Abstract
Recently semivolatile lower chlorinated biphenyls have been identified in inner city air, in public buildings like schools, and at many other sites. Inhalation exposure to these compounds, which are readily metabolized to mono- and dihydroxy-biphenyls and further to quinones, is of great concern in light of new studies revealing that at least one such compound, 4-monochlorobiphenyl (PCB3), has tumor initiating and mutagenic activity in rats. In vitro the quinone metabolites of PCB3 induced gene mutations, whereas its mono-and di-hydroxylated metabolites increased micronuclei frequency. To gain further insight into the genotoxicity and possible structure-activity relationships of the dihydroxy-metabolites, we measured the effects of the 2-chloro-, 3-chloro, and 4-chloro-2′,5′-dihydroxybiphenyl (PCB1-HQ, PCB2-HQ, and PCB3-HQ, respectively), and of 4-chloro-3′,4′-dihydroxybiphenyl (PCB3-Cat) on cytotoxicity, sister chromatid exchange (SCE), cellular proliferation and chromosome number. Notably only PCB3-Cat caused a significant increase in SCE levels. Cell cycle progression during exposure, which is indicated indirectly in this assay by the occurrence of metaphases with Harlequin stained chromosomes (cell underwent 2 S-phases) or uniformly dark stained chromosomes (underwent less than 2 S-phases) was inhibited by PCB2-HQ and PCB3-HQ. Most surprising was the finding that up to 96% of metaphases from cells treated with PCB2- or PCB3-HQ were tetraploid, some of which had dark and some Harlequin stained chromosomes. Neither PCB1-HQ nor PCB3-Cat or the negative (solvent) or positive control (ethylmethane sulfonate, EMS) induced this effect. The mechanism of this polyploidization is unknown. Nearly all cancer cells are hyperdiploid and polyploidization, followed by uneven chromosome loss, is hypothesized as one possible underlying mechanism of carcinogenesis. Thus different PCB metabolites may induce carcinogenesis by different mechanisms, including SCE induction or polyploidization. Understanding the mechanism(s) and structure-activity-relationships of these unexpected effects is needed before we can perform fully data-driven risk assessment of these compounds.
1. Introduction
Polychlorinated Biphenyls (PCBs) are persistent organic pollutants (POPs) and are widespread environmental contaminants (WHO 2003). The worldwide production, which peaked in the 1970s and was terminated in most countries by the mid 1980s, has been estimated to be 2 million tons. PCBs were used in industry as coolants and lubricants in transformers and capacitors, as impregnating and paint agents, as cement and plaster additives, and in sealing liquids, immersion oils, in pesticides, and for many other applications (Holoubek et al. 2001; WHO 2003). Despite the production ban and heavily restricted use, exposure of humans to PCBs still continues, mostly through our food, particularly fish, but also through the inhalation of contaminated air. Vaporization and emission from contaminated landfills and surface water, or from construction materials of public and private buildings are major sources for indoor and outdoor air contamination (Hansen and O’Keefe 1996; Imsilp et al. 2005; Persson et al. 2005). PCB concentrations of 5–16 ng/m3 were measured in Chicago on hot summer days and indoor air concentrations up to 13,000 ng/m3 were detected in some classrooms of contaminated schools (Hu et al. 2008; Kohler et al. 2002).
Airborne PCBs are predominantly lower chlorinated biphenyls. About half (in mole percent) of the PCBs in Chicago air was comprised of congeners with three or fewer chlorines, including the three monochlorinated congeners (Hu et al. 2008; Zhao et al. 2009). PCB3 and other lower chlorinated compounds were found in the air of contaminated sites and in urban areas (Uraki et al. 2004) and can be detected in human blood samples (DeCaprio et al. 2005). PCB1 and PCB3, which are also the major constituents of the commercial PCB mixture Aroclor 1221, were the main congeners in the indoor air of a childcare facility in California, and PCB3 was the major congener coming out of smokestacks of cement factories in Japan (Davis et al. 2002; Ishikawa et al. 2007). These monochlorobiphenyls are more readily converted by xenobiotic-metabolizing enzymes to monohydroxy-PCBs and further to dihydroxy-metabolites, which can be oxidized to quinones (Espandiari et al. 2004; James 2001; McLean et al. 1996). Therefore it must be assumed that these PCB metabolites might contribute to PCB-toxicity. Evidence of the tumor initiating activity of PCB3 and other lower chlorinated PCBs using a modified Solt-Farber protocol in male Fischer 344 rats was recently published and an analysis of nine PCB3 metabolites suggests that a quinone metabolite is the ultimate carcinogen (Espandiari et al. 2003; Espandiari et al. 2004). Another study demonstrated that PCB3 is able to produce gene mutations, predominately transversions, in the livers of male transgenic Fischer 344 rats (Lehmann et al. 2007), but in vitro only the PCB3 quinone metabolites induced gene mutations in Chinese hamster V79 cells, whereas the mono- and dihydroxylated metabolites produced micronuclei (Zettner et al. 2007).
We wished to gain further insight into the genotoxicity of these quinone precursors, the para- (hydroquinone, HQ) and the ortho- (catechol, Cat) dihydroxybiphenyls, and analyze possible structure-activity relationships. We therefore investigated the effects of the hydroquinones of PCB1, PCB2 and PCB3 and of the catechol of PCB3 on sister chromatid exchange (SCE), proliferation, and chromosome number in V79 cells using concentrations ranging from 1–10 μM. We show here that only PCB3-Cat significantly increased the SCE levels, but PCB2-HQ and the PCB3-HQ very efficiently induced a polyploidization of V79 cells, which is suspected to be a mechanism in the process of carcinogenesis.
2. Material and Methods
2.1. Chemical Substances
Reagents, media and media supplements were from Fischer Scientific (Pittsburgh, PA) if not stated otherwise. PCB1-HQ [2-chloro-2′,5′-dihydroxybiphenyl], PCB2-HQ [3-chloro-2′,5′-dihydroxybiphenyl], PCB3-HQ [4-chloro-2′,5′-dihydroxybiphenyl] and PCB3-CAT [4-chloro-3′,4′-dihydroxybiphenyl] (Fig. 1) were synthesized, purified and characterized as described (Espandiari et al. 2003; Tampal et al. 2002). Test compounds were dissolved in dimethylformamide (DMF) for delivery to the cells. The final concentration of the solvent in the culture medium did not exceed 0.5% (v/v) of DMF. This concentration was found to have no effect on either cell viability or sister chromatid exchange (SCE) level in V79 cells. Solvent control samples were treated with 0.5% DMF in medium.
Fig. 1.

