces Ethanol Consumption When Injected into LH
The injection of MCH into sites within or surrounding the LH revealed an unexpected result, a site-specific reduction in ethanol consumption, which was stronger in the lateral LH region compared to the medial PF and not evident in the ZI. This effect was specific to ethanol intake, with no change observed in food or water intake. The site specificity of this effect on ethanol intake is similar to that observed with feeding behavior in ethanol-naïve rats, which exhibited an increase in food intake with injection into the PVN but not in the LH [35]. Although the MCH R1 is only moderately expressed in the LH regions compared to the dense expression in the PVN and various limbic structures [32, 68], several studies demonstrate that neurons in the LH are potently inhibited by MCH [29, 55, 78]. The opposite effects observed here with MCH in the LH compared to PVN regions may be a consequence of regional differences in their physiological functions. While lesions of the LH produce hypophagia [10, 86] and electrical stimulation enhances appetitive behavior [87, 88], lesions of the PVN cause hyperphagia and a loss of feeding control [89, 90]. Since MCH is known to reduce the activity of several excitatory neurochemicals in the LH region, including glutamate and OX [78, 91], it may act through this inhibitory effect to reduce the appetitive behavior associated with ethanol consumption. This indicates that MCH stimulates ethanol intake when injected into the PVN where lesions promote consummatory behavior, but it reduces ethanol intake when injected into the LH where lesions reduce consummatory behavior.
MCH May Enhance Ethanol Consumption through the Mesolimbic DA System
Since MCH-expressing neurons originating in the LH may influence consummatory behavior through projections to the mesolimbic NAc region, we next measured ethanol consumption with MCH injected into this brain region and observed a strong and specific stimulation of drinking behavior at all time points measured (1 h to 4 h), with no change in food or water intake. Although a previous investigation by Georgescu and colleagues [18] suggested that MCH injected into the NAc stimulates food intake, there are several experimental differences between these studies that may explain these different findings. Whereas the test paradigm used in that report was specific for feeding behavior, with chow available for 24 h and injections made at dark onset when food consumption peaks, the present study used a 12-h chow cycling paradigm with ethanol available, and the MCH injections were made 2 hours into the dark cycle when ethanol consumption is highest [92, 93]. These studies also differed in the dose of MCH used, which was lower in our study (0.6 nmol) and possibly below the threshold that stimulates feeding. The availability of ethanol in ethanol-drinking rats has previously been shown to mask the feeding-stimulatory effects of other peptides injected into the NAc [94], suggesting that it may supplant food as the preferred substance for peptide-induced appetitive behavior. Further support for this is provided by findings showing that blockade of MCH R1 suppresses the consumption of preferred substances, such as a palatable high-fat diet or sweetened condensed milk solution, but not of regular chow [40].
The NAc is a main terminal region of the mesolimbic reward circuit, where dopamine (DA) is a primary neurotransmitter strongly associated with consumption of rewarding substances, such as ethanol and other drugs of abuse [83–85]. The LH has prominent connections with the NAc, and MCH cell bodies in the LH are found to project to this region, where MCH R1 is co-expressed with DA receptors on opioidergic neurons [18, 41, 53]. The possibility that MCH stimulates DA release in the NAc region is supported by the finding that centrally injected MCH, while enhancing cocaine reward-associated locomotor activation, potentiates DA agonist-induced spike firing of NAc neurons [41]. In showing a strong, stimulatory effect on ethanol consumption with MCH injections into the NAc similar to the PVN, these results provide support for the involvement of a hypothalamic-mesolimbic circuit in the reinforcing and motivational aspects of ethanol drinking behavior.
Summary and Conclusion
In summary, our results lead us to propose that MCH neurons in the LH that project to the PVN and NAc have a role in stimulating the consumption of ethanol. Our findings demonstrate that, while chronic voluntary consumption of ethanol causes local negative feedback control of MCH expression in the LH, a brief exposure to ethanol stimulates MCH-expressing neurons in this region, which through stimulation of consummatory and reward-related structures such as the PVN and NAc can promote further ethanol drinking behavior.
