Abstract
Objectives
We used a Positron Emission Tomography (PET) paradigm with the D2/3 radiotracer [11C]raclopride and an alcohol challenge to examine the magnitude of alcohol induced dopamine release and compare it between young men and women.
Methods
Twenty-one non-alcohol dependent young social drinkers completed two PET scans on separate days following ingestion of a juice mix containing either ethanol (0.75 mg/kg body water) or trace ethanol only.
The extent of dopamine released after alcohol was estimated by the percent difference in [11C]raclopride binding potential (ΔBPND) between days.
Results
Alcohol administration significantly displaced [11C]raclopride in all striatal subregions indicating dopamine release, with the largest effect observed in the ventral striatum. Linear mixed model analysis across all striatal subregions of regional ΔBPND with region of interest as repeated measure showed a highly significant effect of sex (p < 0.001). Ventrostriatal dopamine release in men, but not in women, showed a significant positive correlation to alcohol-induced measures of subjective activation. Furthermore, we found a significant negative correlation between the frequency of maximum alcohol consumption per 24 hours and ventrostriatal ΔBPND (r=0.739, p=0.009) in men.
Conclusions
This study provides definitive evidence that oral alcohol induces dopamine release in non-alcoholic human subjects, and shows sex differences in the magnitude of this effect. The ability of alcohol to stimulate dopamine release may contribute to its rewarding effects and, thereby, to its abuse liability in humans. Our report further suggests several biological mechanisms that may mediate the difference in vulnerability for alcoholism between men and women.
Introduction
Alcohol is one of the most commonly abused substances, and alcoholism is one of the leading causes of disability in the United States (1, 2). In most developed countries, the lifetime risk for alcohol use disorders is 20% in men (two-fold higher than in women)(3), with a risk of 15% for alcohol abuse and 10% for dependence (4, 5). The heaviest drinking in the general population occurs between the ages of 18 and 22 years (6) and consequently, the highest risk to develop alcohol use disorders is at the beginning of the third decade of life (7).
Little is known about the mechanisms through which alcohol produces its rewarding effects in humans, in part because of the diversity of ethanol targets in the brain (8). Principally based on preclinical studies, primarily the ability of alcohol to stimulate dopaminergic (DA) transmission in the ventral striatum has been hypothesized to contribute to its abuse liability in humans. Alcohol administration induces DA release in the dorsal caudate and nucleus accumbens in rats (9). The rewarding and euphoriant properties of alcohol-induced mesolimbic DA stimulation (10–12) are believed to play a major role in reinforcing its consumption (11, 13). However, in rats habituated to alcohol exposure, self-administration of an ethanol solution raised DA levels in the accumbens only during the early phase after onset of drinking, and there was no DA increase after cue presentation, suggesting that while DA may play a significant role, it is not the only or central substrate producing the reinforcement from alcohol (14).
Alcohol preferring rats have been found to have lower extracellular DA levels at baseline than abstainer rats, decreased D2 receptor density (15), as well as lower DA concentrations in the mesolimbic terminals (16), and intraperitoneal ethanol induced a 2-fold greater increase of DA release in the nucleus accumbens measured by microdialysis (17). Greater magnitude of alcohol induced DA release was also found to be a predictor of degree of alcohol preference in rats in some (18), but not other studies (19).These findings may suggest that both a low DAergic tone and a strong mesolimbic DA response to ethanol are associated with ethanol-seeking behavior.
Human studies have evaluated dopamine transmission in the striatum of both chronic alcohol users and healthy controls. DA release after amphetamine administration is reduced in the ventral striatum (VST) of detoxified subjects with alcohol dependence (20) (21).
Despite this evidence, the DAergic response to alcohol itself has not been extensively studied in humans. Four studies quantifying in vivo alcohol induced displacement of [11C]raclopride from DA D2/3 receptors have reported mixed findings: two studies suggested that alcohol induced DA release within the striatum in humans can be measured with [11C]raclopride displacement (22, 23), one reported no overall effect on binding, but a relationship between subjective effects of alcohol and the magnitude of [11C]raclopride displacement (24), and one found no effect of alcohol on ΔBPND (25).
Here, we present a study designed to evaluate the capacity of oral alcohol to stimulate DA release in the human striatum with a larger sample of subjects providing greater statistical power and robustness to the sources of variance reported in prior studies. We hypothesized that sex is an important moderator of alcohol effects on DA release with greater effect to be expected in men.
Methods
Study population
The study was approved by the Institutional Review Board of the New York State Psychiatric Institute and informed consent was obtained from all subjects. Male and female social drinkers not meeting criteria for alcohol abuse or dependence, aged 21 to 27 years, were included. Subjects were required to have sufficient experience with alcohol to minimize adverse effects associated with the administration of alcohol, based on consumption of at least 10 to 15 standard drinks (standard drinking unit in USA = 14 g alcohol) per week. This was ascertained by self-reported drinking history and Alcohol Time Line Follow Back Interview, (TLFB) (26), used to estimate drinking patterns and the amount consumed over the past 30 days and past 12 months. In addition, all subjects completed questionnaires assessing their prior experiences with alcohol (26). Smoking was not an exclusion criterion.
Study design
Two [11 C]raclopride PET scans on two separate days following consumption of either a placebo or an alcohol drink were obtained in counterbalanced order (11 out of 21 received alcohol on the first day, randomly chosen). The placebo consisted of cranberry-juice and soda alone, while the alcohol drink in addition contained the equivalent of 3 standard drinks of 100 proof vodka designed to deliver an average of 0.75 g alcohol per kg body water. The individual amount of alcohol was calculated based on the subject’s amount of body water according to the equation: TBW (g/liter) = −2.097 + 0.1069 (height in cm) + 0.2466 (weight in kg)(27). For men, the volume of the drink amounted to 500 ml, while women received 350 ml. This difference intended to keep the alcohol concentration per drink similar between groups. Participants were blinded to the drink content. We disguised olfactory cues that might have indicated the nature of the drink before consumption by covering the rim of the drink containers with a paper napkin doused in vodka. The alcohol challenge was administered in a non-fasting condition. Subjects were asked to refrain from alcohol the night before, from smoking tobacco for the 2 hours prior to the PET scan, and from using any recreational drugs after the time of screening. Subjects underwent screening for substances of abuse including alcohol (AlcoMate Pro digital alcohol detector, KHN Solutions, San Francisco, CA) on the first day of screening and on scan days. Oral consumption of alcohol or alcohol-free mixture had to be completed within 5 to 10 min.