Chemical structures of PCB1-HQ, PCB2-HQ, PCB3-HQ, and PCB3-Cat
2.2. Cell culture
Male Chinese hamster V79 lung fibroblasts were kindly provided by Dr. H.R. Glatt (German Institute of Human Nutrition, Nuthetal, Germany). They were grown in Dulbecco’s modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 mg/ml streptomycin at 37°C in a water-saturated atmosphere containing 5% CO2. The cells were screened for mycoplasma using a Hoechst staining method (Mowles 1990). Exponentially growing cells were used throughout this study.
2.3. Analysis of direct cell toxicity using the lactate dehydrogenase (LDH) assay and of reduction in cell number with the Sulforhodamine B (SRB) assay
Cytotoxicity was determined by two methods. For this 1.5 × 104 V79 cells in 200 μl DMEM with 10% FBS and penicillin/streptomycin were seeded in 48-well plates. After 24 h, the medium was changed and the cells were treated with different concentrations of the four test compounds in triplicates for another 26 h, after which the medium was collected for the LDH assay. The cells were processed as described (Skehan et al. 1990) for the SRB assay. Briefly, 10% trichloroacetic acid was added to the cells for fixation at 4°C. After 30 min fixation, cells were washed five times with deionized water, air-dried, and stained for 20 min with 0.4% SRB in 1% aqueous acetic acid. Unbound dye was removed by washing the cells four times with 1% aqueous acetic acid. After air-drying, the SRB dye within cells was dissolved in 300 μl of 10 mM Tris base buffer by shaking the plate rapidly on a microtiter plate shaker for at least 10 min or until all color had been extracted from the cells and formed a homogeneous solution. The optical density of the extracted SRB dye was measured with a microtiter plate reader at 540 nm. The SRB absorbance correlated linearly with the amount of protein in the well measured by both the Lowry and Bradford assays and with the cell number per well.
The release of lactate dehydrogenase (LDH) into the culture medium due to a loss of membrane integrity was used to measure acute cytotoxicity of the test compounds. The assay was performed according to the manual provided with the Cytotoxicity Detection Kit (LDH) (Roche Diagnostics GmbH, Mannheim, GER) with 100 μl medium which was collected after 26 h of exposure from each well. To determine the percent cytotoxicity, the average absorbance values per triplicates were calculated, the background value was subtracted, and the percent toxicity calculated using detergent treated samples as 100% toxicity value.
2.4. Determination of sister chromatid exchange (SCE) and chromosome number per mitosis
The SCE assay was performed as described previously (Lamberti et al. 1983). Briefly, 2 × 105 V79 cells in 4 ml DMEM with 10% FBS and penicillin/streptomycin were seeded in 6 cm Petrie dishes. After 24 h, fresh medium containing 10 μg/ml bromodeoxyuridine (BrdU) and different concentrations of the four test compounds, ethylmethane sulfonate (EMS positive control, final concentration 1 μM) or solvent alone (0.5% DMF) were added for another 26 h. Four h before harvesting, colcemid (final concentration 0.5 μg/ml) was added to the cultures. After 26 h of exposure to test compounds, the cells were washed in phosphate buffered saline (PBS), trypsinized, and incubated in hypotonic solution (0.075 M KCl) for 10 min at 37°C. The cells were fixed by drop-wise addition of ice-cold fixative (glacial acetic acid: methanol, 1:3, v/v) while vortexing. Cells were washed twice in fixative and stored at −20°C for at least 24 h, after which the cell suspension was dropped on chilled, grease-free, coded microscope slides. Samples were aged for at least 2 days and then differentially stained according to the fluorescence-plus-Giemsa (FPG) procedure (Perry and Wolff 1974). Briefly, slides were stained for 20 min with Hoechst 33258 (50 μg/ml), mounted with PBS and exposed to UVA light (366 nm) at a distance of 10 cm for 30 min, treated with 60°C saline-sodium citrate buffer (2 x SSC) for 2 h, stained with Giemsa (5% in PBS), washed with water, and air-dried. For each data point, 50 well-spread, Harlequin-stained mitoses were scored for SCE and chromosome number and the values obtained were calculated as number of SCE per 21 chromosomes. For polyploidy determination 50 well-spread mitoses were scored for staining (dark or Harlequin) and chromosome number (19–24 chromosomes per mitosis = diploid, >24 chromosomes = polyploid).
2.5. Statistics
Data are given as means ± SDs of the means of three independent experiments (n = 3). Statistical analysis of differences was performed with the untransformed data set. Protein content, SCE and chromosome number per mitosis were statistically analyzed with Student’s paired t-test of significance using the Origin program (Microcal Software, Northampton, MA).
3. Results
3.1. Cell toxicity determination with the LDH assay and the SRB assay
The LDH assay provides a measurement of direct cell toxicity based on the release of LDH into the culture medium after loss of membrane integrity. In V79 cells treated for 26 h with 1–10 μM of PCB1-HQ, PCB2-HQ, and PCB3-HQ a substance- and concentration-related significant increase in LDH levels was observed (Fig. 2). PCB2-HQ and PCB3-HQ induced significant cell death at concentrations of 2.5 μM and higher, reaching the equivalence of 41.8 ± 0.1% (PCB2-HQ) and 33.4 ± 0.1% (PCB3-HQ) cell death at 10 μM concentrations. PCB1-HQ induced cell death only at the highest concentration tested, i.e. 7.2 ± 0.0% at 10 μM. No LDH release was observed with PCB3-Cat (Fig. 2).
Fig. 2.
Cell death (LDH leakage) of V79 cells after 26 h treatment with 1–10 μM of PCB metabolites. Data represent mean of one typically experiment done in triplicates ± SDs.
