Abstract
Introduction
Human herpesvirus 8 (HHV8), the infectious cause of Kaposi sarcoma (KS), varies dramatically across Africa, suggesting co-factors correlated with large-area geographical or environmental characteristics may influence risk for infection. Variation of HHV8 seropositivity across small-area regions within countries in Africa is unknown. We investigated this issue in Uganda, where KS distribution is uneven and well-described.
Methods
Archival samples from individuals aged 15–59 years randomly selected from a nationally-representative 2004/05 HIV/AIDS serobehavioral survey were tested for HHV8 seropositivity using enzyme immunoassays based on synthetic peptides from the K8.1 and orf65 viral genes. Adjusted odds ratios (aORs) and 95% confidence intervals (95% CIs) of association of HHV8 seropositivity with demographical risk factors were estimated.
Results
Among 2681 individuals tested, HHV8 seropositivity was 55.4%. HHV8 seropositivity was lower in females than males (aOR 0.82, 95% CI 0.69–0.97), and increased 2.2% (95% 1.0%-3.6%) per year of age in females and 1.2% (95% CI 1.0–2.3%) in males, inversely associated with education (ptrend=0.010), and was elevated in West Nile compared to Central region (aOR 1.49, 95% CI 1.02–2.18) but not in other regions.
Conclusions
Our findings suggest that HHV8 seropositivity in Uganda may be influenced by co-factors correlated with small-area geography, age, gender, and education.
Keywords: Human herpesvirus 8, Kaposi sarcoma, Africa, human immunodeficiency virus, volcanic soil
Introduction
Human herpesvirus 8 (HHV8, also called Kaposi sarcoma-associated herpesvirus) [1] is the infectious cause of Kaposi sarcoma (KS) and is prevalent in Africa but unevenly distributed [2, 3]. HHV8 seropositivity and KS incidence are high in Eastern and Central Africa, but comparatively low in Western, Northern and Southern Africa [2, 3]. The geographical gradient of HHV8 seropositivity and KS incidence has prompted several authors to postulate that geographical or environmental co-factors, including volcanic soils [4], plants [5], and itchy insect bites[6], may influence the transmission of HHV8 and/or the progression to KS, given HHV8 infection. The HHV8-KS geographical correlation, however, is imperfect because high HHV8 seropositivity coupled with low KS incidence has been reported in some countries, such as Botswana [7] and The Gambia [8]. This imperfect HHV8 seropositivity-KS correlation suggests that the effects of geographical or environmental exposures may influence both HHV8 infection and progression to KS, given HHV8 infection, whereas other may influence only one of the processes. Current HHV8-KS data are derived from small, hospital-based studies [2], which may not be representative of the countries or regions where the studies were conducted, thus firm conclusions cannot be made.
To gain insights into small-area variation of HHV8, we investigated HHV8 seroepidemiology in Uganda, where national KS incidence is relatively well described [9], using a large nationally-representative population-based sample.
Methods
Data and archival plasma samples from the Uganda HIV/AIDS serobehavioral survey (UHSBS) conducted in 2004/05 [10, 11] were retrieved for this study. The UHSBS was a weighted nationally-representative population-based sample of people from Uganda. Uganda’s 56 districts were grouped into 9 geographical regions, of which 8 consisted of 5–8 neighboring districts and one region consisted of one district – Kampala, which is also the capital city of Uganda. The UHSBS was designed to be statistically adequate to provide robust estimates for key HIV/AIDS indicators nationally and regionally. Participants were selected using a two-stage non-stratified cluster sample survey design. The first-stage involved randomly selecting 417 census enumeration areas (primary sampling units) from the enumeration area list constructed for the Uganda National Census of 2002 [12]. In the second-stage, 25 households were randomly selected from each enumeration area for a total of 10, 437 households [10, 11]. Household members aged 15–59 years were invited to answer structured pre-coded interviewer-administered household- and individual- questionnaire about their age, gender, residence, religion, marital-status, attained level of formal education, current occupation, and household assets. A venous blood sample was drawn from participants who consented for HIV, syphilis, HSV2, and HBV serology, which were done using standard commercial assays [10], and for storage for future tests.
For the current study, we randomly selected 3097 (17.8%) from 19,656 participants in the UHSBS cohort, of whom 2705 were tested serologically for anti-HHV8 antibodies. We excluded from this study participants who only had filter paper dry bloodspot samples (n=81), whose samples were exhausted (n=3), who refused to be tested in future studies (n=2), and those whose samples could not be found in the repository in Uganda (n=306).
