Abstract
ANP is a peptide released by cardiac myocytes that regulates blood pressure and natriuresis. However, the molecular mechanisms controlling ANP release from cardiac myocytes are not defined. We now identify three components of the exocytic machinery that regulate ANP release from atrial myocytes. We found that cardiac myocytes express N-ethylmaleimide sensitive factor (NSF), soluble NSF attachment protein (α-SNAP), and SNAP receptors (SNAREs). Additionally we found that specific SNARE molecules, VAMP1 and VAMP-2, both co-sediment and co-localize with ANP. Also, one SNARE molecule, syntaxin-4, partially co-sediments and partially co-localizes with ANP. Furthermore, these three SNAREs, sytntaxin-4 and VAMP-1 and VAMP-2 form a SNARE complex inside cardiac myocytes. Finally, knockdown of VAMP1, VAMP-2 or syntaxin-4 blocks regulated release of ANP. In contrast, silencing of VAMP-3 did not have an effect on ANP release. Our data suggest that three specific SNAREs regulate cardiac myocyte exocytosis of ANP. Pathways that modify the exocytic machinery may influence natriuresis and blood pressure.
Introduction
ANP Synthesis
Atrial natriuretic peptide (ANP) is a polypeptide hormone produced, stored and secreted mainly by adult atrial cardiac myocytes.[1, 2] ANP plays a critical role in regulating blood volume and blood pressure by promoting natriuresis and vasodilation.[3–9] In atrial myocytes of adult hearts, ANP is synthesized as a 151 amino-acid pre-pro-hormone (pre-pro-ANP), which is then processed to a 126 amino acid pro-hormone (pro-ANP) and stored in atrial granules.[10] During exocytosis of these atrial granules, enzymatic cleavage of pro-ANP by corin generates the biologically active 28 amino acid peptide ANP that is released into the circulation.[11]
Regulation of ANP Release
ANP is constitutively released at a slow rate at low levels, but it also can be secreted rapidly and at high concentrations after agonist stimulation. The major physiological activator of ANP release from atrial myocytes is increased atrial stretch. Other extracellular activators include catecholamines, prostaglandins, glucocorticoids, angiotensin II and endothelin-1.[12] The intracellular pathways that mediate the exocytosis of ANP containing granules are not well understood. Calcium (Ca2+), protein kinase C (PKC), and G-proteins play a role in exocytic pathways in atrial myocytes. [13–18] The cyclic nucleotides cGMP and cAMP may also inhibit ANP release.[19, 20] However, the precise exocytic pathways regulated by these messenger molecules are not defined.[12]
Exocytic Machinery
The exocytic machinery that mediates ANP granule release is not well characterized. However, cardiac myocytes may share the same sets of exocytic proteins that regulate exocytosis in other cell types, such as neurons, pancreatic acinar cells, and endothelial cells. Intracellular membrane trafficking and exocytosis are controlled by several superfamilies of proteins, including N-ethylmaleimide sensitive factor (NSF) and its co-factor, soluble NSF attachment protein (α-SNAP), SNAP receptors (SNAREs), Sec/Munc18 (SM) and small GTP-binding proteins of the Rab super-family.[21–23] Rab members regulate upstream steps such as budding, delivery, tethering and transport. SNAREs and SM-proteins are involved in fusion events at a later stage of exocytosis. NSF and its cofactors are involved in SNARE complex disassembly. Only a few prior reports has examined the expression of SNARE molecules in cardiac myocytes: a subset of SNARE molecules are expressed in atrial myocytes [24–26]. Although some SNARE molecules have been identified in atrial myocytes, the precise SNARE isoforms that regulate ANP exocytosis are unknown.
Despite the importance of ANP in cardiovascular physiology, several key aspects of ANP biology are not understood. In particular, the molecular machinery that modulates ANP secretion is virtually unknown. Here, we show that exocytosis of ANP granules from neonatal cardiac myocytes is regulated by a set of specific SNARE molecules. Silencing of VAMP1, VAMP2 or Syntaxin-4 by siRNA, resulted in inhibition of ANP release from stimulated cardiac myocytes, while VAMP-3 knockdown did not affect ANP release.
Materials and Methods
Primary culture of neonatal rat cardiac myocytes
Neonatal rat cardiac myocytes (NRCMs) were isolated from the whole heart of 1–2 day old rats as described previously [27]. In brief, the hearts were minced, digested with trypsin overnight at 4°C. The day after, tissue was dissociated by stepwise collagenase treatment for a few minutes at 37°C. Cells were pre-plated twice for 60 minutes to eliminate fibroblasts and enrich the culture for cardiac myocytes. The non adherent myocytes were then plated at a density of 1200 cells/mm2 in plating medium consisting of 199 medium supplemented with HEPES, MEM non-essential amino acids, glucose, glutamine, 10% FBS, vitamin B12, penicillin, streptomycin, on fibronectin coated plates. The next day cells are washed and fresh medium with 2% FCS is added. The cells are maintained at 37°C in the presence of 5% CO2 in a humidified incubator.
Transfection
After isolation myocytes were transfected using an electroporator (Nucleofector, Amaxa, Gaithersburg, MD) following the protocol for neonatal rat myocytes (transfection efficiency, measured by GFP expression using FACS analysis was 62% ± 2.9). Cells were stimulated with ET-1 after 2 or 3 days after transfection with commercial available siRNA for Syntaxin-4 (Santa Cruz, CA), VAMP1, VAMP2, VAMP-3 or control (Applied Biosystems/Ambion, TX).
Sequences of the siRNA used are the following (5′ to 3′):
For Syntaxin-4 the siRNA used was a pool of three different siRNA duplexes
#1, Sense: CAACACAAGGAUGAAGAAAtt
Antisense: UUUCUUCAUCCUUGUGUUGtt
#2, Sense: GUGAGGUGUUUGUGUCUAAtt
Antisense: UUAGACACAAACACCUCACtt
#3, Sense: CCCUAGAGAAUCAGAAGAAtt
Antisense: UUCUUCUGAUUCUCUAGGGtt
VAMP1, sense: GCAAGAAUAGUUGGGAAGUtt,
antisense: ACUUCCCAACUAUUCUUGCtt.
VAMP2, sense: GGGUGAAUGUGGACAAGGUtt,
antisense: ACCUUGUCCACAUUCACCCtc.
VAMP3, sense: GGAUCUUCUUCGAGACUUUTT,
antisense: AAAGUCUCGAAGAAGAUCCTG.
ANP assay
Myocytes were stimulated with endothelin-1 (ET-1) for 1 h and ANP in the supernatant was measured by an ELISA (Peninsula Laboratories Bachem, King of Prussia, Pennsylvania).
Western Blotting
Immunoblotting was performed as described previously.[28] In brief, total cell lysates were separated on SDS-PAGE. Proteins were transferred on PVDF membrane, blocked with 5% milk in PBS and primary antibody was incubated in the same solution overnight at 4°C. Up to 150 μg of proteins were loaded and primary antibodies were used at the following dilutions: SNAPE-25, Syntaxin-1, VAMP3, α-SNAP, Rab3, Rab4 and Rab8 at 1:200 (Santa Cruz, CA). Antibodies for SNAP23, Syntaxin4, VAMP1 and VAMP2 were used at dilution of 1:2000 (Synaptic System, Germany). Antibodies against NSF (BD Pharmingen Inc. San Diego, CA), Synaptotagmin, Munc13 and Munc18 (Synaptic System, Germany) were used at 1:1000 dilution. Antibodies for ANP, 14-3-3 and cadherin were used at 1:500 (Santa Cruz, CA). Antibody against tubulin was used at 1:5000 dilution (Abcam Inc. MA). After washing, membranes were incubated with the secondary horseradish peroxidase-conjugated antibody for 1 hour, washed again, incubated with SuperSignal West Dura (Pierce) and exposed to film.
Cell Fractionation
Cells were placed on ice and then lysed by 15 passages through a cell cracker (H & Y Enterprises, Redwood City, CA) with a 15 um difference between outer diameter of the ball bearing and inner diameter of the barrel.
