Abstract
Calcineurin subunit isoforms are implicated in long term potentiation, long term depression, and structural plasticity. Calcineurin inhibitors benefit axonal damage, cellular dysfunction, and cognitive outcomes in animal models of traumatic brain injury (TBI). Distribution of the catalytic calcineurin A subunit is altered and calcineurin activity increased following fluid percussion injury. Alterations in calcineurin subunit A isoform distribution within the hippocampus also occur post controlled cortical impact (CCI) demonstrating a reduction in catalytic subunit distribution in CA1-2 dendritic fields. Furthermore the effect of TBI on the regulatory subunit, calcineurin B, is unknown. Understanding the role of both subunits is necessary to effectively target alterations in calcineurin signaling as current calcineurin inhibitors, such as cyclosporin A and FK-506, rely upon binding sites on both subunits for complete inhibition. The effect of moderate CCI on the expression and distribution of calcineurin B isoforms within the hippocampus was examined at 2 hours and 2 weeks post injury. Calcineurin B isoforms showed increased expression throughout the CA1 and CA2 while there was a decrease in expression within the ipsilateral dentate gyrus. Alterations in CnB isoform expression within the CA1, CA1-2, and dentate gyrus have significant implications for persistent hippocampal dysfunction following TBI. Regional changes in regulatory subunit expression may alter the effect of calcineurin inhibitors regionally following a traumatic brain injury.
Keywords: Calcineurin, TBI, hippocampus, CCI
1. INTRODUCTION
Traumatic brain injury (TBI) leads to significant behavioral, physical, and cognitive disability. Neuronal cell death occurs both at the site of contusion and in secondary areas due to excitotoxicity and oxidative stress (DeKosky et al., 1998; Kochanek, 1993). Animal models of brain injury have consistently demonstrated cellular death and dysfunction in the hippocampus (Colicos et al., 1996; Tehranian et al., 2008). In addition there is persistent dysfunction in hippocampal potentiation (Sanders et al., 2000). However, the cellular mechanisms which contribute to deficits are not well understood. Protein phosphorylation is a dynamic, rapidly reversible post translational modification, known to be involved in neuronal activation, plastic remodeling, and memory.
Calcineurin (CaN), also known as protein phosphatase-2B (PP2B), is a calcium/calmodulin dependent phosphatase that is highly sensitive to, and preferentially activated by, minor changes in intracellular calcium (Ca2+) (Rusnak and Mertz, 2000). CaN dephosphorylates several key cytoskeletal and synaptic vesicle proteins (Rusnak and Mertz, 2000), modulates the activity of the transcription factors, nuclear factor of activated T-cells (NFAT) and cAMP response element binding (CREB) (Yang and Klee, 2000), and regulates neuronal excitability through modulation of gamma-aminobutyric acid (GABA) (Huang and Dillon, 1998) and N-methyl-D-aspartic acid (NMDA) receptors (Tong et al., 1995).
CaN is composed of a 57–61kDA catalytic subunit (CnA) and a 19kDA regulatory subunit (CnB) (Rusnak and Mertz 2000; Groth et al., 2003; Klee et al., 1979). Following neuronal activation, Ca2+ binds to high affinity EF-hand Ca2+ binding motifs (specialized protein structures composed of a loop-helix-loop conformation) on the CnB subunit, allowing calmodulin (CaM) association with CaM binding domains on CnA (Griffith et al., 1995). This Ca2+ induced interaction of CnB with CnA results in conformational changes in the protein, removal of an autoinhibitory domain and exposure of the CaN active site needed for full activity of the phosphatase (Rusnak and Mertz 2000; Griffith et al., 1995; Guerini and Klee, 1989).
Pathologically, excessive CaN has been linked to mitochondrial dysfunction, and apoptosis (Asai et al., 1999). Alterations in CaN subunit A subcellular distribution (Kurz et al., 2005a) have been reported in the hippocampus following fluid-percussion (FP) TBI which can persist for 2–3 weeks following injury. Following a moderate controlled cortical impact (CCI) injury there are reductions in CaN A isoform distribution within injured hippocampal CA1 and CA2 dendritic fields, with increases in CaN A isoforms in the dentate gyrus (Bales et al., 2010). CaN activity following TBI is generally considered to be pathologically altered and has been associated with increases in cellular death and dysfunction in both ischemia and TBI (Morioka et al., 1999). Inhibition of CaN activity is neuroprotective in ischemia and TBI models (Butcher et al., 1997; Friberg et al., 1998; Okonkwo et al., 1999; Okonkwo et al., 2003).
Both the CnA and CnB subunits consist of several different isoforms (CnAα, CnA β, CnAγ, and CnB1 and CnB2 respectively), which may offer unique substrate specificity, localization and/or recognition properties to the phosphatase.
Mice, which have a selective, forebrain specific knockout of the CnB subunit show impaired LTD and working memory, with LTP, extinction, and memory for multi-trial tasks remaining intact (Zeng et al., 2001). Forebrain CnB knockout mice also show behavioral abnormalities similar to those found in schizophrenia (Miyakawa et al., 2003).
While there is a recognized alteration in CnA subunit isoform distribution within the hippocampus following CCI, little is known about the expression of the CnB isoforms in the hippocampus and how they are altered by trauma. The aims of this study were to determine how the CnB isoforms are distributed in the rat hippocampus and to elucidate how CnB isoforms are affected acutely and chronically following TBI within separate regions of the hippocampus.
2. RESULTS
CnB Isoform Distribution in Rat Hippocampus
CnB isoform distribution within the rat hippocampus was determined utilizing immunohistochemical staining in sham rats. Both isoforms CnB1 (Sham groups in Figs. 1, 3) and CnB2 (Sham groups in Figs. 4, 6) showed similar distribution patterns with higher expression in the CA1 and CA1-2 neuropil and cell bodies than CA3 regions. Within the CA3 the CnB1 and CnB2 isoforms (Sham Figs. 1, 3) appear to be predominantly in the stratum pyramidale with little expression within the dendritic and axonal layers of the stratum radiatum and stratum oriens. In general the two CnB isoforms (Sham Figs. 4, 6) show relatively similar expression within the CA1, CA1-2, and CA3 regions of the hippocampus (Sham Figs. 1, 3). Both isoforms (Sham Figs. 1, 3, 4, 6) also show marked staining within the exposed blade of the dentate gyrus (DG) in both the cell bodies of the stratum granulosum and neuropil of the stratum moleculare layers. There was relatively little expression within the DG hidden blade or hilus of either the CnB1 (Sham Figs. 1, 3) or CnB2 isoforms (Sham Figs. 4, 6).
