Abstract
Background & Aims
Glycochenodeoxycholate (GCDC) and taurolithocholate (TLC) are hepatotoxic and cholestatic bile salts, whereas tauroursodeoxycholate (TUDC) is cytoprotective and anticholestatic. Yet they all act, in part, through phosphatidylinositol-3-kinase(PI3K)-dependent mechanisms (“PI3K-paradox”). Hepatocytes express three catalytic PI3K Class I isoforms (p110α/β/γ), specific functions of which, in liver, are unclear. In other cell types, p110γ is associated with detrimental effects. To shed light on the PI3K enigma, we determined whether hydrophobic and hydrophilic bile salts differentially activate distinct p110 isoforms in hepatocytes, and whether p110γ mediates bile salt-induced hepatocyte cell death.
Methods
Isoform-specific PI3K activity assays were established to determine isoform activation by bile salts in rat hepatocytes. Activation of Akt and JNK were determined by immunoblotting. Following stimulation with hydrophobic bile salts, hepatocellular apoptosis was determined morphologically after Hoechst staining and by analysis of caspase-3/-7 activity or caspase-3 cleavage. Activity or expression of PI3K p110γ was inhibited pharmacologically (AS604850) or by knockdown using specific siRNA.
Results
All bile salts tested activated p110β, while p110α was activated by TUDC and GCDC. Intriguingly, only hydrophobic bile salts activated p110γ. Inhibition of p110γ attenuated GCDC-induced Akt- and JNK-activation, but did not alter TUDC- or cAMP-induced Akt signaling in rat hepatocytes. Inhibition or knockdown of p110γ markedly attenuated hydrophobic bile salt-induced apoptosis in rat hepatocytes and human hepatoma cell lines but did not alter Fas-, tumor necrosis factor α- and etoposide-induced apoptosis. Depletion of Ca++ prevented GCDC-induced toxicity in rat hepatocytes but did not affect GCDC-induced Akt- and JNK-activation.
Conclusions
PI3K p110γ is activated by hydrophobic, but not hydrophilic bile salts. Bile salt-induced hepatocyte apoptosis is partly mediated via a PI3K p110γ signaling pathway, potentially involving JNK.
Keywords: Apoptosis, PI3K, Akt, JNK, Cholestasis, Glycochenodeoxycholate, Taurolithocholate, Tauroursodeoxycholate
Introduction
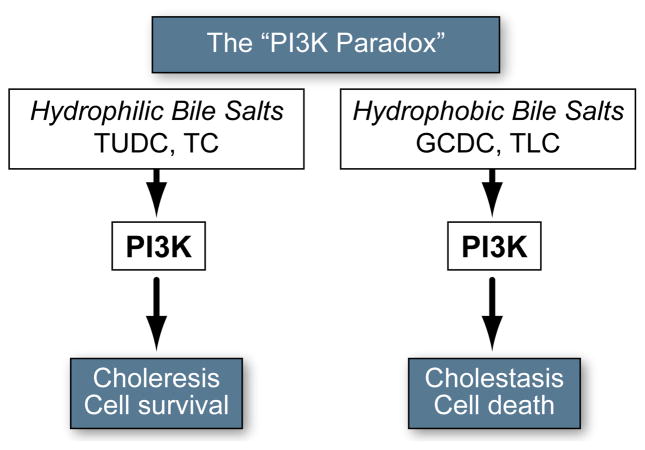
Bile salts are potent signaling molecules involved in the regulation of bile formation and hepatocellular metabolism and survival. Hepatic accumulation of hydrophobic bile salts in cholestasis is a major cause of liver damage, is associated with necrotic/apoptotic cell death, and promotes the development of liver cirrhosis/liver failure [1]. GCDC and TCDC are the predominant hydrophobic bile salts accumulating in cholestatic patients and exert potent necrotic/apoptotic and cholestatic effects in experimental models [2,3]. In contrast, hydrophilic bile salts, such as TUDC and taurocholate (TC), promote cell survival, enhance bile flow, and reconstitute normal liver function in cholestasis [3–6]. Despite these opposing effects, both hydrophobic and hydrophilic bile salts activate phosphatidylinositol 3-kinase(PI3K)-dependent signaling cascades and their effects are, in part, PI3K-dependent (Fig. 1 and Supplementary Table 1). The mechanism behind this “PI3K paradox” is unknown.
Fig. 1. Paradoxical role of PI3K in bile salt signaling.
PI3K seems to mediate the opposing effects of both hydrophilic and hydrophobic bile salts, promoting both cytotoxic and cytoprotective effects. For additional information see Supplementary Table 1.
PI3Ks are a family of lipid kinases (Class I, II, III) that phosphorylate phosphatidylinositides (PtIns) [7]. The Class IA PI3K consists of one of the three catalytic p110 subunits (p110α/p110β/p110δ) and a regulatory subunit, p85. The catalytic subunit is mainly activated by binding of p85 to a phosphotyrosyl peptide in tyrosine kinases [7]. Moreover, it has been shown that p110β can also be activated by G protein coupled receptors (GPCR) [8]. The Class IB PI3K has a single catalytic isoform, p110γ, which interacts with the regulatory subunit p101, and is activated by β/γ subunits of G proteins [7,9]. The in vivo substrate for Class I PI3Ks is PtIns-4,5-diphosphate (PtIns-4,5-P2), which is converted to the signaling molecule PtIns-3,4,5-P3 (PIP3) [7]. PIP3 serves as a docking site for the binding and activation of downstream signaling molecules, the best known being the serine/threonine kinase Akt, a downstream effector of PI3K-mediated survival and hepatobiliary secretion [2,10,11].
Accumulating evidence suggests that different PI3K p110 isoforms are coupled to specific downstream events. Activation of p110α is implicated in cell survival [11,12], physiologic cardiac hypertrophy [13], and insulin signaling [14]; while p110β has been associated with de novo DNA synthesis [12] and carcinogenesis [15]. Both p110α and β are ubiquitously expressed [16]. In contrast, p110δ, associated with induction of inflammatory responses, is confined to the hemopoietic system. p110γ, a regulator of inflammatory responses, was long believed to be restricted to immune cells [17], however, recent studies have shown that p110γ is present in myocytes, pancreas, vascular endothelium, and hepatocytes [16, 18]. In the heart, endothelium, and pancreas, p110γ function has been associated with detrimental effects such as development of atherosclerotic lesions [19], loss of cardiac contractility [13], and induction/mortality of pancreatitis [20,21].
Very little is known about isoform specific functions of PI3Ks in hepatocytes. We have recently shown that cAMP-guanine exchange factors (GEF)-induced cytoprotection is associated with activation of p110α/β in hepatocytes [11]. The aim of the present study was to determine whether hydrophobic and hydrophilic bile salts differentially activate distinct PI3K p110 isoforms and to study the effect of such activation on hepatocyte cell death.