PET data acquisition
Five minutes after the drink, [11 C]raclopride was delivered as a bolus plus constant infusion (28, 29). Emission data were collected using an ECAT EXACT HR+ scanner (Siemens Medical Systems, Knoxville, TN, USA) starting 40 min into the constant infusion. Blood samples for plasma alcohol levels were drawn at 25, 40, 55, and 70 min after the drink (Supplement: Figure S1). Subjective effects of alcohol were assessed with the Biphasic Alcohol Effects Scale (BAES) for rating subjective activation (elation, feeling “up”, energy, excitement, stimulation, vigor, talkativeness,) and sedation (difficulty concentrating, feeling “down”, heavy head, inactive, sedated, slowed thoughts, sluggishness) on scales from 1 to 10 (30), given at baseline and every 30 minutes after drink administration for 90 minutes. Subjects underwent a structural MRI (GE Signa 1.5 or 3 Tesla scanner) on a separate day for coregistration and regions of interest (ROI) analysis.
PET data analysis
Image analysis was performed as described previously (29). PET data were coregistered to the structural MRIs using maximization of mutual information as implemented in the SPM2 software environment (31). ROIs were drawn on each individual’s MRI and applied to the co-registered PET images. ROIs included pre-commissural caudate and putamen (preDCA, preDPU), post-commissural caudate and putamen (postCA, postPU) and ventral striatum (VST) (28). Cerebellum (CER) was used as a reference region to measure free and non-specifically bound [11C]raclopride activity as the concentration of D2 receptors in the cerebellum is negligible (32). Equilibrium analysis was used to derive the specific to non-displaceable equilibrium partition coefficient BPND (unitless) as (ROI activity / CER activity − 1) during steady state. (20).
The primary outcome measure for the study was the percent change in BPND between conditions (ΔBPND), calculated as:
This expresses the relative reduction in DA D2/3 receptor availability for [11C]raclopride binding after alcohol-induced DA release.
Statistical analysis
Comparisons between drink conditions were performed with paired t tests; comparisons between groups were performed with two-group t tests at the ROI level. A linear mixed model across all striatal subregions with regional ΔBPND as the dependent variable and region of interest as repeated measure, was performed to test for a global effect of sex on ΔBPND. Correlation analysis between PET measures and other variables (ΔBPND for all ROIs vs measures of drinking history and subjective response to alcohol) were performed. Data were inspected for normality. Pearson product-moment coefficients were computed for normally distributed data and the Spearman rank correlation coefficient was applied to non-normally distributed data. A two-tailed probability value of p < 0.05 was chosen as statistically significant. The false discovery rate method (FDR) (33) was applied to the correlations between drinking history and VST DA release to correct for multiple comparisons.
Results
Subjects
Twenty-one subjects, ages 24 ± 1.7 (mean ± SD) years, including 11 males and 10 females completed the study (Table 1).
Table 1.
Demographics and scan parameters: Sample composition and scan parameters. Injected dose (ID), injected mass (IM), specific activity (SA), distribution volume of the reference region (VND) and plasma free faction (fp) are shown.
| Demographics | |||
|---|---|---|---|
| Men (n=11) | Women (n=10) | T test (p) | |
| Age (yrs) | 24.4 ± 1.8 | 23.0 ± 1.5 | 0.07 |
| Smokers (<10 cigarettes/day) | 3 | 1 | |
| Ethnicity (C,AA,H,As) | 6,2,2,1 | 7,0,1,2 | |
| Family hx/o EtOH | 2 | 3 | |
| PET parameters (all, n=21) | |||
| Placebo drink | Alcohol | T test (p) | |
| ID (mCi) | 7.99 ± 1.09 | 7.80 ± 1.53 | 0.65 |
| IM (µg) | 3.34 ± 1.93 | 2.99 ± 1.33 | 0.35 |
| SA (Ci/mmol) | 1787 ± 1090 | 1726 ± 1031 | 0.84 |
| VND (ml/cm3) | 0.43 ± 0.08 | 0.45 ± 0.1 | 0.32 |
| fp (unitless) | 4.2 ± 1.6% | 4.1 ± 0.7% | 0.68 |
Drinking history
Table 2 shows drinking history over the last 12 months before enrollment. Regular drinking (i.e. number of drinks per average drinking session), as well as binge-drinking (defined as more than 5 drinks in 2 hours for men and more than 4 drinks for women, (34)) were similar in both groups. The measure M, “maximum number of drinks per 24 hours”(35), a quantitative trait expected to be related to alcohol tolerance (36), showed slightly different patterns. Men had a higher magnitude of M during the past 12 months and when considering lifetime drinking history. Women, on the other hand, consumed M more frequently and with greater variability.
Table 2.
Drinking history: Pattern of drinking behavior for men and women over the past 12 months before enrollment. Drinking binge is defined as >5 drinks in 2 hours for men and >4 drinks in 2 hours for women.
| Men (n=11) | Women (n=10) | Sex diff. | |
|---|---|---|---|
| p | |||
| Age of onset regular alcohol consumption (yrs) | 18.6 ± 1.6 | 18.6 ± 1.4 | 0.96 |
| Duration regular alcohol consumption (yrs) | 5.5 ± 2.7 | 4.2 ± 1.5 | 0.22 |
| Average no. of drinking days/week (past 12 mnths.) | 2.8 ± 1.0 | 3.8 ± 1.0 | 0.05 |
| Average no. drinks/week (past 12 months) | 14.9 ± 5.9 | 17.6 ± 14.4 | 0.57 |
| Average no. drinks/regular drinking occasion | 5.5 ± 2.1 | 4.5 ± 2.5 | 0.32 |
| Number of drinking binges (past 12 months) | 23.5 ± 28.8 | 12.5 ± 15.0 | 0.29 |
| Maximum no. drinks/24 hours (last 12 months): M | 14.4 ± 6.6 | 7.5 ± 2.3 | 0.01 |
| No. days M is consumed (past 12 months): frequency of M | 5.2 ± 3.4 | 33.6 ± 42 | 0.04 |
| Lifetime maximum no. drinks/24 hrs. | 17.6 ± 9.5 | 10.3 ± 3.2 | 0.03 |
Correlations of drinking behavior with ΔBPND
Of 9 measures of drinking behavior tested for correlation with VST DA release (Table 2), only frequency of M over the past 12 months showed a negative correlation in men, i.e. the less frequently men drank their maximal amount, the larger the ΔBPND. As the data for this parameter were not normally distributed, correlations were analyzed with the Spearman’s rank order coefficient: rho = 0.72, p = 0.012; this did not survive FDR multiple comparisons correction at the 0.05 alpha level, but did survive at trend level (corrected p = 0.11). While there was no a priori reason to exclude any subject, it was nonetheless noted that 3 female subjects had a frequency of M that met formal criterion as outliers by leave-one-out analysis (frequency of M = 120, 96 and 48 days). When excluded, the correlation became significant for the overall group (n=18, rho =.613, p = 0.007; at trend level only after multiple comparison correction: p = 0.06) but not for women (n = 7, rho = 0.312, p = 0.50). Because frequency of M was binned into several distinct levels, we also applied ordinal logistic regression with frequency of M as an ordinal dependent variable and ΔBPND in VST as continuous independent variable. This model reached significance for men and the entire cohort, but not for women alone (Figure 1).
