Abstract
Over the past decade, fMRI techniques have been increasingly used to interrogate the neural correlates of successful emotional memory encoding. These investigations have typically aimed to either characterize the contributions of the amygdala and medial temporal lobe (MTL) memory system, replicating results in animals, or delineate the neural correlates of specific behavioral phenomena. It has remained difficult, however, to synthesize these findings into a systems neuroscience account of how networks across the whole brain support the enhancing effects of emotion on memory encoding. To this end, the present study employed a meta-analytic approach using activation likelihood estimates to assess the anatomical specificity and reliability of event-related fMRI activations related to successful memory encoding for emotional versus neutral information. The meta-analysis revealed consistent clusters within bilateral amygdala, anterior hippocampus, anterior and posterior parahippocampal gyrus, the ventral visual stream, left lateral prefrontal cortex and right ventral parietal cortex. The results within the amygdala and MTL support a wealth of findings from the animal literature linking these regions to arousal-mediated memory effects. The consistency of findings in cortical targets, including the visual, prefrontal, and parietal cortices, underscores the importance of generating hypotheses regarding their participation in emotional memory formation. In particular, we propose that the amygdala interacts with these structures to promote enhancements in perceptual processing, semantic elaboration, and attention, which serve to benefit subsequent memory for emotional material. These findings may motivate future research on emotional modulation of widespread neural systems and the implications of this modulation for cognition.
Keywords: neuroimaging, declarative memory, episodic memory, affective, arousal, amygdala
Emotion influences multiple aspects of cognition, and the impact of emotion on memory processes has been particularly well studied. Emotion is known to modulate both non-declarative and declarative forms of memory (see LaBar & Cabeza, 2006b for a review). In particular, declarative memories for emotionally salient information tend to be enhanced relative to their neutral counterparts, marked by both improved memory accuracy for emotional information (Burke, Heuer, & Reisberg, 1992; Cahill, et al., 1996; Dolcos, LaBar, & Cabeza, 2004a, 2004b; LaBar & Phelps, 1998) as well as increased vividness of these memories (Dolcos, Labar, & Cabeza, 2005; Ochsner, 2000; Sharot, Delgado, & Phelps, 2004; Sharot & Yonelinas, 2007). These enhancements are thought to arise from the beneficial influence of emotion on the initial encoding of a memory trace (Canli, Zhao, Brewer, Gabrieli, & Cahill, 2000; Dolcos, et al., 2004a; Kensinger & Corkin, 2004) and its consolidation, or strengthening, over time (Hamann, Ely, Grafton, & Kilts, 1999; Kleinsmith & Kaplan, 1963; LaBar & Phelps, 1998; Ritchey, Dolcos, & Cabeza, 2008).
At the behavioral level, emotion has robust but complex effects on declarative memory. Memory enhancements can be driven by either the intensity of the stimulus’ valence (how emotionally positive or negative it is) or its induced emotional arousal (see Kensinger, 2004 for a review). Arousal has an inverted-U relationship with memory, in that moderate levels of arousal lead to memory enhancements whereas very high levels lead to impairments (Liang, McGaugh, & Yao, 1990; Roozendaal, Nguyen, Power, & McGaugh, 1999). The impact of emotion on memory may also change depending on which features of the memorandum are tested. For example, it has been suggested that emotion differentially impacts the gist versus details of a memory (Burke, et al., 1992; Heuer & Reisberg, 1992; Kensinger, Garoff-Eaton, & Schacter, 2006, 2007b). The influence of emotion on detailed memory may be further modulated by whether details are central versus peripheral to the emotionally-salient features of the stimulus, leading to memory enhancements or impairments, respectively (Christianson & Loftus, 1991; Kensinger, Garoff-Eaton, & Schacter, 2007a; Loftus, 1979). Thus emotion influences memory encoding in a complex manner. For these reasons, further investigation into the cognitive and neural mechanisms that support these processes is warranted.
At the neural level, a wealth of evidence supports the participation of the amygdala and medial temporal lobes (MTL) in promoting emotional memory enhancements in both animals (McGaugh, 2004) and humans (Kensinger, 2004; LaBar & Cabeza, 2006b) for reviews. Recently, human neuroimaging has afforded the opportunity to examine memory processes across the entire brain; thus, there is now emerging evidence regarding the distributed neural systems that support emotional memory encoding. These neuroimaging studies have largely focused on the enhancing effects of emotion on declarative memory formation. The present meta-analysis seeks to quantitatively integrate these results, with an emphasis on how transient activations during encoding predict enhanced memory for emotional information.
The Critical Role of the Amygdala and MTL Memory System
Within the behavioral neuroscience literature, the amygdala has been critically implicated in the acquisition of information about the spatial location, nature and intensity of threat signals in the environment using conditioning paradigms such as avoidance learning (Davis & Whalen, 2001). Other structures in the MTL, including the hippocampus and parahippocampal gyrus, have been more generally linked with declarative memories (Eichenbaum, Yonelinas, & Ranganath, 2007). The memory modulation hypothesis proposes that amygdalar projections to the MTL declarative memory system are critical for consolidating memories for emotionally arousing events (McGaugh, 2004). Within avoidance learning paradigms, pharmacological interventions affecting either the amygdala or MTL are capable of enhancing or mitigating enhancements in emotional memory, even when they occur during the post-acquisition consolidation period (Hatfield & McGaugh, 1999). It has been proposed that these effects are dependent on interactions between the amygdala and MTL, in which amygdalar inputs target the MTL during the formation of declarative memories, although these interactions may additionally be supported by bi-directional connections between the amygdala and MTL (Pape & Pare, 2010).
The amygdala has been repeatedly implicated in emotional memory encoding and consolidation in humans as well. In humans, individuals with damage to the amygdala are impaired relative to controls in declarative memory for arousing versus neutral stimuli (Adolphs, Cahill, Schul, & Babinsky, 1997; Cahill, Babinsky, Markowitsch, & McGaugh, 1995; Phelps, et al., 1998). Consistent with these findings, in healthy subjects amygdala activity during encoding correlates with one’s ability to retrieve emotional but not neutral memories (Cahill, et al., 1996; Hamann, et al., 1999). Early PET and fMRI studies were limited to blocked-design analyses, which provide limited information about the neural systems underlying emotional memory encoding. Because these analyses tested across-subject effects that predicted individual differences in emotional memory, they may be confounded with general differences in the perceptual properties of emotional memoranda, in encoding orientation, or in participants’ reactivity to emotional stimuli. Over the past decade, event-related fMRI has been utilized to identify encoding success activity (ESA) for emotional memoranda by using subsequent memory paradigms (as reviewed by Paller & Wagner, 2002). These paradigms explicitly test the difference between encoding activity time-locked to stimulus onset for subsequently remembered (hits) and forgotten (misses) items by back-sorting fMRI data according to participants’ behavioral performance during later memory retrieval. Thus, these analyses better provide a measure of transient changes in brain activity that predict successful memory encoding on an item-by-item basis. Using this technique, the amygdala has been repeatedly shown to exhibit greater ESA for emotional than neutral memory (LaBar & Cabeza, 2006b).
The relationship between the amygdala and MTL structures has also been investigated in humans. In parallel with animal studies indicating that co-activation of the hippocampus and amygdala is critical to emotional memory formation, neuroimaging results have also shown enhanced functional connectivity between the amygdala and memory-related regions during encoding of emotional information (Dolcos, et al., 2004b; Hamann, et al., 1999; Kensinger & Corkin, 2004; Kilpatrick & Cahill, 2003; Murty, et al., 2009; Ritchey, et al., 2008; St Jacques, Dolcos, & Cabeza, 2009). The localization of these findings has varied across papers, with some enhancements linked to the hippocampal formation (Dolcos, et al., 2004b; Hamann, et al., 1999; Kensinger & Corkin, 2004; Murty, et al., 2009; St Jacques, et al., 2009) and others to the surrounding cortical MTL, including entorhinal and perirhinal cortices in anterior MTL (Dolcos, et al., 2004b; Hamann, et al., 1999; Ritchey, et al., 2008) and parahippocampal cortex in posterior MTL (Kilpatrick & Cahill, 2003). Critically, a neuroimaging study investigating emotional memory encoding in patients with MTL lesions demonstrated that reciprocal connections between the amygdala and MTL were necessary to produce emotional memory enhancements (Richardson, Strange, & Dolan, 2004). Although these findings stem from fMRI data from the encoding period, they have often been interpreted as reflecting the initiation of consolidation processes that unfold over time (Hamann, et al., 1999; Ritchey, et al., 2008), in line with the modulation hypothesis (McGaugh, 2004). Beyond this evidence for MTL-amygdala interactions, findings regarding the MTL memory system’s specific role in emotional memory have been more varied. In one study, ESA within the anterior hippocampus and entorhinal cortex was reported to be greater for emotional than neutral stimuli (Dolcos, et al., 2004b), but other studies have reported that this memory-related activity is shared equally across emotional and neutral stimuli (Cahill, et al., 1996; Hamann, et al., 1999; Kensinger & Corkin, 2004).
Beyond the Amygdala and MTL: Emotional Memory Effects Across the Brain
The majority of fMRI studies regarding the neural correlates of emotional memory formation have focused on interactions between the amygdala and MTL memory system. However, emotion is known to affect many facets of cognition. The amygdala itself is highly interconnected with the rest of the brain (Young, 1993), modulating processing networks which may, in turn, guide memory formation. For example, retrograde tracing studies have revealed direct feedback pathways from the amygdala to the ventral visual pathway (D. G. Amaral, Behniea, & Kelly, 2003). This anatomical relationship between emotional and visual processing is bolstered by a wealth of data detailing emotional enhancements on activation in visual areas and perceptual encoding (Dolan & Vuilleumier, 2003; Vuilleumier & Pourtois, 2007).