Materials and methods
Materials
Antibodies against p110α (SC-7174), p110β (SC-602), p110δ (SC-7176), p110γ (SC-7177), and protein A/G agarose were purchased from Santa Cruz Biotechnology (Santa Cruz, California), antibodies for p110γ (4252), phospho-AktSer-473, total Akt, phospho-JNKThr183Tyr185 (9258) and total JNK (4668), and cleaved caspase-3 (7664) from Cell Signaling Technology (Beverly, MA). Inhibitors AS604850 (p110γ), AS601245 (JNK) and SP600125 (JNK), and anti-actin from Calbiochem (San Diego, CA), and anti-p110β (05-568) from Millipore Corporation (Billerica, MA). Bile salts and actinomycin D were from Sigma-Aldrich (Saint Louis, MO). The PI3K substrates PtIns and PtIns-4,5-P2 from Avanti Polar Lipids (Alabaster, AL), and [γ-32P]-ATP from Perkin Elmer (Waltham, MA). The caspase-3/-7 assay kit was provided by Promega (Madison, WI), siRNA targeted to p110γ (M-005274-02) and random oligonucleotide controls (D-001210-01) by Dharmacon (Lafayette, CO). Oligofectamine and tumor necrosis factor (TNF)α were from Invitrogen (Carlsbad, CA), and Fas ligand from Axxora (San Diego, CA).
Primary hepatocyte cultures
Hepatocytes were isolated from Wistar rats (200–250 g) as previously described [11], plated on culture dishes coated with Type I rat tail collagen in MEM with L-glutamine, 100 nM insulin, and 10% FCS. After 1 hour, medium was changed to insulin free MEM and cells were cultured for 4 h before experiments were initiated. All animals received humane care according to the “Guide for the Care and use of Laboratory Animals” by the National Academy of Sciences (NIH publication 86-23 revised 1985).
Culture of Ntcp-transfected HepG2 and Huh7 cell lines
HepG2-Ntcp [22] and Huh7-Ntcp cells [23], stably transfected with the rat sodium/taurocholate-cotransporting polypeptide (Ntcp) gene, were maintained in MEM containing 10% FCS, 1% non-essential aminoacids, 4 mM L-glutamine, 1 mM sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin, 1mg/ml geneticin.
Determination of Akt- and JNK-phosphorylation levels
Cultured rat hepatocytes were lysed after treatment with diluent (DMSO), 25 μM TLC, 50 μM TUDC, TC or GCDC, or 20 μM of the cAMP-GEF specific cAMP analogue, 4-(4-chlorophenylthio)-2′-O-methyladenosine-3′-5′-cyclic monophosphate (CPT-2-Me-cAMP) for the times indicated. In indicated experiments, cells were pretreated with p110γ inhibitor 2.5 μM AS604580 for 30 min or cultured in Ca++ free medium for 2 h prior to stimulation. Cells were lysed in ice-cold buffer (20 mM Tris-HCl pH 8.0, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1% TritonX-100, 1 mM Na3VO4, 10 μg/ml aprotinin, leupeptin, and pepstatin, 500 nM okadaic acid). Equal amounts of protein were separated in SDS–PAGE, transferred to Immobilon-P membranes and probed with anti-phospho-AktSer-473 or anti-phospho-JNKThr183/Tyr185. Membranes were developed with chemiluminescence after incubation with a peroxidase-conjugated second antibody. Stripped membranes were re-probed with anti-total-Akt or anti-total-JNK for loading control. Blots were digitalized and subjected to densitometric analysis (SigmaGel®).
Expression of PI3K p110 Isoforms
Lysates of cultured hepatocytes and Huh7-Ntcp cells were prepared and subjected to Western Blotting. Expression of p110α, β, δ, and γ was evaluated with isoform specific antibodies; for p110γ two different antibodies were used.
Isoform specific PI3K activity assays
Hepatocyte cultures were treated with diluent (DMSO), 25 μM TLC, 50 μM TUDC, TC, TCDC or GCDC, for 15 min, and lysates were prepared as previously described [11]. Isoform specific lipid kinase assays were performed after immunoprecipitation of the catalytic p110 subunits with antibodies to p110α (SC-7174), p110β (Millipore 05-068) or p110γ (CST 4252 or SC-7177) using the PI3K substrates PtIns, PtIns-4,5-P2, and [γ-32P]-ATP. The PI3K specific reaction product PtIns-3,4,5-P3 (PIP3) was isolated by thin layer chromatography and quantified using liquid scintillation counting. Specificity of the antibodies used for selective immunoprecipitation of PI3K isoforms has been documented in several cell types including hepatocytes [11,15,18,24].
Bile salt uptake
The 30 min accumulation of radiolabeled 3H-taurocholate in hepatocyte cultures was determined as previously described [10] in presence of chemical inhibitors.
Determination of apoptosis
Hepatocyte cultures were treated with diluent (DMSO), 25 μM TLC, 250 μM TCDC, 50 μM GCDC, or 50 ng/ml Fas for 2–4 h. HepG2-Ntcp and Huh7-Ntcp cells were treated with DMSO, 20 μM TLC, 75 μM TCDC or GCDC, 200 μM etoposide or 200 ng/ml TNFα plus 28 ng/ml actinomycin D for 2–4 h. In indicated experiments, inhibitors of JNK, AS601245 (10 μM) or SP600125 (10 μM) or the p110γ inhibitor AS604850 (2.5 μM) were added 30 min prior to stimulation. Selectivity of AS604850 for p110γ over p110α and β has been demonstrated in vitro [25]. The number of apoptotic cells was determined morphologically as previously described using fluorescent staining [10] and expressed as % of cells. Apoptosis was confirmed in human cell lines by determination of caspase-3/-7 activity and in rat hepatocytes by detection of the 17kD proteolytic cleavage fragment of caspase-3 by immunoblotting; equal protein loading was monitored by immunoblotting for actin.
siRNA-mediated gene silencing of p110γ
A pool of four p110γ siRNAs (SMARTpool®) was used to knock down p110γ expression in Huh7-Ntcp and HepG2-Ntcp cells. At 30–40% confluence, cells were transfected with targeted siRNA (10–50 nM) or random oligonucleotides using Oligofectamine. At 72 h, down-regulation of p110γ was confirmed by PCR analysis using sense (ttcctgtgcaggctactgtg) and antisense (gggttagcacaaatggcact) primers, and immunoblotting using anti-p110γ (SC-7177) antibody.
Data analysis
All results are expressed as mean ± SD of at least 3 independent experiments. Data were analyzed using paired student’s t-test with p <0.05 considered significant.
Results
Hydrophilic and hydrophobic bile salts activate PI3K
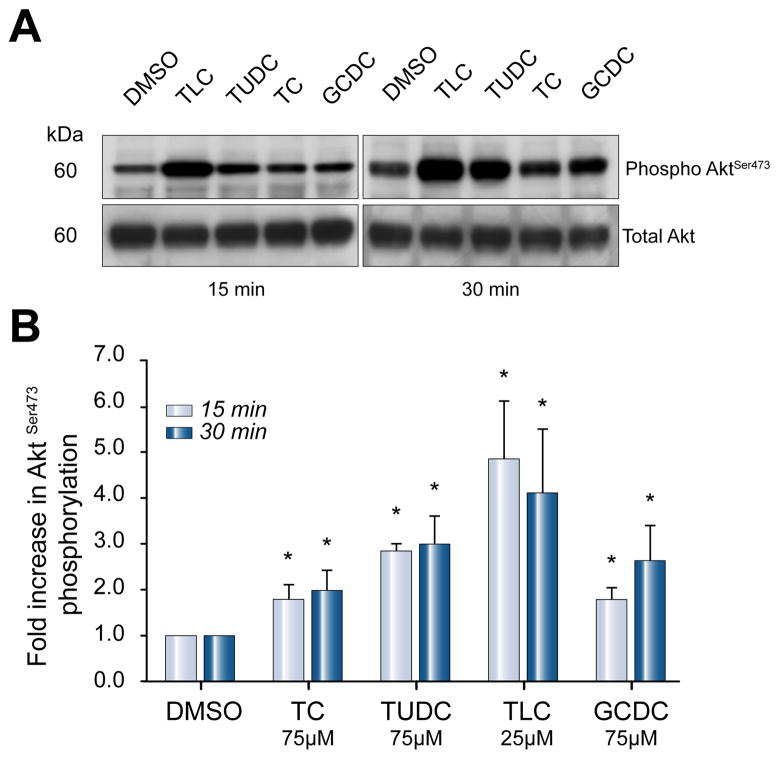
Phosphorylation of Akt was used as readout of total PI3K activity. All bile salts under study, independent of their hydrophobicity, increased Akt phosphorylation 2- to 5-fold at 15 and 30 min when administered at 25 μM (TLC) or 50 μM (TC, TUDC, TCDC, GCDC) (Fig. 2). After 5 min, only TLC activated Akt (1.5 ± 0.3-fold vs. control, n = 4, p <0.05). Only TUDC and GCDC caused a sustained increase in Akt activity at 60 min (2.1 ± 0.9 and 2.4 ± 0.6 vs. control, n = 4, p <0.05). At lower concentrations (10 μM TC/TUDC/GCDC, 5 μM TLC), Akt-phosphorylation was also significantly increased (data not shown).