HHV8 serological testing
Anti-HHV8 antibodies were assayed at the Uganda Virus Research Institute (UVRI) human immunodeficiency virus (HIV) Reference laboratory (HRL), at Entebbe in Uganda using enzyme immunoassays (EIAs) based on synthetic peptides encoded by K8.1 and orf65 viral genes. Peptides were manufactured at the Centers for Disease Control and Prevention (CDC) herpesvirology laboratory [13, 14] and sent to Uganda immediately before testing. Blank uncoated wells were run as negative controls for each plasma specimen to provide background optical density (OD) readings. HHV8 test sample OD readings were adjusted by subtracting the OD reading of the negative well from the test sample OD readings. Samples were considered HHV8 positive when the adjusted OD values were > 0.7 on the K8.1 test or > 2.5 on the orf65 test for samples that were 0.5–0.7 on the K8.1 test. Samples that did not meet these criteria were considered negative. Prior to testing study samples in Uganda, feasibility for large-scale HHV8 testing in Uganda was assessed by parallel testing a panel of 162 well-characterized samples (subjects with KS, asymptomatic Ugandans, and HIV positive and negative Caucasians) at the CDC and at UVRI (Spearman’s rank correlation coefficient for optical density [OD] values = 0.86 for K8.1 and 0.78 for orf65, both p<0.001).
Statistical methods
HHV8 seropositivity patterns were assessed by calculating weighted HHV8 seropositivity for males and females separately as well as combined using the SURVEYFREQ procedure in SAS version 9.2., (SAS Institute Inc, Cary, North Carolina, USA) to account for the survey design. Standard errors and confidence intervals for associations of HHV8 seropositivity with geographical region and demographical variables were estimated using the Rao-Scott Chi-squared statistic [15]. Odds ratios (ORs), 95% confidence intervals (95% CIs) of association of HHV8 seropositivity with geographical region and demographical variables were estimated using PROC SURVEYLOGISTIC. The change in HHV8 seropositivity for each single-year of age was expressed as a percentage change in the odds ratio for each year of age and also tested for statistical interaction between HHV8 seropositivity, gender and age group (<40 years and ≥40 years) in a model specifying age-group, gender, and an age-group*gender interaction term. We also graphically explored change in HHV8 seropositivity across 5 age-groups because HHV8 seropositivity among males and females crossed-over at 40 years. Independent association of HHV8 seropositivity with geographical region and demographical variables was assessed in multivariable models that included variables with established associations with HHV8 seropositivity and those that were significant at a p<0.05 in the SURVEYFREQ two-way table analysis. Variables that became non-significant in the multivariable model were dropped from the final model, except if they were postulated a priori, (e.g., geographical region) or considered confounders (e.g., formal education). Hypothesis testing was based on the Wald test and two-sided p<0.05 were considered statistically significant.
Results
The weighted national HHV8 seropositivity was 55.4% (95% CI 53.0–57.8%) and was slightly higher in males than females (57.5% versus 53.7%, p=0.054) (Table 1). No differences were noted by gender (p=0.80) and age-group (p=0.20) among persons who were excluded (n=416) versus those who were included (n=2681), but HIV infection prevalence was higher in those who were excluded than those who were included (15.7% versus 5.7%, p<0.0001) (not shown).
Table 1.
HHV8 Seropositivity Among Males and Females From The Uganda HIV/AIDS Serobehavioral Survey (2004/05)
| Characteristics | Males | Females | Males and Females | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | |
| All subjects | 712 | 57.5 | 54.3–60.7 | 793 | 53.7 | 50.7–56.7 | 1505 | 55.4 | 53.0–57.8 |
| Age group, years | |||||||||
| 15–19 | 156 | 54.2 | 47.8–60.6 | 142 | 45.5 | 39.5–51.5 | 298 | 49.6 | 45.1–54.1 |
| 20–29 | 198 | 54.9 | 49.3–60.57 | 261 | 50.8 | 45.90–55.8 | 459 | 52.5 | 48.7–56.3 |