Acknowledgments
This research was supported by USPHS Grant AA12882. We would like to thank Ambrose Carr, Jessica Baylan, Siyi Chang and Olga Karatayev for their technical assistance and help with the preparation of this manuscript. We are grateful to Dr. Nicholas A. Tritos (Joslin Diabetes Center, Harvard University) for his generosity in providing the RNA probe used in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thiele TE, et al. Overlapping peptide control of alcohol self-administration and feeding. Alcohol Clin Exp Res. 2004;28(2):288–294. doi: 10.1097/01.alc.0000113777.87190.9c. [DOI] [PubMed] [Google Scholar]
- 2.Thiele TE, et al. Alcoholism and obesity: Overlapping neuropeptide pathways? Neuropeptides. 2003;37(6):321–337. doi: 10.1016/j.npep.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Schneider ER, et al. Orexigenic peptides and alcohol intake: Differential effects of orexin, galanin, and ghrelin. Alcohol Clin Exp Res. 2007;31(11):1858–1865. doi: 10.1111/j.1530-0277.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 4.Barson JR, et al. Opioids in the hypothalamic paraventricular nucleus stimulate ethanol intake. Alcohol Clin Exp Res. 2009;34(2):214–222. doi: 10.1111/j.1530-0277.2009.01084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karatayev O, et al. Galanin knockout mice show disturbances in ethanol consumption and expression of hypothalamic peptides that stimulate ethanol intake. Alcohol Clin Exp Res. 2010;34(1):72–80. doi: 10.1111/j.1530-0277.2009.01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karatayev O, Baylan J, Leibowitz SF. Increased intake of ethanol and dietary fat in galanin overexpressing mice. Alcohol. 2009;43(8):571–580. doi: 10.1016/j.alcohol.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arjune D, Bowen WD, Bodnar RJ. Ingestive behavior following central [d-ala2, leu5, cys6]-enkephalin (dalce), a short-acting agonist and long-acting antagonist at the delta opioid receptor. Pharmacol Biochem Behav. 1991;39(2):429–436. doi: 10.1016/0091-3057(91)90203-e. [DOI] [PubMed] [Google Scholar]
- 8.Lewis MJ, et al. Galanin microinjection in the third ventricle increases voluntary ethanol intake. Alcohol Clin Exp Res. 2004;28(12):1822–1828. doi: 10.1097/01.alc.0000148099.12344.c8. [DOI] [PubMed] [Google Scholar]
- 9.Kelley AE, et al. Corticostriatal-hypothalamic circuitry and food motivation: Integration of energy, action and reward. Physiol Behav. 2005;86(5):773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 10.Willis GL, Hansky J, Smith GC. Ventricular, paraventricular and circumventricular structures involved in peptide-induced satiety. Regul Pept. 1984;9(1–2):87–99. doi: 10.1016/0167-0115(84)90011-9. [DOI] [PubMed] [Google Scholar]
- 11.Olds J, Olds ME. Positive reinforcement produced by stimulating hypothalamus with iproniazid and other compounds. Science. 1958;127(3307):1175–1176. doi: 10.1126/science.127.3307.1175. [DOI] [PubMed] [Google Scholar]
- 12.Sakurai T, et al. Orexins and orexin receptors: A family of hypothalamic neuropeptides and g protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 13.Nahon JL, et al. The rat melanin-concentrating hormone messenger ribonucleic acid encodes multiple putative neuropeptides coexpressed in the dorsolateral hypothalamus. Endocrinology. 1989;125(4):2056–2065. doi: 10.1210/endo-125-4-2056. [DOI] [PubMed] [Google Scholar]
- 14.de Lecea L, et al. The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc.Natl.Acad.Sci.U.S.A. 1998;95(1):322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tritos NA, Maratos-Flier E. Two important systems in energy homeostasis: Melanocortins and melanin-concentrating hormone. Neuropeptides. 1999;33(5):339–349. doi: 10.1054/npep.1999.0055. [DOI] [PubMed] [Google Scholar]