Fig. 2. Hydrophilic and hydrophobic bile salts stimulate Akt-phosphorylation in hepatocytes.
Akt phosphorylation levels were determined in lysates of cultured rat hepatocytes by immunoblotting, after stimulation with 25 μM TLC or 50 μM TC, TUDC or GCDC for 15 and 30 min. Equal loading was confirmed by immunoblotting for total-Akt. (A) Representative immunoblots. (B) Densitometric analysis, expressed as fold increase in phosphorylation compared to cells stimulated with DMSO (*p <0.05 vs. DMSO, n = 4).
Hydrophilic and hydrophobic bile salts differentially activate p110 isoforms
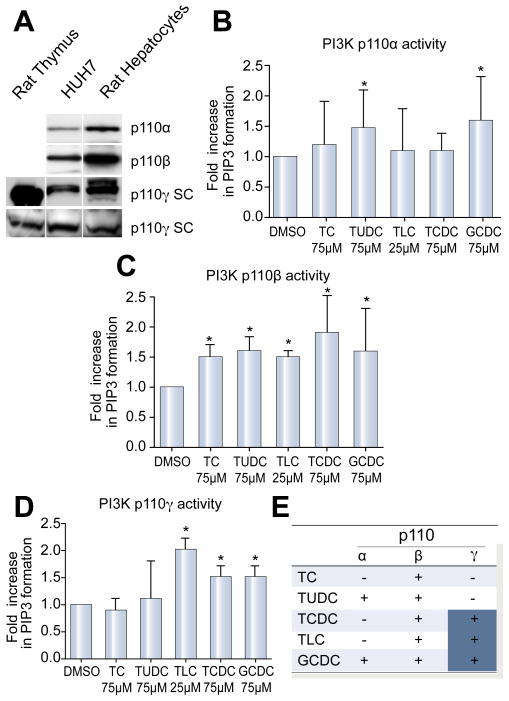
PI3K isoforms p110α and p110β are expressed ubiquitously. p110γ has mainly been described in immune cells but has also been reported in liver [9,18]. We confirmed expression of p110α, β, and γ in rat hepatocytes and Huh7 cells by immunoblotting (Fig. 3A). The p110δ isoform was not present in hepatocytes (data not shown). Subsequently, we studied isoform specific activation of PI3K in rat hepatocytes upon stimulation with hydrophilic and hydrophobic bile salts. By using PtIns-4,5-P2 as substrate, and detecting the reaction product PtIns-3-4-5-P3, the assay is highly specific for PI3K activity. Since we saw maximal activation of Akt at 30 min, we evaluated upstream activation of PI3K at 15 min. Isoform specific lipid kinase assays revealed that p110α activity was increased 1.5 ± 0.7 and 1.6 ± 0.7 fold in TUDC and GCDC treated cells, respectively, compared to controls (n = 6, p <0.05 vs. DMSO). In contrast, p110β was activated 1.5- to 1.9-fold (n = 5, p <0.05 vs. DMSO) by all bile salts tested. Finally, we found that p110γ was activated by hydrophobic bile salts only. TLC, TCDC, and GCDC increased p110γ activity 1.5- to 2.0-fold (n = 4, p <0.05; Figures 3B – E). This level of activation is compatible with that reported previously when PI3K specific formation of PIP3 was used as an endpoint [11]. Thus, all studied bile salts activate PI3K p110β, but differentially activate p110α and p110γ isoforms in hepatocytes.
Fig. 3. Expression and activation of PI3K p110 isoforms by bile salts in hepatocytes.
(A) Expression of p110α, β, and γ in rat hepatocytes and Huh7-Ntcp cells was confirmed by immunoblotting. Two different antibodies (Cell Signaling Technologies(C):CS and Santa Cruz(C):SC) were used for p110γ. Rat thymus served as a positive control for p110γ. Rat hepatocyte cultures were stimulated with bile salts at 25 μM (TLC) to 50 μM (TC, TUDCA, GCDC, TCDC) for 15 min, and isoforms-specific lipid kinase assays were performed. Lipid kinase assays after immunoprecipitation of (B) p110α, (C) p110β, and (D) p110γ (mean ± SD, *p <0.05 vs. DMSO, n = 4–6) are shown. Results represent fold increase in formation of the specific PI3K reaction product PIP3 compared to DMSO. (E) Summary of kinase assays: “+” indicates activation of the respective isoform.
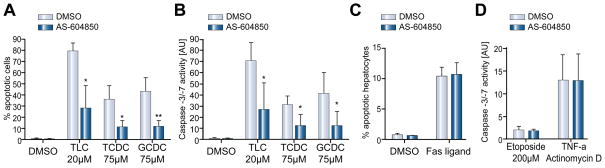
Inhibitor of p110γ activity, AS604850, diminishes GCDC-induced Akt-phosphorylation and apoptosis in rat hepatocytes
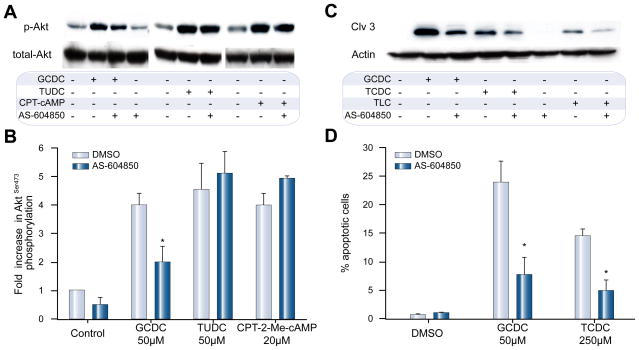
Our data show that p110γ is activated only by hydrophobic bile salts, which are known to be pro-apoptotic. We postulated, therefore, that p110γ might mediate bile salt-induced apoptosis. To test this hypothesis, the effects of a p110γ inhibitor, AS604850, on hepatocyte survival and signaling cascades was studied. AS604850 is highly p110γ specific [25] and at the chosen 2.5 μM concentration had no effect on bile salt uptake as determined by 3H-taurocholate accumulation in hepatocytes (97.7 ± 2.2% vs. 100% in controls, n = 3). Next, we re-evaluated the p110γ specificity of AS604850 in cultured hepatocytes by determining Akt-phosphorylation levels following stimulation with GCDC, TUDC, or CPT-2-Me-cAMP, a known activator of p110α and p110β, but not p110γ [11]. In previous experiments, we have shown that nonspecific PI3K inhibition with wortmannin completely abrogates bile salt (data not shown) and CPT-2-Me-cAMP-induced Akt-phosphorylation [10]. Treatment of rat hepatocytes with GCDC, TUDC or CPT-2-Me-cAMP resulted in a 4.0 ± 0.4, 4.6 ± 0.9, and 4.0 ± 0.4 fold increase in Akt-phosphorylation compared to control. Pretreatment with AS604850 diminished GCDC-induced Akt-phosphorylation by 50% (n = 3, p <0.05 vs. control), but left TUDC and CPT-2-Me-cAMP-induced Akt-phosphorylation unchanged (Fig. 4A and B). This is in line with GCDC-induced signaling via p110γ and TUDC-induced signaling via p110α and p110β.
