Abstract
Objective
Limited data exist to inform clinicians and patients as to the likelihood of long-term endometrial hyperplasia response to progestin therapy, especially for atypical hyperplasia. We evaluated women with complex and atypical endometrial hyperplasia, comparing those prescribed progestin to those not prescribed progestin.
Methods
This retrospective cohort study was conducted in 1985–2005 among women aged 18–88 years at an integrated health plan in Washington State. Women were ineligible if they achieved an outcome (endometrial carcinoma, hysterectomy, or both) within 8 weeks of hyperplasia diagnosis. Exposure was progestin use for at least 14 days, by duration and recency. Outcomes included rate of: 1) endometrial carcinoma; and/or 2) hysterectomy. Analyses performed included Kaplan Meier, incident rate ratios, and Cox proportional hazard ratios.
Results
One thousand four hundred forty-three eligible women were identified. One thousand two hundred one had complex (n=164 no progestin) and 242 had atypical hyperplasia (n=62 no progestin). During follow-up, median 5.3 years (range 8 weeks to 20.8 years), 71 women were diagnosed with endometrial carcinoma (35 complex, 36 atypia) and 323 underwent hysterectomy (216 complex, 107 atypia). Among women with complex and atypical hyperplasia, rates of endometrial carcinoma among progestin users were 3.6 and 20.5 per 1,000 woman-years, respectively (compared with without progestin, 10.8 and 101.4). Among women with complex and atypical hyperplasia, rates of hysterectomy among progestin users were 23.3 and 61.4 per 1,000 woman-years, respectively (compared with without progestin, 55.1 and 297.3).
Conclusion
Endometrial carcinoma risk is diminished approximately 3- to 5-fold in women diagnosed with complex or atypical endometrial hyperplasia and dispensed progestin; hysterectomy risk is also decreased.
Introduction
Endometrial hyperplasia, a noninvasive proliferation of the endometrial epithelium, is generally classified as simple (non-neoplastic) or complex (sometimes neoplastic), with or without atypia (neoplastic), based on architectural complexity and nuclear cytology and is a precursor to endometrial carcinoma. (1) Endometrial hyperplasia with atypia is the least common type of hyperplasia, but is the type most likely to progress to type 1 endometrial carcinoma (1-3) which accounts for 97% of uterine cancers, whereas simple hyperplasia rarely progresses to carcinoma. (1;4) Known risk factors for endometrial hyperplasia are related to an excess of estrogen relative to progesterone; (5;6) therefore progestin is used to treat endometrial hyperplasia.
There are no large population-based studies describing the incidence of progression of endometrial hyperplasia among women treated and not treated with progestin. In 1985, Kurman et al (1) described an increased risk of progression to carcinoma among lesions classified as complex hyperplasia with atypia (23%), in contrast to lesions classified as hyperplasia without atypia (2%), within a mean follow-up of 13.4 years. A recent large study has confirmed the higher risk of progression associated with atypical endometrial hyperplasia. (3) However, these studies combined women treated and not treated with progestin. Data from women who developed endometrial hyperplasia while using postmenopausal hormone therapy have confirmed that progestins uniformly result in regression of endometrial hyperplasia without atypia. (7;8) Others have described regression of atypical endometrial hyperplasia and/or well-differentiated carcinoma with various forms of progestin, although these reports lacked controls. (9-12) Despite this, there are limited data regarding long-term outcomes for women with endometrial hyperplasia treated with progestin therapy. Consequently, endometrial hyperplasia, especially hyperplasia with atypia, is commonly treated with hysterectomy because of fear of progression to endometrial carcinoma and/or concern that unsampled carcinoma may already be present. (13-15)
The objective of this study was to estimate the incidence of endometrial carcinoma and/or hysterectomy associated with complex or atypical endometrial hyperplasia, comparing progestin users to non-users, among women who did not have a hysterectomy and/or a diagnosis of endometrial carcinoma within 8 weeks of endometrial hyperplasia diagnosis.