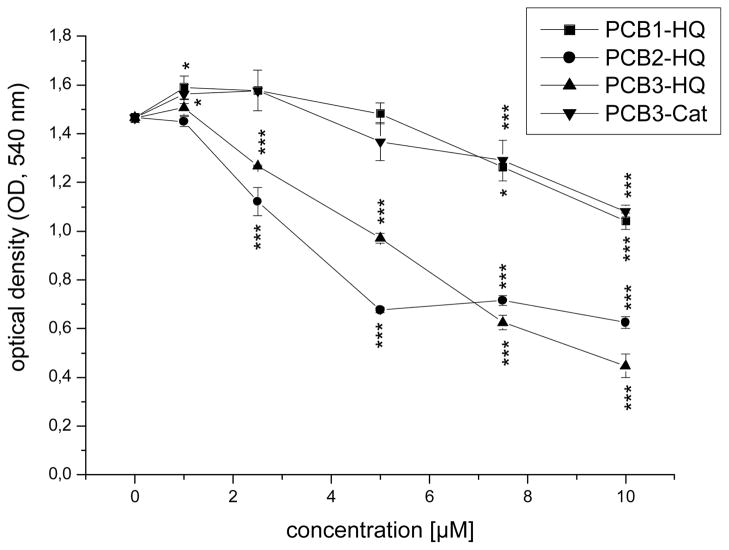
The SRB colorimetric assay determines protein levels per well which can serve as a sensitive measure of drug-induced cytotoxicity and/or cell cycling inhibition. A substance- and concentration-related statistically significant decrease in OD at 540 nm from 1.5 (negative control) to 1.1 ± 0.0 (PCB3-CAT), 1.0 ± 0.0 (PCB1-HQ), 0.6 ± 0.0 (PCB2-HQ) and 0.5 ± 0.1 (PCB3-HQ) was observed at the highest concentration (10 μM) tested, equivalent to a cell mass of 73% (PCB3-Cat), 71% (PCB1-HQ), 43% (PCB2-HQ), and 31% (PCB3-HQ) of the solvent control (Fig. 3). A slight, statistically significant increase in the protein content was observed after treatment with 1 μM PCB1-HQ and PCB3-CAT (Fig. 3).
Fig. 3.
Protein content (OD) per well after 26 h treatment of V79 cells with solvent alone (0.5% DMF) or 1–10 μM of PCB metabolites. Data represent mean of one typically experiment done in triplicates ± SDs. Asterisks indicate significant differences to the corresponding solvent control. Levels of significance (Student’s paired t-test): *p < 0.05; **p < 0.01; ***p < 0.001.
3.2. Sister chromatid exchange (SCE) and chromosome number in Harlequin stained metaphases
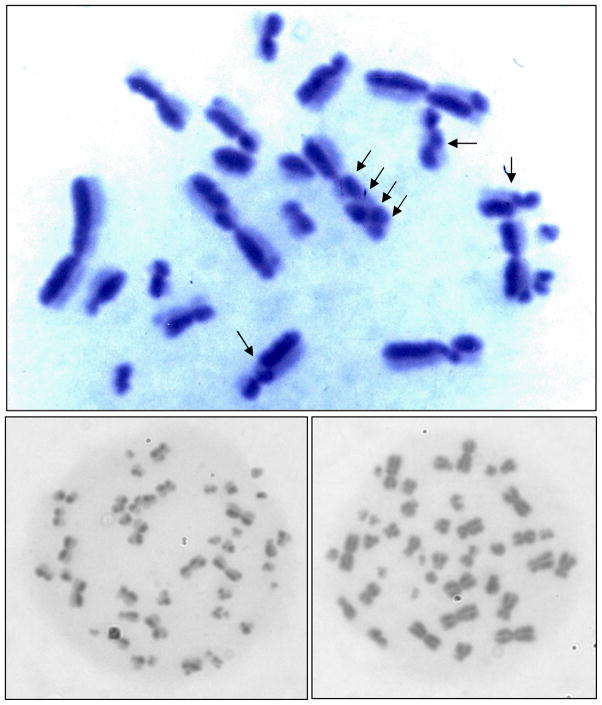
SCE are produced during DNA replication when two sister chromatids break and re-attach with the other one. To visualize this event with FPG-staining, cells have to replicate through two complete S-phases in the presence of BrdU. The Harlequin staining process results in light staining of chromatids with BrdU incorporated in both strands and dark staining of chromatids with BrdU incorporation in only one strand (Fig. 4, top picture). The frequency of spontaneous SCE in our V79 cells exposed to solvent alone (0.5% DMF) ranged from 4.8 ± 0.2 to 7.4 ± 1.0 SCE per 21 chromosomes (Fig 5, diamond symbols at 0 μM). The mutagen EMS served as positive control. Exposure of V79 cells to 1 μM EMS for 26 h caused a statistically significantly increase in the SCE frequency that ranged from 19.4 ± 1.0 to 24.8 ± 3.1 SCE per 21 chromosomes (Fig. 5, star symbols). Among the PCB metabolites (Fig. 5, symbols and line) a statistically significant dose-dependent increase in the SCE frequency was only induced by treatment with PCB3-Cat, resulting in 8.3 ± 1.2 (5 μM), 8.2 ± 0.7 (7.5 μM), and 11.6 ± 0.9 (10 μM) SCE per 21 chromosomes. The very small but significant SCE increase with 5–10 μM PCB2-HQ and 2.5–5 μM PCB3-HQ are results of unusually small standard deviations at these data points and should be regarded with caution. With this consideration neither PCB1-HQ nor PCB2-HQ or PCB3-HQ induced a significant dose-dependent increase in the SCE frequency.
Fig. 4.
Microscope photos of a diploid, harlequin-stained V79 metaphase (top), harlequin-stained tetraploid metaphase (bottom left), and dark-stained tetraploid metaphase (bottom right). Arrows in the top picture indicate some (not all) of the SCE in this metaphase.
Fig. 5.
Chromosomes per metaphase (columns) and SCE per 21 chromosomes (symbols with or without line) in V79 cells after 26 h treatment with solvent alone (0.5% DMF, left) or different PCB metabolites (middle), or EMS (1μM, right). Spontaneous SCE-levels in solvent-treated cells were 4.8–7.4 per 21 chromosomes; metaphases had 20.8–21.6 chromosomes. Data represent mean of three independent experiments ± SDs. Asterisks indicate significant differences to the corresponding solvent control. Levels of significance (Student’s paired t-test): *\+ = p < 0.05; **\++ = p < 0.01; ***\+++ = p < 0.001.
The mean chromosome number per mitosis in the V79 cells exposed to 0.5% DMF ranged from 20.8 ± 0.3 to 21.6 ± 0.1 (Fig. 5, open bars at 0 μM). There was a slight, but non-statistically significant decrease in the chromosome number per mitosis with the highest concentration of PCB1-HQ. The number of chromosomes per metaphase was nearly doubled after exposure to PCB2-HQ, reaching 35.3 ± 3.7 (5 μM), 40.1 ± 1.1 (7.5 μM), and 38.3 ± 1.2 (10μM) chromosomes per metaphase, and with PCB3-HQ reaching 40.9 ± 1.1 (7.5 μM) and 37.4 ± 4.5 (10 μM) chromosomes per metaphase. No change in the chromosome number per metaphase were observed with the PCB3-Cat (1–10 μM) and the positive control compound EMS.
