Abstract
Alzheimer’s Disease (AD) can reduce the effects of emotional content on memory for studied pictures, but less is known about false memory. In healthy adults, emotionally arousing pictures can be more susceptible to false memory effects than neutral pictures, potentially because emotional pictures share conceptual similarities that cause memory confusions. We investigatedthese effects in AD patients and healthy controls. Participants studied pictures and their verbal labels, and then picture recollection was tested using verbal labels as retrieval cues. Some of the test labels had been associated with a picture at study, whereas other had not. On this picture recollection test, we found that both AD patients and controlsincorrectly endorsed some of the test labels that had not been studiedwith pictures. These errors were associated with medium to high levels of confidence, indicating some degree of false recollection. Critically, these false recollection judgments were greater for emotionalcompared to neutral items, especially for positively valenced items, in both AD patients and controls. Dysfunction of the amygdala and hippocampusin early AD may impairrecollection, but ADdid not disrupt the effect of emotion on false recollection judgments.
Keywords: False memory, emotion, Alzheimer’s disease
Contrary to intuition, emotional events are not always remembered more accurately than neutral events. Emotionally arousing information can capture attention and activate specialized neural responses (e.g., amygdala modulation of hippocampus), and these processes can enhance memory for studied items (for reviews see Kensinger, 2009; Mather, 2007). However, emotionally arousing information also can be associated with elevated false recognitionof nonstudied items and more liberal response bias(e.g., Dougal & Rotello, 2007; Herbener, Rosen, Khine,& Sweeney,2007), potentially owingto greaterconceptual relatedness betweenemotional items (e.g., Brainerd, Stein, Silveira et al., 2008; Maratos, Allan, & Rugg 2000). Although different memory tasks tap these different effects of emotion, each pattern has been observed in both younger and older adults(e.g., Kapucu, Rotello, Ready,& Seidl,2008; Kensinger, Brierley, Medford, Growdon, & Corkin 2002), suggesting spared emotional processing withhealthy cognitive aging.
Alzheimer’s disease (AD) tends to reduce the emotional memory effect for studied items, even though AD patients can have normal reactions to emotional stimuli (e.g., Hamann, Monarch, & Goldstein,2000). Hamann et al. (2000) found greater recallfor negative over neutral pictures in healthy controls, but not in ADpatients. The AD group did show elevated recall of positive pictures over negative pictures in this study,but more recently, Kensinger et al. (2002) and Abrisqueta-Gomez, Bueno, Oliveira, and Bertolucci (2002) did not find a memory benefitfor either positive or negative pictures in AD patients. Similarly, while some studies have shown emotional memory benefits for stories in AD patients (e.g., Kazui, Mori, Hashimotoet al., 2000), a large-scale study by Kensinger, Anderson, Growdon, and Corkin (2004) failed to find this effect. It is unclear why some studies have found emotional memory benefits in AD while others have not, but the effect tends to be reduced in AD. These reductionsare often attributed to neuropathological changes in the amygdala(Mori, Ikeda, Hirono et al., 1999; Kensinger et al., 2002), acommon characteristic of early AD (Braak & Braak, 1991).
Surprisingly little is known about emotional false memory in AD, even though emotional memories are an important aspect of healthy aging (Carstensen, Mikels, & Mather 2006). None of the aforementioned studiesanalyzedmemory errors, and we know only threeemotional memory studies in ADthat have directly analyzed false recognition effects. LaBar, Torpey, Cook, et al. (2005) had subjects study neutral and negative emotional pictures from the International Affective Pictures Set (IAPS; Lang, Bradley, & Cuthbert, 2005), and then take an implicit memory test followed by an explicit recognition memory test (re-presenting pictures on the explicit test). They found greater false recognition ofnegative over neutral pictureson the explicit test in both AD patients and healthy older adult controls. Budson, Todman, Chong et al. (2006) used an emotional variant of the Deese-Roediger-McDermott (DRM) false memory task (Roediger & McDermott, 1995), comparing memory for lists of semantically related neutral or negative items. They did not find a significant effect ofemotion on false recognitionin AD patients or in healthy older adults controls, and there was a trend for more liberal bias estimates to emotional items only in control subjects.Brueckner and Moritz (2009) also usedan emotional variant of the DRM task, albeit with different lists of emotional words. In contrast to Budson et al., AD patients and healthy older adult controls tended to falsely recognize more emotional compared to neutral items in this study, and especially in AD patients,these effects were larger for positive than for negative items.
