Abstract
To determine the role of maternal immunity in protecting newborn mice against a C. trachomatis infection female BALB/c mice were immunized intranasally (i.n.) with 104 inclusion forming units (IFU) of the C. trachomatis mouse pneumonitis biovar (MoPn). As a control, another group of female mice was sham-immunized i.n. with HeLa cell extracts. Immunized animals mounted strong immune responses as evidenced by high Chlamydia-specific antibody titers in serum and milk. Newborn mice born from immunized and sham-immunized dams were challenged i.n. with 103 IFU of MoPn at 2-post natal days (PND). Following inoculation, newborn mice were euthanized at 7-PND and 18-PND and the lungs, spleen and intestine were cultured for Chlamydia. Overall, no significant differences were observed between the mice born from and fed by immunized dams and mice born from and fed by sham-immunized dams. Of the mice born from immunized dams, 75% and 25% had positive lung cultures at 7-PND and 18-PND, respectively. Of the mice born from sham-immunized dams, 82% and 50% had positive lung cultures for those same days. When the number of IFU recovered from the lungs and spleens were compared between the two groups no significant differences were observed. However, when the number of IFU recovered from the small intestine were compared, significant differences were observed between the two groups of newborn mice (2×105 versus 32×106 at 7-PND and 9.2×106 versus 85×106 at 18-PND). In conclusion, maternal immunity plays a limited role in protecting newborn mice against a Chlamydia infection.
Keywords: maternal immunity, Chlamydia trachomatis, newborn mice, intranasal infection
INTRODUCTION
In the United States it is estimated that 5% of pregnant women are infected with Chlamydia trachomatis, and as a result, nearly 3.5 million newborns become infected at the time of delivery. Approximately 30% of the infants exposed to C. trachomatis in the birth canal develop conjunctivitis and 10% to 20% show symptoms of pneumonia (Numazaki, et al., 2003; Persson, et al., 1986; Schachter and Caldwell, 1980). A total of 75,000 cases of inclusion conjunctivitis and 30,000 cases of C. trachomatis pneumonia are reported each year (Persson, et al., 1986; Schachter and Caldwell, 1980). In addition, newborns may have vaginal and gastrointestinal tract infections with no known clinical consequences (Schachter and Caldwell, 1980). If diagnosed in time, most of the neonatal infections can be treated with antibiotics, and there are no long-term sequelae. However, some neonates develop persistent infections (Darville, 2005; Persson, et al., 1986).
In general, neonates are highly susceptible to a variety of infectious diseases including Chlamydia. Several factors, such as the immaturity of antigen presenting cells, impaired IgG isotype switching and deficiencies in complement and Th1-type cytokines, are thought to be responsible for the high susceptibility to disease (Forsthuber et al., 1996; Levy 2007; Ridge et al., 1996). Providing passive protection to infants by immunizing mothers could overcome some of these problems and therefore, bypass the limitations of the immature immune system of the neonates. However, during gestation, mainly components of the humoral immune response are transferred from the mother to the fetus (Seigrist, 2001, 2003).
Based on studies performed in the mouse model using live C. trachomatis for immunization, it appears that CD4+ T cells play a predominant role in protective immunity. B cells and/or antibodies also have an important role, while CD8+ T cells are not critical for protection (Morrison et al., 2000). Passive transfer of C. trachomatis MoPn antibodies to adult nu/nu and nu/+ mice has been shown to protect mice against intranasal infections (Williams et al., 1984). Similarly, Pal et al. (2008) have shown that passive immunization with monoclonal antibodies (mAb) recognizing a conformational epitope of the C. trachomatis MoPn MOMP resulted in significant protection in wild type and C.B-17-SCID mice against an intranasal challenge.
Adult mice immunized with live C. trachomatis by the respiratory route develop robust cell-mediated and humoral immune responses and are protected against respiratory and genital challenges (Pal et al., 1994, 2002). Herein, we wanted to investigate whether maternal immunity could protect newborn mice against a respiratory chlamydial infection. To test this hypothesis, mice born from and fed by immunized or sham-immunized dams were challenged intranasally and the course of the infection was assessed.
MATERIALS AND METHODS
Organisms
The Nigg II strain of the C. trachomatis MoPn biovar (Ct-MoPn) (also called Chlamydia muridarum) was obtained from the American Type Culture Collection (Manassas, VA) and was grown in HeLa-229 cells using Eagle’s minimal essential medium (Nigg 1942; Pal et al., 1994). Elementary bodies (EB) were purified and stored at −70°C in sucrose phosphate glutamic acid (SPG) chlamydial transport medium (Caldwell et al., 1981).