3.3. Effect of PCB-treatment on cell cycle progression
Cells in mitosis that underwent DNA synthesis (S-phase) in the presence of BrdU during 2 cell cycles have chromosomes that were half light- and half dark-stained (Harlequin) by FPG-staining (Fig. 4 top picture). Completely dark-stained chromosomes indicate cells which went only through one S-phase. Since V79 cells have a population doubling time (PDT) of about 12 h, all normal proliferating cells should have Harlequin chromosomes after 26 h incubation with BrdU. Indeed the solvent control displayed only metaphases with Harlequin-stained chromosomes, indicating that all cells underwent 2 cell cycles (Table 1). Metaphases from cells exposed to the ortho-hydroxylated PCB3, PCB3-Cat (1–10 μM), also had all Harlequin-stained chromosomes. The same was true for samples treated with up to 10 μM PCB1-HQ; only at the highest PCB1-HQ concentration tested, 20 μM, about a third of the metaphases (30.7 ± 3.1%) had dark chromosomes. In contrast to this, cultures treated with 5, 7.5, or 10 μM PCB2-HQ had 30–40% of metaphases with dark-stained chromosomes, indicating that about a third of the cells underwent only one cell cycle. PCB3-HQ induced a dose-dependent increase in metaphases with dark chromosomes which was visible even at the lowest concentration tested (3.5% at 1μM) and increased to 73% of all metaphases scored at the 10 μM concentration (Table 1).
Table 1.
Percentage of metaphases with 2N (diploid) or 4N (polyploid) chromosome number and Harlequin or dark stained chromosomes after 26 h treatment with solvent alone (0.5% DMF) or different PCB metabolites. Data represent mean of at least three independent experiments ± SDs.
| PCB conc. [μM] | diploid (2N) | polyploid (4N) | ||
|---|---|---|---|---|
| Harlequin | dark | Harlequin | dark | |
| control | ||||
| 0 | 100.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| PCB1-HQ | ||||
| 1 | 100.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 2,5 | 100.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 5 | 100.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 7,5 | 100.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 10 | 100.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 20 | 59.3 ± 3.1 | 40.7 ± 3.1 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| PCB2-HQ | ||||
| 1 | 100.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 2,5 | 100.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 5 | 6.0 ± 2.8 | 10.0 ± 5.7 | 64.0 ± 0.0 | 20.0 ± 8.5 |
| 7,5 | 2.0 ± 2.8 | 2.0 ± 2.8 | 71.0 ± 9.9 | 25.0 ± 4.2 |
| 10 | 3.3 ± 3.1 | 7.3 ± 7.0 | 56.7 ± 5.0 | 32.7 ± 8.1 |
| PCB3-HQ | ||||
| 1 | 96.5 ± 3,4 | 3.5 ± 3.4 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 2,5 | 90.7 ± 7.1 | 9.3 ± 7.1 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 5 | 61.7 ± 7.0 | 9.2 ± 2.1 | 11.3 ± 4.9 | 17.4 ± 3.4 |
| 7,5 | 1.5 ± 1.5 | 11.5 ± 7.7 | 35.9 ± 4.9 | 51.1 ± 12.5 |
| 10 | 1.1 ± 1.9 | 23.2 ± 10.3 | 25.4 ± 2.3 | 49.7 ± 8.5 |
| PCB3-Cat | ||||
| 1 | 100.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 2,5 | 100.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 5 | 100.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 7,5 | 100.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 10 | 100.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
3.4. Induction of polyploidy by PCB metabolites
The currently used strains of V79 cells have on average either 21 or 22 chromosomes. Our V79 cells belong to the 21 chromosomes strain. Solvent control cells, treated during the last 4 h of exposure with colcemid, a spindle poison, to increase the fraction of cells in metaphase, were 100% diploid, i.e. they had between 21 and 22 chromosomes per metaphase (Table 1). Similarly all cultures treated with PCB3-Cat (1–10μM) or PCB1-HQ (1–20μM) were diploid. However, PCB2-HQ and PCB3-HQ caused an increase in chromosome number per metaphase to about 4N (tetraploid) level. After 26 h of exposure to the 3 highest concentrations of PCB2-HQ the majority of metaphases, i.e. 84% (5 μM), 96% (7.5 μM) and 89% (10 μM) were about tetraploid (Table 1). Harlequin stained cells accounted for about 2/3 of this tetraploid population, about 1/3 displayed dark stained chromosomes. With PCB3-HQ a similar, but dose-dependent increase in the percentage of 4N cells was observed, increasing from 0% (2.5 μM), to 29% (5 μM), 37% (7.5 μM), and 75% (10 μM) (Table 1). Of these tetraploid cells the majority, about 60%, had dark stained chromosomes (example shown in Fig 4., bottom right picture), the other had Harlequin-stained chromosomes (see Fig. 4 bottom left for example).
4. Discussion
Recent studies demonstrated that a monochlorinated biphenyl, PCB3, binds to nuclear proteins and can induce gene mutations and preneoplastic foci in the liver of rats (Espandiari et al. 2003; Lehmann et al. 2007; Pereg et al. 2001). The chemical nature of the ultimate reactive metabolite, however, is unknown. In vivo and in vitro experiments with a series of PCB3 metabolites indicate that PCB3 quinone metabolites are prime candidates (Espandiari et al. 2004; Zettner et al. 2007), but earlier metabolites in the metabolic activation pathway, particularly the dihydroxy-derivatives, cannot be excluded, and they therefore were the focus of this study.
4.1. Cytotoxicity and cell cycle delay
The cytotoxicity of our test compounds was determined with two different assays. The LDH assay measures lactate dehydrogenase activity in medium which was released from the cytosol of cells with disintegrated cell membranes. Despite the strong structural similarity of our 4 test compounds, large differences in toxicity were seen with this assay: PCB2-HQ induced a steep increase in dead cells reaching a maximum of 50% at 5 μM concentration followed by a slight reduction in toxicity; PCB3-HQ produced a steady, dose-dependent increase in toxicity reaching 30% with 10 μM, whereas the PCB3-Cat and PCB1-HQ exhibited no or hardly any toxicity, respectively, up to the highest concentration tested. The cause of these differences in toxicity is not clear, but it maybe speculated that the chlorine in ortho-position (PCB1) may alter the transfer of the compound through the cell membrane due to the angled position of the two rings to each other and thus reduce its bioavailability (Luthe et al. 2008; Yoshida and Sekiguchi 1984). Alternatively, the angled position may reduce spontaneous autoxidation or influence its interaction with enzymes like peroxidases that may be involved in the bioactivation of the hydroquinones to more toxic quinones with the formation of also toxic reactive oxygen species (ROS) (McLean et al. 2000). Similarly the PCB3-Cat may be less toxic due to a lower likelihood for (aut)-oxidation to the quinone. The lower toxicity of the catechol compared to the hydroquinone of PCB3 is in agreement with previous observations using counted cell number as the endpoint (Zettner et al. 2007).