After centrifugation at 900 g for 10 min, postnuclear supernatants are centrifuged at 5500 g for 15 min and the resulting supernatant centrifuged at 25000 g for 20 min to obtain in the pellet the granule fraction. The supernatant is centrifuged at 100000 g for 1 h and the supernatant represents the cytoplasmic fraction. All of the steps were carried out at 4°C. Plasma membranes are obtained from the postnuclear pellet which is resuspended and centrifuged at 116000 g for 1 h mixed in a solution of 0.25 M and 2 M sucrose buffer.
Immunocytochemistry
Cells were plated on cover slips coated with fibronectin. Myocytes were fixed in cold methanol for 5 minutes, blocked in 10% FCS for 1 hour at room temperature, and incubated with antibodies at 4°C overnight in PBS containing 5% FCS and 0.1% Tween. After washing, fluorescent label secondary antibody was added for 1 hour at room temperature. Cells were washed again, mounted, and fluorescence was observed with a confocal microscope. Pearson’s correlation coefficient was calculated from the confocal images using Volocity software (Improvision, Coventry, England).
NEM treatment and Immunoprecipitation
Myocytes plated on 10-mm coated dish were washed and incubated with 1mM NEM for 15 min on ice, followed by addition of 10 mM DTT to quench NEM. Control cells were incubated with NEM and DTT at the same time for 30 min on ice. At the same time, some plates were incubated with medium alone for 30 min followed immunoprecipitation.
Myocytes were scraped in immunoprecipitation (IP) buffer (20 mM Tris-HCl, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM b-glycerophosphate) with protease and phosphatase inhibitors on ice.
After centrifugation, samples were pre-cleared for 1 hour at 4°C with protein A agarose beads. Samples were then centrifuged and supernatant was incubated with 10 μl of antibody against synatxin-4 overnight at 4°C. Then, protein A agarose beads was added and further incubation was carried for 1 hour at 4°C. Samples were then centrifuged and washed three time with IP buffer, loading buffer was added and sample boiled. Western blotting was then performed as previously described.
Statistical Analysis
All the data in this study are expressed as mean ± standard deviation (SD). Differences in data between two groups are compared with Student’s 2-tailed t test and between more than two groups with one-way analysis of variance (ANOVA).
Results
SNAREs are expressed in cardiac myocytes
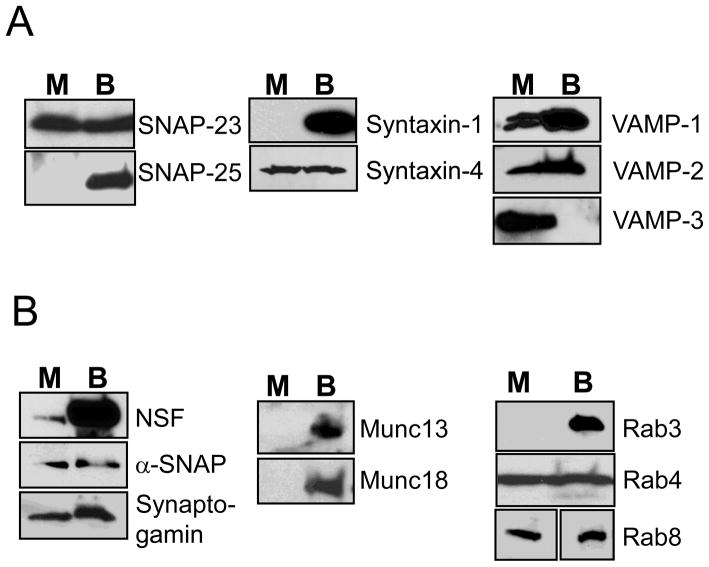
We first characterized the expression of SNARE proteins in neonatal cardiac myocytes. Neonatal cardiac myocytes were selected for this study for two reasons: (1) neonatal rat hearts express equal levels of ANP in both atria and ventricles between 0–3 d after birth [29]; (2) adult cardiac myocytes are difficult to transfect and culture for more than 48 h. Accordingly, cardiac myocytes were harvested from 1–2 d old neonatal rats, plated for 48 h, then lysed, and cell lysates were fractionated by PAGE and analyzed by immunoblotting. Mouse brain was used as a positive control. SNAP-23, Syntaxin-4, VAMP-1, VAMP-2 and VAMP-3 are expressed in cardiac myocytes (Fig. 1A). However, other SNAREs such as SNAP-25 and syntaxin-1 are absent from myocytes (Fig. 1A).
Figure 1. Cardiac myocytes express SNAREs and other proteins involved in vesicle trafficking.
Neonatal rat cardiac myocytes (M) and mouse brains (B) lysates were immunoblotted for (A) SNARE proteins and (B) associated proteins that regulate trafficking.
We found that other proteins involved in vesicle trafficking are also expressed in myocytes. Cardiac myocytes express NSF and its co-factor α-SNAP, the calcium sensor synaptotagmin, and the GTPases Rab8 and Rab4 (Fig. 1B).
ANP is expressed in isolated neonatal cardiac myocytes
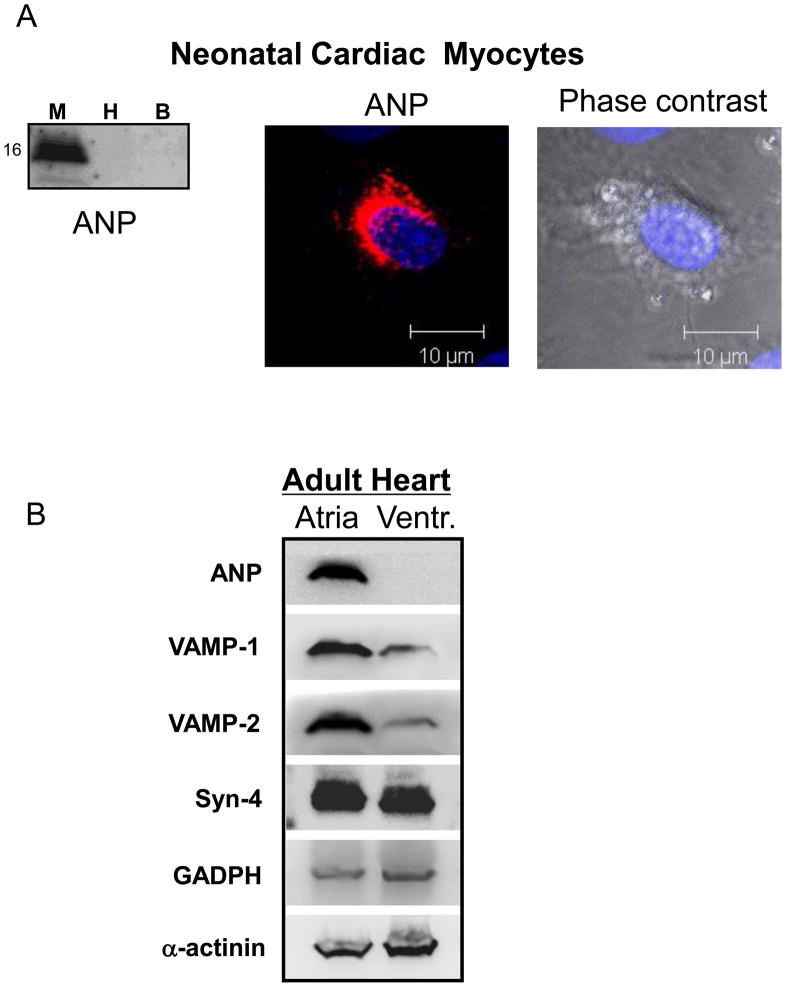
Neonatal myocytes were analyzed for ANP expression by Western blotting analysis and by immunostaining where ANP appears in a granular pattern (Fig. 2A). To validate the use of neonatal cardiac myocytes as an experimental model, we also compared the expression of ANP and SNAREs between adult atria and adult ventricles. The same amount of proteins were loaded for atria and ventricle. Only atria express ANP (Fig. 2B). Atria express more VAMP-1 and VAMP-2 than ventricles; both atria and ventricles express roughly equivalent levels of syntaxin-4 (Fig. 2B).
Figure 2. ANP is expressed in primary cardiac myocytes in a perinuclear granular pattern.
(A) Cell lysates from neonatal rat cardiac myocytes (M), HeLa cells (H), and mouse brain (B) were immunoblotted with antibody against ANP. Neonatal cardiac myocytes were stained with antibody to ANP and a secondary antibody conjugated to Cy3 (red), and counter-stained with DAPI before imaging with a confocal microscope. (B) Adult rat atria and ventricles were homogenized and immunoblotted for ANP and SNARE proteins.