| 30–39 | 170 | 58.7 | 52.5–65.0 | 189 | 53.6 | 48.1–59.1 | 359 | 55.9 | 51.7–60.1 |
| 40–49 | 104 | 56.4 | 49.0–63.9 | 123 | 60.3 | 52.6–68.0 | 227 | 58.4 | 53.0–63.8 |
| 50–59 | 84 | 71.9 | 63.4–80.4 | 78 | 80.6 | 72.3–88.9 | 162 | 76.0 | 69.9–82.0 |
| P value* | 0.022 | <0.0001 | <0.0001 | ||||||
| Residence | |||||||||
| Urban | 114 | 52.3 | 43.2–61.4 | 114 | 45.0 | 38.7–51.3 | 228 | 48.4 | 42.1–54.6 |
| Rural | 598 | 58.4 | 55.0–61.8 | 679 | 55.2 | 51.9–58.5 | 1277 | 56.6 | 54.0–59.2 |
| P value** | 0.212 | 0.005 | 0.015 | ||||||
| Region | |||||||||
| Central | 84 | 60.2 | 51.8–68.7 | 86 | 52.1 | 44.9–59.3 | 170 | 55.8 | 50.1–61.5 |
| Kampala | 71 | 55.5 | 46.2–64.7 | 59 | 39.1 | 30.2–47.9 | 130 | 47.0 | 39.9–54.2 |
| East Central | 66 | 43.6 | 35.7–51.5 | 92 | 53.7 | 45.8–61.6 | 158 | 49.0 | 42.8–55.3 |
| Eastern | 89 | 61.7 | 51.9–71.4 | 75 | 50.7 | 39.9–61.4 | 164 | 56.1 | 47.8–64.4 |
| Northeastern | 77 | 63.2 | 53.5–72.9 | 94 | 54.0 | 44.0–64.1 | 171 | 57.8 | 48.9–66.6 |
| North Central | 76 | 61.6 | 53.1–70.1 | 82 | 57.2 | 48.9–65.5 | 158 | 59.2 | 52.5–65.8 |
| West Nile | 100 | 64.5 | 55.1–74.0 | 123 | 67.0 | 59.1–75.0 | 223 | 65.9 | 59.4–72.4 |
| Western | 84 | 63.9 | 54.9–72.9 | 98 | 58.0 | 49.6–66.5 | 182 | 60.5 | 53.4–67.7 |
| Southwestern | 65 | 52.5 | 43.8–61.2 | 84 | 50.0 | 41.3–58.7 | 149 | 51.0 | 44.5–57.6 |
| P value** | 0.005 | 0.039 | <0.01 | ||||||
| Marital status | |||||||||
| Never-married | 236 | 54.0 | 48.6–59.3 | 149 | 46.1 | 40.1–52.1 | 385 | 50.6 | 46.4–54.8 |
| Married | 420 | 60.2 | 56.2–64.2 | 507 | 55.3 | 51.7–59.0 | 927 | 57.4 | 54.5–60.3 |
| Widowed | 13 | 71.6 | 47.8–95.4 | 82 | 66.2 | 57.2–75.2 | 95 | 66.8 | 58.4–75.3 |
| Divorced/separated | 43 | 51.0 | 39.4–62.6 | 55 | 47.5 | 37.2–57.9 | 98 | 49.1 | 40.9–57.3 |
| P value** | 0.114 | 0.001 | <0.001 | ||||||
| Religion | |||||||||
| Catholic | 310 | 58.6 | 53.5–63.7 | 345 | 54.7 | 50.4–59.1 | 655 | 56.5 | 52.9–60.0 |
| Protestant/Anglican | 268 | 58.4 | 53.5–63.4 | 261 | 51.6 | 46.7–56.6 | 529 | 54.8 | 51.2–58.5 |
| Muslim | 91 | 59.1 | 50.8–67.5 | 104 | 53.2 | 45.2–61.2 | 195 | 55.8 | 49.5–62.1 |
| Other | 41 | 44.5 | 35.3–53.7 | 82 | 57.7 | 49.5–65.9 | 123 | 52.5 | 46.2–58.9 |
| P value** | 0.090 | 0.614 | 0.748 | ||||||
| Education | |||||||||
| None | 82 | 70.8 | 61.9–79.6 | 233 | 60.6 | 54.9–66.4 | 315 | 63.0 | 58.04–68.1 |
| Primary | 432 | 57.9 | 53.8–62.0 | 442 | 53.5 | 49.6–57.5 | 874 | 55.6 | 52.5–58.6 |
| Secondary | 158 | 52.1 | 46.2–58.1 | 100 | 45.8 | 39.4–52.3 | 258 | 49.3 | 44.8–53.8 |
| Higher level | 40 | 55.2 | 42.3–68.1 | 17 | 41.0 | 24.9–57.1 | 57 | 50.2 | 39.7–60.8 |
| P value* | 0.020 | 0.004 | 0.001 | ||||||
| Wealth indexa | |||||||||
| Lowest | 144 | 63.3 | 56.3–70.3 | 176 | 61.4 | 55.1–67.8 | 320 | 62.2 | 57.4–67.1 |
| Low | 151 | 63.1 | 56.8–69.4 | 174 | 54.6 | 48.7–60.5 | 325 | 58.3 | 53.8–62.8 |
| Intermediate | 133 | 56.8 | 50.3–63.3 | 134 | 52.9 | 45.7–60.0 | 267 | 54.7 | 49.4–60.1 |
| High | 127 | 54.1 | 46.8–61.5 | 142 | 51.9 | 45.2–58.6 | 269 | 52.9 | 48.0–57.8 |
| Highest | 157 | 52.8 | 45.9–59.8 | 167 | 49.5 | 44.1–55.0 | 324 | 51.1 | 46.3–55.8 |
| P value* | 0.093 | 0.112 | 0.012 | ||||||
| Occupation | |||||||||
| Professional | 47 | 55.8 | 43.7–67.9 | 11 | 39.1 | 19.1–59.0 | 58 | 51.3 | 41.3–61.3 |
| Semi-skilled | 382 | 60.3 | 56.1–64.6 | 421 | 56.3 | 52.3–60.4 | 803 | 58.1 | 55.1–61.2 |
| Trader/sales | 68 | 55.7 | 46.2–65.3 | 89 | 49.5 | 41.5–57.5 | 157 | 52.0 | 45.9–58.2 |
| Subsistence farmer | 212 | 54.1 | 48.9–59.3 | 272 | 52.3 | 47.8–56.8 | 484 | 53.0 | 49.5–56.6 |
| P value** | 0.283 | 0.144 | 0.048 | ||||||
| HIV status | |||||||||
| Negative | 680 | 57.6 | 54.4–60.9 | 744 | 53.5 | 50.3–56.6 | 1424 | 55.4 | 52.9–57.8 |
| Positive | 32 | 55.9 | 43.7–68.2 | 49 | 57.2 | 45.6–68.8 | 81 | 56.7 | 48.1–65.4 |
| P value** | 0.787 | 0.554 | 0.766 | ||||||
Note. n number of subjects; % percentage; 95% CI 95% confidence internal;
Wealth index was constructed from data on household assets and house characteristics, standardized, and divided into quintiles.