Prefrontal cortex (PFC) is also known to be modulated by emotion(Pessoa, 2009) and recruited during memory encoding (Sergerie, Lepage, & Armony, 2005; Tulving, Kapur, Craik, Moscovitch, & Houle, 1994; Wagner, et al., 1998). In the context of memory encoding, left inferior frontal gyrus (IFG) in particular has been linked to semantic elaboration processes (Kapur, et al., 1994; Otten, Henson, & Rugg, 2001; Otten & Rugg, 2001; Prince, Daselaar, & Cabeza, 2005; Prince, Tsukiura, & Cabeza, 2007), which are known to promote successful memories (Craik and Lockhart 1972). This region has also been identified as showing greater ESA for emotional than neutral memory (Dolcos, et al., 2004a; Kensinger & Corkin, 2004), which has been interpreted as indexing enhanced elaboration of emotional stimuli.
Goals and Questions
Thus far, fMRI studies of emotional memory enhancements in humans have tended to emphasize either 1) replicating the patterns of amygdala and MTL interactions identified by behavioral neuroscience approaches in animals (e.g., Canli, et al., 2000; Dolcos, et al., 2004b; Hamann, et al., 1999), or 2) targeting a specific cognitive process during memory encoding, such as semantic elaboration, and characterizing the neural correlates of emotional effects on this process across the whole brain (e.g., Kensinger, et al., 2007b; Ritchey, LaBar, & Cabeza, in press; Talmi, Anderson, Riggs, Caplan, & Moscovitch, 2008). These approaches have been valuable to the instantiation of behavioral neuroscience and cognitive psychology traditions within a cognitive neuroscience framework. However, given many differences across tasks and analyses, it has remained difficult to synthesize these varied findings with regard to the neural circuits underlying behavioral phenomena. In this quantitative meta-analysis, we aim to characterize the neural systems that have been consistently associated with emotional memory formation. To this end, we used Activation Likelihood Estimates (Laird, et al., 2005) to identify regions which reliably show greater ESA for emotional versus neutral encoding, within the subset of event-related fMRI studies that performed this contrast. We additionally propose systems-neuroscience accounts for the organization of these findings, as well as how their organization may predict specific behavioral phenomena.
Specifically, we have three main goals. Although the critical role of the MTL in promoting emotional memories has been well documented, there has been little attempt to characterize the organization of these effects across the MTL (but see Dolcos, et al., 2004b; Dougal, Phelps, & Davachi, 2007). First, we test the reliability of emotional ESA across the MTL, as well as its localization and organization within MTL. Second, we test the reliability of emotional ESA in regions associated with perceptual processing, as well as its specificity to the ventral visual stream. Third, we test the reliability of emotional ESA in prefrontal targets, as well as its localization within PFC. Because our meta-analysis includes fMRI studies of emotional memory encoding across a range of experimental manipulations, the latter two goals also test the reliability of these activation patterns regardless of explicit attempts to manipulate recruitment of these regions.
Methods
Study Selection
A literature search was conducted to identify fMRI studies of successful emotional memory encoding for potential inclusion in the meta-analysis. This search was conducted on Pubmed using the following search terms in mid-December 2009: (1) memory + encoding + fMRI + (affective OR arousal OR emotion OR emotional) and (2) subsequent memory + fMRI + (affective OR arousal OR arousing OR emotion OR emotional). This search yielded 195 papers from which a subset of papers were selected with the following inclusion requirements: (1) collection of fMRI data during encoding, (2) inclusion of emotional stimuli (either negative, positive, or both), (3) behavioral testing of successful declarative/episodic memory, (4) analysis directly relating fMRI data with successful memory performance (e.g. ESA analyses, regression), (5) direct comparisons of emotional and neutral memory encoding, (6) inclusion of a healthy, young adult sample (reported separately in the case of articles studying special populations), and (7) inclusion of a voxel-wise analysis with coordinates reported across the whole brain or restricted to the medial temporal lobe (MTL) region of interest. These studies were additionally inspected to verify that they reported results from independent samples. If overlapping samples were noted, data from only one report were included. This conservative set of inclusion criteria identified studies of successful emotional memory encoding within young, healthy volunteers. Fifteen studies with 16 sets of coordinates across the whole brain and an additional 3 studies with 4 sets of coordinates within the MTL met criteria for inclusion in the meta-analysis. A complete list of these studies with an overview of their design characteristics is included in Table 1.
Table 1.
Studies Included in the Meta-Analysis
| 1st Author | Year | N (N female) | Stimuli | Valence | Encoding | Ret. Delay | Ret. Task | EEM: Hits/CR |
|---|---|---|---|---|---|---|---|---|
| Full Brain | ||||||||
| Cahill (Men) | 2004 | 12 (0) | scenes | neg | incidental: emotion | 2 weeks | recognition/rem, know | Yes/- |
| Cahill (Women) | 2004 | 11 (11) | scenes | neg | incidental: emotion | 2 weeks | recognition/rem, know | Yes/- |
| Dougal | 2006 | 14 (9) | words | neg/pos | incidental | 1 day | recognition/source | Yes/No |
| Harvey | 2007 | 17 (8) | scenes | neg/pos | incidental | immediate | old/new | No/- |
| Kensinger | 2004 | 28 (14) | words | neg/pos | incidental | 10 minutes | recognition/rem, know | Yes/- |
| Kensinger | 2005 | 16 (9) | words/objects | neg/pos | incidental | 1–2 days | recognition/source | Yes/- |
| Kensinger | 2007 | 20 (10) | objects | neg | incidental | 30 minutes | recognition | Yes/- |
| Kensinger | 2008 | 17 (12) | objects | neg/pos | incidental | 30 minutes | recognition | Yes/- |
| Mickley | 2008 | 20 (10) | words/scenes | neg/pos | incidental | 30 minutes | recognition/rem, know | Yes/Yes |
| Mickley Steinmetz | 2009 | 21 (9) | scenes | neg/pos | incidental | 30 minutes | recognition/rem, know | Yes/Yes |
| Rasch | 2009 | 57 (41) | scenes | neg/pos | incidental: emotion | 10 minutes | recall | Yes/- |
| Ritchey | 2008 | 13 (7) | scenes | neg | incidental | 20 minutes/1 week | recognition/confidence | -/Yes* |
| Sergerie | 2006 | 18 (9) | faces | neg/pos | intentional | immediate | recognition | -/Yes |
| Sommer | 2008 | 17 (17) | words | neg | intentional | immediate | recognition/confidence | No/No |
| St. Jacques | 2009 | 15 (15) | scenes | neg | incidental: emotion | 45 minutes | cued recall | Yes/- |
| Talmi | 2008 | 11 (5) | scenes | neg | incidental | 25 minutes | recognition/confidence | -/Yes |
| MTL | ||||||||
| Canli (Men) | 2002 | 12 (0) | scenes | neg | incidental: emotion | 3 weeks | recognition/rem, familiar | Yes/- |
| Canli (Women) | 2002 | 12 (12) | scenes | neg | incidental: emotion | 3 weeks | recognition/rem, familiar | Yes/- |
| Mackiewicz | 2006 | 40 (18) | scenes | neg | incidental | immediate, 2 weeks | recognition | Yes/No |
| Kensinger | 2006 | 21 (10) | words/scenes | neg/pos | incidental | 30 minutes | recognition/source | Yes/- |
neg = negative; pos = positive; ret = retrieval; incidental: emotion = explicit judgments about the valence and/or arousal of stimuli; rem = remember; EEM = enhancement in emotional memory; CR = corrected recognition data
Recollection effects for long versus short delay
Meta-Analysis Techniques
Activation likelihood estimate (ALE) meta-analysis was performed using BrainMap GingerALE 2.0 (Laird, et al., 2005), a coordinate-based random-effects meta-analysis for neuroimaging data. This method has been shown to produce meta-analytical results that are the most similar to gold-standard mega-analyses, which incorporate unthresholded data from each study that are typically unfeasible to obtain (Salimi-Khorshidi, Smith, Keltner, Wager, & Nichols, 2009). For each study foci of activation were entered from a contrast of successful memory encoding of emotional greater than neutral stimuli into GingerALE 2.0 in Talairach space. Studies that originally listed coordinates in MNI space were transformed into Talairach space using icbm2tal (Lancaster, et al., 2007) as implemented in GingerALE 2.0. Studies that originally listed coordinates in Talairach space derived within SPM (which uses the Brett transform) were back-transformed to MNI space using tal2mni as implemented in GingerALE 2.0, then re-transformed into Talairach space also using icbm2tal. More details regarding these transformations can be found on the BrainMap website (www.brainmap.org). Foci from each individual study were smoothed by an FWHM value empirically determined as function of the study’s sample size (Eickhoff, et al., 2009). Across all studies activation likelihood estimates (ALE statistics) were computed for each voxel in the brain, reflecting the likelihood that a given voxel was activated across studies in a contrast of emotional > neutral encoding success. All reported data survived p < 0.05 significance FDR-corrected for multiple comparisons (Genovese, Lazar, & Nichols, 2002), as determined by permutation testing (Eickhoff, et al., 2009), with a spatial extent greater than 100 mm3. Of note, given that the statistical significance of these analyses are determined by comparing ALE values with a histogram of randomly generated foci, often it may take only a few overlapping foci to contribute to a significant cluster. ALE was first performed on the 15 sets of coordinates from the full-brain analysis resulting in 141 foci. The analysis was performed again adding the 4 additional sets of coordinates from the medial temporal lobe to reconfirm the finding within the MTL with a larger sample resulting in 157 foci. All the data in the figures are presented on a standard template in Talairach Space. Furthermore, spherical masks surrounding each activation foci (with a radius of the smoothing kernel used in the meta-analysis) from each individual study was compared with the output of the meta-analysis to determine the percentage of studies contributing to each significant cluster in the meta-analysis (reported in Table 1).
Results
Behaviorally, emotional stimuli were remembered better than neutral stimuli across the studies included in the meta-analysis, with 90% of the studies reporting significant emotional memory enhancements in either hits and/or corrected recognition (Table 1). One striking aspect of this table is the paucity of studies that reported statistical comparisons of corrected recognition scores (e.g., hits minus false alarms, d′) for quantifying behavioral effects. Given that emotional stimuli can induce high false alarm rates relative to neutral (Dougal & Rotello, 2007; Maratos, Allan, & Rugg, 2000; Windmann & Kutas, 2001), future studies should report whether their results persist after correction for false alarms.