Co-localization of ANP and SNAREs
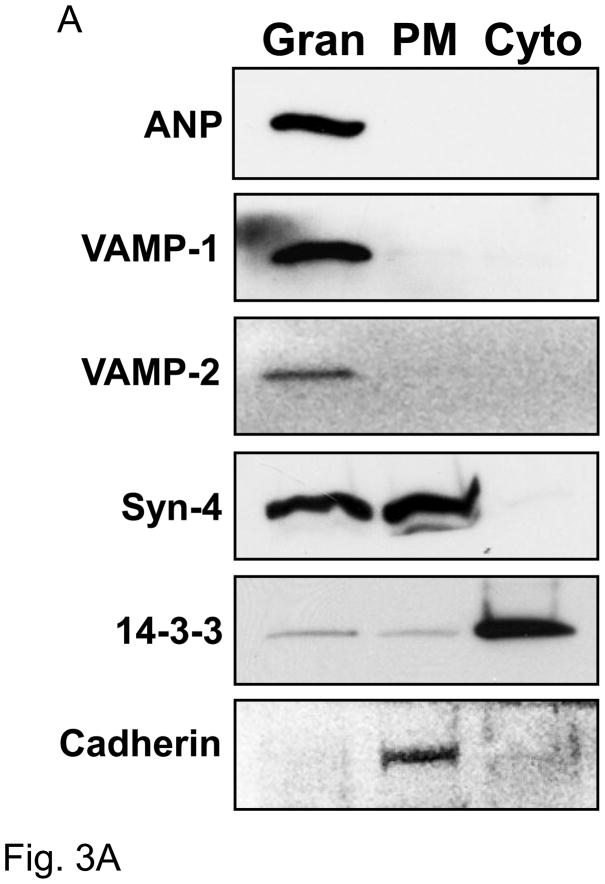
To analyze the subcellular distribution of ANP and SNARE proteins, we performed ultracentrifugation. Cardiac myocyte lysates were fractionated by ultracentrifugation into three main fractions: 1) granules (Gran), 2) plasma membrane (PM), 3) cytoplasm (Cyto). Fractions were collected and immunoblotted.
ANP is found in the granular compartment, but not in the plasma membrane or cytoplasm (Fig. 3A). VAMP-1 and VAMP-2 are also found entirely in the granular fraction. In addition, syntaxin-4 is present in part in the granular fraction with ANP and in part in the plasma membrane fraction. None of these SNAREs are found in the cytoplasm. Cadherin and 14-3-3 proteins were used as markers of plasma membrane and cytosol fractions respectively.
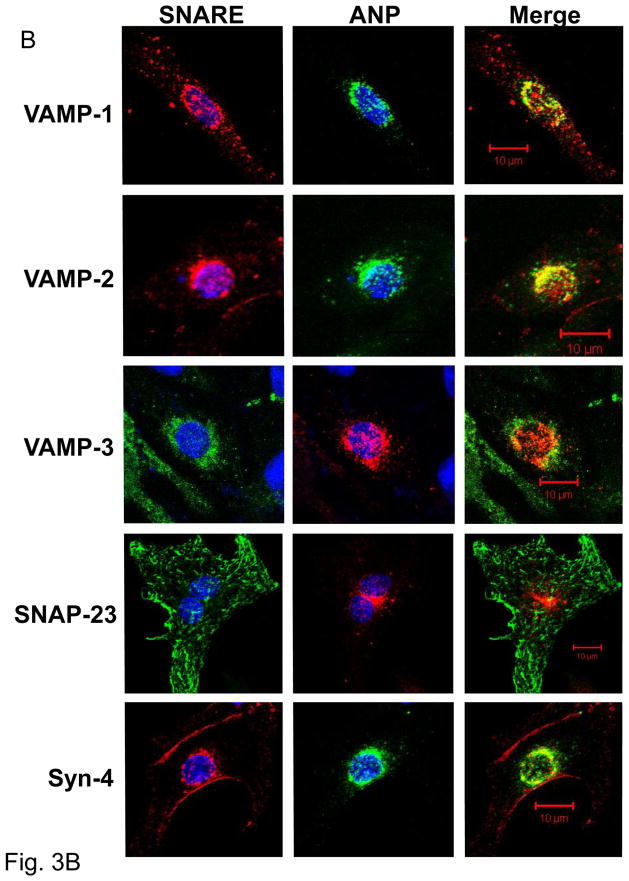
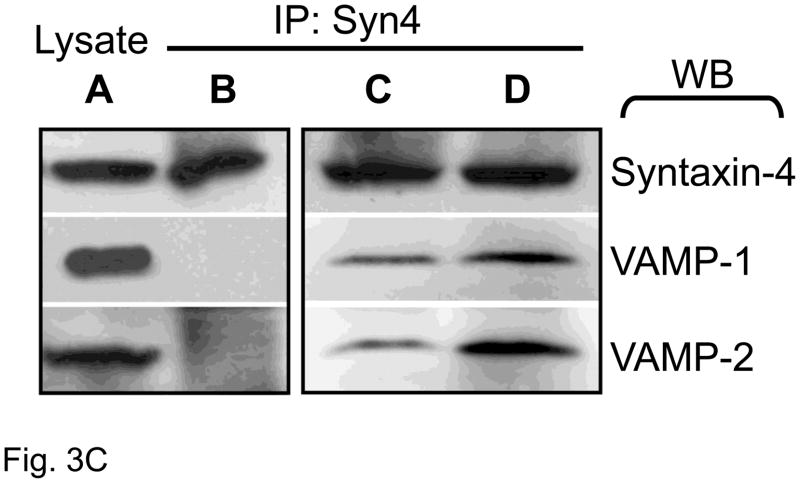
Figure 3. Co-localization of ANP and SNAREs.
(A) Sucrose density centrifugation. Co-sedimentation in the granular fraction of ANP, VAMP-1, and VAMP-2. Syntaxin-4 partially sediments with ANP. The proteins 14-3-3 and cadherin were used as control for cytosolic and plasma membrane fractions respectively. Granular fraction: Gran, plasma membrane fraction: PM, and cytoplasmic fraction: Cyto. (B) Confocal microscopy of ANP with SNARE molecules in cardiac myocytes. (C) Co-immunoprecipitation of Syntaxin-4 with SNARE molecules. Left panel: Lysates (A) were immunoprecipitated with an antibody against synatxin-4 (IP: Syn4), and precipitants were immunoblotted (WB). Cardiac myocytes were pre-treated with buffer (B), or with NEM and DTT simultaneously for 30 min (C), or with NEM for 15 min followed by DTT for 15 min (D).
We also performed confocal immunocytochemistry to visualize the distribution of ANP and SNARE proteins (Fig. 3B). ANP is distributed in cardiac myocytes in a punctate perinuclear pattern, matching prior reports.[26, 30] [31]. VAMP-1 partially overlaps with ANP, with a Pearson’s correlation coefficient of 0.52 ± 0.07 (Fig. 3B, top). VAMP-2 also co-localizes with ANP, with a Pearson’s coefficient is 0.81 ± 0.04 (Fig. 3B, second row). The presence and localization of VAMP-3 was also examined. VAMP-3 appears in a punctuate fashion around the nuclei and in the cytoplasm. Only a small portion of VAMP-3 localizes with ANP with a Pearson’s coefficient of 0.14 ±0.05 (Fig. 3B, third row).
SNAP-23 is also expressed in cardiac myocytes and localizes in the periphery of cells with the plasma membrane. SNAP-23 is also visible also within the cardiac myocyte associated with linear structures of the cells (Fig. 3B fourth row). Although SNAP-23 is a plasma membrane protein, it has also been shown to associate with the cytoskeleton in adipocytes [32]. Syntaxin-4 is distributed mainly at the periphery of the cardiac myocyte to the plasma membrane with some presence in the perinuclear region; the majority of syntaxin-4 does not overlap with ANP, with a Pearson’s correlation coefficient of 0.35 ± 0.01 (Fig. 3B, bottom).
Taken together these sedimentation and imaging data suggest that ANP co-localizes with a subset of SNARE molecules.
