Abstract
Purpose
To study and correlate corneal sensation in patients with herpes simplex keratitis (HSK) with density and morphology of subbasal corneal nerves by in vivo confocal microscopy (IVCM).
Design
Prospective, cross-sectional, controlled, single-center study.
Participants
Thirty-one eyes with the diagnosis of acute (n=7) or chronic (n=24) HSK and the contralateral clinically unaffected eyes were studied and compared to normal controls (n=15).
Methods
IVCM (Confoscan 4, Nidek) and corneal esthesiometry (Cochet-Bonnet) of the central cornea were performed bilaterally in all patients and controls. Patients were grouped into normal (>5.5 cm), mild (>2.5 to 5.5cm) and severe (≤2.5 cm) loss of sensation.
Main Outcome Measures
Changes in corneal nerve density, total nerve number, main nerve trunks, branching and tortuosity were evaluated after IVCM and correlated to corneal sensation, disease duration, and number of recurrences.
Results
HSK eyes, as compared to controls, demonstrated significant (p<0.001) decrease in mean nerve density (448.9±409.3 vs. 2,258.4±989.0 μm/frame), total nerve number (5.2±4.5 vs. 13.1±3.8), main nerve trunks (2.3±1.6 vs. 4.7±1.2) and nerve branches (3.2 ± 4.3 vs. 9.8±3.3). In contralateral unaffected eyes, mean nerve density (992.7±465.0 μm/frame), total nerve number (7.8±3.3), and branches (4.5±2.3) were significantly decreased as compared to controls (p<0.002). Reduced nerve density, total nerve count and main trunks in HSK eyes were significantly correlated with corneal sensation across all subgroups (p<0.001). Nerve density decreased within days of infection and was correlated to frequency of episodes in patients with HSK (p<0.02).
Conclusions
In vivo confocal microscopy reveals that the loss of corneal sensation in HSK correlates strongly with profound diminishment of the subbasal nerve plexus after herpes simplex virus (HSV) infection. Surprisingly, the contralateral clinically unaffected eyes also demonstrated a diminishment of the subbasal nerve plexus, as compared to normal subjects, revealing bilateral nerve alteration in an apparently unilateral disease.
INTRODUCTION
Herpes simplex keratitis (HSK) is the most common infectious cause of corneal blindness in the industrialized world, primarily because of its recurrent nature.1 Liesegang et al., demonstrated an incidence of 20.7 cases per 100,000 per patient year, and a prevalence of 149 cases per 100,000 population.2 Herpes simplex virus (HSV) has been detected in close to 100% of the population over the age of 60 years by polymerase chain reaction of trigeminal ganglia of cadavers, where HSV remains latently.3
Herpes simplex keratitis often presents clinically with decreased corneal sensation. One of the sequelae of HSK is neurotrophic keratopathy (NTK), resulting from impaired corneal nerve function. NTK may be clinically manifested by persistent corneal epithelial defects that can in turn lead to corneal melts as a result of excessive degradation of underlying stromal collagen, inflammation, and toxicity from topical medication. Finally, advanced stages of NTK present with decreased visual acuity, stromal scarring, corneal neovascularization, and perforation. In individuals with NTK, an insufficient supply of trophic neural factors and impairment of the tear and blink reflex is caused by loss of corneal sensation, which in turn may lead to the development of corneal epithelial defects.4, 5 The management of NTK is challenging, lacking a definitive treatment for advanced stages.
Corneal nerves penetrate the corneal periphery in a radial distribution and form the subbasal nerve plexus between the Bowman’s layer and the basal epithelium. Corneal innervation provides protective and trophic functions, regulates epithelial integrity, proliferation and wound healing.5, 6 Their complex stromal and epithelial branching is not visible by conventional slit-lamp biomicroscopy, but can be visualized by in vivo confocal microscopy (Figure 1).
Figure 1.
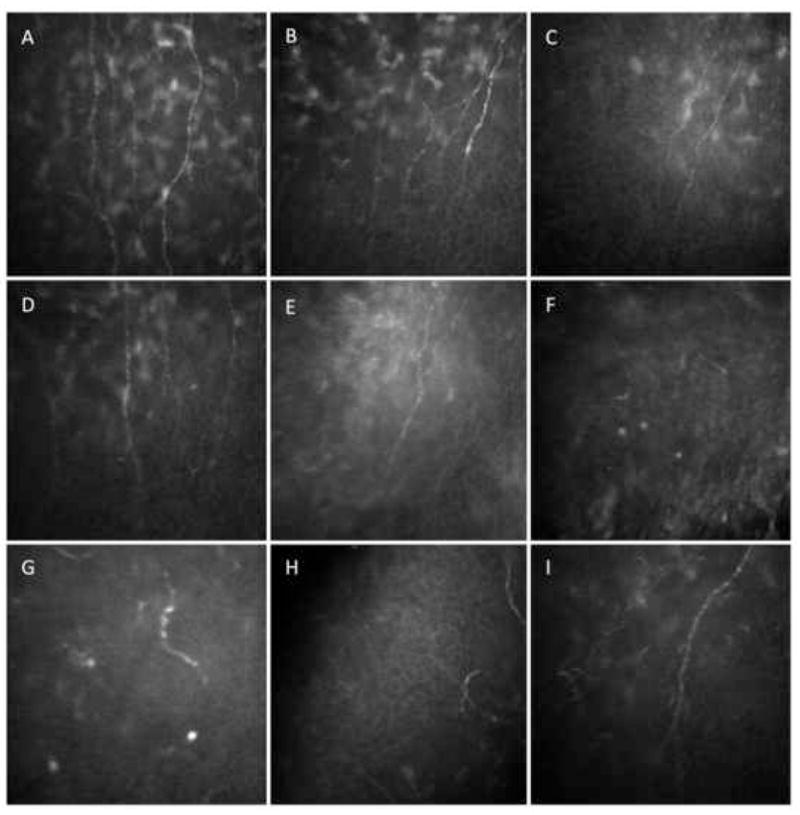
In vivo confocal microscopy at the level of the corneal subbasal nerve plexus in patients with herpes simplex keratitis (HSK). A) Normal cornea. B) HSK with normal sensation with decrease in nerve density. C, D) HSK with mild loss of sensation, decrease in nerve density, number of nerves and branching. E) HSK with severe loss of sensation with decrease in total nerve count, density and branches. F, G, H) HSK with severe loss of sensation. Partially regenerated nerve stubs can be seen, without any branches. I) Contralateral eye of HSK patient.