It is unclear why these three prior studies of emotional false recognition in AD have obtained different results, as there were numerous methodological differences (e.g., different types of materials). The most importantmethodological detail for present purposes, though, was that each of these studies used standard old/new recognition memory tests. As a result, correct recognition of studied itemscould have been driven by vague feelings of familiarity towards the test item or by specific recollections of prior presentation (Yonelinas, 2002). Similarly, false recognition of nonstudied itemsmay have reflectedfamiliarity-based guessing.This possibility is especially likely for AD patients in the DRM studies, which used multiple lists of semantically related words to elicit false recognition. Under these conditions it has been argued that AD patients are especially prone to responding on the basis of gist or similarities, producing many familiarity-based errors (Gallo, Shahid, Olson, Solomon, Schacter & Budson, 2006). This is less of a concern forthe task used by LaBar et al. (2005), which involved complex IAPS pictures that were relatively unrelated. However,in thattaskthe studied pictures were re-presented at test, so that subjects could have relied on feelings of familiarity or perceptual fluency towards studied pictures at test,as opposed to specific recollections. This possibility may have resulted in similar performance between AD patients and controls, becauseAD patients can perform relatively well on memory tests that are sensitive to the perceptual fluency or familiarity of complex visual stimuli (cf. Westerberg, Paller, Weintraub et al., 2006).
In the current study we were specifically interested in the effects of emotion on false recollection. We therefore attempted to avoid the interpretative issues with prior studies by using IAPS pictures and a cued recollection test. We also took steps to minimize the usefulness of familiarity-based responding at test, as described next.
Current Experiment
The present study was designed toinvestigate the effects ofemotion on false recollection judgments in AD. Pictureswere paired with a descriptive label at study, and these labels were used to cue picture recollections at test. Importantly, some test labels had been studied with pictures (studied targets), whereas others had been studiedwithout pictures (studied lures). Thus, both types of labels should have elicited a sense of familiarity at test, but subjects were explicitly instructed to accept labels only if theycued a picture recollection.The ability to discriminate between studied targets and studied lures would be maximized if participants relied on picture recollections to make their test decisions, as opposed to more vague feelings of familiarity elicited by the test label. We also included test labels that were not presented at study (nonstudied lures), in order to measure the baserate for incorrectly accepting lures that should not elicit strong recollection or familiarity.
Using this task, Gallo, Foster, and Johnson (2009) found that both younger and healthy older adults were more likely to incorrectly accept studied lures compared to nonstudied lures. Moreover, many of the incorrectly accepted studied lures were accompanied with relatively high levels of confidence. Because this task emphasized recollection-based responding, it was assumed that these high-confidence errors were at least partly based on false recollection. Critically, these errors were greater forpositive and negativelurescompared to neutral lures, suggesting elevated false recollection for emotional pictures in both age groups.
In the current study we used this task to testthe effects of emotion on false recollection judgments in AD. Because AD patients tend to be more impaired in recollection than familiarity (e.g., Ally, Gold, & Budson, 2009), we simplified the task to help AD patients discriminate between studied targets and studied lures. The task was divided into three separate study/test blocks, corresponding to emotionally positive, negative, and neutral materials. We tested a group of patients in the early stages of AD as well as two control groups of cognitive healthy older adults. One control group was tested under identical conditions as the AD patients, in order to compare the effects of AD on task performance. Because the task was relatively easy for this control group, we also tested a separate control group under conditions of divided attention during study. This manipulationincreased the overall level of memory errors in control subjects, thereby providing an additional opportunity to observe the effects of emotion on false recollection judgments.
Method
Participants
The participants included18 AD patientsfrom the Rush Alzheimer’s Disease Center (RADC, mean age = 77.3 years; SD = 7.5; mean education = 14.2 years, SD = 2.6; 11 females) and 18 healthy controls approximately matched on age, sex, and education (mean age = 72.8 years; SD = 7.8; mean education = 15.3 years, SD = 2.1, 12 females; all p’s > .05). We also tested 18 healthy controls in an unmatcheddivided attention condition (mean age = 79.2 years; SD = 7.7; mean education = 16.2 years, SD = 3.3, 11 females). These participants were similar in age to the AD patients, but had more years of education (p < .05). Patients were clinically diagnosed with probable ADbased on NINCDS-ADRDA criteria (McKhann, Drachman, Folstein et al., 1984), following procedures reported by Bennett, Schneider, Aggarwal et al. (2006). Scores on the MiniMental State Examination (Folstein, Folstein, & McHugh, 1975) were significantly lower in the AD patients (mean = 23.9, SD = 1.5, range = 22–27) compared to the matched controls (29.3, SD = 1.2, p < .001) and divided attention controls (28.8, SD = 1.0, p < .001), whereas the two control groups did not differ. (One control participant did not complete the MMSE.) Exclusion criteria includedcomorbid neurodegenerative disease,stroke, severe head trauma, cerebrovascular disease, alcohol or drug abuse, untreated depression, poor or uncorrected vision, or if English was not a primary language. Prior to the study phase, the AD patients and full attention controls rated their current mood as relatively positive (both means = 5.3 on a 1–7 scale). All participants (and caregivers, where appropriate) gave written informed consent and were paid, andreview boards at the University of Chicago and the RADC approved the study.