Figure 1.
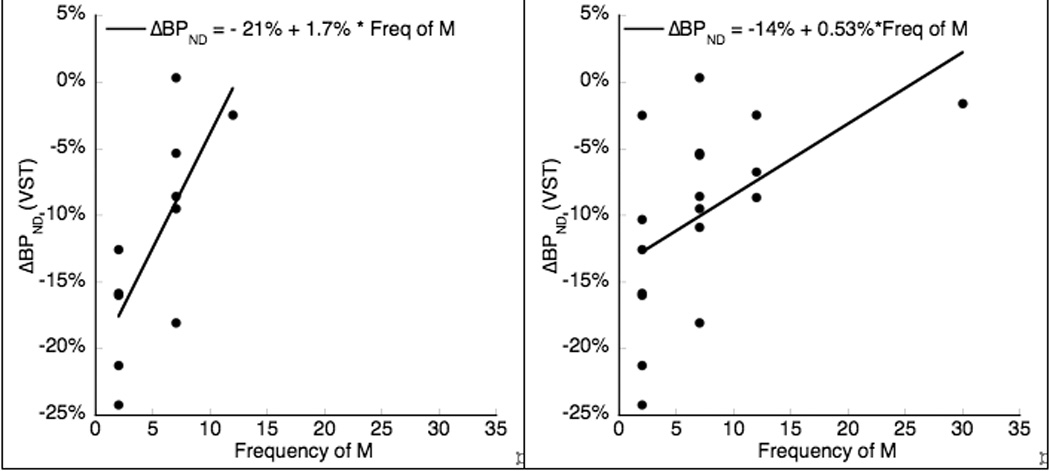
Frequency of M (maximum number of drinks per 24 hours) versus ΔBPND in VST. Left panel is in men only, n = 11, rho = 0.73, p = 0.012 (Spearman’s rank order coefficient). Right panel includes all men and 7 women, 3 outlier female subjects with very high frequency of M excluded, n = 18, rho = 0.613, p = 0.007. Ordinal logistic regression with frequency of M as an ordinal dependent variable and ΔBPND in VST as continuous independent variable reached significance for men alone (n = 11, χ2 = 8.28, p = 0.004) and for the entire cohort (n = 21, χ2 = 5.46, p = 0.019) but not for women alone.
Blood alcohol levels
Subjects were distinctly, but not heavily intoxicated with blood alcohol levels slightly above the legal limit. BAL peaked at 55 min after drink (1.15 ± 0.3 mg/ml in men and 1.02 ± 0.4 mg/ml in women, p = 0.37, see Supplement: Figure S1) and did not differ between groups at any time point (25, 40, 55 and 70 min). There was no correlation between BAL at any of the time points and ventrostriatal DA release. Table S1 (see Supplement) shows mean alcohol content of the drink.
Subjective effects of alcohol
The group as a whole showed a significant increase in total scores (sum of scores for each item) of subjective activation and sedation, at all time points (alcohol vs. placebo condition, Supplement: Table S2). Baseline scores for activation or sedation did not differ significantly between conditions, however, activation at baseline was higher on the first scan day, independent of the nature of the drink (35 ± 14 first day vs. 28 ± 12 second day, p < 0.01). For men, difference in activation scores between conditions was significant after 30 minutes, for women only after 60 minutes.
Total scores for subjective sedation differed between conditions: Peak total scores (at 60 min) were 27.8 ± 9.5 for alcohol and 19.2 ± 11.7 for placebo drink (p < 0.01), but were not different between groups.
Correlations of subjective effects with VST DA release
The difference in activation total scores between conditions over 90 min was significantly correlated to VST DA release for the group as a whole at all time points. For men, there was significant correlation at 30 and 60 min, but not for women (Fig. 2; Supplement: Table S2). There was no significant correlation between subjective sedation and ΔBPND at any time.
Figure 2.