Fig. 4. Inhibitor of p110γ, AS604850, diminishes GCDC-induced PI3K activation and apoptosis but does not alter TUDC- and CPT-2-Me-cAMP induced signaling in hepatocytes.
Rat hepatocytes were stimulated with 50 μM GCDC or TUDC, or 20 μM CPT-2-Me-cAMP, an activator of p110α and p110β, with and without pre-incubation with 2.5 μM AS604850. (A) Akt-phosphorylation was determined by immunoblotting for phosphorylated AktSer-473 and total Akt (representative blots) (B) Densitometric analysis of 3 independent experiments (*p <0.05 vs. control). (C) Rat hepatocytes were pre-treated with AS604850 and then exposed to DMSO, 50 μM GCDC or 250 μM TCDC for 2–4 h, respectively, and apoptosis determined by immunoblotting for the 17 kd cleavage product of active caspase-3 or (D) following staining with Hoechst 33258 (*p <0.05 vs. control, n = 3). Equal loading was confirmed by immunoblotting for actin on the same membrane.
Next, we evaluated the effect of AS604850 on bile salt-induced apoptosis. Cultured rat hepatocytes were stimulated with hydrophobic bile salts after preincubation with AS604850. Cleavage of caspase-3 was determined as readout of apoptosis induction. GCDC, TCDC, and TLC increased caspase-3 cleavage (Fig. 4C) which was markedly decreased by AS604850. Morphologic determination of apoptosis confirmed the hepatotoxic effect of GCDC and TCDC with 24.0 ± 3.7 and 14.6 ± 0.9% apoptotic cells, respectively. Inhibition of p110γ profoundly diminished this apoptotic effect by 67.5% and 66.4% (n = 3, p <0.05; Fig. 4D). These results indicate that bile salt-induced apoptosis in rat hepatocytes is mediated, in part, via PI3K p110γ.
Inhibition of p110γ diminishes bile salt-induced apoptosis in HepG2-Ntcp and Huh7-Ntcp cells
We then tested whether inhibition of p110γ would also prevent bile salt-induced apoptosis in human hepatoma cell lines. HepG2-Ntcp and Huh7-Ntcp cells were stimulated with hydrophobic bile salts in the presence or absence of the p110γ inhibitor AS604850. In HepG2-Ntcp cells, all bile salts tested induced marked increase in the percentage of apoptotic cells to 79.5±6.9 (TLC), 36.1 ± 11.5 (TCDC), and 43.2 ± 11.5% (GCDC) (Fig. 5A). Inhibition of p110γ significantly diminished this apoptotic effect to 35.6%, 31.2%, and 27.2% of the initial values (n = 4, p <0.05; Fig. 5A). These morphologic results were confirmed by determination of caspase-3/-7 activity, which was increased after bile salt stimulation to 71.2 ± 16.5 (TLC), 31.5 ± 7.4 (TCDC), and 41.6 ± 18.9 (GCDC) arbitrary units (AU) and was diminished to 38.3%, 39.9%, and 30.2%, respectively, of the initial values after p110γ inhibition (n = 4, p <0.05 vs. control; Fig. 5B). Comparable effects were observed in Huh7-Ntcp cells (data not shown). Thus, p110γ may also mediate bile salt-induced apoptosis in human liver cells.
Fig. 5. Inhibition of p110γ selectively inhibits bile salt-induced apoptosis in hepatocytes but does not alter the effects of independent apoptotic stimuli.
HepG2-Ntcp cells were stimulated with DMSO, 20 μM TLC or 75 μM of TCDC or GCDC. The p110γ inhibitor AS604850 (2.5 μM) was added 30 min prior to stimulation. (A) Apoptotic cells were quantified morphologically after Hoechst staining. Rate of apoptosis was expressed as % of apoptotic cells. (B) In separate experiments, apoptosis in identically stimulated HepG2-Ntcp was determined by caspase-3/-7 activity (*p <0.05 vs. control, n = 3–6). (C) Rat hepatocyte cultures were treated with 50 ng/ml Fas ligand for 4 hours and apoptotic cells were determined morphologically. AS604850 was added at 2.5 μM 30 min prior to the addition of Fas ligand (n = 3). (D) HepG2-Ntcp cells were treated with 200 μM etoposide or 200 ng/ml TNFα plus 28 ng/ml actinomycin D for 4 h and apoptotic effects were quantified in caspase-3/-7 assays. Prior to stimulation, cells were treated with 2.5 μM AS604850 for 30 min (n = 3).
Bile salt independent stimuli of apoptosis are p110γ independent
To test whether the role of p110γ in mediating apoptosis is specific for bile salt-induced cell death, we evaluated the effect of p110γ inhibition on Fas-, etoposide-, and TNFα/actinomycin D-induced apoptosis. These pro-apoptotic agents, unlike bile salts, do not activate the PI3K/Akt pathway. Fas-induced apoptosis in cultured rat hepatocytes was not altered by inhibition of p110γ (Fig. 5C). Likewise, etoposide- and TNFα-induced caspase-3/-7 activity was unaffected by AS604850 (Fig. 5D) in HepG2-Ntcp. Thus, PI3K p110γ specifically mediates bile salt-induced apoptosis.
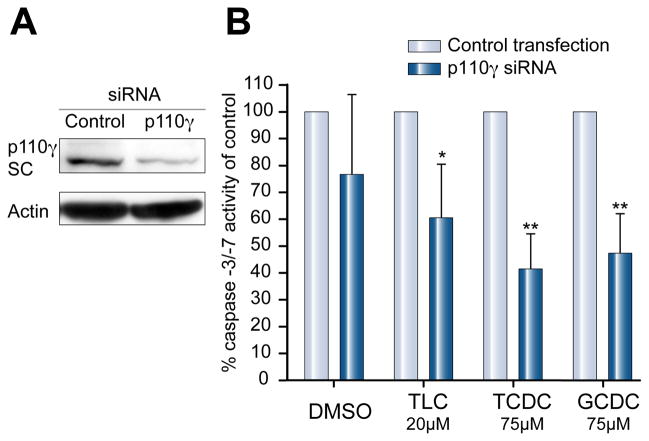
Knockdown of p110γ by siRNA diminishes bile salt-induced apoptosis
Huh7-Ntcp cells were transfected with siRNA against p110γ or nonsense siRNA for 72 h. Knock-down of p110γ in Huh7-Ntcp was confirmed by PCR (data not shown) and Western Blot (Fig. 6A). Expression of the control gene GAPDH and basal cell viability was not affected (data not shown). Cells transfected with nonspecific siRNA showed a clear apoptotic response to treatment with hydrophobic bile salts, as expected. In p110γ depleted Huh7-Ntcp cells, the activity of caspases-3/-7 was decreased to 60.5%, 41.8%, 47.6% respectively, of control transfected cells (Fig. 6B). Gene silencing experiments in HepG2-Ntcp cells showed similar results (data not shown). Since primary rat hepatocytes dedifferentiate within days and lose their capacity for bile salt uptake, this model could not be used for gene silencing techniques. These results show that silencing of PI3K class I p110γ protects against bile salt-induced apoptosis in human liver cells.