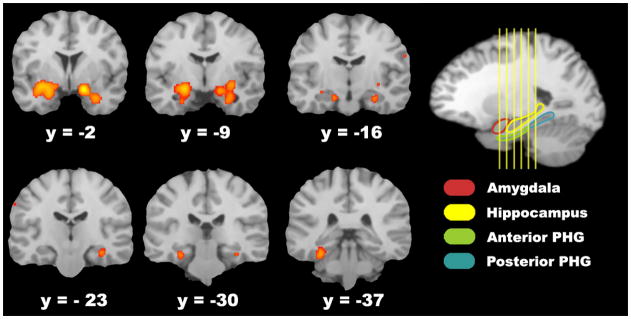
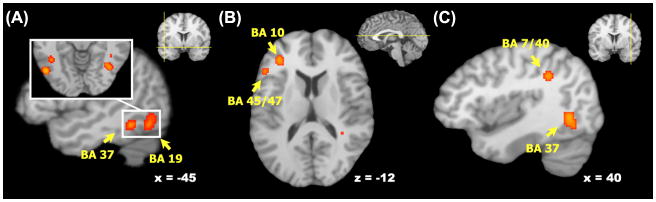
ALE meta-analysis revealed a network of regions showing greater activation during successful encoding of emotional stimuli compared to neutral stimuli (Table 2). Within the MTL, consistent activations were seen across studies bilaterally in the amygdala and anterior hippocampus, with clusters extending into the surrounding entorhinal and perirhinal cortex as well as the left posterior parahippocampus (Figure 1), during emotional memory encoding. Similarly, activations related to successful emotional versus neutral encoding were seen throughout the ventral visual stream including the middle temporal, middle occipital, and fusiform gyri (BAs 19 and 37, Figure 2.A) and in the left prefrontal cortex in the middle and inferior frontal gyri (BAs 10 and 45/47, Figure 2.B). Significant effects were also identified in right parietal cortex/supramarginal gyrus (Figure 2.C), the left claustrum, and the right caudate. When including the studies that reported clusters only in the MTL, meta-analysis revealed a nearly identical pattern of activation in the MTL as the whole-brain meta-analysis, with significant activations bilaterally in the amygdala and anterior hippocampus, as well as the left parahippocampus.
Table 2.
Consistently activated clusters across full-brain studies during the successful encoding of emotional greater than neutral stimuli (p < 0.05, FDR corrected)
| Region | BA | Hemi | Volume (mm3) | x | y | z | % Overlap |
|---|---|---|---|---|---|---|---|
| Amygdala/Anterior Hippocampus | - | L | 4224 | −22 | −6 | −12 | 68.75 |
| - | R | 3712 | 16 | −2 | −14 | 81.25 | |
| Middle Temporal Gyrus | 37 | R | 1080 | 42 | −62 | −4 | 31.25 |
| Middle Occipital Gyrus | 19 | L | 1064 | −50 | −68 | −8 | 25 |
| Parahippocampus | 36 | L | 808 | −28 | −34 | −18 | 31.25 |
| Middle Frontal Gyrus | 10 | L | 616 | −34 | 36 | 10 | 25 |
| Fusiform Gyrus | 37 | L | 472 | −42 | −52 | −12 | 18.75 |
| Inferior Frontal Gyrus | 45,47 | L | 352 | −50 | 22 | 10 | 18.75 |
| Hippocampus/Parahippocampus | - | R | 296 | 34 | −24 | −18 | 25 |
| Supramarginal Gyrus | 7/40 | R | 256 | 40 | −44 | 32 | 12.5 |
| Claustrum | - | R | 160 | 28 | 14 | −6 | 12.5 |
| Caudate | - | R | 104 | 20 | −14 | 30 | 12.5 |
% overlap refers to the number of studies whose activation foci overlapped with the meta-analysis results (see methods). BA = Brodmann Area; Hemi = hemisphere. Coordinates are in Talairach space.
Figure 1.
Regions showing reliable activations within the amygdalae and MTL memory system. Activation likelihood map from the full-brain analysis (p<.05, FDR-corrected) is overlaid on the Colin template. Coordinates are in Talairach space. PHG = parahippocampal gyrus
Figure 2.
Regions showing reliable cortical activations in the ventral visual stream (a; also visible in b and c), the left prefrontal cortex (b), and the right parietal cortex (c). Activation likelihood map from the full-brain analysis (p<.05, FDR-corrected) is overlaid on the Colin template. Coordinates are in Talairach space. BA = Brodmann Area.
Discussion
The meta-analysis revealed a network of regions that are consistently activated across studies of emotional memory encoding. Importantly, these studies all used event-related fMRI techniques; thus, our results reflect transient, emotion-related changes in the neural systems supporting successful encoding operations. We now aim to characterize the contributions of each identified region based on our knowledge of its functional anatomy, as well as how its processing is modulated by emotion. Furthermore, we attempt to use this integrated framework to generate hypotheses regarding the behavioral and neural consequences of emotion on memory.
Amygdala
The meta-analysis revealed consistent bilateral activations in the amygdala during the successful encoding of emotional memories, which is consistent with data from both human lesion studies and neuroscientific investigations in non-human animals. In fact, every study included here (Table 1) reported peaks that overlapped with at least one of the amygdala clusters identified by the meta-analysis. Human patients with damage to the amygdala exhibit deficits in arousal-mediated memory consolidation (Adolphs, Denburg, & Tranel, 2001; Adolphs, Tranel, & Buchanan, 2005; Adolphs, Tranel, & Denburg, 2000; Cahill & McGaugh, 1998a; LaBar & Phelps, 1998). Furthermore, the severity of human amygdala damage significantly correlates with deficits in emotional but not neutral declarative memory (Richardson, et al., 2004). In line with these human studies, rodent studies have reliably demonstrated that lesions to the amygdala disrupt MTL-dependent memory (for a review, see McGaugh, 2004), and that stimulation of the amygdala reduces the threshold for hippocampal long-term potentiation (Akirav & Richter-Levin, 2002; Frey, Bergado-Rosado, Seidenbecher, Pape, & Frey, 2001; Ikegaya, Nakanishi, Saito, & Abe, 1997; Nakao, Matsuyama, Matsuki, & Ikegaya, 2004) and increases expression of immediate early genes critical in mediating synaptic plasticity in the hippocampus (Huff, et al., 2006; McIntyre, et al., 2005) via noradrenergic neurotransmission (Roozendaal, McEwen, & Chattarji, 2009). Interestingly, recent human neuroimaging studies have shown that encoding-success activity in the amygdala is sensitive to pharmacological challenges that modulate noradrenergic tone (van Stegeren, et al., 2005; van Stegeren, Roozendaal, Kindt, Wolf, & Joels, 2010). Together these findings suggest a necessary role for the amygdala in mediating emotional memory enhancements, and our meta-analysis results suggest that the amygdala is reliably engaged during fMRI studies of human emotional memory encoding.
It is worth noting that reliable activations were identified in both left and right amygdalae. There have been prior suggestions of lateralization effects in the amgydala, typically proposed in the context of sex differences during emotional memory encoding (Cahill, Uncapher, Kilpatrick, Alkire, & Turner, 2004; Canli, Desmond, Zhao, & Gabrieli, 2002) or differences in automatic versus evaluative processing (Glascher & Adolphs, 2003; Phelps, et al., 2001). The present meta-analysis did not reveal any patterns indicative of amygdala lateralization, with no apparent differences in activation likelihood between left and right amygdalae across a wide range of studies. However, because only two studies explicitly investigated sex differences in emotional memory encoding, and the other studies did not report coordinates separately across gender, this meta-analysis did not allow for direct comparisons of sex groups or encoding demands. We cannot discount the possibility that differences would emerge under these direct assessments. A final caveat to the present amygdala findings is that the meta-analysis included all coordinates reported within amygdala, regardless of the probability threshold chosen by the original authors. Because of the relatively low percent signal change within the amygdala, the relatively high rate of signal drop-out (LaBar, Gitelman, Mesulam, & Parrish, 2001), and its small volume, along with the strong evidence linking amygdala activity with emotional memory benefits, some authors have used a priori hypotheses to justify relaxing thresholds within the anatomical boundaries of the amygdala (Cahill, et al., 2004; Rasch, et al., 2009) while using more stringent thresholds across the rest of the brain. While these relaxed thresholds are appropriate within the amygdala during emotional memory encoding, it is possible that the fMRI meta-analysis results may reflect some degree of amygdala over-reporting as statistical thresholds varied across studies. Future studies will need to investigate this potential confound using mega-analysis techniques (Salimi-Khorshidi, et al., 2009), which analyze whole-brain, un-thresholded statistical maps.
MTL Memory System
Given its necessary role in declarative memory formation (Scoville & Milner, 1957), the hippocampus and the surrounding MTL cortex have been identified as the primary target for amygdala-mediated emotional memory enhancement. However, multiple structures within the MTL could support declarative memory formation. The current meta-analysis identified reliable activations bilaterally in the anterior hippocampus and the surrounding entorhinal and perirhinal cortices. These findings are well supported by a wealth of animal studies investigating structural and functional interactions between the amygdala and the anterior MTL. Anatomical studies provide evidence of rich connections from the amygdala to the anterior hippocampus and rhinal cortices in non-human primates (D.G. Amaral, Price, Pitkänen, & Carmichael, 1992; Burwell, Witter, & Amaral, 1995; Pitkanen, Savander, & LeDoux, 1997; Suzuki & Amaral, 1994). These same regions have a high density of noradrenergic and glucocorticoid receptors which are thought to mediate the amygdala’s modulatory role on declarative memory (Roozendaal, et al., 2009). Functionally, rodent studies have shown that spontaneous activity in the BLA and anterior MTL (including the hippocampus HPC and rhinal cortices) exhibit similar patterns of activation (Pare, 2003; Pelletier & Pare, 2004) and increases in synchrony through gamma oscillations (Bauer, Paz, & Pare, 2007). Furthermore, the firing of amygdala neurons increases synaptic transmission from perirhinal to entorhinal neurons (Paz, Pelletier, Bauer, & Pare, 2006) during MTL-dependent memory, and blocking amygdala activity disrupts emotional memory enhancements mediated by the entorhinal cortex (Roesler, Roozendaal, & McGaugh, 2002).