In vivo confocal microscopy (IVCM) has become an increasingly popular non-invasive tool to image the cornea at the cellular or microstructural level in normal, diseased, and post-surgical eyes, increasing our understanding of corneal nerve anatomy in healthy and diseased human corneas.7 Recent IVCM studies have demonstrated the feasibility of this technology and analyzed the subbasal nerve plexus in normal corneas.5, 8, 9 In addition, studies in patients with keratoconus and dry eye syndrome have revealed a significantly lower subbasal nerve density in the central cornea.10–14 Moreover, IVCM studies in penetrating keratoplasty, LASIK or photorefractive keratectomy (PRK) have documented the loss of subbasal plexus and the regenerative capacity of corneal nerves.15–17 Furthermore, in systemic diseases with peripheral neuropathy such as diabetes, IVCM has been shown to be an accurate non-invasive tool to diagnose and assess the progression of the disease.18
The correlation between corneal innervation and corneal sensation has recently been studied in normal human corneas, as well as in patients with dry eye syndrome, keratoconus, and diabetes.19–23 Further, the association between corneal nerve regeneration and esthesiometry has been observed in penetrating keratoplasty and laser refractive surgery.24, 25 Although loss of sensation is a common feature in HSK, to date there have been no systematic studies evaluating the correlation of corneal nerve density and morphology. The purpose of this study was to correlate the degree of corneal nerve loss and morphological changes in HSK to corneal sensation by in vivo confocal microscopy.
PATIENTS AND METHODS
This was a prospective, cross-sectional study, conducted in a controlled, single-blinded fashion. Thirty-one eyes of 28 patients with diagnosis of HSK (3 bilateral), as well as the contralateral clinically unaffected eyes, were included in the study. Fifteen eyes of 15 normal volunteers constituted the control group. All subjects were recruited from the Cornea Service of the Department of Ophthalmology of the Massachusetts Eye & Ear Infirmary, Boston, Massachusetts, between 2006 and 2008. Subjects with a history of ocular trauma, ocular surgery, contact lens use, or diabetes were excluded from the study. Patients were subdivided into acute HSK (n=7) and chronic HSK (n=24). The diagnosis of acute HSK was made according to epithelial dendritic lesions characteristic of epithelial HSV keratitis, and no stromal involvement. The chronic HSK group included patients that were followed after one or more acute episodes of epithelial or stromal HSV keratitis. Further, patients were divided according to the clinical location of the herpetic lesion, in central and peripheral herpetic keratitis.
Institutional Review Board (IRB)/Ethics Committee approval was obtained. This study was Health Insurance Portability and Accountability Act (HIPAA)-compliant and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all subjects after a detailed explanation of the nature of the study. All patients and normal subjects were examined by slit lamp biomicroscopy. Central corneal sensation was measured bilaterally with a Cochet-Bonnet esthesiometer (Luneau Ophthalmlogie, Chartres, France). This test mechanically stimulates corneal nerves, by pressing a retractable 6 cm length monofilament nylon thread of 0.12 mm diameter against the anterior corneal surface, shortening in steps of 1.0 cm if a positive response is not obtained. If a positive response was obtained, the thread was advanced by 0.5 cm. The longest filament length resulting in a positive response was considered the corneal sensitivity threshold, which was verified 2 times.
HSK patients were grouped into normal (>5.5 cm), mild (>2.5 cm to ≤5.5 cm), and severe (≤2.5 cm) loss of corneal sensation, according to the corneal sensitivity threshold measurements, and results were correlated to corneal nerve density and morphology. In vivo slit scanning confocal microscopy (Confoscan 4; Nidek Technologies, Gamagori, Japan) was performed bilaterally in the central cornea of all subjects. The microscope was equipped with a 40x/0.75 objective lens. One drop of topical anesthesia of 0.5% proparacaine hydrochloride (Alcaine, Alcon) was instilled in both eyes. A drop of 0.3% hypromellose (GenTeal gel, Novartis) was placed on the tip of the objective lens as an optical coupling medium, and the lens was manually advanced until the gel contacted the central surface of the cornea. Full thickness confocal scans were acquired at a speed of 25 frames per second, obtaining 350 images per scan, every 7μm. A second scan was obtained for the anterior cornea obtaining sections every 3μm. Each image represented a coronal section of 460 × 345 μm with a minimum axial step of 1 μm, magnification of 500x and lateral resolution of 1 μm/pixel. A total of 4 to 8 scans were obtained for each cornea by the same experienced operator in all subjects, depending on full thickness or anterior scan mode.