Fig. 6. p110γ knockdown inhibits hydrophobic bile acid-induced increases in caspase-3/-7 activity.
(A) Transfection with p110γ siRNA effectively silenced protein expression in Huh7-Ntcp as shown by immunoblots from control transfected cells and cells transfected with specific p110γ siRNA. (B) p110γ-knockdown cells were stimulated with DMSO, 20 μM TLC or 75 μM TCDC or GCDC. After 4 h, activity of caspases-3/-7 was determined and the data are expressed as % of caspase-3/-7 activity of control transfected cells (n = 4, *p <0.05/**p <0.005 vs. control).
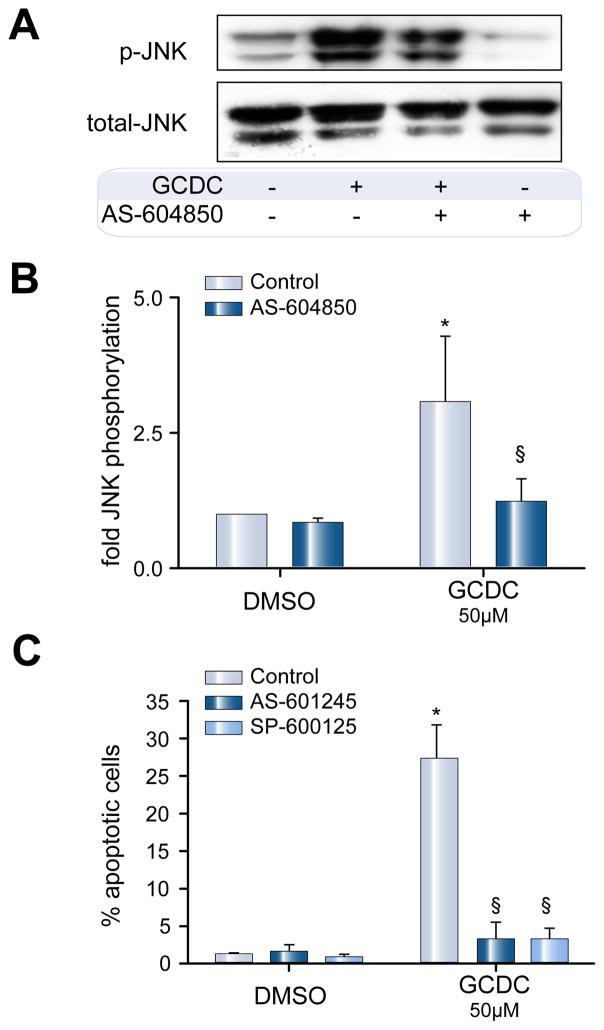
GCDC-induced apoptosis involves p110γ-dependent activation of JNK
We and others have previously documented a role for JNK activation in the cytotoxic effects of hydrophobic bile salts in rat hepatocytes [26–28] and Huh7 cells [29]. Treatment of rat hepatocytes, with 50 μM GCDC for 30 min, induced a 3.1 ± 1.2-fold increase in JNK phosphorylation (n = 4, p <0.05 vs. DMSO, Fig. 7A and B). Pretreatment with AS604850 prevented this phosphorylation. GCDC induced apoptosis in 27.1 ± 4.6% of cells vs. 1.1 ± 0.3 for DMSO. Pretreatment with JNK inhibitors AS601245 or SP600125 decreased GCDC-induced apoptosis by 88.0 ± 7.1% and 86.0 ± 6.4%, respectively (Fig. 7C). Inhibition of GCDC-induced JNK activation in Huh7-Ntcp cells with AS601245 produced comparable results (data not shown). Thus, p110γ-mediated bile salt-induced apoptosis appears to involve downstream activation of JNK.
Fig. 7. GCDC-induced apoptosis involves PI3K p110γ-dependent activation of JNK.
Rat hepatocytes were pretreated for 30 min with 2.5 μM of the p110γ inhibitor AS604850 and then exposed to 50 μM GCDC for 30 min after which the amount of phosphorylated JNK was determined by immunoblotting. (A) Representative blot and (B) Densitometric analysis of 4 independent experiments (*p <0.05 vs. DMSO, §p <0.05 vs. GCDC control ). (C) Rat hepatocytes were pretreated with either of 2 JNK inhibitors, SP600125 or AS-612245 (10 μM, each) for 30 min and then exposed to 50 μM GCDC for 2 h. Apoptosis was determined morphologically and expressed as % apoptotic cells (n = 3, *p <0.05 vs. DMSO, §p <0.05 vs. GCDC control).
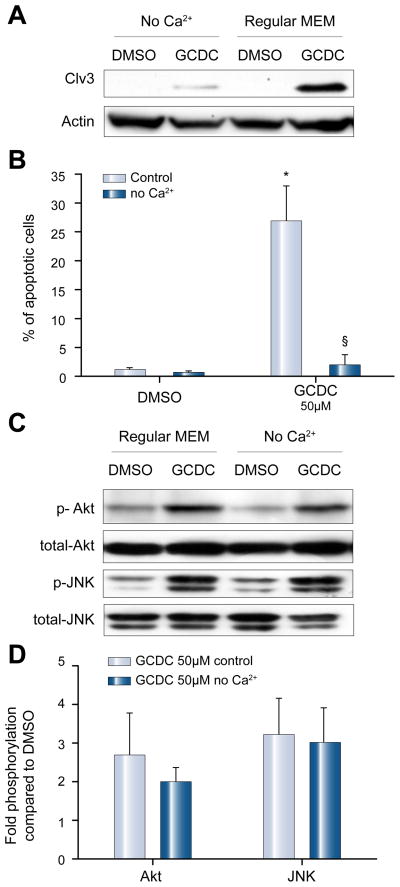
GCDC-induced hepatotoxicity, but not Akt- and JNK-signaling is Ca++ dependent
Several studies have shown that bile salt-induced signaling in hepatocytes is linked to increases in intracellular calcium [30]. In addition, the action of PI3K p110γ in non-hepatic cells has been linked to Ca++ signaling [20,31,32]. Therefore, we tested the hypothesis that bile salt-induced cytotoxicity might be calcium dependent. We cultured rat hepatocytes in Ca++ free medium for 2 h, an interval considered sufficient to deplete intracellular Ca++ stores [33]. Subsequently, apoptosis was induced by stimulation with 50 μM GCDC for 2 h. Depletion of Ca++ reduced the number of apoptotic cells by 92.3 ± 9.0% (n = 4, p <0.05, Fig. 8A) and blocked GCDC-induced caspase-3 cleavage (Fig. 8B). Similar results were seen in the Huh7-Ntcp cells (data not shown). Calcium free conditions, however, had no effect on GCDC-induced PI3K/Akt or JNK phosphorylation in rat hepatocytes (Fig. 8C and D), suggesting that the Ca++-dependent effect on cell death is downstream of PI3Kp110γ/JNK or that it is involved in a complementary cell death pathway.