Materials
Stimuli were 144 pictures from the IAPS. Based on norms (1–9 scale), 48 were neutral (range 4.18–5.99), 48 were negative (range 1.91–3.91), and 48 were positive (range 6.18–8.34). Negative and positive pictures were equally arousing (means = 5.62 and 5.58), and were more arousing than neutral pictures (3.31). Picturesof nudity and mutilation were excluded. Each picture was given a unique two to three word label (e.g., tourist with bookfor neutral images; toxic waste cleanupfor negative images; astronaut in space for positive images). Although a variety of content was portrayed in each category (e.g., people, activities, objects), the emotional items were judged as more conceptually related than the neutral itemsin an independent rating task with older adults (see Gallo et al., 2009).
Procedure
All stimuli were presented via computer, and the experimenter entered responses. Thetask was divided into three study/test blocks, one block per emotional category (order counterbalanced). Within a block, the test phase immediately followed the study phase.
Each study phasecontained16labels with their picture and16labels without their picture (randomly intermixed). In the full attention condition, item presentation was self-paced. On each trial, subjects were presented with a label, read it aloud, and rated it for emotional arousal (1 [low], 2 [medium], 3 [high]). They then were presented with the corresponding picture (if any) and rated it for arousal. In the divided attention condition,items were presentedevery second. Rather than making arousal judgments, subjects repeatedrandom digits spokenevery two seconds (not locked to stimulus onset).Errors on the digit task were rare (mean = 3.4 per block).
Each test phase usedthe labels as retrieval cues. Each test contained 16 labels that had been studied with their picture (targets), 16 labelsthat had been studied without their picture (studied lures), and 16 labels that were not studied (nonstudied lures).Items were counterbalanced across these conditions.Participantswere instructed to respond “yes” to test labels only if they recollected a corresponding picture, and “no” to labels that did not elicit such recollections, followed by a confidence judgment (1 [low], 2 [medium], 3 [high]).Test items were randomized and self-paced.
Results and Discussion
Unless noted otherwise, results were significant at the conventional p< .05 (two-tailed), and effect sizes usedηp 2(F-tests) or Cohen’s d (t-tests).
Study Ratings
Analysis of arousal ratings revealed an effect of item, F (1, 34) = 13.31, MSE = .019, ηp 2 = .28, as pictures were more arousing than their labels (2.14 and 2.07), and an effect of emotion, F (2, 68) = 49.14, MSE = .194, ηp 2 = .59, as negative items (2.40) were more arousing than positive (2.21), and each was more arousing than neutral (1.70, all p’s < .05). That older adults rated the negative images as more arousing than the positive images is inconsistent with the IAPS norms, which were based on younger adults, but this valence effect replicates the older adult ratings from Gallo et al. (2009) and was not associated with any systematic effects in false recollection judgments in that study.More importantly, there was no effect of group and no interactions in the current study, indicating that AD patients had normal reactions to the stimuli.
Test Performance
Collapsingacross the emotional conditions, the overall patterns of recollection judgments were very consistent (Table 1).Studied targets were endorsed more than studied lures in each group (all p’s < .001), demonstrating accurate recollection judgments even in AD patients, but studied lures were endorsed more than nonstudied luresin each group (all p’s < .05), demonstrating memory confusions. As expected, AD patients endorsed fewer targets and more lures (studied and nonstudied) compared to the other two groups, and similarly fordivided compared to full attentionin controls (all p’s < .05).
Table 1.
Mean Recollection Judgments on Each Test for Each of the Three Groups
| Older Adults | Older Adults | AD Patients | |
|---|---|---|---|
| Full Attention | Divided Attention | Full Attention | |
| Neutral Picture Test | |||
| Neutral Targets | .88 (.03) | .74 (.04) | .64 (.06) |
| Studied Lures | .07 (.04) | .24 (.04) | .49 (.08) |
| Nonstudied Lures | .01 (.00) | .15 (.03) | .34 (.07) |
| Positive Picture Test | |||
| Positive Targets | .85 (.03) | .82 (.03) | .67 (.05) |
| Studied Lures | .12 (.02) | .37 (.05) | .57 (.07) |
| Nonstudied Lures | .03 (.01) | .23 (.04) | .44 (.07) |
| Negative Picture Test | |||
| Negative Targets | .89 (.03) | .69 (.04) | .58 (.06) |
| Studied Lures | .09 (.03) | .28 (.06) | .42 (.07) |
| Nonstudied Lures | .01 (.00) | .18 (.05) | .33 (.06) |
Note. Standard errors are in parenthesis.