SNARE interactions in cardiac myocytes
SNARE molecules interact to form SNARE complexes composed of three SNAREs necessary for membrane fusion. We hypothesized that syntaxin-4, VAMP-1, and VAMP-2 form a ternary SNARE complex inside cardiac myocytes. To test this idea, we immunoprecipitated syntaxin-4, and then probed precipitants for VAMP-1 and VAMP-2. Our initial studies showed that syntaxin-4 does not interact with VAMP-1 and VAMP-2 (Fig. 3C, lane B). Although SNARE molecules can assemble into a complex, the protein N-ethylmaleimide sensitive factor (NSF) can associate with SNAREs and rapidly disassemble the complex into separate SNARE components. We therefore treated cardiac myocytes with N-ethylmaleimide to block NSF, and then searched again for an interaction between SNAREs. After NSF inhibition, syntaxin-4 forms a complex containing both VAMP-1 and VAMP-2 (Fig. 3C, lanes C and D). Thus three specific SNAREs form a complex inside cardiac myocytes.
Endothelin Induces ANP Release
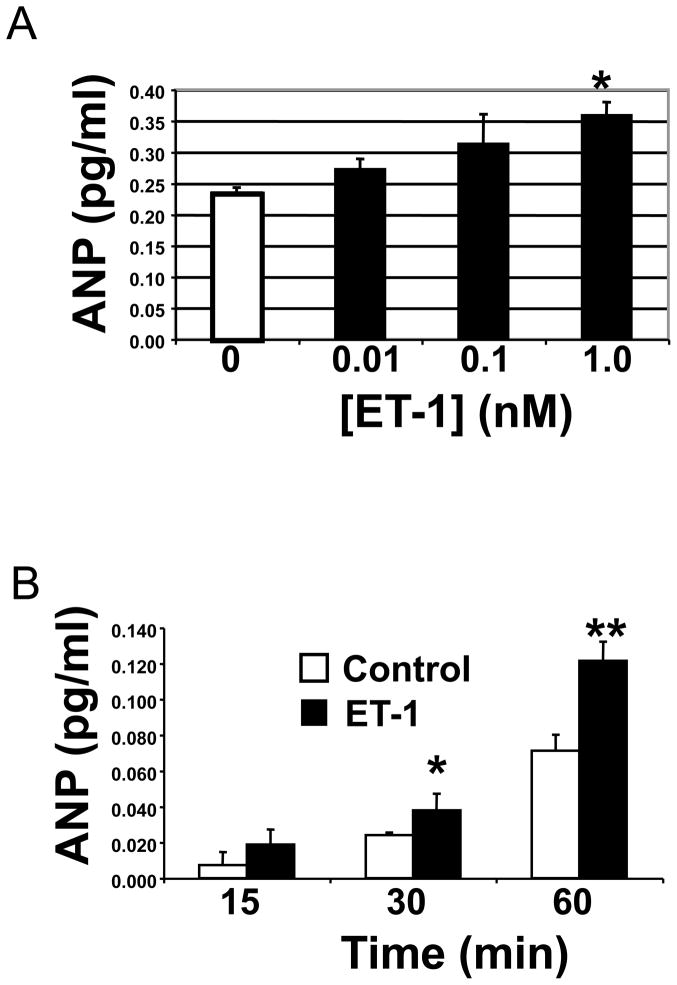
We confirmed that endothelin activates cardiac myocyte exocytosis of ANP in vitro.[12, 33, 34] We added increasing concentrations of endothelin-1 (ET-1) for 1 h to cardiac myocytes and measured ANP release by an ELISA. ET-1 induces ANP release in a dose-dependent manner (Fig. 4A). Cardiac myocytes continue to release ANP after exposure to ET-1 over 60 min (Fig. 4B).
Figure 4. Endothelin-1 induces ANP release.
(A) Primary cardiac myocytes were incubated with media alone or increasing concentrations of endothelin-1 (ET-1) for 1 h. Supernatants were harvested and ANP measured by ELISA (n = 4 ± S.D. and repeated twice with similar results; *P = 0.01 vs. 0 nM). (B) Primary cardiac myocytes were incubated with media alone (white) or with 1 nM endothelin-1 (black) for 15, 30 and 60 minutes. Supernatants were harvested and assessed for ANP by ELISA (n = 4 ± S.D. and repeated twice; *P = 0.05 vs. control; **P < 0.001 vs. control).
SNAREs Regulate ANP Release
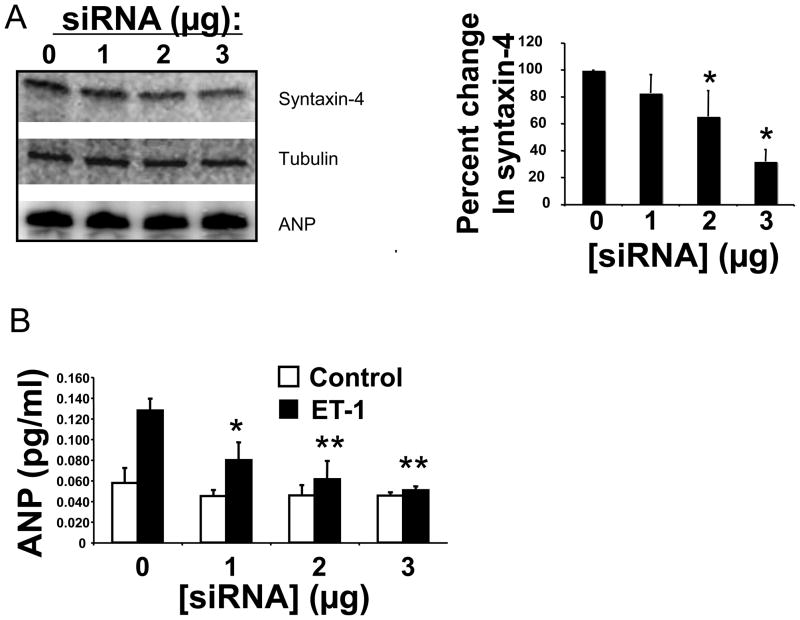
Syntaxin-4 is expressed mostly on the plasma membrane of cardiac myocytes, as well as the perinuclear region (Fig. 3B). In other cells, syntaxin-4 interacts with vesicle associated SNARE proteins, leading to exocytosis of the granules. We used RNA silencing to assess the role of syntaxin-4 in ANP exocytosis. We transfected cardiac myocytes with oligonucleotides directed against syntaxin-4. In cardiac myocytes, increasing amounts of siRNA against syntaxin-4 decreases syntaxin-4 expression without affecting ANP intracellular expression (Fig. 5A). We then stimulated these transfected cardiac myocytes with ET-1. Silencing of syntaxin-4 decreases ANP release in a dose-dependent manner (Fig. 5B). These data support the idea that syntaxin-4 regulates ANP release from cardiac myocytes.
Figure 5. Syntaxin-4 regulates ANP release.
(A) Cardiac myocytes were transfected with an oligonucleotide directed against syntaxin-4, and cell lysates were immunoblotted for syntaxin-4 or ANP. Densitometry was used to quantify the immunoblot (n = 3–4) (right panel). (B) Knockdown of syntaxin-4 in cardiac myocytes blocks ANP exocytosis. Neonatal cardiac myocytes were transfected with 0, 1, 2 or 3 μg of siRNA directed against syntaxin-4. After 48 h cells were stimulated with ET-1 for 1 h and the release of ANP was measured (n=3; *P = 0.05 vs. 0 siRNA; **P < 0.01 vs. siRNA).
