Abstract
Classical activating stimuli like LPS drive macrophages to secrete a battery of inflammatory cytokines, including interleukin (IL)-12/23, through toll-like receptor (TLR) signaling. TLR activation in the presence of some factors, including prostaglandin E2 (PGE2), promotes an anti-inflammatory cytokine profile, with production of IL-10 and suppression of IL-12/23 secretion. Extracellular signal-regulated kinase (ERK) is a key regulator of macrophage IL-10 production. Since it inhibits ERK, we investigated the impact of Sorafenib on the cytokine profile of macrophages. In the presence of PGE2, Sorafenib restored the secretion of IL-12 and suppressed IL-10 production. Moreover, IL-12 secretion was enhanced by Sorafenib under conditions of TLR ligation alone. Furthermore, the impact of tumor culture supernatants, cholera toxin, and cAMP analogs (which suppress IL-12 secretion), was reversed by Sorafenib. Sorafenib inhibited the activation of the MAP-kinase p38 and its downstream target mitogen and stress activated protein kinase (MSK), and partially inhibited protein kinase B (AKT) and its subsequent inactivation of the downstream target glycogen synthase kinase 3-β (GSK-3β). Interference with these pathways, which are pivotal in determining the balance of inflammatory versus anti-inflammatory cytokines, provides a potential mechanism by which Sorafenib can modulate the macrophage cytokine phenotype. These data raise the possibility that the use of Sorafenib as cancer therapy could potentially reverse the immunosuppressive cytokine profile of tumor-associated macrophages, rendering the tumor microenvironment more conducive to an anti-tumor immune response.
Keywords: Sorafenib, IL-12, p38, MSK, macrophages
1. Introduction
A regulatory macrophage population that secretes relatively high levels of anti-inflammatory interleukin (IL)-10 and low levels of pro-inflammatory IL-12/23 has been previously described[1]. Prostaglandin E2, extracellular adenosine, immune complexes, vascular endothelial growth factor (VEGF), IL-10, and transforming growth factor (TGF)-β, can all drive the evolution of a regulatory macrophage phenotype [1]. The mitogen-activated protein kinase (MAPK) ERK plays a key role in this process [2,3,4]. Under conditions of strong ERK activation, the anti-inflammatory cytokine IL-10 is upregulated and pro-inflammatory IL-12/23 is suppressed.
Sorafenib (BAY 43-9006) is a multikinase inhibitor that has anti-tumor activity in a wide variety of tumor models (reviewed in [5]). It was developed as a Raf-1 kinase inhibitor that inhibits the Raf/MEK/ERK signaling pathway [6]. Subsequently, a number of off-target effects emerged, including the inhibition of both wild-type and mutant BRAF, STAT3, and a number of pro-angiogenic receptor tyrosine kinases, (including VEGFR2, VEFGR3, and PDGFR-β) [7]. Sorafenib is FDA-approved for the treatment of hepatocellular carcinoma and renal cell carcinoma[5].
Accumulating data suggest that, in addition to inhibiting tumor cell proliferation and angiogenesis, Sorafenib can modulate immune cell function. First, it can inhibit dendritic cell phenotype and function[8]. Second, it can impair T-cell responses in a MAPK independent fashion, inhibiting the phosphorylation of LCK. [9]. Third, Sorafenib also inhibits natural killer (NK) cell cytotoxicity and interferon (IFN)-γ secretion [10].
Due to its known effects on the ERK/MAPK pathway, we explored the impact of Sorafenib on cytokine production by macrophages. Here, we demonstrate three new findings related to the activity of Sorafenib on macrophages. First, Sorafenib suppresses the expression of IL-10 induced by TLR activation in the presence of PGE2, with concomitant restoration of IL-12 expression. Second, Sorafenib can promote the upregulation of IL-12 expression with TLR activation alone. Finally, inhibition of the MAPK p38 and its downstream kinase MSK-1 and partial inhibition of AKT/GSK3-β activation are associated with these effects. These observations suggest that Sorafenib influences the cytokine profile of macrophages by an ERK-independent mechanism.
2. Materials and Methods
2.1. Materials
Sorafenib was purchased from LC Laboratories (Woburn, MA). The p38 pathway inhibitor SB203580, AKT inhibitor IV, and Cholera toxin were purchased from Sigma-Aldrich (St. Louis, MO). The ERK pathway inhibitor U0126 was purchased from Invitrogen (Carlsbad, CA). Ultra-Pure LPS (Escherichia coli K12) was purchased from Invivogen (San Diego, CA). Prostaglandin E2 (PGE2) was purchased from Caymen Chemicals (Ann Arbor, Michigan). Antibodies for p-STAT3 (Tyr705 and Ser727), STAT3, p-ERK1/2 (Thr202/Tyr204), ERK1/2, p-p38 (Thr180/Tyr182), p38, p-GSK3α/β(Ser21/9), p-AKT (Ser473), AKT, p-MSK1 (Thr581), MSK1, p-MEK1/2 (Ser217/221), and phospho-histone H3 (Ser10) were all purchased from Cell Signaling Technologies (Beverley, MA). The cAMP analogs, N6-Benzoyl-Adenosine 3′,5′-cyclic Monophosphate (6-BNZ-cAMP), 8-(4-Chlorophenylthio)-2′-O-Methyl-Adenosine 3′,5′-cyclic Monophosphate (8-CPT-2′-O-Me-cAMP), 8-Bromo-Adenosine 3′,5′-cyclic Monophosphate (8-Bromo-cAMP), and actin antibody were purchased from Calbiochem (San Diego, CA).
2.2. Cell Lines
4T1 cells were obtained from the ATCC and grown in DMEM supplemented with 10% FBS, penicillin/streptomycin, and glutamine. The NT2.5 breast tumor cell line is derived from a spontaneous tumor explanted from a neu-N mouse and grown as previously described[11]. Prior to collecting culture supernatants, NT2.5 cells were washed in PBS and media was changed to DMEM supplemented with 10% FBS, penicillin/streptomycin, and glutamine. Media was collected for macrophage stimulations after 24 hours of culture.
2.3. Mice
FVB mice were purchased from Harlan (Frederick, MD). IL-10−/− mice were purchased from The Jackson Laboratory. Experiments were performed with 6- to 10-week-old mice. Animals were kept in pathogen-free conditions and were treated in accordance with institutional and AAALAC policies. All protocols were approved by the Animal Care and Use Committee of Johns Hopkins University.
2.4. Macrophages
Bone marrow-derived macrophages (BMMΦ) were generated as previously described[12]. Briefly, bone marrow was flushed from the femurs and tibias of mice using PBS+2% penicillin/ streptomycin. Cells were plated in Petri dishes in DMEM supplemented with 10% FBS, penicillin/streptomycin, glutamine, and 20% conditioned medium from the supernatants of macrophage colony stimulating factor secreting L929 (LC14) fibroblasts (L cell-conditioned medium). Cells were re-fed on day 2. Cells were used at 7–14 days for experiments. Peritoneal macrophages were obtained as previously described[13]. Briefly, peritoneal lavage was performed and peritoneal exudate cells were allowed to adhere to 48-well plate in complete media for 90’ at 37°, and then washed three times in warm PBS to remove non-adherent cells.
2.5. Macrophage Stimulations
Macrophages were harvested from Petri dishes by incubating cells in CellStripper (Mediatech). 2×106 and 2×105 macrophages were seeded into 6- or 48- well plates, respectively, overnight in media absent of L-cell conditioned media. Several fold less macrophages were used for cytokine experiments involving resident peritoneal macrophages. 48-well plates were used for ELISAs and 6-well plates were used for harvesting protein or RNA from macrophages. For inhibitor experiments, macrophages were pre-treated with inhibitors one hour prior to stimulation. Final concentrations of DMSO were normalized within each experiment. Macrophages were stimulated with LPS alone (10ng/ml) or combined with PGE2 (10−8M) for the periods of time indicated in each figure before cell lysis, or overnight for ELISAs. Throughout these studies, the concentration of Sorafenib required to maximally restore the production of IL-12p40 and suppress IL-10 under the conditions of LPS+PGE2 was carefully titrated for each lot of drug used. The concentration required for maximal effect was typically between 5–7µM. Prior to experiments testing the effect of Sorafenib during stimulation with LPS+tumor-conditioned supernatants, concentrations of tumor culture supernatants were titrated step-wise with increasing doses of culture supernatants for maximum suppression of IL-12p40 (4T1 and NT2.5) and enhancement of IL-10 (4T1).