To assessemotional memory effectswe analyzed each item type separately. For studied lures there was an effect of group, F (2, 51) = 16.11, MSE = .134, ηp 2 = .39, and emotion, F (2, 102) = 10.73, MSE = .014, ηp 2 = .17, with no interaction. The effect of emotion indicates that positive lures were endorsed more than neutral lures or negative lures (both p’s < .001), with no difference between the latter two. Similar results were obtained for nonstudied lures, with an effect of group, F (2, 51) = 18.64, MSE = .019, ηp 2 = .42, and emotion, F (2, 102) = 8.68, MSE = .010, ηp 2 = .15, and no interaction. Positive lures were endorsed more than neutral lures or negative lures (both p’s < .001), with no difference between the latter two. Analysis of studied targets revealed an effect of group and emotion, and an interaction between the two, F (4, 102) = 2.66, MSE = .013, ηp 2 = .09. Positive targets were endorsed more than neutral or negative targets in divided attention controls, and more than negative targets in AD patients (all p’s < .05), with no other significant differences.
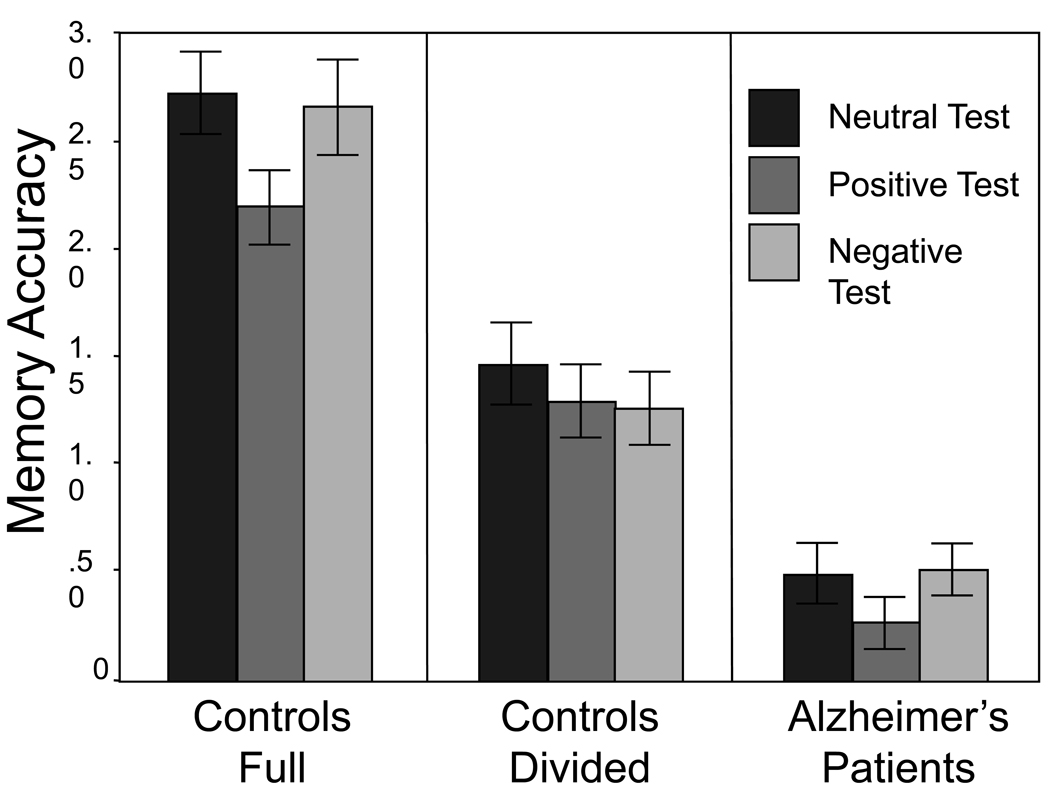
We also computed signal detection estimates of accuracy (d'), using the correction described in Snodgrass and Corwin (1988) to avoid ceiling and floor effects in the raw data. Our primary interest was in the ability to discriminate between studied targets and studied lures (Figure 1). Each of these labels was presented during the study phase, but only the targets had been associated with a picture. We therefore assume that this discrimination was primarily driven by recollection of the picture for studied targets, although picture presentation also may have increased familiarity.
Figure 1.
Mean accuracy (d')and standard errors, comparing studied targets and studied lures.