Beyond the studies included in our meta-analysis, other human neuroimaging studies have supported the notion that the amygdala targets the anterior MTL during emotional memory encoding. These findings have typically emerged from functional connectivity analyses, rather than differences in ESA. Human neuroimaging results have shown enhanced functional connectivity during encoding of emotional information between the amygdala and the hippocampus (Dolcos, et al., 2004b; Hamann, et al., 1999; Kensinger & Corkin, 2004; Murty, et al., 2009; St Jacques, et al., 2009) as well as the parahippocampal cortices (Dolcos, et al., 2004b; Hamann, et al., 1999; Kilpatrick & Cahill, 2003; Ritchey, et al., 2008). A subset of these findings was restricted to anterior hippocampus (Dolcos, et al., 2004b; Kensinger & Corkin, 2004) and the anterior parahippocampal cortices (Dolcos, et al., 2004b; Hamann, et al., 1999; Ritchey, et al., 2008). Interestingly, although some previous studies have claimed that ESA in the MTL is common to both emotional and neutral encoding (Hamann, et al., 1999; Kensinger & Corkin, 2004), the present results identified MTL regions that reliably distinguished between emotional and neutral ESA, supporting the presence of emotion effects in these regions.
The meta-analysis revealed reliable activations not only in anterior MTL but also in the left posterior parahippocampus during successful emotional memory encoding within the MTL. This region within the MTL has been strongly implicated in the perceptual processing, encoding, and retrieval of scenes and places (Epstein, 2008; Epstein, Harris, Stanley, & Kanwisher, 1999; Rudy, 2009). More specific to emotional memory, it has been strongly implicated in contextual fear conditioning in both rodents (Rudy, 2009; Rudy, Huff, & Matus-Amat, 2004) and humans (Alvarez, Biggs, Chen, Pine, & Grillon, 2008) suggesting it may have a specific role in emotional memory enhancements for scenes and locations. However, qualitative assessment of the studies included in the meta-analysis revealed that those studies that showed activations in the posterior parahippocampal gyrus employed a broad set of memoranda, including scenes, words, and objects. This observation suggests that the posterior parahippocampal gyrus may play a more domain-general role in emotion-driven learning. Future studies will need to investigate the specific contributions of this region during emotional memory encoding, beyond the stimulus properties of the memoranda.
Ventral Stream
The meta-analysis identified regions throughout the ventral visual pathway, including the middle occipital and middle temporal gyri. These regions have been linked with the processing of visual object and form representation (Ungerleider & Haxby, 1994). These regions may play a critical role in emotional memory encoding given the amygdala’s role in modulating the extent of visual processing. Retrograde tracing studies have revealed direct feedback pathways from the amygdala to the ventral visual pathway (D. G. Amaral, et al., 2003). Furthermore, human neuroimaging studies have revealed significant connectivity between the amygdala and the ventral visual stream using both resting-state fMRI (Roy, et al., 2009), diffusion tensor imaging (Cohen, Elger, & Weber, 2008), and metaanalytic connectivity modeling (Robinson, Laird, Glahn, Lovallo, & Fox, 2010). This anatomical relationship between emotional and visual processing is bolstered by a wealth of behavioral and functional imaging data detailing emotional enhancements of visual processes supporting perceptual encoding. Indeed, emotionally arousing visual stimuli capture greater visual attention (Bishop, Duncan, & Lawrence, 2004; Ishai, Pessoa, Bikle, & Ungerleider, 2004; Most, Chun, Widders, & Zald, 2005; Smith, Most, Newsome, & Zald, 2006), dominate visual awareness (Alpers & Gerdes, 2007), and trigger heightened responses in perceptual processing areas compared to neutral stimuli (Damaraju, Huang, Barrett, & Pessoa, 2009; Lane, Chua, & Dolan, 1999; Lang, et al., 1998; Vuilleumier, Armony, Driver, & Dolan, 2001; Vuilleumier & Driver, 2007). Furthermore, exposure to emotional stimuli has been shown to modulate the perceptual processing of unrelated neutral stimuli (Phelps, Ling, & Carrasco, 2006) via modulating activity throughout the ventral visual pathway (Padmala & Pessoa, 2008; Schmitz, De Rosa, & Anderson, 2009).
The extant literature indicates that emotional arousal impacts visual processing via back projections from the amygdala that prime further perceptual processing across the ventral visual pathway. This interaction may result in more detailed perceptual analysis of emotional items, improving the quality of information being sent to memory systems and ultimately resulting in superior memory for emotional stimuli compared to neutral. The present meta-analysis corroborates this hypothesis. Although most of the studies included in the meta-analysis did not explicitly study perceptual effects on emotional memory, reliable activations were seen throughout the ventral visual stream suggesting significant differences in visual processing of emotional encoded items. Furthermore, qualitatively, all of the studies contributing to the clusters in the ventral visual stream used visually-rich stimuli (i.e., objects or scenes) and a relatively short retrieval delay, which may promote reliance on perceptual information (Uncapher & Rugg, 2005). This activation pattern makes several predictions with regard to the behavioral phenomena associated with emotional memory. One such hypothesis, which recently has been directly tested, is that memory for specific visual details should be better for arousing compared to neutral items (Kensinger, et al., 2006, 2007b). Not surprisingly, neuroimaging results revealed that both the amygdala and components of the ventral visual stream participated in this effect (Kensinger, et al., 2007b), though these results may be specific to negatively-valenced stimuli. Another hypothesis that may be derived from these findings is that memory structures most tightly linked with the ventral stream may likewise exhibit preferential encoding effects for emotional than neutral information. The ventral stream is most densely interconnected with anterior, relative to posterior, portions of cortical MTL, particularly the perirhinal and lateral entorhinal cortices (Eichenbaum, et al., 2007), thus yielding the prediction that memory-related activity in these regions may be coupled with emotion-related memory enhancements in the ventral stream. Although this pattern of findings seems to be borne out within the literature, as described in our discussion of anterior MTL findings, future studies will need to follow up on these findings to explicitly test the mechanisms and consequences of the contribution of the ventral visual stream to emotional memory, explicitly testing the role of perceptual characteristics of the memoranda. Furthermore, future studies should investigate sensory modalities besides vision in order to test the generalizability of these findings.
Prefrontal Cortex
The meta-analysis identified two distinct regions in left PFC that were consistently activated for emotional ESA relative to neutral ESA: a region in inferior frontal gyrus (BA 45/47) and another in anterior middle frontal gyrus (BA 10). Although there are dense bidirectional projections between the amygdala and orbitofrontal and medial sectors of PFC (D. G. Amaral & Price, 1984; Ghashghaei & Barbas, 2002; Porrino, Crane, & Goldman-Rakic, 1981) projections from the amygdala to lateral PFC regions are relatively sparse (D. G. Amaral & Price, 1984; D.G. Amaral, et al., 1992; Ghashghaei & Barbas, 2002). This anatomical arrangement suggests that medial PFC regions are likely to mediate the relationship between the lateral PFC and amygdala, an idea supported by functional connectivity analyses in humans (Lieberman, et al., 2007). Notably, the meta-analysis did not identify any consistent activations in medial PFC. It should be noted, however, that signal dropoff in the ventromedial PFC and orbitofrontal cortex may hinder observing activation in these areas. With this caveat in mind, emotional memory effects in the lateral PFC may arise even in the absence of corresponding processing in the medial PFC, suggesting that these areas serve some function other than simple emotion processing. Indeed, the inferior frontal gyrus (BAs 45/47) has been linked with memory encoding processes even in the absence of emotion. These processes include semantic elaboration, known to benefit subsequent memory (Otten & Rugg, 2001; Prince, et al., 2005; Prince, et al., 2007), as well as the promotion of recollection-based or associative memories (Henson, Rugg, Shallice, Josephs, & Dolan, 1999; Prince, et al., 2005). Studies reporting subsequent emotional memory effects in the IFG have emphasized this region’s role in semantic elaboration (Dolcos, et al., 2004a; Kensinger & Corkin, 2004; Mickley & Kensinger, 2008; Steinmetz & Kensinger, 2009), with the implication that emotional information tends to command deeper forms of processing than neutral information (Christianson, 1992). In keeping with this interpretation, one recent study manipulated the level of semantic elaboration during encoding and demonstrated that emotion effects on ESA in the IFG were greater during semantic-based encoding than perceptual-based encoding (Ritchey, et al., in press).
The meta-analysis also identified a cluster in an anterior lateral portion of the PFC (BA 10). Interestingly, a recent meta-analysis of the neuroimaging studies of episodic memory for neutral stimuli demonstrated that this region is typically not engaged during the encoding of neutral information (Spaniol, et al., 2009). Meta-analyses have also identified the anterior lateral portions of the PFC during working memory and multi-task processes (Gilbert, et al., 2006; Owen, McMillan, Laird, & Bullmore, 2005; Schmidt, et al., 2009). Hierarchical models of PFC function have posited a role for anterior PFC in representing abstract goals (Badre, 2008; Badre & D’Esposito, 2009) or in shifting attention from internally generated sources to external information (Burgess, Dumontheil, & Gilbert, 2007). It remains unclear whether emotional memory encoding specifically recruits these types of processes more than neutral memory encoding, leaving the role of anterior PFC during emotional memory formation an open question for future research. Regions within BA 10 have also been implicated in social cognitive processes, such as representation of self, retrieval of emotional autobiographical memories, making inferences of others, and social perception (Amodio & Frith, 2006); however these regions are located medially, and do not overlap with the clusters identified in this meta-analysis.