2.6. Western Blotting
After stimulation, macrophages were lysed in ice-cold CellLytic cell lysis reagent (Sigma) supplemented with Phosphatase Inhibitor Cocktail 2 (Sigma) and EDTA-free protease inhibitor cocktail from Roche Diagnostics (Basel, Switzerland) for 5–10 minutes on ice. Cell lysates were scraped from 6-well plates, collected and centrifuged for 10’ at 10,000 RPM. Lysates were mixed 1:1 with Laemmli sample buffer and boiled for 8 minutes. Samples were subjected to SDS-PAGE on 4–15% gradient gels (BioRad, Hercules, CA) and transferred to Amersham Hybond-ECL (GE Healthcare, Piscataway, NJ). Membranes were blocked for 1 hour in 5% Milk in TBS-Tween (w/v), then incubated overnight with primary antibodies in 5% BSA in TBS-Tween (w/v). After washing, membranes were incubated with HRP-conjugated Goat-α-Rabbit IgG (Cell Signaling Technologies) for 30’ at room temperature, washed, and developed using HyGLO Quickspray (Denville Scientific, Metuchen, NJ). Membranes were stripped with Restore Western Blot Stripping Buffer (Thermo Scientific, Rockford, IL) according to the manufacturer’s instructions, then blocked and reprobed. Image quantitation was performed using the TotalLab Quant software (TotalLab Limited).
2.7. ELISAs
ELISAs were performed for mouse IL-10 and IL12/23p40 using BD OptEIA ELISA sets according to the manufacturer’s instructions. ELISAs were developed using BD OptEIA substrate reagents and stopped with 2N H2SO4. IL-10 was assayed directly from supernatants, whereas supernatants were diluted 1:5 to 1:20 to determine IL-12/23p40 concentrations.
2.8. RNA isolation and real-time PCR
BMMΦ were subject to RNA extraction using TRIzol (Invitrogen). Contaminating DNA was removed by treatment with RNase-free DNase I (Roche). The ThermoScript RT-PCR system (Invitrogen) was used to generate cDNA from RNA using oligo(dT)20 primers. Real-time PCR was conducted with the Applied Biosystems (Foster City, CA) ABI Prism 7900 sequence detection system using iQ SYBR Green Supermix (Bio-Rad) following the manufacturer’s instructions. The following primer pairs were used in this study: GAPDH, 5'-TGTTCCTACCCCCAATGTGT-3' and 5'-GGTCCTCAGTGTAGCCCAAG-3'; IL-10, 5'-AAGGACCAGCTGGACAACAT-3' and 5'-TCTCACCCAGGGAATTCAAA-3'; IL-12/23p40, 5′-CACACTGGACCAAAGGGACT-3′ and 5′-TGGTTTGATGATGTCCCTGA-3′. For data analysis, the comparative threshold cycle (Ct) value for GAPDH was used to normalize loading variations in the real-time PCR reactions. A ΔΔCt value was then obtained by subtracting control ΔCt values from the corresponding experimental ΔCt. The ΔΔCt values were converted to fold difference compared with the control by raising two to the ΔΔCt power.
3. Results
3.1. Sorafenib Restores IL-12 and Suppresses IL-10 Expression in Prostaglandin-E2 Conditioned Bone Marrow-Derived Macrophages
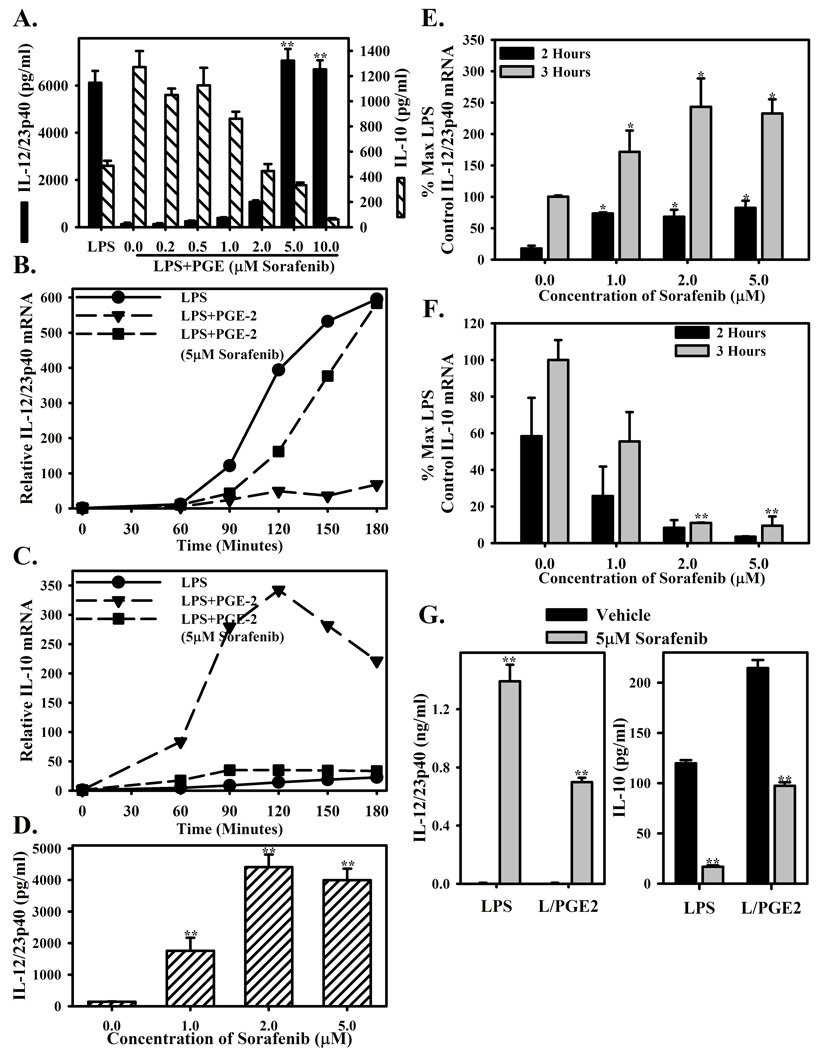
As previously demonstrated, macrophages stimulated with LPS alone produce relatively high levels of IL-12/23p40 (black bars) and relatively low levels of IL-10 (hatched bars). Additionally, macrophages activated in the presence of PGE2 display suppressed IL-12/23p40 and enhanced IL-10 production by ELISA (Figure 1A)[14,15]. Pretreatment with Sorafenib restores the production of IL-12/23p40 to levels comparable to LPS stimulation alone and abrogates IL-10 secretion (Figure 1A). This was confirmed at the mRNA level by real-time PCR. Macrophages stimulated with LPS alone express relatively high levels of IL-12/23p40, and relatively low levels of IL-10 (Figures 1B and 1C, solid circles). Stimulation with both LPS and PGE2 reverses cytokine expression with high IL-10 and low IL-12/23p40 (Figures 1B and 1C, solid triangles). This suppression and enhancement of IL-12/23p40 and IL-10, respectively is reversed by the presence of Sorafenib (Figures 1B and 1C, solid squares). The differences in mRNA levels were not due to Sorafenib-induced apoptosis (data not shown).
Figure 1. Sorafenib reverses PGE2 mediated suppression of LPS induced IL-12 expression.