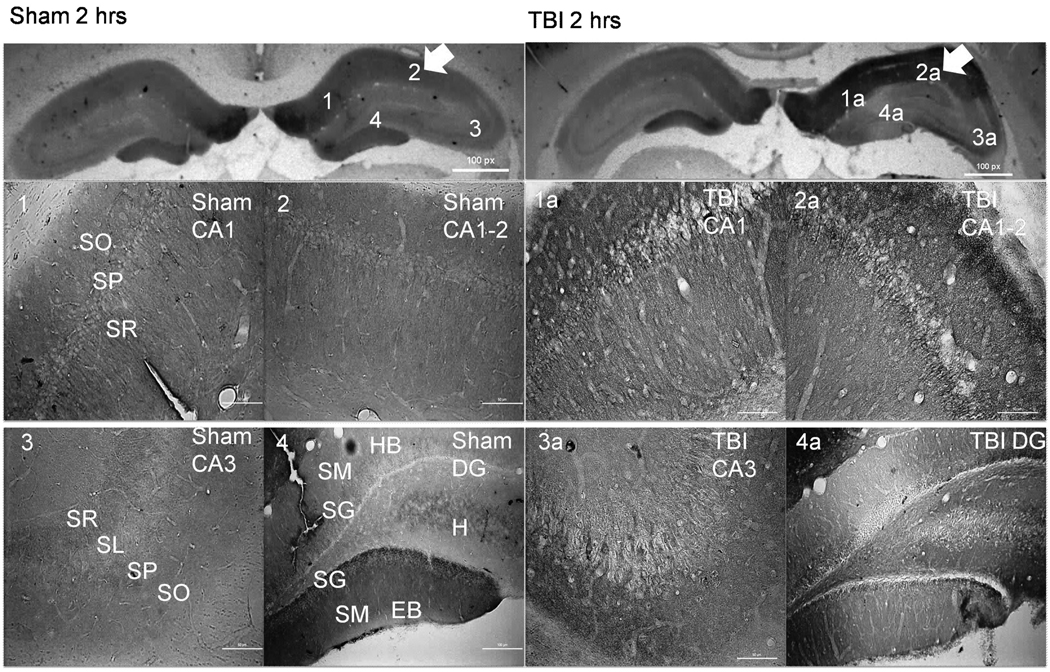
Figure 1.
CnB1 immunohistochemistry demonstrating alterations in CnB1 isoform distribution 2 hours following TBI (right panels). Arrows indicate side of cortex ipsilateral to injury or sham surgery. There is a noticeable increase of staining within CA1 and CA1-2 in TBI (1a and 2a) versus sham (1 and 2). There is a significant loss of CnB1 expression within the EB of the DG in TBI (4a) versus sham (4). Scale bars, 50 µm for regional sections. Abbreviations: SO= Stratum Oriens; SP= Stratum Pyramidale; SR= Stratum Radiatum; SL= Stratum Lucidum; SM= Stratum Moleculare; SG= Stratum Granulosum; HB= Hidden Blade; H=Hilus; EB= Exposed Blade.
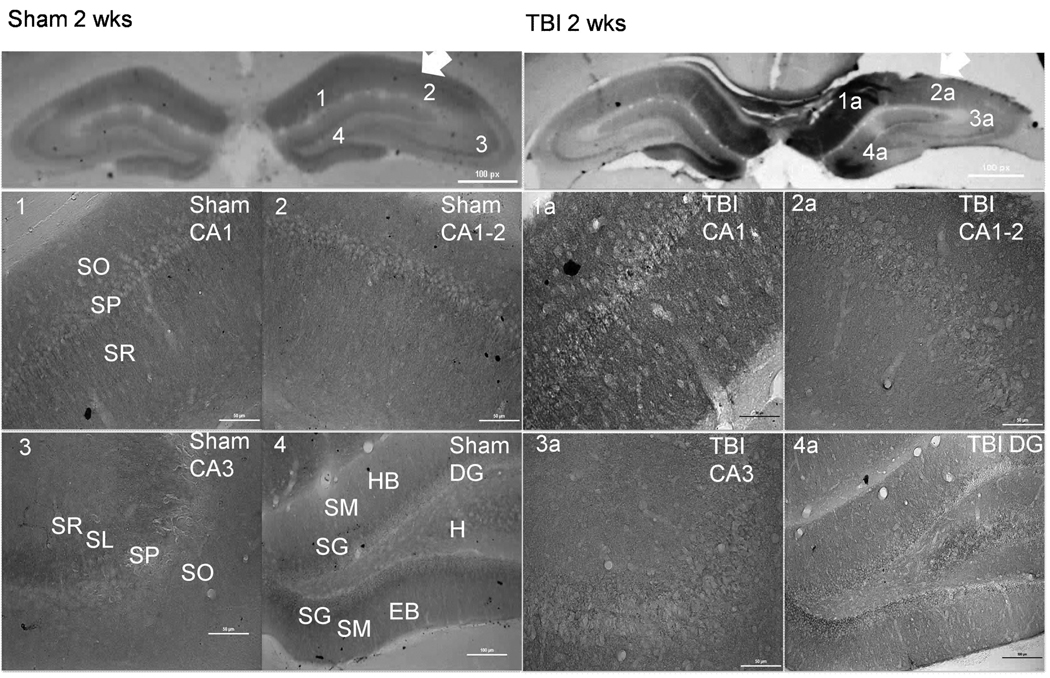
Figure 3.
CnB1 immunohistochemistry demonstrating alterations in CnB1 isoform distribution 2 weeks following TBI (right panels). Arrows indicate side of cortex ipsilateral to injury or sham surgery. There is an increase in staining within the CA1, CA1-2, and CA3 in TBI (1a, 2a, 3a) versus sham (1, 2, 3). There is a significant loss of CnB1 expression within the EB of the DG in TBI (4a) versus sham (4). Scale bars, 50 µm for regional sections. Abbreviations: SO= Stratum Oriens; SP= Stratum Pyramidale; SR= Stratum Radiatum; SL= Stratum Lucidum; SM= Stratum Moleculare; SG= Stratum Granulosum; HB= Hidden Blade; H=Hilus; EB= Exposed Blade.