- 16.Shimada M, et al. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396(6712):670–674. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- 17.Qu D, et al. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380(6571):243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 18.Georgescu D, et al. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. J Neurosci. 2005;25(11):2933–2940. doi: 10.1523/JNEUROSCI.1714-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clegg DJ, et al. Eating elicited by orexin-a, but not melanin-concentrating hormone, is opioid mediated. Endocrinology. 2002;143(8):2995–3000. doi: 10.1210/endo.143.8.8977. [DOI] [PubMed] [Google Scholar]
- 20.Viale A, et al. The melanin-concentrating hormone gene in human: Flanking region analysis, fine chromosome mapping, and tissue-specific expression. Brain Res Mol Brain Res. 1997;46(1–2):243–255. doi: 10.1016/s0169-328x(97)00018-1. [DOI] [PubMed] [Google Scholar]
- 21.Presse F, et al. Melanin-concentrating hormone is a potent anorectic peptide regulated by food-deprivation and glucopenia in the rat. Neuroscience. 1996;71(3):735–745. doi: 10.1016/0306-4522(95)00481-5. [DOI] [PubMed] [Google Scholar]
- 22.Hahn JD. Comparison of melanin-concentrating hormone and hypocretin/orexin peptide expression patterns in a current parceling scheme of the lateral hypothalamic zone. Neurosci Lett. 468(1):12–17. doi: 10.1016/j.neulet.2009.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flier JS, Maratos-Flier E. Obesity and the hypothalamus: Novel peptides for new pathways. Cell. 1998;92(4):437–440. doi: 10.1016/s0092-8674(00)80937-x. [DOI] [PubMed] [Google Scholar]
- 24.Wang S, et al. Identification and pharmacological characterization of a novel human melanin-concentrating hormone receptor, mch-r2. J Biol Chem. 2001;276(37):34664–34670. doi: 10.1074/jbc.M102601200. [DOI] [PubMed] [Google Scholar]
- 25.Chambers J, et al. Melanin-concentrating hormone is the cognate ligand for the orphan g-protein-coupled receptor slc-1. Nature. 1999;400(6741):261–265. doi: 10.1038/22313. [DOI] [PubMed] [Google Scholar]
- 26.Bachner D, et al. Identification of melanin concentrating hormone (mch) as the natural ligand for the orphan somatostatin-like receptor 1 (slc-1) FEBS Lett. 1999;457(3):522–524. doi: 10.1016/s0014-5793(99)01092-3. [DOI] [PubMed] [Google Scholar]
- 27.Shimomura Y, et al. Isolation and identification of melanin-concentrating hormone as the endogenous ligand of the slc-1 receptor. Biochem Biophys Res Commun. 1999;261(3):622–626. doi: 10.1006/bbrc.1999.1104. [DOI] [PubMed] [Google Scholar]
- 28.Saito Y, et al. Molecular characterization of the melanin-concentrating-hormone receptor. Nature. 1999;400(6741):265–269. doi: 10.1038/22321. [DOI] [PubMed] [Google Scholar]
- 29.Gao XB, van den Pol AN. Melanin-concentrating hormone depresses l-, n-, and p/q-type voltage-dependent calcium channels in rat lateral hypothalamic neurons. J Physiol. 2002;542(Pt 1):273–286. doi: 10.1113/jphysiol.2002.019372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pissios P, et al. Melanin-concentrating hormone receptor 1 activates extracellular signal-regulated kinase and synergizes with g(s)-coupled pathways. Endocrinology. 2003;144(8):3514–3523. doi: 10.1210/en.2002-0004. [DOI] [PubMed] [Google Scholar]
- 31.Hawes BE, et al. The melanin-concentrating hormone receptor couples to multiple g proteins to activate diverse intracellular signaling pathways. Endocrinology. 2000;141(12):4524–4532. doi: 10.1210/endo.141.12.7833. [DOI] [PubMed] [Google Scholar]