(A.) Bone marrow-derived macrophages were pre-treated for 1 hour with increasing concentrations (0.2–10µM) of Sorafenib or vehicle control (DMSO, 0µM), then stimulated with LPS (10ng/ml) with PGE2 (10−8M). 16 hour culture supernatants were measured for IL-12p40 (black bars) or IL-10 (hatched bars) by ELISA. LPS stimulation alone is present as a control. (B.,C.) BMMΦ were pre-treated and stimulated as in (A). The presence of IL-12/23p40 (B.) and IL-10 (C.) mRNA was measured at 0’, 60’, 90’, 120’, 150’, and 180’ post-stimulation by real-time PCR after stimulation with LPS (circles), LPS+PGE (triangles), or LPS+PGE in the presence of Sorafenib (5µM, squares). mRNA levels are presented as relative to the expression at 0’. (D.) BMMΦ were pre-treated with vehicle or 1–5µM Sorafenib then stimulated with LPS alone. IL-12/23p40 protein was measured by ELISA. Culture supernatants were diluted 1:50 prior to ELISA. (E., F.) BMMΦ were pretreated and simulated as in (D.), and then IL-12/23p40 and IL-10 expression was measured by real-time PCR 2 and 3 hours after stimulation. (G.) Peritoneal macrophages were pretreated with vehicle or 5µM Sorafenib as in (A) and stimulated with LPS or LPS+ PGE2. ELISAs were performed from 16h culture supernatants. Each bar represents the mean ± SD triplicate samples. *Indicates a p-value <0.05 and **indicates a p-value <0.01 as determined by student’s T-test comparing drug to vehicle control. Figures are representative of three independent experiments.
To determine if pretreatment was required for Sorafenib to modulate cytokine production by macrophages, cytokine production from macrophages treated with Sorafenib prior to stimulation or given concomitantly with stimulation by LPS+PGE2 was assessed. Similar to pre-treatment with Sorafenib, the production of IL-12p40 was restored and the production of IL-10 was diminished with concomitant treatment (Supplemental Figure 1).
To determine if the drug target is inherently important in controlling excessive inflammatory cytokine production, we tested the effect of Sorafenib on macrophage cytokine profiles under LPS stimulation alone (Figures 1D–F). Sorafenib enhanced IL-12/23p40 secretion by macrophages stimulated with LPS alone in a dose dependent manner (Figure 1D). This was confirmed by real-time PCR, with enhanced IL-12/23p40 mRNA measured at 2h and 3h after stimulation (Figure 1E). Additionally, IL-10 mRNA was suppressed by the presence of Sorafenib (Figure 1F).
Similar observations were made using resident peritoneal macrophages. Peritoneal macrophages were treated with drug vehicle or Sorafenib, then stimulated with LPS or LPS+PGE2 overnight. IL-12/23p40 concentrations were nearly undetectable under either stimulation condition in the presence of the drug vehicle. IL-12/23p40 secretion was greatly enhanced by the presence of Sorafenib upon stimulation with either LPS or LPS+ PGE2 (Figure 1G, left). The secretion of IL-10 was diminished by the presence of Sorafenib (Figure 1G, right).
3.2. Sorafenib Reverses the Suppressive Effect of Tumor Culture Supernatants and cAMP Analogs
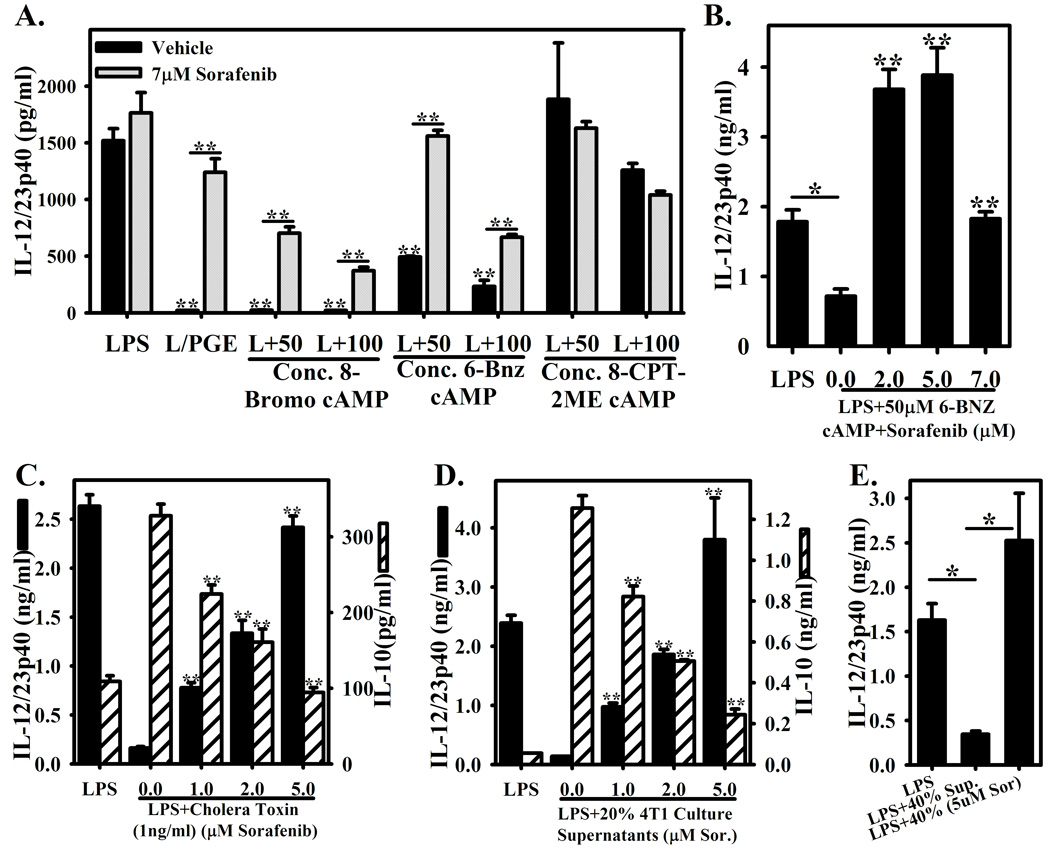
PGE2 is a well-established and important modulator of inflammatory cytokine production by macrophages. Many other factors can manipulate this balance, including several soluble factors produced by tumors (among them prostaglandin E2). Many of these molecules mediate their effects via enhanced intracellular cAMP[16]. Accordingly, we explored whether Sorafenib could reverse the inhibitory effects of distinct cAMP analogs (Figures 2A–B), the generic cAMP-activating agent Cholera toxin (Figure 2C), and culture supernatants from the mouse mammary tumor cell lines 4T1 (Figure 2D) and NT2.5 (Figure 2E).
Figure 2. Sorafenib reverses tumor culture supernatant and cAMP analogue and tumor culture supernatant mediated suppression of IL-12 expression.
(A.) BMMΦ stimulated with LPS in the presence of cAMP analogs at 50–100µM. A general cAMP analog (8-Bromo cAMP), a PKA-activating analog (6-BNZ-cAMP), or an EPAC activating analog (8-CPT-2′-O-Me-cAMP) were used. Macrophages were either pretreated with vehicle (black bars) or 7µM Sorafenib (hatched bars). Concentrations of IL-12/23p40 were measured by ELISA. Stimulation with LPS alone and LPS+PGE are present as controls. (B.) Macrophages with stimulated with LPS+50µM 6-BNZ-cAMP in the presence of various doses of Sorafenib (2–7µM) or vehicle (0µM). Concentrations of IL-12/23p40 were measured by ELISA. LPS stimulation alone is present as a control. (C.) BMMΦ were stimulated with LPS or LPS+Cholera Toxin (1ng/ml) in the presence of increasing concentrations of Sorafenib (0–5µM). Concentrations of IL-12/23p40 and IL-10 are presented as in (A.). (D.–E.) 4T1 and NT2.5 mouse mammary tumor cells were cultured overnight at approximately 106 cells/ml of macrophage culture media overnight as a source of tumor culture supernatants. Media was collected and centrifuged, then added to BMMΦ cultures at final concentrations of 20% (D.) and 40% (E.). (D.) BMMΦ were stimulated with LPS or LPS+20% 4T1 culture supernatants in the presence or absence of increasing concentrations of Sorafenib (0–5µM). Concentrations of IL-12/23p40 (black bars) or IL-10 (hatched bars) were measured by ELISA. (E.) BMMΦ were stimulated with LPS or LPS+40% NT2.5 culture supernatants in the presence or absence of 5µM Sorafenib. Each bar represents the mean ± SD of triplicate samples. *Indicates a p-value <0.01 in (B.) and (E.). **Indicates a p-value <0.001, comparing drug to vehicle control in A., B., C., and D., as well as comparing LPS alone to LPS+PGE, LPS+8-Bromo cAMP, and LPS+6-Bnz cAMP in (A.). Figures are representative of three independent experiments.