Consistent with prior work on recollection-based responding (Yonelinas, 2002), discrimination(d’) was considerably reduced by both divided attention and by AD in each emotional condition (all p’s < .001). Analysis ofpositive and neutral items confirmed an effect of emotion, F (1, 51) = 11.57, MSE = .217, ηp 2 = .19, and group, F (2, 51) = 48.65, MSE = .811, ηp 2 = .66, and no interaction. Overall discrimination was lower for positive (1.25) than neutral items (1.56). Similarly, discrimination was lower for positive (1.25) than negative items (1.47), F (1, 51) = 6.62, MSE = .199, ηp 2 = .12, with an effect of group, F (2, 51) = 45.55, MSE = .838, ηp 2 = .64. There also was a marginal interaction, F (2, 51) = 2.83, MSE = .199, ηp 2 = .10, primarily driven by the lack of a difference between positive and negative items in the divided attention condition (see Figure 1). Analysis of discrimination for neutral (1.56) and negative (1.47) items revealed only the effect of group, F (2, 51) = 46.65, MSE = .953, ηp 2 = .65.Discriminationbetween studied targets and nonstudied lures also was computed, and the resulting analyses were mostly similar to those for studied lures. In addition to the effects of group (all p’s < .001), discrimination was lower for positive (1.70) than neutral items (1.89), F (1, 51) = 4.37, MSE = .233, ηp 2 = .08, with no difference between positive and negative items (1.82) or between neutral and negative items, and no interactions.
Bias estimates (C) also were calculatedusing studied targets and studied lures, given that prior memory studies have found elevated bias estimates for emotional items (e.g., Dougal & Rotello, 2007). Analysis of neutral and positive items revealed only an effect of emotion, F (1, 51) = 15.42, MSE = .099, ηp 2 = .23, as estimates were more liberal to positive (−.19) than neutral items (.04). Estimates also were more liberal to positive (−19) than negative items (.08), F (1, 51) = 24.83, MSE = .08, ηp 2 = .33, along with a group interaction, F (2, 51) = 4.75, MSE = .08, ηp 2 = .16. The interaction was driven by the lack of an effect in full attention controls, with significant effects in the other two groups (both p’s < .01). There were no differences in bias estimates for neutral (.04) and negative (.08) items.Bias estimates calculated with studied targets and nonstudied lures yielded similar results. Bias estimates were more liberal to positive (.03) than neutral items (.21), F (1, 51) = 12.81, MSE = .07, ηp 2 = .20, with no other effects or interactions, and also were more liberal to positive (.03) than negative items (.25), F (1, 51) = 17.71, MSE = .074, ηp 2 = .26, along with a group interaction, F (2, 51) = 4.87, MSE = .074, ηp 2 = .16. The interaction again was driven by the lack of an effect in full attention controls, with significant effects in the other two groups (both p’s < .01). Bias estimates for neutral and negative items did not differ. As we elaborate in the General Discussion,the theoretical implications of these bias estimates are subject to interpretation, but in general these bias effects tracked the effects of emotion observed in false recollection judgments.
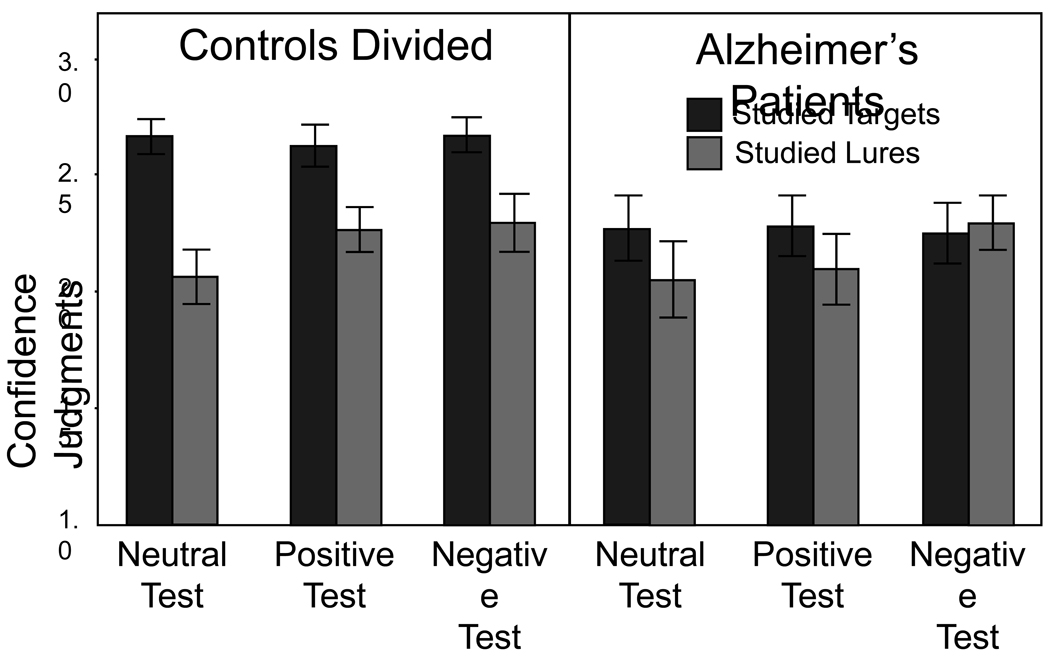
Confidence Judgments
Confidence was analyzed for correct endorsements of targets and false endorsements of studied lures, excluding full attention controls and nonstudied lures owing to limited lure observations (Figure 2). In general subjects were more confident for targets than lures, but this difference was reduced in AD patients. Moreover, both groups endorsed studied lures with medium to high levels of confidence (i.e., judgments greater than 2), suggesting some degree of false recollection. Analysis of targets revealed only an effect of group, F (1, 34) = 8.72, MSE = .458, ηp 2 = .204, indicating that AD patients were less confident in their recollection judgments compared to controls. Analysis of studied lures revealed a trend for an effect of emotion, F (2, 52) = 2.44, MSE = .120, ηp 2 = .09, p = .097, which primarily was driven by significantly greater confidence for negative over neutral items, t (27) = 2.24, SEM = .091, d = .37.