P value is for trend.
P values for association is for heterogeneity.
Among males, HHV8 seropositivity was stable among those aged 15–19 years to 40–49 years (54.2% – 56.4%), and rose steeply to 71.9% among those aged 50–59 years (ptrend=0.022) (Table 1). No difference was noted in HHV8 seropositivity among those residing in urban and rural areas, but it was different in men residing in different geographical regions. HHV8 seropositivity was lowest in men from East Central region (43.6%) and highest in those from the Western and from the West Nile regions (63.9% and 64.5%, respectively, pheterogeneity=0.005). HHV8 seropositivity was unrelated to marital status, religion, wealth index, occupational group and HIV seropositivity, but it was inversely related with attained level of ormal education (p=0.020).
Among females, HHV8 seropositivity increased from 45.5% among those aged 15–19 years to 60.3% among those aged 40–49 years and rose steeply to 80.6% among females aged 50–59 years (ptrend<0.0001) (Table 1). HHV8 seropositivity was lower in women from urban areas than in those from rural areas (45.0% versus 55.2, p=0.005). Similar to findings among men, HHV8 seropositivity was different among women residing in different geographical regions. HHV8 seropositivity was lowest in women from Kampala (39.1%) and highest in women from Western and from West Nile regions (58.0% and 67.0%, respectively, pheterogeneity=0.039). In contrast to findings among men, HHV8 seropositivity in women differed by marital status (pheterogeneity =0.001). Similar to findings in men, HHV8 seropositivity was unrelated to religion, wealth index, occupational group, or HIV seropositivity, but it was inversely associated with attained level of formal education (p=0.004).
Among participants aged <40 years, HHV8 seropositivity was higher in men than women. Conversely, among those aged ≥40 years, it was higher in women than men (Figure 1). In a model including gender, age-group, and an interaction term for gender and age-group, HHV8 seropositivity showed significant statistical interaction with age-group and gender (pheterogenity=0.034). In stratified multivariable models, HHV8 seropositivity increased 1.2% per year of adult age (95% CI 1.0–2.3%) among men, was significantly different by region (p=0.027), and was marginally inversely associated with level of attained formal education (0.062) (Table 2). Among women, HHV8 seropositivity increased 2.2% per year of adult age (95% CI 1.0 –3.6%), but was not independently different by geographical region (p=0.101) or by level of attained formal education (p=0.224). In multivariable models combining men and women to gain statistical stability, HHV8 seropositivity was lower among women than men (aOR 0.82, 95% CI 0.69–0.97), was inversely associated with attained level of formal education (p<0.010), and varied by geographical region (pheterogeneity =0.021). Specifically, HHV8 seropositivity was highest in the West Nile than Central region (aOR 1.49, 95% CI 1.02–2.18), Table 2, but it was not different in the other 8 regions.
Figure 1.
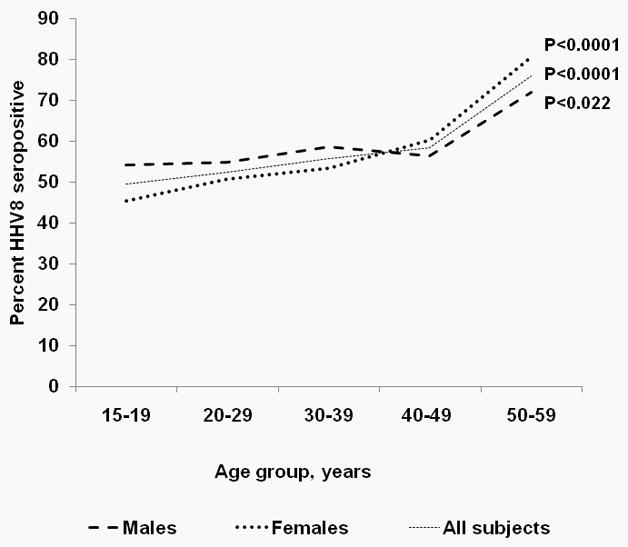
Percent HHV8 seropositivity among males and females aged 15–59 years selected from the Uganda HIV/AIDS serobehavioral Survey 2004/05. Note. HHV88 seropositivity for men and women crosses over at about 40 years of age (P = 0.034 for interaction between HHV8 seropositivity, age group (<40 years versus ≥40 years), and gender.)
Table 2.