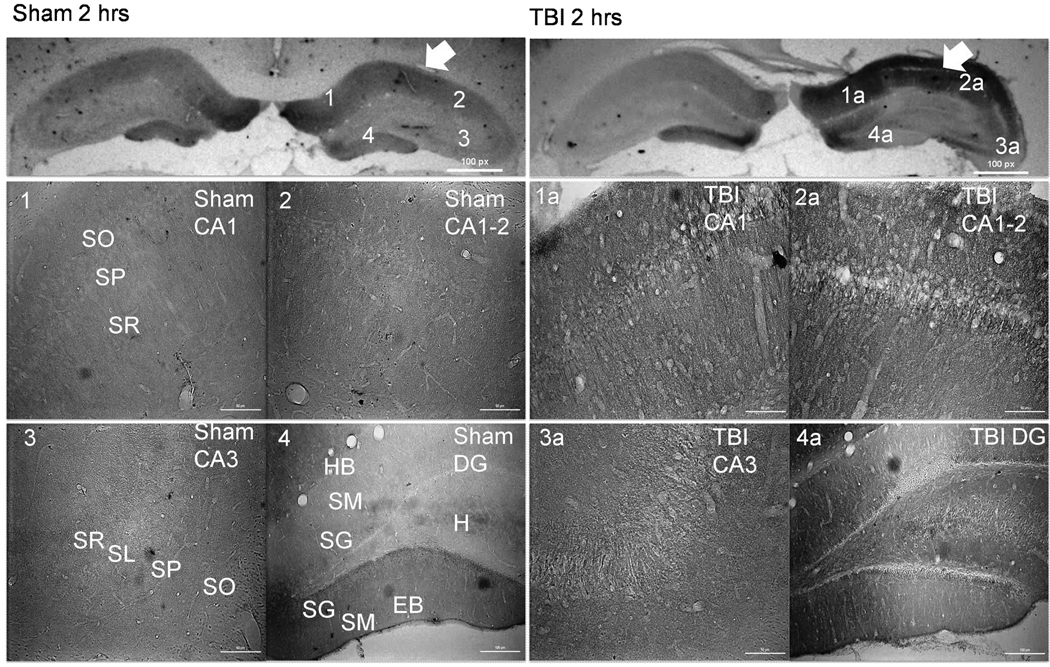
Figure 4.
CnB2 immunohistochemistry demonstrating alterations in CnB2 isoform distribution 2 hours following TBI (right panels). Arrows indicate side of cortex ipsilateral to injury or sham surgery. There is a significant increase in staining within the CA1 and CA1-2 in TBI (1a and 2a) versus sham (1 and 2). There is a significant loss of CnB2 expression within the EB of the DG in TBI (4a) versus sham (4). Scale bars, 50 µm for regional sections. Abbreviations: SO= Stratum Oriens; SP= Stratum Pyramidale; SR= Stratum Radiatum; SL= Stratum Lucidum; SM= Stratum Moleculare; SG= Stratum Granulosum; HB= Hidden Blade; H=Hilus; EB= Exposed Blade.
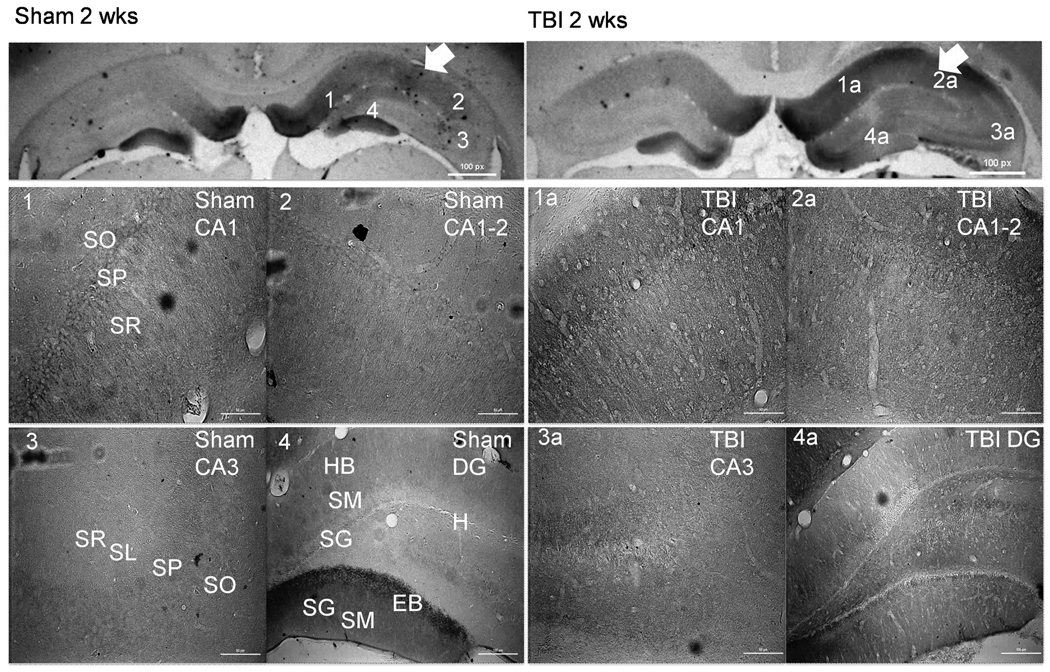
Figure 6.
CnB2 immunohistochemistry demonstrating alterations in CnB2 isoform distribution 2 weeks following TBI (right panels). Arrows indicate side of cortex ipsilateral to injury or sham surgery. There is a significant increase in CnB2 isoform expression within both the CA1 and CA1-2 regions in injured (1a and 2a) compared to shame (1 and 2). There is a significant loss of CnB2 expression within the EB of the DG in TBI (4a) versus sham (4). Scale bars, 50 µm for regional sections. Abbreviations: SO= Stratum Oriens; SP= Stratum Pyramidale; SR= Stratum Radiatum; SL= Stratum Lucidum; SM= Stratum Moleculare; SG= Stratum Granulosum; HB= Hidden Blade; H=Hilus; EB= Exposed Blade.
TBI Induced Alterations in CnB Isoform Expression within the Rat Hippocampus
There were changes in CnB isoform staining within regions of the rat hippocampus at 2 hours post TBI with persistent alterations lasting until 2 weeks post TBI as determined by sham versus injury comparison using the Kruskal-Wallis rank-sum test with a Bonferroni corrected p value for significance set at p≤0.005. (See Summary of Significant Changes in Table 1).
Table 1.
Summary of statistical analysis utilizing Kruskal-Wallis rank-sum test to assess median rank differences of scored DAB stained histological sections. Analysis of regional alterations in CnB isoform immunoreactivity within the rat hippocampus demonstrates significant alterations in the regional immunoreactivity of both isoforms 2 hours following injury. Many of these alterations persist to 2 weeks following injury.
| CnB1 | 2 hours | 2 weeks | CnB2 | 2 hours | 2 weeks | ||||
|---|---|---|---|---|---|---|---|---|---|
| Injury Compared to Sham |
Injury Compared to Sham |
Injury Compared to Sham |
Injury Compared to Sham |
||||||
| I-CA1 | ↑ | p≤0.005 | ↑ | p≤0.005 | I-CA1 | ↑ | p≤0.005 | NC | |
| I-CA1-2 | ↑ | p≤0.005 | ↑ | p≤0.005 | I-CA1-2 | ↑ | p≤0.005 | ↑ | p≤0.005 |
|
C-CA1-2- SR |
NC | NC |
C-CA1-2- SR |
↑ | p≤0.005 | ↑ | p≤0.005 | ||
| I-CA3 | ↑ | p≤0.005 | NC | I-CA3 | ↑ | p≤0.005 | NC | ||
| I-DG-H | NC | NC | I-DG-H | ↑ | p≤0.005 | ↑ | p≤0.005 | ||
| I-DG-EB | ↓ | p≤0.005 | ↓ | p≤0.005 | IDG-EB | ↓ | p≤0.005 | ↓ | p≤0.005 |
(p values represent Bonferroni corrected p values for pairwise comparisons within regions).