A minimum of 3 representative images of the subbasal nerve plexus were selected for analysis for each eye. Two masked observers evaluated the confocal images for corneal nerve morphology and analyzed the subbasal nerve plexus as described before.16 Briefly, nerve density was assessed by measuring the total length of the nerve fibers in micrometers per frame (158,700 μm2). Main nerve trunks were defined as the total number of main nerves in one image. Nerve branching was defined as the total number of nerve branches in one image. The number of total nerves measured was defined as the number of all nerves, including main nerve trunks and branches in one image. The grade of nerve tortuosity was classified in four grades according to a tortuosity grading scale reported by Oliveira-Soto et al.5 Statistical analysis was performed by student’s T-test, analysis of variance (ANOVA), Pearson correlation coefficient, and multivariate linear regression analysis. Differences were considered statistically significant for p-values less than 0.05. Analyses were performed with SAS software version 9.2 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Thirty-one eyes of 28 patients, with HSK and 25 contralateral unaffected eyes were included for analysis in the study and were compared with 15 eyes of 15 normal volunteers. Of the 28 patients with HSK, 3 had bilateral clinical infection (10.7%).
Eyes with HSK were subdivided further into acute HSK (7 eyes) and chronic HSK (24 eyes), and into central (20 eyes) and peripheral (11 eyes) location of the herpetic lesion. Demographics of the HSK group and control group are presented in Table 1.
Table 1.
Demographic data of normal controls and patients with herpes simplex keratitis.
| Controls | Herpes simplex keratitis | |
|---|---|---|
| Number of patients (n) | 15 | 28 (25 unilateral disease 3 bilateral disease) |
| Age (mean ± SD) (years) | 59 ± 17 | 62.1 ± 19.1 |
| Gender (male/female) | 8/7 | 8/20 |
| Sensation (mean ± SD) (cm) | 6.0 ± 0 | 3.3 ± 1.9 |
| Disease duration (mean ± SD) (years) | - | 14.8 ± 15.1 |
| Number of episodes (mean ± SD) (n) | - | 3.0 ± 2.1 |
| Clinical location of herpetic lesions | - | 20 central/11 peripheral |
Values are mean ± standard deviation (SD).
Of the 28 patients with herpes simplex keratitis, 3 had bilateral clinical infection.
Comparison of Nerve Density and Morphology by IVCM
Mean nerve parameters and corneal sensation for eyes with HSK, contralateral, and normal control groups are reported in Table 2. In HSK eyes the mean nerve density (448.9±409.3 vs. 2258.4±989.0; p< 0.0001), total nerve number (5.2±4.5 vs. 13.1±3.8; p<0.0001), main nerve trunks (2.3±1.6 vs. 4.7±1.2; p<0.0001) and number of branches (3.2±4.3 vs. 9.8±3.3; p<0.0001) were significantly lower as compared to controls. Nerve tortuosity was slightly increased in HSK compared to controls, but did not reach statistical significance (p=0.22). In the contralateral unaffected eyes the mean nerve density (992.7±465.0; p<0.0001), total nerve number (7.8±3.3; p<0.0001), main nerve trunks (3.5±1.6; p<0.01) and number of branches (4.5±2.3; p<0.0001) revealed a significant decrease compared to controls. These results demonstrate not only a diminishment of the subbasal corneal nerve plexus in eyes with HSK, but also a diminishment in the contralateral unaffected eyes of patients.
Table 2.
Corneal sensation and corneal subbasal nerve plexus parameters in herpes simplex keratitis, contralateral clinically unaffected eyes, and control groups.
| HSK patients |
Controls | ||
|---|---|---|---|
| HSK eye | Contralateral unaffected eye | ||
| Eyes (n) | 31 | 25 | 15 |
| Mean central corneal sensation (cm) | 3.4 ± 1.9* | 5.6 ± 0.6 | 6.0 ± 0 |
| Mean subbasal nerve density (μm) | 448.9 ± 409.3* | 992.7 ± 465.0* | 2258.4 ± 989.0 |
| Total nerves (n) | 5.2 ± 4.5* | 7.8 ± 3.3* | 13.1 ± 3.8 |
| Main nerve trunks (n) | 2.3 ± 1.6* | 3.5 ± 1.6* | 4.7 ± 1.2 |
| Total nerve branches | 3.2 ± 4.3* | 4.5 ± 2.3* | 9.8 ± 3.3 |
| Grade of tortuosity | 1.74 ± 0.8 | 1.9 ± 0.6 | 1.6 ± 0.3 |
Values reported as mean ± standard deviation. HSK: herpes simplex keratitis.
Statistically significant (p<0.05) compared to controls.