The second toxicity test, the sulforhodamine B assay, measures protein levels in the wells after treatment. As before in the LDH assay, PCB2-HQ had the highest efficacy, causing about 57% reduction of protein compared to control at 5 μM, which plateaued at that level. With 7.5 and 10 μM, PCB3-HQ produced a linear dose-response curve, reaching a protein reduction of nearly 70% at the highest concentration tested; PCB3-Cat and PCB1-HQ slightly reduced protein levels in a dose-dependent way by less than 30% at the highest concentration tested. Thus the effect of all compounds on protein levels is a little stronger than on cell death measured by LDH release, which could be due to the fact that the SRB assay is sensitive for cell killing and an arrest or slowing of the cell cycling, i.e. cytotoxic and cytostatic effects of the test compound. Indeed, both PCB2- and PCB3-HQ strongly reduced cell cycle progression as indicated by the increase in metaphases with dark chromosomes in the SCE assay, but with different dose-responses (Table 1). With PCB2-HQ the cytostatic effect is only visible with 5, 7.5, and 10 μM concentrations and similarly strong at all three concentrations, i.e. 30–40% dark metaphases. Moreover, the majority of these metaphases were tetraploid. It is not clear what caused this plateau effect. PCB3-HQ on the other hand produced a dose-dependent increase in the % of dark metaphases, indicating that this compound induced both simultaneously, cell death and cell cycle delay, the effect on both related to the concentration. PCB3-Cat and PCB1-HQ obviously had such a minor effect on cell cycle progression that it did not lead to dark chromosomes. A small effect on cell viability and slowing or stop in proliferation should be enough to cause the small reduction in protein per well. Overall, all three endpoints of toxicity, cell membrane integrity, protein levels, and BrdU incorporation, confirm that even the small differences in chemical structure among these 4 isomers results in measurable differences in their effects on cells.
4.2. Sister chromatid exchange
The sister chromatid exchange assay has been used extensively as an endpoint in cytogenetic and genotoxic research and mutagenicity testing. SCEs only occur during replication (S-phase) and are caused by a double strand break of both chromatid arms at the replication fork and reannealing to the opposite strand. Thus these exchanges presumably require enzymatic incision, translocation and ligation of at least two DNA helices. SCEs are visualized during the metaphase portion of the cell cycle. To differentiate the parental strand and the newly synthesized strand, BrdU is added for two cell cycles. Second mitosis chromosomes are “Harlequin stained” after having undergone FPG staining that removes nucleotides from the double BrdU-substituted chromatid resulting in a light Giemsa-stained strand, thereby allowing the determination of the number of SCEs in that metaphase (Latt 1974; Latt and Gerald 1973; Perry and Wolff 1974). Of all dihydroxylated PCBs that we tested, only the ortho-dihydroxy PCB3, PCB3-Cat, significantly increased SCE levels, none of the para-dihydroxy PCBs did so. An analysis of the genotoxicity of benzene metabolites in V79 cells had shown that the hydroquinone (1,4-para-dihydroxybenzene) is only a weak SCE inducer, whereas the catechol (1,2-ortho-dihydroxybenzene) is a much more potent and efficient inducer of SCE (Glatt et al. 1989). This is in agreement with our finding that an ortho-position of hydroxyl groups is needed for SCE-inducing activity of PCBs. Interestingly, 1,2,4-trihydroxybenzene was an even stronger SCE inducer than the catechol, whereas 1,2,3-trihydroxybenzene was weaker than the hydroquinone (Glatt et al. 1989). V79 cells do not express cytochromes P450 (McGregor et al. 1991) and we can therefore assume that in our experiments the PCB3-Cat itself, and not a secondary metabolite, is responsible for the SCE-inducing activity. Previously we reported that in V79 cells neither the PCB3-HQ nor the PCB3-Cat induces gene mutations at the hrpt locus, whereas their quinone metabolites were both very efficacious mutagens (Zettner et al. 2007). The fact that these two types of genotoxicity are not related to each other supports the need for batteries of tests to uncover the genotoxic potential of a given compound. It has been postulated that a small minority of SCE may not be exact exchanges, leading to deletion, insertion, or frameshift mutagenesis (Wolff 1977). Alternatively, SCE may simply be an indication of perturbation and distortion at the replication fork due to DNA or protein adduction of the carcinogen. Although the mechanism of SCE induction or role in carcinogenesis is not well understood, the occurrence of high SCE levels in certain cancer-prone disorders like Blooms syndrome (Yoshida and Sekiguchi 1984) and good correlation between SCE-induction and carcinogenic activity(Tucker et al. 1993) implies that an SCE-inducing compound should be considered as a potential carcinogen. Therefore PCB3-Cat could play a role in carcinogenesis thru induction of SCE.
4.3. Polyploidization
Application of the SCE assay resulted in two unrelated and unexpected discoveries: 1. some of our test compounds caused tetraploidization, 2. some of these tetraploid metaphases had dark chromosomes, which technically should be impossible with our assay protocol. As Table 1 shows, we had four distinct populations of metaphases: (a) diploid-Harlequin are normal cells that underwent two cell cycles in the 26h as expected for V79; (b) diploid-dark cells are normal cells that underwent only one S-phase with BrdU because of a strong retardation of cell cycle progression, resulting in cells in 1st mitosis at the end of the assay. This would be expected, based on the results in the SRB toxicity assay; (c) polyploid-Harlequin cells: these may have been generated by cells going through mitosis but without cytokinesis (division of the mother cell into two daughter cells) or by mitotic slippage, a process where prometaphase cells return to interphase without finishing mitosis. Mitotic slippage or mitosis without cell division must have happened during the first mitosis followed by a second S-phase, resulting in tetraploid-Harlequin metaphases in the second mitosis. This does not explain the occurrence of (d) tetraploid-dark metaphases. Inhibition of cell cycle progression alone can only result in diploid-dark metaphases. Tetraploidy can only be observed in the second mitosis, which would result in Harlequin staining. We can imagine two possible mechanisms that could generate tetraploid-dark cells in our system: a) cell fusion of cells that progress very slowly through the cell cycle; b) inhibition of BrdU-incorporation in cells that experiences cytokinesis inhibition or mitotic slippage in their 1st mitosis. The second possibility seems unlikely, since 1/3 to 2/3 of the tetraploid metaphases are Harlequin stained. If BrdU uptake or incorporation into the DNA were inhibited, we would expect it in all cells. Cell fusion seems to be the more likely mechanism. We did not observe hexaploid or higher ploid cells indicating that this process or the resulting product may have limitations with respect to viability or functioning of the cells.