Abbreviations: C = Contralateral, I = Ipsilateral, SR = Stratum Radiatum (Dendrite Counts), DG = Dentate Gyrus, H = Hilus, HB = Hidden Blade, EB = Exposed Blade, NC = No Change, ↓ = Denotes a Decrease, ↑ = Denotes an Increase.
TBI Resulted in Acute Regionally Specific Alterations in CnB1 Distribution
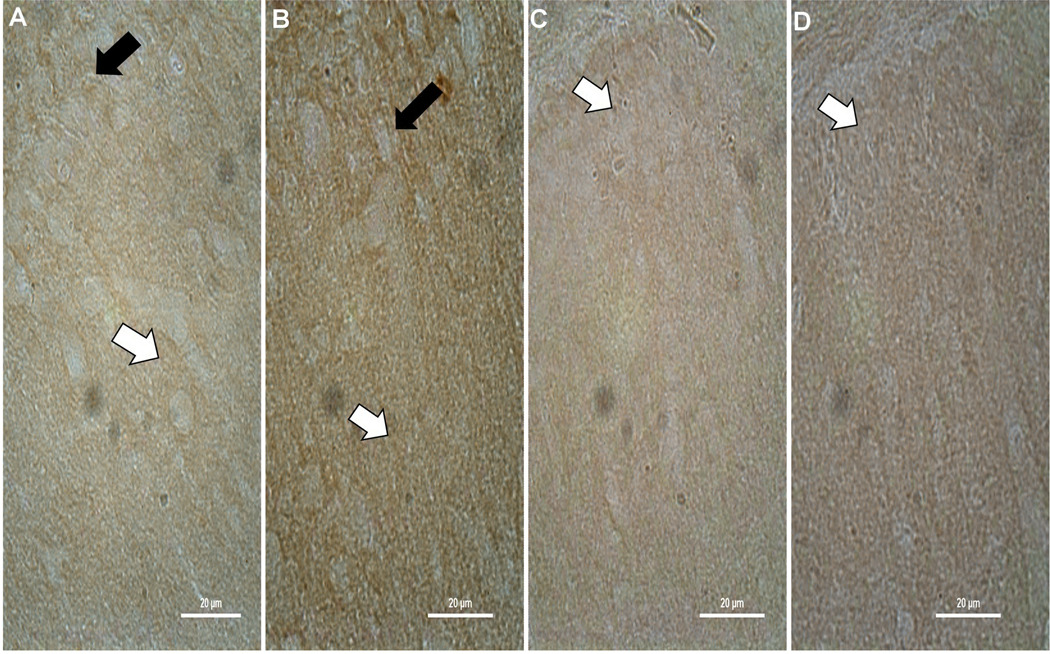
CnB1 staining in the injured ipsilateral CA1 region was in cell bodies within the stratum pyramidale (SP) layer and in the stratum oriens (SO) layer without localization to cell bodies (Figure 1). The columnar distribution of CnB1 staining within dendrites of the stratum radiatum (SR) layer remained intact in injured animals compared to sham and there appeared to be increased expression (Figure 2). CnB1 staining within the contralateral CA1 of injured animals did not differ from sham animals (not pictured) with significant staining of dendrites and cell bodies throughout the SR and SP layers.
Figure 2.
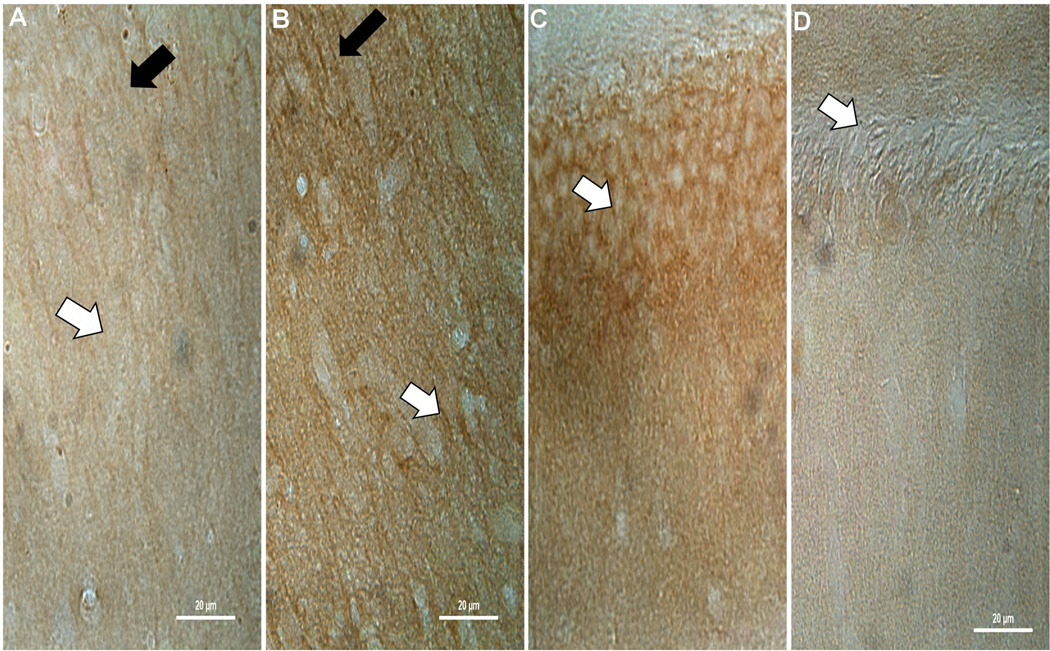
Representative higher power images demonstrating the increase of CnB1 isoform staining in CA1 of TBI animals (B – white arrow) compared to sham (A – white arrow) at 2 hours post injury, with a relative sparing of dendrites (A and B – black arrows). There is also a noticeable decrease in the exposed blade of the dentate in the SG cell body layer as seen in TBI animals (D – white arrow) compared to sham (C – white arrow). There appears to be some cell loss in the dentate which may explain some of the decreases with both isoforms. Similar results can be seen at 2 weeks for CnB1 and at 2 hours (Figure 6) and 2 weeks post injury for CnB2.