Correlation of Corneal Sensation and Subbasal Corneal Nerve Alterations
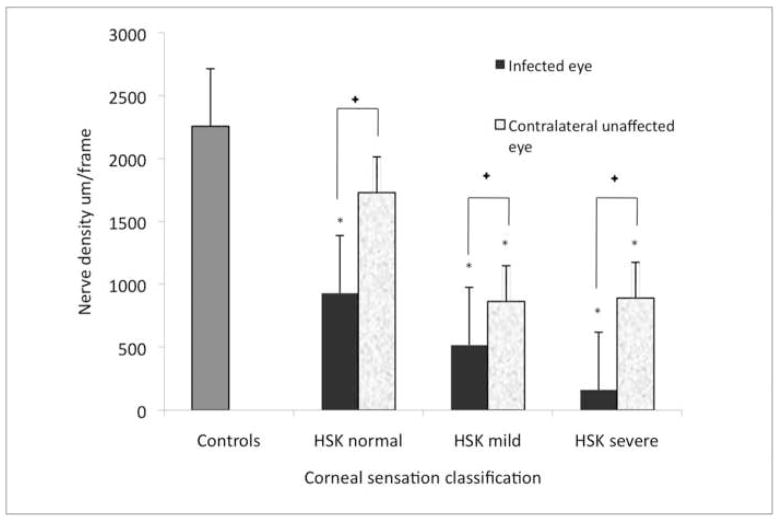
To correlate corneal sensation to IVCM findings, 31 eyes with HSK were subcategorized based on central corneal sensation into 3 groups. Eyes were classified as normal sensation, mild sensation loss and severe sensation loss. This grouping allowed for comparison of affected and unaffected eyes between groups and their comparison to normal controls. The correlation of corneal sensation loss to subbasal nerve alterations for all groups, are presented in Table 3 (available at http://aaojournal.org). IVCM scans in all 3 groups (normal, mild, and severe sensation loss) revealed a significant decrease in mean nerve density (p<0.003, p<0.0001, p<0.0001, respectively), total nerve number (p<0.002, p<0.0001, p<0.0001, respectively), main nerve trunks (p=0.2, p<0.0001, p<0.0001, respectively) and number of branches (p<0.004, p<0.0003, p<0.0001, respectively) as compared to the normal control group (Figure 1). The differences in corneal nerve parameters between the HSK sub-groups were significant (p<0.05) (Table 3 available at http://aaojournal.org, Figure 2).
Table 3.
Corneal subbasal nerve alterations in the control group and in herpes simplex keratitispatients according to grade of hypoesthesia.
| Controls and HSK eyes by Sensitivity Subcategory | Eyes n (%) | Age | Mean central corneal sensitivity (cm) | Mean subbasal nerve density (μm/μm2) | Total nerves (n) | Main nerve trunks (n) | Total branches (n) |
|---|---|---|---|---|---|---|---|
| Controls | 15 | 59±17 | 6.0 ± 0 | 2258.4 ±989.0 | 13.1 ± 3.8 | 4.7 ± 1.2 | 9.8 ± 3.3 |
| HSK Normal sensation (>5.5cm) | 6 (19%) | 43.2 ± 23.1 | 6.0 ± 0 | 930.9 ± 312.2* (p<0.003) | 7.5 ± 3.0* (p<0.002) | 3.9 ± 1.8 (p=0.2) | 3.6 ± 1.4* (p<0.004) |
| HSK Mild hypoesthesia (>2.5cm to ≤5.5cm) | 12 (39%) | 66.5 ± 14 | 4.1 ± 0.9* (p<0.0001) | 518.8 ± 394.6* (p<0.0001) | 5.2 ± 2.8* (p<0.0001) | 2.3 ± 1.2* (p<0.0001) | 4.7 ± 6.1* (p<0.0003) |
| HSK Severe hypoesthesia (≤2.5cm) | 13 (42%) | 66.7 ± 17 | 1.4 ± 1.0* (p<0.0001) | 161.8 ± 158.8* (p<0.0001) | 3.1 ± 2.7* (p<0.0001) | 1.5 ± 1.3* (p<0.0001) | 1.5 ± 2.3* (p<0.0001) |
|
p-value within HSK sensation groups: |
|||||||
| normal - mild | p<0.004 | p<0.0002 | p<0.01 | p<0.04 | p<0.003 | p=0.32 | |
| normal - severe | p<0.02 | p<0.0001 | p<0.001 | p<0.002 | p<0.001 | p<0.002 | |
| severe-mild | p=0.48 | p<0.0001 | p<0.004 | p<0.04 | p=0.059 | p<0.008 | |
Herpes simplex keratitis (HSK) patients were grouped into normal (>5.5 cm), mild (>2.5 cm to ≤5.5 cm), and severe (≤2.5 cm) loss of corneal sensation according to the corneal sensitivity threshold measurements with the Cochet-Bonnet esthesiometer. Values reported as mean ± standard deviation. Statistical analysis performed by ANOVA (analysis of variance between groups).
Statistically significant compared to controls, P-values shown in parenthesis. P-values compared within sensation groups at the bottom of the table.
Figure 2.
Corneal nerve density in herpes simplex keratitis (HSK) according to corneal sensation loss classification and their respective contralateral clinically unaffected eye. Error bars represents standard error from the mean. *p<0.01 compared to control group. +p<0.02 between HSK eye and the unaffected eye in normal sensation, mild sensation loss and severe sensation loss. Statistiscal analysis by ANOVA (analysis of variance between groups).
Acute versus Chronic Herpes Simplex Keratitis
Significant changes in nerve morphology and density, as compared to normal controls, were noted within days after the onset of disease. However, when we analyzed nerve alterations between acute and chronic HSK eyes, no statistical difference was detected, indicating that the changes noted in patients with HSK are induced during the acute phase of disease and are long-lasting.