- 32.Saito Y, et al. Expression of the melanin-concentrating hormone (mch) receptor mrna in the rat brain. J Comp Neurol. 2001;435(1):26–40. doi: 10.1002/cne.1191. [DOI] [PubMed] [Google Scholar]
- 33.Rossi M, et al. Melanin-concentrating hormone acutely stimulates feeding, but chronic administration has no effect on body weight. Endocrinology. 1997;138(1):351–355. doi: 10.1210/endo.138.1.4887. [DOI] [PubMed] [Google Scholar]
- 34.Ludwig DS, et al. Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance. J Clin Invest. 2001;107(3):379–386. doi: 10.1172/JCI10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abbott CR, et al. Identification of hypothalamic nuclei involved in the orexigenic effect of melanin-concentrating hormone. Endocrinology. 2003;144(9):3943–3949. doi: 10.1210/en.2003-0149. [DOI] [PubMed] [Google Scholar]
- 36.Guesdon B, et al. Effects of intracerebroventricular and intra-accumbens melanin-concentrating hormone agonism on food intake and energy expenditure. Am J Physiol Regul Integr Comp Physiol. 2009;296(3):R469–R475. doi: 10.1152/ajpregu.90556.2008. [DOI] [PubMed] [Google Scholar]
- 37.Sakamaki R, et al. Melanin-concentrating hormone enhances sucrose intake. Int J Mol Med. 2005;15(6):1033–1039. [PubMed] [Google Scholar]
- 38.Duncan EA, Proulx K, Woods SC. Central administration of melanin-concentrating hormone increases alcohol and sucrose/quinine intake in rats. Alcohol Clin. Exp. Res. 2005;29(6):958–964. doi: 10.1097/01.alc.0000167741.42353.10. [DOI] [PubMed] [Google Scholar]
- 39.Benoit SC, et al. The role of previous exposure in the appetitive and consummatory effects of orexigenic neuropeptides. Peptides. 2005;26(5):751–757. doi: 10.1016/j.peptides.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 40.Morens C, et al. Effects of mch and a mch1-receptor antagonist on (palatable) food and water intake. Brain Res. 2005;1062(1–2):32–38. doi: 10.1016/j.brainres.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Chung S, et al. The melanin-concentrating hormone system modulates cocaine reward. Proc Natl Acad Sci U S A. 2009;106(16):6772–6777. doi: 10.1073/pnas.0811331106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duncan EA, et al. The regulation of alcohol intake by melanin-concentrating hormone in rats. Pharmacol Biochem Behav. 2006;85(4):728–735. doi: 10.1016/j.pbb.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duncan EA, et al. Alcohol drinking in mch receptor-1-deficient mice. Alcohol Clin Exp Res. 2007;31(8):1325–1337. doi: 10.1111/j.1530-0277.2007.00427.x. [DOI] [PubMed] [Google Scholar]
- 44.Chang G-Q, et al. Maternal high-fat diet and fetal programming: Increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J Neurosci. 2008;28(46):12107–12119. doi: 10.1523/JNEUROSCI.2642-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang GQ, et al. Circulating triglycerides impact on orexigenic peptides and neuronal activity in hypothalamus. Endocrinology. 2004;145(8):3904–3912. doi: 10.1210/en.2003-1582. [DOI] [PubMed] [Google Scholar]
- 46.Chang G-Q, et al. Effect of ethanol on hypothalamic opioid peptides, enkephalin, and dynorphin: Relationship with circulating triglycerides. Alcohol Clin Exp Res. 2007;31(2):249–259. doi: 10.1111/j.1530-0277.2006.00312.x. [DOI] [PubMed] [Google Scholar]
- 47.Leibowitz SF, et al. Ethanol intake increases galanin mrna in the hypothalamus and withdrawal decreases it. Physiol Behav. 2003;79(1):103–111. doi: 10.1016/s0031-9384(03)00110-0. [DOI] [PubMed] [Google Scholar]
- 48.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Sydney: Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- 49.Wortley KE, et al. Peptides that regulate food intake: Orexin gene expression is increased during states of hypertriglyceridemia. Am J Physiol Regul. Integr. Comp Physiol. 2003;284(6):R1454–R1465. doi: 10.1152/ajpregu.00286.2002. [DOI] [PubMed] [Google Scholar]