Materials and Methods
Following institutional review board approval from the Group Health Research Institute, this retrospective cohort study was conducted among female enrollees at Group Health (GH), a mixed-model integrated health plan with over 500,000 enrollees in Washington State. Automated pathology, enrollment, pharmacy, inpatient and outpatient databases were linked for data on all women over age 18, diagnosed with complex and/or atypical endometrial hyperplasia, between January 1, 1985 and April 1, 2005. Women were followed from the time of hyperplasia diagnosis until an outcome occurred (endometrial carcinoma and/or hysterectomy), disenrollment, death, or until September 30, 2005. Eligibility criteria included no prior diagnosis of endometrial carcinoma and an intact uterus. Because the most commonly recommended progestin treatment duration for endometrial hyperplasia is at least 8 weeks,(16-18) women who had outcomes prior to 8 weeks were excluded. In addition, women who took primarily unopposed estrogen for greater than 6 months at any time during the study period or who left GH for over 2 months during the study period were excluded.
Automated databases were linked through a unique identifier assigned to each woman when she first joined GH, and reassigned upon each subsequent enrollment. Disenrollment was ascertained by computerized membership files.
The pathology database includes unique pathology accession numbers, specimen collection dates, and test results, entered as text fields. Text searches indicating possible diagnoses of complex or atypical hyperplasia were conducted to identify women with the conditions of interest. Details of this methodology have been previously described. (19) The primary goal of this study was to answer the question, “in a given population of women with a clinical diagnosis of either complex or atypical endometrial hyperplasia, what is the risk of endometrial carcinoma or hysterectomy occurring at least 8 weeks after the endometrial hyperplasia diagnosis, with and without progestin exposure?”
A diagnosis of endometrial carcinoma was ascertained from linkage with records of the Cancer Surveillance System of Western Washington, a population-based cancer registry that participates in the Surveillance Epidemiology and End Results (SEER) cancer registry. Hysterectomy, including date, was determined during review of the medical record (yes/no) and was confirmed by the presence of a uterine specimen in the pathology record.
The exposure of interest, progestin prescriptions, was ascertained from the GH pharmacy database. GH pharmacy databases capture all medications dispensed to enrollees through GH pharmacies including the specific drug, drug class, date and amount dispensed, and dosing instructions. Surveys among female GH members aged 50-80 years have shown that 97% of HT prescriptions are filled at GH pharmacies. (20)
All progestin dispensings from one week before the index biopsy up until the outcome or censoring date were identified. Women were classified as progestin “ever users” if they were dispensed at least 15 days of progestin and as “never users” if they had ≤ 14 days of progestin dispensed. Duration of exposure (<56 days and ≥ 56 days of progestin dispensed) was evaluated in sub-analyses.
For time-dependent analyses, days of use was calculated from the time of the index biopsy to the outcome or censoring, within 6-month blocks. Women were classified as progestin “ever users” or as “never users” as described above, within each 6-month block. We created progestin and estrogen exposure variables for each time period that reflected exposure in the previous 6 month period.
Progestins were categorized by type - megesterol acetate, medroxyprogesterone acetate, and norethindrone acetate. Women who were given oral contraceptives were included in the progestin user group as all of these formulations are progestin dominant. We classified women as unopposed progestin users (PT) if they were dispensed progestin without an estrogen or if the number of estrogen pills dispensed was less than 1/3 the number of progestin pills dispensed. Women were classified as estrogen plus progestin users (EPT) if the number of estrogen pills dispensed was at least 1/3 of the number of progestin pills dispensed. We classified women as unopposed estrogen users (ET) if they were dispensed estrogen alone or if the number of progestin pills dispensed was less than 1/3 of the estrogen pills dispensed.
Three trained abstractors reviewed archived paper charts and the electronic medical records. Variables ascertained included: medical and family history; demographic, reproductive, and physical characteristics, including height and weight at the time of the index biopsy; bleeding patterns preceding the biopsy; ultrasound findings; age at menopause; race; parity; history of breast, colon, or ovarian cancer; diabetes; hypertension; and smoking status. Indications for hysterectomy and endometrial biopsies were recorded. Last clinical contact date (including date of death) and if deceased, whether death was related to endometrial carcinoma, were assessed.
Analyses were performed separately for complex and atypical hyperplasia. We computed the proportion of women with each type of hyperplasia (complex; atypical) who subsequently had a diagnosis of endometrial carcinoma and/or a hysterectomy. We calculated the adjusted rates of endometrial carcinoma and hysterectomy by computing the number of events by person years at risk for “ever users versus “never users” and by duration of progestin use (< 56 days, ≥ 56 days). Absolute risk differences were calculated. Time to event was estimated using Kaplan Meier survival functions. We computed adjusted Cox proportional hazard ratios with progestin exposure as a time-dependent variable. The time between index and censoring was divided into 6 month periods. Only cohort members who had been followed for one year or more were included in the analyses using time-dependent variables.