The majority of CnB1 staining in ipsilateral CA1-2 and CA3 regions of injured animals are in the surrounding neuropil of the SO and SR. There was no change in dendritic staining in the SR of the CA1-2 region compared to sham. A significant decrease in CnB1 staining within the exposed blade of the DG was localized to cell bodies within the SG layer and neuropil of the stratum molecular (SM) layer (Figure 2). The loss of CnB1 isoform staining within the exposed blade of the DG is predominantly lateral to midline with a relative sparing of staining in the genu of the DG.
TBI Resulted in Chronic Regionally Specific Alterations in CnB1 Distribution
At 2 weeks post injury staining within the ipsilateral CA1 appears to be localized to the SP layer within the cell somas and dendrites of the SR layer (Figure 3). CnB1 staining is increased in injured CA1-2 region of the ipsilateral hippocampus in the neuropil of the SO and SR. In the exposed blade of the ipsilateral DG CnB1 demonstrates reduced staining predominantly within cell somas of the SG with some decreases in the surrounding neuropil compared to sham (Figure 3).
TBI Resulted in Acute Regionally Specific Alterations in CnB2 Distribution
Acute changes in staining of the CnB2 subunit are similar to alterations seen with CnB1 subunit staining. CnB2 staining is in the neuropil of the SO and SR of both the CA1 and CA1-2 regions with a noticeable increase in staining in the SO and SR of the CA1-2 region and within CA3 (Figure 4). There is less CnB2 staining of both the cell somas of the SG and neuropil of the SM layer in the exposed blade of the injured ipsilateral DG compared to sham (Figure 5).
Figure 5.
Representative higher power images demonstrating the increase of CnB2 isoform staining in the deep SR of TBI animals (B – white arrow) compared to sham (A – white arrow) at 2 hours post injury, with a relative sparing of dendrites (A and B – black arrows). Unlike CnB1 (Figure 2), the decrease in the exposed blade of the dentate in the SG cell body layer in TBI animals (D – white arrow) compared to sham (C – white arrow) is not as striking.
Unlike CnB1 there are significant increases in CnB2 in contralateral CA1-2 SR dendritic staining acutely (not pictured).
TBI Resulted in Chronic Regionally Specific Alterations in CnB2 Distribution
There is more CnB2 staining within dendrites of the SR of both the ipsilateral and contralateral CA1-2 as well as increases in CnB2 staining within the SO and SP regions of both the ipsilateral CA1 and CA1-2 regions (Figure 6). At 2 weeks changes within the CA3 region of the CnB2 subunit seen at 2 hours are no longer present. Similar to 2 hours post injury there is less CnB2 staining within the exposed blade of the injured ipsilateral DG compared to sham, predominantly in the SG layer, and an increase in CnB2 staining within the ipsilateral hilus of injured animals compared to sham.
Changes in Subunit Staining Appreciated with Immunohistochemistry are in Part Due to Changes in Protein Concentration within Hippocampal Regions
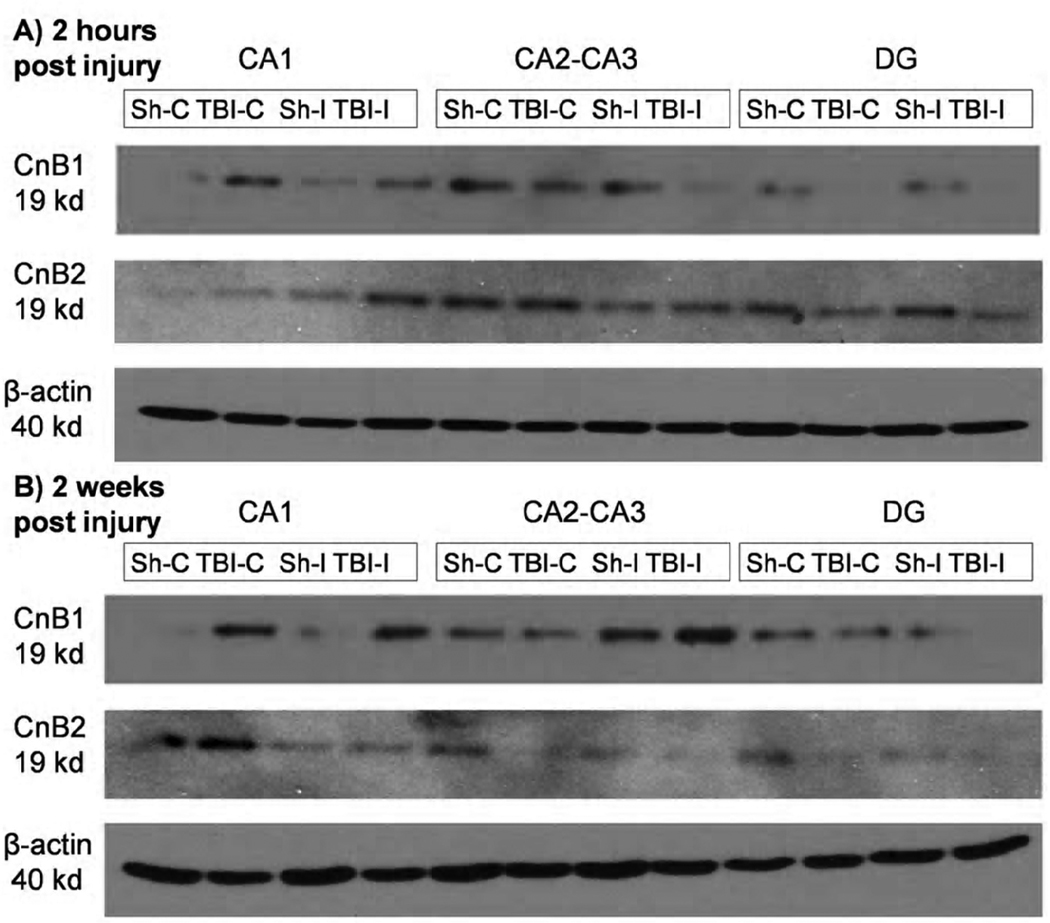
Western blot analysis of ipsilateral and contralateral DG, CA1, and CA1-2/CA3 regions indicates a visible difference between sham and injured animals in the protein concentrations of the CnB1 and CnB2 isoforms relevant to each region. At both 2 hours and 2 weeks post injury there is an increase in the expression of both CnB isoforms within the injured contralateral and ipsilateral CA1 regions compared to sham. There is also a decrease in both isoforms expression within the injured contralateral and ipsilateral DG compared to sham at 2 hours and 2 weeks. This suggests that the alterations in CnB isoform staining seen in immunohistochemistry is due in part to changes in protein expression and possibly also to a redistribution in the regions of interest (Figure 7).
Figure 7.
