Abstract
The volatile anesthetic isoflurane is capable of inducing preconditioning and postconditioning effects in the brain. However, the mechanisms for these neuroprotective effects are not fully understood. Here, we showed that rat hippocampal neuronal cultures exposed to 2% isoflurane for 30 min at 24 h before a 1-h oxygen-glucose deprivation (OGD) and a 24-h simulated reperfusion had a reduced lactate dehydrogenase release. Similarly, this OGD and simulated reperfusion-induced lactate dehydrogenase release was attenuated by exposing the neuronal cultures to 2% isoflurane for 1 h at various times after the onset of the simulated reperfusion (isoflurane postconditioning). The combination of isoflurane preconditioning and postconditioning induced a better neuroprotection than either alone. Inhibition of the calcium/calmodulin-dependent protein kinase II (CaMKII), inhibition of N-methyl D-aspartate (NMDA) receptors, or activation of adenosine A2A receptors resulted in reduction of the OGD and simulated reperfusion-induced cell injury. The combination of CaMKII inhibition and isoflurane preconditioning or postconditioning did not provide better protection than CaMKII inhibition, isoflurane preconditioning, or isoflurane postconditioning alone. The combination of NMDA receptor inhibition and isoflurane postconditioning was not better than NMDA receptor inhibition or isoflurane postconditioning alone for neuroprotection. However, the combination of adenosine A2A receptor activation with either isoflurane preconditioning or isoflurane postconditioning induced a better neuroprotective effect than adenosine A2A receptor activation, isoflurane preconditioning, or isoflurane postconditioning alone. The combination of NMDA receptor inhibition and isoflurane preconditioning caused a better neuroprotective effect than NMDA receptor inhibition or isoflurane preconditioning alone. These results suggest that isoflurane preconditioning- and postconditioning-induced neuroprotection can be additive. Isoflurane preconditioning and isoflurane postconditioning may involve CaMKII inhibition, but may not involve adenosine A2A receptor activation. Inhibition of NMDA receptors may mediate the effects of isoflurane postconditioning, but not isoflurane preconditioning.
Keywords: calcium/calmodulin-dependent protein kinase II, isoflurane, neuron, preconditioning, postconditioning
1. INTRODUCTION
Stroke afflicts nearly 800,000 people each year in the United States and is the third leading cause of death (Lloyd-Jones et al., 2009). In addition, many diseases and injuries are thought to be mediated, at least in part, by ischemic conditions in the central nervous system, such as traumatic brain injury, cerebral palsy and spinal cord injury. Despite extensive research efforts in the past decades, few safe and effective interventions have been implemented in producing significant advancements in outcomes after ischemia of the central nervous tissues.
One interesting avenue developed in recent years for neuroprotection is the concept of “conditioning”. The term originally described a phenomenon seen in organs and tissues where brief exposures to ischemic conditions enabled cells to survive subsequent prolonged ischemic conditions. This phenomenon was termed “ischemic preconditioning” (Murry et al., 1986). A similar phenomenon can also be induced with hypoxia as the preconditioning stimulus (Schurr et al., 1986). It has been shown that there are other conditioning stimuli apart from short episodes of ischemia or hypoxia. For example, volatile anesthetics have been shown to provide protection against ischemia, in both cardiac and neural tissues, when used as pre-conditioning stimuli in animal models (Belhomme et al., 1999; Kitano et al., 2007; Li and.Zuo, 2009; Payne et al., 2005; Wang et al., 2008; Zheng and Zuo, 2004). In 2003, the concept of “ischemic postconditioning” was introduced (Zhao et al., 2003). Ischemic postconditioning described a phenomenon in which interruption of the early reperfusion phase by short episodes of ischemia provided protection against cell injury (Zhao, 2007; Zhao et al., 2003; Zhao and Vinten-Johansen, 2006). We have shown that volatile anesthetics can also induce a postconditioning effect (Lee et al., 2008).
The mechanisms of conditioning induced by volatile anesthetics are not fully understood. Glutamate receptor over-activation is a major mechanism contributing to ischemic brain injury (Lipton, 1999). Among the glutamate receptors, over-excitation of the N-methyl D-aspartate (NMDA) receptor can cause fast excitotoxicity (Gwag et al., 2001; Lipton, 1999). We have shown that isoflurane preconditioning reduces cell death induced by NMDA receptor over-activation (Zheng and Zuo, 2005). However, the role of NMDA receptor over-activation in isoflurane preconditioning- and postconditioning-induced neuroprotection against ischemia is not yet known.
The calcium/calmodulin-dependent protein kinase II (CaMKII) is a major mediator of calcium signaling in neurons (Domanska-Janik, 1996). Increased intracellular calcium binds to calmodulin to form a complex, which then binds to and activates CaMKII (Anderson, 2009; Domanska-Janik, 1996). Previous work has shown that anesthetic preconditioning effects are at least in part mediated by intracellular calcium signaling (Bickler and Fahlman, 2010). Interestingly, CaMKII levels in neurons have been shown to decrease during ischemia, and this decrease is attenuated by isoflurane preconditioning (Blanck et al., 2000). Also, anesthetics have been shown to affect CaMKII activity as measured by phosphorylation of CaMKII (Cui et al., 2009). In addition, the isoflurane preconditioning effect on neurons was abolished by calmidazolium, a compound that inhibits calcium-bound calmodulin (Bickler et al., 2005). However, the involvement of CaMKII in the anesthetic preconditioning and postconditioning effects on the brain has not been examined directly.
Adenosine, an endogenous purine nucleoside, binds and activates its receptors (designated A1, A2A, A2B, and A3). The A2A receptors are a major type of adenosine receptor in the brain and have been shown to modulate the release of neurotransmitters, such as glutamate and dopamine (Cunha et al., 2008; Fuxe et al., 2007). Although adenosine receptors have been shown to be involved in volatile anesthetic preconditioning-induced cardioprotection (Roscoe et al., 2000; Toller et al., 2000), the role of adenosine receptors in ischemic brain injury and volatile anesthetic preconditioning and postconditioning effects on the brain is not established.