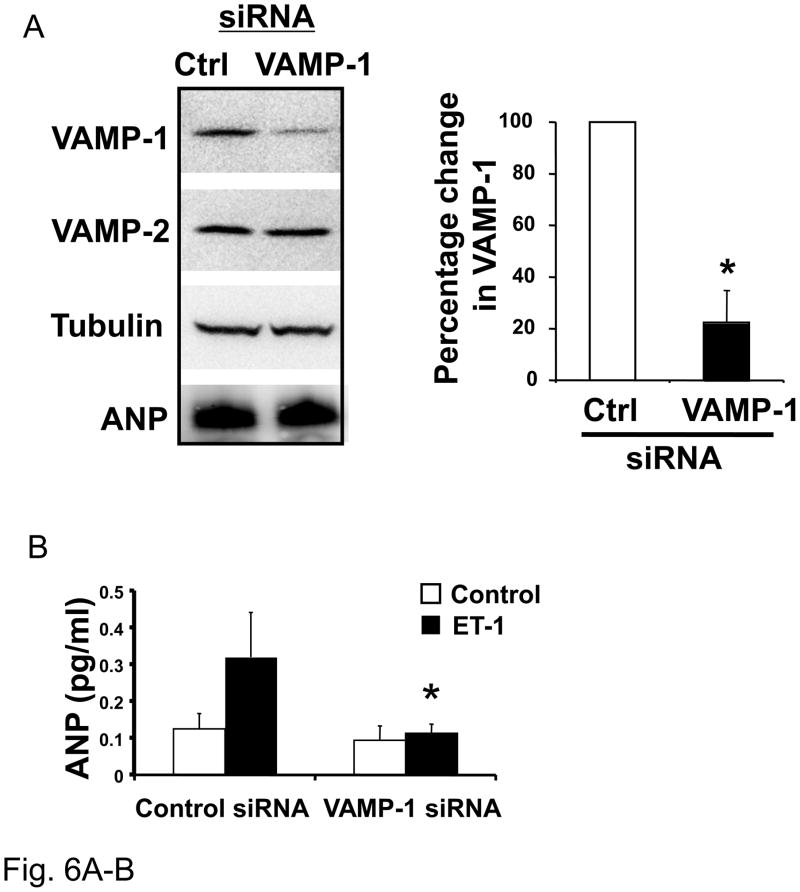
In other cell types, VAMP-1 or VAMP-2 or VAMP-3 is present on the surface of granules and interact with specific SNARE proteins on the plasma membrane, such as syntaxin-4, leading to membrane fusion. Since we found that VAMP-1, VAMP-2 and VAMP-3 are expressed in cardiac myocytes, we next explored the role of these proteins in the release of ANP from cardiac myocytes. We transfected cardiac myocytes with a control scrambled oligonucleotide or with an oligonucleotide directed against VAMP-1 (Fig. 6A). RNA silencing decreases VAMP-1 expression only in the cells transfected with the specific siRNA and does not affect the expression of VAMP-2 nor the expression of intracellular content of ANP (Fig. 6A). We then stimulated these transfected cells with ET-1. Silencing of VAMP1 decreases stimulated ANP release (Fig. 6B). These data suggest that VAMP1 mediates cardiac myocytes exocytosis.
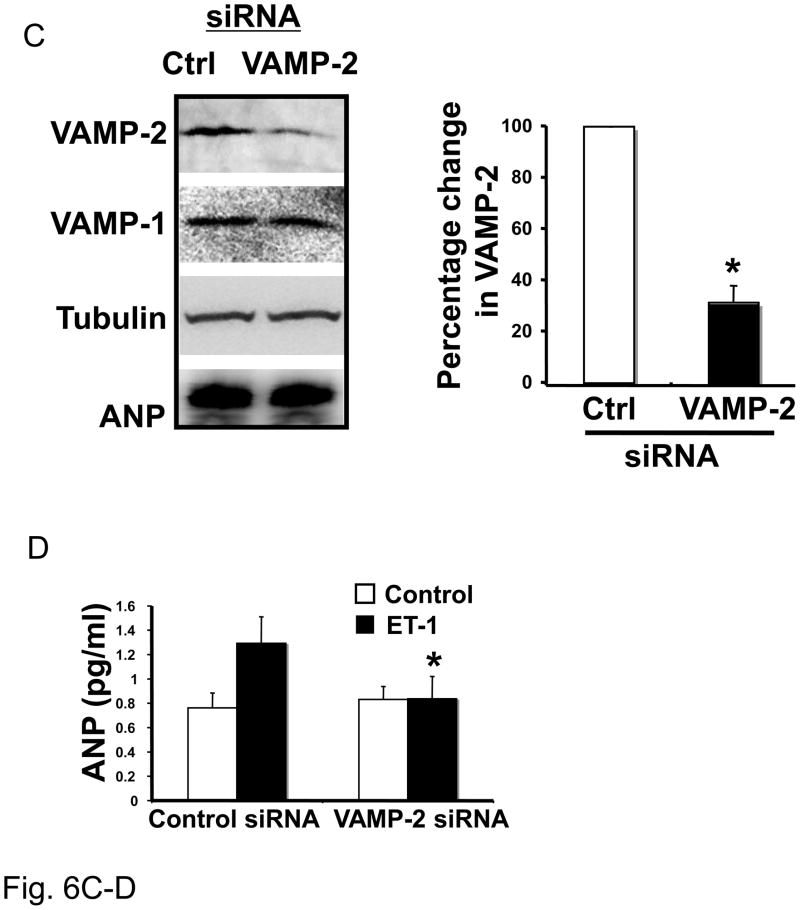
Figure 6. VAMP-1 and VAMP-2 regulate ANP release.
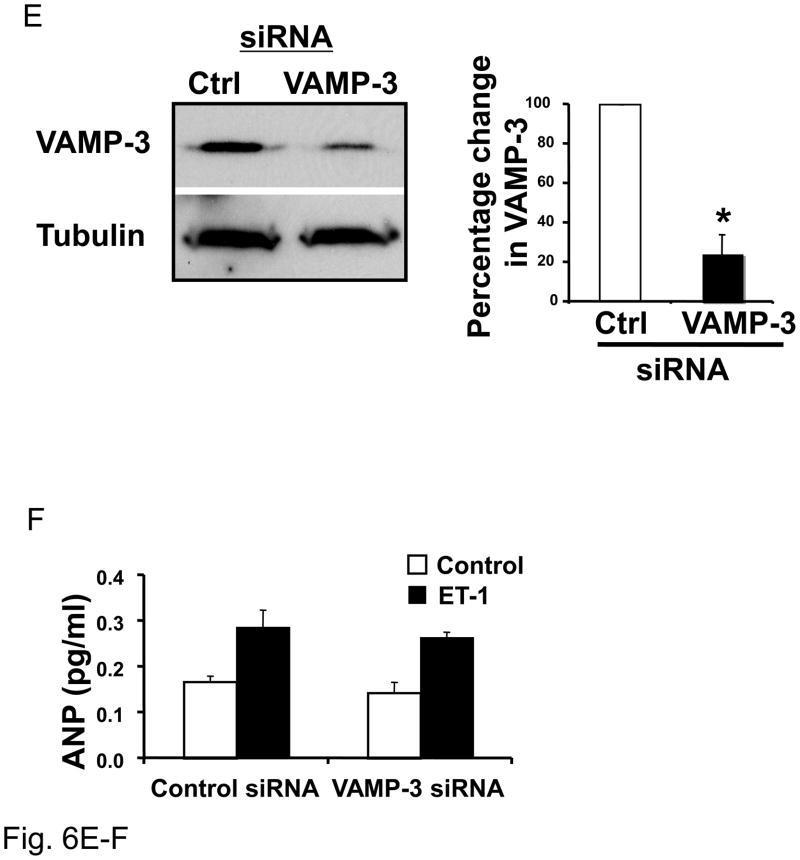
(A) Knockdown of VAMP-1 in cardiac myocytes. Neonatal cardiac myocytes were transfected with 1 nM siRNA directed against VAMP-1 or a control siRNA and cultured for 72 hours. Cell lysates were immunoblotted for VAMP-1, VAMP-2, tubulin and ANP. Densitometry with n = 3 (right panel). (B) Knockdown of VAMP-1 in cardiac myocytes. Neonatal cardiac myocytes were transfected with 1 nM oligonucleotide directed against VAMP-1 or a control oligonucleotide for 72 hours. Cells were then stimulated with medium alone (Control) or ET-1 for 1 hour and ANP released in the medium was assessed by ELISA (n=3; *P < 0.01 vs. control/ET-1). (C) Knockdown of VAMP-2 decreases VAMP-2 but not VAMP-1, tubulin or ANP. Densitometric analysis (right panel, n = 3). (D) After transfection cells were stimulated with ET-1 for 1 hour and ANP measured in the medium by ELISA (n=3). (E) Knockdown of VAMP-3 does not affect ANP exocytosis. After transfection with siRNA for VAMP-3 or control siRNA proteins were immunoblotted for VAMP-3 or tubulin (representative immunoblot in left panel) and densitometric analysis is shown on the right (n=3). (F) After transfection, cells were stimulated with ET-1 for 1 hour before the assessment of ANP in the media (n = 3 P > 0.05 for ET-1 treatment comparing control siRNA vs. VAMP-3 siRNA).