Multivariable Associations of HHV8 seropositivity in The Uganda HHV8/AIDS Serobehavioral Survey (2004/05)a
| Population | Males | Females | Males and females | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | aOR | 95% CI | P value* | aOR | 95% CI | P value* | aOR | 95% CI | P value* |
| Gender | 0.82 | 0.69–0.97 | 0.019 | ||||||
| Age, per year | 1.012 | 1.0–1.023 | 0.033 | 1.022 | 1.01–1.036 | 0.002 | 1.017 | 1.01–1.025 | <0.0001** |
| Residence | |||||||||
| Urban | Ref. | ||||||||
| Rural | 1.16 | 0.78–1.72 | 0.475 | ||||||
| Region | 0.027 | 0.101 | 0.021 | ||||||
| Central | Reference | 1.00 | Reference | Reference | 1.00 | ||||
| Kampala | 0.92 | 0.55–1.55 | 0.762 | 0.76 | 0.43–1.34 | 0.190 | 0.79 | 0.54–1.14 | 0.204 |
| East Central | 0.53 | 0.32–0.85 | 0.009 | 1.05 | 0.68–1.62 | 0.988 | 0.76 | 0.54–1.06 | 0.106 |
| Eastern | 1.04 | 0.60–1.78 | 0.899 | 0.90 | 0.53–1.54 | 0.466 | 0.96 | 0.64–1.45 | 0.853 |
| Northeastern | 1.00 | 0.59–1.71 | 0.991 | 1.00 | 0.59–1.69 | 0.805 | 0.99 | 0.65–1.50 | 0.953 |
| North Central | 1.07 | 0.65–1.75 | 0.801 | 1.12 | 0.71–1.76 | 0.714 | 1.07 | 0.75–1.53 | 0.689 |
| West Nile | 1.23 | 0.70–2.16 | 0.467 | 1.86 | 1.15–2.99 | 0.001 | 1.49 | 1.02–2.18 | 0.038 |
| Western | 1.15 | 0.68–1.94 | 0.601 | 1.21 | 0.75–1.94 | 0.408 | 1.18 | 0.81–1.72 | 0.400 |
| Southwestern | 0.72 | 0.44–1.18 | 0.195 | 0.86 | 0.53–1.39 | 0.251 | 0.79 | 0.55–1.13 | 0.196 |
| Marital status | 0.251 | ||||||||
| Never-married | Ref. | 1.00 | |||||||
| Married | 0.97 | 0.70–1.35 | 0.981 | ||||||
| Widowed | 1.27 | 0.75–2.17 | 0.115 | ||||||
| Divorced/separated | 0.71 | 0.43–1.18 | 0.066 | ||||||
| Education | 0.062** | 0.224** | <0.010** | ||||||
| None | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | |||
| Primary | 0.63 | 0.39–1.02 | 0.061 | 0.92 | 0.67–1.26 | 0.444 | 0.79 | 0.61–1.03 | 0.087 |
| Secondary | 0.55 | 0.32–0.92 | 0.024 | 0.84 | 0.57–1.24 | 0.999 | 0.68 | 0.51–0.92 | 0.011 |
| Higher level | 0.55 | 0.29–1.05 | 0.071 | 0.64 | 0.31–1.32 | 0.296 | 0.63 | 0.39–1.02 | 0.057 |
Note: 95% CI 95% confidence interval; aOR adjusted odds ratio;
all associations are adjusted for each other.
P values for association is for heterogeneity.
P value for trend.
Discussion
We found moderate but significant regional variation of HHV8 seropositivity, which has not been noted in Uganda before. We also found significant association of HHV8 seropositivity with male gender, older age, and lower level of attained education and significant statistical interaction of HHV8 seropositivity with age-group and gender. Our findings suggest that factors correlated with small-area geography, gender, age, and formal education may influence risk for HHV8 infection.
The geographical pattern of HHV8 seropositivity resembled the pattern of standardized KS morbidity in Uganda before the AIDS epidemic (Figure 2) [9]. Ecological comparisons of HHV8 and KS regional distributions are risky because HHV8 seropositivity and KS data are derived from non-contemporaneous time-periods and KS data are probably not accurate. Both conditions, however, were coincidentally highest in West Nile and western regions of Uganda and lower elsewhere. Notable differences were also apparent. HHV8 seropositivity varied 1.5-fold from the lowest to highest prevalence region, whereas standardized KS incidence varied 3–6-fold from low to highest region [9]. This HHV8-KS disparity suggests that geographical co-factors may influence risk for HHV8 seropositivity separately from risk for KS, given HHV8 infection. In contrast to KS, whose incidence has dramatically increased in Uganda during the AIDS epidemic, based on data from Kampala region [16], HHV8 seropositivity was unrelated to HIV seropositivity in our study. Similar findings have been reported in some [17, 18], but not all studies [19–22]. The pattern of HHV8 seropositivity does not resemble the HIV pattern in Uganda, i.e., HIV seropositivity is high in Kampala and low in the West Nile region [10, 11], which suggests that our HHV8 seropositivity patterns are more comparable to pre-AIDS era KS patterns.
Figure 2.