We designed this study to determine the role of NMDA receptors, adenosine receptors, and the CaMKII enzyme in the isoflurane preconditioning- and postconditioning-induced neuroprotection. We used primary hippocampal neuronal cultures and simulated ischemia by oxygen-glucose deprivation (OGD) in vitro. Exposure to 2% isoflurane for 30 min or 1 h was chosen to be the preconditioning or postconditioning stimulus, respectively, because our previous studies show that preconditioning- and postconditioning-induced neuroprotection can be maximized with the exposure to 2% isoflurane for longer than 20 min (Lee et al., 2008; Zheng and Zuo, 2003).
2. RESULTS
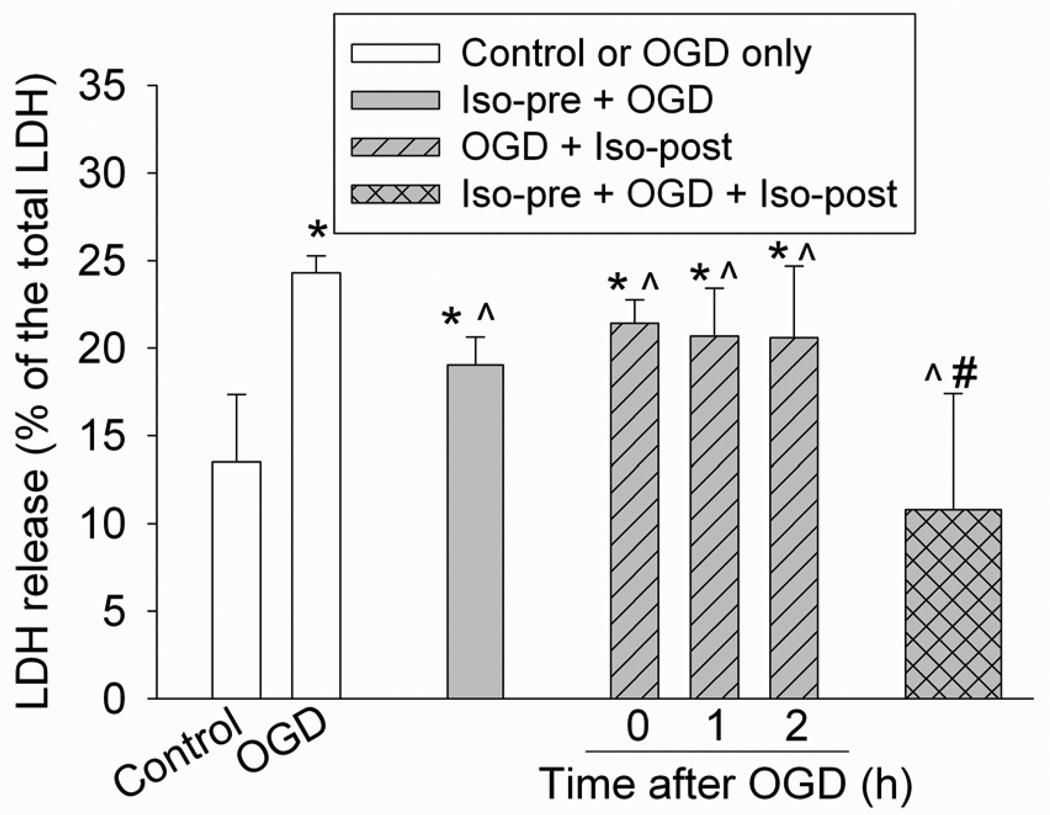
Our OGD and simulated reperfusion condition caused a significant increase of LDH release from cells (Fig. 1), suggesting that this condition induced cell injury. This cell injury was reduced by isoflurane preconditioning for 30 min at 24 h before the OGD and by isoflurane postconditioning applied immediately, 1 h, or 2 h after the onset of the simulated reperfusion. The combination of isoflurane preconditioning and isoflurane postconditioning applied at 1 h after the onset of the simulated reperfusion also attenuated the OGD and simulated reperfusion-induced neuronal injury. This attenuation was greater than the attenuation induced by isoflurane preconditioning or postconditioning alone. In fact, this combination therapy abolished the increased LDH release caused by the OGD and simulated reperfusion (Fig. 1).
Fig. 1. Isoflurane preconditioning- and postconditioning-induced neuroprotection.
Rat hippocampal neurons were preconditioned with 2% isoflurane for 30 min at 24 h before a 1-h oxygen-glucose deprivation (OGD) and a 24-h simulated reperfusion. Postconditioning was performed by exposing rat hippocampal neurons to 2% isoflurane for 1 h at various times after the onset of OGD. The combination of isoflurane preconditioning and postconditioning was performed by isoflurane preconditioning at 24 hr before the OGD and then isoflurane postconditioning at 1 h after the onset of the simulated reperfusion. Results are mean ± S.D. (n = 8 – 16). * P < 0.05 compared with control, ^ P < 0.05 compared with OGD only, # P < 0.05 compared with isoflurane preconditioning or isoflurane postconditioning at 1 h after the onset of simulated reperfusion. Iso-pre: isoflurane preconditioning; Iso-post: isoflurane postconditioning; LDH: lactate dehydrogenase.