Central versus Peripheral Location of Herpes Keratitis Lesions
Stratification of HSK patients based on central versus peripheral location of HSK lesions, demonstrated no statistically significant changes in the affected or contralateral eyes, for sensation or nerve alterations, although a trend towards lower nerve density was noted in eyes with central lesions.
Correlation and Regression Analysis
Pearson correlation was performed to determine significant relationships between the IVCM findings and patient age, number of recurrences, and total length of disease. We found a significant correlation between patient age and the reduction of nerve density and main nerve trunks, (R= −0.45, p<0.02; R= −0.41, p<0.02, respectively). To rule out patient age as a factor in the decrease in sensation or the diminishment of the subbasal nerve plexus, the data was adjusted for age.
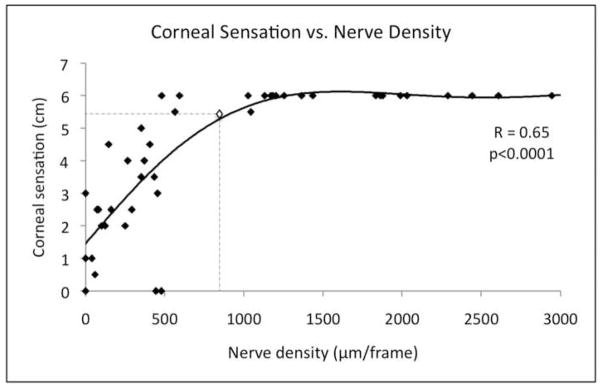
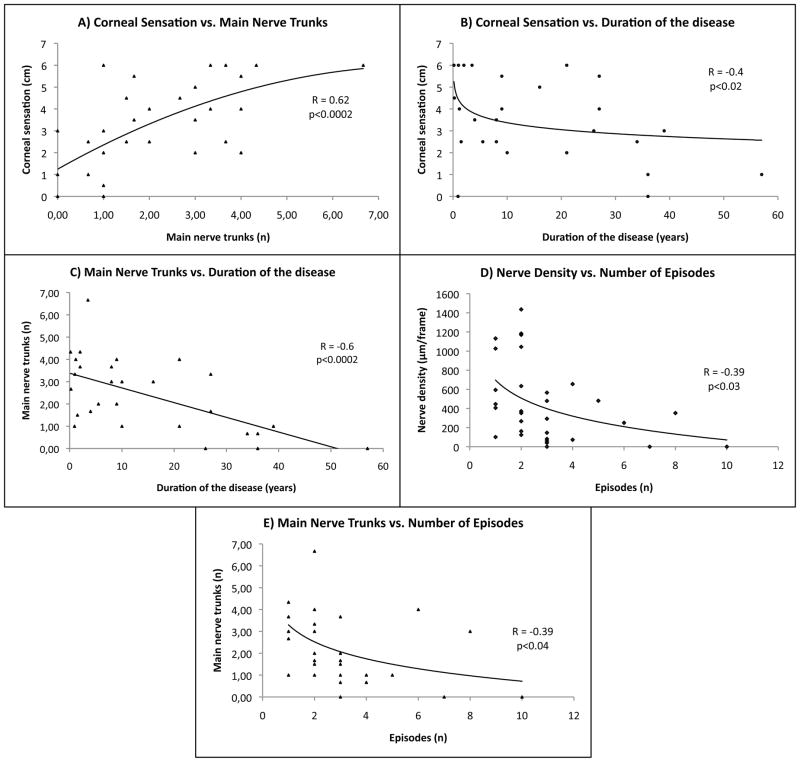
In HSK eyes, corneal sensation was significantly correlated to nerve density and main nerve trunks (R=0.65, p<0.0001; R=0.62, p<0.0002, respectively) (Figures 3 and 4A available at http://aaojournal.org). In addition, long-term disease duration was inversely correlated to corneal sensation (R= −0.42, p<0.02) and main nerve trunks (R= −0.6, p<0.0002) (Figures 4B-C available at http://aaojournal.org). Further, we found a significantly negative correlation between the number of total episodes and nerve density (R= −0.39, p<0.03) and main nerve trunks (R= −0.39, p<0.04) (Figure 4D-E available at http://aaojournal.org). In the contralateral unaffected eyes of HSK patients, corneal sensation was significantly correlated to the number of total nerves and main nerve trunks as well (R=0.43, p<0.04; R=0.5, p<0.03, respectively). We also found that the number of total episodes correlated to a decrease in corneal sensation in the contralateral unaffected eyes. (R= −0.44, p<0.04).
Figure 3.
Corneal sensation is significantly correlated to nerve density. Dashed lines show the cutoff point calculated through quantitative regression of corneal sensation from nerve density. Abnormal sensation (≤ 5.5cm) is noted with a nerve density of 835 μm/frame or lower. Multivariate regression factor and p-values are showed.
Figure 4.
Stascal significant correlaons affecng corneal nerve parameters and/or corneal sensaon adjusted by age. Mulvariate regression factor and p-values are shown. Corneal sensaon is significantly correlated to main nerve trunks (3A) and to the duraon of the disease (3B). The number of main nerve trunks is significantly affected by the duraon of the disease (3C) and the number of herpes simplex keras (HSK) episodes (3D). The number of main nerve trunks is significantly correlated to the number of HSK episodes (3E).
Moreover, in order to calculate the approximate corneal nerve density needed for normal sensation, quantitative regression analysis was applied. Results demonstrated that abnormal sensation (≤5.5cm) is noted with a nerve density of 835 μm/frame or lower (Figure 3). Finally, in order to confirm the appropriate sample size for our study, we performed power calculations, which demonstrated a power of >98% for all statistically significant variables.