We considered and evaluated confounding factors and adjusted for variables that influenced the risk estimates associated with progestin dispensing by more than 10%, specifically, age (<50, ≥50 years) and body mass index (BMI) (<30, ≥ 30 kg/m2). Analyses were performed using STATA 9.2 (STATA Corporation, College Station, Texas). All reported p-values are 2-sided.
Results
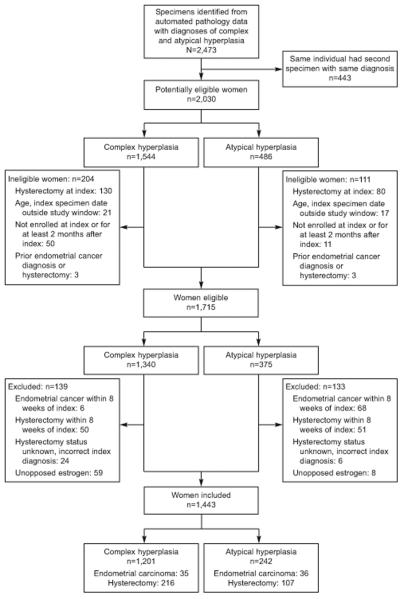
A total of 2030 potentially eligible women, ages 18-88 years, (1544 complex; 486 atypia) were identified from the automated pathology database (Figure 1). Of these, 315 were ineligible (204 complex; 111 atypia) and an additional 272 women (139 complex; 133 atypia) were excluded; 74 had a diagnosis of endometrial carcinoma within 8 weeks of index diagnosis (6 complex, 68 atypia) and 67 took unopposed estrogen during the study period (59 complex, 8 atypia). The remaining 1443 women (1201 complex, 242 atypia) were followed for a median of 5.3 years (range 8 weeks - 20.8 years).
Figure 1.
Study design – ineligibility and exclusions. Numbers of women ineligible and excluded are mutually exclusive and were determined in the order shown. “Unopposed estrogen” use was defined as at least 6 months of unopposed estrogen or combined estrogen and progestin use where progestin was used less than 1/3 of the time. Women using unopposed estrogen prior to index date were not excluded.
Cohort characteristics (Table 1) did not differ by progestin exposure (ever versus never) with the exception that those women dispensed progestin were more likely to have been dispensed ET or EPT in the 6 months prior to index diagnosis. During follow-up, 42% (45% complex; 25% atypia) were dispensed EPT for at least one 6 month period and 24% (25% complex; 15% atypia) were dispensed EPT during at least 50% of their follow-up period. Among women dispensed EPT or PT, 80% used medroxyprogesterone acetate (>80% of the time), 11.5% used megesterol acetate (> 80% of the time), and 8.5% used other progestins or combinations of progestins such that there was no predominant type used.
Table 1.