Western blot analysis of specific hippocampal regions. Homogenates were made from pooled resection tissue from N=3 rats per group. There is a noticeable increase in both CnB1 and CnB2 expression within the CA1 region at both timepoints examined. There is also a noticeable decrease in both CnB subunit isoforms expression within the DG of injured animals compared to sham, which is consistent with immunohistochemistry. Results of western blot analysis of the CA2-CA3 region do not completely agree with immunohistochemistry suggesting that there may be an alteration in distribution within these regions as well as changes in total expression. Abbreviations: Sh-C= Sham Contralateral; TBI-C= Traumatic Brain Injury Contralateral; Sh-I= Sham Ipsilateral; TBI-I= Traumatic Brain Injury Ipsilateral.
3. DISCUSSION
Following CCI there are regionally specific alterations in CnB subunit isoform distribution. Acutely there is decreased IR in both CnB1 and CnB2 within the exposed blade of the DG and an increase in IR within the CA1, CA1-2, and CA3 regions of the ipsilateral hippocampus in CCI versus sham. Alterations in IR within the DG persist chronically as do changes in the CA1-2 region. There are also significant increases in the IR of the CnB2 isoform in the contralateral CA1-2 region that persists chronically.
Acutely, following a TBI, elevations in Ca2+ (Shapira et al., 1989) can lead to abnormal neuronal activation leading to excitoxicity, increased superoxide levels, oxidative stress and cell death (Hovda et al., 1992). Although CaN appears be a key mediator in many of these processes there needs to be a greater understanding of how CaN is specifically modulated after injury, particularly with regards to the regulatory subunit, CnB. This is important given the increased use of drugs designed to block CaN activity which typically inhibit activity by limiting the ability of the regulatory subunit (CnB) to bind to and activate the catalytic subunit (CnA). The present study showed that CnB isoforms are altered in the hippocampus in a regionally specific manner after TBI. This suggests that individual CaN regulatory isoforms may have different roles within select hippocampal areas in the post-injury period and may have implications for regional effects of CaN inhibitors.
The present study illustrates that changes in the CnB regulatory subunit appear as early as 2 hours post TBI and persist until at least 2 weeks post injury. Changes in hippocampal IR, as assessed utilizing immunohistochemical staining, were noticeable in both of the CnB isoforms. Western blot data suggest that the visualized alterations in IR were associated with changes in protein concentrations within gross hippocampal regions, however this does not rule out possible changes in cellular distribution or perhaps in divergent expression within subsets of different cell types throughout the hippocampus (Figure 7).
Changes in CnB expression have been shown to cause alterations in hippocampal cellular potentiation and depression (Zeng et al., 2001) and have been implicated in schizophrenia (Miyakawa et al., 2003). A previous study demonstrated significant alterations in the CnA subunit within the same regions of interest (Bales et al., 2010). Cellular death pathways may be affected by these alterations, however the regionally specific and variable alteration of both the CnA and CnB subunits within the CA1, CA1-2, CA3 and DG acutely and chronically may have broader implications (Figures 1–6). Mbye et al., 2009 demonstrated that a non-CaN inhibitory analog of cyclosporin A demonstrates cellular protection by acting upon mitochondrial integrity.
Changes in CnB distribution and expression profiles throughout the hippocampus have important implications for cellular potentiation and depression. Unlike the CnA subunit, CnB isoforms are generally upregulated throughout the CA1 and CA1-2 regions ipsilaterally and the acute changes in the regulatory CnB subunit isoforms nearly all persist into the chronic phase (Table 1). The CnB2 isoform also shows a significant increases in dendritic IR within the CA1-2 region both acutely and chronically (Figures 4 and 6). Zeng and colleagues (2001) suggest from their studies that the selective loss of the CnB subunit hypothetically impairs bidirectional signaling mechanisms between LTP and LTD during very specific memory tasks.
The down-regulation of the CnB regulatory subunit isoforms (Figure 7) may be a counter regulatory response to eliminate non-specific neuronal activity after TBI occurring within the DG. This may be more pertinent in TBI given that following a brain injury the CnA subunit can become constitutively active if proteolytic cleavage removes the autoinhibitory site (Manalan et al., 1983). Perhaps downregulation of the regulatory subunit is a cellular response to the increases in catalytic CaN activity seen following a TBI FP (Kurz et al., 2005b). This would suggest that there may be multiple dysfunctions in CaN signaling following a TBI, both an increase in potentially harmful activity, and an alteration in subunit distribution important for dendritic stability (Halpain et al., 1998) and the growth of neurites (Chang et al., 1995).
For normal CaN activity it is necessary, in the presence of Ca2+, for the regulatory CnB subunit to bind to its binding site on the catalytic CnA subunit (Griffith et al., 1995). Both of the calcineurin inhibitors, FK-506 and cyclosporin A, rely upon the disruption of this interaction to limit CaN activity (Kay et al., 1989; Wiederrecht et al., 1993). Regionally distinct changes in the two subunits create a situation whereby these two drugs may have different effects within different regions of the hippocampus, which have yet to be appreciated. Given the loss of regulatory expression within the DG (Figure 7), CaN inhibitors may not be effective in curtailing DG damage post TBI. Understanding how CaN activity and structure is altered post injury may help to better develop and target treatment strategies for TBI.
In summary, CnB isoform expression is altered by injury in a regionally specific manner within the hippocampus. Regional alterations in CnB isoforms within the ipsilateral hippocampus persist chronically. While studies have shown cellular potentiation and depression dependent upon CnB expression, it remains unclear what the CnB subunit’s function is independent of the activity of the CaN heterodimer. These regionally specific alterations in isoform expression also have important implications for the clinical use of CaN inhibitors in TBI. This study is the first step in identifying these alterations and characterizing specific CaN subunit changes following TBI that will allow for better targeted therapeutic strategies to assist in patient recovery from TBI.
4. EXPERIMENTAL PROCEDURE
Animals
Adult male Sprague-Dawley rats (n=36) were used in the study. Rats were purchased from Hilltop Laboratories (Scottsdale, PA, USA) and housed in pairs under a 12:12 light/dark cycle. Rats were given food and water with ad libitum throughout the study. All experiments were carried out in accordance with the University of Pittsburgh’s guidelines for the Care and Use of Laboratory Animals. All experiments were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
Surgery
On the day of surgery anesthesia was initiated with 4% isoflurane (IsoFlo; Abbott Laboratories, North Chicago, IL, USA) and 2:1 N2O/O2. Rats were intubated and maintained on 1.5–2% isofluorane during the surgical procedure. Following intubation, rats were placed on a thermal blanket to regulate body temperature (37°C) and the animals head placed in a stereotaxic frame. Topical analgesia, EMLA Cream (lidocaine and pilocaine 2.5%), was applied to the ear bars prior to insertion to reduce discomfort. Surgical tools and area were sterilized prior to and in-between each surgery. An incision was made down the midline of the skull and the soft tissues and periosteum deflected. A craniotomy was then performed over the right parietal bone to expose the dura. Controlled cortical injury (CCI, Pittsburgh Precision Instruments, Inc.) at a depth of 2.4mm at 4m/sec was carried out as previously reported (Dixon et al., 1991; Bales et al., 2010). A total of 18 rats were injured, and the remaining 18 rats were shams. Righting reflex (Dixon et al., 1991) was monitored in the immediate post-surgical period to assess acute recovery. Rats surviving for two weeks were returned to their housing 30 mins post recovery, weighed daily, and monitored for signs of distress (piloerection, ruffled hair coat, hunched posture, rapid respirations, and porphyrin secretion). Ibuprofen (Advil) was used for post-operative analgesia at 0.3 mg/mL dissolved in the water for at least 3 days. Any rat that showed significant distress and weight loss (below 60% of pre-operative weight) was sacrificed. No rats met the criteria for premature sacrifice for this study.