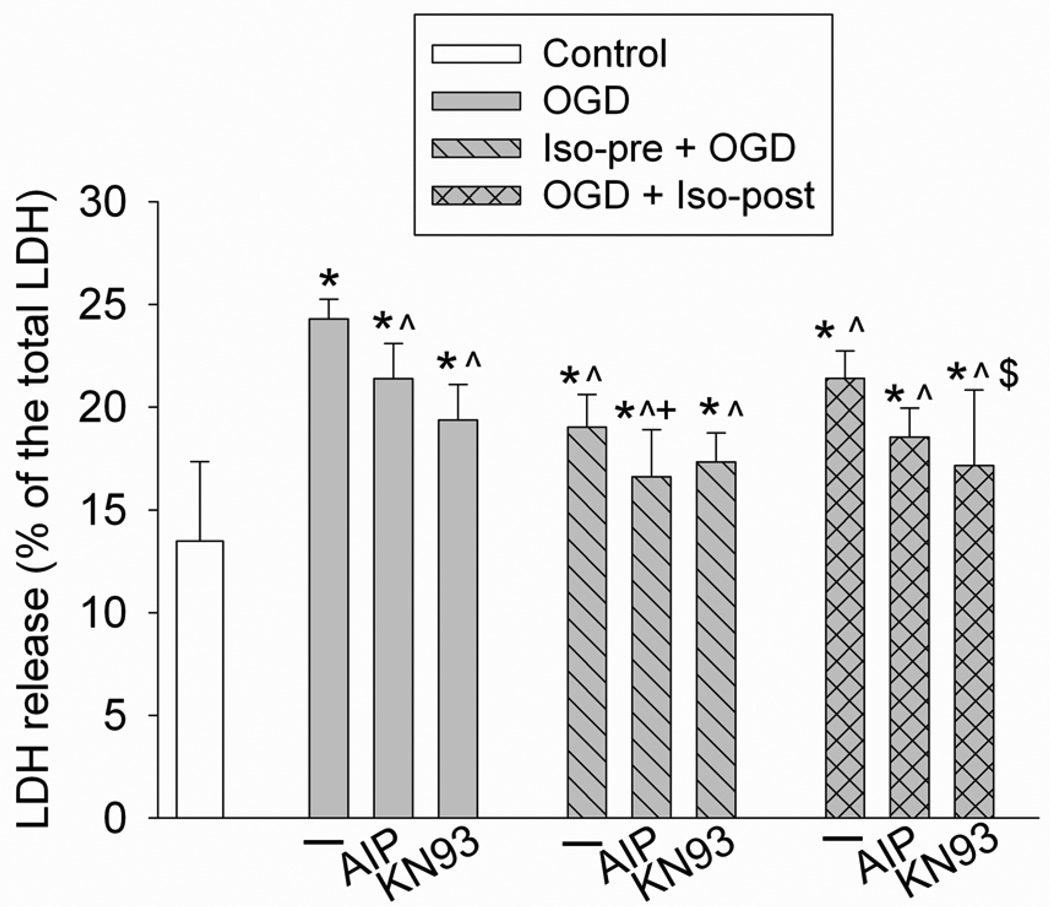
The CaMKII inhibitors AIP and KN93 inhibited the OGD and simulated reperfusion-induced LDH release (Fig. 2), suggesting that inhibition of CaMKII is neuroprotective. The combination of AIP and isoflurane preconditioning provided a better protection than AIP alone, but the protection caused by this combination was similar to that induced by isoflurane preconditioning. The combination of KN93 and isoflurane postconditioning induced a better protection than postconditioning alone, but had a similar level of protection to that of KN93 alone (Fig. 2). These results suggest that the CaMKII inhibitors, and isoflurane preconditioning and postconditioning, may share mechanisms to provide neuroprotection.
Fig. 2. Effects of calcium/calmodulin-dependent protein kinase II inhibition on isoflurane preconditioning- and postconditioning-induced neuroprotection.
Rat hippocampal neurons were preconditioned with 2% isoflurane for 30 min at 24 h before a 1-h oxygen-glucose deprivation (OGD) and a 24-h simulated reperfusion. Postconditioning was performed by exposing rat hippocampal neurons to 2% isoflurane for 1 h immediately after the onset of OGD. Results are mean ± S.D. (n = 8 – 16). * P < 0.05 compared with control, ^ P < 0.05 compared with OGD only, + P < 0.05 compared with OGD plus AIP, $ P < 0.05 compared with OGD plus isoflurane postconditioning. Iso-pre: isoflurane preconditioning; Iso-post: isoflurane postconditioning; LDH: lactate dehydrogenase.
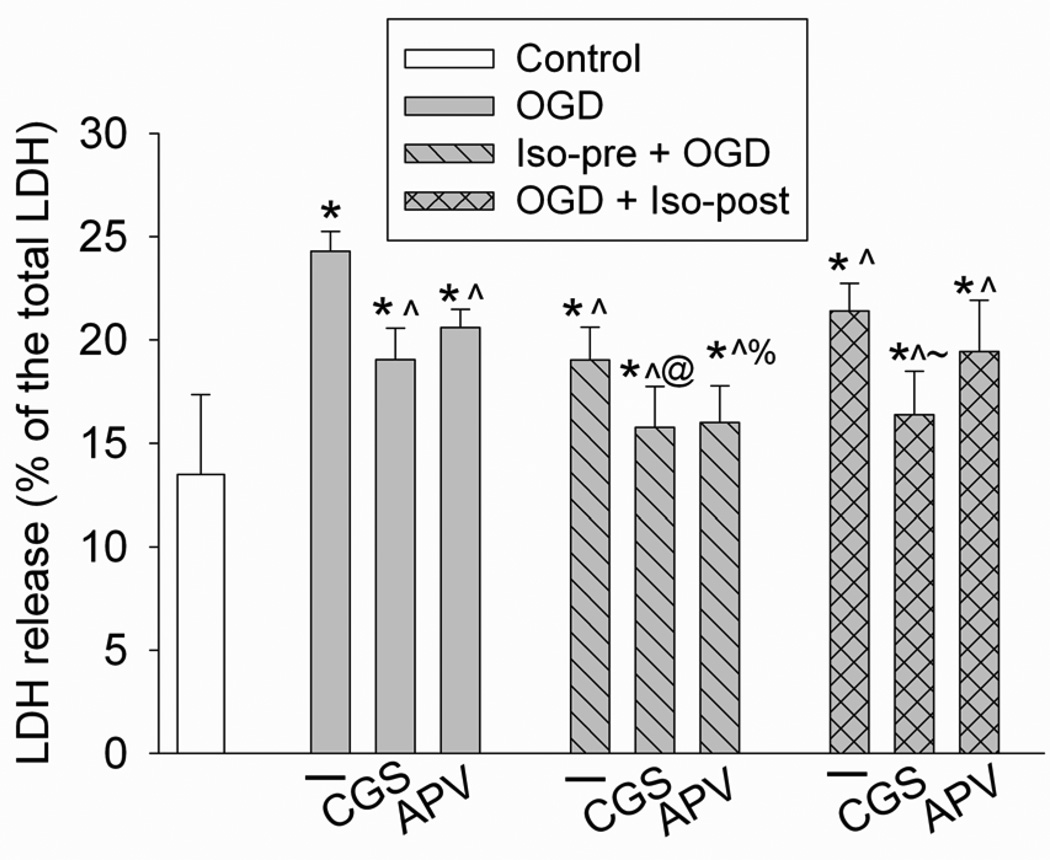
The adenosine A2A receptor agonist CGS-21680 and the NMDA receptor antagonist APV reduced the OGD and simulated reperfusion-induced LDH release (Fig. 3), suggesting that activation of the A2A receptors and inhibition of NMDA receptors have a neuroprotective effect. The combination of CGS-21680 and isoflurane preconditioning provided a better neuroprotective effect than CGS-21680 or isoflurane preconditioning alone. The combination of CGS-21680 and isoflurane postconditioning also had a better neuroprotective effect than CGS-21680 or isoflurane postconditioning alone (Fig. 3). These results indicate that the A2A receptor agonist CGS-21680 and isoflurane preconditioning and postconditioning may not share mechanisms for neuroprotection. The combination of APV and isoflurane preconditioning induced a better protective effect than either APV or isoflurane preconditioning alone (Fig. 3), suggesting that APV and isoflurane preconditioning may not share mechanisms for neuroprotection. However, the combination of APV and isoflurane postconditioning induced a similar level of protection to APV alone or isoflurane postconditioning alone (Fig. 3), indicating that APV and isoflurane postconditioning may work through the same mechanism for neuroprotection.