The same approach was used to study the role of VAMP-2 and VAMP-3 in ANP release from stimulated cardiac myocytes. Transfection of cardiac myocytes with siRNA directed against VAMP-2 decreased VAMP-2 expression but not VAMP-1 or ANP expression (Fig. 6C), and control siRNA did not have any effect on expression of these proteins. Cells were then stimulated with ET-1 and ANP release in the extracellular medium assessed by ELISA. Knock-down of VAMP-2 in cardiac myocytes resulted in reduced ANP release from stimulated cells to basal level (Fig. 6D).
In contrast to VAMP-1 and VAMP-2, the SNARE protein VAMP-3 does not play any role in stimulated exocytosis of ANP in cardiac myocytes (Fig. 6E and F). Instead, silencing of VAMP-3 did not change the levels of ANP released from cardiac myocytes after stimulation with ET-1.
Discussion
Our major finding is that three SNAREs molecules that regulate cardiac myocyte exocytosis of ANP: VAMP-1, VAMP-2, and syntaxin-4. VAMP-1 and VAMP-2 partially co-localize with ANP granules. Knockdown of VAMP-1 or VAMP-2 decreases ANP release after stimulation with endothelin-1. Cardiac myocytes also express another SNARE, syntaxin-4; syntaxin-4 is found mainly in the plasma membrane, and knockdown of syntaxin-4 also inhibits ANP secretion. Finally, these three SNARE molecules VAMP-1, VAMP-2, and syntaxin-4 form a complex. These results suggest that VAMP-1, VAMP-2 and syntaxin-4 modulate exocytosis of ANP from cardiac myocytes.
Identification of SNAREs that regulate ANP release
We identified VAMP-1, VAMP-2 and syntaxin-4 as three potential SNAREs regulating ANP release. Although others have found that VAMP-1 and VAMP-2 are associated with ANP secretory granules,[26] our data extend these findings by demonstrating the functional importance of VAMP-1, VAMP-2 and syntaxin-4 in exocytosis of ANP. The role of these proteins inside cardiac myocytes was previously unknown, and the identity of proteins controlling ANP exocytosis was also previously unknown..
VAMP-2 regulates secretion of neurotransmitters from neurons and GLUT4 trafficking in muscle and adipose cells.[35–37] Our study adds ANP granule trafficking as another process controlled by VAMP-2. Previous work suggest an important role for VAMP-3 in general receptor recycling from endosome compartment to the plasma membrane and in phagocytosis [38–40]. Conflicting results have emerged regarding the role of VAMP-3 on platelet secretion [41, 42]. Our studies show that VAMP-3 does not play a role in ANP release. We found that syntaxin-4 is a third SNARE that regulates ANP secretion from cardiac myocytes. Others have found that syntaxin-4 regulates vesicular transport of the glucose transporter 4 (GLUT4) [43, 44], and trafficking of membrane-type 1 matrix metalloproteinase (MT1-MMP) to the plasma membrane [45].
Components of the exocytic machinery in cardiac myocytes
In addition to the SNARE molecules, we found that cardiac myocytes express additional components of the exocytic machinery, including NSF and its adaptor molecule alpha-SNAP, synaptotagmin, and rab4 and rab8 (Fig. 1B). These proteins and related isoforms regulate exocytosis from a variety of other cells such as neurons, platelets, and endothelial cells. Therefore NSF and specific rab proteins may also mediate ANP release.
Intracellular localization of ANP granules
Our immunostaining shows that ANP granules are present mostly in the perinuclear area of cardiac myocytes (Fig. 3). This result confirms previous findings from other investigators [26, 30, 31, 46, 47]. However, in other secretory cell types, granules are more widely distributed throughout the cell, in the perinuclear area, the cytoplasm, and in proximity of the plasma membrane. Why is ANP granule distribution in myocytes different compared to granule distribution in other cell types? One possibility is that a structural difference between cardiac myocytes and other exocytic cells accounts for differing localization of granules. Cardiac cells contain a transverse-axial tubular system to control calcium influx. In ventricular myocytes, this is a well developed system of transverse tubules (T-tubules) that play an important role in spreading uniform depolarization in the cell with Ca2+ release from the sarcoplasmic reticulum (SR). Atrial cells as well as rat neonatal myocytes do not posses a well developed T-tubules system but they contain a form of transverse oriented tubules formed from the internal SR[48–52]. It is possible that this system of internal invaginations and compartments can play a functional role in docking of ANP granules that are found in the perinuclear region of the cells. (In this study we used neonatal cardiac myocytes, and the structure of T-tubules is very similar between adult atrial myocytes and neonatal cardiac myocytes [53].)
The location of the ANP granules in the center of the cell suggests a solution to the problem: how does normal calcium cycling within a cardiac myocyte cause contraction without exocytosis? Calcium sparks localized to the periphery of atrial myocytes can stimulate contraction [50, 54]. Since calcium can also trigger exocytosis, sequestering granules in the center of the cell away from calcium sparks at the periphery of the cell might prevent continuous granule secretion.
How do external triggers activate ANP exocytosis?
ANP exocytosis can be triggered by a variety of physiological stimuli such as atrial distension or ischemia.[7, 12] Paracrine factors such as endothelin and angiotensin II can directly activate atrial myocytes to release ANP; other neurohormonal factors such as vasopressin or some catecholamines can also activate ANP secretion, perhaps directly or indirectly through hemodynamic changes. All of these various agents trigger unique signal transduction pathways, but our studies suggest that VAMP-1, VAMP-2 and Syntaxin-4 are part of exocytic machinery that is responsible for the final step of ANP release.
One second messenger that many of these pathways have in common is Ca2+. The role of Ca2+ in atrial myocyte exocytosis is complex.[12] Ca2+ may be involved in many steps along the pathway of myocyte exocytosis, including transduction of stretching of the myocytes, intracellular signaling, and exocytosis. Ca2+ plays a role in exocytosis by regulating the Ca2+ sensor synaptotagmin. Recent studies suggest that the SNARE complex by itself can drive membrane fusion. However, the accessory molecule complexin serves as a clamp around the SNARE complex to block membrane fusion. During stimulation, synaptotagmin removes the complexin clamp, permitting membrane fusion and exocytosis.[55–57] Thus the many different activators of exocytosis might ultimately converge on the exocytic machinery by regulating synaptotagmin and complexin.
Regulated vs. constitutive ANP exocytosis
Our data show that three specific SNAREs control regulated ANP release. Although silencing each of these SNAREs decreases stimulated ANP release, it does not affect constitutive ANF release (Figs. 5–6). These results suggest that ANP can be secreted through two distinct pathways, and that these pathways do not share SNAREs syntaxin-4, VAMP-1, and VAMP-2. These results are similar to studies in other systems that note distinct constitutive and regulated secretory pathways. For example, platelets from VAMP8 knockout mice release fewer dense and lysosomal and secretory granules than platelets from wild-type mice after stimulation, but platelets from both VAMP8 knockout and wild-type mice release the same levels of granules in the absence of stimulation [58].
Acknowledgments
Supported by grants from the NIH (R01 HL63706, R01 HL074061, P01 HL65608, P01 HL56091), and the Paul N. Yu Professorship to CJL. Supported by grants from the AHA (0830395N) to MF.