Fig. 8. GCDC-induced apoptosis but not Akt- and JNK-signaling is calcium dependent.
Rat hepatocytes were cultured in Ca++ free medium for 2 h, stimulated with 50 μM GCDC for 2 h and apoptosis was determined by (A) immunoblotting for the active cleavage fragment of caspase-3, and by (B) morphologic evaluation of Hoechst stained cells (n = 4, *p <0.05 vs. DMSO, §p <0.05 vs. control). (C) Rat hepatocytes were cultured in Ca++ free media for 2 h and then exposed to GCDC for 30 min. The amount of phosphorylated Akt and JNK was determined by immunoblotting. Representative immunoblots are shown in (C) and the results of densitometric analysis representing 3 separate experiments are shown in (D). Equal loading was controlled by immunoblotting for total-JNK or total-Akt.
Discussion
While hydrophobic and hydrophilic bile salts exert opposing effects on hepatobiliary secretion and survival, they all appear to act partly via PI3K (Supplementary Table 1), a paradoxical finding that has never been explained mechanistically. In light of the emergence of information on the diverse functions attributed to specific PI3K isoforms in other tissues, we studied whether distinct catalytic PI3K isoforms might be involved in this PI3K paradox in liver, as we had speculated in the past [2,24].
In the present study, we observed that hydrophobic and hydrophilic bile salts stimulate distinct isoforms of the catalytic p110 subunit of class I PI3K in hepatocytes. All bile salts tested (TUDC, TC, TCDC, TLC, and GCDC) activate p110β, rendering it an unlikely candidate for the discrimination of bile salt signaling pathways. Only TUDC and GCDC activate p110α, a finding that cannot explain the PI3K paradox as TUDC is cytoprotective and GCDC is cytotoxic. However, we found that p110γ is exclusively activated by hydrophobic, hepatotoxic bile salts (TLC, TCDC, and GCDC). Furthermore, we showed that selective inhibition of p110γ by chemical inhibitors or gene silencing partially protects rat hepatocytes and human hepatic cell lines from bile salt-induced apoptosis. We also presented evidence that GCDC-induced apoptosis is JNK dependent, an observation in line with previous studies in rat hepatocytes [26–28]. We showed for the first time that bile salt-induced JNK activation is p110γ-dependent. Collectively, our results support the existence of a bile salt-induced pro-apoptotic PI3K p110γ-dependent signaling pathway in human and rat hepatocytes, that involves downstream activation of JNK. This pathway seems to be specific for bile salt-induced apoptosis, as Fas-, Etoposide- and TNFα-dependent cell death are not affected by inhibition of p110γ.
We are just beginning to understand the roles of PI3K isoforms in liver. Recently, we reported that the cytoprotective effect of cAMP-GEF in hepatocytes is associated with PI3K p110α/p110β activation [11] and p110α is known to be necessary for insulin signaling in liver [14]. A role for PI3K in bile salt signaling has been established using PI3K inhibitors, LY294002 and wortmannin. While both are considered non-selective, LY294002 exhibits a 10- and 23-fold specificity for p110α and p110β, respectively, over p110γ (Supplementary Table 2). This is of interest, as studies demonstrating the PI3K-dependence of the hepatoprotective effects of TUDC used LY294002 (Supplementary Table 1). In light of selectivity data of this inhibitor, these findings support a role for p110α in TUDC-induced hepatoprotection, in line with the described p110α/p110β-dependent hepatoprotection by cAMP-GEF. The cytotoxic/apoptotic effect of GCDC, however, is enhanced when this potentially cytoprotective p110α pathway is blocked by LY294002 [34]. Administration of wortmannin, which effectively blocks all PI3K class I isoforms, attenuates GCDC-induced hepatocyte apoptosis in perfused rat livers [2]. These reports are consistent with a role for PI3K p110γ in cytotoxic signaling in hepatocytes, as identified in this study.
The present finding that bile salt-induced apoptosis is mediated via PI3K p110γ-dependent signaling is in contrast to the established concept of PI3K/Akt signaling as a promoter of cell survival in many cell types including hepatocytes [10,11,35]. Although it has been shown that PI3K p110γ-mediated Akt-signaling can have detrimental effects in other tissues [36], it is unlikely that the Akt pathway is the apoptotic downstream effector of p110γ-dependent bile salt-induced apoptosis. Indeed, we present evidence that p110γ-mediated JNK phosphorylation may be a major downstream target. This finding is supported by several studies which have linked hydrophobic bile salt-induced hepatotoxicity to JNK signaling [26–28] and showed that p110γ can induce JNK mediated signaling cascades in non-hepatic cells [37].
Hydrophobic bile salts stimulate Ca++ mobilization through extracellular calcium influx and/or calcium release from intracellular organelles [30]. In angiotensin II-induced vascular toxicity, Ca++ flux is regulated by p110γ [36]. In pancreatic cells, inhibitors of PI3K or genetic deletion of p110γ prevent both bile salt-induced Ca++ responses and cytotoxicity [20]. In our study, GCDC-induced toxicity was abolished under Ca++ free conditions. These results are in line with previous studies demonstrating Ca++ dependence of bile salt-induced death pathways [34–37]. In contrast, GCDC-mediated PI3K activation, as determined by Akt phosphorylation, as well as JNK phosphorylation were not altered under Ca++ free conditions. This suggests that PI3K activation in bile salt-induced hepatotoxicity is independent of bile salt-induced Ca++ flux. Furthermore, the Ca++-dependent step in transduction of bile salt-induced apoptosis seems to be a later event, occurring downstream of PI3K/JNK signaling. How hydrophobic bile salts induce activation of p110γ remains enigmatic. Activation of p110γ is tied to activation of GPCR [7,9], and some signaling cascades of bile salts involve GPCR [38,39]. Two lines of evidence, however, suggest that bile salts do not directly activate GPCR. Bile salts need to enter the cell in order to induce apoptosis [34,40], thus it is unlikely that they mediate their hepatotoxic effects by directly binding to cell surface GPCR. Moreover, the major bile salt responsive GPCR identified so far, TGR5, is not present in rat or human hepatocytes [38,41]. TGR5, however, was identified in a hepatoma cell line and mediates deoxycholate-induced apoptosis and JNK phosphorylation in these cells [42]. It may be possible that bile salt signaling is differently regulated in primary hepatocytes versus hepatoma cell lines. Alternatively, bile salts may bind to a yet unidentified GPCR in the liver. However, we speculate that bile salts may mediate their effects via p110γ activation from an intracellular platform. There is some evidence to suggest that this platform may involve mitochondrial and/or NADPH oxidase mediated generation of ROS [30].
In conclusion, we provide experimental evidence that bile salts differentially activate PI3K class I isoforms. In particular, we showed that activation of the p110γ isoform by hydrophobic bile salts contributes to their hepatotoxicity. These findings have two potential clinical implications. First, if in vivo studies substantiate the protective effect of p110γ inhibition in animal models of cholestatic disease, these compounds may offer a novel approach to ameliorate liver injury in cholestasis. Second, since PI3K inhibitors are under clinical development for the treatment of inflammatory disorders (p110γ) [43], cancer (p110α) [44], and thrombosis (p110β) [45], it will be crucial to understand the implications of PI3K isoform specific action in the liver, in order to predict potential beneficial or harmful effects in normal and diseased liver.