Fig. 3. Effects of adenosine A2A receptor activation and N-methyl D-aspartate (NMDA) receptor inhibition on isoflurane preconditioning- and postconditioning-induced neuroprotection.
Rat hippocampal neurons were preconditioned with 2% isoflurane for 30 min at 24 h before a 1-h oxygen-glucose deprivation (OGD) and a 24-h simulated reperfusion. Postconditioning was performed by exposing rat hippocampal neurons to 2% isoflurane for 1 h immediately after the onset of OGD. Results are mean ± S.D. (n = 8 – 16). * P < 0.05 compared with control, ^ P < 0.05 compared with OGD only, @ P < 0.05 compared with OGD plus CGS-21680 or OGD plus isoflurane preconditioning, % P < 0.05 compared with OGD plus APV or OGD plus isoflurane preconditioning, ~ P < 0.05 compared with OGD plus CGS-21680 or OGD plus isoflurane postconditioning. CGS: CGS-21680; Iso-pre: isoflurane preconditioning; Iso-post: isoflurane postconditioning; LDH: lactate dehydrogenase.
3. DISCUSSION
Consistent with previous studies (Lee et al., 2008; Zheng and Zuo, 2003; Zheng and Zuo, 2004), we showed here that isoflurane induced a preconditioning and postconditioning effect in rat hippocampal neurons. The neuroprotective effects were apparent even when the postconditioning isoflurane exposure was at 2 h after the onset of simulated reperfusion. An important question is whether preconditioning and postconditioning effects are additive. It has been shown that there are two phases of preconditioning-induced protection. The effective time-window of the early phase starts minutes after the preconditioning stimulus and continues for a few hours. The delayed phase begins a few hours after the preconditioning stimulus and can last for a few days or longer (Dirnagl et al., 2003; Nandagopal et al., 2001). Previous human and animal studies have suggested that there are no additional benefits to combining ischemic postconditioning and the acute phase of ischemic preconditioning in heart (Halkos et al., 2004; Manintveld et al., 2009). It has also been shown that the combination of sevoflurane postconditioning, and the acute phase of sevoflurane preconditioning, does not provide greater protection than either alone in rat hearts (Deyhimy et al., 2007). The only study on central nervous tissues showed that the combination of stimuli, which were ineffective when applied alone to induce neuroprotection via the mechanisms of ischemic postconditioning or the acute phase of ischemic preconditioning, significantly improved the neurological functions of rabbits after spinal cord ischemia (Jiang et al., 2008). Our study clearly showed that the combination of isoflurane postconditioning and the delayed phase of isoflurane preconditioning provided greater protection than either alone, suggesting that these two interventions can be combined to further improve the neurological outcomes after a detrimental insult, such as ischemia.
It has been shown that the adenosine A2A receptor can regulate the release of neurotransmitters, such as glutamate (Cunha et al., 2008; Fuxe et al., 2007). The overall effects of adenosine receptor activation in the brain may be inhibitory. Thus, activation of adenosine receptors should provide neuroprotection because neuronal over-excitation after a detrimental insult, such as ischemia, contributes significantly to cell injury in the brain (Lipton, 1999). Consistent with this assumption, multiple studies have shown the neuroprotective effects of adenosine receptor activation (Jones et al., 1998; Rudolphi et al., 1992). In particular, activation of A2A receptors has been shown to be neuroprotective, although controversy exists (Chen et al., 1999; Jones et al., 1998). Our results showed that the CGS-21680, a specific A2A receptor agonist, reduced the OGD and simulated reperfusion-induced LDH release, suggesting that activation of the A2A receptors is neuroprotective. Our results also showed that the CGS-21680 may not share mechanisms with isoflurane preconditioning and postconditioning for neuroprotection, suggesting that activation of the A2A receptors may not be involved in the isoflurane preconditioning- and postconditioning-induced neuroprotection. Similar to this situation, our previous study suggests that the adenosine A1 receptor does not mediate the acute phase of isoflurane preconditioning-induced neuroprotection (Zheng and Zuo, 2005).
CaMKII is one of the most abundant protein kinases in neurons and an important molecule to transduce calcium signaling (Anderson, 2009; Domanska-Janik, 1996). It has been proposed that activation of CaMKII can lead to cell apoptosis, hypertrophy, and inflammation in the heart (Anderson, 2009). Although multiple studies have documented the decreased expression of CaMKII after brain ischemia (Domanska-Janik, 1996; Lipton, 1999), it is interesting to note that the role of CaMKII in ischemic brain injury is not yet known. Our results showed that inhibition of CaMKII by KN93, a CaMKII inhibitor, or AIP, a very specific peptide inhibitor, reduced the OGD and simulated reperfusion-induced LDH release, suggesting that inhibition of CaMKII is neuroprotective. Our results also suggest that AIP and KN93 may share mechanisms with isoflurane preconditioning and postconditioning for neuroprotection, indicating that inhibition of CaMKII may play a role in isoflurane preconditioning- and postconditioning-induced neuroprotection. The intravenous anesthetics propofol and ketamine have been shown to inhibit CaMKII phosphorylation/activation (Cui et al., 2009). It is not known yet whether volatile anesthetics, including isoflurane, also reduce CaMKII activation. Isoflurane has been shown to attenuate the decrease of ischemia-induced CaMKII expression (Blanck et al., 2000). Calmidazolium, a compound that inhibits calcium-bound calmodulin, inhibited the isoflurane preconditioning-induced neuroprotection in hippocampal slices (Bickler et al., 2005), implying that activation of CaMKII, an event that is downstream of the calcium/calmodulin binding, is involved in the isoflurane preconditioning-induced neuroprotection. Consistent with this finding, isoflurane has been shown to activate inositol 1,4,5-trisphosphate (IP3) receptors on the endoplasmic reticulum membrane, which then leads to increased cytosolic calcium concentration (Wei et al., 2008). Isoflurane preconditioning-induced neuroprotection may require a moderate increase of intracellular calcium concentrations and activation of IP3 receptor (Bickler and Fahlman, 2010; Bickler et al., 2005). These findings regarding the role of IP3 receptor-calcium-CaMKII pathway in isoflurane preconditioning-induced neuroprotection are different from ours. The reasons for these different findings are not known. However, consistent with our findings, it has been proposed in a recent study that inhibition of CaMKII trafficking to the plasma membrane, a possible activation process for CaMKII, is a mechanism for isoflurane-induced neuroprotection (Matsumoto et al., 2008). Interestingly, there is no report on the role of IP3 receptor-calcium-CaMKII pathway in isoflurane postconditioning-induced neuroprotection. Our results suggest the involvement of CaMKII inhibition in isoflurane postconditioning-induced neuroprotection. However, it is not known yet whether this effect is due to a direct inhibition of CaMKII or inhibition of its upstream molecules, such as IP3 receptors.