Animals
Seven to eight week-old adult female and proven fertile breeder male BALB/c (H-2d) mice were purchased from Charles River Laboratories (Wilmington, MA.). To determine the infectious dose (ID)50 for newborn mice, newborn mice born from naïve dams were infected with 102, 103, and 104 C. trachomatis MoPn IFU per mouse at 2-post natal day (PND). Inoculates were administered in 3 μl of MEM-0 onto the nostrils of newborn mice without anesthesia. Mice were euthanized at 11-PND, their lungs were harvested, and the number of IFU were determined (Pal et al., 2002).
For immunization, female mice were immunized with 104 IFU of Ct-MoPn or sham-immunized with HeLa cell extracts as described by (Pal et al., 1994, 2002). Newborn mice born from immunized or sham-immunized dams were challenged with 103 IFU of Ct-MoPn at 2-PND. To examine the course of the infection, newborn mice were euthanized at 7- and 18-PND and their lungs, small intestines and spleens were harvested and homogenized for the isolation of Chlamydia. The University of California Irvine, Animal Care and Use Committee approved animal protocols.
Collection of milk from mice
Milk was collected from lactating dams at various days PND to determine antibody titers as described by Parr et al. (1995). Briefly, dams were separated from newborns for 4 to 5 h in order to accumulate milk in the mammary glands. The dams were euthanized with a xylazine and ketamine solution and then inoculated i.p. with 1.5 U of oxytocin (Sigma Chemicals; St. Louis, MO) in 150 μl of PBS. Whey samples from the milk were prepared and stored at −70°C.
Immunoassays
An enzyme linked immunosorbent assay (ELISA) was used to determine the antibody responses in serum and whey samples (Pal et al., 1994). At the time of euthanization, blood and milk were collected from dams and blood was collected from newborn mice by cardiac puncture. The following class or subclass-specific antibodies were used: immunoglobulin G (IgG), IgG1, IgG2a, IgG2b, IgG3, IgA, and IgM (Southern Biotechnology Associates, Inc., Birmingham, AL). T cell proliferation assays were performed using splenocytes as previously described (Pal et al., 1994).
In vitro neutralization assays were performed using HeLa-229 cell monolayers as previously described (Pal et al., 1994). Western blot analyses were performed using nitrocellulose membranes as described (Schagger and Jagow 1987).
Organ culture
Following euthanasia the lungs, small intestines, and spleens were collected, homogenized in 2 ml of SPG and inoculated onto McCoy cell monolayers grown in 48-well tissue culture plates (Pal et al., 2002, 2008).
Statistics
All statistical analyses were performed with the SigmaStat software except multivariate analysis. A Multivariate regression analysis using generalized estimating equation (PROC GENMOD) was performed employing the SAS 9.2 software program.
RESULTS
Determination of the ID50 for newborn mice
To determine the ID50 for newborn mice, animals born from naïve dams were inoculated at 2-PND with doses of Ct-MoPn ranging from 102 to 104 IFU per newborn mice. Table 1 shows the results of the lungs cultures at 11-PND. When newborn mice were inoculated with 102 Ct-MoPn IFU, 4 out of 8 mice (50%) had positive cultures. Of the 12 newborn mice that received 103 IFU, 10 (83%) had positive cultures. Of the newborn animals that received 104 IFU per mouse, 16 out of 18 (89%) had positive cultures. Thus for BALB/c mice, the ID50 determined by the Reed-Muench formula was 102 IFU of Ct-MoPn per newborn mice.
Table 1.
Determination of C. trachomatis ID50 for newborn micea
| Dose (IFU/newborn mice) | No. newborn mice + cultures/total (%+) | Median (range) no. of IFU recovered from lungs |
|---|---|---|
| 102 | 4/8 (50) | 400 (<20–4,400) |
| 103 | 10/12 (83) | 305 (<20–4,900,000) |
| 104 | 16/18 (89) | 490,000 (<20–8,800,000) |
Newborn mice born from sham-immunized-dams were inoculated at 2-PND and were euthanized at 11-PND.
Limit of detection: <20 IFU/culture.
Characterization of the immune responses in dams
Table 2 shows antibody titers in serum and milk at 0-, 7- and 18-PND. Overall, high IgG antibody titers in serum and milk were detected in the dams inoculated i.n. with live EB. Immunized dams had a predominant Th1-immune response as indicated by the IgG2a/IgG1 ratio. For example, at day 0-PND, the IgG2a/IgG1 ratio in sera from immunized dams was 8 (6,400/800). On the same day the IgA titer was 1,600. High antibody titers to Ct-MoPn were detected in the milk samples of immunized dams. At 7-PND, milk had high levels of IgG (6,400) with a high IgG2a/IgG1 ratio (6,400/<100). Very high titers of IgA were also detected in the milk (25,600).
Table 2.