Finally, the emotion effects in the PFC have been proposed to follow patterns of hemispheric lateralization. It has been suggested that, in general, the right hemisphere is more involved in emotional processing than the left hemisphere (Schwartz, Davidson, & Maer, 1975). Another theory regarding hemispheric specialization in PFC posits that whereas the left PFC tends to be preferentially engaged for positive, approach-related emotions, the right PFC tends to be preferentially activated in response to negative, withdrawal-related emotions (Davidson, 1992, 1998). The present meta-analysis did not find support for either of these patterns of hemispheric specialization in PFC during emotional versus neutral memory encoding. Instead, reliable activations were identified in the left PFC but not the right PFC, regardless of stimulus valence. Similarly, a meta-analysis of emotion processing failed to identify consistent lateralization effects within lateral PFC, although overall, there tended to be emotion-related activations in left lateral PFC more often than in right, contrary to previous theories (Wager, Phan, Liberzon, & Taylor, 2003). Thus, it may be that emotional lateralization effects are not robust in lateral PFC, or that they do not extend to predicting subsequent memory performance. In the present meta-analysis, left lateralization is consistent with the role of left PFC in memory encoding processes (Tulving, et al., 1994; Wagner, et al., 1998) and additionally highlight the influence of emotion on encoding processes, such as semantic elaboration, that are mediated by this region.
Parietal Cortex
Although the parietal cortex is not discussed very often within the context of emotional memory encoding, the meta-analysis revealed a cluster in right supramarginal gyrus (BAs 7/40) within ventral parietal cortex. Parietal activations have been most often interpreted as the deployment of attentional resources, with dorsal parietal corresponding to goal-directed attention and ventral parietal to reflexive attention (Corbetta & Shulman, 2002). Within the context of memory encoding, activations in ventral parietal cortex have been associated with subsequent memory reductions, interpreted as the negative consequences of reflexive orienting to task-irrelevant thoughts or stimuli (Uncapher & Wagner, 2009). Furthermore it has been speculated that there exist situations in which reflexive orienting may prove beneficial to memory, such as when a salient feature of the memorandum itself captures attention (Uncapher & Wagner, 2009). This may provide a preliminary explanation for the present results linking ventral parietal cortex with successful emotional versus neutral memory encoding. Emotional salience acts as a pop-out feature in visual search paradigms (Ohman, Flykt, & Esteves, 2001), and emotional scenes are more likely to attract the initial saccade and longer viewing times during free viewing when paired with neutral scenes (LaBar, Mesulam, Gitelman, & Weintraub, 2000). Emotional items are also more likely to be detected during attentional blink paradigms (Anderson & Phelps, 2001; Ishai, et al., 2004), and results from amygdala lesion patients show that this effect is amygdala-dependent (Anderson & Phelps, 2001). Finally, it is apparent that the effects of emotion on attention have positive consequences for subsequent memory: memory for emotional items is less disrupted than memory for neutral items when attention is divided during encoding (Kensinger & Corkin, 2004; Kern, Libkuman, & Otani, 2005; Talmi, Schimmack, Paterson, & Moscovitch, 2007). Although this interpretation is speculative, it sets forth a testable hypothesis for future research: does the ventral parietal cortex mediate beneficial effects of reflexive attention on emotional memory encoding?
Questions for Future Research
There remain several unanswered questions regarding neural systems predicting successful emotional memory encoding. One unresolved issue is the level of involvement assigned to the cortical targets identified above. As described above, the memory modulation hypothesis has typically highlighted amygdala-MTL interactions that are specifically elicited by emotional arousal, emphasizing direct, inflexible modulation of memory consolidation (Cahill & McGaugh, 1998b; McGaugh, 2004). Alternatively, emotional memory benefits may also arise as the indirect consequence of emotional modulation of other neural systems that influence memory encoding, such as attention, working memory, and semantic elaboration (Hamann, 2001; Kensinger & Corkin, 2004; Kilpatrick & Cahill, 2003; LaBar & Cabeza, 2006a; Talmi, et al., 2008; Talmi, et al., 2007). We assume that both direct and indirect mechanisms are likely to promote emotional memory formation, an idea supported by the distribution of regions identified by the meta-analysis. However, the present findings are unable to distinguish whether these cortical targets actually mediate the link between emotion and memory or whether they are epiphenomenal to subsequent memory benefits instantiated in MTL. Future investigations may be able to disentangle these accounts via mediation analysis (c.f., Talmi, et al., 2008) and/or direct manipulation of specific cortically-mediated functions. Another remaining question is whether the indirect pathways reflect upregulation of standard encoding pathways (Kensinger & Corkin 2004), or whether they may also be seen as specialized mechanisms that distinguish emotional memory formation (Hamann 2001). A qualitative comparison of the present results with those of Spaniol et al. (2009) reveals that many of the regions identified by our emotion comparison are also identified when neutral memory encoding is considered alone, excluding the present findings in the bilateral amygdala and BA 10. A valuable topic for future research is the circumstances under which emotional memory formation not only quantitatively but qualitatively diverges from neutral memory formation.
Another lingering issue regards the consistency of findings across the MTL. This current analysis was able to identify a limited set of regions that were reliably activated throughout the MTL during emotional memory encoding; however the current study was limited by the spatial resolution of the current methods. Recent studies within the field of declarative memory in rodents and humans have determined that engagement of different MTL subcomponents lead to differential encoding of the environment. One theory posits that the perirhinal and parahippocampal cortex support representations of items and scenes, respectively, whereas the hippocampus binds this information into a flexible memory trace (Davachi, 2006; Eichenbaum, et al., 2007; but see Squire, Wixted, & Clark, 2007). However, in humans, very few studies have explicitly tested the differential contributions of substructures within the MTL during emotional memory encoding (Dolcos, et al., 2004b; Dougal, et al., 2007). Future studies, using high-resolution fMRI, anatomical region of interest methods, and intracranial EEG methods could better delineate the respective contributions of subcomponents of the MTL.
Another open question is the role of valence versus arousal on mediating emotional ESA. Many emotional memory studies exclusively use highly arousing, valenced memoranda, suggesting that many of the regions identified in the meta-analysis could be driven independently by arousal or valence, or could be mediated by their interaction. It has been proposed that memory effects in the amygdala are driven primarily by arousal (Hamann, et al., 1999; Kensinger & Corkin, 2004; Phelps, et al., 1998), whereas memory effects in the prefrontal cortex are influenced by emotional valence (Dolcos, et al., 2004a; Kensinger & Corkin, 2004). For example, left posterior IFG predicts memory for negatively valenced stimuli, even in the absence of arousal, more than neutral stimuli (Kensinger & Corkin, 2004). There are also findings of differential PFC effects for positive versus negative memory: regions in the medial PFC and left IFG exhibit greater ESA for positive versus negative stimuli, though other regions in left IFG seems to promote memory for both equally (Dolcos, et al., 2004a; Mickley & Kensinger, 2008). Finally, it has recently been hypothesized that reliance on controlled processes in the PFC versus perceptual processes in the ventral stream during memory encoding varies by valence, with prefrontal networks predicting positive memory and ventral stream networks predicting negative memory (Steinmetz & Kensinger, 2009). A full analysis of the influence of valence on the activation likelihood, similar to those conducted in previous meta-analyses of emotion (Phan, Wager, Taylor, & Liberzon, 2002; Wager, et al., 2003) would have been desirable; however, due to our explicit interest in successful emotional memory encoding, the data available for such a comparison were limited in the present meta-analysis because many of studies that used both positive and negative stimuli did not report ESA separately for each valence. Similarly, the influence of task design on subsequent emotional memory effects has yet to be resolved across the literature reviewed here. Individual studies have demonstrated that the behavioral and neural effects of emotion on memory are influenced by such elements of task design as encoding task (e.g., Jay, Caldwell-Harris, & King, 2008; Ritchey, et al., in press; Talmi, et al., 2008; Talmi, et al., 2007), retrieval task (Adolphs, et al., 2001; Kensinger & Schacter, 2006a), retention interval (e.g., Kleinsmith & Kaplan, 1963; LaBar & Phelps, 1998; Mackiewicz, Sarinopoulos, Cleven, & Nitschke, 2006; Ritchey, et al., 2008) as well as the properties of the memoranda (Keightley, Chiew, Anderson, & Grady, 2010; Kensinger & Schacter, 2006b). The present study lacked the power to meta-analytically quantify these effects. As more emotional memory fMRI studies are conducted, meta-analytical techniques can be utilized to compare whether these variables systematically modulate the recruitment of differential neural circuitry.
Future studies of successful emotional memory encoding could be particularly informative in understanding processing changes in certain populations (i.e. healthy aging, psychiatric disorders). Recent investigations of healthy aging have revealed that older adults recruit different brain networks during emotional memory encoding compared to younger adults, tending to rely less on amygdala-MTL and more on amygdala-prefrontal cortical networks (Murty, et al., 2009; St Jacques, et al., 2009). Some evidence also suggests that these age-related differences are specific to memory for positive items (Addis, Leclerc, Muscatell, & Kensinger, 2010; Kensinger & Schacter, 2008). As a complement to the valence shifts identified in older adults, negativity biases in patients with major depressive have been linked to over-recruitment of amygdala-MTL networks during negative memory encoding, which also correlated with depressive severity (Hamilton & Gotlib, 2008). Furthermore, deficits in emotional memory have been implicated in the diagnosis and symptoms of other mood and anxiety disorders, such as PTSD and phobia; however relatively few studies have investigated emotional memory encoding using functional imaging in these patient populations (Dickie, Brunet, Akerib, & Armony, 2008; Thomaes, et al., 2009; Wolfensberger, et al., 2008). With the future accumulation of neuroimaging studies of emotional encoding within these populations, meta-analysis could determine whether these group-related changes are simple differences in magnitude (i.e. more or less activity in the same network) or localization (i.e. engagement of different networks). Understanding how emotional memory effects vary in these populations may be important to the development of future therapeutic interventions, as they could provide information regarding the neural targets for both emotion regulation strategies and psychopharmacological interventions.