It has been well recognized that glutamate receptor over-activation-induced cell injury contributes significantly to the ischemic brain injury (Lipton, 1999). Consistent with this recognition, APV, a NMDA receptor antagonist, reduced the OGD and reperfusion-induced LDH release. Our results also suggest that isoflurane postconditioning, but not isoflurane preconditioning, shares mechanisms with APV for neuroprotection, indicating that isoflurane postconditioning, but not isoflurane preconditioning involves inhibition of NMDA receptor over-activation for its neuroprotection. Consistent with this finding, it is generally believed that postconditioning-induced protection may involve effects on existing proteins and that the delayed phase of preconditioning-induced protection requires synthesis of protective proteins (Dirnagl et al., 2003; Lee et al., 2008). Our previous studies have suggested that the delayed phase of isoflurane preconditioning-induced neuroprotection may depend on the synthesis of B-cell lymphoma-2 protein, a protective protein (Li et al., 2008; Li and .Zuo, 2009; Zhao et al., 2007). These different final effectors for the postconditioning, and delayed phase of preconditioning, may explain the additive neuroprotective effects observed in our study.
The results of our experiments have important implications for ischemic stroke. If some cells in the ischemic penumbra, or perhaps even some cells in the ischemic core, can be salvaged, outcomes after a stroke could be improved significantly. It remains unknown, however, whether volatile anesthetics can induce preconditioning or postconditioning effects in human brain. Protective effects via preconditioning mechanisms have already been shown in the human heart with volatile anesthetics during cardiac surgery (Belhomme et al., 1999; Julier et al., 2003). If preconditioning could also provide clinically significant neuroprotection, this would have a significant impact on the perioperative management of procedures that involve a risk of stroke, such as carotid endarterectomies, cardiac surgeries, and many other neurovascular procedures.
In addition, if postconditioning can be shown to achieve neuroprotective effects in humans, this would have a significant impact on the management of spontaneous and acute strokes. Acute stroke is one of the leading causes of morbidity, mortality, and disability in the world; and, thus far medical interventions that have shown both safety and efficacy in improving long-term outcomes are extremely limited. Volatile anesthetics are an inexpensive drug that can be administered by anesthesiologists. There may be difficulties with administering a general anesthetic to an acute stroke patient who requires intensive neurological monitoring and who is possibly hemodynamically unstable, but it appears that the application of isoflurane would not need to be long to induce these protective effects, and that the required concentrations for these effects are clinically relevant. Ultimately, the hope would be to reveal the mechanisms of the neuroprotective effects of these agents and then to design more specific neuroprotective agents.
4. Experimental Procedures
4.1. Hippocampal neuronal culture
Hippocampal neuronal cultures were obtained from Invitrogen Life Technologies (Catalog number: A1084101, Carlsbad, CA). The neurons were harvested from Fischer 344 day 18 rat embryos. Neurons were plated in 24 well plates at approximately 38,000 cells per well or 30,000 cells/cm2. Cells were cultured per company’s instruction. Briefly, cells were cultured in Invitrogen Neurobasal A media (Catalog Number: 21103-049) supplemented with (for each 500 mL of Neurobasal A media) 5 mL glutamax-1, 10 mL B-27, and 5 mL penn/strep antibiotic solution. The solution was also supplemented with 25 µM glutamate from DIV 0–4 and with (for each 500 mL Neurobasal A media) 2.5 mg 2’-deoxy-5-fluorouridine, 5 mg uridine and 1.5 mL Dulbecco’s modified Eagle medium from DIV 4–7. The cells were fed on DIV 1 and DIV 4 by replacing half of the culture media with new media that had the appropriate supplements as specified above, and were kept in a cell culture incubator with 95% air-5% CO2 at 37°C.
4.2. Oxygen-glucose deprivation
Oxygen-glucose deprivation (OGD) experiments were performed on DIV 7. The culture medium was removed and cells were washed with Invitrogen Neurobasal A media without glucose, aspartic acid, glutamate, glutamine, or sodium pyruvate (Invitrogen Catalog Number: 0050128DJ). As we described previously (Kim et al., 2009), this medium was pregassed with 95% N2-5% CO2 for 20 min to get rid of oxygen in the solution. The neurons were then stored in this pregassed glucose-free medium in sealed Billups-Rothenburg containers that were flushed with 95% N2-5% CO2 for approximately 8 min until O2 levels in the outlet gases were < 2% for longer than 2 min. The outlet gases were continuously monitored by a Datex™ infrared analyzer (Capnomac, Helsinki, Finland). Cells were then kept under this OGD condition for 1 h at 37°C. At the end of the OGD period, cells were re-exposed to room air. Neurobasal A media (100 µL) with concentrated glucose (175 mM) were added to the 600-µL incubation solution in each well to bring the glucose concentration to the concentration (25mM) found in the standard Neurobasal A media. This simulated reperfusion lasted for 24 h at 37°C. For the cells that served as controls, their growth media were replaced with normal Neurobasal A media (i.e., with glucose) instead of the oxygen-glucose deprivation solution. The media changes not only served to control for the changes caused by media replacement, but also removed any lactate dehydrogenase (LDH) released during prior culture period.