Antibody titers in serum and milka of dams during post natal period
| Type of dams | Sample | PND | Anti-C. trachomatis MoPn ELISA titer |
Neutralizing titer | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IgG | IgG1 | IgG2a | IgG2b | IgG3 | IgA | IgM | ||||
| Immunizedb | Serum | 0c | 12,800 | 800 | 6,400 | 12,800 | 800 | 1,600 | <100 | 1,250 |
| Serum | 7 | 12,800 | 800 | 6,400 | 12,800 | 1,600 | 1,600 | 400 | 1,250 | |
| Serum | 18 | 12,800 | 1,600 | 12,800 | 6,400 | 1,600 | 3,200 | 100 | 1,250 | |
| Sham-immunized | Serum | 0c | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 |
| Serum | 7 | 200 | 200 | <100 | <100 | <100 | 100 | <100 | <100 | |
| Serum | 18 | 100 | 100 | <100 | <100 | <100 | 100 | 100 | <100 | |
| Immunized | Milk | 0c | ND | ND | ND | ND | ND | ND | ND | ND |
| Milk | 7 | 6,400 | <100 | 6,400 | 3,200 | 100 | 25,600 | <100 | <10 | |
| Milk | 18 | 3,200 | 200 | 3,200 | 3,200 | 200 | 25,600 | <100 | 50 | |
| Sham-immunized | Milk | 0c | ND | ND | ND | ND | ND | ND | ND | ND |
| Milk | 7 | 100 | 100 | <100 | <100 | <100 | 100 | <100 | <10 | |
| Milk | 18 | 100 | 100 | <100 | <100 | <100 | <100 | <100 | <10 | |
Serum and milk samples were pooled from 5 to 6 dams.
Dams were immunized i.n. with 104 C. trachomatis MoPn IFU per mouse
Day before caging together with male mice.
ND = Not done
In the sham-immunized dams, at 0-PND no chlamydial antibodies were measured. However, at 7-PND and 18-PND low levels (100) of anti-Chlamydia IgG, antibodies were detected in the serum and milk samples. The appearance of antibodies in milk and serum from the sham-immunized animals suggests that the dams were infected by their offspring.
A high neutralizing antibody titer of 1,250 to Ct-MoPn was measured in the sera of immunized dams at 0-PND, 7-PND and 18-PND (Table 2). In contrast, no significant Chlamydia-specific neutralizing antibodies in the whey of the immunized dams were observed when compared to the milk of sham-immunized dams. This suggests that the normal milk might contain components that non-specifically inhibit the infectivity of Chlamydia.
Western blot analyses of the serum and whey samples of immunized dams are shown in Fig. 1. Serum and whey antibodies from the immunized dams at 7-PND and 18-PND bound predominantly to the 100- and 150-kDa polymorphic membrane proteins, MOMP (40-kDa), the 60-kDa crp, the 60-kDa hsp, the 28-kDa protein (pgp3), and the lipopolysaccharide (LPS). Sera or whey from sham-immunized dams showed no specific reactivity with Ct-MoPn EB in the Western blot assay.
Figure 1. Immunoblot analysis of the sera and whey of immunized- and sham-immunized dams.
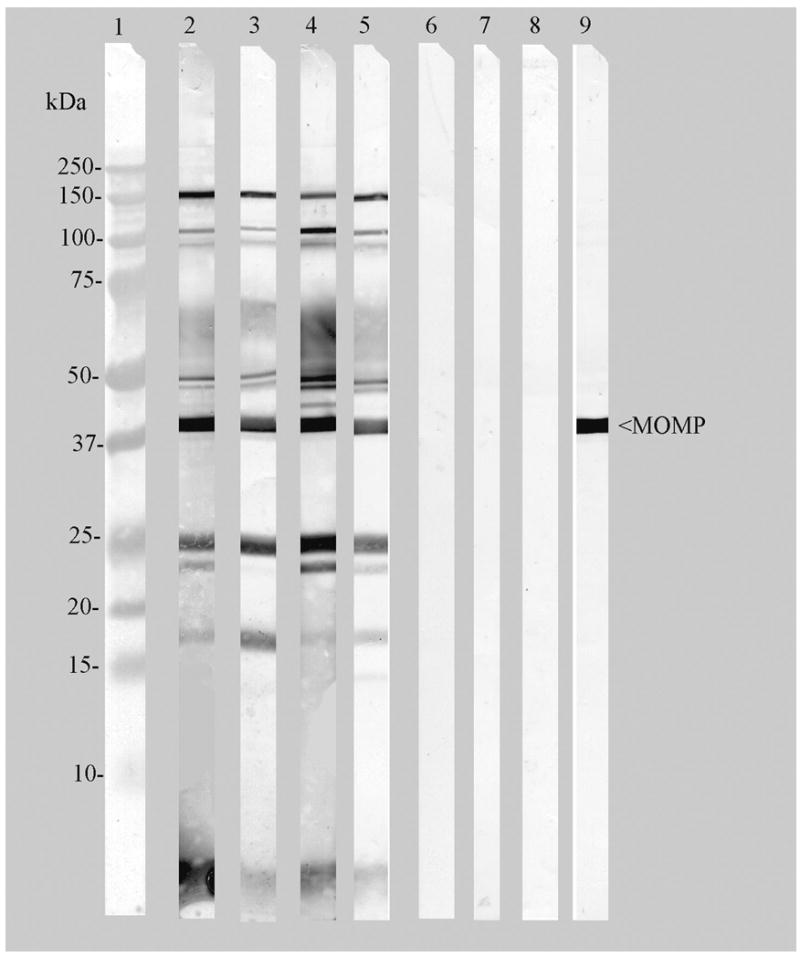
C. trachomatis MoPn EB were used as the antigen. Lane 1, Molecular weight standards, Lanes 2, 4, sera from immunized dams collected at 7- and 18-PND. Lanes 3, 5, whey from immunized dams collected at 7- and 18-PND. Lanes 6, 8 sera from sham-immunized dams collected at 7- and 18-PND. Lanes 7, 9, whey collected from sham-immunized dams at 7- and 18-PND. Lane 10, Positive control: mAb-40 to the Ct-MoPn MOMP. Serum or whey samples were diluted 1:100 to examine their reactivities.