Only two of our four test compounds caused polyploidization, PCB2-HQ and PCB3-HQ, and again PCB2-HQ had the higher efficacy (≥90% of metaphases polyploid) whereas PCB3-HQ had the higher potency (polyploidization starting with 2.5 μM). No polyploid cells were seen with PCB1-HQ, PCB3-Cat, EMS or in the solvent controls, indicating that this cell line is not prone to spontaneous polyploidization. It is striking that both compounds that induced polyploidization (PCB2-HQ, PCB3-HQ) also had strong cell cycle effects at the same concentrations, whereas the other test compounds had neither effect at concentrations up to 10 μM (PCB1-HQ, PCB3-Cat).
There are several other reports about high to near complete polyploidization induction by a compound which may provide clues about the underlying mechanisms. 2-mercaptobenzothiazole induced polyploidization in V79 cells, but it was accompanied by endoreduplication, which is characterized by diplochromosomes since two S-phases are interrupted only by a G-phase, no M-phase (Matsuoka et al. 2005). Similarly, Topo II inhibitors such as etoposite and amsacrine are known to induce endoreduplication by preventing the decatenation of replicated chromosomes which then fail to segregate in mitosis (Sumner 1998). However, we did not observe diplochromosomes in our samples. Two important factors during mitosis are the microtubules of the spindle and actin, which is needed for cytokinesis. Noscapine is believed to induce polyploidization through direct effect upon the spindle structure (Gatehouse et al. 1991). Microtubulin-interference is also believed to be the mechanism of polyploidization by nocodazole and taxol (Jordan et al. 1993). However, demecolzin, another spindle inhibitor, induces polyploidization in V79 cells with occurrance of multi-nucleization (Fujikawa-Yamamoto et al. 2000). Multiple nuclei were also observed with pectinotoxin-2, a polyether macrolite found in certain Dinoflagellates, sponges, and shellfish. This compound induced polyploidization by depolarization of actin, not inhibition of tubulin (Moon et al. 2008). Post-mitotic dinucleated cells were not prominent in our samples, suggesting that these mechanisms were not the major once in our experiments. The kinase inhibitor K-252a, a staurosporine analogue, induced polyploidization with nuclei that were multi-lobed (Fujikawa-Yamamoto et al. 2000). Nothing was reported about cell cycle effects. We did not observe multi-lobed nuclei. Matsuoka and co-worker reported that exposure of V79 cells for 26 h to dimethylbenzanthracene (DMBA) resulted in nearly 100% polyploidization, but all of the metaphases had dark chromosomes, which they described as not-M2 (second mitoses) (Matsuoka et al. 1997). No explanation was given how dark tetraploid cells can be produced. It was hypothesized that a complete inhibition of spindle formation was the cause of the polyploidization (Matsuoka et al. 1998). An overlay of PCB3-HQ over DMBA shows the structural similarity between these two compounds (Fig. 5). Whether this indicates a similar mechanism needs to be determined. Thus none of these reports can solve the problem of how dark polyploidy cells occurred after treatment with PCB2- and PCB3-HQ.
Chromosomal instability is believed to be a common feature of most human cancers (Shih et al. 2001) and nearly all of more than 20,000 solid human cancers that have been analyzed to date were aneuploid (Kops et al. 2005; Li et al. 1997; Sandberg 1990). Indeed, it is questioned whether aneuploidy or gene mutation causes cancer (Duesberg and Rasnick 2000; Li et al. 2000). Almost any non-lethal aneuploidy unbalances proteins that are involved in segregation, synthesis and repair of chromosomes or maintenance of the integrity of genes. Thus aneuploidy catalyzes a chain reaction of aneuploidizations and mutations. Therefore the transition of a stable diploid to a unstable aneuploid cell is considered a primary cause of preneoplastic and neoplastic genomic instability and cancer (Duesberg et al. 2004). One major route to aneuploid cancer cells is through an unstable tetraploid intermediate. Uneven chromosome distribution during mitosis of tetraploid cells might facilitate genetic changes that lead to aneuploid cancers (Ganem et al. 2007). Thus PCB2-and PCB3-HQ may be involved in cancer initiation through induction of polyploidization.
4.4. Summary
We have shown here that in a group of monochlorinated biphenyls, the HQs were more toxic then the catechol, but only the catechol induced SCE, whereas the HQs with chlorines in meta- or para positions produced cell cycle delay and tetraploidization. Although these differences in the structure of our 4 test compounds seem small, they were significant with respect to the toxic effect caused by a specific compound in V79 cells. This illustrates the difficulty to understand and predict structure-activity relationships. Since both, SCE and polyploidizations, have been implicated as mechanisms in carcinogenesis, different PCB metabolites may be carcinogenic through different pathways. We have to understand these mechanisms and structure-activity-relationships to be able to make judgments about the danger of exposure to these and other compounds for human health.
Fig. 6.

Overlay of DMBA (blue) over PCB3-HQ (green) structures
Acknowledgments
This publication was made possible by grant number P42 ES013661 from the National Institute of Environmental Health Sciences (NIEHS) and DOD DAMD17-02-1-0241. Funds were also available from ES 05605. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS and DOD. We thank Dr. Larry W. Robertson and Dr. Hans-Joachim Lehmler for providing PCB3-HQ, PCB3-Cat, PCB2-HQ and PCB1-HQ and Dr. Larry W. Robertson for a careful reading of the manuscript.
