Abstract
Studies of immunity typically focus on understanding how hematopoietic cells interact within conventional secondary lymphoid tissues. However, immune reactions and their regulation occur in various environments within the body. Adipose tissue is one context that can influence and be influenced by adjacent and embedded lymphocytes. Despite the abundance and wide distribution of such tissue, and despite a growing obesity epidemic, studies of these interactions have been only marginally appreciated in the past. Here, we review advances in understanding of lymphoid structures within adipose tissue, the relationship between adipose tissue and adaptive immune function, and evidence for how this relationship contributes to obesity-associated diseases.
Keywords: Adipose, FALC, Leptin, Lymphocytes, Milky spots, VAT
Adipose and immune cells
Adipose tissue (Box 1) mediates energy storage and energy expenditure [1,2]. When energy intake exceeds expenditure, this tissue's major cell type, the adipocyte, increases in size, leading to increased fat mass, and ultimately to obesity [2,3]. Adipocytes secrete and respond to soluble factors that directly influence metabolism, as well as the nervous and immune systems [2,4].
Box 1. Types of adipose tissues.
Adipose tissues in different anatomical locations are unequal in their metabolic activity, and in their relationship with immune cells. Adipose is generally separated into visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) [2,4]. Although SAT accounts for ~80% of human adipose, VAT is more metabolically active [20], and VAT accumulation is a greater predictor of obesity-associated mortality [2,4,79]. VAT refers to adipose within the peritoneal cavity, including depots such as the gonadal fat pad, the omentum, and the intestinal mesentery [4]. Most studies focus on comparisons between lean and obese subjects, or on comparisons between SAT and VAT, with VAT being represented by either the gonadal fat pad or by the omentum as a source of adipocytes, implying that the two depots are functionally interchangeable. Although gonadal and omental adipocytes may be more similar to each other than to those from subcutaneous adipocytes, it is important to distinguish between the various types of VAT, particularly the omentum and the mesentery, as they contain distinctly organized lymphocyte areas.
Adipose-derived soluble factors include leptin (Box 2), which suppresses appetite in mice and humans [5,6]. High circulating levels of leptin [4,7–9] may contribute to the inflammatory state of adipose tissue associated with obesity [4,8,10]. Adipocytes and the adipose stromovasculature also secrete classical cytokines (e.g., interleukins) and chemokines that regulate immune cells [4,10]. Compared with lean1 individuals, adipose in obese subjects has more macrophages per gram of tissue and has higher inflammatory cytokine levels [4,8,11–14]. Soluble factors released from leukocytes reciprocally influence the activity of adipocytes, as well as muscle and endocrine cells, in ways that in-turn affect nutrient levels (particularly glucose), insulin, and other adipokines, all of which contribute to a complex feedback-sensitive metabolic network [10,15–18]. As the effects of adipose on inflammation and innate immunity have been reviewed elsewhere [7,19], here we focus on the relationship between adipose and adaptive immunity. Recent advances such as identifying fat-associated lymphoid structures, the role of B lymphocytes in regulating atherosclerosis progression, and the contribution of fat-associated regulatory T cells (Tregs) in preventing autoimmunity, emphasize the importance of investigating this association to understand their contributions to obesity-associated diseases.
Box 2. Leptin in obesity.
Several considerations should be noted when interpreting results from the leptin-deficient ob/ob mouse strain, which has wide-ranging phenotypes [7]. Despite the hyperphagy and obesity observed in the ob/ob mice [5,6], leptin levels are elevated in other rodent models for obesity [9], and in human obesity [6,18], which is associated with leptin resistance [6]. Furthermore, apart from appetite control, leptin has direct effects on metabolism in mice, but to a much lesser degree in humans, and the leptin receptor is ubiquitously expressed, with six splice variants whose products differentially affect how cells respond to the ligand [5–7,69].
Lymphoid structures in visceral adipose
The omentum is a fatty tissue connecting the spleen, stomach, pancreas, and colon [20,21]. The omentum is enriched in macrophages and B1 B cells [22–27], but it also possesses dendritic cells [28–30] and NKT cells [31]. To varying degrees, these leukocytes are organized into clusters called “milky spots” (MS, Box 3) [21–24,32]. Reports of omental T cell composition vary, likely due to species difference, basal antigen exposure, and analysis methods [22,23,31].
Box 3. Development of adipose-associated lymphoid structures.
The development, architecture, and function of omental MS and mesenteric FALCs have characteristics that are both shared with and distinct from those of “conventional” secondary lymphoid organs, such as spleen and lymph nodes (LN). MS develop independently of Lymphoid Tissue inducer (LTi) cells [33], which are required for development of most other lymphoid structures [80]. MS development requires the chemokine CXCL13. However, unlike conventional lymphoid organs, which express this chemokine on FDC, CXCL13 expression localizes around the outside of the MS follicle [33]. Since MS lack FDC [22,23,33], it is unknown which cells produce CXCL13 in the omentum. The possibility that FALC cells might participate in FALC development is suggested by gene expression of lymphotoxin and its receptor [37], which are known to contribute to development of other lymphoid structures [81,82]. FALC cell development requires IL7 signaling and the transcriptional regulator Id2 [37], initially suggesting a developmental program akin to lymphocytes and to LTi cells. However, FALC cells develop independently of recombination-activating gene 2 and lack discernible T or B cell differentiation potential in vitro [37]. FALC cells also develop independently of RORγ t, which distinguishes them from LTi cells [37]. Interestingly, IL5-competent FALC cells can stimulate division of B1 B cells in culture, and can rescue poor B1 B cell division in cytokine signaling-deficient (and therefore FALC cell-deficient) hosts [37]. These data strongly suggest that it is indeed FALC cells that support B1 B cells self-renewal in the peritoneum.