Conclusions
In this meta-analysis, we aimed to characterize the neural systems that have been consistently associated with emotional memory formation. To this end, we used Activation Likelihood Estimates to identify regions that reliably show greater ESA for emotional versus neutral stimuli. These regions included regions within bilateral amygdala, anterior hippocampus, parahippocampal gyrus, ventral visual stream, ventral parietal cortex, and PFC. Some of these regions, such as the amygdala, anterior hippocampus, and parahippoccampal gyrus, have been significantly highlighted in previous animal, patient, and neuroimaging studies of emotional memory. The specific roles of the PFC, parietal cortex, and ventral stream during emotional memory encoding, however, have been seldom investigated, even though these regions have been extensively studied within other domains of cognitive neuroscience. The consistency of findings in these cortical targets underscores the importance of generating hypotheses regarding their participation in emotional memory formation. In particular, we propose that the amygdala interacts with these structures to promote enhancements in perceptual processing, semantic elaboration, and attention, which serve to benefit subsequent memory for emotional material. Because of the complexities inherent in understanding the intersection of emotion and memory, more research is warranted that breaks down these gross constructs into more detailed analysis of cognitive subprocesses and corresponding neural mechanisms.
Acknowledgments
This work was supported in part by grants NIH R01 DA027802, NIH F31 085384, and NSF 0745919.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addis DR, Leclerc CM, Muscatell KA, Kensinger EA. There are age-related changes in neural connectivity during the encoding of positive, but not negative, information. Cortex. 2010;46(4):425–433. doi: 10.1016/j.cortex.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Cahill L, Schul R, Babinsky R. Impaired declarative memory for emotional material following bilateral amygdala damage in humans. Learning & Memory. 1997;4(3):291–300. doi: 10.1101/lm.4.3.291. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Denburg NL, Tranel D. The amygdala’s role in long-term declarative memory for gist and detail. Behav Neurosci. 2001;115(5):983–992. doi: 10.1037//0735-7044.115.5.983. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Buchanan TW. Amygdala damage impairs emotional memory for gist but not details of complex stimuli. Nat Neurosci. 2005;8(4):512–518. doi: 10.1038/nn1413. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Denburg N. Impaired emotional declarative memory following unilateral amygdala damage. Learn Mem. 2000;7(3):180–186. doi: 10.1101/lm.7.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akirav I, Richter-Levin G. Mechanisms of amygdala modulation of hippocampal plasticity. J Neurosci. 2002;22(22):9912–9921. doi: 10.1523/JNEUROSCI.22-22-09912.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpers GW, Gerdes AB. Here is looking at you: emotional faces predominate in binocular rivalry. Emotion. 2007;7(3):495–506. doi: 10.1037/1528-3542.7.3.495. [DOI] [PubMed] [Google Scholar]
- Alvarez RP, Biggs A, Chen G, Pine DS, Grillon C. Contextual fear conditioning in humans: cortical-hippocampal and amygdala contributions. J Neurosci. 2008;28(24):6211–6219. doi: 10.1523/JNEUROSCI.1246-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Behniea H, Kelly JL. Topographic organization of projections from the amygdala to the visual cortex in the macaque monkey. Neuroscience. 2003;118(4):1099–1120. doi: 10.1016/s0306-4522(02)01001-1. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) The Journal of Comparative Neurology. 1984;230(4):465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkänen A, Carmichael T. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. New York: Wiley-Liss; 1992. pp. 1–66. [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411(6835):305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends in Cognitive Sciences. 2008;12(5):193–200. doi: 10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Badre D, D’Esposito M. Is the rostro-caudal axis of the frontal lobe hierarchical? Nat Rev Neurosci. 2009;10(9):659–669. doi: 10.1038/nrn2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer EP, Paz R, Pare D. Gamma oscillations coordinate amygdalo-rhinal interactions during learning. J Neurosci. 2007;27(35):9369–9379. doi: 10.1523/JNEUROSCI.2153-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ, Duncan J, Lawrence AD. State Anxiety Modulation of the Amygdala Response to Unattended Threat-Related Stimuli. Journal of Neuroscience. 2004;24(46):10364–10368. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PW, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends in Cognitive Sciences. 2007;11(7):290–298. doi: 10.1016/j.tics.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Burke A, Heuer F, Reisberg D. Remembering Emotional Events. Memory & Cognition. 1992;20(3):277–290. doi: 10.3758/bf03199665. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Witter MP, Amaral DG. Perirhinal and postrhinal cortices of the rat: a review of the neuroanatomical literature and comparison with findings from the monkey brain. Hippocampus. 1995;5(5):390–408. doi: 10.1002/hipo.450050503. [DOI] [PubMed] [Google Scholar]
- Cahill L, Babinsky R, Markowitsch HJ, McGaugh JL. The amygdala and emotional memory. Nature. 1995;377(6547):295–296. doi: 10.1038/377295a0. [DOI] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, Fallon J, Alkire MT, Tang C, Keator D, et al. Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proc Natl Acad Sci U S A. 1996;93(15):8016–8021. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trends Neurosci. 1998a;21(7):294–299. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trends in Neurosciences. 1998b;21(7):294–299. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- Cahill L, Uncapher M, Kilpatrick L, Alkire MT, Turner J. Sex-related hemispheric lateralization of amygdala function in emotionally influenced memory: an FMRI investigation. Learning and Memory. 2004;11(3):261–266. doi: 10.1101/lm.70504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Desmond JE, Zhao Z, Gabrieli JD. Sex differences in the neural basis of emotional memories. Proc Natl Acad Sci U S A. 2002;99(16):10789–10794. doi: 10.1073/pnas.162356599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Brewer J, Gabrieli JDE, Cahill L. Event-related activation in the human amygdala associates with later memory for individual emotional experience. Journal of Neuroscience. 2000;20(19):5. doi: 10.1523/JNEUROSCI.20-19-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson S. Emotional stress and eyewitness memory: A critical review. Psychological Bulletin. 1992;112(2):284–309. doi: 10.1037/0033-2909.112.2.284. [DOI] [PubMed] [Google Scholar]
- Christianson S, Loftus EF. Remembering emotional events: The fate of detailed information. Cognition & Emotion. 1991;5(2):81–108. [Google Scholar]
- Cohen MX, Elger CE, Weber B. Amygdala tractography predicts functional connectivity and learning during feedback-guided decision-making. Neuroimage. 2008;39(3):1396–1407. doi: 10.1016/j.neuroimage.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Damaraju E, Huang YM, Barrett LF, Pessoa L. Affective learning enhances activity and functional connectivity in early visual cortex. Neuropsychologia. 2009;47(12):2480–2487. doi: 10.1016/j.neuropsychologia.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Current Opinion in Neurobiology. 2006;16(6):693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Emotion and Affective Style: Hemispheric Substrates. Psychological Science. 1992;3(1):39–43. [Google Scholar]
- Davidson RJ. Anterior electrophysiological asymmetries, emotion, and depression: Conceptual and methodological conundrums. Psychophysiology. 1998;35(05):607–614. doi: 10.1017/s0048577298000134. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Dickie EW, Brunet A, Akerib V, Armony JL. An fMRI investigation of memory encoding in PTSD: influence of symptom severity. Neuropsychologia. 2008;46(5):1522–1531. doi: 10.1016/j.neuropsychologia.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Vuilleumier P. Amygdala automaticity in emotional processing. Annals of the New York Academy of Sciences. 2003;985(1):348–355. doi: 10.1111/j.1749-6632.2003.tb07093.x. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaulation and subsequent memory: an event-related fMRI study. Neuroimage. 2004a;23:64–74. doi: 10.1016/j.neuroimage.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004b;42:855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- Dolcos F, Labar KS, Cabeza R. Remembering one year later: Role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proceedings of the National Academy of Sciences. 2005;102(7):2626–2631. doi: 10.1073/pnas.0409848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougal S, Phelps EA, Davachi L. The role of medial temporal lobe in item recognition and source recollection of emotional stimuli. Cognitive, Affective, & Behavioral Neuroscience. 2007;7:233–242. doi: 10.3758/cabn.7.3.233. [DOI] [PubMed] [Google Scholar]
- Dougal S, Rotello CM. Remembering emotional words is based on response bias, not recollection. Psychonomic Bulletin & Review. 2007;14(3):423–429. doi: 10.3758/bf03194083. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. [review] Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30(9):2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R. Parahippocampal and retrosplenial contributions to human spatial navigation. Trends Cogn Sci. 2008;12(10):388–396. doi: 10.1016/j.tics.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, Harris A, Stanley D, Kanwisher N. The parahippocampal place area: recognition, navigation, or encoding? Neuron. 1999;23(1):115–125. doi: 10.1016/s0896-6273(00)80758-8. [DOI] [PubMed] [Google Scholar]
- Frey S, Bergado-Rosado J, Seidenbecher T, Pape HC, Frey JU. Reinforcement of early long-term potentiation (early-LTP) in dentate gyrus by stimulation of the basolateral amygdala: heterosynaptic induction mechanisms of late-LTP. J Neurosci. 2001;21(10):3697–3703. doi: 10.1523/JNEUROSCI.21-10-03697.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115(4):1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, et al. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. J Cogn Neurosci. 2006;18(6):932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Glascher J, Adolphs R. Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. Journal of Neuroscience. 2003;23(32):10274–10282. doi: 10.1523/JNEUROSCI.23-32-10274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann SB. Cognitive and neural mechanisms of emotional memory. Trends in Cognitive Sciences. 2001 doi: 10.1016/s1364-6613(00)01707-1. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Grafton ST, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nature Neuroscience. 1999;2(3):289–293. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Gotlib IH. Neural substrates of increased memory sensitivity for negative stimuli in major depression. Biological Psychiatry. 2008;63(12):1155–1162. doi: 10.1016/j.biopsych.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PO, Fossati P, Lepage M. Modulation of memory formation by stimulus content: specific role of the medial prefrontal cortex in the successful encoding of social pictures. J Cogn Neurosci. 2007;19(2):351–362. doi: 10.1162/jocn.2007.19.2.351. [DOI] [PubMed] [Google Scholar]