- 50.Lucas LR, et al. Effects of adrenal steroids on basal ganglia neuropeptide mrna and tyrosine hydroxylase radioimmunoreactive levels in the adrenalectomized rat. J.Neurochem. 1998;71(2):833–843. doi: 10.1046/j.1471-4159.1998.71020833.x. [DOI] [PubMed] [Google Scholar]
- 51.Reagan LP, et al. Chronic restraint stress up-regulates glt-1 mrna and protein expression in the rat hippocampus: Reversal by tianeptine. Proc.Natl.Acad.Sci.U.S.A. 2004;101(7):2179–2184. doi: 10.1073/pnas.0307294101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paxinos G, Watson C. The rat brain, in stereotaxic coordinates. Compact Third Edition. San Diego, C.A: Academic Press, Inc; 1997. [Google Scholar]
- 53.Rossi M, et al. Investigation of the feeding effects of melanin concentrating hormone on food intake--action independent of galanin and the melanocortin receptors. Brain Res. 1999;846(2):164–170. doi: 10.1016/s0006-8993(99)02005-3. [DOI] [PubMed] [Google Scholar]
- 54.Morganstern I, et al. Differential effects of acute and chronic ethanol exposure on orexin expression in the perifornical lateral hypothalamus. Alc Clin Exp Res. 2010 doi: 10.1111/j.1530-0277.2010.01161.x. (Accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rao Y, et al. Regulation of synaptic efficacy in hypocretin/orexin-containing neurons by melanin concentrating hormone in the lateral hypothalamus. J Neurosci. 2008;28(37):9101–9110. doi: 10.1523/JNEUROSCI.1766-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seilicovich A, et al. Effect of ethanol on gaba uptake and release from hypothalamic fragments. Psychopharmacology (Berl) 1988;95(3):418–422. doi: 10.1007/BF00181959. [DOI] [PubMed] [Google Scholar]
- 57.Kaneyuki T, Morimasa T, Shohmori T. Neurotransmitter interactions in the striatum and hypothalamus of mice after single and repeated ethanol treatment. Acta Med Okayama. 1995;49(1):13–17. doi: 10.18926/AMO/30415. [DOI] [PubMed] [Google Scholar]
- 58.Moragues N, et al. Gabaa receptor epsilon subunit expression in identified peptidergic neurons of the rat hypothalamus. Brain Res. 2003;967(1–2):285–289. doi: 10.1016/s0006-8993(02)04270-1. [DOI] [PubMed] [Google Scholar]
- 59.Backberg M, et al. Cellular localization of gaba receptor alpha subunit immunoreactivity in the rat hypothalamus: Relationship with neurones containing orexigenic or anorexigenic peptides. J Neuroendocrinol. 2004;16(7):589–604. doi: 10.1111/j.1365-2826.2004.01207.x. [DOI] [PubMed] [Google Scholar]
- 60.Backberg M, et al. Chemical coding of gaba(b) receptor-immunoreactive neurones in hypothalamic regions regulating body weight. J Neuroendocrinol. 2003;15(1):1–14. doi: 10.1046/j.1365-2826.2003.00843.x. [DOI] [PubMed] [Google Scholar]
- 61.Alam MN, et al. Gaba-mediated control of hypocretin- but not melanin-concentrating hormone-immunoreactive neurones during sleep in rats. J Physiol. 2005;563(Pt 2):569–582. doi: 10.1113/jphysiol.2004.076927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tritos NA, et al. Characterization of expression of hypothalamic appetite-regulating peptides in obese hyperleptinemic brown adipose tissue-deficient (uncoupling protein-promoter-driven diphtheria toxin a) mice. Endocrinology. 1998;139(11):4634–4641. doi: 10.1210/endo.139.11.6308. [DOI] [PubMed] [Google Scholar]