Omental MS collect fluids, particulates, bacteria, and cells from the peritoneal cavity [21,29,30,33], and depending on the antigen, may induce a productive immune response or promote immune suppression [29,30,33–35]. Since the omentum also occludes wounds and other disturbances in the peritoneum [21], this organ is capable of coupling physical tissue repair with immunological defense. MS increase in size and in number in response to intraperitoneal immunization, intestinal perforations, burst appendices, dialysis-induced peritonitis, peritoneal catheter implantation, and trauma - any of which may introduce bacteria into the peritoneal cavity [21,22,24,26,35,36].
In naïve animals, there is a constant cell flux between the omentum and peritoneal cavity. Some, but not all, of this migration is mediated by pertussis toxin-sensitive receptors (typically chemokine receptors) and integrins (particularly LFA-1) [29,33]. When the intestinal barrier is breached, B1 B cells employ CXCL13, integrins, and CD9 to exit the peritoneal cavity and enter tissues such as omentum, mesentery, and spleen [36]. Although B1 B cells predominate in MS, conventional (B2) B cell responses can be identified in the omentum as well. Intraperitoneal immunization with protein (“T-dependent”) antigen results in organization of B cell follicles within the MS [22–24] with characteristics of germinal center formation, including antibody somatic hypermutation [23,33]. In mice lacking spleen, lymph nodes, and Peyer’s patches (“SLP” mice), such immunization still induces strong and sustained affinity-matured serum IgG correlating with these omental B cell reactions [33]. Therefore, the omentum can accommodate B2 B cell responses that may be competent to provide a degree of systemic humoral immunity, although contributions from other adipose (such as gonadal fat pad or mesentery) cannot be excluded. When activated T cells are injected intraperitoneally, they preferentially home to the mouse omentum, out-competing their naïve counterparts [29]. Additionally, activated T cells are readily detected in the omentum of antigen-experienced SLP mice [33]. These reactions include CD4 responses to protein given intraperitoneally, CD4 responses to orally fed helminth larvae, and CD8 responses to inhaled influenza virus [33]. Thus, antigen-experienced T cells recirculate through the peritoneal cavity and omentum, even when these cells are primed at distal locations. Whether distally primed B cells also do this is unknown.
Lymphoid clusters reminiscent of omental MS have recently been identified in human mesentery as well as in mouse mesenteric, adrenal, and gonadal adipose [37]. These fat-associated lymphoid clusters (FALCs) have proportionally fewer B cells than Peyer's patches or MS [37]. However, FALCs contain a substantial population of hematopoietic lineage marker-negative cells expressing markers associated with progenitor potential (e.g., c-kit, sca1, and IL7 receptor) together with mature leukocyte activation markers (e.g., CD69 and CD44) [37].
These “FALC cells” (or “natural helper cells”) can express multiple cytokines, particularly IL4, 5, 6, and 13 [37], which are considered “type 2” (Th2) T cell effector molecules. Correspondingly, these cells can coordinate cytokine production and intestinal goblet cell hyperplasia in response to parasitic worms [37]. It is unknown whether parasite expulsion is influenced FALC-derived cells per se, and if so, whether the mechanism involves Th2 CD4 T cells. However, recently described “nuocytes” from the mesenteric LNs bear a striking resemblance to FALC cells, and do promote parasite expulsion in a lymphocyte-dependent manner [38]. Another mesenteric LN population (“MPPtype2”) resembling FALC cells can differentiate into several myeloid lineages and into antigen-presenting cells capable of driving Th2 responses in vitro [39]. Although the gene expression profiles of MPPtype2 and FALC cells are not identical, these two populations and nuocytes all share a surface phenotype and the propensity to produce Th2 cytokines, particularly in response to IL25 [37–39]. It remains to be clarified whether the three populations are distinct, or instead are common with non-identical characteristics when situated in FALC versus LNs. Understanding the relationship between mesenteric FALC with the biology of the LN and gut will likely be valuable in optimizing prevention and treatment of parasite infections and food allergies.
B cell relationships with adipose
During ontogeny, mouse and human omenta are sources of B lineage cells, particularly of the B1 subset [40,41]. The adult omentum is also enriched in B1 B cells, as is the surrounding peritoneal cavity [25,36]. B1 B cells respond to pathogen-associated molecules both more rapidly than “conventional” (B2) B cells and with a less-diverse but more polyreactive antibody repertroire [42]. B1 B cells confer resistance to some bacterial [43] and viral infections [44], likely through antibody production, which tends to be of the IgM isotype. Therefore, regulation of B1 B cells by adipose-associated cells, such as FALC (see Box 3), might then influence immunity to these microorganisms. Omental and peritoneal B cells also contribute to the generation of natural IgA in the gut mucosa [45]. Intestinal leakage or intraperitoneal delivery of bacteria leads to rapid activation of B1 cells and promotes T-independent antibody responses [25]. Cells in the MS are highly responsive to bacterial products like LPS [36], thus, omental B1 cells provide natural immunity to bacterial pathogens. Correspondingly, Cxcl13−/ − mice lacking detectable MS have very few peritoneal B1 B cells, have low levels of natural antibody, and make poor antibody responses to bacterial antigens in the peritoneal cavity [25,33]. B1 B cells can also be a major source IL10 [46,47]. Given the contribution of such IL10-producing “Bregs” in modulating autoimmune manifestations [46,47], it will be important to determine whether such cells are altered in the omentum and other adipose of obese subjects with high susceptibility to autoimmunity.