8-Bromo cAMP, a broad activator of cAMP-dependent signaling, suppressed IL-12p40 expression (Figure 2A and [17]) while enhancing the production of IL-10 (data not shown and [17]). Sorafenib blunted but did not completely reverse its effect on IL-12p40 and IL-10 (Figure 2A and data not shown). To further dissect the pathway, we used 6-BNZ-cAMP and 8-CPT-2′-O-Me-cAMP as specific activators of protein kinase A (PKA) and exchange protein directly activated by cAMP (EPAC), respectively, which mediate cAMP dependent signaling[18]. 6-BNZ-cAMP, but not 8-CPT-2′-O-Me-cAMP, suppressed the production of IL-12/23p40 in a dose dependent manner (Figure 2A). 6-BNZ-cAMP, but not 8-CPT-2′-O-Me-cAMP was essential for the suppression of IL-12p40, implying a critical role for PKA signaling in this effect (Figure 2A). At 7µM Sorafenib, the effect of 8-Bromo-cAMP or 6-BNZ-cAMP on IL-12/23p40 production could be at least partially reversed (Figure 2A). Closer examination of macrophages activated in the presence of 50µM 6-BNZ-cAMP revealed that Sorafenib could completely restore or enhance IL-12/23p40 production above that of LPS alone (Figure 2B). Similar observations were made using Cholera toxin, a generic activator of cAMP which suppresses IL-12 and enhances IL-10 [19] (Figure 2C).
To extend these observations to tumor immunology, we explored the stimulation of macrophages with LPS in the presence of increasing concentrations of 4T1 and NT2.5-derived tumor culture supernatants. Consistent with prior observations, IL-12/23p40 secretion was diminished with in the presence of either culture supernatant, while IL-10 production was enhanced with 4T1 culture supernatants and was largely unchanged with NT2.5 culture supernatants (data not shown and Figure 2D–E). Pretreatment with Sorafenib reversed the suppression of IL-12/23p40 mediated by the presence of both tumor culture supernatants (Figure 2D–E), and suppressed the enhanced secretion of IL-10 with 4T1 culture supernatants (Figure 2D).
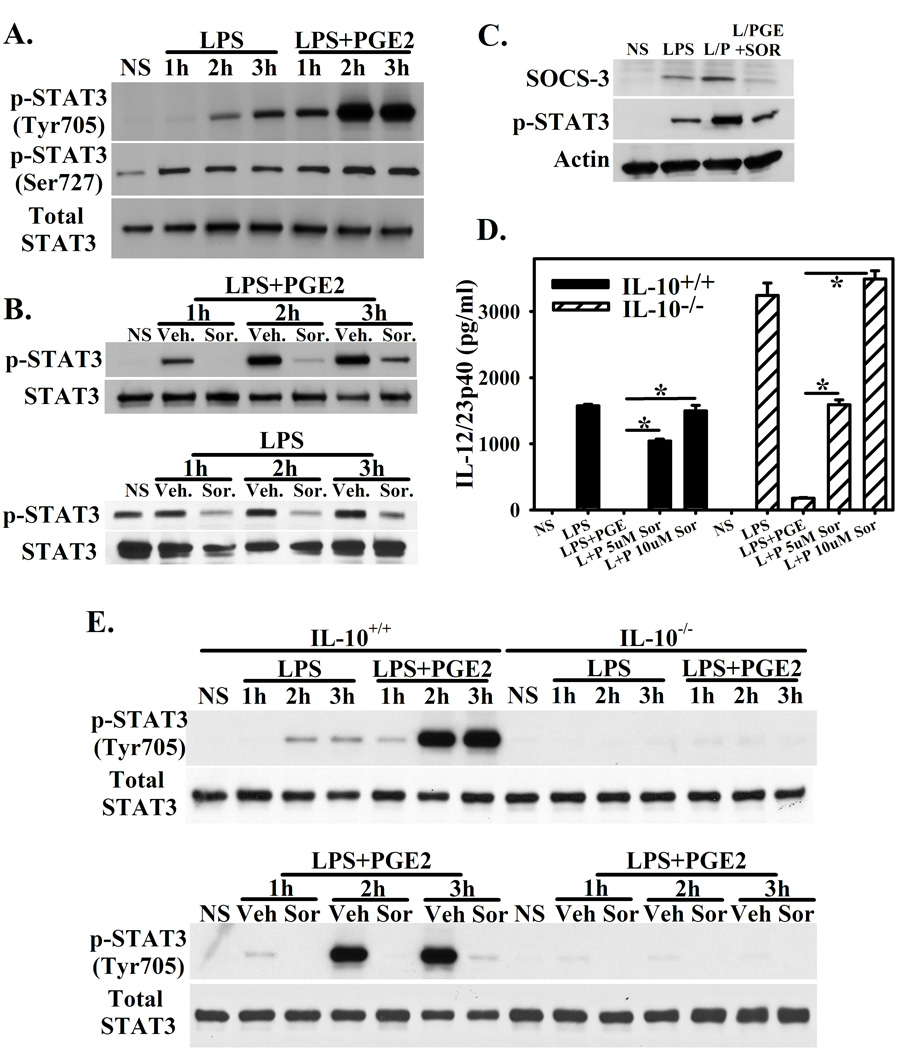
3.3. Sorafenib Reverses IL-10 Mediated Activation of STAT-3 and Expression of SOCS-3
Activated STAT3 suppresses inflammatory cytokine production and serves as a transcription factor for IL-10[20,21]. Additionally, constitutively activated STAT3 is a potential target of Sorafenib[22,23]. Stimulation of macrophages with LPS results in STAT3 phosphorylation at Tyr705 and Ser727. This is enhanced by the presence of PGE2 during activation with LPS (Figure 3A). Consistent with our findings that Sorafenib inhibits IL-10 expression, the enhanced STAT3 activation mediated by both the presence of LPS+PGE2 (Figure 3B, top) and LPS alone (Figure 3B, bottom) is blocked by Sorafenib. Additionally, LPS-stimulation results in the up-regulation of SOCS-3, a downstream target of STAT3. This is enhanced by the presence of PGE2. This effect is reversed by the presence of Sorafenib (Figure 3C).
Figure 3. Sorafenib Reverses IL-10 Mediated Activation of STAT-3.
(A.) BMMΦ were stimulated for 1, 2, and 3 hours with LPS or LPS+PGE and assayed for STAT3 phosphorylation at Tyr705 and Ser727. Total STAT3 is present as a loading control. (B.) BMMΦ were pretreated with vehicle or Sorafenib (5µM) for one hour, then stimulated with LPS+ PGE2. STAT3 phosphorylation was measured at Tyr705 at 1, 2, and 3h by western blot. Total STAT3 is present as a loading control. (C.) BMMΦ were stimulated with LPS, LPS+ PGE2 (L/P), or LPS+PGE in the presence of Sorafenib for three hours. The presence of SOCS-3, STAT-3 phosphorylation at Tyr705, and actin were measured by western blotting. (D.) BMMΦ from wild type or IL-10−/− BMMΦ were stimulated with LPS, LPS+PGE, and LPS+PGE in the presence of Sorafenib (5 and 10µM). Concentrations of IL-12/23p40 were measured by ELISA from 16h stimulated cultures. Each bar represents the mean ± SD of triplicate samples. *Indicates a p-value <0.0001. (E.) STAT3 phosphorylation at Tyr705 was assay by western blotting as in (A. and B.). Upper. Wild-type or IL-10−/− BMMΦ were stimulated with LPS or LPS+PGE for 1, 2, or 3h. Lower. Wild-type or IL-10−/− BMMΦ pretreated for 1 hour with vehicle or Sorafenib (5µM) were stimulated with LPS+PGE. Total STAT3 is present as a loading control. Figures are representative of at least two independent experiments.