Serum antibody and cell mediated immune (CMI) responses in newborn mice
Newborn mice were challenged i.n. with 103 IFU of Ct-MoPn at 2-PND and the geometric mean serum antibody titers determined from three pooled serum samples collected from three separate experiments (Table 3). Animals born from immunized dams had high antibody titers as determined by an ELISA. At 0-PND, newborn mice had IgG, IgG1, IgG2a, IgG2b, IgG3, and IgA geometric titers of 10,159, 400, 5,080, 6,400, 1,600 and 504, respectively. No IgM antibody titer was detected at that time or at 7-PND or 18 -PND. Like the dams, the newborns had a high IgG2a/IgG1 ratio. The newborn mice born from and fed by sham-immunized dams had no detectable anti-chlamydial antibodies until 11-PND (data not shown). At 18-PND, the geometric mean titers of IgG and IgA rose to 673 and 168, respectively. Of the four IgG isotypes, newborn mice developed IgG2a (126), IgG2b (238) and IgG3 (566) but no IgG1 antibodies were detected.
Table 3.
Geometric mean serum antibody titers in newborn mice before and after an i.n. challenge with C. trachomatis MoPn at 2 PND
| Newborn mice born from and fed by: | PND | Anti-C. trachomatis MoPn ELISA titer |
||||||
|---|---|---|---|---|---|---|---|---|
| IgG | IgG1 | IgG2a | IgG2b | IgG3 | IgA | IgM | ||
| Immunized-dams | 0 | 10,159 | 400 | 5,080 | 6,400 | 1,600 | 504 | <100 |
| 7 | 32,254 | 5,080 | 51,200 | 25,600 | 3,200 | 4,032 | <100 | |
| 18 | 32,254 | 5,080 | 81,275 | 16,127 | 2,540 | 4,032 | 100 | |
| Sham-immunized-dams | 0 | <100 | <100 | <100 | <100 | <100 | <100 | <100 |
| 7 | <100 | <100 | <100 | <100 | <100 | <100 | <100 | |
| 18 | 673 | <100 | 126 | 238 | 566 | 168 | 100 | |
Geometric mean titers were obtained from pooled serum samples of newborn mice collected from three separate experiments.
As shown in Table 4 no CMI responses to Ct-MoPn were detected in the pups born from Chlamydia immunized- or sham-immunized dams at 9- or 16-PND. These results the immaturity of T cells in both groups of newborn mice. Significant, high responses to Ct-MoPn were noticed at 28-PND in both groups of pups. Responses to Con A started at 16-PND and increased at 28-PND.
Table 4.
T-cell responses of newborn mice following an i.n. challenge with C. trachomatis MoPn at 2-PND
| Newborn mice born from and fed by: | PND | T-cell proliferative responsea (cpm) to: |
||
|---|---|---|---|---|
| EBb | Con Ac | Medium | ||
| Immunized-dams | 9 | 360±63 | 264±23 | 209±52 |
| 16 | 956±298 | 16,533±4,198 | 279±57 | |
| 28 | 2,657±882 | 29,999±1,423 | 426±123 | |
| Sham-immunized-dams | 9 | 536±126 | 298±45 | 150±25 |
| 16 | 217±24 | 9,975±727 | 187±59 | |
| 28 | 2,282±374 | 31,393±1,697 | 218±8 | |
The values are means ± SE for triplicate cultures. The data are from one of the experiments representative of duplicate experiments.
UV-inactivated C. trachomatis MoPn EB were added at a ratio of 10:1 to Tcells.
Con A was added: 5 μg per ml.