B1 B cells express a unique antibody repertoire, including the T15 idiotype [48,49]. T15 antibody cross-reacts with phosphorylcholine on Streptococcus pneumoniae, with modified plasma membrane lipids on apoptotic mammalian host cells, and with modified forms of circulating lipids, such as oxidized low-density lipoprotein (oxLDL) [49,50]. A growing body of data suggest that B cell responses to these lipids might both positively and negatively influence progression of atherosclerosis. A high-fat/high cholesterol diet, as well as genetic factors, can result in high levels of modified LDLs, which promote the activation of vascular endothelia and macrophages, in-turn forming and exacerbating atherosclerotic lesions [12,50,51]. Interestingly, B cell deficiency increases vascular lesion size in a model of mouse atherosclerosis [52]. Furthermore, prior vaccination of normal mice with either S. pneumoniae or with modified LDL can reduce such vascular lesions [53]. This protective effect correlates with an increased secretion of IL5 and IL13 from modified LDL-restimulated spleen cells, an anti-modified LDL IgG response biased toward IgG1, as well as oxLDL-reactive IgM and immune complexes of IgM (specifically T15) with the protein component of LDL [53]. Additionally, IL5 deficiency enhances atherosclerotic lesions induced by a high-fat diet (HFD2) in susceptible mice, correlating with an inability to produce basal T15 and an inability to make an IgM response to modified LDL immunization [53]. These results collectively suggest that B cells, possibly through production of antibody reactive with modified LDLs, can be protective against lesion progression. However, oxLDL-reactive human antibody can enhance macrophage uptake of cholesterol products in culture, correlating with increased TNFα secretion [54]. Furthermore, mice deficient in activating Fc receptors for IgG have reduced symptoms of aortic stress when kept on HFD [55]. These apparently paradoxical observations likely reflect both protective and pathological roles of B cells in atherosclerosis, which may be determined by relative antibody isotype levels, involvement of T cell help, and differential responses of B1 and B2 B cells (Figure 1). Intriguingly, lesion formation in atherosclerotic mice is followed by formation of tertiary lymphoid structures in the surrounding adventitia [56]. These structures have characteristics of true germinal centers, including CD35-expressing follicular dendritic cells (FDC). It remains to be determined whether antibody contributes to this formation (e.g., by forming immune complexes that bind matured FDC, possibly through the CD35 complement receptor) and whether antibody derived from the B cells in this reaction further exacerbate the lesion.
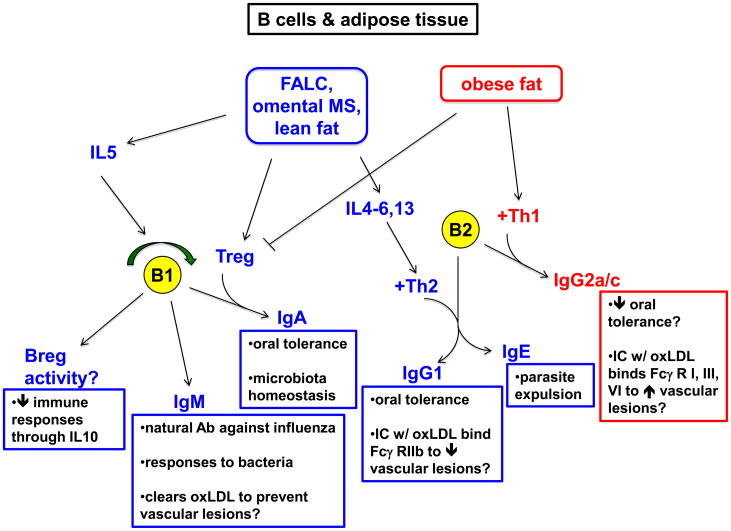
Figure 1. B cells and adipose tissue.
Fat-associated lymphoid clusters (FALC), omental milky spots (MS), and lean fat in general (blue boxes) have features favoring immune regulation. IL5 production by FALC sustains self-renewal of B1 B cells. B cells in this subset may produce IL10 and exert regulatory activity (Breg) [46,47]. B1 B cells also produce IgM, known to provide protection against influenza virus and also against bacteria such as Borrelia hermsii and S. pneumoniae. B1 cell-derived IgM against oxLDL may prevent this metabolite from exacerbating vascular atherosclerotic lesions. Omental and peritoneal B1 B cells also contribute to gut IgA production [45], important for oral tolerance and microbiota homeostasis. Incidentally, lean fat is enriched in TGFβ-producing regulatory T cells (Treg), which may provide this critical factor for IgA class switching. A Th2 bias in lean fat, including type 2 cytokine production by FALC cells, likely favors IgG1 production by conventional, B2 B lymphocytes. IgG1 against oral antigens contributes to oral tolerance in mice. Additionally, IgG1 against oxLDL may prevent or dampen vascular lesion formation by engaging the inhibitory Fc receptor (Fcγ RIIB) [83]. Th2 cytokine influence of B2 B cells may also promote IgE production favoring parasite expulsion. By contrast, obese fat (red boxes) tends to be in a pro-inflammatory state favoring exacerbation and/or initiation of autoimmune pathologies. This state includes a paucity of Tregs and a Th1 bias that may cooperate with B2 B cells to generate IgG2a/c. IgG2a/c against oral antigens in mice may contribute to poor oral tolerance (e.g., by complement fixation). IgG2a/c immune complexes with modified LDL may engage activating FcR (e.g., on macrophages) and exacerbate atherosclerotic lesions.