To explore the role of autocrine IL-10 in the suppression of IL-12/23p40 due to PGE2, we compared cytokine production of wild type BMMΦ with IL-10−/− BMMΦ. Upon stimulation with LPS, both wild type and IL-10−/− macrophages produced relatively high levels of IL-12/23p40, which could be suppressed by the presence of PGE2. This was true even in the absence of IL-10, although the overall production of IL-12/23p40 was higher from IL-10−/− macrophages (Figure 3D). Additionally, IL-12/23p40 expression was restored by the presence of Sorafenib (Figure 3D). Examination of STAT3 phosphorylation revealed that as in Figure 3A–B, STAT3 phosphorylation was enhanced by the presence of PGE2 and blocked by the presence of Sorafenib (Figure 3E). However, STAT3 phosphorylation was almost completely absent in IL-10−/− macrophages, regardless of the stimulation conditions (Figure 3E). As a result, despite being a potential target in certain tumors, the absence of STAT3 phosphorylation in the presence of Sorafenib is not the mechanism resulting in the restoration of IL-12/23p40 production in macrophages. Sorafenib blocks enhanced IL-10 production, resulting in minimal STAT3 activation.
3.4. Sorafenib Manipulates the Activation of MAP Kinase Signaling in Macrophages
Because the MAPK signaling pathways play a pivotal role in manipulating inflammatory versus anti-inflammatory cytokine expression, we investigated the impact of Sorafenib on MAP kinase signaling. Macrophages were stimulated with LPS+ PGE2 in the presence or absence of Sorafenib. Whereas LPS+ PGE2 induced phosphorylation of MEK1/2 and ERK1/2, the presence of Sorafenib had no impact (Figure 4A). Despite the lack of an effect on ERK signaling, the phosphorylation of MSK-1, which is a distinct MAPK-activated kinase downstream of both ERK and p38 signaling[24], was diminished (Figure 4A). Accordingly, we compared the effect of Sorafenib on p38 activation. The presence of Sorafenib suppressed the activation of p38, while not having a measurable effect on ERK1/2 phosphorylation (Figure 4B). Based on these results, we hypothesized that inhibition of the negative regulator MSK-1 could be the mechanism by which IL-12p40 expression is restored. Stimulation of macrophages with LPS or LPS+ PGE2 resulted in the phosphorylation of MSK-1, peaking around 30’–45’ (Figure 4C). The presence of PGE2 did not enhance the phosphorylation of MSK-1 (Figure 4C).
Figure 4. Sorafenib manipulates MAPK activation in BMMΦ.
(A.) BMMΦ pretreated with Sorafenib were stimulated with LPS+PGE for 15’, 30’, or 45’. Lysates were assayed for MEK1/2 (Ser217/221), ERK1/2 (Thr202/Tyr204), and MSK-1 (Thr581) phosphorylation by western blotting. Total ERK is present as a loading control. (B) BMMΦ were pretreated and stimulated as in (A.). p-ERK1/2, p-p38 (Thr180/Tyr182), ERK1/2, and p38 were measured by western blotting. (C.) BMMΦ were stimulated with LPS or LPS+PGE for 15’, 30’, 45’, or 60’. Lysates were assayed for MSK1 phosphorylation or total MSK1 by western blotting. (D.) BMMΦ pretreated with vehicle or Sorafenib were stimulated with LPS alone. Phospho- or total ERK1/2, p38, and MSK1 were measured by western blotting. (E.) BMMΦ were pretreated with vehicle or Sorafenib, then stimulated with LPS+PGE for 30’ or 60’. Phospho-histone H3 Ser10 was determined by western blotting. Figures are representative of three independent experiments.
We next determined if the inhibition of p38 and MSK-1 activation was specific to LPS activation in the presence of PGE2. Therefore, macrophages were stimulated with LPS alone in the presence or absence of Sorafenib. As was observed for macrophages stimulated with LPS+ PGE2, when macrophages were stimulated with LPS alone in the presence Sorafenib both p38 and MSK-1 phosphorylation were diminished (Figure 4D). Activation of ERK1/2 was unaffected by the presence of Sorafenib.
In order to determine if the kinase activity of the MSKs was inhibited, we investigated the phosphorylation status of histone H3 at serine 10, which is modulated by MSK-1/2[25,26]. Macrophages have some constitutive phosphorylation at S10 on histone H3, which is enhanced by stimulation with LPS+ PGE2 (Figure 4E). The presence of Sorafenib diminished the phosphorylation of histone H3, in parallel with the phosphorylation of p38 and MSK-1 (Figure 4E).
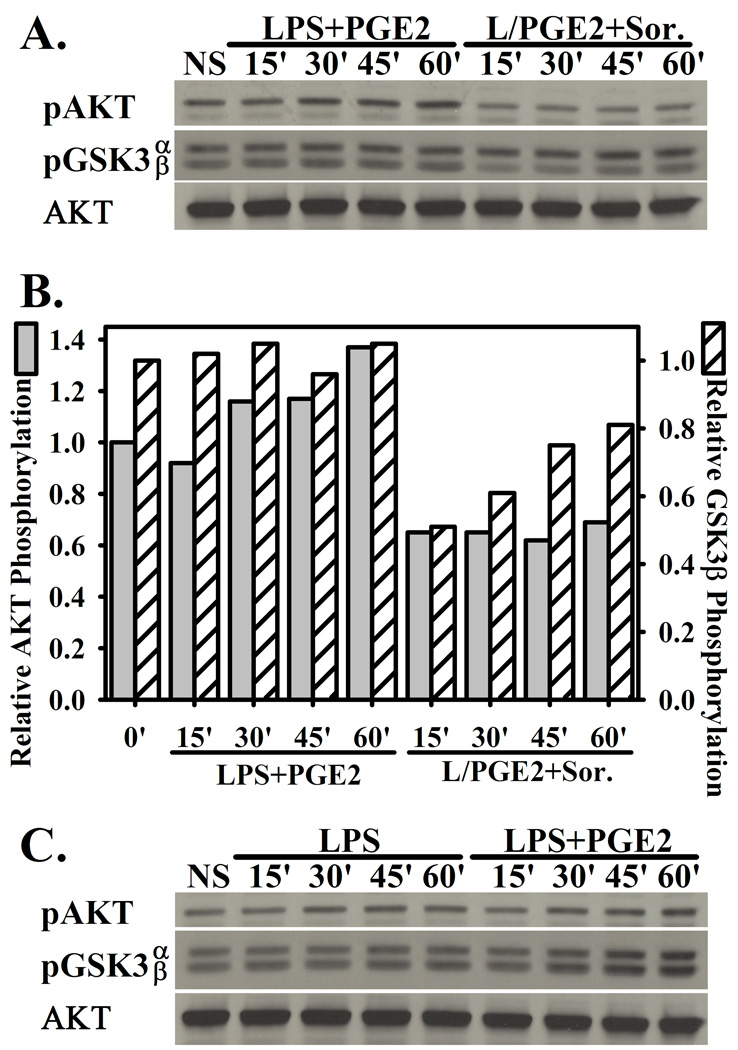
3.5. Sorafenib partially inhibits activation of the AKT/GSK3-β axis
Glycogen synthase kinase 3 (GSK3)-β is an important regulator of TLR-induced cytokine production. GSK3-β in its constitutively active un-phosphorylated form promotes proinflammatory cytokine expression. Upon pharmacological inhibition or inactivation via AKT-mediated phosphorylation, the production of pro-inflammatory cytokines is suppressed, while IL-10 production is enhanced [27]. Furthermore, inhibition of AKT and consequent GSK-3β inactivation promotes excessive inflammatory cytokine production. Because AKT activation can be inhibited by Sorafenib in tumor lines[28,29], we explored the effects of Sorafenib on the activation of AKT in macrophages.