Immunohistochemistry
Following a 2 hr (6 shams; 6 TBI) or 2 week (6 shams; 6 TBI) recovery period, animals were given an overdose of sodium pentobarbital (100 mg/kg; i.p.), and perfused intra-aortically with 0.1M heparinized PBS in 4% PFA/0.1M PBS. Brains were dissected, submerged in increasing concentrations of sucrose, and stored at −80°C. Brains were then sectioned at 35µm in a cryostat, and free floating sections collected in tissue plate wells containing 0.1M TBS (pH 7.5).
All immunohistochemical procedures and incubations were carried out with agitation with the exception of the chromogen step. All treatment groups were stained together within each immunohistochemical session. Sections were matched by region, rinsed 3× 5 min in washing buffer (0.1%Triton-X in 0.1M TBS) and blocked in a mixture of 10% normal donkey serum in washing buffer for 2 hours at room temperature. Sections were then incubated overnight at 4°C in (one only) primary goat antibodies (Santa Cruz Biotechnology, CA) specific to CnB1 (1:100), CnB2 (1:100) subunit isoforms diluted in washing buffer/5% normal donkey serum. Following incubation, sections were then washed 3 × 8 minutes in washing buffer and endogenous peroxidase activity was quenched with 0.3% H2O2 in methanol for 10 minutes. Following 5 × 5 minutes washing in TBS, sections were incubated for 1hr at RT in HRP conjugated donkey anti-goat secondary antibody (Jackson ImmunoResearch, PA) at 1:200 in 0.1M TBS. Immunoreactivity (IR) was then visualized using 0.01% 3’ 3’ Diaminobenzidine (DAB) after an extensive wash. The DAB reaction was terminated with dH2O and sections were rinsed in 0.1M TBS, mounted onto slides, air dried and cover-slipped for light microscope analysis. All sections within the reaction were exposed to each of the reagents for the same time period. Control sections pre-adsorbed with their homologous peptides were negative for IR.
Western Blot Analysis
Tissue collection for western blot analysis was performed as reported in Bales et al., 2010. Briefly at 2 hours or 2 weeks (3 sham; 3 TBI per timepoint) animals were deeply anesthetized with pentobarbital (Nembutal, 80–100 mg/kg; Abbott Laboratories, North Chicago, IL). Animals were decapitated and the brains quickly removed and chilled on ice. Both the right and left hippocampi were exposed by careful dissection and the dentate gyrus, CA1, and CA1-2/CA3 regions were excised separately and combined across animals for adequate protein yield. Tissue was immediately placed into liquid nitrogen and then into a −80°C freezer until processed. Tissue was homogenized in Lysis buffer (suspension buffer) and protein concentrations were determined using a BCA protein Assay Kit (PIERCE, Rockford, IL). Samples containing 20 µg of protein were subjected to SDS-polyacrylamide gel electrophoresis through a 10% acrylamide gel, and then transferred to nitrocellulose membranes and immunolabeled with antiserum followed by donkey anti-goat immunoglobulin G conjugated to peroxidase (1:5,000; PIERCE, Rockford, IL). Proteins were visualized with a chemiluminescence detection system (SuperSignal, PIERCE, Rockford, IL). To assure equal loading, the membrane was restriped and reblotted with rabbit anti-actin antibody (1:15,000, Sigma, St. Louis, MO).
Selection of Timepoints for Analysis
Two hours corresponds to acute alterations after TBI, including rapid increases in Ca2+ that occur immediately post injury and persist (Hovda et al., 1990; Shapira et al., 1989). Given the role of Ca2+ in the control of CaN activity an acute examination of isoform alterations at 2 hours post injury was chosen to examine the affect of acute Ca2+ increases. Two weeks corresponds to the timepoint used to assess cognitive outcomes following CCI in rats (Kline et al., 2002; Yan et al., 2000).
Scoring for Wilcoxon-Mann-Whitney Rank-Sum Analysis
A six point scoring scale (0 = no IR, 6 = heavy IR) was utilized to assess the level of CnB isoform expression in DAB stained sections from separate subregions (CA1, CA1-2, CA3, dentate gyrus hilus, dentate gyrus hidden blade, dentate gyrus exposed blade) of the ipsilateral and contralateral hippocampi as described in Bales et al., 2010. To assess dendritic staining in the stratum radiatum of the ipsilateral and contralateral CA1 and CA1-2 a three point scoring scale was employed (1 = few, 3 = numerous). An independent observer blinded to timepoint and isoform stain scored each tissue section utilizing both scoring paradigms. Following ranking, sham and TBI designations were revealed. Analysis of outcomes of injury × time were compared (independently for each region and side) using the Kruskal-Wallis rank-sum test with a Bonferroni corrected p value for significance set at p≤0.005.