- 63.Elliott JC, et al. Increases in melanin-concentrating hormone and mch receptor levels in the hypothalamus of dietary-obese rats. Brain Res Mol Brain Res. 2004;128(2):150–159. doi: 10.1016/j.molbrainres.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 64.Pickering C, et al. The role of hypothalamic peptide gene expression in alcohol selfadministration behavior. Peptides. 2007;28(12):2361–2371. doi: 10.1016/j.peptides.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 65.Presse F, et al. Rat melanin-concentrating hormone messenger ribonucleic acid expression: Marked changes during development and after stress and glucocorticoid stimuli. Endocrinology. 1992;131(3):1241–1250. doi: 10.1210/endo.131.3.1505462. [DOI] [PubMed] [Google Scholar]
- 66.Green JA, Baker BI, Kawauchi H. The effect of rearing rainbow trout on black or white backgrounds on their secretion of melanin-concentrating hormone and their sensitivity to stress. J Endocrinol. 1991;128(2):267–274. doi: 10.1677/joe.0.1280267. [DOI] [PubMed] [Google Scholar]
- 67.Qi Y, et al. Characterization of the projections to the hypothalamic paraventricular and periventricular nuclei in the female sheep brain, using retrograde tracing and immunohistochemistry. Neuroendocrinology. 2009;90(1):31–53. doi: 10.1159/000221304. [DOI] [PubMed] [Google Scholar]
- 68.Hervieu GJ, et al. The distribution of the mrna and protein products of the melanin-concentrating hormone (mch) receptor gene, slc-1, in the central nervous system of the rat. Eur J Neurosci. 2000;12(4):1194–1216. doi: 10.1046/j.1460-9568.2000.00008.x. [DOI] [PubMed] [Google Scholar]
- 69.Supko DE, Uretsky NJ, Wallace LJ. Activation of ampa/kainic acid glutamate receptors in the zona incerta stimulates locomotor activity. Brain Res. 1991;564(1):159–163. doi: 10.1016/0006-8993(91)91367-a. [DOI] [PubMed] [Google Scholar]
- 70.Milner KL, Mogenson GJ. Electrical and chemical activation of the mesencephalic and subthalamic locomotor regions in freely moving rats. Brain Res. 1988;452(1–2):273–285. doi: 10.1016/0006-8993(88)90031-5. [DOI] [PubMed] [Google Scholar]
- 71.Nadal R, Armario A, Janak PH. Positive relationship between activity in a novel environment and operant ethanol self-administration in rats. Psychopharmacology (Berl) 2002;162(3):333–338. doi: 10.1007/s00213-002-1091-5. [DOI] [PubMed] [Google Scholar]
- 72.Heise CE, Mitrofanis J. Evidence for a glutamatergic projection from the zona incerta to the basal ganglia of rats. J Comp Neurol. 2004;468(4):482–495. doi: 10.1002/cne.10971. [DOI] [PubMed] [Google Scholar]
- 73.Lembo PM, et al. The receptor for the orexigenic peptide melanin-concentrating hormone is a g-protein-coupled receptor. Nat Cell Biol. 1999;1(5):267–271. doi: 10.1038/12978. [DOI] [PubMed] [Google Scholar]
- 74.Shen EH, Phillips TJ. Mk-801 potentiates ethanol's effects on locomotor activity in mice. Pharmacol Biochem Behav. 1998;59(1):135–143. doi: 10.1016/s0091-3057(97)00389-4. [DOI] [PubMed] [Google Scholar]
- 75.Araujo NP, et al. Sleep deprivation abolishes the locomotor stimulant effect of ethanol in mice. Brain Res Bull. 2006;69(3):332–337. doi: 10.1016/j.brainresbull.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 76.Elias CF, Bittencourt JC. Study of the origins of melanin-concentrating hormone and neuropeptide ei immunoreactive projections to the periaqueductal gray matter. Brain Res. 1997;755(2):255–271. doi: 10.1016/s0006-8993(97)00104-2. [DOI] [PubMed] [Google Scholar]
- 77.Chang GQ, et al. Effect of ethanol on hypothalamic opioid peptides, enkephalin, and dynorphin: Relationship with circulating triglycerides. Alcohol Clin Exp Res. 2007;31(2):249–259. doi: 10.1111/j.1530-0277.2006.00312.x. [DOI] [PubMed] [Google Scholar]