Stimulation of macrophages with LPS+PGE modestly enhanced phosphorylation of AKT (Figure 5A and 5B). The presence of Sorafenib partially inhibited phosphorylation of AKT, and consequently the inactivation of its downstream target GSK-3β via phosphorylation of serine 9 (Figure 5A and 5B). The presence of Sorafenib did not inhibit the phosphorylation of GSK-3α, which is not a target of AKT (Figure 5A and 5B). The phosphorylation of AKT and GSK-3β is not specific to macrophage activation with LPS+PGE, as stimulation with LPS alone led to comparable levels of AKT and GSK-3β phosphorylation (Figure 5B). Under conditions of LPS stimulation alone, the presence of Sorafenib did not significantly inhibit the phosphorylation of AKT and its target GSK3β (data not shown). Thus, although the activation of AKT and inhibition of GSK3β activity does not appear to be a mechanism specific to LPS+PGE stimulation (Figure 5B), the presence of Sorafenib is partially able to inhibit this fundamental mechanism of inflammatory cytokine regulation (Figure 5A and 5B) during stimulation with LPS+PGE2.
Figure 5. Sorafenib partially inhibits AKT activation and GSK3β phosphorylation.
(A.) BMMΦ pretreated with vehicle or Sorafenib (5µM) were stimulated with LPS+PGE for 15’, 30’, 45’, or 60’. Lysates were assayed for pAKT (Ser473), p-GSK3α/β (Ser21/9), and total AKT by western blotting. (B.) Image quantitation of AKT and GSK3β phosphorylation from (A.) (C.) BMMΦ were stimulated for LPS or LPS+PGE for 15’, 30’, 45’, 60’. Western blotting was performed as in (A.). Figures are representative of at least two independent experiments.
3.6. Use of MAPK, but not AKT inhibitors reproduces the activity of Sorafenib
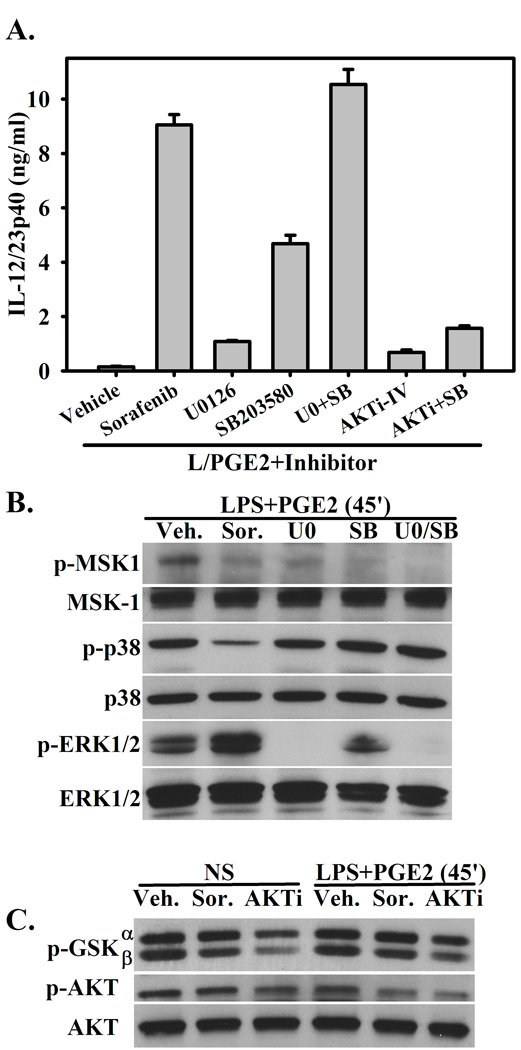
Sorafenib appears to have substantial activity against the phosphorylation of both p38 MAPK and AKT. Therefore, we wished to determine whether pharmacological inhibition of one or both of these pathways could reproduce the effects of Sorafenib. Macrophages were stimulated with LPS+PGE in the presence of Sorafenib, the MEK1/2 inhibitor U0126 (ERK pathway inhibitor), the p38 inhibitor SB203580, or both (U0/SB). As in figure 1A, the presence of Sorafenib restores the expression of IL-12/23p40 (Figure 6A). The presence of the ERK inhibitor marginally restores IL-12/23p40 expression, while the p38 inhibitor further restores IL-12/23p40, albeit at ~50% of the level observed in the presence of Sorafenib (Figure 6A). Inhibition of both the p38 and ERK pathways restores the expression of IL-12/23 to the levels of observed in the presence of Sorafenib (Figure 6A).
Figure 6. The restoration of IL-12/23 secretion is similar to MAPK inhibition.
(A.) BMMΦ were pretreated with Sorafenib (5µM), the MEK1/2 inhibitor U0126 (5µM), the p38 inhibitor SB203580 (5µM), U0+SB (5µM each), AKTi inhibitor IV (1µM), or AKTi+SB (1µM and 5µM, respectively), then stimulated with LPS+ PGE2. The concentration of IL-12/23p40 was determined by ELISA 16 hours after stimulation. Concentrations of inhibitors were titrated based on the highest IL-12/23p40 concentration after stimulation with LPS+ PGE2. (B.) BMMΦ were pretreated with Sorafenib or MAPK inhibitors and stimulated with LPS+PGE as in (A.) for 45’. Lysates were blotted for phospho- and total MSK-1, p38, and ERK-1/2. (C.) BMMΦ were pretreated with Sorafenib or the AKT inhibitor and stimulated with LPS+PGE as in (A.) or left unstimulated for 45’. Lysates were blotted for p-GSK3α/β, p-AKT, and total AKT. Figures are representative of three independent experiments.
The activity of these inhibitors was compared to the activity of Sorafenib by western blot. Inhibition of MEK1/2 and/or p38 via the presence of U0126 and SB203580 respectively led to the inhibition of MSK-1 phosphorylation, similar to the activity of Sorafenib. Furthermore, while U0126 inhibited the phosphorylation of ERK1/2, Sorafenib did not (Figure 4). Unlike the p38 inhibitor SB203580, which directly inhibits the kinase activity of p38 itself, Sorafenib inhibited the phosphorylation of p38 (Figure 6B).
Finally, we determined whether inhibition of AKT by the AKT inhibitor IV, which inhibits a kinase upstream of AKT but does not inhibit PI3K, could also restore IL-12/23p40 expression. The presence of AKTi IV only marginally restored the expression of IL-12/23p40 (Figure 6A). Because Sorafenib appears to inhibit both p38 and AKT activation, the AKT and p38 inhibitors were used in combination. The expression of IL-12/23p40 was only marginally enhanced when compared with AKT inhibition alone, while it was diminished when compared to p38 inhibition alone (Figure 6A). By western blot, as in figure 5, Sorafenib was able to partially inhibit the phosphorylation of AKT and GSK3β, either with or without stimulation with LPS+PGE. This inhibition was fairly marginal when compared to the inhibition observed in the presence of AKTi-IV (Figure 6C). This difference in inhibition may be due to AKT isoform specificity with Sorafenib or inefficient inhibition.
4. Discussion
The immunological effects of multikinase inhibitors routinely used in cancer treatment are emerging. The present study was undertaken to investigate the potential ability of Sorafenib to manipulate cytokine expression by macrophages.
Many previous studies have shown the biological effects of PGE2 and other cAMP elevating substances on the cytokine profile of macrophages, namely the suppression of several inflammatory cytokines and enhancement of the anti-inflammatory cytokine IL-10[14,15,30]. We wished to investigate the impact that Sorafenib could potentially have in the presence of PGE2 and other cAMP elevating extracellular mediators. We determined that Sorafenib could restore the expression of IL-12 and suppress IL-10 in a dose-dependent manner. Furthermore, activation with LPS alone in the presence of Sorafenib led to high levels of IL-12 secretion. These data suggest that Sorafenib interferes with a global mechanism by which inflammatory cytokine expression is suppressed and IL-10 expression is induced in macrophages.