Baseline characteristics of 1443 women with complex hyperplasia with and without atypia by progestin exposure during the study
| Complex N=1201 | Atypia N=242 | |||
|---|---|---|---|---|
| No Progestin N=164 |
Progestin > 14d N=1037 |
No Progestin N=62 |
Progestin > 14d N=180 |
|
| Age (years) | ||||
| <39 | 17(10.4) | 79(7.6) | 4(6.5) | 13(7.2) |
| 40-49 | 50(30.5) | 314(30.3) | 16(25.8) | 35(19.4) |
| 50-59 | 59(36.0) | 415(40.0) | 16(25.8) | 64(35.6) |
| 60-69 | 23(14.0) | 145(14.0) | 15(24.2) | 43(23.9) |
| ≥70 | 15(9.1) | 84(8.1) | 11(17.7) | 25(13.9) |
| Caucasian1 | 128(82.1) | 907(89.3) | 55(90.2) | 156(90.2) |
| Diabetes1 | 13(8.3) | 82(8.1) | 6(9.8) | 21(12.1) |
|
Breast/Colon Cancer1 |
15(9.1) | 23(2.2) | 3(4.8) | 13(7.2) |
| Current Smoker1 | 12(8.3) | 117(12.1) | 9(15.5) | 21(12.6) |
| BMI (kg/m2)1 | ||||
| <25 | 42(27.5) | 329(33.1) | 19(31.2) | 56(32.2) |
| 25 – 29.9 | 32(20.9) | 260(26.2) | 14(23.0) | 39(22.4) |
| ≥30 | 79(51.6) | 404(40.7) | 28(45.8) | 79(45.4) |
| Nulliparous1 | 32(20.5) | 158(15.6) | 13(21.3) | 32(18.5) |
| Oral contraceptive2 | 4(2.4) | 13(1.3) | 1(1.6) | 1(0.6) |
|
Estrogen + Progestin2,3 |
0(0) | 165(15.9) | 4(6.5) | 24(13.3) |
|
Unopposed Estrogen2,4 |
6(3.7) | 143(13.8) | 7(11.3) | 28(15.6) |
| Progestin only2 | 0(0) | 11(1.1) | 0(0) | 4(2.2) |
| Index biopsy year | ||||
| 1985 – 1989 | 32(19.5) | 233(22.5) | 19(30.7) | 56(31.1) |
| 1990 – 1994 | 37(22.6) | 364(35.1) | 14(22.6) | 52(28.9) |
| 1995 – 1999 | 46(28.0) | 291(28.0) | 19(30.7) | 42(23.3) |
| 2000 – 2005 | 49(29.9) | 149(14.4) | 10(16.1) | 30(16.7) |
Missing data. d = days. BMI = body mass index.
Dispensed for at least 2 months in the 6 months preceding diagnosis of endometrial hyperplasia.
Estrogen + progestin = postmenopausal hormone therapy (the progestin was dispensed for at least 1/3 of the time that the estrogen was dispensed).
Unopposed Estrogen = postmenopausal hormone therapy (estrogen alone or estrogen plus progestin where progestin was dispensed less than 1/3 of the time that estrogen was dispensed).
Among women with complex and atypical hyperplasia, the rate of hysterectomy in progestin users was less than that in non-users; 23.3 and 61.4 per 1,000 woman-years, respectively (vs. without progestin, 55.1 and 297.3) (Table 2). Adjusted relative rates associated with the use of a progestin were aIRR = 0.47 (95%CI 0.33-0.67) for complex and aIRR =0.23 (95% CI 0.16-0.34) for atypical hyperplasia. Absolute rate differences between progestin users and non-users were 7.2 and 80.9 per 1,000 women years for women with complex and atypical hyperplasia, respectively. Adjusted relative rates associated with the use of a progestin were aIRR = 0.35 (95% CI 0.16-0.78) for complex and aIRR = 0.23 (95% CI 0.12-0.44) for atypical hyperplasia.
Table 2.
Incidence rate ratios for endometrial cancer and hysterectomy among women diagnosed with complex and atypical hyperplasia
| CARCINOMA | HYSTERECTOMY | ||||||
|---|---|---|---|---|---|---|---|
| Progestin Exposure |
Person years |
Cases | Rate1 | aIRR (95%CI)2 | Cases | Rate1 | aIRR (95%CI)2 |
| COMPLEX | |||||||
| None | 744 | 8 | 10.75 | 1.0 | 41 | 55.11 | 1.0 |
| P >14 d | 7524 | 27 | 3.59 | 0.35(.16-.78) | 175 | 23.26 | 0.47 (.33-.67) |
| P 15-56d | 530 | 3 | 5.66 | 0.39 (.08-2.02) | 32 | 60.38 | 1.13 (.70-1.82) |
| P >56 d | 6994 | 24 | 3.43 | 0.35 (.16-.77) | 143 | 20.45 | 0.42(.29-.60) |
| Total | 8268 | 35 | 4.23 | 216 | 26.12 | ||
| ATYPIA | |||||||
| None | 148 | 15 | 101.35 | 1.0 | 44 | 297.30 | 1.0 |
| P >14 d | 1026 | 21 | 20.47 | 0.23 (.12-.44) | 63 | 61.40 | 0.23 (.16-.34) |
| P 15-56d | 68 | 9 | 132.35 | 1.61(.71-3.64) | 18 | 264.71 | 0.98 (.58-1.66) |
| P >56 d | 958 | 12 | 12.53 | 0.15(.07-.31) | 45 | 46.97 | 0.19 (.12-.28) |
| Total | 1174 | 36 | 30.66 | 107 | 91.14 | ||
Per 1000 person years
Adjusted for age (<50, 50+ years) and body mass index (<30, 30+ kilograms per meter squared)
aIRR = adjusted Incidence Rate Ratio
CI = Confidence Interval
P = Progestin
d = days
Among women with complex and atypical hyperplasia, the rate of hysterectomy in progestin users was less than that in non-users; aIRR = 0.47 (95%CI 0.33-0.67) and aIRR =0.23 (95% CI 0.16-0.34), respectively.