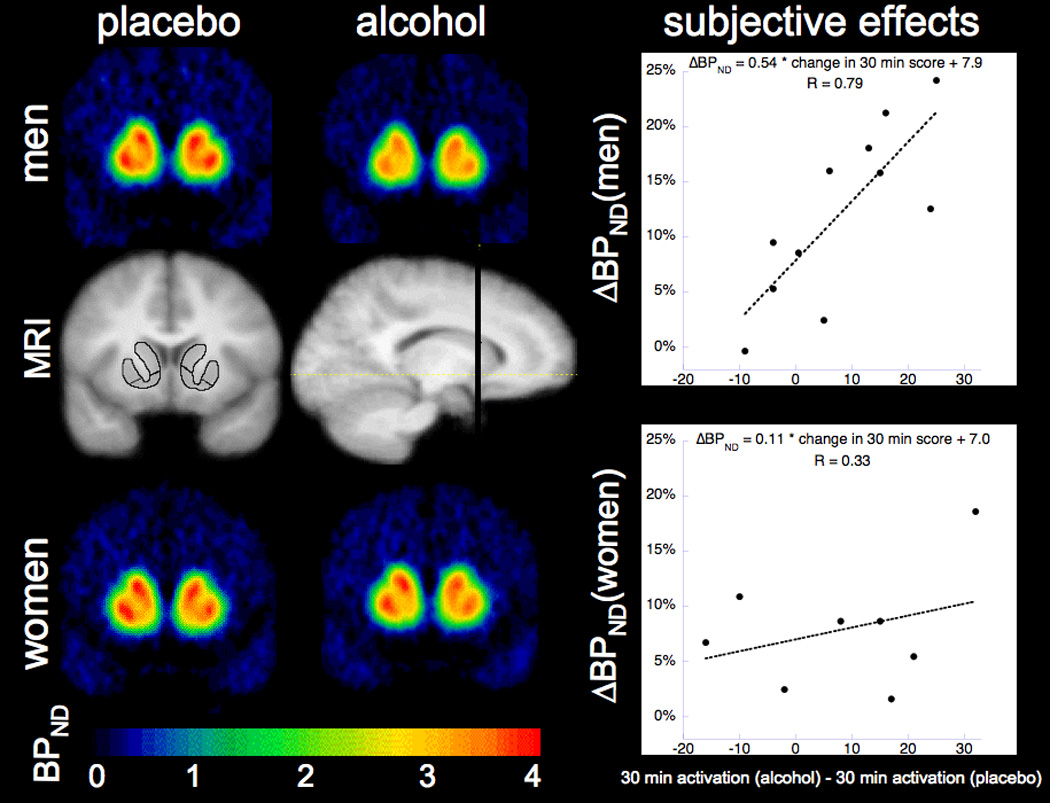
Striatal change in [11C]raclopride binding potential maps and subjective activation in response to alcohol
Binding potential maps averaged across men (n=11, top) and women (n = 10, bottom) following placebo drink (left) and alcohol drink (right). The MRI images (center) are averaged across all 21 subjects. Images were all non-linearly warped into MNI space in the SPM2 software environment (31). The ROIs on the coronal MRI image (left) are the preDCA, preDPU and VST. The line through the sagittal MRI slice (right) shows the coronal slice level of the other images. The graphs on the right show the correlation between subjective activation at 30 minutes after drink (total score post-alcohol minus total score post-placebo, not adjusted for baseline) and absolute ΔBPND. The relationship is stronger for men (top). Note that the absolute value of ΔBPND is presented here.
Imaging results
There were no differences in ROI volumes or scan parameters. See Table 1 (all subjects).
Effect of alcohol on DA release
The effect of alcohol on DA release in the group as a whole was significant for all striatal substructures with the greatest effect observed in the VST (ΔBPND = −9 ± 8%, p < 0.0001). ΔBPND were −7 ± 8% in the preDPU, −5 ± 8% in the preDCA, −6 ± 8% in the postCA, −5 ± 6% in the postPU, −6 ± 7% for AST, −6 ± 6% in the DPU and −6 ± 7% for the striatum as a whole (p<0.05 for all ROI). When separated by sex, men showed a significant effect of alcohol on ΔBPND in all ROIs, (VST: − 12 ± 8%, p < 0.001), and an overall greater magnitude of change than women (VST: − 6 ± 8%, p = 0.02; statistically significant also in preDPU: − 5 ± 7%, p < 0.05). See table 3.
Table 3.
Binding potential: [11C]raclopride binding (BPND) for all regions of interest (ROI) after each condition (placebo vs. alcohol) and percent change of [11C]raclopride displacement (ΔBPND) for both men and women.
| Men (n=11) | Women (n=10) | men vs. women |
|||||
|---|---|---|---|---|---|---|---|
| ROI | BPND (placebo) | BPND (alcohol) | % ΔBPND* | BPND(placebo) | BPND (alcohol) | %ΔBPND** | p (ttest)*** |
| VST | 2.28 ± 0.23 | 2.00 ± 0.18 | −12.1% ± 8% | 2.23 ± 0.21 | 2.09 ± 0.19 | −6.2% ± 8% | 0.10 |
| preDCA | 2.51 ± 0.31 | 2.32 ± 0.29 | −7.3% ± 8% | 2.49 ± 0.24 | 2.43 ± 0.28 | −2.2% ± 6% | 0.12 |
| preDPU | 3.02 ± 0.33 | 2.75 ± 0.28 | −8.5% ± 8% | 2.99 ± 0.31 | 2.85 ± 0.38 | −4.7% ± 7% | 0.26 |
| postCA | 1.83 ± 0.25 | 1.68 ± 0.26 | −8.5% ± 7% | 2.09 ± 0.28 | 2.03 ± 0.25 | −2.4% ± 9% | 0.08 |
| postPU | 3.17 ± 0.23 | 2.93 ± 0.25 | −7.6% ± 6% | 3.35 ± 0.25 | 3.24 ± 0.20 | −3.0% ± 5% | 0.08 |
changes were significant for all regions in men. VST: p<0.001
changes were only significant for VST and preDPU in women. VST: p=0.02, preDPU: p=0.04
analysis with a linear mixed model across all striatal subregions showed a highly significant effect of sex (p < 0.001) with significantly greater Δ BPND in men
Two group t-tests for effect of sex on ΔBPND did not reach statistical significance in individual ROIs (for VST, p = 0.10), but application of a linear mixed model across all striatal subregions with regional ΔBPND as the dependent variable and regions of interest as repeated measures showed a highly significant effect of sex (p < 0.001) with larger DA release in men. Because drink order was balanced for the group as a whole, but not across sex (3 women and 7 men had alcohol on the first day), the model was repeated with drink order as a covariate. Effect of sex remained significant (p = 0.027). There was also a significant independent effect of scan order (p<0.001), but there was no sex-by-order interaction (p=0.35). Figure 2 illustrates the sex difference with binding potential maps averaged across subjects.
BPND for the placebo condition only was not significantly different between men and women for any ROI apart from the postCA (BPND men = 1.83 ± 0.25, BPND women = 2.09 ± 0.28, p=0.04).
There was no difference in ΔBPND between smokers (n=4) and non-smokers (n=17) in any ROI. A two-way test with sex and smoking as covariates and ΔBPND as the dependent variable showed no effect of smoking status (p = 0.92).