Figure 2.
Mean confidence for recollection judgments and standard errors.
General Discussion
AD patients showed a similar emotionaleffect on false recollection judgments as healthy controls, despite considerably reduced memory for studied pictures. Emotional effects on memory for studied informationcan be reduced in AD patients (e.g., Kensinger et al., 2002), potentially due to dysfunction of the amygdala and hippocampus, but the early stages of AD do not appear to disrupt the effect of emotional content on false recollection judgments. These effects may have significant clinical implications because many important life events recalled by older adults are emotional (e.g., Berntsen & Rubin, 2002). To the extent that AD spares emotional processing of these events, but limits the ability to monitor memory for accuracy, AD patients may be especially prone to emotional autobiographical memory distortion (see Budson, Simons, Sullivan, et al., 2004).
The current results are consistent withprior AD studies that reported elevated false recognition of emotional compared to neutral items (Brueckner & Moritz, 2009; LaBar et al., 2005), and they extend these findings to false recollection judgments. Our results also are consistent with the valence effects of Brueckner and Moritz(2009), whofound greater effects of positive over negative words in AD patients.(LaBar et al. only used negative items.) We found valence effects in false recollection judgments for AD patients and healthy controls (positive > negative), and we also found that positive targets were more likely to be correctly recognized than negative targets in divided attention controls and in AD patients. In contrast, Budson et al. (2006) found no emotional effect on false recognition in AD patients, although there was a trend for more liberal bias estimates in controls. Numerous methodological differences may underlie this discrepancy, but it is worth reiterating that we used highly emotional pictures, and even with these stimuli the false memory effects were stronger for positive than for negative items. Thus, the use of relatively less arousing word lists and only negative items may have limited the ability of Budson et al. to detect emotional memory effects in AD patients.
Our finding that positive valence influenced both true and false responses is consistent with the source monitoring framework, which assumes that the same biases and confusions that drive memory errors also can influence responding for studied items (Johnson, 2006). These effects also may be related to a more general preference for positive information with aging (Carstensen et al., 2006). However, such positivity effects are not always found (see Murphy, & Isaacowitz, 2008), and in the current study, negative information enhanced confidence judgments to errors for studied lures. Considered overall, our findingssuggest that both positive and negativepictures can enhance false memory effects (also see Gallo et al., 2009).
Emotion might enhance false recollection judgmentsby increasing conceptual relatednessbetween items.Enhanced relatedness could result from item-specific semantic associations or from the emotional category itself (e.g., happy items, sad items, etc.). Our results are more consistent with the latter, because AD patients showed similar effects of emotion on memory errors as healthy controls. If semantic associations were the critical factor then these effects should have been reduced in AD,analogous to AD effects on association-based false recognition (e.g., Budson et al., 2006; Gallo et al., 2006). In contrast, both groups weresensitive to the emotional category of the items, as indicated by their similar arousal responses at study. Further support for an emotional category effect comes from studies demonstrating liberal bias estimates and enhanced false recognition for emotional items even after equating for item associations or content (e.g., Herbener et al., 2007;Kapucu et al., 2008).
In addition to false recollection judgments, we also found effects of emotion on estimates of response bias. These bias estimates tended to track the emotional effects observed on false recollection judgments, with more liberal bias estimates for emotional items. These effects are consistent with prior results (e.g., Dougal & Rotello, 2007; Gallo et al., 2009; Kapucu et al., 2008), and they extend these findings to AD patients. However, these effects should be interpreted with caution for two reasons. First, because our primary interest was in false recollection judgments, we tested more lures than targets. This factor was held constant across our emotional conditions and therefore is unlikely to have caused the observed effects of emotion. Nevertheless, including more lures may have had an overall effect on bias estimates. Second, bias estimates are based on the hypothetical relationship between a response criterion and the memory distributions for targets and lures. As a result, changes in bias estimates might be cased by the creation of false memories, which could influence the lure distribution instead of the response criterion (see Wixted & Stretch, 2000). Ifemotional content increases false recollections for test lures, then this could increase false recollection judgments and corresponding estimates of bias independent of changes in response criterion. Thus, the observed effects of emotion on bias estimatesare theoretically ambiguous. They may be driven by differences in response criterion orfalse recollection.
