It is commonly believed that cancer arises as a consequence of an imbalance in cellular homeostasis. The discovery of microRNAs (miRNAs) has opened a new era in the understanding of this balance. A growing body of evidence indicates that miRNAs, such as let-7, regulate a large variety of cellular processes. Consequently, deregulation of miRNAs results in many diseases including cancer. The let-7 family consists of 13 sequence-conserved members located on nine different chromosomes and is functionally conserved from c. elegans to humans. Let-7s are down-regulated in many malignancies and widely viewed as tumor suppressors. Given the functional redundancy and co-repression of let-7s in tumors, the presence of a common mechanism to coordinately inhibit let-7s was anticipated. Consistently, several studies revealed that the lin28 family members, i.e., Lin28 and Lin28B, are both targets and inhibitors of let-7s [1–3]. Moreover, high expression levels of lin28 and lin28B is often found in tumors. Taken together, these results indicate that lin28s may promote transformation by targeting let-7 and that the intricate balance between lin28 and let-7 may be critical for regulating tumor development and progression.
In this manuscript, Viswanathan et al examined the role of lin28 in cellular transformation and its functional link with let-7 [4]. They first demonstrated that lin28 facilitates cellular transformation in different cell lines. Consistently, lin28 is overexpressed in various human cancer cell lines and in different types of primary tumors. Moreover, lin28 expression is associated with advanced disease progression across multiple tumor types including hepatocellular carcinoma (HCC). Interestingly, all of the lin28-associated activities are tightly linked to its suppression of let-7 expression and consequently an up-regulation of let-7 targets. Furthermore, they observed that the lin28/let-7 signaling link is only applicable to a subset of HCC patients with high-grade tumors and a high incidence of early recurrence [4]. This study is encouraging and is the first example that convincingly demonstrates the critical role of the intricate balance between lin28 and let-7 in cellular transformation and tumor progression.
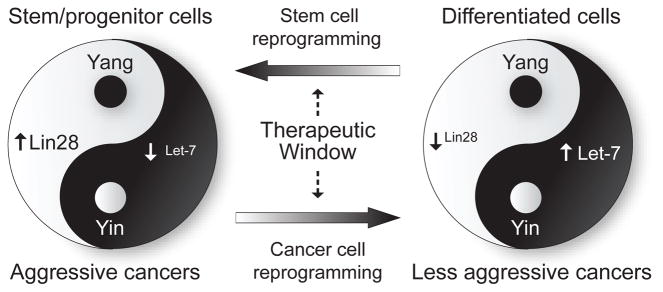
We suggest that lin28/let-7 signaling link can be viewed much like a Yin-Yang balancing act (Fig. 1), based on the ancient Chinese scientific thinking of how things work. The Yin-Yang theory suggests that the ‘universe’ is governed by the balance of Yin and Yang, where Yin represents the negative element while Yang represents the positive element. Consistent with this concept, lin28 and let-7 have been shown to have opposing expression patterns and functions in development and tumor progression.
Figure 1.
The opposing expression patterns and the functional roles of Lin28 and let-7 are found throughout development, from embryo to adult. In embryonic stem cells, the level of lin28 is very high while let-7 is extremely low. Lin28 is then gradually reduced while let-7 is gradually increased during development. Functionally forced expression of let-7 in stem cells leads to differentiation while exogenous lin28 and other factors can reprogram differentiated cells towards “stemness”. It is conceivable that loss of this balance would be detrimental, which would result in abnormal differentiation or cancer. Consistent with the above view, there is growing evidence that an aberrant balance of lin28/let-7, as in the data presented by Viswanathan et al, is linked to human malignancies. Consequently, down-regulation of let-7 along with up-regulation of lin28 is frequently found in various human tumors including HCC and is associated with HCC metastasis [4–6]. Interestingly, the opposing expression pattern of lin28 and let-7 is only found in HCC with an advanced stage and in poorly differentiated ovarian tumors, suggesting that this Yin-Yang imbalance is mainly associated with tumor progression [4,7]. Encouragingly, silencing of lin28 or re-induction of let-7 in HCC or ovarian cancer cells can inhibit tumor cell proliferation [4,6,7].