Recovery of C. trachomatis from newborns following the i.n. challenge
Mice born from immunized- or sham-immunized dams were challenged i.n. with 103 IFU of Ct-MoPn at 2-PND. This dose resulted in positive lung cultures from 83% of the pups at 11 PND born from sham-immunized dams (Table 1). To monitor the infection, the animals were euthanized at 7-PND and 18-PND. The lungs, spleen and intestine were harvested and tissue homogenates cultured in McCoy cell monolayers. Table 5 shows that the number of newborn mice with positive Chlamydia cultures was not significantly different between the newborn mice born from and fed by immunized dams and the newborn mice born from and fed by sham-immunized dams. For example, at 7-PND 75% (9/12) of newborn mice born from and fed by immunized dams had positive Chlamydia cultures from the lungs while 82% (9/11) of newborn mice born from and fed by sham-immunized dams had positive cultures (P>0.05). A similar trend was observed at 18-PND. In addition, when the number of Ct-MoPn IFU recovered from the lungs was analyzed, no significant reduction was observed between the newborn mice born from and fed by immunized dams and those born from and fed by sham-immunized dams. For example, at 7-PND the median number of IFU recovered from the newborn mice of immunized dams was 743 while it was 350 for the newborn mice of sham-immunized dams. The infection rate declined in both groups of newborn mice by 18-PND. Similar results were observed from the spleen cultures.
Table 5.
Recovery of C. trachomatis MoPn from the newborn mice
| Newborn mice born and fed by: | PND | No. culture + newborn mice (%+) and median (range) no. of IFU recovered from organs: |
|||||
|---|---|---|---|---|---|---|---|
| Lungs (×106) | Intestine (×106) | Spleen | |||||
| Immunized-dams | 7 | 9 (75) | 743 (<20–0.8) | 12 (100) | 0.2 (0.002–1,049)a | 4 (33) | <20 (<20–11,000) |
| Sham-immunized-dams | 7 | 9 (82) | 350 (<20–5.3) | 11 (100) | 32 (2.9–270) | 8 (73) | 80 (<20–17,600) |
| Immunized-dams | 18 | 6 (38) | <20 (<20–0.007) | 16 (100) | 9.2 (0.9–128)b | 4 (25) | <20 (<20–3,387) |
| Sham-immunized-dams | 18 | 6 (50) | 30 (<20–2.3) | 12 (100) | 85 (1.2–2,975) | 2 (17) | <20 (<20–21,400) |
P<0.05 by the multivariate regression analysis compared with the newborn mice born from and fed by sham-immunized-dams.
P<0.1 by the multivariate regression analysis compared with the newborn mice born from and fed by sham-immunized-dams Limit of detection: <20 IFU/culture.
The small intestines of all newborn mice from both groups were consistently positive for Chlamydia at 7- and 18-PND. Interestingly, a significantly lower number of Ct-MoPn IFU was recovered from the mice born from and fed by the immunized dams compared to the mice born from and fed by the sham-immunized dams. However, the level of significance waned down from 0.05 to 0.1 by 18-PND. For example, at 7-PND the median number of IFU recovered from the newborn mice of immunized dams was 0.2×106 whereas the median IFU number for the newborn mice from the sham-immunized dams was 32×106 (P=0.014). Similarly, at 18-PND there was a 10-fold difference in the number of IFU recovered from the intestines of the two groups (P<0.0924). Cultures from non-challenged newborn mice, born from immunized-dam, always had negative Chlamydia cultures, indicating that no viable Chlamydia was transferred from previously infected immune mothers to newborn pups.
DISCUSSION
Herein we evaluated the role of maternal immunity in protection of newborn mice against a respiratory Ct-MoPn challenge. Our results showed that maternal immunity only confers a limited organ specific protection. The number of newborn mice with positive lung and spleen cultures was not significantly different between those born from the immunized- and sham-immunized dams. Furthermore, the number of IFU recovered from the lungs and spleens were similar between the two groups. However, the mice born from and fed by immunized dams had significantly reduced number of chlamydial IFU in the intestines when compared to the newborn animals born from and fed by sham-immunized dams.
During gestation the fetus develops in an environment protected from pathogenic organisms and other immunological challenges (Adkins and Du 1998; Levy 2007, Seigrist 2001). Both the immune systems of the mother and the fetus have to develop tolerance towards each other so that the pregnancy can come to a successful end. However, once the fetal membranes break the newborn is immediately exposed to multiple foreign antigens including the normal and pathogenic flora present in the birth canal. At that point, the immune system of the newborn is immature and is not ready to quickly respond to the new environment. During gestation the mother supplies immunological components, in particular antibodies, which can help protect the newborn for several months. In addition, through breast-feeding, the mother can transfer to the newborn a broad range of anti-microbial, anti-inflammatory and immunoregulatory agents (Xanthou 1998). In human newborns, serum immunoglobulin concentrations equal to those of their mothers (Newburg 2005). However, in mice maternal antibodies are mainly acquired by the newborn by intestinal absorption from colostrum or milk and only very limited of maternal antibody is transmitted to the fetus prenatally (Brambell 1970).