- Hatfield T, McGaugh JL. Norepinephrine Infused into the Basolateral Amygdala Posttraining Enhances Retention in a Spatial Water Maze Task. Neurobiology of Learning and Memory. 1999;71:232–239. doi: 10.1006/nlme.1998.3875. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: An event-related functional magnetic resonance imaging study. The Journal of Neuroscience. 1999;19(10):3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer F, Reisberg D. Emotion, arousal, and memory for detail. In: Christianson S-Ãk., editor. The handbook of emotion and memory: Research and theory. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1992. pp. 151–180. [Google Scholar]
- Huff NC, Frank M, Wright-Hardesty K, Sprunger D, Matus-Amat P, Higgins E, et al. Amygdala regulation of immediate-early gene expression in the hippocampus induced by contextual fear conditioning. J Neurosci. 2006;26(5):1616–1623. doi: 10.1523/JNEUROSCI.4964-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegaya Y, Nakanishi K, Saito H, Abe K. Amygdala beta-noradrenergic influence on hippocampal long-term potentiation in vivo. Neuroreport. 1997;8(14):3143–3146. doi: 10.1097/00001756-199709290-00027. [DOI] [PubMed] [Google Scholar]
- Ishai A, Pessoa L, Bikle PC, Ungerleider LG. Repetition suppression of faces is modulated by emotion. Proc Natl Acad Sci U S A. 2004;101(26):9827–9832. doi: 10.1073/pnas.0403559101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay T, Caldwell-Harris C, King K. Recalling taboo and nontaboo words. American Journal of Psychology. 2008;121(1):83–103. [PubMed] [Google Scholar]
- Kapur S, Craik FIM, Tulving E, Wilson AA, Houle S, Brown G. Neuroanatomical correlates of encoding in episodic memory: Levels of processing effect. Proceedings of the National Academy of Sciences of USA. 1994;91:2008–2011. doi: 10.1073/pnas.91.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keightley ML, Chiew KS, Anderson JA, Grady CL. Neural correlates of recognition memory for emotional faces and scenes. Soc Cogn Affect Neurosci. 2010 doi: 10.1093/scan/nsq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA. Remembering emotional experiences: The contribution of valence and arousal. [review] Reviews in the Neurosciences. 2004;15(4):241–253. doi: 10.1515/revneuro.2004.15.4.241. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. Two routes to emotional memory: Distinct neural processes for valence and arousal. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(9):3310–3315. doi: 10.1073/pnas.0306408101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. Memory for specific visual details can be enhanced by negative arousing content. Journal of Memory and Language. 2006;54(1):99–112. [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. Effects of emotion on memory specificity: Memory trade-offs elicited by negative visually arousing stimuli. Journal of Memory and Language. 2007a;56(4):575–591. [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. How Negative Emotion Enhances the Visual Specificity of a Memory. Journal of Cognitive Neuroscience. 2007b;19(11):1872–1887. doi: 10.1162/jocn.2007.19.11.1872. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Retrieving accurate and distorted memories: neuroimaging evidence for effects of emotion. Neuroimage. 2005;27(1):167–177. doi: 10.1016/j.neuroimage.2005.03.038. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Amygdala activity is associated with the successful encoding of item, but not source, information for positive and negative stimuli. Journal of Neuroscience. 2006a;26(9):2564–2570. doi: 10.1523/JNEUROSCI.5241-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Processing emotional pictures and words: Effects of valence and arousal. Cognitive, Affective, & Behavioral Neuroscience. 2006b;6(2):110–126. doi: 10.3758/cabn.6.2.110. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Neural processes supporting young and older adults’ emotional memories. J Cogn Neurosci. 2008;20(7):1161–1173. doi: 10.1162/jocn.2008.20080. [DOI] [PubMed] [Google Scholar]
- Kern RP, Libkuman TA, Otani H. Emotional stimuli, divided attention, and memory. Emotion. 2005;5(4):408–417. doi: 10.1037/1528-3542.5.4.408. [DOI] [PubMed] [Google Scholar]
- Kilpatrick L, Cahill L. Amygdala modulation of parahippocampal and frontal regions during emotionally influenced memory storage. Neuroimage. 2003;20(4):2091–2099. doi: 10.1016/j.neuroimage.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Kleinsmith LJ, Kaplan S. Paired-associate learning as a function of arousal and interpolated interval. Journal of Experimental Psychology. 1963;65:190–193. doi: 10.1037/h0040288. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. [review] Nature Reviews Neuroscience. 2006a;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nat Rev Neurosci. 2006b;7(1):54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Mesulam MM, Parrish TB. Impact of signal-to-noise on functional MRI of the human amygdala. Neuroreport. 2001;12(16):3461–3464. doi: 10.1097/00001756-200111160-00017. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Mesulam MM, Gitelman DR, Weintraub S. Emotional curiosity: modulation of visuospatial attention by arousal is preserved in aging and early-stage Alzheimer’s disease. Neuropsychologia. 2000;38(13):1734–1740. doi: 10.1016/s0028-3932(00)00077-4. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Phelps EA. Arousal-mediated memory consolidation: Role of the medial temporal lobe in humans. Psychological Science. 1998;9(6):490–493. [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, et al. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005;25(1):155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28(11):1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RD, Chua PML, Dolan RJ. Common effects of emotional valence, arousal and attention on neural activation during visual processing of pictures. Neuropsychologia. 1999;37(9):989–997. doi: 10.1016/s0028-3932(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Fitzsimmons JR, Cuthbert BN, Scott JD, Moulder B, et al. Emotional arousal and activation of the visual cortex: an fMRI analysis. Psychophysiology. 1998;35(2):199–210. [PubMed] [Google Scholar]
- Liang KC, McGaugh JL, Yao HY. Involvement of amygdala pathways in the influence of post-training intra-amygdala norepinephrine and peripheral epinephrine on memory storage. Brain Research. 1990;508(2):225–233. doi: 10.1016/0006-8993(90)90400-6. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychological Science. 2007;18(5):421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Loftus EF. Eyewitness Testimony. Cambridge, MA: Harvard University Press; 1979. [Google Scholar]
- Mackiewicz KL, Sarinopoulos I, Cleven KL, Nitschke JB. The effect of anticipation and the specificity of sex differences for amygdala and hippocampus function in emotional memory. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(38):14200–14205. doi: 10.1073/pnas.0601648103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maratos EJ, Allan K, Rugg MD. Recognition memory for emotionally negative and neutral words: An ERP study. Neuropsychologia. 2000;38:1452–1465. doi: 10.1016/s0028-3932(00)00061-0. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Miyashita T, Setlow B, Marjon KD, Steward O, Guzowski JF, et al. Memory-influencing intra-basolateral amygdala drug infusions modulate expression of Arc protein in the hippocampus. Proc Natl Acad Sci U S A. 2005;102(30):10718–10723. doi: 10.1073/pnas.0504436102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickley K, Kensinger EA. Emotional Valence Influences the Neural Corerlates Associated with Remembering and Knowing. Cognitive, Affective and Behavioral Neuroscience. 2008;8(2):143–152. doi: 10.3758/cabn.8.2.143. [DOI] [PubMed] [Google Scholar]
- Mickley Steinmetz KR, Kensinger EA. The effects of valence and arousal on the neural activity leading to subsequent memory. Psychophysiology. 2009;46(6):1190–1199. doi: 10.1111/j.1469-8986.2009.00868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Most SB, Chun MM, Widders DM, Zald DH. Attentional rubbernecking: cognitive control and personality in emotion-induced blindness. Psychon Bull Rev. 2005;12(4):654–661. doi: 10.3758/bf03196754. [DOI] [PubMed] [Google Scholar]
- Murty VP, Sambataro F, Das S, Tan HY, Callicott JH, Goldberg TE, et al. Age-related alterations in simple declarative memory and the effect of negative stimulus valence. J Cogn Neurosci. 2009;21(10):1920–1933. doi: 10.1162/jocn.2009.21130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao K, Matsuyama K, Matsuki N, Ikegaya Y. Amygdala stimulation modulates hippocampal synaptic plasticity. Proc Natl Acad Sci U S A. 2004;101(39):14270–14275. doi: 10.1073/pnas.0405709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN. Are affective events richly recollected or simply familiar? The experience and process of recognizing feelings past. Journal of Experimental Psychology: General. 2000;129(2):242–261. doi: 10.1037//0096-3445.129.2.242. [DOI] [PubMed] [Google Scholar]
- Ohman A, Flykt A, Esteves F. Emotion drives attention: detecting the snake in the grass. Journal of Experimental Psychology: General. 2001;130(3):466–478. doi: 10.1037/0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Henson RN, Rugg MD. Depth of processing effects on neural correlates of memory encoding: Relationship between findings from across- and within-task comparisons. Brain. 2001;124:399–412. doi: 10.1093/brain/124.2.399. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Rugg MD. Task-dependency of the Neural Correlates of Episodic Encoding as Measured by fMRI. Cerebral Cortex. 2001;11(12):1150–1160. doi: 10.1093/cercor/11.12.1150. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25(1):46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmala S, Pessoa L. Affective learning enhances visual detection and responses in primary visual cortex. J Neurosci. 2008;28(24):6202–6210. doi: 10.1523/JNEUROSCI.1233-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller KA, Wagner AD. Observing the transformation of experience into memory. Trends Cogn Sci (Regul Ed) 2002;6(2):93–102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90(2):419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D. Role of the basolateral amygdala in memory consolidation. Prog Neurobiol. 2003;70(5):409–420. doi: 10.1016/s0301-0082(03)00104-7. [DOI] [PubMed] [Google Scholar]
- Paz R, Pelletier JG, Bauer EP, Pare D. Emotional enhancement of memory via amygdala-driven facilitation of rhinal interactions. Nat Neurosci. 2006;9(10):1321–1329. doi: 10.1038/nn1771. [DOI] [PubMed] [Google Scholar]