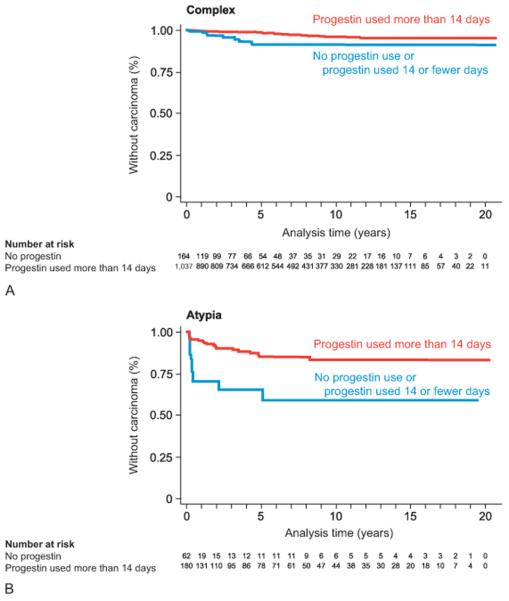
There were 1159 women remaining in the cohort after 1 year (Figure 2). There were too few women with atypical hyperplasia (n=150) to assess the impact of duration or recency of progestin use on risk for endometrial carcinoma or hysterectomy among women who remained in the cohort at 1 year. However, among the 1009 women with complex hyperplasia who remained in the cohort at 1 year, the risk of endometrial carcinoma was decreased among women who used progestin ≥ 56 days, RR = 0.29 (95% CI 0.12-0.68) [data not shown], and among recent users, HR = 0.42 (95% CI 0.18-1.01) [data not shown]. The incidence of hysterectomy was not decreased among women with complex hyperplasia who used progestin who remained in the cohort at 1 year, either for ≥ 56 days, RR = 0.66 (95% CI 0.37-1.14) or among recent users, HR = 1.08 (95% CI 0.74-1.58) [data not shown].
Figure 2.
Time from index diagnosis of complex (A) and atypical (B) hyperplasia to endometrial carcinoma diagnosis, by progestin exposure. Women who were diagnosed with endometrial carcinoma within 8 weeks of index endometrial hyperplasia diagnosis were excluded (6 complex, 68 atypia).
A total of 71 women were diagnosed with endometrial carcinoma (35 complex, 36 atypia) during follow-up (Figure 2, Table 3) and 30 of these were diagnosed in the first year (8 complex, 22 atypia). Median interval between index biopsy and carcinoma diagnosis was 1.3 years (range 8 weeks – 11.6 years). Of the women who developed carcinoma, 49 (67.1%) had at least 14 days of progestin treatment. Median time to diagnosis of endometrial carcinoma among women diagnosed 1 year after index diagnosis of endometrial hyperplasia was longer for women with complex (5.1, range 1.1 – 11.6 years) than for women with atypical hyperplasia (2.5, range 1.01 – 7.9 years).
Table 3.