Several pathogenic organisms including viruses, such as herpes simplex virus and human papillomavirus, and bacteria such as Chlamydia trachomatis, Neisseria gonorrhoeae and Streptococcus agalactiae (group B streptoccoci) can be present in the birth canal and can infect the newborn at the time of delivery. At that stage maternal antibodies transferred during gestation are the main immunological component protecting the newborn against infection. Defining the role that maternal antibodies play in protection of the newborn against these pathogens at the time of delivery has proven to be a difficult task (Nahmias et al., 1971; Prober et al., 1987, Whitley et al., 1980). Contradictory findings have been reported in the various mouse models of genital infections. For example, Hayashi et al., (1983) indicated that prenatally acquired maternal IgG HSV antibodies protected newborn mice, while Kohl and Loo (1984) only found protection from antibodies present in the maternal breast milk. Interestingly, Evans and Jones (2002) showed that maternal immunization with a HSV-2 replication-defective mutant protected newborn mice against disseminated disease after an oral challenge, but did not protect them against spread of the virus to the central nervous system. Similarly, in Group B streptococcal infection maternal antibodies appear to play a more definitive role in the protection of the newborn (Baker and Kasper 1976; Magliani et al., 1998; Martin et al., 2002).
Few investigators have addressed the role of maternal antibodies in protection of neonates from C. trachomatis infections. Persson et al. (1986) determined maternal chlamydial antibodies in cord sera of 41 infants with nenonatal C. trachomatis conjunctivitis. As a control, they used 16 infants with no conjunctivitis but whose mothers had genital chlamydial genital infection at the time of delivery. Investigators concluded that transferred maternal serum antibodies did not confer significant protection against chlamydial conjunctivitis. Brunham et al. (1990) studied the prevalence of C. trachomatis infection among mothers of children with trachoma and concluded that mothers with antibody titers to the genital serovars D, F, and G were unable to prevent transmission of the genital serovars to their babies. The culture results from the lungs and spleen we report here using the mouse model support these findings in humans suggesting that maternal antibodies do not protect newborn mice against a chlamydial infection at the time of delivery.
In contrast to the culture results from the lungs and spleen, we found a significant difference in the yields of Chlamydia from the intestines of mice born and fed by immunized dams versus those born and fed from sham-immunized dams. This observation suggests that the inhibition of Chlamydia in the intestine was mediated by components present in the maternal breast milk. The identification and characterization of specific and non-specific anti-microbial components in the colostrum and breast milk have been pursued for several years (Hosea-Blewett et al., 2008; Chirico et al., 2008). Ramsey et al. (1998) characterized in vitro the anti-chlamydial activities of colostrum and breast milk from females not infected with Chlamydia. Incubation of chlamydial IFU with colostrums and breast-milk resulted in a significant decrease in infectivity. Therefore, the decrease in the number of IFU recovered from the intestines of the newborns fed by immunized dams reported here maybe the results of both specific and non-specific anti-microbial activity of breast milk. Our data obtained with whey from sham-immunized dams are in agreement with the results of Ramsey et al. (1998).
Of the specific components immunoglobulins represent 88 to 94% of the total milk protein (Chirico et al., 2008; Hosea-Blewett et al., 2008). Among these, secretory IgA (SIgA) is the major immunoglobulin in breast milk and may be the most important component blocking pathogens (Kaetzel et al., 1991). Mucosal defense functions of SIgA have been mostly studied in viral infections. Three mechanisms have been described to account for the antiviral activity of SIgA: immune exclusion, virus excretion, and intracellular neutralization. Of these three mechanisms most likely the one more relevant for chlamydial infections is immune exclusion. Intracelluar neutralization is highly improbable since Chlamydia replicates inside an intracytoplasmic inclusion and therefore, penetration of SIgA in that compartment is unlikely to happen. Excretion from the submucosa is also not a likely possibility since Chlamydia is a mucosal pathogen that does not appear to penetrate into the submucosal layer. Therefore, the finding that the number of chlamydial IFU recovered from the lumen of the small intestine were significantly decreased in the animals born form immunized dams can be explained by postulating that EB are neutralized in the lumen of the intestine by IgA.
In conclusion, maternal immunity to Chlamydia conferred no protection in the lungs and spleen and only limited protection in the gut of neonatal mice challenged intranasally immediately after birth. Although the transfer of maternal antibodies during gestation differs between humans and mice, these results suggest that maternal immunity may not protect newborns from a chlamydial infection at the time of delivery. This murine model of neonatal infection may be used for the development of therapeutic vaccines for treating offspring born from infected mothers. A therapeutic vaccine may be more desirable than antibiotic treatment since it has been shown that, with antibiotics, there is a loss of natural protective immunity against chlamydial infections (Brunham et al., 2005).