In order to further explore the effect of drinking history on DA release, we used general linear model analysis of ΔBPND in VST with “frequency of M” as a covariate and grouping by sex as fixed factor. Whether all subjects (n=21) or only n=18 were included (3 female outliers with higher frequency of M removed), we found a significant sex by frequency interaction (p=0.016, n = 21; p=0.009, n = 18), as well as a significant effect of “frequency of M” (p=0.02, n = 21; p = 0.002, n = 18); sex remained a significant factor in this model (p=0.005; p = 0.007 after outliers removed).
Discussion
This report presents conclusive evidence in a large group of young adults for alcohol induced DA release measured in vivo, and shows, for the first time, sex differences in the magnitude of release. Although exposed to similar levels of alcohol, men had greater DA release than women. Furthermore, we show that alcohol stimulates DA release throughout the human striatum, but most significantly in striatal regions implicated in reward and motivation. Whereas large effects were seen in both VST and postPU following amphetamine (28, 37, 38) with smaller effects in other striatal subregions, only the VST displayed large ΔBPND after alcohol. We can estimate the level of fractional increase in ventrostriatal DA induced by our alcohol administration by utilizing the simplifying assumptions that a) the interaction between DA and [11C]raclopride at the D2/3 receptor is purely competitive, b) DA KD for D2/3 receptors does not change between conditions and c) receptor-bound DA during the placebo condition is comparable to baseline values. Using the baseline occupancy of D2/3 receptors by DA in healthy volunteers estimated by Laruelle et al (10%, 39) and in vivo estimates of the fraction of D2/3 receptors in a high affinity state for agonists (80%, 40), we estimate that the alcohol challenge increased extracellular DA levels by 138% in men, and 69% in women. The magnitude of the effect of alcohol is comparable to that measured with a low dose of amphetamine in young subjects (41, 42), and similar to that reported for challenge with nicotine or smoking (43). Similar sex differences have been previously reported after an amphetamine challenge (38) showing greater change in [11C]raclopride binding in men in several striatal subregions (VST: 12 ±6% in men, 7±5% in women, p=0.01), but no difference in baseline D2 binding.
The effect of sex was apparent across the whole striatum, suggesting that alcohol affects a broader dopaminergic pathway than the classic VTA-VST circuit.
While amphetamine works by a mechanism of facilitated exchange diffusion at the DA transporter (44, 45), it is not clear how alcohol stimulates dopamine release and it may have direct and indirect effects. Ethanol has been reported to remove GABAergic inhibition of DA neurons (46), to directly excite DA VTA neurons and reduce the afterhyperpolarization (AHP) that follows spontaneous action potentials by reducing a quinidine-sensitive K+ current (47). Additionally, alcohol promotes DA release by a local calcium-dependent effect at the DA terminals in the striatum and accumbens (48, 49, 50) possibly mediated by an effect on DA transporters (51). In animals, ethanol administered at doses typically associated with human drinking enhances DA release in the accumbens via actions at other brain sites (52, 53). In rats habituated to alcohol exposure, this may be limited to the early phase after the onset of drinking, suggesting a blunted striatal DA release as an effect of habituation as seen in chronically alcohol dependent humans (20, 21), but also that DA may not be the central substrate producing the reinforcement in habituated rats (14). While passively administered i.v. ethanol can stimulate DA release, ethanol-related cues evoke an additional component of DA release (54, 55). Repeated alcohol intake may induce salience attribution to alcohol-associated cues.
In this study, we did not test for the effect of cues, but endeavored to minimize olfactory cues. Comparison of the placebo condition BPND in our study to baseline [11C] raclopride BPND values from a cohort of age- and sex-matched healthy controls (n = 20, mean age 24.8 ± 3 years, 11 men, 9 women, unpublished observations) shows no statistically significant differences in binding potential in any region: BPND in the baseline cohort was 2.21 ± 0.3 in the VST (vs. 2.26 ± 0.2 after placebo drink in this study, p=0.57), and 2.8 ± 0.3 for the striatum as a whole (vs. 2.7 ± 0.2 after placebo, p=0.31). This suggests that the placebo drink in our hands was associated with negligible or no change in DA release and provided a neutral stimulus rather than an appetitive cue. This interpretation is limited by the fact that we are comparing different cohorts. A better paradigm would include an additional baseline scan to test the effects of all sensory cues.
The alcoholic drink supplied both the sensory properties of alcohol (taste and smell) as well as the pharmacological effects once absorbed, which may both contribute to dopamine release and are not easily separated in this study design.
The fact that women received drinks with slightly lower concentrations of alcohol may support the contribution of sensory stimuli to the difference in VST DA release, however, as sensory organs generally respond logarithmically to increase in stimuli intensity, rather than linearly (56), it is unlikely that the absolute difference in concentrations of 10% (49% in women and 59% in men) was detectable. We consider it unlikely that sensory properties of alcohol alone are able to explain the large effect on ΔBPND and the significant sex differences in the striatum as a whole. The effects sizes for alcohol (1.125 total group, 1.5 for men, 0.75 for women) and sex (=0.75) here are comparable to those in Boileau et al.’s study : alcohol effect size of 1.03 in an all male sample (22).
As a further caveat, we did not control for estrogen levels among subjects and its possible effect on the magnitude of DA release in women. However, so far only behavioral and biochemical studies in animals indicate central dopaminergic neurotransmission may be modulated by sex steroids, while human studies have not confirmed these findings (57, 58).
Correlations with clinical measures
We observed correlations in men between magnitude of release and subjective activation, as well as with maximal number of drinks per 24 hours. These observations should be regarded as preliminary but they allow us to speculate on the functional significance of the observed DA release.
Alcohol induced greater subjective activation than placebo and the difference in activation scores across days between conditions correlated with greater DA release in the VST (p < 0.05). Greater activation between alcohol and placebo was no longer observed when the ratings were corrected for baseline for each day, due to an order effect where subjective activation at baseline on the first day was higher than second day regardless of the nature of the drink. This effect is possibly related to the novelty of the situation on the first day. This is an unexpected effect of the two-day paradigm and presents a limitation in our study. To bypass this order effect, we compared ratings of subjective activation at specific timepoints across days, and we observed that men showed greater activation in the early phase after alcohol consumption (Fig. 2), which correlated with ΔBPND in VST only in men. It is tempting to speculate, based on this observation, that the larger effect on DA transmission may contribute to the initial reinforcing properties of alcohol and may be related to the higher incidence of alcoholism in men.