Incidence of endometrial carcinoma (stage, grade and type), by time since diagnosis and type of hyperplasia
| Outcome >8 weeks < 1 yr n=1443 |
Outcome ≥ 1 yr n=1159 |
|||
|---|---|---|---|---|
| Complex N=1201 |
Atypia N=242 |
Complex N=1009 |
Atypia N= 150 |
|
| Type 1 (endometrioid) | ||||
| St 1Gr 1 | 3 | 8 | 5 | 2 |
| St 1 Gr 2 | 2 | 6 | 10 † † | 7 |
| St 1 Gr 3 | 1 | 1 | 2 | 4 |
| St 1 unk Gr | 1 | 4 | 4 | 0 |
| St 2 Gr 1 | 0 | 1 | 0 | 0 |
| St 2 Gr 2 | 0 | 0 | 2 | 0 |
| St 2 Gr 3 | 0 | 0 | 2 † | 0 |
| St unk Gr2 | 0 | 0 | 2 | 0 |
| Type 2 (papillary serous) | ||||
| St 1 | 1 | 2 | 0 | 0 |
| St 4 | 0 | 0 | 0 | 1 † |
|
Total Endometrial carcinomas |
8 | 22 | 27 | 14 |
6 women with an index diagnosis of complex and 68 women with an index diagnosis of atypical hyperplasia with subsequent diagnosis of endometrial carcinoma between index date and 8 weeks were excluded from the analyses.
Death from endometrial carcinoma.
There were 131 deaths during follow up including 11 in women with endometrial carcinoma. Of these 11 women, 4 died with documented complications related to endometrial carcinoma; 2 had stage 1 grade 2 endometrial carcinoma, one of whom was considered too high risk for surgery due to multiple comorbidities; 2 had either a type 2 endometrial carcinoma (serous carcinoma) or a poorly differentiated adenocarcinoma (both with normal BMI). Of the 4 women who died from their disease, 2 had a family history of breast or endometrial carcinoma.
Discussion
In this cohort study, among women who did not have a diagnosis of carcinoma and/or hysterectomy within 8 weeks of hyperplasia diagnosis, 2.9% of women with complex hyperplasia and 14.9% of women with atypical hyperplasia were subsequently diagnosed with endometrial carcinoma during a median follow-up of 5.5 years. Of the 71 women who developed endometrial carcinoma, 30 were diagnosed between 8 weeks and one year after the endometrial hyperplasia diagnosis, suggesting they may have had concomitant carcinoma at the time of index biopsy; the majority of these cases (73.3%) had atypical endometrial hyperplasia at index. Whereas, among the remaining endometrial carcinomas diagnosed at least one year after index, the majority (65.9%) had complex hyperplasia at index. Any use of progestin decreased the risk of endometrial carcinoma by approximately 65% and 77% in women with complex or atypical hyperplasia, respectively. Four women (0.3%) died from endometrial carcinoma.
Although endometrial carcinoma is undoubtedly the most important outcome, the rates of hysterectomy in our study were considerable and thus have significant societal and economic impact. Others estimate that hysterectomy is performed in 75-80% of women with atypical hyperplasia. (21) Progestin therapy decreased the risk of hysterectomy in our study by 53% and 77% in women with complex and atypical hyperplasia, respectively. It should be noted that among those women excluded from this study, a larger proportion of women with atypia had hysterectomy within 8 weeks of index hyperplasia diagnosis (119/376, 31.7%) than women with complex hyperplasia (56/1340, 4.2%).
For women with complex hyperplasia, a low risk of progression to endometrial carcinoma supports current clinical standards for non-surgical treatment. (17) Findings from earlier studies using the current WHO classification scheme for endometrial hyperplasia (4) also support this management strategy for complex hyperplasia. (1;7;22-25) However, only one of these studies compared women with complex hyperplasia treated with progestin to those untreated, and women were followed for only a median of 4.8 months. (22) Among 208 women with complex hyperplasia treated with progestin for 3-5 months, 2 (1%) were diagnosed with endometrial carcinoma, whereas 6 (3.3%) out of 182 not treated with progestin developed endometrial carcinoma.
More controversial is whether clinicians should use non–surgical approaches to treat women with atypical endometrial hyperplasia. Currently, in the United States, hysterectomy is commonly recommended for atypical hyperplasia rather than a trial of hormonal therapy, due to concern for development of carcinoma or concurrent carcinoma (13;16) although many clinicians do treat and follow women who desire fertility conservation. There are no other studies with substantial numbers of women with atypical hyperplasia treated with progestin versus untreated to comment on endometrial cancer risk. Our data suggest that among women who did not have a hysterectomy and/or a diagnosis of endometrial carcinoma within 8 weeks of their atypical hyperplasia diagnosis, the risk of endometrial carcinoma in women treated with progestin was 4-5 fold lower than in women not treated with progestin. The majority of these women were diagnosed with carcinoma in the year following their atypical hyperplasia diagnosis and none of those women died from complications related to their disease. Therefore, our data suggest that with close follow-up, progestin therapy may be safely used to treat atypical endometrial hyperplasia in select patients.