Our task emphasized recollection by using verbal retrieval cuesand explicitpicture recollection instructions, and the finding that false recollection judgments were made with relatively high confidence under these conditions suggests that these memory errors were at least partly based on the subjective experience of false recollection. Evidence that our task involved recollection-based responding was that accuracy was considerably reduced by both divided attention at study and by AD, both of which tend to affect recollection more than familiarity (Yonelinas, 2002). This is not to say, though, thatfamiliarity plays no role in the subjective experience of false recollectionor in false recollection judgments. According to the familiarity/corroboration hypothesis(Lampinen, Neuschatz, & Payne, 1999), the familiarity of misleading retrieval cues may motivate subjects tosearch memory for corroborating evidence, causing them toreconstruct afalse recollectionfrom partially recollected features.Emotion may enhance this reconstructive process byincreasing conceptual similarities between studied and nonstudied items, thereby enhancing familiarity as well as the availability of potentially confusable features in memory. AD also may enhance this reconstructive process, by degrading recollection and increasing reliance on familiarity, although these effects may be offset by reduced recollection of the features contributing to reconstruction.To more fully understand the processes that contribute to false recollection, and how Alzheimer’s disease may affect these processes, additional research is needed that uses tasks emphasizing recollection-based responding.
Research Highlights
Emotion increases false recollection judgments
Alzheimer’s disease increases false recollection judgments
Alzheimer’s disease spares emotional false memory effects
Acknowledgments
We are grateful to Celia Berdes, Barbara Eubeler, Raj Shah, and Presbyterian Homes.This work was supported by National Institute of Aging grantsAG30345 and P30AG10161.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrisqueta-Gomez J, Bueno OFA, Oliveira MGM, Bertolucci PHF. Recognition memory for emotional picture in Alzheimer’s patients. Acta Neurologica Scandinavica. 2002;105:51–54. doi: 10.1034/j.1600-0404.2002.00035.x. [DOI] [PubMed] [Google Scholar]
- Ally BA, Gold CA, Budson AE. An evaluation of recollection and familiarity in Alzheimer’s disease and mild cognitive impairment using receiver operating characteristics. Brain and Cognition. 2009;69:504–513. doi: 10.1016/j.bandc.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Aggarwal NT, Arvanitakis Z, Shah RC, Kelly JF, Fox JH, Cochran EJ, Arends D, Treinkman AD, Wilson RS. Decision rules guiding the clinical diagnosis of Alzheimer's disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006;27:169–176. doi: 10.1159/000096129. [DOI] [PubMed] [Google Scholar]
- Berntsen D, Rubin DC. Emotionally charged autobiographical memories across the life span: The recall of happy, sad, traumatic, and involuntary memories. Psychology and Aging. 2002;17:636–652. doi: 10.1037//0882-7974.17.4.636. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathologia. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brainerd CJ, Stein LM, Silveira RA, Rohenkohl G, Reyna VF. How does negative emotion cause false memories? Psychological Science. 2008;19:919–925. doi: 10.1111/j.1467-9280.2008.02177.x. [DOI] [PubMed] [Google Scholar]
- Brueckner K, Moritz S. Emotional valence and semantic relatedness differentially influence false recognition in mild cognitive impairment, Alzheimer’s disease, and healthy elderly. Journal of the International Neuropsychological Society. 2009;15:268–276. doi: 10.1017/S135561770909047X. [DOI] [PubMed] [Google Scholar]
- Budson AE, Simons JS, Sullivan AL, Beier JS, Solomon PR, Scinto LF, Daffner KR, Schacter DL. Memory and emotions for the September 11, 2001, terrorist attacks in patients with Alzheimer’s disease, patients with Mild Cognitive Impairment, and healthy older adults. Neuropsychology. 2004;18:315–327. doi: 10.1037/0894-4105.18.2.315. [DOI] [PubMed] [Google Scholar]
- Budson AE, Todman RW, Chong H, Adams EH, Kensinger EA, Krangel TS, Wright CI. False recognition of emotional word lists in aging and Alzheimer disease. Cognitive and Behavioral Neurology. 2006;19:71–78. doi: 10.1097/01.wnn.0000213905.49525.d0. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Mikels JA, Mather M. Aging and the intersection of cognition, motivation, and emotion. In: Birren J, Schaie KW, editors. Handbook of the psychology of aging. 6th ed. San Diego, CA: Academic Press; 2006. pp. 343–362. [Google Scholar]