Obesity might influence B2 B cell responses, as suggested by the observation that overweight children and mice have poor antibody responses to tetanus toxin immunization [57,58]. However, it is unknown if this effect is due to dysregulated B cell responses directly, or to dysregulated T cell help, or simply a lower vaccine-to-body mass ratio, to as initially suggested. This issue could be addressed by normalization of vaccine doses in experimental animals. Mice on a HFD make an IgG2a response to protein antigen despite prior oral feeding of the same protein, which in lean mice suppresses this inflammatory isotype in favor of IgG1 (oral tolerance) [59]. Further investigation is needed to determine whether obesity influences susceptibility to food allergies.
T cell relationships with adipose
Adipose-resident T lymphocyte populations differ in location with host metabolic status. Mouse and human visceral adipose tissue (VAT; see Box 1) have a greater quantity of T lymphocytes per gram in obese subjects [13,14,60–62]. Such increases could result from T cell proliferation and from T cell influx. T cell proliferation can be potentiated by purified leptin [7] and by VAT from mice on HFD [13]. Additionally, body mass index (BMI) correlates with CD3 mRNA levels in human VAT, which correlate with message for the chemokine RANTES [60,63]. RANTES is known to recruit leukocytes, including T cells, to sites of inflammation [64,65]. Similarly, VAT from mice on HFD produces more RANTES compared with VAT from lean mice (on standard chow) [13,60]. However, RANTES expression in situ has also been localized to the stromovasculature [60]. RANTES production is likely influenced by the overall inflammatory condition of obese adipose tissue [4,8,10], as its production by cultured mouse adipocytes can be stimulated by cytokines such as TNFα, IFNγ , and IL1β [10,14,60]. Reciprocally, activated T cells can inhibit maturation of an adipocyte cell line [60], and VAT CD8 T cells can stimulate macrophage differentiation from monocytes in vitro [13]. Correspondingly, macrophage infiltration into VAT of mice on HFD temporally lags behind, and is dependent upon, CD8 cells [13]. Furthermore, mouse CD4 T cells can negatively regulate weight gain, VAT mass, high blood glucose, and elevated circulating TNFα and IL6 induced by HFD [62]. Thus, T cells both respond to and regulate the inflammatory state of obese fat (Figure 2).
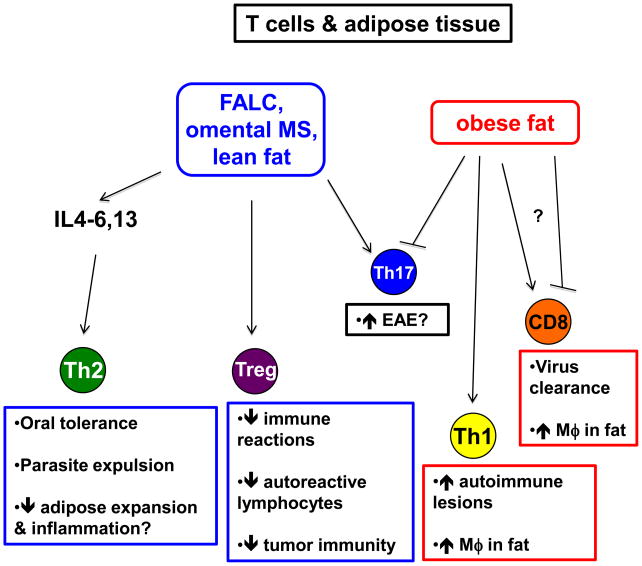
Figure 2. T cells and adipose tissue.
Fat-associated lymphoid clusters (FALC), omental milky spots (MS), and lean fat in general (blue boxes) have features favoring immune regulation. A Th2 bias in lean fat may promote oral tolerance, parasite expulsion, and also prevent adipose expansion and inflammation. Lean fat is also enriched with regulatory T cells (Treg) that suppress multiple immune reactions, including expansion and activity of autoreactive lymphocytes, and also suppression of tumor immunity. Th17 cells are differentially regulated by obesity in distinct anatomical locations, and may contribute to autoimmune manifestations such as EAE. By contrast, obese fat (red boxes) has fewer Treg and a bias toward Th1 differentiation that likely contribute to the inflammatory state of obese adipose by stimulating macrophages and exacerbating autoimmune lesions. CD8 T cells may be differentially regulated by obese fat, depending on their involvement in autoimmunity versus infections.