Abbreviations
- PCBs
polychlorinated biphenyls
- DMF
dimethylformamide
- SCE
sister chromatid exchange
- EMS
ethylmethane sulfonate
- FBS
fetal bovine serum
- DMEM
Dulbecco’s modified Eagle medium
- BrdU
bromodeoxyuridine
- LDH
lactate dehydrogenase
- FPG
fluorescence-plus-Giemsa
- PBS
phosphate buffered saline
- SRB
sulforhodamine B
- PCB1-HQ
2-chloro-2′,5′-dihydroxybiphenyl
- PCB2-HQ
3-chloro-2′,5′-dihydroxybiphenyl
- PCB3-HQ
4-chloro-2′,5′-dihydroxybiphenyl
- PCB3-CAT
4-chloro-3′,4′-dihydroxybiphenyl
- PDT
population doubling time
- SSC
saline-sodium citrate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature
- Davis B, Beach JMW, Klein AK, Hoch K. Risk Assessment of Polychlorinated Biphenyls (PCBs) in Indoor Air. The Toxicologist, Supplement to Toxicological Sciences. 2002;66:516. [Google Scholar]
- DeCaprio AP, Johnson GW, Tarbell AM, Carpenter DO, Chiarenzelli JR, Morse GS, et al. Polychlorinated biphenyl (PCB) exposure assessment by multivariate statistical analysis of serum congener profiles in an adult Native American population. Environ Res. 2005;98:284–302. doi: 10.1016/j.envres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Duesberg P, Fabarius A, Hehlmann R. Aneuploidy, the primary cause of the multilateral genomic instability of neoplastic and preneoplastic cells. IUBMB Life. 2004;56:65–81. doi: 10.1080/15216540410001667902. [DOI] [PubMed] [Google Scholar]
- Duesberg P, Rasnick D. Aneuploidy, the somatic mutation that makes cancer a species of its own. Cell motility and the cytoskeleton. 2000;47:81–107. doi: 10.1002/1097-0169(200010)47:2<81::AID-CM1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Espandiari P, Glauert HP, Lehmler HJ, Lee EY, Srinivasan C, Robertson LW. Polychlorinated biphenyls as initiators in liver carcinogenesis: resistant hepatocyte model. Toxicology and applied pharmacology. 2003;186:55–62. doi: 10.1016/s0041-008x(02)00018-2. [DOI] [PubMed] [Google Scholar]
- Espandiari P, Glauert HP, Lehmler HJ, Lee EY, Srinivasan C, Robertson LW. Initiating activity of 4-chlorobiphenyl metabolites in the resistant hepatocyte model. Toxicol Sci. 2004;79:41–46. doi: 10.1093/toxsci/kfh097. [DOI] [PubMed] [Google Scholar]
- Fujikawa-Yamamoto K, Yamagishi H, Zong ZP, Ohdoi C, Wang SY. Different responses of polyploidized V79 cells after removal of two drugs, demecolcine and K-252a. Cell Struct Funct. 2000;25:41–46. doi: 10.1247/csf.25.41. [DOI] [PubMed] [Google Scholar]
- Ganem NJ, Storchova Z, Pellman D. Tetraploidy, aneuploidy and cancer. Curr Opin Genet Dev. 2007;17:157–162. doi: 10.1016/j.gde.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Gatehouse DG, Stemp G, Pascoe S, Wilcox P, Hawker J, Tweats DJ. Investigations into the induction of aneuploidy and polyploidy in mammalian cells by the anti-tussive agent noscapine hydrochloride. Mutagenesis. 1991;6:279–283. doi: 10.1093/mutage/6.4.279. [DOI] [PubMed] [Google Scholar]
- Glatt H, Padykula R, Berchtold GA, Ludewig G, Platt KL, Klein J, et al. Multiple activation pathways of benzene leading to products with varying genotoxic characteristics. Environ Health Perspect. 1989;82:81–89. doi: 10.1289/ehp.898281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen LG, O’Keefe PW. Polychlorinated dibenzofurans and dibenzo-p-dioxins in subsurface soil, superficial dust, and air extracts from a contaminated landfill. Arch Environ Contam Toxicol. 1996;31:271–276. doi: 10.1007/BF00212377. [DOI] [PubMed] [Google Scholar]
- Holoubek I, Kocan A, Holoubkova I, Hilscherova K, Kohoutek J, Falandysz J, et al. Persistent, bioaccumulative, and toxic compounds in central and eastern Europe--hot spots. Arh Hig Rada Toksikol. 2001;52:239–251. [PubMed] [Google Scholar]
- Hu D, Martinez A, Hornbuckle KC. Discovery of non-aroclor PCB (3,3′-dichlorobiphenyl) in Chicago air. Environ Sci Technol. 2008;42:7873–7877. doi: 10.1021/es801823r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imsilp K, Schaeffer DJ, Hansen LG. PCB disposition and different biological effects in rats following direct soil exposure vs. PCBs off-gassed from the soil. Toxicol Environ Chem. 2005;87:267–285. [Google Scholar]
- Ishikawa Y, Noma Y, Mori Y, Sakai S. Congener profiles of PCB and a proposed new set of indicator congeners. Chemosphere. 2007;67:1838–1851. doi: 10.1016/j.chemosphere.2006.05.075. [DOI] [PubMed] [Google Scholar]
- James MO. Polychlorinated biphenyls: metabolism and metabolites. Lexington, KY: University of Kentucky; 2001. [Google Scholar]
- Jordan MA, Toso RJ, Thrower D, Wilson L. Mechanism of mitotic block and inhibition of cell proliferation by taxol at low concentrations. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:9552–9556. doi: 10.1073/pnas.90.20.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler M, Zennegg M, Waeber R. Coplanar polychlorinated biphenyls (PCB) in indoor air. Environ Sci Technol. 2002;36:4735–4740. doi: 10.1021/es025622u. [DOI] [PubMed] [Google Scholar]
- Kops GJ, Weaver BA, Cleveland DW. On the road to cancer: aneuploidy and the mitotic checkpoint. Nature reviews. 2005;5:773–785. doi: 10.1038/nrc1714. [DOI] [PubMed] [Google Scholar]
- Lamberti L, Bigatti Ponzetto P, Ardito G. Cell kinetics and sister-chromatid-exchange frequency in human lymphocytes. Mutat Res. 1983;120:193–199. doi: 10.1016/0165-7992(83)90163-x. [DOI] [PubMed] [Google Scholar]
- Latt SA. Sister chromatid exchanges, indices of human chromosome damage and repair: detection by fluorescence and induction by mitomycin C. Proc Natl Acad Sci U S A. 1974;71:3162–3166. doi: 10.1073/pnas.71.8.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latt SA, Gerald PS. Staining of human metaphase chromosomes with fluorescent conjugates of polylysine. Exp Cell Res. 1973;81:401–406. doi: 10.1016/0014-4827(73)90529-6. [DOI] [PubMed] [Google Scholar]
- Lehmann L, HLE, PAK, LWR, Ludewig G. 4-monochlorobiphenyl (PCB3) induces mutations in the livers of transgenic Fisher 344 rats. Carcinogenesis. 2007;28:471–478. doi: 10.1093/carcin/bgl157. [DOI] [PubMed] [Google Scholar]