Supplementary Material
Acknowledgments
Financial support
This work was supported in part by grants from NIH, R01 DK065975 to CRL Webster and R01 DK033436 to MS Anwer and from DFG, Be-1242/5-5 to U Beuers and Ru-743/3-1 to C Rust.
Abbreviations
- CPT-2-Me-cAMP
4-(4-chloro-phenylthio)-2′-O-methyladenosine-3′-5′-cyclic monophosphate
- JNK
c-Jun N-terminal kinase
- GCDC
Glycochenodeoxycholate
- GPCR
G protein coupled receptors
- PtIns
phosphatidylinositides
- PI3K
phosphatidylinositol 3-kinase
- PI4K
phosphatidylinositol 4-kinase
- PI5K
phosphatidylinositol 5-kinase
- PIP3
PtIns-3,4,5-triphosphate
- ROS
reactive oxygen species
- Ntcp
sodium/taurocholate cotransporting polypeptide
- TCDC
taurochenodeoxycholate
- TC
taurocholate
- TLC
taurolithocholate
- TUDC
tauroursodeoxycholate
- TNFα
tumor necrosis factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guicciardi ME, Gores GJ. Bile acid-mediated hepatocyte apoptosis and cholestatic liver disease. Dig Liver Dis. 2002;34:387–392. doi: 10.1016/s1590-8658(02)80033-0. [DOI] [PubMed] [Google Scholar]
- 2.Rust C, Bauchmuller K, Fickert P, Fuchsbichler A, Beuers U. Phosphatidylinositol 3-kinase-dependent signaling modulates taurochenodeoxycholic acid-induced liver injury and cholestasis in perfused rat livers. Am J Physiol Gastrointest Liver Physiol. 2005;289:G88–94. doi: 10.1152/ajpgi.00450.2004. [DOI] [PubMed] [Google Scholar]
- 3.Perez MJ, Briz O. Bile-acid-induced cell injury and protection. World J Gastroenterol. 2009;15:1677–1689. doi: 10.3748/wjg.15.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wimmer R, Hohenester S, Pusl T, Denk GU, Rust C, Beuers U. Tauroursodeoxycholic acid exerts anticholestatic effects by a cooperative cPKC alpha-/PKA-dependent mechanism in rat liver. Gut. 2008;57:1448–1454. doi: 10.1136/gut.2007.140871. [DOI] [PubMed] [Google Scholar]
- 5.Hohenester S, Oude-Elferink RP, Beuers U. Primary biliary cirrhosis. Semin Immunopathol. 2009 doi: 10.1007/s00281-009-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amaral JD, Viana RJ, Ramalho RM, Steer CJ, Rodrigues CM. Bile acids: regulation of apoptosis by ursodeoxycholic ccid. J Lipid Res. 2009 doi: 10.1194/jlr.R900011-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 8.Guillermet-Guibert J, Bjorklof K, Salpekar A, Gonella C, Ramadani F, Bilancio A, et al. The p110beta isoform of phosphoinositide 3-kinase signals downstream of G protein-coupled receptors and is functionally redundant with p110gamma. Proc Natl Acad Sci U S A. 2008;105:8292–8297. doi: 10.1073/pnas.0707761105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoyanov B, Volinia S, Hanck T, Rubio I, Loubtchenkov M, Malek D, et al. Cloning and characterization of a G protein-activated human phosphoinositide-3 kinase. Science. 1995;269:690–693. doi: 10.1126/science.7624799. [DOI] [PubMed] [Google Scholar]
- 10.Webster CR, Usechak P, Anwer MS. cAMP inhibits bile acid-induced apoptosis by blocking caspase activation and cytochrome c release. Am J Physiol Gastrointest Liver Physiol. 2002;283:G727–738. doi: 10.1152/ajpgi.00410.2001. [DOI] [PubMed] [Google Scholar]
- 11.Gates A, Hohenester S, Anwer MS, Webster CR. cAMP-GEF cytoprotection by Src tyrosine kinase activation of phosphoinositide -3 -kinase p110 {beta}/{alpha} in rat hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2009 doi: 10.1152/ajpgi.90622.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benistant C, Chapuis H, Roche S. A specific function for phosphatidylinositol 3-kinase alpha (p85alpha-p110alpha) in cell survival and for phosphatidylinositol 3-kinase beta (p85alpha-p110beta) in de novo DNA synthesis of human colon carcinoma cells. Oncogene. 2000;19:5083–5090. doi: 10.1038/sj.onc.1203871. [DOI] [PubMed] [Google Scholar]
- 13.Pretorius L, Owen KL, McMullen JR. Role of phosphoinositide 3-kinases in regulating cardiac function. Front Biosci. 2009;14:2221–2229. doi: 10.2741/3374. [DOI] [PubMed] [Google Scholar]
- 14.Foukas LC, Claret M, Pearce W, Okkenhaug K, Meek S, Peskett E, et al. Critical role for the p110alpha phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature. 2006;441:366–370. doi: 10.1038/nature04694. [DOI] [PubMed] [Google Scholar]
- 15.Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee SH, et al. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature. 2008;454:776–779. doi: 10.1038/nature07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kok K, Geering B, Vanhaesebroeck B. Regulation of phosphoinositide 3-kinase expression in health and disease. Trends Biochem Sci. 2009;34:115–127. doi: 10.1016/j.tibs.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Hawkins PT, Stephens LR. PI3Kgamma is a key regulator of inflammatory responses and cardiovascular homeostasis. Science. 2007;318:64–66. doi: 10.1126/science.1145420. [DOI] [PubMed] [Google Scholar]
- 18.Misra S, Varticovski L, Arias IM. Mechanisms by which cAMP increases bile acid secretion in rat liver and canalicular membrane vesicles. Am J Physiol Gastrointest Liver Physiol. 2003;285:G316–324. doi: 10.1152/ajpgi.00048.2003. [DOI] [PubMed] [Google Scholar]
- 19.Chang JD, Sukhova GK, Libby P, Schvartz E, Lichtenstein AH, Field SJ, et al. Deletion of the phosphoinositide 3-kinase p110gamma gene attenuates murine atherosclerosis. Proc Natl Acad Sci U S A. 2007;104:8077–8082. doi: 10.1073/pnas.0702663104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer L, Gukovskaya AS, Penninger JM, Mareninova OA, Friess H, Gukovsky I, et al. Phosphatidylinositol 3-kinase facilitates bile acid-induced Ca(2+) responses in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G875–886. doi: 10.1152/ajpgi.00558.2005. [DOI] [PubMed] [Google Scholar]
- 21.Lupia E, Goffi A, De Giuli P, Azzolino O, Bosco O, Patrucco E, et al. Ablation of phosphoinositide 3-kinase-gamma reduces the severity of acute pancreatitis. Am J Pathol. 2004;165:2003–2011. doi: 10.1016/s0002-9440(10)63251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glasova H, Berghaus TM, Kullak-Ublick GA, Paumgartner G, Beuers U. Tauroursodeoxycholic acid mobilizes alpha-PKC after uptake in human HepG2 hepatoma cells. Eur J Clin Invest. 2002;32:437–442. doi: 10.1046/j.1365-2362.2002.01002.x. [DOI] [PubMed] [Google Scholar]