The mechanism of PGE2-induced suppression of inflammatory cytokines has partially been elucidated. PGE2 has been shown to partially inhibit LPS-induced degradation of NF-κB p105 via the prostaglandin E receptor EP4. This mechanism is responsible for the inhibition of TNF-α, MCP-1, and a number of other cytokines[31]. It does not appear to be responsible for the inhibition of IL-12p40[31]. We have shown that Sorafenib reverses the inhibition of IL-12p40 mediated by the presence of PGE2, but not the inhibition of TNF-α (data not shown). Furthermore, we have observed that the degradation of p105 that is inhibited by PGE2 is not restored by the presence of Sorafenib (data not shown).
Two pathways that can differentially regulate inflammatory versus anti-inflammatory cytokine production are the MSK and GSK3-β kinase pathways. Interrogating the p38 and ERK MAP kinase signaling pathways revealed that Sorafenib can inhibit p38 activation and activation of its downstream target protein kinase MSK while having no effect on the activation of ERK. The lack of an effect on the activation of ERK, however, is not entirely unexpected as LPS induced activation of ERK1/2 is through a RAF-independent mechanism via the MAP3K TPL-2 (MAP3K8 [32]). Furthermore, inhibition of p38/MSK pathway disrupted the phosphorylation of histone H3 at serine 10, a downstream phosphorylation target of the MSKs. The MAPK p38α is integral in dampening inflammation via the MSKs in macrophages and other cells of myeloid origin [33]. Furthermore, the deletion of p38α leads to enhanced expression of a number of pro-inflammatory mediators, including IL-12p40, and severely diminished expression of anti-inflammatory IL-10[33]. The MSKs have been previously described as negative regulators of Toll-like receptor signaling and integral to regulating excessive expression of inflammatory cytokines, including IL-12p40. In addition, they have been shown to be required for transcription factor association to the il10 promoter[25]. Inhibition of p38/MSK appears to a be a major mechanism contributing to the ability of Sorafenib to promote excessive IL-12p40 expression in LPS simulated macrophages and restoring its expression in macrophages stimulated with LPS+ PGE2 via an IL-10 independent mechanism. Furthermore, inhibition of the MSKs also provides a potential mechanism for the inhibition of IL-10 expression.
Cytokine expression induced by Toll-like receptor engagement has previously been shown to be differentially regulated by glycogen synthase kinase (GSK) 3-β. GSK3-β is a constitutively active downstream kinase of the PI3K/Akt pathway which is inactivated upon phosphorylation at Ser9[27]. Direct inhibition of GSK3-β via the presence of the inhibitors LiCl or SB216763 diminishes the expression of IL-12p40 and enhances IL-10 production. Interference with AKT meditated inhibition of GSK3-β activity via Akt or PI3K inhibitors led to enhanced expression of IL-12p40 and suppression of IL-10 expression[27]. As we saw a similar pattern with macrophages stimulated in the presence of Sorafenib, we investigated the potential inhibitory activity of Sorafenib on the inactivation of GSK3-β. Sorafenib did show modest inhibition of both AKT activation and GSK3-β phosphorylation when macrophages were stimulated with LPS+PGE2. However, inhibition of AKT prior to stimulation with LPS+PGE2 did not lead to the restoration of IL-12p40 expression. Therefore, inhibition of the Akt/GSK3-β did not appear to the major mechanism leading to the restoration of IL-12p40 expression.
Due to the promiscuity of Sorafenib as an inhibitor it may have some unintentional targets which could boost its potential for successful anti-cancer therapy. Tumor associated macrophages have increasingly been recognized as tumor promoting. They appear to share many properties with regulatory macrophages and aid in tumor metastasis, tumor growth, down-regulation of adaptive immunity, and further drive the differentiation of recruited monocytes to a regulatory-like phenotype. They produce abundant IL-10 and are devoid of IL-12[34]. For certain tumors it is possible that Sorafenib may not only contribute to tumor resolution though its established mechanisms of vascular endothelial growth factor receptor (VEGFR) signaling blockade and direct tumor toxicity, but potentially also by transitioning macrophages from an regulatory-like to tumor-resolving inflammatory phenotype via the suppression of IL-10 and restoration of IL-12 production. The restoration of IL-12 and inhibition of IL-10 expression by tumor associated macrophages have been considered to be potentially valuable anticancer targets which could potentially have a profound impact on the tumor microenvironment[35]. Enhancing the presence of IL-12 within the tumor environment has been shown to contribute to tumor clearance through a variety of mechanisms, including restoring the cytotoxicity of tumor-resident CD8+ T-cells[36,37] and stimulating IFN-γ mediated inhibition of tumor-induced regulatory T-cell proliferation[38]. Future studies addressing the use of Sorafenib either in mono- or combinatorial therapy should potentially examine the influence it may have on macrophages within the tumor environment in addition to its well established tumoricidal and anti-angiogenic effects.
Supplementary Material
Macrophages were pretreated with 0–5µM Sorafenib then stimulated with LPS+PGE2 or treated with Sorafenib concomitant with stimulation. Macrophages treated LPS+PGE2 alone is present as a control (0µM Sorafenib). Concentrations of IL-12p40 (top) or IL-10 (bottom) were measured by ELISA. *Indicates a p-value <0.01, comparing drug to vehicle control.
Acknowledgements
This study was supported in part by grants DOD Center of Excellence Award (W81XWH-04-1-0595) and Johns Hopkins Breast Cancer Spore (P50 CA88843).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interests.
References
- 1.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chandra D, Naik S. Leishmania donovani infection down-regulates TLR2-stimulated IL-12p40 and activates IL-10 in cells of macrophage/monocytic lineage by modulating MAPK pathways through a contact-dependent mechanism. Clin Exp Immunol. 2008;154:224–234. doi: 10.1111/j.1365-2249.2008.03741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Figueiredo AS, Hofer T, Klotz C, Sers C, Hartmann S, Lucius R, et al. Modelling and simulating interleukin-10 production and regulation by macrophages after stimulation with an immunomodulator of parasitic nematodes. FEBS J. 2009;276:3454–3469. doi: 10.1111/j.1742-4658.2009.07068.x. [DOI] [PubMed] [Google Scholar]
- 4.Lucas M, Zhang X, Prasanna V, Mosser DM. ERK activation following macrophage FcgammaR ligation leads to chromatin modifications at the IL-10 locus. J Immunol. 2005;175:469–477. doi: 10.4049/jimmunol.175.1.469. [DOI] [PubMed] [Google Scholar]
- 5.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–3140. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilhelm S, Chien DS. BAY 43-9006: preclinical data. Curr Pharm Des. 2002;8:2255–2257. doi: 10.2174/1381612023393026. [DOI] [PubMed] [Google Scholar]
- 7.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 8.Hipp MM, Hilf N, Walter S, Werth D, Brauer KM, Radsak MP, et al. Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses. Blood. 2008;111:5610–5620. doi: 10.1182/blood-2007-02-075945. [DOI] [PubMed] [Google Scholar]
- 9.Zhao W, Gu YH, Song R, Qu BQ, Xu Q. Sorafenib inhibits activation of human peripheral blood T cells by targeting LCK phosphorylation. Leukemia. 2008;22:1226–1233. doi: 10.1038/leu.2008.58. [DOI] [PubMed] [Google Scholar]