Footnotes
Disclosures. The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Bold AJ. Atrial natriuretic factor of the rat heart. Studies on isolation and properties. Proc Soc Exp Biol Med. 1982 Jun;170(2):133–8. doi: 10.3181/00379727-170-41408. [DOI] [PubMed] [Google Scholar]
- 2.de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981 Jan 5;28(1):89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 3.Munagala VK, Burnett JC, Jr, Redfield MM. The natriuretic peptides in cardiovascular medicine. Curr Probl Cardiol. 2004 Dec;29(12):707–69. doi: 10.1016/j.cpcardiol.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 4.John SW, Veress AT, Honrath U, Chong CK, Peng L, Smithies O, et al. Blood pressure and fluid-electrolyte balance in mice with reduced or absent ANP. Am J Physiol. 1996 Jul;271(1 Pt 2):R109–14. doi: 10.1152/ajpregu.1996.271.1.R109. [DOI] [PubMed] [Google Scholar]
- 5.Steinhelper ME, Cochrane KL, Field LJ. Hypotension in transgenic mice expressing atrial natriuretic factor fusion genes. Hypertension. 1990 Sep;16(3):301–7. doi: 10.1161/01.hyp.16.3.301. [DOI] [PubMed] [Google Scholar]
- 6.Cataliotti A, Burnett JC., Jr Natriuretic peptides: novel therapeutic targets in heart failure. J Investig Med. 2005 Nov;53(7):378–84. doi: 10.2310/6650.2005.53711. [DOI] [PubMed] [Google Scholar]
- 7.Lee CY, Burnett JC., Jr Natriuretic peptides and therapeutic applications. Heart Fail Rev. 2007 Jun;12(2):131–42. doi: 10.1007/s10741-007-9016-3. [DOI] [PubMed] [Google Scholar]
- 8.Rose RA, Giles WR. Natriuretic peptide C receptor signalling in the heart and vasculature. J Physiol. 2008 Jan 15;586(2):353–66. doi: 10.1113/jphysiol.2007.144253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahluwalia A, MacAllister RJ, Hobbs AJ. Vascular actions of natriuretic peptides. Cyclic GMP-dependent and -independent mechanisms. Basic Res Cardiol. 2004 Mar;99(2):83–9. doi: 10.1007/s00395-004-0459-6. [DOI] [PubMed] [Google Scholar]
- 10.de Bold AJ, de Bold ML. Determinants of natriuretic peptide production by the heart: basic and clinical implications. J Investig Med. 2005 Nov;53(7):371–7. doi: 10.2310/6650.2005.53710. [DOI] [PubMed] [Google Scholar]
- 11.Yan W, Wu F, Morser J, Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci U S A. 2000 Jul 18;97(15):8525–9. doi: 10.1073/pnas.150149097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dietz JR. Mechanisms of atrial natriuretic peptide secretion from the atrium. Cardiovasc Res. 2005 Oct 1;68(1):8–17. doi: 10.1016/j.cardiores.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Doubell AF. The effect of calcium antagonists on atrial natriuretic peptide (ANP) release from the rat heart during rapid cardiac pacing. J Mol Cell Cardiol. 1989 May;21(5):437–40. doi: 10.1016/0022-2828(89)90783-9. [DOI] [PubMed] [Google Scholar]
- 14.Doubell AF, Thibault G. Calcium is involved in both positive and negative modulation of the secretory system for ANP. Am J Physiol. 1994 May;266(5 Pt 2):H1854–63. doi: 10.1152/ajpheart.1994.266.5.H1854. [DOI] [PubMed] [Google Scholar]
- 15.Glembotski CC, Irons CE, Sprenkle AB, Sei CA. Studies of ANF processing and secretion using a primary cardiocyte culture model. Can J Physiol Pharmacol. 1991 Oct;69(10):1525–36. doi: 10.1139/y91-228. [DOI] [PubMed] [Google Scholar]
- 16.Veress AT, Milojevic S, Yip C, Flynn TG, Sonnenberg H. In vitro secretion of atrial natriuretic factor: receptor-mediated release of prohormone. Am J Physiol. 1988 May;254(5 Pt 2):R809–14. doi: 10.1152/ajpregu.1988.254.5.R809. [DOI] [PubMed] [Google Scholar]
- 17.Han JH, Cao C, Kim SZ, Cho KW, Kim SH. Decreases in ANP secretion by lysophosphatidylcholine through protein kinase C. Hypertension. 2003 Jun;41(6):1380–5. doi: 10.1161/01.HYP.0000071317.98004.B3. [DOI] [PubMed] [Google Scholar]
- 18.Bensimon M, Chang AI, de Bold ML, Ponce A, Carreras D, De Bold AJ. Participation of G proteins in natriuretic peptide hormone secretion from heart atria. Endocrinology. 2004 Nov;145(11):5313–21. doi: 10.1210/en.2004-0698. [DOI] [PubMed] [Google Scholar]
- 19.Iida H, Page E. Inhibition of atrial natriuretic peptide secretion by forskolin in noncontracting cultured atrial myocytes. Biochem Biophys Res Commun. 1988 Nov 30;157(1):330–6. doi: 10.1016/s0006-291x(88)80051-2. [DOI] [PubMed] [Google Scholar]
- 20.Muir TM, Hair J, Inglis GC, Dow JW, Lindop GB, Leckie BJ. Hormonal control of atrial natriuretic peptide synthesis and secretion from cultured atrial myocytes. J Mol Cell Cardiol. 1993 May;25(5):509–18. doi: 10.1006/jmcc.1993.1061. [DOI] [PubMed] [Google Scholar]
- 21.Jahn R, Lang T, Sudhof TC. Membrane fusion. Cell. 2003 Feb 21;112(4):519–33. doi: 10.1016/s0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 22.Jahn R, Sudhof TC. Membrane fusion and exocytosis. Annu Rev Biochem. 1999;68:863–911. doi: 10.1146/annurev.biochem.68.1.863. [DOI] [PubMed] [Google Scholar]
- 23.Mellman I, Warren G. The road taken: past and future foundations of membrane traffic. Cell. 2000 Jan 7;100(1):99–112. doi: 10.1016/s0092-8674(00)81687-6. [DOI] [PubMed] [Google Scholar]
- 24.Sevilla L, Tomas E, Munoz P, Guma A, Fischer Y, Thomas J, et al. Characterization of two distinct intracellular GLUT4 membrane populations in muscle fiber. Differential protein composition and sensitivity to insulin. Endocrinology. 1997 Jul;138(7):3006–15. doi: 10.1210/endo.138.7.5235. [DOI] [PubMed] [Google Scholar]
- 25.Kang Y, Leung YM, Manning-Fox JE, Xia F, Xie H, Sheu L, et al. Syntaxin-1A inhibits cardiac KATP channels by its actions on nucleotide binding folds 1 and 2 of sulfonylurea receptor 2A. J Biol Chem. 2004 Nov 5;279(45):47125–31. doi: 10.1074/jbc.M404954200. [DOI] [PubMed] [Google Scholar]
- 26.Peters CG, Miller DF, Giovannucci DR. Identification, localization and interaction of SNARE proteins in atrial cardiac myocytes. J Mol Cell Cardiol. 2006 Mar;40(3):361–74. doi: 10.1016/j.yjmcc.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Cingolani E, Ramirez Correa GA, Kizana E, Murata M, Cho HC, Marban E. Gene therapy to inhibit the calcium channel beta subunit: physiological consequences and pathophysiological effects in models of cardiac hypertrophy. Circ Res. 2007 Jul 20;101(2):166–75. doi: 10.1161/CIRCRESAHA.107.155721. [DOI] [PubMed] [Google Scholar]
- 28.Ferlito M, Irani K, Faraday N, Lowenstein CJ. Nitric oxide inhibits exocytosis of cytolytic granules from lymphokine-activated killer cells. Proc Natl Acad Sci U S A. 2006 Aug 1;103(31):11689–94. doi: 10.1073/pnas.0600275103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei YF, Rodi CP, Day ML, Wiegand RC, Needleman LD, Cole BR, et al. Developmental changes in the rat atriopeptin hormonal system. J Clin Invest. 1987 May;79(5):1325–9. doi: 10.1172/JCI112957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doyle DD, Ambler SK, Upshaw-Earley J, Bastawrous A, Goings GE, Page E. Type B atrial natriuretic peptide receptor in cardiac myocyte caveolae. Circ Res. 1997 Jul;81(1):86–91. doi: 10.1161/01.res.81.1.86. [DOI] [PubMed] [Google Scholar]