DISCUSSION
The assessment of corneal innervation, until recently, has only been possible through measurement of corneal sensation. The use of in vivo confocal microscopy now allows systematic studies of corneal nerve morphology and density in normal subjects and patients. This method is rapid, noninvasive, and precise, with good interobserver variability. Our study presented herein, is the largest IVCM study of corneal nerves in patients with herpes simplex keratitis, correlating IVCM findings to corneal sensation and clinical history of HSK. In addition to studying the eyes with HSK, our data compares diseased eyes with unaffected contralateral eyes, as well as to normal controls.
Previously, Rosenberg et al. have described pathologic corneal changes by IVCM in 16 patients with a history of HSV keratitis as compared to their contralateral eyes finding no significant difference in the subbasal nerve plexus between the two eyes. However, there was no normal control group unaffected by HSK in this study.26 However, our current study demonstrates that a significant reduction of the corneal subbasal nerve plexus is present not only in eyes with HSK, but surprisingly also in unaffected contralateral eyes, as compared to normal controls. However, nerve alterations were not as severe in contralateral unaffected eyes, as compared to eyes with HSK. Although Martone et al,27 had described bilateral subbasal nerve changes in patients with bilateral HSK, our study is the first to show a decrease of subbasal nerves in clinically unaffected contralateral eyes as compared to normal controls. This finding explains why the previous study by Rosenberg et al did not find a significant difference in subbasal nerve changes between the affected and unaffected contralateral eyes.
The incidence of bilateral HSK has been reported to be between 1.3% to 12%.1, 28, 29 The contralateral subbasal nerve plexus reduction described herein, could theoretically reflect asymmetric bilateral disease without clinically evident manifestations. It has been well established that HSV, once entering through a facial portal, travels by retrograde axoplasmic flow to the trigeminal ganglia, the main source of virus in recurrent disease.30, 31 It is theoretically possible that HSV travels peripherally between nerve anastomosis or from the trigeminal ganglia to the mesencephalic trigeminal nucleus, causing contralateral damage to the distal nerve plexus despite lack of contralateral clinical manifestations. However, contralateral nerve alterations might also be caused by central neural downregulation in the contralateral eye. Current studies are currently underway in the laboratory to elucidate this mechanism.
In the present study, we further show that reduced corneal subbasal nerve density, total nerve count and main nerve trunks were significantly correlated with the loss of corneal sensation in eyes with HSK. Although previous studies have correlated corneal innervation with corneal sensation in patients with dry eye, diabetes mellitus or refractive surgery, to our knowledge, this study is the first that correlates the morphological and quantitative findings of the corneal subbasal nerve plexus with corneal sensation in herpes simplex keratitis patients.
In contrast to our present findings in HSK, Patel et al. found no strong correlation between the density of the subbasal nerve plexus and corneal sensation in healthy corneas.19 As we have demonstrated, a nerve density of 1064 μm/frame is sufficient for a patient to have near normal sensation (Figure 3). Therefore, it is not surprising that in the study by Patel et al., no correlation was demonstrated, as normal subjects have a nerve density much higher than this. Nevertheless, the lack of correlation in their study could potentially be due to different esthesiometers employed to evaluate corneal sensation as well. Moreover, the fact that abnormal sensation is noted with a nerve density of 835 μm/frame or lower, explains why the sensation in the contralateral unaffected eyes was perceived as normal, despite significant decrease in nerve density and number.
In our study, we found a significant and inverse correlation between age and the reduction of nerve density and main nerve trunks. Decreased nerve density by IVCM32, as well as a higher incidence of HSK has been described in older patients.33 Therefore, in order to rule out age as a confounding factor in our study, results were adjusted for age. After adjustment for age, we found that in HSK patients the loss of corneal sensation correlated strongly with decreased nerve density and numbers.
Another important facet in loss of sensation and subbasal nerves was the clinical course of the disease. We observed that the total duration of the disease correlated with progressive loss of sensation and main nerve trunks. In addition, the total number of recurrences, correlated with decrease in nerve density and main nerve trunks. These findings may suggest that more aggressive treatment in patients with frequent number of recurrences or longer disease duration may be warranted in order to prevent sequelae of neurotrophic keratopathy.
Interestingly, when we compared acute to chronic cases of HSK, we found very similar alterations of the subbasal nerve plexus regardless of onset of disease, when grouping patients according to sensation. Induced changes in nerve morphology and density were evident within days after the onset of the disease. These findings demonstrate that the main insult or loss of the subbasal nerve plexus takes place immediately after the acute HSV infection, but continues gradually. However, longitudinal studies, following the same patients over time would need to be performed to corroborate this data.
The significant reduction of corneal nerve fibers in patients with HSK implies not only loss of sensation in these patients, as we have demonstrated, but may potentially affect other layers of the cornea. Quantification of corneal innervation by IVCM demonstrates an objective methodology for monitoring patients with HSK, potentially predicting the risk for NTK. Since NTK initially often lacks of signs and symptoms, preventing epithelial breakdown is crucial, as this can progress to inflammation, corneal melting and corneal perforation.