Supplementary Material
Acknowledgments
This work was supported by Public Health Service grant AI-64853 to S. Pal and AI-32248 to L. M. de la Maza from the National Institute of Allergy and Infectious Diseases. We thank Dr. Wesley O. Johnson, Professor, Department of Statistic, University of California, Irvine, for his help on multivariate analyses.
Footnotes
Disclosure Statement
All authors disclose that there is no actual or potential conflict of interest including any financial, personal or other relationships with other people or organizations within three (3) years of beginning the work submitted.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adkins B, Du RQ. Newborn mice develop balanced Th1/Th2 primary effector responses in vivo but are biased to Th2 secondary responses. J Immunol. 1998;160:4217–4224. [PubMed] [Google Scholar]
- 2.Baker CJ, Kasper DL. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N Eng J Med. 1976;294:753–756. doi: 10.1056/NEJM197604012941404. [DOI] [PubMed] [Google Scholar]
- 3.Brambell FWR. In: The transmission of passive immunity from mother to young. Neuberger A, Tatum EL, editors. North Holland Publishing Co; Amsterdam, London: 1970. [Google Scholar]
- 4.Brunham RC, Laga M, Simonsen JN, Cameron DW, Peeling R, McDowell J, Pamba H, Ndinya-Achola JO, Maitha G, Plummer FA. The prevalence of Chlamydia trachomatis infection among mothers of children with trachoma. Am J Epidemiol. 1990;132:946–952. doi: 10.1093/oxfordjournals.aje.a115737. [DOI] [PubMed] [Google Scholar]
- 5.Brunham RC, Pourbohloul B, Mak S, White, Rekart R, ML The unexpected impact of a Chlamydia trachomatis infection control program on susceptibility to reinfection. J Infect Dis. 2005;192:1836–1844. doi: 10.1086/497341. [DOI] [PubMed] [Google Scholar]
- 6.Caldwell HD, Kromhout J, Schachter J. Purification and characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chirico G, Marzollo R, Cortinovis S, Fonte C, Gasparoni A. Antiinfective properties of human milk. J Nutr. 2008;138:1801S–1806S. doi: 10.1093/jn/138.9.1801S. [DOI] [PubMed] [Google Scholar]
- 8.Darville T. Chlamydia trachomatis infections in neonates and young children. Semin Pediatr Infect Dis. 2005;16:235–244. doi: 10.1053/j.spid.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Evans IA, Jones CA. Maternal immunization with a herpes simplex virus type 2 replication-defective virus reduces visceral dissemination but not lethal encephalitis in newborn mice after oral challenge. J Infect Dis. 2002;185:1550–60. doi: 10.1086/340572. [DOI] [PubMed] [Google Scholar]
- 10.Forsthuber T, Yip HC, Lehmann PV. Induction of Th1 and Th2 immunity in neonatal mice. Science. 1996;271:1728–1730. doi: 10.1126/science.271.5256.1728. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi Y, Wada T, Mori R. Protection of newborn mice against herpes simplex virus infection by prenatal and postnatal transmission of antibody. J Gen Virol. 1983;64:1007–1012. doi: 10.1099/0022-1317-64-5-1007. [DOI] [PubMed] [Google Scholar]
- 12.Hosea-Blewett HJ, Cicalo MC, Holland CD, Field CJ. Immunological components of human milk. Adv in Food and Nutr Res. 2008;54:45–80. doi: 10.1016/S1043-4526(07)00002-2. [DOI] [PubMed] [Google Scholar]
- 13.Kaetzel CS, Robinson JK, Chintalacharuvu KR, Vaerman J-P, Lamm ME. The polymeric immunoglobulin receptor (secretory component) mediates transport of immune complexes across epithelial cells: A local defense function for IgA. Proc Natl Acad Sci USA. 1991;88:8796–8800. doi: 10.1073/pnas.88.19.8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohl S, Loo LS. The relative role of transplacental and milk immune transfer in protection against lethal neonatal herpes simplex virus infection in mice. J Infect Dis. 1984;149:38–42. doi: 10.1093/infdis/149.1.38. [DOI] [PubMed] [Google Scholar]
- 15.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nature Rev Immunol. 2007;7:379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 16.Magliani W, Polonelli L, Conti S, Salati A, Rocca PF, Cusumano V, Mancuso G, Teti G. Neonatal mouse immunity against group B streptococcal infection by maternal vaccination with recombinant anti-idiotypes. Nat Med. 1998;4:705–709. doi: 10.1038/nm0698-705. [DOI] [PubMed] [Google Scholar]