- 31.Jamieson JD, Palade GE. Specific Granules in Atrial Muscle Cells. J Cell Biol. 1964 Oct;23:151–72. doi: 10.1083/jcb.23.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foster LJ, Yaworsky K, Trimble WS, Klip A. SNAP23 promotes insulin-dependent glucose uptake in 3T3-L1 adipocytes: possible interaction with cytoskeleton. Am J Physiol. 1999 May;276(5 Pt 1):C1108–14. doi: 10.1152/ajpcell.1999.276.5.C1108. [DOI] [PubMed] [Google Scholar]
- 33.Lew RA, Baertschi AJ. Endothelial cells stimulate ANF secretion from atrial myocytes in co-culture. Biochem Biophys Res Commun. 1989 Sep 15;163(2):701–9. doi: 10.1016/0006-291x(89)92280-8. [DOI] [PubMed] [Google Scholar]
- 34.Fukuda Y, Hirata Y, Taketani S, Kojima T, Oikawa S, Nakazato H, et al. Endothelin stimulates accumulations of cellular atrial natriuretic peptide and its messenger RNA in rat cardiocytes. Biochem Biophys Res Commun. 1989 Nov 15;164(3):1431–6. doi: 10.1016/0006-291x(89)91830-5. [DOI] [PubMed] [Google Scholar]
- 35.Deitcher DL, Ueda A, Stewart BA, Burgess RW, Kidokoro Y, Schwarz TL. Distinct requirements for evoked and spontaneous release of neurotransmitter are revealed by mutations in the Drosophila gene neuronal-synaptobrevin. J Neurosci. 1998 Mar 15;18(6):2028–39. doi: 10.1523/JNEUROSCI.18-06-02028.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schoch S, Deak F, Konigstorfer A, Mozhayeva M, Sara Y, Sudhof TC, et al. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science. 2001 Nov 2;294(5544):1117–22. doi: 10.1126/science.1064335. [DOI] [PubMed] [Google Scholar]
- 37.Montecucco C, Schiavo G. Mechanism of action of tetanus and botulinum neurotoxins. Mol Microbiol. 1994 Jul;13(1):1–8. doi: 10.1111/j.1365-2958.1994.tb00396.x. [DOI] [PubMed] [Google Scholar]
- 38.Teter K, Chandy G, Quinones B, Pereyra K, Machen T, Moore HP. Cellubrevin-targeted fluorescence uncovers heterogeneity in the recycling endosomes. J Biol Chem. 1998 Jul 31;273(31):19625–33. doi: 10.1074/jbc.273.31.19625. [DOI] [PubMed] [Google Scholar]
- 39.Daro E, van der Sluijs P, Galli T, Mellman I. Rab4 and cellubrevin define different early endosome populations on the pathway of transferrin receptor recycling. Proc Natl Acad Sci U S A. 1996 Sep 3;93(18):9559–64. doi: 10.1073/pnas.93.18.9559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hackam DJ, Rotstein OD, Sjolin C, Schreiber AD, Trimble WS, Grinstein S. v-SNARE-dependent secretion is required for phagocytosis. Proc Natl Acad Sci U S A. 1998 Sep 29;95(20):11691–6. doi: 10.1073/pnas.95.20.11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schraw TD, Rutledge TW, Crawford GL, Bernstein AM, Kalen AL, Pessin JE, et al. Granule stores from cellubrevin/VAMP-3 null mouse platelets exhibit normal stimulus-induced release. Blood. 2003 Sep 1;102(5):1716–22. doi: 10.1182/blood-2003-01-0331. [DOI] [PubMed] [Google Scholar]
- 42.Polgar J, Chung SH, Reed GL. Vesicle-associated membrane protein 3 (VAMP-3) and VAMP-8 are present in human platelets and are required for granule secretion. Blood. 2002 Aug 1;100(3):1081–3. doi: 10.1182/blood.v100.3.1081. [DOI] [PubMed] [Google Scholar]
- 43.Volchuk A, Wang Q, Ewart HS, Liu Z, He L, Bennett MK, et al. Syntaxin 4 in 3T3-L1 adipocytes: regulation by insulin and participation in insulin-dependent glucose transport. Mol Biol Cell. 1996 Jul;7(7):1075–82. doi: 10.1091/mbc.7.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tellam JT, Macaulay SL, McIntosh S, Hewish DR, Ward CW, James DE. Characterization of Munc-18c and syntaxin-4 in 3T3-L1 adipocytes. Putative role in insulin-dependent movement of GLUT-4. J Biol Chem. 1997 Mar 7;272(10):6179–86. doi: 10.1074/jbc.272.10.6179. [DOI] [PubMed] [Google Scholar]
- 45.Miyata T, Ohnishi H, Suzuki J, Yoshikumi Y, Ohno H, Mashima H, et al. Involvement of syntaxin 4 in the transport of membrane-type 1 matrix metalloproteinase to the plasma membrane in human gastric epithelial cells. Biochem Biophys Res Commun. 2004 Oct 8;323(1):118–24. doi: 10.1016/j.bbrc.2004.08.064. [DOI] [PubMed] [Google Scholar]
- 46.Thibault G, Haile-Meskel H, Wrobel-Konrad E, Ballak M, Garcia R, Genest J, et al. Processing of the atrial natriuretic factor propeptide by atrial cardiocytes as revealed by immunocryoultramicrotomy. Endocrinology. 1989 Jun;124(6):3109–16. doi: 10.1210/endo-124-6-3109. [DOI] [PubMed] [Google Scholar]
- 47.Slot JW, Garruti G, Martin S, Oorschot V, Posthuma G, Kraegen EW, et al. Glucose transporter (GLUT-4) is targeted to secretory granules in rat atrial cardiomyocytes. J Cell Biol. 1997 Jun 16;137(6):1243–54. doi: 10.1083/jcb.137.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berlin JR. Spatiotemporal changes of Ca2+ during electrically evoked contractions in atrial and ventricular cells. Am J Physiol. 1995 Sep;269(3 Pt 2):H1165–70. doi: 10.1152/ajpheart.1995.269.3.H1165. [DOI] [PubMed] [Google Scholar]
- 49.Huser J, Lipsius SL, Blatter LA. Calcium gradients during excitation-contraction coupling in cat atrial myocytes. J Physiol. 1996 Aug 1;494( Pt 3):641–51. doi: 10.1113/jphysiol.1996.sp021521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mackenzie L, Bootman MD, Berridge MJ, Lipp P. Predetermined recruitment of calcium release sites underlies excitation-contraction coupling in rat atrial myocytes. J Physiol. 2001 Feb 1;530(Pt 3):417–29. doi: 10.1111/j.1469-7793.2001.0417k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamasaki Y, Furuya Y, Araki K, Matsuura K, Kobayashi M, Ogata T. Ultra-high-resolution scanning electron microscopy of the sarcoplasmic reticulum of the rat atrial myocardial cells. Anat Rec. 1997 May;248(1):70–5. doi: 10.1002/(SICI)1097-0185(199705)248:1<70::AID-AR8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 52.Franzini-Armstrong C, Protasi F, Tijskens P. The assembly of calcium release units in cardiac muscle. Ann N Y Acad Sci. 2005 Jun;1047:76–85. doi: 10.1196/annals.1341.007. [DOI] [PubMed] [Google Scholar]
- 53.Bootman MD, Higazi DR, Coombes S, Roderick HL. Calcium signalling during excitation-contraction coupling in mammalian atrial myocytes. J Cell Sci. 2006 Oct 1;119(Pt 19):3915–25. doi: 10.1242/jcs.03223. [DOI] [PubMed] [Google Scholar]
- 54.Kockskamper J, Sheehan KA, Bare DJ, Lipsius SL, Mignery GA, Blatter LA. Activation and propagation of Ca(2+) release during excitation-contraction coupling in atrial myocytes. Biophys J. 2001 Nov;81(5):2590–605. doi: 10.1016/S0006-3495(01)75903-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giraudo CG, Eng WS, Melia TJ, Rothman JE. A clamping mechanism involved in SNARE-dependent exocytosis. Science. 2006 Aug 4;313(5787):676–80. doi: 10.1126/science.1129450. [DOI] [PubMed] [Google Scholar]
- 56.Schaub JR, Lu X, Doneske B, Shin YK, McNew JA. Hemifusion arrest by complexin is relieved by Ca2+-synaptotagmin I. Nat Struct Mol Biol. 2006 Aug;13(8):748–50. doi: 10.1038/nsmb1124. [DOI] [PubMed] [Google Scholar]
- 57.Tang J, Maximov A, Shin OH, Dai H, Rizo J, Sudhof TC. A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell. 2006 Sep 22;126(6):1175–87. doi: 10.1016/j.cell.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 58.Ren Q, Barber HK, Crawford GL, Karim ZA, Zhao C, Choi W, et al. Endobrevin/VAMP-8 is the primary v-SNARE for the platelet release reaction. Mol Biol Cell. 2007 Jan;18(1):24–33. doi: 10.1091/mbc.E06-09-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]