We also observed an effect of scan order on ΔBPND: alcohol administered in the first PET session evoked greater DA release. However, sex was an independent factor: men still had greater alcohol-evoked DA release than did women after controlling for the order effect; there was no sex by order interaction.
Finally, we observed that larger DA release was associated with smaller frequency of maximum number of drinks per 24 hours (M), a strong relationship that survived correction for multiple comparisons. This observation is interesting as it could suggest that habitual drinking of large numbers of alcoholic drinks at individual occasions, as measured by M, a parameter proposed to indicate greater potential for addiction (35), and withdrawal symptoms (36), is associated with smaller release. In other terms, the beginning of a transition to habit, detected here by frequent drinking, may be associated with a decrease in the magnitude of DA release in men. In women this relationship was not significant, possibly due to lack of power in the presence of large variance. When outliers among women were removed, the same relationship of lower DA release with higher frequency was true for the group as a whole, but not for women. Our interpretation of lowered DA release as a correlate of transition to habit is consistent with preclinical animal models of addiction (59).
In summary, the current findings indicate that alcohol stimulates DA release in humans, and this effect is greater in men than in women. We also observe that DA release is associated with subjective activation in men, and inversely related to the frequency of heavy drinking. Together, these findings suggest that the ability of alcohol to stimulate DA release may play an important and complex role in its rewarding effects and abuse liability in humans. Our report further suggests a biological mechanism that may mediate the difference in vulnerability for alcoholism between men and women.
Supplementary Material
Acknowledgements
Financial disclosures:
L.S. Kegeles: Amgen, Pfizer (research grants); M.Slifstein: Amgen, GlaxoSmithKline (consultant); S.S. O’Malley: member ACNP workgroup sponsored by Eli Lilly, Janssen, Schering Plough, Lundbeck, Glaxo-Smith Kline and Alkermes; partner Applied Behavioral Research; Scientific Panel Butler Center for Research at Hazelden; Brown University and Medical College of South Carolina (consultant); NABI pharmaceuticals (research contract); Controlled Release Society, AMERSA (travel reimbursement/award); J.H. Krystal reports the following: Consultant: Aisling Capital, LLC, AstraZeneca, Brintnall & Nicolini, Inc., GlaxoSmithKline, Janssen, Merz, Pfizer, F. Hoffman-La Roche, Ltd., SK Holdings Co., Ltd., Teva Pharmaceuticals, Ltd.; Scientific Advisory Board/Consultant: Abbott Laboratories, Bristol-Myers Squibb, Eli Lilly and Co., Lohocla Research Corporation, Takeda Industries,Transcept; ExercisableWarrant Options (value less than $500): Tetragenex Pharmaceuticals; Research/Study Drug Support: Janssen Research Foundation (to the Department of Veterans Affairs); Board of Directors: American College of Neuropsychopharmacology; Editor: Biological Psychiatry; Inventions: 1) Seibyl JP, Krystal JH, Charney DS. Dopamine and noradrenergic reuptake inhibitors in treatment of schizophrenia. Patent #:5,447,948.September 5, 1995; 2) Co-inventor with Dr. Gerard Sanacora on a filed patent application by Yale University related to targeting the glutamatergic system for the treatment of neuropsychiatric disorders (PCTWO06108055A1); and, 3) Intranasal Administration of Ketamine to Treat Depression (pending). A.Abi-Dargham: BristolNina Myers Squibb-Otsuka (consultant and speaker), Bohringer-Engelheim (consultant), GlaxoSmithKline (research grant).
Funding/Support: This research was carried out at New York State Psychiatric Institute/Columbia University Medical Center under a subcontract from the Center for Translational Neuroscience of Alcoholism (CTNA) at Yale University, supported by grant number P50AA-012870-09 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA).
Role of the Sponsor: The funding agency had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All other authors reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64(7):830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- 2.Keyes KM, Geier T, Grant BF, Hasin DS. Influence of a drinking quantity and frequency measure on the prevalence and demographic correlates of DSM-IV alcohol dependence. Alcohol Clin Exp Res. 2009;33(5):761–771. doi: 10.1111/j.1530-0277.2009.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saxena S. Alcohol, Europe and the developing countries. Addiction. 1997;92 Suppl 1:S43–S48. [PubMed] [Google Scholar]
- 4.Teesson M, Baillie A, Lynskey M, Manor B, Degenhardt L. Substance use, dependence and treatment seeking in the United States and Australia: a cross-national comparison. Drug Alcohol Depend. 2006;81(2):149–155. doi: 10.1016/j.drugalcdep.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Schuckit MA. Alcohol-use disorders. Lancet. 2009;373(9662):492–501. doi: 10.1016/S0140-6736(09)60009-X. [DOI] [PubMed] [Google Scholar]
- 6.Kuperman S, Chan G, Kramer JR, Bierut L, Bucholz KK, Fox L, et al. Relationship of age of first drink to child behavioral problems and family psychopathology. Alcohol Clin Exp Res. 2005;29(10):1869–1876. doi: 10.1097/01.alc.0000183190.32692.c7. [DOI] [PubMed] [Google Scholar]
- 7.Clark DB. The natural history of adolescent alcohol use disorders. Addiction. 2004;99 Suppl 2:5–22. doi: 10.1111/j.1360-0443.2004.00851.x. [DOI] [PubMed] [Google Scholar]
- 8.Krystal JH, Tabakoff B. Ethanol abuse, dependence, and withdrawal: neurobiology and clinical implications. In: Davis KL, editor. Psychopharmacology: a fifth generation of progress, e.a. Philadelphia: Lippincott Williams and Wilkins; 2002. pp. 1425–1443. [Google Scholar]