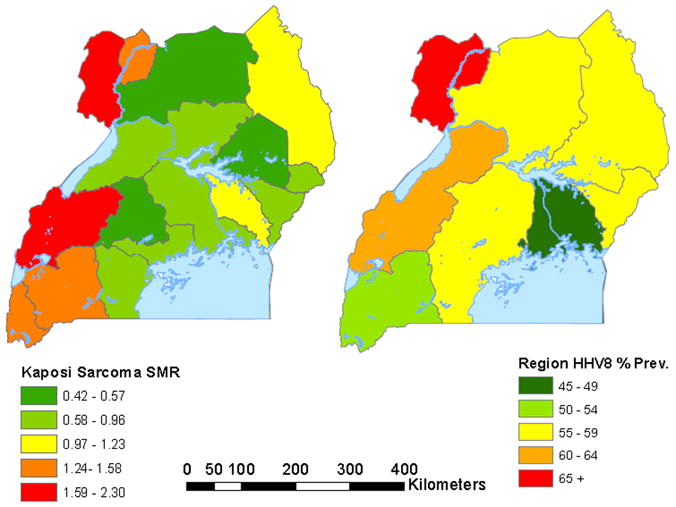
Map of Uganda showing the standardized morbidity ratio (SMR) for Kaposi sarcoma for 17 regions in Uganda during 1964 to 1968 based on published data in reference [9] (left panel) and HHV8 seropositivity for 9 regions based on HHV8 serological testing in a sample from the Uganda HIV/AIDS Serobehavioral survey 2004/05 (right panel).
Geographical co-factors, including soil types [4], exposure to plants [5], and behaviors, such as transferring HHV8 in saliva to denuded surfaces when saliva is used to soothe itchy insect bite wounds [6] have been postulated, but most remain untested in individual-level studies [23]. Our finding of higher HHV8 seropositivity in West Nile region reinforces the notion that geographical co-factors may influence risk for HHV8 infection and/or KS. Interestingly, the small-area variation we observed is reminiscent of the modest but significant positive association we found between HHV8 seropositivity and low village elevation in Tanzania [24]. The West Nile region differs from other regions in Uganda in lying at the center of the Congo-Nile river basin, where climate is favorable for colonization and dispersion of diverse vectors for helminth parasites, such as snails for schistosomal parasites and simulium flies for filarial worms [25]. This ecologic niche, coupled with traditional economic activity, such as fishing, subsistence farming, and gathering firewood, places humans in direct regular contact with numerous parasites. Given that chronic exposure to helminth parasites is associated with immune perturbations[26], we speculate that this small-area variation in HHV8 seropositivity and KS, similar to HHV8 variation in Tanzania [24], may be a surrogate for biological effects of parasites that influence HHV8 spread and/or progression to KS. Interestingly, intestinal heminths have been associated with KS [27, 28] and schistosomal infection with HHV8 seropositivity [29], lending some support to the hypothesis that parasites may influence HHV8 and/or KS risk.
Our finding of age-related increase of HHV8 seropositivity agrees with some [24, 30], but not all studies [18, 20]. This finding is interpreted as an epidemiological clue to ongoing low-grade HHV8 transmission, possibly, via sexual contact, although this is controversial in Africa [31]. HHV8 infection is thought to spread mostly via person-to-person contact with saliva during childhood [32, 33]. Our finding of higher HHV8 seropositivity in older women than men in Uganda contrasts with findings in Tanzania [24]. The Tanzanian study was designed to investigate intra-familial HHV8 patterns and HHV8 seropositivity was higher in older men than women and women with seropositive husbands were more likely to be seropositive, suggesting sexual transmission was possible. Perhaps, women are infected by children who are shedding HHV8 virions in their saliva [32, 33], to whom they are exposed culturally through sharing of mothering responsibilities. Alternatively, older women may be more prone than older men to lytic HHV8 infection [8], which could also explain our findings. The HHV8 seropositivity disparity in by gender, while significant, is substantially less in magnitude than the disparity of endemic and of epidemic KS by gender (M:F 10:1 and 3:1, respectively) [16]. HHV8- KS disparities by gender and geography suggest that co-factors may influence risk for HHV8 infection and for KS separately or through different mechanisms.
Our study has some limitations. HHV8 serology is imperfect [34], so HHV8 seropositivity could have been misclassified. Misclassification of HHV8 seropositivity, however, would be random and would bias the results towards the null. The strengths of our study include being a large nationally-representative population-based sample with detailed demographical data from all regions in Uganda. This allowed us to explore small-area geographical HHV8 patterns. Our study demonstrated a model for international collaboration and feasibility of technology transfer to Africa for large-scale HHV8 serology testing.
To summarize, HHV8 seropositivity showed significant variation by geography, age, gender, and was inversely related to level of attained formal education. Our findings point to an interplay of factors correlated with geography, gender, and age in HHV8 infection and KS risk.
Acknowledgments
We thank Mathew Airola at Westat (Rockville, Md) for drawing GIS maps to illustrate geographic variation of Kaposi sarcoma and human herpesvirus 8 seropositivity in Uganda, and Sandra Mora (infections and Immunoepidemiology Branch, NCI, Bethesda, Md) for computerizing HHV8 results from the feasibility study. We thank Drs. Wilford Kirungi, Joshua Musinguzi and Alex Opio of the Ministry of Health, Kampala, Uganda, the survey teams, laboratory staff, data managers, analysts for UHSBS 2004/05; the Uganda Bureau of Statistics, the Joint United Nations Program on HIV/AIDS; ORC Macro and the World Health Organization, United States Agency for International Development, CDC who sponsored and/or implemented, and supervised the UHSBS 2004/05.