The adipose environment may also influence T cell effector cytokine polarization. Compared with spleen, mouse VAT has more CD4 T cells expressing the Th2-regulating transcription factor Gata3 [62]. However, HFD reduces Gata3+ CD4 cells in VAT and reciprocally increases IFNγ-expressing CD4 (Th1-phenotype) cells [62]. Obese human children also have elevated frequencies of IFNγ-expressing peripheral blood CD4 cells, which correlates with increased circulating insulin and with signs of fatty liver disease [66]. In mice, IFNγ is needed for macrophage accumulation in obese fat and for elevated expression of TNFα, MCP1, and RANTES [14], although it is unknown whether the IFNγ is derived from a particular subset of T cells or from another cell type. Nonetheless, these data are generally consistent with the association of inflammation and obese fat [4,8,10]. The results further suggest that resident Th2 cells may be somehow important for maintaining immune homeostasis. Like Th1 cells, Th17 (IL17-expressing CD4 T cells) contribute to inflammatory autoimmunity [67]. Although there are more IL17-expressing CD4s in VAT versus subcutaneous adipose tissue (SAT) and spleen in mice on standard chow, on HFD, Th17 cells are reduced in VAT, yet increased in SAT [62]. Therefore, the broad generalization that obese inflammatory VAT promotes/exacerbates autoimmune manifestations may not be all-encompassing. However, splenic skewing to the Th17 phenotype in HFD mice correlates with an accelerated and higher clinical score after experimentally induced encephalomyelitis (EAE) [68].
T cells can be directly and indirectly affected by leptin [7]. These effects include increased thymic output [7], repression of TCR signaling in regulatory T cells [69], increased sensitivity to TCR signaling in “conventional” (non-regulatory) T cells [7,69,70], increased IL2 secretion [70], Th1 polarization [7,70], inhibition of apoptosis [7], and increased delayed-type hypersensitivity [70]. Correspondingly, leptin levels are elevated in several human T cell-mediated autoimmune manifestations including inflammatory bowel disease, type 1 diabetes, vasculitis, and sometimes rheumatoid arthritis [7]. Leptin-deficient ob/ob mice have reduced susceptibility to EAE, arthritis, colitis, hepatitis, nephrotoxic nephritis, glomerulonephritis, and NOD-associated diabetes [7]. In several of these cases, leptin administration can restore susceptibility to such pathologies [7]. Thus, high leptin in obese individuals [4,7–9] may contribute to further health complications by promoting general inflammation, which directly (through the leptin receptor on T cells) and indirectly (through general inflammation associated with obesity) results in enhanced and sustained effector/inflammatory T cell responses that may initiate and/or exacerbate tissue pathology (Figure 2).
Dysregulation of Tregs might also contribute to obesity-associated clinical complications. Tregs suppress immune responses [71] and are enriched in VAT of non-obese humans and mice compared with spleen and SAT from the same individuals [10]. However, these cells are reduced in VAT of both obese mice and humans [10,13,62], in the colon of HFD mice [72], and in the peripheral blood of children with metabolic syndrome [73]. These reductions may result from leptin suppressing Treg proliferation directly [69], although additional causes cannot be excluded.
Tregs from mouse spleen, lean fat, and obese fat can inhibit conventional splenic T cell proliferation equally well in vitro [10,69]. However, phenotypic and gene expression profiling show differences among Tregs from these sources [10,73]. Since Tregs can be suppressive using multiple mechanisms [71], it remains to be determined how much altered Treg numbers and altered functions each contribute to the immune dysregulation associated with obesity. Nonetheless, the proportion of Treg-phenotype CD4 cells in mouse VAT inversely correlates with insulin resistance, a manifestation of type 2 diabetes [10]. Additional evidence suggests that Treg can suppress atherosclerotic lesions in mice [74]. Interestingly, leptin receptor-deficient Tregs are highly efficient at suppressing atherosclerotic lesions compared with receptor-sufficient Tregs [75]. These data would argue in favor of a model in which a paucity of Tregs, and not necessarily impaired function, contributes to disease exacerbation in obesity, although the situation may be distinct for particular complications. Additionally, these observations, together with the association between inflammatory T cell responses and obese fat, may suggest that the enrichment Th2 cells and Tregs in lean fat may play a valuable role in immune and physiological homeostasis. Whether these cells are needed for preventing excessive inflammatory responses to lipid oxidation byproducts during metabolism and/or for some other purpose will be an intriguing line of investigation to follow.
Tregs can suppress numerous immune reactions, including CD8 T cell responses [71]. Therefore, the combination of reduced Tregs in obese fat with the ability of VAT CD8 T cells to promote macrophage recruitment during obesity-associated inflammation [13] may provide a positive feedback loop that ultimately progresses to autoimmune pathology. It might then be expected that infections regulated by CD8 T cells (such as influenza virus) would be more efficiently eliminated and the pathology avoided in the context of obese fat. However, in primary and in secondary heterosubtypic infections, HFD mice have an increased susceptibility to normally sub-lethal doses of influenza [76,77]. This susceptibility (increased mortality) correlates with paradoxically reduced induction of IFNαβ , RANTES, TNFα, and IL6 mRNA in the lung [76,77]. The T cells from such mice also respond poorly to antigen in vitro [77], and the mice have somewhat dampened CD8 responses after secondary challenge [77]. Adoptive transfer experiments of CD8 cells could determine the relative contribution of the T cells compared with the environment influenced by HFD. Further studies are needed to establish how the network of various immune cells (e.g., Treg, Th1, Th17, and CD8 T cells) is altered in HFD mice on autoimmune-prone backgrounds versus HFD mice given infections.