- Pelletier JG, Pare D. Role of amygdala oscillations in the consolidation of emotional memories. Biol Psychiatry. 2004;55(6):559–562. doi: 10.1016/j.biopsych.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Pessoa L. How do emotion and motivation direct executive control? Trends in Cognitive Sciences. 2009;13(4):160–166. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional Neuroanatomy of Emotion: A Meta-Analysis of Emotion Activation Studies in PET and fMRI. NeuroImage. 2002;16(2):331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LaBar KS, Anderson AK, O’Connor KJ, Fulbright RK, Spencer DD. Specifying the contributions of the human amygdala to emotional memory: A case study. Neurocase. 1998;4:527–540. [Google Scholar]
- Phelps EA, Ling S, Carrasco M. Emotion facilitates perception and potentiates the perceptual benefits of attention. Psychol Sci. 2006;17(4):292–299. doi: 10.1111/j.1467-9280.2006.01701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, O’Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. [Article] Nature Neuroscience. 2001;4(4):437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20(11):517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Crane AM, Goldman-Rakic PS. Direct and indirect pathways from the amygdala to the frontal lobe in rhesus monkeys. The Journal of Comparative Neurology. 1981;198(1):121–136. doi: 10.1002/cne.901980111. [DOI] [PubMed] [Google Scholar]
- Prince SE, Daselaar SM, Cabeza R. Neural correlates of relational memory: Successful encoding and retrieval of semantic and perceptual associations. Journal of Neuroscience. 2005;25:1203–1210. doi: 10.1523/JNEUROSCI.2540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince SE, Tsukiura T, Cabeza R. Distinguishing the neural correlates of episodic memory encoding and semantic memory retrieval. Psychological Science. 2007;18(2):144–151. doi: 10.1111/j.1467-9280.2007.01864.x. [DOI] [PubMed] [Google Scholar]
- Rasch B, Spalek K, Buholzer S, Luechinger R, Boesiger P, Papassotiropoulos A, et al. A genetic variation of the noradrenergic system is related to differential amygdala activation during encoding of emotional memories. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(45):19191–19196. doi: 10.1073/pnas.0907425106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson MP, Strange BA, Dolan RJ. Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nat Neurosci. 2004;7(3):278–285. doi: 10.1038/nn1190. [DOI] [PubMed] [Google Scholar]
- Ritchey M, Dolcos F, Cabeza R. Role of Amygdala Connectivity in the Persistence of Emotional Memories Over Time: An Event-Related fMRI Investigation. Cerebral Cortex. 2008:bhm262. doi: 10.1093/cercor/bhm262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M, LaBar KS, Cabeza R. Level of processing modulates the neural correlates of emotional memory formation. Journal of Cognitive Neuroscience. doi: 10.1162/jocn.2010.21487. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Lovallo WR, Fox PT. Metaanalytic connectivity modeling: delineating the functional connectivity of the human amygdala. Hum Brain Mapp. 2010;31(2):173–184. doi: 10.1002/hbm.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesler R, Roozendaal B, McGaugh JL. Basolateral amygdala lesions block the memory-enhancing effect of 8-Br-cAMP infused into the entorhinal cortex of rats after training. European Journal of Neuroscience. 2002;15(5):905–910. doi: 10.1046/j.1460-9568.2002.01924.x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10(6):423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Nguyen BT, Power AE, McGaugh JL. Basolateral amygdala noradrenergic influence enables enhancement of memory consolidation induced by hippocampal glucocorticoid receptor activation. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(20):11642–11647. doi: 10.1073/pnas.96.20.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, et al. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45(2):614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW. Context representations, context functions, and the parahippocampal-hippocampal system. Learn Mem. 2009;16(10):573–585. doi: 10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: insights from a two-process model. Neurosci Biobehav Rev. 2004;28(7):675–685. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Salimi-Khorshidi G, Smith SM, Keltner JR, Wager TD, Nichols TE. Meta-analysis of neuroimaging data: a comparison of image-based and coordinate-based pooling of studies. Neuroimage. 2009;45(3):810–823. doi: 10.1016/j.neuroimage.2008.12.039. [DOI] [PubMed] [Google Scholar]
- Schmidt H, Jogia J, Fast K, Christodoulou T, Haldane M, Kumari V, et al. No gender differences in brain activation during the N-back task: an fMRI study in healthy individuals. Hum Brain Mapp. 2009;30(11):3609–3615. doi: 10.1002/hbm.20783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz TW, De Rosa E, Anderson AK. Opposing influences of affective state valence on visual cortical encoding. J Neurosci. 2009;29(22):7199–7207. doi: 10.1523/JNEUROSCI.5387-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz G, Davidson R, Maer F. Right hemisphere lateralization for emotion in the human brain: interactions with cognition. Science. 1975;190(4211):286–288. doi: 10.1126/science.1179210. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20(1):11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergerie K, Lepage M, Armony JL. A face to remember: emotional expression modulates prefrontal activity during memory formation. Neuroimage. 2005;24(2):580–585. doi: 10.1016/j.neuroimage.2004.08.051. [DOI] [PubMed] [Google Scholar]
- Sergerie K, Lepage M, Armony JL. A process-specific functional dissociation of the amygdala in emotional memory. J Cogn Neurosci. 2006;18(8):1359–1367. doi: 10.1162/jocn.2006.18.8.1359. [DOI] [PubMed] [Google Scholar]
- Sharot T, Delgado MR, Phelps EA. How emotion enhances the feeling of remembering. Nature Neuroscience. 2004;7(12):1376–1380. doi: 10.1038/nn1353. [DOI] [PubMed] [Google Scholar]
- Sharot T, Yonelinas AP. Differential time-dependent effects of emotion on recollective experience and memory for contextual information. Cognition. 2007 doi: 10.1016/j.cognition.2007.03.002. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- Smith SD, Most SB, Newsome LA, Zald DH. An emotion-induced attentional blink elicited by aversively conditioned stimuli. Emotion. 2006;6(3):523–527. doi: 10.1037/1528-3542.6.3.523. [DOI] [PubMed] [Google Scholar]
- Sommer T, Glascher J, Moritz S, Buchel C. Emotional enhancement effect of memory: removing the influence of cognitive factors. Learn Mem. 2008;15(8):569–573. doi: 10.1101/lm.995108. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47(8–9):1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci. 2007;8(11):872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques PL, Dolcos F, Cabeza R. Effects of aging on functional connectivity of the amygdala for subsequent memory of negative pictures: a network analysis of functional magnetic resonance imaging data. Psychol Sci. 2009;20(1):74–84. doi: 10.1111/j.1467-9280.2008.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz KRM, Kensinger EA. The effects of valence and arousal on the neural activity leading to subsequent memory. Psychophysiology. 2009;46(6):1190–1199. doi: 10.1111/j.1469-8986.2009.00868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J Comp Neurol. 1994;350(4):497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Talmi D, Anderson AK, Riggs L, Caplan JB, Moscovitch M. Immediate memory consequences of the effect of emotion on attention to pictures. Learning & Memory. 2008;15(3):172–182. doi: 10.1101/lm.722908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmi D, Schimmack U, Paterson T, Moscovitch M. The role of attention and relatedness in emotionally enhanced memory. Emotion. 2007;7(1):89–102. doi: 10.1037/1528-3542.7.1.89. [DOI] [PubMed] [Google Scholar]
- Thomaes K, Dorrepaal E, Draijer NP, de Ruiter MB, Elzinga BM, van Balkom AJ, et al. Increased activation of the left hippocampus region in Complex PTSD during encoding and recognition of emotional words: a pilot study. Psychiatry Res. 2009;171(1):44–53. doi: 10.1016/j.pscychresns.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Tulving E, Kapur S, Craik FI, Moscovitch M, Houle S. Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(6):2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uncapher MR, Rugg MD. Encoding and the durability of episodic memory: A functional magnetic resonance imaging study. Journal of Neuroscience. 2005;25(31):7260–7267. doi: 10.1523/JNEUROSCI.1641-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uncapher MR, Wagner AD. Posterior parietal cortex and episodic encoding: Insights from fMRI subsequent memory effects and dual-attention theory. Neurobiology of Learning and Memory. 2009;91(2):139–154. doi: 10.1016/j.nlm.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Haxby JV. ‘What’ and ‘where’ in the human brain. Curr Opin Neurobiol. 1994;4(2):157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- van Stegeren AH, Goekoop R, Everaerd W, Scheltens P, Barkhof F, Kuijer JP, et al. Noradrenaline mediates amygdala activation in men and women during encoding of emotional material. Neuroimage. 2005;24(3):898–909. doi: 10.1016/j.neuroimage.2004.09.011. [DOI] [PubMed] [Google Scholar]
- van Stegeren AH, Roozendaal B, Kindt M, Wolf OT, Joels M. Interacting noradrenergic and corticosteroid systems shift human brain activation patterns during encoding. Neurobiol Learn Mem. 2010;93(1):56–65. doi: 10.1016/j.nlm.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: An event-related fMRI study. Neuron. 2001;30:829–841. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Driver J. Modulation of visual processing by attention and emotion: windows on causal interactions between human brain regions. Philosophical Transactions of the Royal Society B-Biological Sciences. 2007;362(1481):837–855. doi: 10.1098/rstb.2007.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: Evidence from functional neuroimaging. Neuropsychologia. 2007;45(1):174–194. doi: 10.1016/j.neuropsychologia.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. NeuroImage. 2003;19(3):513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, et al. Building Memories: Remembering and Forgetting of Verbal Experiences as Predicted by Brain Activity. Science. 1998;281(5380):1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Windmann S, Kutas M. Electrophysiological correlates of emotion-induced recognition bias. Journal of Cognitive Neuroscience. 2001;13(5):577–592. doi: 10.1162/089892901750363172. [DOI] [PubMed] [Google Scholar]
- Wolfensberger SP, Veltman DJ, Hoogendijk WJ, De Ruiter MB, Boomsma DI, de Geus EJ. The neural correlates of verbal encoding and retrieval in monozygotic twins at low or high risk for depression and anxiety. Biol Psychol. 2008;79(1):80–90. doi: 10.1016/j.biopsycho.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Young MP. The organization of neural systems in the primate cerebral cortex. Proc Biol Sci. 1993;252(1333):13–18. doi: 10.1098/rspb.1993.0040. [DOI] [PubMed] [Google Scholar]