4.3. Isoflurane exposure
In the isoflurane pre-conditioning experiments, cells were placed in the Billups-Rothenburg containers that were flushed with 2% isoflurane in 95% air-5% CO2 at 6 L/min until the gasses exited from the container were measured to contain 2% isoflurane consistently for 2 min. This process took approximately 7 – 8 min. The containers were then sealed with this gas mixture for 30 min at 37°C at 24 h prior to subjecting them to OGD. In isoflurane post-conditioning experiments, immediately or at the preset times (for time course experiments) after cells were bathed in the glucose and oxygen containing solution, they were placed in the Billups-Rothenburg containers that were flushed with 2% isoflurane in 95% air-5% CO2 at 6 L/min for ~7 – 8 min and then sealed for 1 h at 37°C. The isoflurane concentrations in the outlet gases from the containers were confirmed to be 2% in both the preconditioning and postconditioning experiments by the Datex™ infrared analyzer. The durations and concentrations of the isoflurane exposure for preconditioning and postconditioning were decided based on our previous studies (Lee et al., 2008; Zheng and Zuo, 2003). In addition, these exposure conditions alone did not cause toxicity to neurons in previous studies (Wise-Faberowski et al., 2005; Zhao and Zuo, 2004; Zuo et al., 1999).
4.4. Application of various agents
To study the mechanisms involved in the isoflurane preconditioning and postconditioning effects, the following chemicals were used: autocamide-2 related inhibitory peptide (AIP, a specific peptide inhibitor of CaMKII), 2-[N-(2-hydroxyethyl)]-N-(4-methoxybenzenesulfonyl)]amino-N-(4-chlorocinnamyl)-N-methylbenzylamine)(KN93, a CaMKII inhibitor), 3-[4-[2-[[6-amino-9-[(2R,3R,4S,5S)-5-(ethylcarbamoyl)-3,4-dihydroxy-oxolan-2-yl]purin-2-yl]amino]ethyl]phenyl]propanoic acid (CGS-21680, an agonist of the adenosine A2A receptor) and (2R)-amino-5-phosphonovaleric acid; (2R)-amino-5-phosphonopentanoate (APV, an antagonist of the NMDA receptor). These compounds were added 2 h prior to OGD, or 2 h prior to preconditioning isoflurane exposure, and remained in the media at the end of the reperfusion phase. The final concentrations in the media were based on previous reported studies and pilot studies in the lab: 133 µM for AIP (Ishida et al., 1995; Laabich and Cooper, 2000), 10 µM for KN93 (Gao et al., 2006; Vest et al., 2007), 1 µM for CGS-21680 (Li and Henry, 1998; Ruan and Brown, 2009), and 100 µM for APV (Goldberg et al., 1987; MacGregor et al., 2003).
4.5. Lactate dehydrogenase activity assay
Cell injury was quantified by measuring LDH release from the cells using an LDH cytotoxicity detection kit as we did before (Kim et al., 2009). Briefly, the incubation media harvested from the cells at the end of the experiments was centrifuged at 13,000 rpm for 10 min and 100 µl of the cell-free supernatant was transferred to 96-well plates. The supernatant was incubated with the same amount of reaction mixture. LDH activity was determined by a colorimetric assay with the absorbance wavelength at 492 nm and the reference wavelength at 655 nm in a spectrophotometry (Bio-Rad Laboratories, Hercules, CA). Intracellular LDH activity was measured in the same way after cells were lysed by 0.5% triton for 30 min. LDH release was calculated as a percentage of total LDH in each well of cells. The calculation required first subtracting the value of “blank” samples of neurobasal media with no cells from all values measured from samples. The LDH release in percentage was calculated using the following equation: LDH level in the incubation media x 100/(intracellular LDH level + LDH level in the incubation media).
4.6. Statistical analysis
Results are presented as mean ± S.D. (n ≥ 8 for each experimental condition). Results were analyzed by one-way analysis of variance followed by the Student-Newman-Keuls Method for post hoc analysis after confirmation of normal distribution of the data. A P value < 0.05 was considered statistically significant.
Research Highlights
-
*
Isoflurane induces preconditioning and postconditioning effects in hippocampal neurons.
-
*
Isoflurane preconditioning- and postconditioning-induced neuroprotection can be additive.
-
*
Isoflurane preconditioning and isoflurane postconditioning may involve inhibition of calcium/calmodulin-dependent protein kinase II.