Sources of funding: The study was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute (NCI), National Institutes of Health, Department of Health and Human Services (contract HHSN2612009004060P and Support Services contract NO2-CP-31003) and by an Inter-Agency Agreement between NCI and the Centers for Disease Control and Prevention (IAA Y1CP903801). The content is the responsibility of the authors alone and does not necessarily reflect the views or the policies of the United States Department of Health and Human Services or participating entities.
Footnotes
Presentation at meetings: The findings have not been presented at an international meeting.
Informed consent and ethical approval: The Uganda National Council of Science and Technology (UNCS&T) gave ethical permission to conduct the Uganda HIV/AIDS serobehavioral survey. Ethical permission to test for human herpesvirus 8 antibodies was given by the Uganda Virus Research Institute (UVRI), the Centers for Disease Control, and Prevention, and by the Office of Human Subject Research at the National Institutes of Health.
Conflict of interest: None declared
References
- 1.Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–9. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 2.Dedicoat M, Newton R. Review of the distribution of Kaposi’s sarcoma-associated herpesvirus (KSHV) in Africa in relation to the incidence of Kaposi’s sarcoma. Br J Cancer. 2003;88:1–3. doi: 10.1038/sj.bjc.6600745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook-Mozaffari P, Newton R, Beral V, Burkitt DP. The geographical distribution of Kaposi’s sarcoma and of lymphomas in Africa before the AIDS epidemic. Br J Cancer. 1998;78:1521–8. doi: 10.1038/bjc.1998.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ziegler JL. Endemic Kaposi’s sarcoma in Africa and local volcanic soils. Lancet. 1993;342:1348–51. doi: 10.1016/0140-6736(93)92252-o. [DOI] [PubMed] [Google Scholar]
- 5.Whitby D, Marshall VA, Bagni RK, et al. Reactivation of Kaposi’s sarcoma-associated herpesvirus by natural products from Kaposi’s sarcoma endemic regions. Int J Cancer. 2007;120:321–8. doi: 10.1002/ijc.22205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coluzzi M, Calabro ML, Manno D, Chieco-Bianchi L, Schulz TF, Ascoli V. Saliva and the transmission of human herpesvirus 8: potential role of promoter-arthropod bites. J Infect Dis. 2004;190:199–200. doi: 10.1086/420890. author reply 200-1. [DOI] [PubMed] [Google Scholar]
- 7.Engels EA, Sinclair MD, Biggar RJ, et al. Latent class analysis of human herpesvirus 8 assay performance and infection prevalence in sub-saharan Africa and Malta. Int J Cancer. 2000;88:1003–8. doi: 10.1002/1097-0215(20001215)88:6<1003::aid-ijc26>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 8.Ariyoshi K, Schim van der Loeff M, Cook P, et al. Kaposi’s sarcoma in the Gambia, West Africa is less frequent in human immunodeficiency virus type 2 than in human immunodeficiency virus type 1 infection despite a high prevalence of human herpesvirus 8. J Hum Virol. 1998;1:193–9. [PubMed] [Google Scholar]
- 9.Taylor JF, Smith PG, Bull D, Pike MC. Kaposi’s sarcoma in Uganda: geographic and ethnic distribution. Br J Cancer. 1972;26:483–97. doi: 10.1038/bjc.1972.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bunnell R, Opio A, Musinguzi J, et al. HIV transmission risk behavior among HIV-infected adults in Uganda: results of a nationally representative survey. AIDS. 2008;22:617–24. doi: 10.1097/QAD.0b013e3282f56b53. [DOI] [PubMed] [Google Scholar]
- 11.Mermin J, Musinguzi J, Opio A, et al. Risk factors for recent HIV infection in Uganda. JAMA. 2008;300:540–9. doi: 10.1001/jama.300.5.540. [DOI] [PubMed] [Google Scholar]
- 12.2002 Uganda Population and Housing Census. Kampala: Government of Uganda (Uganda Bureau of Statistics); 2007. [Google Scholar]
- 13.Pau CP, Lam LL, Spira TJ, et al. Mapping and serodiagnostic application of a dominant epitope within the human herpesvirus 8 ORF 65-encoded protein. J Clin Microbiol. 1998;36:1574–7. doi: 10.1128/jcm.36.6.1574-1577.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spira TJ, Lam L, Dollard SC, et al. Comparison of serologic assays and PCR for diagnosis of human herpesvirus 8 infection. J Clin Microbiol. 2000;38:2174–80. doi: 10.1128/jcm.38.6.2174-2180.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao JNK, Scott AJ. The analysis of categorical data from complex surveys: Chi-squared test for goodness of fit and independence in two-way tables. Journal of American Statistical Association. 1981;76:221–230. [Google Scholar]