A limitation of the current study is the evaluation of only the center of the cornea for corneal sensitivity and for in vivo confocal microscopy. One cannot necessarily extrapolate our findings to the peripheral cornea. In addition, poor topographic reproducibility and the difficulty to ensure the exact same locations tested with both techniques are currently not optimal. Further, the Cochet-Bonnet esthesiometer, although considered the gold standard for measuring corneal sensation, only measures mechanical nociceptors.19
In conclusion, IVCM enables a direct and reproducible comparison between corneal sensory innervation and sensation as measured by esthesiometry, and demonstrates strong correlation between corneal sensation and subbasal nerve plexus changes. Based on our findings of bilateral corneal nerve changes, comparison between affected and unaffected eyes for determination of sensation loss needs to be reconsidered, as it could lead to erroneous results. Rather, quantitative measurement of corneal sensation is suggested (e.g., by the Cochet-Bonnet esthesiometer or the Belmonte esthesiometer).34 Currently, new surgical and pharmacological treatments are being investigated for nerve regeneration in NTK.4 IVCM could allow objective evaluation of treatment response through quantification of cellular and nerve changes in the cornea.
Acknowledgments
Financial Support: NIH K12-EY016335, NIH K24-EY019098, New England Corneal Transplant Research Fund, Falk Medical Research Foundation.
The funding organizations had no role in the design or conduct of this research.
Footnotes
Conflict of Interest: The authors have no financial/conflicting interests to disclose.
This work was presented in part at the Association of Research in Vision and Ophthalmology in Ft. Lauderdale, Florida, April 2007, and at the Ocular Microbiology and Immunology Group in New Orleans, Louisiana, November 2007.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20:1–13. doi: 10.1097/00003226-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Liesegang TJ, Melton LJ, III, Daly PJ, Ilstrup DM. Epidemiology of ocular herpes simplex: incidence in Rochester, Minn, 1950 through 1982. Arch Ophthalmol. 1989;107:1155–9. doi: 10.1001/archopht.1989.01070020221029. [DOI] [PubMed] [Google Scholar]
- 3.Liedtke W, Opalka B, Zimmermann CW, Lignitz E. Age distribution of latent herpes simplex virus 1 and varicella-zoster virus genome in human nervous tissue. J Neurol Sci. 1993;116:6–11. doi: 10.1016/0022-510x(93)90082-a. [DOI] [PubMed] [Google Scholar]
- 4.Nishida T, Yanai R. Advances in treatment for neurotrophic keratopathy. Curr Opin Ophthalmol. 2009;20:276–81. doi: 10.1097/icu.0b013e32832b758f. [DOI] [PubMed] [Google Scholar]
- 5.Oliveira-Soto L, Efron N. Morphology of corneal nerves using confocal microscopy. Cornea. 2001;20:374–84. doi: 10.1097/00003226-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Guthoff RF, Wienss H, Hahnel C, Wree A. Epithelial innervation of human cornea: a three-dimensional study using confocal laser scanning fluorescence microscopy. Cornea. 2005;24:608–13. doi: 10.1097/01.ico.0000154384.05614.8f. [DOI] [PubMed] [Google Scholar]
- 7.Patel DV, McGhee CN. In vivo confocal microscopy of human corneal nerves in health, in ocular and systemic disease, and following corneal surgery: a review. Br J Ophthalmol. 2009;93:853–60. doi: 10.1136/bjo.2008.150615. [DOI] [PubMed] [Google Scholar]
- 8.Grupcheva CN, Wong T, Riley AF, McGhee CN. Assessing the sub-basal nerve plexus of the living healthy human cornea by in vivo confocal microscopy. Clin Experiment Ophthalmol. 2002;30:187–90. doi: 10.1046/j.1442-9071.2002.00507.x. [DOI] [PubMed] [Google Scholar]
- 9.Patel DV, McGhee CN. Mapping of the normal human corneal sub-basal nerve plexus by in vivo laser scanning confocal microscopy. Invest Ophthalmol Vis Sci. 2005;46:4485–8. doi: 10.1167/iovs.05-0794. [DOI] [PubMed] [Google Scholar]
- 10.Patel DV, McGhee CN. Mapping the corneal sub-basal nerve plexus in keratoconus by in vivo laser scanning confocal microscopy. Invest Ophthalmol Vis Sci. 2006;47:1348–51. doi: 10.1167/iovs.05-1217. [DOI] [PubMed] [Google Scholar]
- 11.Simo Mannion L, Tromans C, O’Donnell C. An evaluation of corneal nerve morphology and function in moderate keratoconus. Cont Lens Anterior Eye. 2005;28:185–92. doi: 10.1016/j.clae.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Tuominen IS, Konttinen YT, Vesaluoma MH, et al. Corneal innervation and morphology in primary Sjögren’s syndrome. Invest Ophthalmol Vis Sci. 2003;44:2545–9. doi: 10.1167/iovs.02-1260. [DOI] [PubMed] [Google Scholar]
- 13.Benitez del Castillo JM, Wasfy MA, Fernandez C, Garcia-Sanchez J. An in vivo confocal masked study on corneal epithelium and subbasal nerves in patients with dry eye. Invest Ophthalmol Vis Sci. 2004;45:3030–5. doi: 10.1167/iovs.04-0251. [DOI] [PubMed] [Google Scholar]