Acknowledgement
This study was supported by a grant (R01 GM065211 to Z Zuo) from the National Institute of Health, Bethesda, Maryland, by a grant from the International Anesthesia Research Society (2007 Frontiers in Anesthesia Research Award to Z Zuo), Cleveland, Ohio and the Robert M. Epstein Professorship endowment, University of Virginia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson ME. CaMKII and a failing strategy for growth in heart. J. Clin. Invest. 2009;119:1082–1085. doi: 10.1172/JCI39262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belhomme D, Peynet J, Louzy M, Launay J-M, Kitakaze M, Menasche P. Evidence for preconditioning by isoflurane in coronary artery bypass graft surgery. Circulation. 1999;100 Suppl II:II340–II344. doi: 10.1161/01.cir.100.suppl_2.ii-340. [DOI] [PubMed] [Google Scholar]
- Bickler PE, Fahlman CS. Enhanced hypoxic preconditioning by isoflurane: signaling gene expression and requirement of intracellular Ca2+ and inositol triphosphate receptors. Brain Res. 2010;1340:86–95. doi: 10.1016/j.brainres.2010.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickler PE, Zhan X, Fahlman CS. Isoflurane preconditions hippocampal neurons against oxygen-glucose deprivation: role of intracellular Ca2+ and mitogen-activated protein kinase signaling. Anesthesiology. 2005;103:532–539. doi: 10.1097/00000542-200509000-00016. [DOI] [PubMed] [Google Scholar]
- Blanck TJ, Haile M, Xu F, Zhang J, Heerdt P, Veselis RA, Beckman J, Kang R, Adamo AI, Hummings H. Isoflurane pretreatment ameliorates postischemic neurological dysfunction and preserves hippocampal Ca2+/calmodulin-dependent protein kinase in a canine cardiac arrest model. Anesthesiology. 2000;93:1285–1293. doi: 10.1097/00000542-200011000-00023. [DOI] [PubMed] [Google Scholar]
- Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, Moskowitz MA, Fink JS, Schwarzschild MA. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J. Neurosci. 1999;19:9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Li J, Li T, Ji F, Bu X, Zhang N, Zhang B. Propofol and ketamine-induced anesthetic depth-dependent decrease of CaMKII phosphorylation levels in rat hippocampus and cortex. J. Neurosurg. Anesthesiol. 2009;21:145–154. doi: 10.1097/ANA.0b013e31819ac2c0. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Ferre S, Vaugeois JM, Chen JF. Potential therapeutic interest of adenosine A2A receptors in psychiatric disorders. Curr. Pharm. Des. 2008;14:1512–1524. doi: 10.2174/138161208784480090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyhimy DI, Fleming NW, Brodkin IG, Liu H. Anesthetic preconditioning combined with postconditioning offers no additional benefit over preconditioning or postconditioning alone. Anesth. Analg. 2007;105:316–324. doi: 10.1213/01.ane.0000267524.71445.e7. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Domanska-Janik K. Protein serine/threonine kinases (PKA, PKC and CaMKII) involved in ischemic brain pathology. Acta Neurobiol Exp (Wars) 1996;56:579–585. doi: 10.55782/ane-1996-1163. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Ferre S, Genedani S, Franco R, Agnati LF. Adenosine receptor-dopamine receptor interactions in the basal ganglia and their relevance for brain function. Physiol. Behav. 2007;92:210–217. doi: 10.1016/j.physbeh.2007.05.034. [DOI] [PubMed] [Google Scholar]
- Gao L, Blair LA, Marshall J. CaMKII-independent effects of KN93 and its inactive analog KN92: reversible inhibition of L-type calcium channels. Biochem. Biophys. Res. Commun. 2006;345:1606–1610. doi: 10.1016/j.bbrc.2006.05.066. [DOI] [PubMed] [Google Scholar]
- Goldberg MP, Weiss JH, Pham PC, Choi DW. N-methyl-D-aspartate receptors mediate hypoxic neuronal injury in cortical culture. J. Pharmacol Exp Ther. 1987;243:784–791. [PubMed] [Google Scholar]
- Gwag BJ, Won SJ, Kim DY. Excitotoxicity, oxidative stress, and apoptosis in ischemic neuronal death. In: Lin CS, editor. New Concepts in Cerebral Ischemia. CRC Press; 2001. pp. 79–112. (Ed.)^(Eds.) [Google Scholar]
- Halkos ME, Kerendi F, Corvera JS, Wang NP, Kin H, Payne CS, Sun HY, Guyton RA, Vinten-Johansen J, Zhao ZQ. Myocardial protection with postconditioning is not enhanced by ischemic preconditioning. Ann. Thorac. Surg. 2004;78:961–969. doi: 10.1016/j.athoracsur.2004.03.033. discussion 969. [DOI] [PubMed] [Google Scholar]
- Ishida A, Kameshita I, Okuno S, Kitani T, Fujisawa H. A novel highly specific and potent inhibitor of calmodulin-dependent protein kinase II. Biochem. Biophys. Res. Commun. 1995;212:806–812. doi: 10.1006/bbrc.1995.2040. [DOI] [PubMed] [Google Scholar]
- Jiang X, Shi E, Li L, Nakajima Y, Sato S. Co-application of ischemic preconditioning and postconditioning provides additive neuroprotection against spinal cord ischemia in rabbits. Life Sci. 2008;82:608–614. doi: 10.1016/j.lfs.2007.12.026. [DOI] [PubMed] [Google Scholar]
- Jones PA, Smith RA, Stone TW. Protection against kainate-induced excitotoxicity by adenosine A2A receptor agonists and antagonists. Neuroscience. 1998;85:229–237. doi: 10.1016/s0306-4522(97)00613-1. [DOI] [PubMed] [Google Scholar]
- Julier K, da Silva R, Garcia C, Bestmann L, Frascarolo P, Zollinger A, Chassot PG, Schmid ER, Turina MI, von Segesser LK, Pasch T, Spahn DR, Zaugg M. Preconditioning by sevoflurane decreases biochemical markers for myocardial and renal dysfunction in coronary artery bypass graft surgery: a double-blinded, placebo-controlled, multicenter study. Anesthesiology. 2003;98:1315–1327. doi: 10.1097/00000542-200306000-00004. [DOI] [PubMed] [Google Scholar]
- Kim JA, Li L, Zuo Z. Isoflurane induces a postconditioning effect on bovine pulmonary arterial endothelial cells exposed to oxygen-glucose deprivation. Eur. J. Pharmacol. 2009;615:144–149. doi: 10.1016/j.ejphar.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano H, Kirsch JR, Hurn PD, Murphy SJ. Inhalational anesthetics as neuroprotectants or chemical preconditioning agents in ischemic brain. J Cereb Blood Flow Metab. 2007;27:1108–1128. doi: 10.1038/sj.jcbfm.9600410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laabich A, Cooper NG. Neuroprotective effect of AIP on N-methyl-D-aspartate-induced cell death in retinal neurons. Brain Res. Mol. Brain Res. 2000;85:32–40. doi: 10.1016/s0169-328x(00)00226-6. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Li L, Jung H-H, Zuo Z. Postconditioning with isoflurane reduced ischemia-induced brain injury in rats. Anesthesiology. 2008;108:1055–1062. doi: 10.1097/ALN.0b013e3181730257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Henry JL. Adenosine A2 receptor mediation of pre- and postsynaptic excitatory effects of adenosine in rat hippocampus in vitro. Eur. J. Pharmacol. 1998;347:173–182. doi: 10.1016/s0014-2999(98)00105-8. [DOI] [PubMed] [Google Scholar]