- 78.Gao XB, van den Pol AN. Melanin concentrating hormone depresses synaptic activity of glutamate and gaba neurons from rat lateral hypothalamus. J Physiol. 2001;533(Pt 1):237–252. doi: 10.1111/j.1469-7793.2001.0237b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meister B, et al. Glutamic acid decarboxylase- and gamma-aminobutyric acid-like immunoreactivities in corticotropin-releasing factor-containing parvocellular neurons of the hypothalamic paraventricular nucleus. Neuroendocrinology. 1988;48(5):516–526. doi: 10.1159/000125058. [DOI] [PubMed] [Google Scholar]
- 80.Kennedy AR, et al. Effect of direct injection of melanin-concentrating hormone into the paraventricular nucleus: Further evidence for a stimulatory role in the adrenal axis via slc-1. J Neuroendocrinol. 2003;15(3):268–272. doi: 10.1046/j.1365-2826.2003.00997.x. [DOI] [PubMed] [Google Scholar]
- 81.Rodaros D, et al. Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience. 2007;150(1):8–13. doi: 10.1016/j.neuroscience.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 82.Wanat MJ, et al. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase c-dependent enhancement of ih. J Physiol. 2008;586(8):2157–2170. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weiss F, et al. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: Genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267(1):250–258. [PubMed] [Google Scholar]
- 84.Koob GF, Nestler EJ. The neurobiology of drug addiction. J Neuropsychiatry Clin Neurosci. 1997;9(3):482–497. doi: 10.1176/jnp.9.3.482. [DOI] [PubMed] [Google Scholar]
- 85.Fitzgerald LW, Nestler EJ. Molecular and cellular adaptations in signal transduction pathways following ethanol exposure. Clin Neurosci. 1995;3(3):165–173. [PubMed] [Google Scholar]
- 86.Oltmans GA, Harvey JA. Lateral hypothalamic syndrome in rats: A comparison of the behavioral and neurochemical effects of lesions placed in the lateral hypothalamus and nigrostriatal bundle. J Comp Physiol Psychol. 1976;90(11):1051–1062. doi: 10.1037/h0078660. [DOI] [PubMed] [Google Scholar]
- 87.Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. Journal of Comparative and Physiological Psychology. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- 88.Olds J. Self-stimulation of the brain; its use to study local effects of hunger, sex, and drugs. Science. 1958;127(3294):315–324. doi: 10.1126/science.127.3294.315. [DOI] [PubMed] [Google Scholar]
- 89.Shor-Posner G, et al. Deficits in the control of food intake after hypothalamic paraventricular nucleus lesions. Physiol Behav. 1985;35(6):883–890. doi: 10.1016/0031-9384(85)90255-0. [DOI] [PubMed] [Google Scholar]
- 90.Leibowitz SF, Hammer NJ, Chang K. Feeding behavior induced by central norepinephrine injection is attenuated by discrete lesions in the hypothalamic paraventricular nucleus. Pharmacol Biochem Behav. 1983;19(6):945–950. doi: 10.1016/0091-3057(83)90396-9. [DOI] [PubMed] [Google Scholar]
- 91.van den Pol AN, et al. Physiological properties of hypothalamic mch neurons identified with selective expression of reporter gene after recombinant virus infection. Neuron. 2004;42(4):635–652. doi: 10.1016/s0896-6273(04)00251-x. [DOI] [PubMed] [Google Scholar]
- 92.Goldstein DB, Kakihana R. Circadian rhythms of ethanol consumption by mice: A simple computer analysis for chronopharmacology. Psychopharmacology (Berl) 1977;52(1):41–45. doi: 10.1007/BF00426598. [DOI] [PubMed] [Google Scholar]
- 93.Agabio R, et al. Circadian drinking pattern of sardinian alcohol-preferring rats. Alcohol Alcohol. 1996;31(4):385–388. doi: 10.1093/oxfordjournals.alcalc.a008166. [DOI] [PubMed] [Google Scholar]
- 94.Barson JR, et al. Opioids in the nucleus accumbens stimulate ethanol intake. Physiol Behav. 2009;98(4):453–459. doi: 10.1016/j.physbeh.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]