- 16.Wabinga HR, Parkin DM, Wabwire-Mangen F, Nambooze S. Trends in cancer incidence in Kyadondo County, Uganda, 1960–1997. Br J Cancer. 2000;82:1585–92. doi: 10.1054/bjoc.1999.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bestetti G, Renon G, Mauclere P, et al. High seroprevalence of human herpesvirus-8 in pregnant women and prostitutes from Cameroon. AIDS. 1998;12:541–3. [PubMed] [Google Scholar]
- 18.Hladik W, Dollard SC, Downing RG, et al. Kaposi’s sarcoma in Uganda: risk factors for human herpesvirus 8 infection among blood donors. J Acquir Immune Defic Syndr. 2003;33:206–10. doi: 10.1097/00126334-200306010-00015. [DOI] [PubMed] [Google Scholar]
- 19.Baeten JM, Chohan BH, Lavreys L, et al. Correlates of human herpesvirus 8 seropositivity among heterosexual men in Kenya. AIDS. 2002;16:2073–8. doi: 10.1097/00002030-200210180-00013. [DOI] [PubMed] [Google Scholar]
- 20.Wawer MJ, Eng SM, Serwadda D, et al. Prevalence of Kaposi sarcoma-associated herpesvirus compared with selected sexually transmitted diseases in adolescents and young adults in rural Rakai District, Uganda. Sex Transm Dis. 2001;28:77–81. doi: 10.1097/00007435-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Malope BI, MacPhail P, Mbisa G, et al. No evidence of sexual transmission of Kaposi’s sarcoma herpes virus in a heterosexual South African population. AIDS. 2008;22:519–26. doi: 10.1097/QAD.0b013e3282f46582. [DOI] [PubMed] [Google Scholar]
- 22.Sitas F, Carrara H, Beral V, et al. Antibodies against human herpesvirus 8 in black South African patients with cancer. N Engl J Med. 1999;340:1863–71. doi: 10.1056/NEJM199906173402403. [DOI] [PubMed] [Google Scholar]
- 23.Pelser C, Dazzi C, Graubard BI, Lauria C, Vitale F, Goedert JJ. Risk of classic Kaposi sarcoma with residential exposure to volcanic and related soils in Sicily. Ann Epidemiol. 2009;19:597–601. doi: 10.1016/j.annepidem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mbulaiteye SM, Pfeiffer RM, Whitby D, Brubaker GR, Shao J, Biggar RJ. Human herpesvirus 8 infection within families in rural Tanzania. J Infect Dis. 2003;187:1780–5. doi: 10.1086/374973. [DOI] [PubMed] [Google Scholar]
- 25.Brooker S, Kabatereine NB, Smith JL, et al. An updated atlas of human helminth infections: the example of East Africa. Int J Health Geogr. 2009;8:42. doi: 10.1186/1476-072X-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3:733–44. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- 27.Lin CJ, Katongole-Mbidde E, Byekwaso T, Orem J, Rabkin CS, Mbulaiteye SM. Intestinal parasites in Kaposi sarcoma patients in Uganda: indication of shared risk factors or etiologic association. Am J Trop Med Hyg. 2008;78:409–12. [PubMed] [Google Scholar]
- 28.Williams EH, Williams PH. A note on an apparent similarity in distribution of onchocerciasis, femoral hernia and Kaposi’s sarcoma in the West Nile district of Uganda. East Afr Med J. 1966;43:208–9. [PubMed] [Google Scholar]
- 29.Mbulaiteye SM, Pfeiffer RM, Dolan B, et al. Seroprevalence and risk factors for human herpesvirus 8 infection, rural Egypt. Emerg Infect Dis. 2008;14:586–91. doi: 10.3201/eid1404.070935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olsen SJ, Chang Y, Moore PS, Biggar RJ, Melbye M. Increasing Kaposi’s sarcoma-associated herpesvirus seroprevalence with age in a highly Kaposi’s sarcoma endemic region, Zambia in 1985. AIDS. 1998;12:1921–5. doi: 10.1097/00002030-199814000-00024. [DOI] [PubMed] [Google Scholar]
- 31.Mbulaiteye SM, Goedert JJ. Transmission of Kaposi sarcoma-associated herpesvirus in sub-Saharan Africa. AIDS. 2008;22:535–7. doi: 10.1097/QAD.0b013e3282f4352e. [DOI] [PubMed] [Google Scholar]
- 32.Mbulaiteye SM, Pfeiffer RM, Engels EA, et al. Detection of kaposi sarcoma-associated herpesvirus DNA in saliva and buffy-coat samples from children with sickle cell disease in Uganda. J Infect Dis. 2004;190:1382–6. doi: 10.1086/424489. [DOI] [PubMed] [Google Scholar]
- 33.Mbulaiteye S, Marshall V, Bagni RK, et al. Molecular evidence for mother-to-child transmission of Kaposi sarcoma-associated herpesvirus in Uganda and K1 gene evolution within the host. J Infect Dis. 2006;193:1250–7. doi: 10.1086/503052. [DOI] [PubMed] [Google Scholar]
- 34.Rabkin CS, Schulz TF, Whitby D, et al. Interassay correlation of human herpesvirus 8 serologic tests. HHV-8 Interlaboratory Collaborative Group. J Infect Dis. 1998;178:304–9. doi: 10.1086/515649. [DOI] [PubMed] [Google Scholar]


