Abstract
There is evidence that nitric oxide, an endothelium-derived relaxing factor, may be produced by the macula densa, as well as by blood vessels, within the kidney. To examine the role of nitric oxide in macula densa control of glomerular hemodynamics directly, we performed in vitro microperfusions of both rabbit afferent arterioles (with the glomerulus intact) and adherent tubular segments consisting of portions of the thick ascending limb, macula densa, and early distal tubule. While keeping afferent arteriolar pressure constant at 60 mmHg, we examined the effect of Nw-nitro-L-arginine methyl ester (L-NAME), an inhibitor of nitric oxide synthesis, added to a macula densa perfusate. When the macula densa perfusate was changed from low to high NaCl, the diameter of the arterioles decreased from 16.3 +/- 1.0 to 14.0 +/- 1.1 microns (n = 10; P < 0.001). Addition of 10(-5) M L-NAME to the high NaCl solution further decreased the diameter to 11.9 +/- 1.1 microns (P < 0.001). In contrast, when macula densa perfusion was maintained with the low NaCl solution, addition of L-NAME had no effect. L-NAME-induced constriction was completely reversed by adding 10(-3) M L-arginine (the precursor of nitric oxide) but not D-arginine (an inactive isomer) to the macula densa perfusate. We confirmed that perfusing the macula densa with L-NAME did not affect the vasodilator action of acetylcholine added to the lumen of the afferent arteriole, indicating that NO synthesis by the arteriole was not altered. Thus, our findings suggest that the macula densa may produce nitric oxide, which in turn modulates the afferent arteriolar constriction induced by high concentrations of NaCl at the macula densa.
Full text
PDF
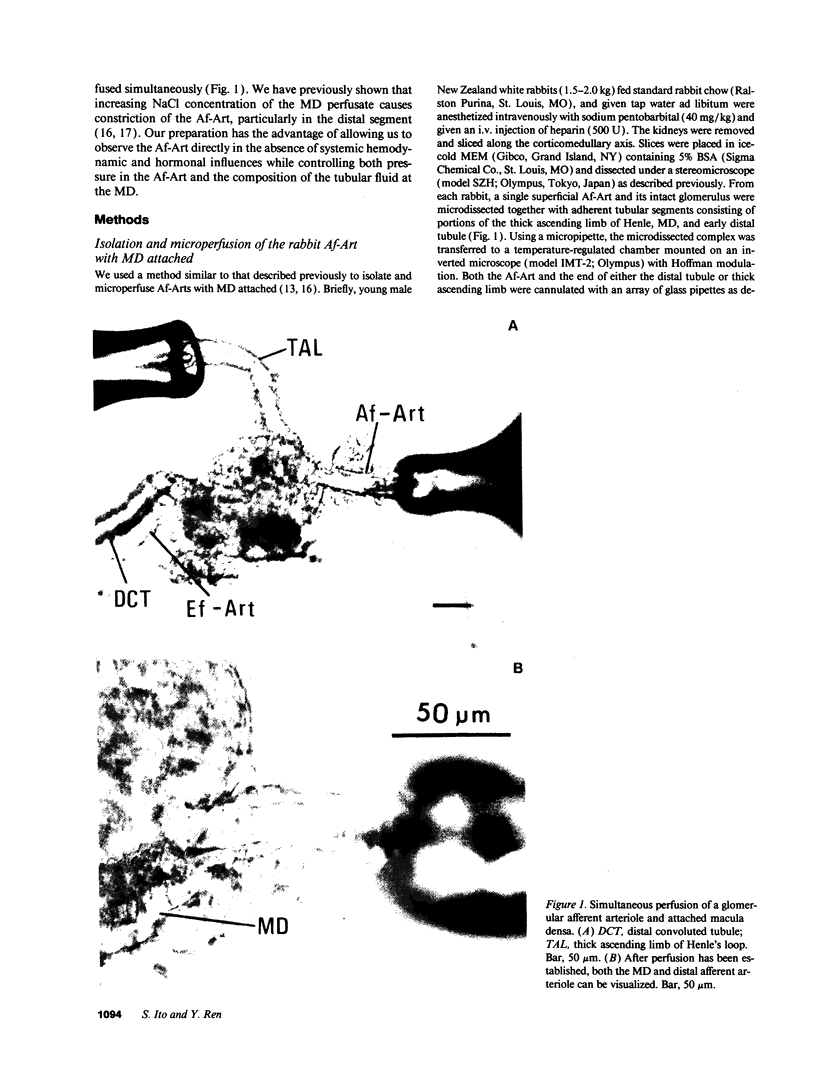


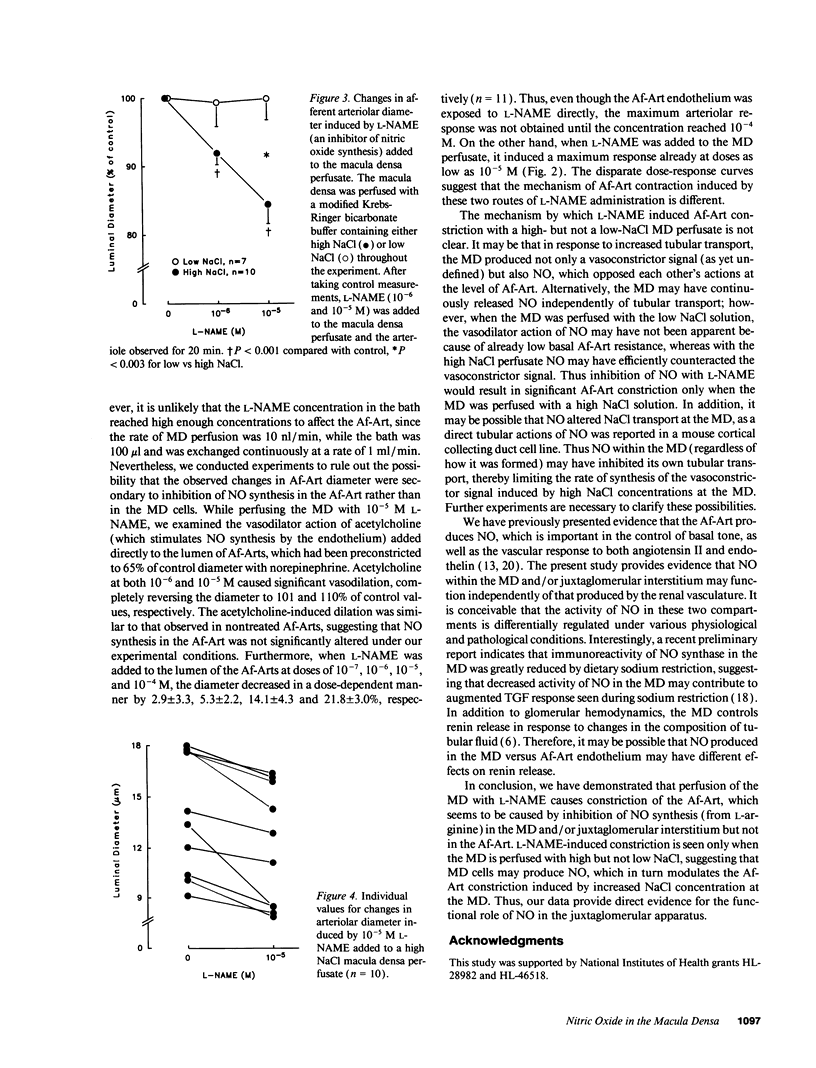

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arendshorst W. J. Altered reactivity of tubuloglomerular feedback. Annu Rev Physiol. 1987;49:295–317. doi: 10.1146/annurev.ph.49.030187.001455. [DOI] [PubMed] [Google Scholar]
- Bell P. D., Franco M., Navar L. G. Calcium as a mediator of tubuloglomerular feedback. Annu Rev Physiol. 1987;49:275–293. doi: 10.1146/annurev.ph.49.030187.001423. [DOI] [PubMed] [Google Scholar]
- Briggs J. P., Schnermann J. The tubuloglomerular feedback mechanism: functional and biochemical aspects. Annu Rev Physiol. 1987;49:251–273. doi: 10.1146/annurev.ph.49.030187.001343. [DOI] [PubMed] [Google Scholar]