- 9.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85(14):5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samson HH, Tolliver GA, Haraguchi M, Hodge CW. Alcohol self-administration: role of mesolimbic dopamine. Ann N Y Acad Sci. 1992;654:242–253. doi: 10.1111/j.1749-6632.1992.tb25971.x. [DOI] [PubMed] [Google Scholar]
- 11.Wise R, Romprè P. Brain dopamine and reward. Ann Rev Psychol. 1989;40 doi: 10.1146/annurev.ps.40.020189.001203. 191-. [DOI] [PubMed] [Google Scholar]
- 12.Le Moal M, Simon H. Mesocorticolimbic dopaminergic network: functional and regulatory roles. Physiol Rev. 1991;71(1):155–234. doi: 10.1152/physrev.1991.71.1.155. [DOI] [PubMed] [Google Scholar]
- 13.Fibiger HC. Drugs and reinforcement mechanisms: a critical review of the catecholamine theory. Annu Rev Pharmacol Toxicol. 1978;18:37–56. doi: 10.1146/annurev.pa.18.040178.000345. [DOI] [PubMed] [Google Scholar]
- 14.Nurmi M, Sinclair JD, Kiianmaa K. Dopamine release during ethanol drinking in AA rats. Alcohol Clin Exp Res. 1998;22(8):1628–1633. [PubMed] [Google Scholar]
- 15.McBride WJ, Chernet E, Dyr W, Lumeng L, Li TK. Densities of dopamine D2 receptors are reduced in CNS regions of alcohol-preferring P rats. Alcohol. 1993;10(5):387–390. doi: 10.1016/0741-8329(93)90025-j. [DOI] [PubMed] [Google Scholar]
- 16.Murphy JM, McBride WJ, Lumeng L, Li TK. Regional brain levels of monoamines in alcohol-preferring and -nonpreferring lines of rats. Pharmacol Biochem Behav. 1982;16(1):145–149. doi: 10.1016/0091-3057(82)90026-0. [DOI] [PubMed] [Google Scholar]
- 17.Bustamante D, Quintanilla ME, Tampier L, Gonzalez-Lira V, Israel Y, Herrera-Marschitz M. Ethanol induces stronger dopamine release in nucleus accumbens (shell) of alcohol-preferring (bibulous) than in alcohol-avoiding (abstainer) rats. Eur J Pharmacol. 2008;591(1–3):153–158. doi: 10.1016/j.ejphar.2008.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katner SN, Weiss F. Neurochemical characteristics associated with ethanol preference in selected alcohol-preferring and -nonpreferring rats: a quantitative microdialysis study. Alcohol Clin Exp Res. 2001;25(2):198–205. [PubMed] [Google Scholar]
- 19.Ramachandra V, Phuc S, Franco AC, Gonzales RA. Ethanol preference is inversely correlated with ethanol-induced dopamine release in 2 substrains of C57BL/6 mice. Alcohol Clin Exp Res. 2007;31(10):1669–1676. doi: 10.1111/j.1530-0277.2007.00463.x. [DOI] [PubMed] [Google Scholar]
- 20.Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, et al. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry. 2005;58(10):779–786. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 21.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, et al. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007;27(46):12700–12706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, et al. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49(4):226–231. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- 23.Yoder KK, Morris ED, Constantinescu CC, Cheng TE, Normandin MD, O'Connor SJ, et al. When what you see isn't what you get: alcohol cues, alcohol administration, prediction error, and human striatal dopamine. Alcohol Clin Exp Res. 2009;33(1):139–149. doi: 10.1111/j.1530-0277.2008.00821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoder KK, Constantinescu CC, Kareken DA, Normandin MD, Cheng TE, O'Connor SJ, et al. Heterogeneous effects of alcohol on dopamine release in the striatum: a PET study. Alcohol Clin Exp Res. 2007;31(6):965–973. doi: 10.1111/j.1530-0277.2007.00390.x. [DOI] [PubMed] [Google Scholar]
- 25.Salonen I, Hietala J, Laihinen A, Lehikonen P, Leino L, Nagren K, et al. A PET Study on the Acute E€ ffect of Ethanol on Striatal D2 Dopamine Receptors with [11C]Raclopride in Healthy Males. Human Psychopharmacology: Clinical and Experimental. 1997;12(2):145–152. [Google Scholar]
- 26.Maisto SA, Sobell LC, Cooper AM, Sobell MB. Comparison of two techniques to obtain retrospective reports of drinking behavior from alcohol abusers. Addict Behav. 1982;7(1):33–38. doi: 10.1016/0306-4603(82)90022-3. [DOI] [PubMed] [Google Scholar]
- 27.Watson PE, Watson ID, Batt RD. Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr. 1980;33(1):27–39. doi: 10.1093/ajcn/33.1.27. [DOI] [PubMed] [Google Scholar]
- 28.Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23(3):285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- 29.Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21(9):1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17(1):140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 31.Ashburner J. Computational anatomy with the SPM software. Magn Reson Imaging. 2009;27(8):1163–1174. doi: 10.1016/j.mri.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Hall H, Sedvall G, Magnusson O, Kopp J, Halldin C, Farde L. Distribution of D1- and D2-dopamine receptors, and dopamine and its metabolites in the human brain. Neuropsychopharmacology. 1994;11(4):245–256. doi: 10.1038/sj.npp.1380111. [DOI] [PubMed] [Google Scholar]
- 33.Benjamini Y, Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B-methodological. 1995;57(1):289–300. [Google Scholar]
- 34.NIAAA. NIAAA Council Approves Definition of Binge Drinking. Bethesda, MD: National Institute of Alcohol Abuse and Alcoholism; 2004. p. 3. [Google Scholar]
- 35.Begleiter HRT, Hesselbrock V, Porjesz B, Li T-K, Schucki MA, Edenberg HJ, Rice JP. The collaborative study on the genetics of alcoholism. Alcohol Health Res World. 1995;19:228–236. [PMC free article] [PubMed] [Google Scholar]
- 36.Schuckit MA, Tipp JE, Reich T, Hesselbrock VM, Bucholz KK. The histories of withdrawal convulsions and delirium tremens in 1648 alcohol dependent subjects. Addiction. 1995;90(10):1335–1347. doi: 10.1046/j.1360-0443.1995.901013355.x. [DOI] [PubMed] [Google Scholar]