- 23.Rust C, Wild N, Bernt C, Vennegeerts T, Wimmer R, Beuers U. Bile acid-induced apoptosis in hepatocytes is caspase-6-dependent. J Biol Chem. 2009;284:2908–2916. doi: 10.1074/jbc.M804585200. [DOI] [PubMed] [Google Scholar]
- 24.Beuers U, Denk GU, Soroka CJ, Wimmer R, Rust C, Paumgartner G, et al. Taurolithocholic acid exerts cholestatic effects via phosphatidylinositol 3-kinase-dependent mechanisms in perfused rat livers and rat hepatocyte couplets. J Biol Chem. 2003;278:17810–17818. doi: 10.1074/jbc.M209898200. [DOI] [PubMed] [Google Scholar]
- 25.Camps M, Ruckle T, Ji H, Ardissone V, Rintelen F, Shaw J, et al. Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med. 2005;11:936–943. doi: 10.1038/nm1284. [DOI] [PubMed] [Google Scholar]
- 26.Graf D, Kurz AK, Fischer R, Reinehr R, Haussinger D. Taurolithocholic acid-3 sulfate induces CD95 trafficking and apoptosis in a c-Jun N-terminal kinase-dependent manner. Gastroenterology. 2002;122:1411–1427. doi: 10.1053/gast.2002.32976. [DOI] [PubMed] [Google Scholar]
- 27.Usechak P, Gates A, Webster CR. Activation of focal adhesion kinase and JNK contributes to the extracellular matrix and cAMP-GEF mediated survival from bile acid induced apoptosis in rat hepatocytes. J Hepatol. 2008;49:251–261. doi: 10.1016/j.jhep.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta S, Natarajan R, Payne SG, Studer EJ, Spiegel S, Dent P, et al. Deoxycholic acid activates the c-Jun N-terminal kinase pathway via FAS receptor activation in primary hepatocytes. Role of acidic sphingomyelinase-mediated ceramide generation in FAS receptor activation. J Biol Chem. 2004;279:5821–5828. doi: 10.1074/jbc.M310979200. [DOI] [PubMed] [Google Scholar]
- 29.Park SC, Yoon JH, Kim W, Gwak GY, Kim KM, Lee SH, et al. Eupatilin attenuates bile acid-induced hepatocyte apoptosis. J Gastroenterol. 2006;41:772–778. doi: 10.1007/s00535-006-1854-6. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen A, Bouscarel B. Bile acids and signal transduction: role in glucose homeostasis. Cell Signal. 2008;20:2180–2197. doi: 10.1016/j.cellsig.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 31.Kerfant BG, Zhao D, Lorenzen-Schmidt I, Wilson LS, Cai S, Chen SR, et al. PI3Kgamma is required for PDE4, not PDE3, activity in subcellular microdomains containing the sarcoplasmic reticular calcium ATPase in cardiomyocytes. Circ Res. 2007;101:400–408. doi: 10.1161/CIRCRESAHA.107.156422. [DOI] [PubMed] [Google Scholar]
- 32.Quignard JF, Mironneau J, Carricaburu V, Fournier B, Babich A, Nurnberg B, et al. Phosphoinositide 3-kinase gamma mediates angiotensin II-induced stimulation of L-type calcium channels in vascular myocytes. J Biol Chem. 2001;276:32545–32551. doi: 10.1074/jbc.M102582200. [DOI] [PubMed] [Google Scholar]
- 33.Beuers U, Nathanson MH, Isales CM, Boyer JL. Tauroursodeoxycholic acid stimulates hepatocellular exocytosis and mobilizes extracellular Ca++ mechanisms defective in cholestasis. J Clin Invest. 1993;92:2984–2993. doi: 10.1172/JCI116921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoemaker MH, Conde de la Rosa L, Buist-Homan M, Vrenken TE, Havinga R, Poelstra K, et al. Tauroursodeoxycholic acid protects rat hepatocytes from bile acid-induced apoptosis via activation of survival pathways. Hepatology. 2004;39:1563–1573. doi: 10.1002/hep.20246. [DOI] [PubMed] [Google Scholar]
- 35.Duronio V. The life of a cell: apoptosis regulation by the PI3K/PKB pathway. Biochem J. 2008;415:333–344. doi: 10.1042/BJ20081056. [DOI] [PubMed] [Google Scholar]
- 36.Vecchione C, Patrucco E, Marino G, Barberis L, Poulet R, Aretini A, et al. Protection from angiotensin II-mediated vasculotoxic and hypertensive response in mice lacking PI3Kgamma. J Exp Med. 2005;201:1217–1228. doi: 10.1084/jem.20040995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Go YM, Park H, Maland MC, Darley-Usmar VM, Stoyanov B, Wetzker R, et al. Phosphatidylinositol 3-kinase gamma mediates shear stress-dependent activation of JNK in endothelial cells. Am J Physiol. 1998;275:H1898–1904. doi: 10.1152/ajpheart.1998.275.5.H1898. [DOI] [PubMed] [Google Scholar]
- 38.Fang Y, Studer E, Mitchell C, Grant S, Pandak WM, Hylemon PB, et al. Conjugated bile acids regulate hepatocyte glycogen synthase activity in vitro and in vivo via Galphai signaling. Mol Pharmacol. 2007;71:1122–1128. doi: 10.1124/mol.106.032060. [DOI] [PubMed] [Google Scholar]
- 39.Dent P, Fang Y, Gupta S, Studer E, Mitchell C, Spiegel S, et al. Conjugated bile acids promote ERK1/2 and AKT activation via a pertussis toxin-sensitive mechanism in murine and human hepatocytes. Hepatology. 2005;42:1291–1299. doi: 10.1002/hep.20942. [DOI] [PubMed] [Google Scholar]
- 40.Rust C, Bauchmuller K, Bernt C, Vennegeerts T, Fickert P, Fuchsbichler A, et al. Sulfasalazine reduces bile acid induced apoptosis in human hepatoma cells and perfused rat livers. Gut. 2006;55:719–727. doi: 10.1136/gut.2005.077461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keitel V, Donner M, Winandy S, Kubitz R, Haussinger D. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem Biophys Res Commun. 2008;372:78–84. doi: 10.1016/j.bbrc.2008.04.171. [DOI] [PubMed] [Google Scholar]
- 42.Yang JI, Yoon JH, Myung SJ, Gwak GY, Kim W, Chung GE, et al. Bile acid-induced TGR5-dependent c-Jun-N terminal kinase activation leads to enhanced caspase 8 activation in hepatocytes. Biochem Biophys Res Commun. 2007;361:156–161. doi: 10.1016/j.bbrc.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Ghigo A, Hirsch E. Isoform selective phosphoinositide 3-kinase gamma and delta inhibitors and their therapeutic potential. Recent Pat Inflamm Allergy Drug Discov. 2008;2:1–10. doi: 10.2174/187221308783399270. [DOI] [PubMed] [Google Scholar]
- 44.Jia S, Roberts TM, Zhao JJ. Should individual PI3 kinase isoforms be targeted in cancer? Curr Opin Cell Biol. 2009;21:199–208. doi: 10.1016/j.ceb.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jackson SP, Schoenwaelder SM, Goncalves I, Nesbitt WS, Yap CL, Wright CE, et al. PI 3-kinase p110beta: a new target for antithrombotic therapy. Nat Med. 2005;11:507–514. doi: 10.1038/nm1232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.