Concluding remarks
Human obesity has risen alarmingly in the past three decades, and is predicted to become a worldwide pandemic, even in developing nations [78,79]. This phenomenon will inevitably be associated with widespread increases in obesity-associated diseases, namely, autoimmunity and cancer [4,78,79]. Because such diseases are regulated by adaptive immune cells, fully understanding their relationship with adipose tissue will be needed to optimize clinical interventions and lifestyle changes to improve human health. Additionally, the results reviewed here strongly suggest that metabolic status (e.g., BMI) should be considered more frequently in analysis of human clinical data sets, and that analysis of adipose tissue can provide insight into immune regulation that is not appreciated when only “conventional” lymphoid organs are examined.
Outstanding questions.
How does obesity affect the functions and reactions of omental MS and of mesenteric FALC?
Do lymphocytes in inflamed, obese fat organize into tertiary lymphoid structures?
Is the impaired response to influenza virus in HFD mice affected by altered B1 B cell-derived IgM and by altered CD8 T cells?
Does adipose tissue also support regulatory IL10-producing B cells, and if so, are these “Bregs” altered in obesity as Tregs are?
How do responses to various current human vaccines, and the efficacy and consequences of B cell depletion in autoimmune therapy, vary with metabolic status?
Acknowledgments
The authors thank Javier Rangel-Moreno, Tulin Dadali, and John Kearney for reading the manuscript. D.A.K. is supported by NIH AI079537. T.D.R. is supported by HL69409, AI072689, and AI061511.
Footnotes
Body mass index (BMI) ≤ 25
Also referred to as “Western diet”, typically ~50% of calories from fat
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Enerback S. Human brown adipose tissue. Cell Metab. 2010;11:248–252. doi: 10.1016/j.cmet.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2009 doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 3.Johnson PR, Hirsch J. Cellularity of adipose depots in six strains of genetically obese mice. J Lipid Res. 1972;13:2–11. [PubMed] [Google Scholar]
- 4.John BJ, et al. Systematic review: adipose tissue, obesity and gastrointestinal diseases. Aliment Pharmacol Ther. 2006;23:1511–1523. doi: 10.1111/j.1365-2036.2006.02915.x. [DOI] [PubMed] [Google Scholar]
- 5.Halaas JL, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 6.Hukshorn CJ, Saris WH. Leptin and energy expenditure. Curr Opin Clin Nutr Metab Care. 2004;7:629–633. doi: 10.1097/00075197-200411000-00007. [DOI] [PubMed] [Google Scholar]
- 7.La Cava A, Matarese G. The weight of leptin in immunity. Nat Rev Immunol. 2004;4:371–379. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- 8.Maury E, et al. Adipokines oversecreted by omental adipose tissue in human obesity. Am J Physiol Endocrinol Metab. 2007;293:E656–665. doi: 10.1152/ajpendo.00127.2007. [DOI] [PubMed] [Google Scholar]
- 9.Rausch ME, et al. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 2008;32:451–463. doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- 10.Feuerer M, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tordjman J, et al. Adipose tissue inflammation and liver pathology in human obesity. Diabetes Metab. 2008;34:658–663. doi: 10.1016/S1262-3636(08)74601-9. [DOI] [PubMed] [Google Scholar]
- 12.Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. 2007;153:907–917. doi: 10.1016/j.ahj.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Nishimura S, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 14.Rocha VZ, et al. Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res. 2008;103:467–476. doi: 10.1161/CIRCRESAHA.108.177105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hotamisligil GS, et al. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 16.Uysal KT, et al. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 17.Kirchgessner TG, et al. Tumor necrosis factor-alpha contributes to obesity-related hyperleptinemia by regulating leptin release from adipocytes. J Clin Invest. 1997;100:2777–2782. doi: 10.1172/JCI119824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mantzoros CS, et al. Leptin concentrations in relation to body mass index and the tumor necrosis factor-alpha system in humans. J Clin Endocrinol Metab. 1997;82:3408–3413. doi: 10.1210/jcem.82.10.4323. [DOI] [PubMed] [Google Scholar]
- 19.Schaffler A, Scholmerich J. Innate immunity and adipose tissue biology. Trends Immunol. 2010 doi: 10.1016/j.it.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Yang YK, et al. Human mesenteric adipose tissue plays unique role versus subcutaneous and omental fat in obesity related diabetes. Cell Physiol Biochem. 2008;22:531–538. doi: 10.1159/000185527. [DOI] [PubMed] [Google Scholar]
- 21.Platell C, et al. The omentum. World J Gastroenterol. 2000;6:169–176. doi: 10.3748/wjg.v6.i2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Vugt E, et al. Omental milky spots in the local immune response in the peritoneal cavity of rats. Anat Rec. 1996;244:235–245. doi: 10.1002/(SICI)1097-0185(199602)244:2<235::AID-AR11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 23.Krist LF, et al. Cellular composition of milky spots in the human greater omentum: an immunochemical and ultrastructural study. Anat Rec. 1995;241:163–174. doi: 10.1002/ar.1092410204. [DOI] [PubMed] [Google Scholar]