- 37.Slifstein M, Kegeles L, Xu X, Thompson J, Urban N, Castrillon J, et al. Striatal and extrastriatal dopamine release measured with PET and [18F] fallypride. Synapse. 2010;64(5):350–362. doi: 10.1002/syn.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munro CA, McCaul ME, Wong DF, Oswald LM, Zhou Y, Brasic J, et al. Sex differences in striatal dopamine release in healthy adults. Biol Psychiatry. 2006;59(10):966–974. doi: 10.1016/j.biopsych.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Laruelle M, D'Souza CD, Baldwin RM, Abi-Dargham A, Kanes SJ, Fingado CL, et al. Imaging D2 receptor occupancy by endogenous dopamine in humans. Neuropsychopharmacology. 1997;17(3):162–174. doi: 10.1016/S0893-133X(97)00043-2. [DOI] [PubMed] [Google Scholar]
- 40.Narendran R, et al. Measurement of in vivo affinity of [11C]NPA and the proportion of D2 receptors configured in agonist high affinity state (%Rhigh) in baboons using PET. Neuroimage. 2004;22:T19–T20. [Google Scholar]
- 41.Anand A, Verhoeff P, Seneca N, Zoghbi SS, Seibyl JP, Charney DS, et al. Brain SPECT imaging of amphetamine-induced dopamine release in euthymic bipolar disorder patients. Am J Psychiatry. 2000;157(7):1108–1114. doi: 10.1176/appi.ajp.157.7.1108. [DOI] [PubMed] [Google Scholar]
- 42.Abi-Dargham A, Kegeles LS, Martinez D, Innis RB, Laruelle M. Dopamine mediation of positive reinforcing effects of amphetamine in stimulant naive healthy volunteers: results from a large cohort. Eur Neuropsychopharmacol. 2003;13(6):459–468. doi: 10.1016/j.euroneuro.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 43.Brody AL, Mandelkern MA, Olmstead RE, Allen-Martinez Z, Scheibal D, Abrams AL, et al. Ventral striatal dopamine release in response to smoking a regular vs a denicotinized cigarette. Neuropsychopharmacology. 2009;34(2):282–289. doi: 10.1038/npp.2008.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sulzer D, Rayport S. Amphetamine and other psychostimulants reduce pH gradients in midbrain dopaminergic neurons and chromaffin granules: a mechanism of action. Neuron. 1990;5(6):797–808. doi: 10.1016/0896-6273(90)90339-h. [DOI] [PubMed] [Google Scholar]
- 45.van Rossum JM, van der Schoot J, Hurkmans JA. Mechanism of action of cocaine and amphetamine in the brain. Experientia. 1962;18:229–231. doi: 10.1007/BF02148316. [DOI] [PubMed] [Google Scholar]
- 46.Mereu G, Gessa G. Low doses of ethanol inhibit the firing of neurons in the substantia nigra, pars reticulata, a GABAergic effect? Brain Res. 1997;360:325–330. doi: 10.1016/0006-8993(85)91249-1. [DOI] [PubMed] [Google Scholar]
- 47.Appel SB, Liu Z, McElvain MA, Brodie MS. Ethanol excitation of dopaminergic ventral tegmental area neurons is blocked by quinidine. Journal of Pharmacology & Experimental Therapeutics. 2003;306(2):437–446. doi: 10.1124/jpet.103.050963. [DOI] [PubMed] [Google Scholar]
- 48.Russell VA, Lamm MC, Taljaard JJ. Effect of ethanol on [3H]dopamine release in rat nucleus accumbens and striatal slices. Neurochem Res. 1988;13(5):487–492. doi: 10.1007/BF01268885. [DOI] [PubMed] [Google Scholar]
- 49.Snape BM, Engel JA. Ethanol enhances the calcium-dependent stimulus-induced release of endogenous dopamine from slices of rat striatum and nucleus accumbens in vitro. Neuropharmacology. 1988;27(11):1097–1101. doi: 10.1016/0028-3908(88)90003-2. [DOI] [PubMed] [Google Scholar]
- 50.Wozniak KM, Pert A, Mele A, Linnoila M. Focal application of alcohols elevates extracellular dopamine in rat brain: a microdialysis study. Brain Res. 1991;540(1–2):31–40. doi: 10.1016/0006-8993(91)90489-i. [DOI] [PubMed] [Google Scholar]
- 51.Eshleman AJ, Henningsen RA, Neve KA, Janowsky A. Release of dopamine via the human transporter. Mol Pharmacol. 1994;45(2):312–316. [PubMed] [Google Scholar]
- 52.Yim HJ, Schallert T, Randall PK, Gonzales RA. Comparison of local and systemic ethanol effects on extracellular dopamine concentration in rat nucleus accumbens by microdialysis. Alcohol Clin Exp Res. 1998;22(2):367–374. [PubMed] [Google Scholar]
- 53.Yim HJ, Schallert T, Randall PK, Bungay PM, Gonzales RA. Effect of ethanol on extracellular dopamine in rat striatum by direct perfusion with microdialysis. J Neurochem. 1997;68(4):1527–1533. doi: 10.1046/j.1471-4159.1997.68041527.x. [DOI] [PubMed] [Google Scholar]
- 54.Doyon WM, Anders SK, Ramachandra VS, Czachowski CL, Gonzales RA. Effect of operant self-administration of 10% ethanol plus 10% sucrose on dopamine and ethanol concentrations in the nucleus accumbens. J Neurochem. 2005;93(6):1469–1481. doi: 10.1111/j.1471-4159.2005.03137.x. [DOI] [PubMed] [Google Scholar]
- 55.Howard EC, Schier CJ, Wetzel JS, Duvauchelle CL, Gonzales RA. The shell of the nucleus accumbens has a higher dopamine response compared with the core after non-contingent intravenous ethanol administration. Neuroscience. 2008;154(3):1042–1053. doi: 10.1016/j.neuroscience.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Young PT. The role of affective processes in learning and motivation. Psychol Rev. 1959;66(2):104–125. doi: 10.1037/h0045997. [DOI] [PubMed] [Google Scholar]
- 57.Kaasinen V, Kemppainen N, Nagren K, Helenius H, Kurki T, Rinne JO. Age-related loss of extrastriatal dopamine D(2) -like receptors in women. J Neurochem. 2002;81(5):1005–1010. doi: 10.1046/j.1471-4159.2002.00895.x. [DOI] [PubMed] [Google Scholar]
- 58.Nordstrom AL, Olsson H, Halldin C. A PET study of D2 dopamine receptor density at different phases of the menstrual cycle. Psychiatry Res. 1998;83(1):1–6. doi: 10.1016/s0925-4927(98)00021-3. [DOI] [PubMed] [Google Scholar]
- 59.Murphy JM, McBride WJ, Gatto GJ, Lumeng L, Li K. Effects of acute ethanol administration on monoamine and metabolite content in forebrain regions of ethanol-tolerant and -nontolerant alcohol-preferring (P) rats. Pharmacol Biochem Behav. 1988;29(1):169–174. doi: 10.1016/0091-3057(88)90291-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