- Dougal S, Rotello CM. “Remembering” emotional words is based on response bias, not recollection. Psychonomic Bulletin and Review. 2007;14:423–429. doi: 10.3758/bf03194083. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the mental state of patients for the clinician. JournalofPsychiatricResearch. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gallo DA, Foster KT, Johnson EL. Elevated false recollection of emotional pictures in younger and older adults. Psychology and Aging. 2009;24:981–988. doi: 10.1037/a0017545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo DA, Shahid KR, Olson MA, Solomon TM, Schacter DL, Budson AE. Overdependence on degraded gist memory in Alzheimer’s disease. Neuropsychology. 2006;20:625–632. doi: 10.1037/0894-4105.20.6.625. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Monarch ES, Goldstein FC. Memory enhancement for emotional stimuli is impaired in early Alzheimer's disease. Neuropsychology. 2000;14:82–92. [PubMed] [Google Scholar]
- Herbener ES, Rosen C, Khine T, Sweeney JA. Failure of positive but not negative emotional valence to enhance memory in schizophrenia. Journal of Abnormal Psychology. 2007;115:43–55. doi: 10.1037/0021-843X.116.1.43. [DOI] [PubMed] [Google Scholar]
- Johnson MK. Memory and reality. American Psychologist. 2006;61:760–771. doi: 10.1037/0003-066X.61.8.760. [DOI] [PubMed] [Google Scholar]
- Kazui H, Mori E, Hashimoto M, Hirono N, Imamura T, Tanimukai S, Hanihara T, Cahill L. Impact of emotion on memory: Controlled study of the influence of emotionally charged material on declarative memory in Alzheimer’s Disease. British Journal of Psychiatry. 2000;177:343–347. doi: 10.1192/bjp.177.4.343. [DOI] [PubMed] [Google Scholar]
- Kensinger EA. Emotional memory across the adult lifespan. New York: Psychology Press; 2009. [Google Scholar]
- Kensinger EA, Anderson A, Growdon JH, Corkin S. Effects of Alzheimer’s disease on memory for verbal emotional information. Neuropsychologia. 2004;42:791–800. doi: 10.1016/j.neuropsychologia.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Brierley B, Medford N, Growdon JH, Corkin S. Effects of normal aging and Alzheimer's disease on emotional memory. Emotion. 2002;2:118–134. doi: 10.1037/1528-3542.2.2.118. [DOI] [PubMed] [Google Scholar]
- Kapucu A, Rotello CM, Ready RE, Seidl KN. Response bias in “remembering” emotional stimuli: A new perspective on age differences. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2008;34:703–711. doi: 10.1037/0278-7393.34.3.703. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Torpey DC, Cook CA, Johnson SR, Warren LH, Burke JR, Welsh-Bohmer KA. Emotional enhancement of perceptual priming is preserved in aging and early-stage Alzheimer’s disease. Neuropsychologia. 2005;43:1824–1837. doi: 10.1016/j.neuropsychologia.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Lampinen JM, Neuschatz JS, Payne DG. Source attributions and false memories: A test of the demand characteristics account. Psychonomic Bulletin & Review. 1999;6:130–135. doi: 10.3758/bf03210820. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-6. Gainesville, FL: University of Florida; 2005. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- Mather M. Emotional arousal and memory binding: An object-based framework. Perspectives on Psychological Science. 2007;2:33–52. doi: 10.1111/j.1745-6916.2007.00028.x. [DOI] [PubMed] [Google Scholar]
- Maratos EJ, Allan K, Rugg MD. Recognition memory for emotionally negative and neutral words: An ERP study. Neuropsychologia. 2000;38:1452–1465. doi: 10.1016/s0028-3932(00)00061-0. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mori E, Ikeda M, Hirono N, Kitagaki H, Imamura T, Shimomura T. Amygdalar volume and emotional memory in Alzheimer’s disease. American Journal of Psychiatry. 1999;156:216–222. doi: 10.1176/ajp.156.2.216. [DOI] [PubMed] [Google Scholar]
- Murphy NA, Isaacowitz DM. Preferences for emotional information in older and younger adults: A meta-analysis of memory and attention tasks. Psychology and Aging. 2008;23:263–286. doi: 10.1037/0882-7974.23.2.263. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: Applications to dementia and amnesia. Journal of Experimental Psychology: General. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Westerberg CE, Paller KA, Weintraub S, Mesulam M-M, Holdstock JS, Mayes AR, Reber PJ. When memory does not fail: Familiarity-based recognition in mild cognitive impairment and Alzheimer's disease. Neuropsychology. 2006;20:193–205. doi: 10.1037/0894-4105.20.2.193. [DOI] [PubMed] [Google Scholar]
- Wixted JT, Stretch V. The case against a criterion-shift account of false memory. Psychological Review. 2000;107:368–376. doi: 10.1037/0033-295x.107.2.368. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]