- Li L, Peng L, Zuo Z. Isoflurane preconditioning increases B-cell lymphoma-2 expression and reduces cytochrome c release from the mitochondria in the ischemic penumbra of rat brain. Eur. J. Pharmacol. 2008;586:106–113. doi: 10.1016/j.ejphar.2008.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zuo Z. Isoflurane preconditioning improves short-term and long-term neurological outcome after focal brain ischemia in adult rats. Neuroscience. 2009;164:497–506. doi: 10.1016/j.neuroscience.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol. Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2009;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- MacGregor DG, Avshalumov MV, Rice ME. Brain edema induced by in vitro ischemia: causal factors and neuroprotection. J. Neurochem. 2003;85:1402–1411. doi: 10.1046/j.1471-4159.2003.01772.x. [DOI] [PubMed] [Google Scholar]
- Manintveld OC, Hekkert ML, van der Ploeg NT, Verdouw PD, Duncker DJ. Interaction between pre- and postconditioning in the in vivo rat heart. Exp Biol Med (Maywood) 2009;234:1345–1354. doi: 10.3181/0903-RM-121. [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Murozono M, Nagaoka D, Matsuoka S, Takeda A, Narita H, Watanabe S, Isshiki A, Watanabe Y. Isoflurane inhibits protein kinase Cgamma and calcium/calmodulin dependent protein kinase ii-alpha translocation to synaptic membranes in ischemic mice brains. Neurochem. Res. 2008;33:2302–2309. doi: 10.1007/s11064-008-9727-4. [DOI] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Nandagopal K, Dawson TM, Dawson VL. Critical role for nitric oxide signaling in cardiac and neuronal ischemic preconditioning and tolerance. The Journal of Pharmacology and Experimental Therapeutics. 2001;297:474–478. [PubMed] [Google Scholar]
- Payne RS, Akca O, Roewer N, Schurr A, Kehl F. Sevoflurane-induced preconditioning protects against cerebral ischemic neuronal damage in rats. Brain Res. 2005;1034:147–152. doi: 10.1016/j.brainres.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Roscoe AK, Christensen JD, Lynch C., III Isoflurane, but not halothane, induces protection of human myocardium via adenosine A1 receptors and adenosine triphosphate-sensitive potassium channels. Anesthesiology. 2000;92:1692–1701. doi: 10.1097/00000542-200006000-00029. [DOI] [PubMed] [Google Scholar]
- Ruan M, Brown CH. Feedback inhibition of action potential discharge by endogenous adenosine enhancement of the medium afterhyperpolarization. J Physiol. 2009;587:1043–1056. doi: 10.1113/jphysiol.2008.167239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolphi KA, Schubert P, Parkinson FE, Fredholm BB. Neuroprotective role of adenosine in cerebral ischaemia. Trends Pharmacol. Sci. 1992;13:439–445. doi: 10.1016/0165-6147(92)90141-r. [DOI] [PubMed] [Google Scholar]
- Schurr A, Reid KH, Tseng MT, West C, Rigor BM. Adaptation of adult brain tissue to anoxia and hypoxia in vitro. Brain Res. 1986;374:244–248. doi: 10.1016/0006-8993(86)90418-x. [DOI] [PubMed] [Google Scholar]
- Toller WG, Kersten JR, Gross ER, Pagel PS, Warltier DC. Isoflurane Preconditions Myocarduim Against Infarction via Activation of Inhibitory Guanine Nucleotide Binding Proteins. Anesthesiology. 2000;92:1400–1407. doi: 10.1097/00000542-200005000-00031. [DOI] [PubMed] [Google Scholar]
- Vest RS, Davies KD, O'Leary H, Port JD, Bayer KU. Dual mechanism of a natural CaMKII inhibitor. Mol. Biol. Cell. 2007;18:5024–5033. doi: 10.1091/mbc.E07-02-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Traystman RJ, Murphy SJ. Inhalational anesthetics as preconditioning agents in ischemic brain. Curr Opin Pharmacol. 2008;8:104–110. doi: 10.1016/j.coph.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Liang G, Yang H, Wang Q, Hawkins B, Madesh M, Wang S, Eckenhoff RG. The common inhalational anesthetic isoflurane induces apoptosis via activation of inositol 1,4,5-trisphosphate receptors. Anesthesiology. 2008;108:251–260. doi: 10.1097/01.anes.0000299435.59242.0e. [DOI] [PubMed] [Google Scholar]
- Wise-Faberowski L, Zhang H, Ing R, Pearlstein RD, Warner DS. Isoflurane-induced neuronal degeneration: an evaluation in organotypic hippocampal slice cultures. Anesth. Analg. 2005;101:651–657. doi: 10.1213/01.ane.0000167382.79889.7c. table of contents. [DOI] [PubMed] [Google Scholar]
- Zhao H. The protective effect of ischemic postconditioning against ischemic injury: from the heart to the brain. J Neuroimmune Pharmacol. 2007;2:313–318. doi: 10.1007/s11481-007-9089-8. [DOI] [PubMed] [Google Scholar]
- Zhao P, Peng L, Li L, Xu X, Zuo Z. Isoflurane preconditioning improves long-term neurological outcome after hypoxic-ischemic brain injury in neonatal rats. Anesthesiology. 2007;107:963–970. doi: 10.1097/01.anes.0000291447.21046.4d. [DOI] [PubMed] [Google Scholar]
- Zhao P, Zuo Z. Isoflurane preconditioning induces neuroprotection that is inducible nitric oxide synthase-dependent in the neonatal rats. Anesthesiology. 2004;101:695–702. doi: 10.1097/00000542-200409000-00018. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Vinten-Johansen J. Postconditioning: reduction of reperfusion-induced injury. Cardiovasc. Res. 2006;70:200–211. doi: 10.1016/j.cardiores.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Zheng S, Zuo Z. Isoflurane preconditioning reduces Purkinje cell death in an in vitro model of rat cerebellar ischemia. Neuroscience. 2003;118:99–106. doi: 10.1016/s0306-4522(02)00767-4. [DOI] [PubMed] [Google Scholar]
- Zheng S, Zuo Z. Isoflurane preconditioning induces neuroprotection against ischemia via activation of p38 mitogen-activated protein kinase. Mol. Pharmacol. 2004;65:1172–1180. doi: 10.1124/mol.65.5.1172. [DOI] [PubMed] [Google Scholar]
- Zheng S, Zuo Z. Isoflurane preconditioning decreases glutamate receptor overactivation-induced Purkinje neuronal injury in rat cerebellar slices. Brain Res. 2005;1054:143–151. doi: 10.1016/j.brainres.2005.06.064. [DOI] [PubMed] [Google Scholar]
- Zuo Z, Tichotsky A, Johns RA. Inhibition of excitatory neurotransmitter-nitric oxide signaling pathway by inhalational anesthetics. Neuroscience. 1999;93:1167–1172. doi: 10.1016/s0306-4522(99)00194-3. [DOI] [PubMed] [Google Scholar]