The connection between the lin28/let-7 signaling link and oncogenesis is further supported by the observation that this signaling pathway is also associated with several oncogenes such as c-myc and NF-κB in inducing cell transformation [8,9]. Importantly, the activation of Lin28 by c-myc and NF-κB is necessary and sufficient for oncogene mediated let-7 repression. Thus, an imbalance between lin28 and let-7 induced by Myc and NF-κB can result in cellular transformation.
Mechanistically, let-7s suppresses the expression of lin28 through let-7-binding sites in the lin28 3′UTR [3]. Moreover, lin28 suppresses the production of mature let-7s at multiple levels. For example, lin28 binds to the loop region of the precursor of let-7, which blocks the let-7 processing at both the Drosha and Dicer steps [1]. In addition, lin28 induces 3′-terminal uridylation of let-7 precursors, leading to the failure of Dicer processing and finally degradation [2]. Therefore, both lin28 and let-7 are involved in the same negative feedback loop to regulate cellular processes.
The lin28/let-7 signaling link plays a critical role in regulating cellular homeostasis during human development. An imbalance in this link leads to abnormal differentiation and cellular transformation. Therefore, reprogramming of the lin28/let-7 signaling link would undoubtedly show therapeutic benefits. It is interesting to point out that lin28 may be a key player leading to oncogenesis via its locus amplification [4] or myc and NF-κB mediated signaling [8,9]. Therefore, lin28 may have additional functions other than suppressing let-7. It is of interest to further explore additional downstream signaling molecules of lin28 and its association with the let-7 network. These studies may also help us understand other developmental processes, such as aging. In addition, the lin28/let-7 signaling link may be applicable to cancer stem cells since down-regulation of let-7 in HCC with cancer stem cell features has been reported [6,10]. Taken together, research into the molecular networks of lin28 and let-7 has improved our understanding of the intricate microRNA regulatory circuit. The exploitation of such a circuit with the use of the Yin-Yang concept may allow us to identify novel therapeutic strategies useful in human cancer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Viswanathan SR, Daley GQ, Gregory Rl. Selective blockade of microRNA processing by Lin28. Science. 2008;320:97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heo I, Joo C, Cho J, Ha M, Han J, Kim VN. Lin28 mediates the terminal uridylation of let-7 precursor MicroRNA. Mol Cell. 2008;32:276–284. doi: 10.1016/j.molcel.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Guo Y, Chen Y, Ito H, Watanabe A, Ge X, Kodama T, et al. Identification and characterization of lin28 homolog B (LIN28B) in human hepatocellular carcinoma. Gene. 2006;384:51–61. doi: 10.1016/j.gene.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Viswanathan SR, Powers JT, Einhorn W, Hoshida Y, Ng TL, Toffanin S, et al. Lin28 promotes transformation and is associated with advanced human malignancies. Nat Genet. 2009;41:843–848. doi: 10.1038/ng.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, et al. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 6.Ji J, Zhao L, Budhu A, Forgues M, Jia HL, Qin LX, et al. Let-7g targets collagen type I alpha2 and inhibits cell migration in hepatocellular carcinoma. J Hepatol. 2010;52:690–697. doi: 10.1016/j.jhep.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng S, Maihle NJ, Huang Y. Pluripotency factors Lin28 and Oct4 identify a subpopulation of stem cell-like cells in ovarian cancer. Oncogene. 2010;29:2153–2159. doi: 10.1038/onc.2009.500. [DOI] [PubMed] [Google Scholar]
- 8.Chang TC, Zeitels LR, Hwang HW, Chivukula RR, Wentzel EA, Dews M, et al. Lin-28B transactivation is necessary for Myc-mediated let-7 repression and proliferation. Proc Natl Acad Sci U S A. 2009;106:3384–3389. doi: 10.1073/pnas.0808300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji J, Yamashita T, Budhu A, Forgues M, Jia HL, Li C, et al. Identification of microRNA-181 by genome-wide screening as a critical player in EpCAM-positive hepatic cancer stem cells. Hepatology. 2009;50:472–480. doi: 10.1002/hep.22989. [DOI] [PMC free article] [PubMed] [Google Scholar]