- 14.Hosal BM, Ornek N, Zilelioglu G, Elhan AH. Morphology of corneal nerves and corneal sensation in dry eye: a preliminary study. Eye (Lond) 2005;19:1276–9. doi: 10.1038/sj.eye.6701760. [DOI] [PubMed] [Google Scholar]
- 15.Niederer RL, Perumal D, Sherwin T, McGhee CN. Corneal innervation and cellular changes after corneal transplantation: an in vivo confocal microscopy study. Invest Ophthalmol Vis Sci. 2007;48:621–6. doi: 10.1167/iovs.06-0538. [DOI] [PubMed] [Google Scholar]
- 16.Calvillo MP, McLaren JW, Hodge DO, Bourne WM. Corneal reinnervation after LASIK: prospective 3-year longitudinal study. Invest Ophthalmol Vis Sci. 2004;45:3991–6. doi: 10.1167/iovs.04-0561. [DOI] [PubMed] [Google Scholar]
- 17.Erie JC, McLaren JW, Hodge DO, Bourne WM. Recovery of corneal subbasal nerve density after PRK and LASIK. Am J Ophthalmol. 2005;140:1059–64. doi: 10.1016/j.ajo.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 18.Malik RA, Kallinikos P, Abbott CA, et al. Corneal confocal microscopy: a non–invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia. 2003;46:683–8. doi: 10.1007/s00125-003-1086-8. [DOI] [PubMed] [Google Scholar]
- 19.Patel DV, Tavakoli M, Craig JP, et al. Corneal sensitivity and slit scanning in vivo confocal microscopy of the subbasal nerve plexus of the normal central and peripheral human cornea. Cornea. 2009;28:735–40. doi: 10.1097/ICO.0b013e318193e0e3. [DOI] [PubMed] [Google Scholar]
- 20.Benítez-Del-Castillo JM, Acosta MC, Wassfi MA, et al. Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye. Invest Ophthalmol Vis Sci. 2007;48:173–81. doi: 10.1167/iovs.06-0127. [DOI] [PubMed] [Google Scholar]
- 21.Tuisku IS, Konttinen YT, Konttinen LM, Tervo TM. Alterations in corneal sensitivity and nerve morphology in patients with primary Sjögren’s syndrome. Exp Eye Res. 2008;86:879–85. doi: 10.1016/j.exer.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Patel DV, Ku JY, Johnson R, McGhee CN. Laser scanning in vivo confocal microscopy and quantitative aesthesiometry reveal decreased corneal innervation and sensation in keratoconus. Eye (Lond) 2009;23:586–92. doi: 10.1038/eye.2008.52. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg ME, Tervo TM, Immonen IJ, et al. Corneal structure and sensitivity in type 1 diabetes mellitus. Invest Ophthalmol Vis Sci. 2000;41:2915–21. [PubMed] [Google Scholar]
- 24.Richter A, Slowik C, Somodi S, Vick HP, Guthoff R. Corneal reinnervation following penetrating keratoplasty--correlation of esthesiometry and confocal microscopy. Ger J Ophthalmol. 1996;5:513–7. [PubMed] [Google Scholar]
- 25.Linna TU, Vesaluoma MH, Perez-Santonja JJ, et al. Effect of myopic LASIK on corneal sensitivity and morphology of subbasal nerves. Invest Ophthalmol Vis Sci. 2000;41:393–7. [PubMed] [Google Scholar]
- 26.Rosenberg ME, Tervo TM, Müller LJ, et al. In vivo confocal microscopy after herpes keratitis. Cornea. 2002;2:265–9. doi: 10.1097/00003226-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Martone G, Alegente M, Balestrazzi A, et al. In vivo confocal microscopy in bilateral herpetic keratitis: a case report. Eur J Ophthalmol. 2008;18:994–7. doi: 10.1177/112067210801800622. [DOI] [PubMed] [Google Scholar]
- 28.Souza PM, Holland EJ, Huang AJ. Bilateral herpetic keratoconjunctivitis. Ophthalmology. 2003;110:493–6. doi: 10.1016/S0161-6420(02)01772-4. [DOI] [PubMed] [Google Scholar]
- 29.Wilhelmus KR, Falcon MG, Jones BR. Bilateral herpetic keratitis. Br J Ophthalmol. 1981;65:385–7. doi: 10.1136/bjo.65.6.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bastian FO, Rabson AS, Yee CL, Tralka TS. Herpesvirus hominus: isolation from human trigeminal ganglion. Science. 1972;178:306–7. doi: 10.1126/science.178.4058.306. [DOI] [PubMed] [Google Scholar]
- 31.Baringer J, Swoveland P. Recovery of herpes-simplex virus from human trigeminal ganglions. N Engl J Med. 1973;288:648–50. doi: 10.1056/NEJM197303292881303. [DOI] [PubMed] [Google Scholar]
- 32.Niederer RL, Perumal D, Sherwin T, McGhee CN. Age-related differences in the normal human cornea: a laser scanning in vivo confocal microscopy study. Br J Ophthalmol. 2007;91:1165–9. doi: 10.1136/bjo.2006.112656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Messaoudi I, Lemaoult J, Guevara-Patino JA, et al. Age-related CD8 T cell clonal expansions constrict CD8 T cell repertoire and have the potential to impair immune defense. J Exp Med. 2004;200:1347–58. doi: 10.1084/jem.20040437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belmonte C, Acosta MC, Schmelz M, Gallar J. Measurement of corneal sensitivity to mechanical and chemical stimulation with a CO2 esthesiometer. Invest Ophthalmol Vis Sci. 1999;40:513–9. [PubMed] [Google Scholar]