- 17.Martin D, Rioux S, Gagnon E, Boyer M, Hamel J, Charland N, Brodeur BR. Protection from group B streptococcal infection in neonatal mice by maternal immunization with recombinant Sip protein. Infect Immun. 2002;70:4897–901. doi: 10.1128/IAI.70.9.4897-4901.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison SG, Su H, Caldwell HD, Morrison RP. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4(+) T cells but not CD8(+) T cells. Infect Immun. 2000;68:6979–6987. doi: 10.1128/iai.68.12.6979-6987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nahmias AJ, Josey WE, NAIB ZM, Freeman MG, Fernandez RJ, Wheeler JH. Perinatal risk associated with maternal genital herpes simplex virus infection. Am J Obstetr and Gynecol. 1971;110:825–834. doi: 10.1016/0002-9378(71)90580-1. [DOI] [PubMed] [Google Scholar]
- 20.Nigg C. An unidentified virus which produces pneumonia and systemic infection in mice. Science. 1942;99:49–50. doi: 10.1126/science.95.2454.49-a. [DOI] [PubMed] [Google Scholar]
- 21.Newburg DS. Innate immunity and human milk. J Nutr. 2005;135:1308–1312. doi: 10.1093/jn/135.5.1308. [DOI] [PubMed] [Google Scholar]
- 22.Numazaki K, Asanuma H, Niida Y. Chlamydia trachomatis infection in early neonatal period. BMC Infect Dis. 2003;3:2. doi: 10.1186/1471-2334-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pal S, Fielder TJ, Peterson EM, de la Maza LM. Protection against infertility in a BALB/c mouse salpingitis model by intranasal immunization with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect Immun. 1994;62:3354–3362. doi: 10.1128/iai.62.8.3354-3362.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pal S, Davis HL, Peterson EM, de la Maza LM. Immunization with the Chlamydia trachomatis mouse pneumonitis major outer membrane protein by use of CpG oligodeoxynucleotides as an adjuvant induces a protective immune response against an intranasal chlamydial challenge. Infect Immun. 2002;70:4812–4817. doi: 10.1128/IAI.70.9.4812-4817.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pal S, Bravo J, Peterson EM, de la Maza LM. Protection of wild-type and severe combined immunodeficiency mice against an intranasal challenge by passive immunization with monoclonal antibodies to the Chlamydia trachomatis mouse pneumonitis major outer membrane protein. Infect Immun. 2008;76:5581–5587. doi: 10.1128/IAI.00574-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parr EL, Bozzola JJ, Parr MB. Purification and measurement of secretory IgA in mouse milk. J Immunol Meth. 1995;180:147–157. doi: 10.1016/0022-1759(94)00310-s. [DOI] [PubMed] [Google Scholar]
- 27.Persson K, Ronnerstam R, Svanberg L, Polberger S. Neonatal chlamydial conjunctivitis. Arch Dis Child. 1986;61:565–568. doi: 10.1136/adc.61.6.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prober CG, Sullender WM, Yasukawa LL, Au DS, Yeager AS, Arvin AM. Low risk of herpes simplex virus infections in neonates exposed to the virus at the time of vaginal delivery to mothers with recurrent genital herpes simplex virus infections. N Engl J Med. 1987;316:240–244. doi: 10.1056/NEJM198701293160503. [DOI] [PubMed] [Google Scholar]
- 29.Ramsey KH, Poulsen CE, Motiu PP. The in vitro antimicrobial capacity of human colostrum against Chlamydia trachomatis. J Reprod Immunol. 1998;38:155–67. doi: 10.1016/s0165-0378(98)00010-2. [DOI] [PubMed] [Google Scholar]
- 30.Ridge JP, Fuchs EJ, Matzinger P. Neonatal tolerance revisited: turing on newborn T cells with dendritic cells. Science. 1996;271:1723–1730. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- 31.Schachter J, Caldwell HD. Chlamydiae. Annu Rev Microbiol. 1980;34:285–309. doi: 10.1146/annurev.mi.34.100180.001441. [DOI] [PubMed] [Google Scholar]
- 32.Schagger H, Von Jagow G. Tricine-sodium dodecyl sulphate polyacrylamide gel electrophoresis for the separation of protein range 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 33.Seigrist CA. Neonatal and early life vaccinology. Vaccine. 2001;19:3331–3346. doi: 10.1016/s0264-410x(01)00028-7. [DOI] [PubMed] [Google Scholar]
- 34.Seigrist CA. Mechanisms by which maternal antibodies influence infant vaccine responses: review of hypotheses and definition of main determinants. Vaccine. 2003;21:3406–3412. doi: 10.1016/s0264-410x(03)00342-6. [DOI] [PubMed] [Google Scholar]
- 35.Whitley RJ, Nahmias AJ, Visintine AM, Fleming CL, Alford CA. The natural history of herpes simplex virus infection of mother and newborn. Pediatrics. 1980;66:489–494. [PubMed] [Google Scholar]
- 36.Williams DM, Schachter J, Weiner MH, Grubbs B. Antibody in host defense against mouse pneumonitis agent (murine Chlamydia trachomatis) Infec Immun. 1984;45:674–678. doi: 10.1128/iai.45.3.674-678.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xanthou M. Immune protection of human milk. Biol Neonate. 1998;74:121–33. doi: 10.1159/000014018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