- Li R, Sonik A, Stindl R, Rasnick D, Duesberg P. Aneuploidy vs. gene mutation hypothesis of cancer: recent study claims mutation but is found to support aneuploidy. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:3236–3241. doi: 10.1073/pnas.040529797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Yerganian G, Duesberg P, Kraemer A, Willer A, Rausch C, et al. Aneuploidy correlated 100% with chemical transformation of Chinese hamster cells. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:14506–14511. doi: 10.1073/pnas.94.26.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthe G, Garcia Boy R, Jacobus J, Smith BJ, Rahaman A, Robertson LW, et al. Xenobiotic geometry and media pH determine cytotoxicity through solubility. Chemical research in toxicology. 2008;21:1017–1027. doi: 10.1021/tx700214p. [DOI] [PubMed] [Google Scholar]
- Matsuoka A, Isama K, Tsuchiya T. In vitro induction of polyploidy and chromatid exchanges by culture medium extracts of natural rubbers compounded with 2-mercaptobenzothiazole as a positive control candidate for genotoxicity tests. Journal of biomedical materials research. 2005;75:439–444. doi: 10.1002/jbm.a.30442. [DOI] [PubMed] [Google Scholar]
- Matsuoka A, Matsuura K, Sakamoto H, Hayashi M, Sofuni T. Spindle disturbances induced by benzo[a]pyrene and 7, 12-dimethylbenz[a]anthracene in a Chinese hamster cell line (V79-MZ) and the stability of the numerical chromosome aberrations that follow. Mutation research. 1998;419:1–12. doi: 10.1016/s1383-5718(98)00069-2. [DOI] [PubMed] [Google Scholar]
- Matsuoka A, Ozaki M, Takeshita K, Sakamoto H, Glatt HR, Hayashi M, et al. Aneuploidy induction by benzo[a]pyrene and polyploidy induction by 7,12-dimethylbenz[a]anthracene in Chinese hamster cell lines V79-MZ and V79. Mutagenesis. 1997;12:365–372. doi: 10.1093/mutage/12.5.365. [DOI] [PubMed] [Google Scholar]
- McGregor DB, Edwards I, Wolf CR, Forrester LM, Caspary WJ. Endogenous xenobiotic enzyme levels in mammalian cells. Mutat Res. 1991;261:29–39. doi: 10.1016/0165-1218(91)90095-4. [DOI] [PubMed] [Google Scholar]
- McLean MR, Bauer U, Amaro AR, Robertson LW. Identification of catechol and hydroquinone metabolites of 4-monochlorobiphenyl. Chem Res Toxicol. 1996;9:158–164. doi: 10.1021/tx950083a. [DOI] [PubMed] [Google Scholar]
- McLean MR, Twaroski TP, Robertson LW. Redox cycling of 2-(x′-mono, -di, -trichlorophenyl)-1, 4-benzoquinones, oxidation products of polychlorinated biphenyls. Arch Biochem Biophys. 2000;376:449–455. doi: 10.1006/abbi.2000.1754. [DOI] [PubMed] [Google Scholar]
- Moon DO, Kim MO, Kang SH, Lee KJ, Heo MS, Choi KS, et al. Induction of G2/M arrest, endoreduplication, and apoptosis by actin depolymerization agent pextenotoxin-2 in human leukemia cells, involving activation of ERK and JNK. Biochem Pharmacol. 2008;76:312–321. doi: 10.1016/j.bcp.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Mowles JM. Mycoplasma Detection. Humana Press; New Jersey: 1990. [DOI] [PubMed] [Google Scholar]
- Pereg D, Tampal N, Espandiari P, Robertson LW. Distribution and macromolecular binding of benzo[a]pyrene and two polychlorinated biphenyl congeners in female mice. Chem Biol Interact. 2001;137:243–258. doi: 10.1016/s0009-2797(01)00256-3. [DOI] [PubMed] [Google Scholar]
- Perry P, Wolff S. New Giemsa method for the differential staining of sister chromatids. Nature. 1974;251:156–158. doi: 10.1038/251156a0. [DOI] [PubMed] [Google Scholar]
- Persson NJ, Pettersen H, Ishaq R, Axelman J, Bandh C, Broman D, et al. Polychlorinated biphenyls in polysulfide sealants--occurrence and emission from a landfill station. Environ Pollut. 2005;138:18–27. doi: 10.1016/j.envpol.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Sandberg AA. The Chromosomes in Human Cancer and Leukemia. New York: Elsevier Science; 1990. [Google Scholar]
- Shih IM, Zhou W, Goodman SN, Lengauer C, Kinzler KW, Vogelstein B. Evidence that genetic instability occurs at an early stage of colorectal tumorigenesis. Cancer Res. 2001;61:818–822. [PubMed] [Google Scholar]
- Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- Sumner AT. Induction of diplochromosomes in mammalian cells by inhibitors of topoisomerase II. Chromosoma. 1998;107:486–490. doi: 10.1007/s004120050333. [DOI] [PubMed] [Google Scholar]
- Tampal N, Lehmler HJ, Espandiari P, Malmberg T, Robertson LW. Glucuronidation of hydroxylated polychlorinated biphenyls (PCBs) Chem Res Toxicol. 2002;15:1259–1266. doi: 10.1021/tx0200212. [DOI] [PubMed] [Google Scholar]
- Tucker JD, Auletta A, Cimino MC, Dearfield KL, Jacobson-Kram D, Tice RR, et al. Sister-chromatid exchange: second report of the Gene-Tox Program. Mutation Research. 1993;297:101–180. doi: 10.1016/0165-1110(93)90001-4. [DOI] [PubMed] [Google Scholar]
- Uraki Y, Suzuki S, Yasuhara A, Shibamoto T. Determining sources of atmospheric polychlorinated biphenyls based on their fracturing concentrations and congener compositions. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2004;39:2755–2772. [PubMed] [Google Scholar]
- WHO. Polychlorinated biphenyls: human health aspects. 2003. [Google Scholar]
- Wolff S. Sister chromatid exchange. Annu Rev Genet. 1977;11:183–201. doi: 10.1146/annurev.ge.11.120177.001151. [DOI] [PubMed] [Google Scholar]
- Yoshida MC, Sekiguchi T. Correction of sister chromatid exchanges in Bloom’s syndrome fibroblasts after fusion with cytoplasts from a Chinese hamster cell line. Experimental cell research. 1984;155:315–319. doi: 10.1016/0014-4827(84)90797-3. [DOI] [PubMed] [Google Scholar]
- Zettner MA, Flor S, Ludewig G, Wagner J, Robertson LW, Lehmann L. Quinoid metabolites of 4-monochlorobiphenyl induce gene mutations in cultured Chinese hamster v79 cells. Toxicol Sci. 2007;100:88–98. doi: 10.1093/toxsci/kfm204. [DOI] [PubMed] [Google Scholar]
- Zhao HX, Adamcakova-Dodd A, Hu D, Hornbuckle KC, Just CL, Robertson LW, et al. Development of a synthetic PCB mixture resembling the average polychlorinated biphenyl profile in Chicago air. Environ Int. 2009 doi: 10.1016/j.envint.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]