ACKNOWLEDGEMENTS
We would like to thank Melanie Grubisha for her invaluable advice regarding this research. This work supported by NIH grant 2P50-NS030318.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Asai A, et al. High level calcineurin activity predisposes neuronal cells to apoptosis. Journal of Biological Chemistry. 1999;274:34450–34458. doi: 10.1074/jbc.274.48.34450. [DOI] [PubMed] [Google Scholar]
- Bales JW, et al. Expression of protein phosphatase 2B (calcineurin) subunit A isoforms in rat hippocampus after traumatic brain injury. Journal of Neurotrauma. 2010;27:109–120. doi: 10.1089/neu.2009.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher SP, et al. Neuroprotective actions of FK506 in experimental stroke: in vivo evidence against an antiexcitotoxic mechanism. Journal of Neuroscience. 1997;17:6939–6946. doi: 10.1523/JNEUROSCI.17-18-06939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, et al. Asymmetric retraction of growth cone filopodia following focal inactivation of calcineurin. Nature. 1995;376:686–690. doi: 10.1038/376686a0. [DOI] [PubMed] [Google Scholar]
- Colicos MA, et al. Delayed, selective neuronal death following experimental cortical impact injury in rats: possible role in memory deficits. Brain Res. 1996;739:111–119. doi: 10.1016/s0006-8993(96)00819-0. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, et al. Secondary Injury After Head Trauma: Subacute and Long-term Mechanisms. Semin Clin Neuropsychiatry. 1998;3:176–185. [PubMed] [Google Scholar]
- Dixon CE, et al. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Friberg H, et al. Cyclosporin A, but not FK 506, protects mitochondria and neurons against hypoglycemic damage and implicates the mitochondrial permeability transition in cell death. Journal of Neuroscience. 1998;18:5151–5159. doi: 10.1523/JNEUROSCI.18-14-05151.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith JP, et al. X-ray structure of calcineurin inhibited by the immunophilin-immunosuppressant FKBP12-FK506 complex. Cell. 1995;82:507–522. doi: 10.1016/0092-8674(95)90439-5. [DOI] [PubMed] [Google Scholar]
- Groth RD, et al. Calcineurin regulation of neuronal plasticity. Biochemical and Biophysical Research Communications. 2003;311:1159–1171. doi: 10.1016/j.bbrc.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Guerini D, Klee CB. Cloning of human calcineurin A: evidence for two isozymes and identification of a polyproline structural domain. Proc Natl Acad Sci U S A. 1989;23:9183–9187. doi: 10.1073/pnas.86.23.9183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpain S, et al. Regulation of F-actin stability in dendritic spines by glutamate receptors and calcineurin. Journal of Neuroscience. 1998;18:9835–9844. doi: 10.1523/JNEUROSCI.18-23-09835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovda DA, et al. Secondary injury and acidosis. Journal of Neurotrauma. 1992;9 Suppl 1:S47–S60. [PubMed] [Google Scholar]
- Huang RQ, Dillon GH. Maintenance of recombinant type A gamma-aminobutyric acid receptor function: role of protein tyrosine phosphorylation and calcineurin. J Pharmacol Exp Ther. 1998;286:243–255. [PubMed] [Google Scholar]
- Kay JE, et al. The mechanism of action of he immunosuppresive drug FK-506. Cell Immunol. 1989;124:175–181. doi: 10.1016/0008-8749(89)90121-4. [DOI] [PubMed] [Google Scholar]
- Klee CB, et al. Calcineurin: a calcium- and calmodulin-binding protein of the nervous system. Proceedings of the National Academy of Sciences of the United States of America. 1979;76:6270–6273. doi: 10.1073/pnas.76.12.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline AE, et al. Attenuation of working memory and spatial acquisition deficits after a delayed and chronic bromocriptine treatment regimen in rats subjected to traumatic brain injury by controlled cortical impact. J Neurotrauma. 2002;19:415–425. doi: 10.1089/08977150252932370. [DOI] [PubMed] [Google Scholar]
- Kochanek PM. Ischemic and traumatic brain injury: pathobiology and cellular mechanisms. Crit Care Med. 1993;21:S333–S335. doi: 10.1097/00003246-199309001-00016. [DOI] [PubMed] [Google Scholar]
- Kurz JE, et al. A persistent change in subcellular distribution of calcineurin following fluid percussion injury in the rat. Brain Research. 2005a;1048:153–160. doi: 10.1016/j.brainres.2005.04.062. [DOI] [PubMed] [Google Scholar]
- Kurz JE, et al. A significant increase in both basal and maximal calcineurin activity following fluid percussion injury in the rat. J Neurotrauma. 2005b;22:476–490. doi: 10.1089/neu.2005.22.476. [DOI] [PubMed] [Google Scholar]
- Manalan AS, Klee CB. Activation of calcineurin by limited proteolysis. Proceedings of the National Academy of Sciences of the United States of America. 1983;80:4291–4295. doi: 10.1073/pnas.80.14.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbye LH, et al. Comparative neuroprotective effects of cyclosporin A and NIM811, a nonimmunosuppressive cyclosporin A analog, following traumatic brain injury. Journal of Cerebral Blood Flow and Metabolism. 2009;29:87–97. doi: 10.1038/jcbfm.2008.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa T, et al. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:8987–8992. doi: 10.1073/pnas.1432926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morioka M, et al. Potential role of calcineurin for brain ischemia and traumatic injury. Progress in Neurobiology. 1999;58:1–30. doi: 10.1016/s0301-0082(98)00073-2. [DOI] [PubMed] [Google Scholar]
- Okonkwo DO, et al. Cyclosporin A limits calcium-induced axonal damage following traumatic brain injury. Neuroreport. 1999;10:353–358. doi: 10.1097/00001756-199902050-00026. [DOI] [PubMed] [Google Scholar]
- Okonkwo DO, et al. Dose-response of cyclosporin A in attenuating traumatic axonal injury in rat. Neuroreport. 2003;14:463–466. doi: 10.1097/00001756-200303030-00033. [DOI] [PubMed] [Google Scholar]
- Rusnak F, Mertz P. Calcineurin: form and function. Physiological Reviews. 2000;80:1483–1521. doi: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- Sanders MJ, et al. Chronic failure in the maintenance of long-term potentiation following fluid percussion injury in the rat. Brain Res. 2000;861:69–76. doi: 10.1016/s0006-8993(00)01986-7. [DOI] [PubMed] [Google Scholar]
- Shapira Y, et al. Accumulation of calcium in the brain following head trauma. Neurological Research. 1989;11:169–172. doi: 10.1080/01616412.1989.11739885. [DOI] [PubMed] [Google Scholar]
- Tehranian R, et al. Disruption of Bax protein prevents neuronal cell death but produces cognitive impairment in mice following traumatic brain injury. Journal of Neurotrauma. 2008;25:755–767. doi: 10.1089/neu.2007.0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong G, Shepherd D, Jahr CE. Synaptic desensitization of NMDA receptors by calcineurin. Science. 1995;267:1510–1512. doi: 10.1126/science.7878472. [DOI] [PubMed] [Google Scholar]
- Wiederrecht G, et al. The mechanism of action of FK-506 and cyclosporin A. Ann N Y Acad Sci. 1993:9–19. doi: 10.1111/j.1749-6632.1993.tb17137.x. [DOI] [PubMed] [Google Scholar]
- Yan HQ, et al. Evaluation of combined fibroblast growth factor-2 and moderate hypothermia therapy in traumatically brain injured rats. Brain Research. 2000;887:134–143. doi: 10.1016/s0006-8993(00)03002-x. [DOI] [PubMed] [Google Scholar]
- Yang SA, Klee CB. Low affinity Ca2+-binding sites of calcineurin B mediate conformational changes in calcineurin A. Biochemistry. 2000;39:16147–16154. doi: 10.1021/bi001321q. [DOI] [PubMed] [Google Scholar]
- Zeng H, et al. Forebrain-specific calcineurin knockout selectively impairs bidirectional synaptic plasticity and working/episodic-like memory. Cell. 2001;107:617–629. doi: 10.1016/s0092-8674(01)00585-2. [DOI] [PubMed] [Google Scholar]