- Briggs J. P., Wright F. S. Feedback control of glomerular filtration rate: site of the effector mechanism. Am J Physiol. 1979 Jan;236(1):F40–F47. doi: 10.1152/ajprenal.1979.236.1.F40. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Ito S., Carretero O. A. An in vitro approach to the study of macula densa-mediated glomerular hemodynamics. Kidney Int. 1990 Dec;38(6):1206–1210. doi: 10.1038/ki.1990.335. [DOI] [PubMed] [Google Scholar]
- Ito S., Carretero O. A. Macula densa control of glomerular hemodynamics. Kidney Int Suppl. 1991 Jun;32:S83–S85. [PubMed] [Google Scholar]
- Ito S., Johnson C. S., Carretero O. A. Modulation of angiotensin II-induced vasoconstriction by endothelium-derived relaxing factor in the isolated microperfused rabbit afferent arteriole. J Clin Invest. 1991 May;87(5):1656–1663. doi: 10.1172/JCI115181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Juncos L. A., Nushiro N., Johnson C. S., Carretero O. A. Endothelium-derived relaxing factor modulates endothelin action in afferent arterioles. Hypertension. 1991 Jun;17(6 Pt 2):1052–1056. doi: 10.1161/01.hyp.17.6.1052. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Mundel P., Bachmann S., Bader M., Fischer A., Kummer W., Mayer B., Kriz W. Expression of nitric oxide synthase in kidney macula densa cells. Kidney Int. 1992 Oct;42(4):1017–1019. doi: 10.1038/ki.1992.382. [DOI] [PubMed] [Google Scholar]
- Navar L. G., Ploth D. W., Bell P. D. Distal tubular feedback control of renal hemodynamics and autoregulation. Annu Rev Physiol. 1980;42:557–571. doi: 10.1146/annurev.ph.42.030180.003013. [DOI] [PubMed] [Google Scholar]
- Romero J. C., Lahera V., Salom M. G., Biondi M. L. Role of the endothelium-dependent relaxing factor nitric oxide on renal function. J Am Soc Nephrol. 1992 Mar;2(9):1371–1387. doi: 10.1681/ASN.V291371. [DOI] [PubMed] [Google Scholar]
- Stoos B. A., Carretero O. A., Farhy R. D., Scicli G., Garvin J. L. Endothelium-derived relaxing factor inhibits transport and increases cGMP content in cultured mouse cortical collecting duct cells. J Clin Invest. 1992 Mar;89(3):761–765. doi: 10.1172/JCI115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox C. S., Welch W. J., Murad F., Gross S. S., Taylor G., Levi R., Schmidt H. H. Nitric oxide synthase in macula densa regulates glomerular capillary pressure. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11993–11997. doi: 10.1073/pnas.89.24.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright F. S., Okusa M. D. Functional role of tubuloglomerular feedback control of glomerular filtration. Adv Nephrol Necker Hosp. 1990;19:119–133. [PubMed] [Google Scholar]