- 10.Krusch M, Salih J, Schlicke M, Baessler T, Kampa KM, Mayer F, et al. The kinase inhibitors sunitinib and sorafenib differentially affect NK cell antitumor reactivity in vitro. J Immunol. 2009;183:8286–8294. doi: 10.4049/jimmunol.0902404. [DOI] [PubMed] [Google Scholar]
- 11.Manning EA, Ullman JG, Leatherman JM, Asquith JM, Hansen TR, Armstrong TD, et al. A vascular endothelial growth factor receptor-2 inhibitor enhances antitumor immunity through an immune-based mechanism. Clin Cancer Res. 2007;13:3951–3959. doi: 10.1158/1078-0432.CCR-07-0374. [DOI] [PubMed] [Google Scholar]
- 12.Edwards JP, Zhang X, Mosser DM. The expression of heparin-binding epidermal growth factor-like growth factor by regulatory macrophages. J Immunol. 2009;182:1929–1939. doi: 10.4049/jimmunol.0802703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Chapter 14: Unit. Curr Protoc Immunol. 2008 doi: 10.1002/0471142735.im1401s83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Pouw Kraan TC, Boeije LC, Smeenk RJ, Wijdenes J, Aarden LA. Prostaglandin-E2 is a potent inhibitor of human interleukin 12 production. J Exp Med. 1995;181:775–779. doi: 10.1084/jem.181.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams JA, Pontzer CH, Shacter E. Regulation of macrophage interleukin-6 (IL-6) and IL-10 expression by prostaglandin E2: the role of p38 mitogen-activated protein kinase. J Interferon Cytokine Res. 2000;20:291–298. doi: 10.1089/107999000312423. [DOI] [PubMed] [Google Scholar]
- 16.Su Y, Huang X, Raskovalova T, Zacharia L, Lokshin A, Jackson E, et al. Cooperation of adenosine and prostaglandin E2 (PGE2) in amplification of cAMP-PKA signaling and immunosuppression. Cancer Immunol Immunother. 2008;57:1611–1623. doi: 10.1007/s00262-008-0494-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng W, Wang Y, Zhang J, Wang X, Li C, Chang Z. Effects of CTx and 8-bromo-cAMP on LPS-induced gene expression of cytokines in murine peritoneal macrophages. Biochem Biophys Res Commun. 2000;269:570–573. doi: 10.1006/bbrc.2000.2341. [DOI] [PubMed] [Google Scholar]
- 18.Bryn T, Mahic M, Enserink JM, Schwede F, Aandahl EM, Tasken K. The cyclic AMP-Epac1-Rap1 pathway is dissociated from regulation of effector functions in monocytes but acquires immunoregulatory function in mature macrophages. J Immunol. 2006;176:7361–7370. doi: 10.4049/jimmunol.176.12.7361. [DOI] [PubMed] [Google Scholar]
- 19.Braun MC, He J, Wu CY, Kelsall BL. Cholera toxin suppresses interleukin (IL)-12 production and IL-12 receptor beta1 and beta2 chain expression. J Exp Med. 1999;189:541–552. doi: 10.1084/jem.189.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benkhart EM, Siedlar M, Wedel A, Werner T, Ziegler-Heitbrock HW. Role of Stat3 in lipopolysaccharide-induced IL-10 gene expression. J Immunol. 2000;165:1612–1617. doi: 10.4049/jimmunol.165.3.1612. [DOI] [PubMed] [Google Scholar]
- 21.Moore KW, de Waal MR, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 22.Yang F, Van Meter TE, Buettner R, Hedvat M, Liang W, Kowolik CM, et al. Sorafenib inhibits signal transducer and activator of transcription 3 signaling associated with growth arrest and apoptosis of medulloblastomas. Mol Cancer Ther. 2008;7:3519–3526. doi: 10.1158/1535-7163.MCT-08-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blechacz BR, Smoot RL, Bronk SF, Werneburg NW, Sirica AE, Gores GJ. Sorafenib inhibits signal transducer and activator of transcription-3 signaling in cholangiocarcinoma cells by activating the phosphatase shatterproof 2. Hepatology. 2009;50:1861–1870. doi: 10.1002/hep.23214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arthur JS. MSK activation and physiological roles. Front Biosci. 2008;13:5866–5879. doi: 10.2741/3122. [DOI] [PubMed] [Google Scholar]
- 25.Ananieva O, Darragh J, Johansen C, Carr JM, McIlrath J, Park JM, et al. The kinases MSK1 and MSK2 act as negative regulators of Toll-like receptor signaling. Nat Immunol. 2008;9:1028–1036. doi: 10.1038/ni.1644. [DOI] [PubMed] [Google Scholar]
- 26.Thomson S, Clayton AL, Hazzalin CA, Rose S, Barratt MJ, Mahadevan LC. The nucleosomal response associated with immediate-early gene induction is mediated via alternative MAP kinase cascades: MSK1 as a potential histone H3/HMG-14 kinase. EMBO J. 1999;18:4779–4793. doi: 10.1093/emboj/18.17.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen KF, Yu HC, Liu TH, Lee SS, Chen PJ, Cheng AL. Synergistic interactions between sorafenib and bortezomib in hepatocellular carcinoma involve PP2A-dependent Akt inactivation. J Hepatol. 2009 doi: 10.1016/j.jhep.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Yu C, Friday BB, Lai JP, Yang L, Sarkaria J, Kay NE, et al. Cytotoxic synergy between the multikinase inhibitor sorafenib and the proteasome inhibitor bortezomib in vitro: induction of apoptosis through Akt and c-Jun NH2-terminal kinase pathways. Mol Cancer Ther. 2006;5:2378–2387. doi: 10.1158/1535-7163.MCT-06-0235. [DOI] [PubMed] [Google Scholar]
- 30.Kunkel SL, Spengler M, May MA, Spengler R, Larrick J, Remick D. Prostaglandin E2 regulates macrophage-derived tumor necrosis factor gene expression. J Biol Chem. 1988;263:5380–5384. [PubMed] [Google Scholar]
- 31.Minami M, Shimizu K, Okamoto Y, Folco E, Ilasaca ML, Feinberg MW, et al. Prostaglandin E receptor type 4-associated protein interacts directly with NF-kappaB1 and attenuates macrophage activation. J Biol Chem. 2008;283:9692–9703. doi: 10.1074/jbc.M709663200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beinke S, Robinson MJ, Hugunin M, Ley SC. Lipopolysaccharide activation of the TPL-2/MEK/extracellular signal-regulated kinase mitogen-activated protein kinase cascade is regulated by IkappaB kinase-induced proteolysis of NF-kappaB1 p105. Mol Cell Biol. 2004;24:9658–9667. doi: 10.1128/MCB.24.21.9658-9667.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim C, Sano Y, Todorova K, Carlson BA, Arpa L, Celada A, et al. The kinase p38 alpha serves cell type-specific inflammatory functions in skin injury and coordinates pro- and anti-inflammatory gene expression. Nat Immunol. 2008;9:1019–1027. doi: 10.1038/ni.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porta C, Larghi P, Rimoldi M, Totaro MG, Allavena P, Mantovani A, et al. Cellular and molecular pathways linking inflammation and cancer. Immunobiology. 2009;214:761–777. doi: 10.1016/j.imbio.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 35.Sica A, Saccani A, Mantovani A. Tumor-associated macrophages: a molecular perspective. Int Immunopharmacol. 2002;2:1045–1054. doi: 10.1016/s1567-5769(02)00064-4. [DOI] [PubMed] [Google Scholar]
- 36.Gu T, Rowswell-Turner RB, Kilinc MO, Egilmez NK. Central role of IFNgamma-indoleamine 2,3-dioxygenase axis in regulation of interleukin-12-mediated antitumor immunity. Cancer Res. 2010;70:129–138. doi: 10.1158/0008-5472.CAN-09-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kilinc MO, Aulakh KS, Nair RE, Jones SA, Alard P, Kosiewicz MM, et al. Reversing tumor immune suppression with intratumoral IL-12: activation of tumor-associated T effector/memory cells, induction of T suppressor apoptosis, and infiltration of CD8+ T effectors. J Immunol. 2006;177:6962–6973. doi: 10.4049/jimmunol.177.10.6962. [DOI] [PubMed] [Google Scholar]
- 38.Cao X, Leonard K, Collins LI, Cai SF, Mayer JC, Payton JE, et al. Interleukin 12 stimulates IFN-gamma-mediated inhibition of tumor-induced regulatory T-cell proliferation and enhances tumor clearance. Cancer Res. 2009;69:8700–8709. doi: 10.1158/0008-5472.CAN-09-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Macrophages were pretreated with 0–5µM Sorafenib then stimulated with LPS+PGE2 or treated with Sorafenib concomitant with stimulation. Macrophages treated LPS+PGE2 alone is present as a control (0µM Sorafenib). Concentrations of IL-12p40 (top) or IL-10 (bottom) were measured by ELISA. *Indicates a p-value <0.01, comparing drug to vehicle control.