- 24.Beelen RH, et al. The cellular composition of omentum milky spots and the ultrastructure of milky spot macrophages and reticulum cells. J Reticuloendothel Soc. 1980;28:585–599. [PubMed] [Google Scholar]
- 25.Ansel KM, et al. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity. 2002;16:67–76. doi: 10.1016/s1074-7613(01)00257-6. [DOI] [PubMed] [Google Scholar]
- 26.Di Paolo N, et al. Omental milky spots and peritoneal dialysis--review and personal experience. Perit Dial Int. 2005;25:48–57. [PubMed] [Google Scholar]
- 27.Harman-Boehm I, et al. Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metab. 2007;92:2240–2247. doi: 10.1210/jc.2006-1811. [DOI] [PubMed] [Google Scholar]
- 28.Bedford PA, et al. Adipose tissue of human omentum is a major source of dendritic cells, which lose MHC Class II and stimulatory function in Crohn's disease. J Leukoc Biol. 2006;80:546–554. doi: 10.1189/jlb.0905501. [DOI] [PubMed] [Google Scholar]
- 29.Carlow DA, et al. Lymphocytes in the peritoneum home to the omentum and are activated by resident dendritic cells. J Immunol. 2009;183:1155–1165. doi: 10.4049/jimmunol.0900409. [DOI] [PubMed] [Google Scholar]
- 30.Creusot RJ, et al. Lymphoid-tissue-specific homing of bone-marrow–derived dendritic cells. Blood. 2009;113:6638–6647. doi: 10.1182/blood-2009-02-204321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lynch L, et al. Invariant NKT cells and CD1d(+) cells amass in human omentum and are depleted in patients with cancer and obesity. Eur J Immunol. 2009;39:1893–1901. doi: 10.1002/eji.200939349. [DOI] [PubMed] [Google Scholar]
- 32.Cui L, et al. Biodefense function of omental milky spots through cell adhesion molecules and leukocyte proliferation. Cell Tissue Res. 2002;310:321–330. doi: 10.1007/s00441-002-0636-6. [DOI] [PubMed] [Google Scholar]
- 33.Rangel-Moreno J, et al. Omental milky spots develop in the absence of lymphoid tissue-inducer cells and support B and T cell responses to peritoneal antigens. Immunity. 2009;30:731–743. doi: 10.1016/j.immuni.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerber SA, et al. Preferential attachment of peritoneal tumor metastases to omental immune aggregates and possible role of a unique vascular microenvironment in metastatic survival and growth. Am J Pathol. 2006;169:1739–1752. doi: 10.2353/ajpath.2006.051222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beelen RH, et al. Omental milky spots in peritoneal pathophysiology (spots before your eyes) Perit Dial Int. 2005;25:30–32. [PubMed] [Google Scholar]
- 36.Ha SA, et al. Regulation of B1 cell migration by signals through Toll-like receptors. J Exp Med. 2006;203:2541–2550. doi: 10.1084/jem.20061041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moro K, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 38.Neill DR, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saenz SA, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Solvason N, Kearney JF. The human fetal omentum: a site of B cell generation. J Exp Med. 1992;175:397–404. doi: 10.1084/jem.175.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solvason N, et al. An embryonic source of Ly1 but not conventional B cells. Int Immunol. 1991;3:543–550. doi: 10.1093/intimm/3.6.543. [DOI] [PubMed] [Google Scholar]
- 42.Martin F, Kearney JF. B1 cells: similarities and differences with other B cell subsets. Curr Opin Immunol. 2001;13:195–201. doi: 10.1016/s0952-7915(00)00204-1. [DOI] [PubMed] [Google Scholar]
- 43.Alugupalli KR, Gerstein RM. Divide and conquer: division of labor by B-1 B cells. Immunity. 2005;23:1–2. doi: 10.1016/j.immuni.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Choi YS, Baumgarth N. Dual role for B-1a cells in immunity to influenza virus infection. J Exp Med. 2008;205:3053–3064. doi: 10.1084/jem.20080979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kunisawa J, et al. Sphingosine 1-phosphate regulates peritoneal B-cell trafficking for subsequent intestinal IgA production. Blood. 2007;109:3749–3756. doi: 10.1182/blood-2006-08-041582. [DOI] [PubMed] [Google Scholar]
- 46.Mauri C, Ehrenstein MR. The 'short' history of regulatory B cells. Trends Immunol. 2008;29:34–40. doi: 10.1016/j.it.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 47.Lemoine S, et al. Regulatory B cells in autoimmune diseases: how do they work? Ann N Y Acad Sci. 2009;1173:260–267. doi: 10.1111/j.1749-6632.2009.04651.x. [DOI] [PubMed] [Google Scholar]
- 48.Vakil M, et al. Antigen-independent selection of T15 idiotype during B-cell ontogeny in mice. Dev Immunol. 1991;1:203–212. doi: 10.1155/1991/45352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kearney JF. Immune recognition of OxLDL in atherosclerosis. J Clin Invest. 2000;105:1683–1685. doi: 10.1172/JCI10426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Binder CJ, Silverman GJ. Natural antibodies and the autoimmunity of atherosclerosis. Springer Semin Immunopathol. 2005;26:385–404. doi: 10.1007/s00281-004-0185-z. [DOI] [PubMed] [Google Scholar]
- 51.Gu L, et al. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 52.Major AS, et al. B-lymphocyte deficiency increases atherosclerosis in LDL receptor-null mice. Arterioscler Thromb Vasc Biol. 2002;22:1892–1898. doi: 10.1161/01.atv.0000039169.47943.ee. [DOI] [PubMed] [Google Scholar]
- 53.Binder CJ, et al. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J Clin Invest. 2004;114:427–437. doi: 10.1172/JCI20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Virella G, et al. Proatherogenic and proinflammatory properties of immune complexes prepared with purified human oxLDL antibodies and human oxLDL. Clin Immunol. 2002;105:81–92. doi: 10.1006/clim.2002.5269. [DOI] [PubMed] [Google Scholar]
- 55.Sumiyoshi K, et al. Deletion of the Fc receptors gamma chain preserves endothelial function affected by hypercholesterolaemia in mice fed on a high-fat diet. Cardiovasc Res. 2008;80:463–470. doi: 10.1093/cvr/cvn206. [DOI] [PubMed] [Google Scholar]
- 56.Grabner R, et al. Lymphotoxin beta receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE−/ − mice. J Exp Med. 2009;206:233–248. doi: 10.1084/jem.20080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eliakim A, et al. Reduced tetanus antibody titers in overweight children. Autoimmunity. 2006;39:137–141. doi: 10.1080/08916930600597326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roberts DW, et al. Differential effects of the mottled yellow and pseudoagouti phenotypes on immunocompetence in Avy/a mice. Proc Natl Acad Sci U S A. 1984;81:2152–2156. doi: 10.1073/pnas.81.7.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mito N, et al. Oral-tolerance induction in diet-induced obese mice. Life Sci. 2006;79:1056–1061. doi: 10.1016/j.lfs.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 60.Wu H, et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–1038. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- 61.Duffaut C, et al. Interplay between human adipocytes and T lymphocytes in obesity: CCL20 as an adipochemokine and T lymphocytes as lipogenic modulators. Arterioscler Thromb Vasc Biol. 2009;29:1608–1614. doi: 10.1161/ATVBAHA.109.192583. [DOI] [PubMed] [Google Scholar]
- 62.Winer S, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Madani R, et al. RANTES release by human adipose tissue in vivo and evidence for depot-specific differences. Am J Physiol Endocrinol Metab. 2009;296:E1262–1268. doi: 10.1152/ajpendo.90511.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levy JA. The unexpected pleiotropic activities of RANTES. J Immunol. 2009;182:3945–3946. doi: 10.4049/jimmunol.0990015. [DOI] [PubMed] [Google Scholar]
- 65.Appay V, Rowland-Jones SL. RANTES: a versatile and controversial chemokine. Trends Immunol. 2001;22:83–87. doi: 10.1016/s1471-4906(00)01812-3. [DOI] [PubMed] [Google Scholar]
- 66.Pacifico L, et al. Increased T-helper interferon-gamma-secreting cells in obese children. Eur J Endocrinol. 2006;154:691–697. doi: 10.1530/eje.1.02138. [DOI] [PubMed] [Google Scholar]
- 67.Dardalhon V, et al. Role of Th1 and Th17 cells in organ-specific autoimmunity. J Autoimmun. 2008;31:252–256. doi: 10.1016/j.jaut.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Winer S, et al. Obesity predisposes to Th17 bias. Eur J Immunol. 2009;39:2629–2635. doi: 10.1002/eji.200838893. [DOI] [PubMed] [Google Scholar]
- 69.De Rosa V, et al. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–255. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 70.Lord GM, et al. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 71.Vignali DA, et al. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma X, et al. High-fat diet modulates non-CD1d-restricted natural killer T cells and regulatory T cells in mouse colon and exacerbates experimental colitis. Clin Exp Immunol. 2008;151:130–138. doi: 10.1111/j.1365-2249.2007.03530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luczynski W, et al. Disturbances in some gene expression in T regulatory cells separated from children with metabolic syndrome. Clinical Immunology. 2010;71:115–122. doi: 10.1111/j.1365-3083.2009.02354.x. [DOI] [PubMed] [Google Scholar]
- 74.Ait-Oufella H, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 75.Taleb S, et al. Defective leptin/leptin receptor signaling improves regulatory T cell immune response and protects mice from atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2691–2698. doi: 10.1161/ATVBAHA.107.149567. [DOI] [PubMed] [Google Scholar]
- 76.Smith AG, et al. Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J Nutr. 2007;137:1236–1243. doi: 10.1093/jn/137.5.1236. [DOI] [PubMed] [Google Scholar]
- 77.Karlsson EA, et al. Diet-induced obesity impairs the T cell memory response to influenza virus infection. J Immunol. 2010;184:3127–3133. doi: 10.4049/jimmunol.0903220. [DOI] [PubMed] [Google Scholar]
- 78.Hossain P, et al. Obesity and diabetes in the developing world--a growing challenge. N Engl J Med. 2007;356:213–215. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 79.Cali AM, Caprio S. Obesity in children and adolescents. J Clin Endocrinol Metab. 2008;93:S31–36. doi: 10.1210/jc.2008-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Randall TD, et al. Development of secondary lymphoid organs. Annu Rev Immunol. 2008;26:627–650. doi: 10.1146/annurev.immunol.26.021607.090257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Togni P, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- 82.Harmsen A, et al. Cutting edge: organogenesis of nasal-associated lymphoid tissue (NALT) occurs independently of lymphotoxin-alpha (LT alpha) and retinoic acid receptor-related orphan receptor-gamma, but the organization of NALT is LT alpha dependent. Journal of Immunology. 2002;168:986–990. doi: 10.4049/jimmunol.168.3.986. [DOI] [PubMed] [Google Scholar]
- 83.Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]