Of concern to patients and clinicians is a concomitant endometrial carcinoma at the time of diagnosis of atypical endometrial hyperplasia. Of women who had a hysterectomy performed within 12 weeks of atypical endometrial hyperplasia diagnosis with no intervening therapy, up to 46% had concomitant endometrial carcinoma. (14;21;26-32) A second related concern is the reliability of the pathologist's diagnosis of atypical hyperplasia versus well-differentiated carcinoma. (3;14;15;33-36) In studies by Kurman (1) and others, (3) the presence of cytologic atypia has been associated with the highest risk of developing carcinoma; hence the current WHO terminology divides endometrial hyperplasia into typical (simple and complex hyperplasia) and atypical hyperplasia. (4) Regrettably, others have shown that the diagnosis of atypical hyperplasia is one of the least reproducible in the current WHO scheme. (14;15;33-36)
More important than diagnostic accuracy may be the ability to predict therapeutic response to progestin therapy. Multiple studies have assessed progestin treatment of atypical hyperplasia and well-differentiated endometrial carcinoma. (9-12) A literature review of women diagnosed with endometrial carcinoma showed an overall histologic response of 76% in 81 patients at a median time of 12 weeks; 15 of the women who had an initial response (24%) recurred at a median of 19 months. No patients died of their disease. (10)
There are limitations to our study. We were unable to control for unmeasured factors related to whether a patient and her physician opted for progestin therapy and those related to the incidence of carcinoma or hysterectomy. In addition, the study was designed to only include those women who did not undergo hysterectomy or have a diagnosis of endometrial carcinoma within 8 weeks of their endometrial hyperplasia diagnosis. The challenges with standardization of diagnostics in endometrial tissues are well established. (33) We could not control for the method of endometrial sampling. The number of women included in our study limited our ability to fully assess the possible impact of progestin duration, dose and type on the likelihood of progression to endometrial carcinoma, particularly for women with atypical hyperplasia. Finally, we were unable to assess compliance and a central pathology review was not utilized for these analyses.
Several study strengths bear mentioning. Few studies have compared risks of endometrial carcinoma among women treated and untreated with progestin and the number of women in our cohort is much greater than has been previously studied. The pharmacy data has been shown to be reliable at our institution (20) and the methods used for case and outcome identification were rigorous. (19) We had extensive data on multiple potential covariates and controlled for age, BMI, ET use during the study, and prior HT exposure.
In summary, the decision whether or not to attempt hormonal therapy with progestins or to proceed immediately to hysterectomy is influenced by the perceived risk of progression to invasive carcinoma that each histology-based diagnosis carries. Our work would suggest that among women with a diagnosis of complex or atypical hyperplasia who do not choose immediate hysterectomy, a 3-month trial of progestin with strict surveillance for recurrence is relatively safe with regard to risk of endometrial carcinoma. This strategy does not completely negate endometrial carcinoma risk. Whether women with endometrial hyperplasia need continued ongoing progestin therapy for a number of years remains unanswered.
PRECIS.
Endometrial carcinoma risk is diminished 3- to 5-fold in women diagnosed with complex or atypical endometrial hyperplasia and prescribed progestin; hysterectomy risk is also decreased.
Acknowledgments
The authors appreciate the assistance of Mr. Kevin Beverly and Dr. Walter Clinton with data management, Ms. Kay Hager and Ms. Kathy Plant with data collection, and Ms. Anne Oswald and Ms. Carolyn Howard with administrative assistance.
Study supported by peer-reviewed funding from the Eunice Kennedy Shriver National Institute of Child Health and Development (NICHD) Grant number 5 R01 HD44813-02.
Footnotes
Financial Disclosure: The authors did not disclose any potential conflicts of interest.
Presented as a poster at the Annual American Association for Cancer Research (AACR), April 18-22, 2009, in Denver, CO.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia. A long-term study of “untreated” hyperplasia in 170 patients. Cancer. 1985;56:403–12. doi: 10.1002/1097-0142(19850715)56:2<403::aid-cncr2820560233>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 2.Ferenczy A, Gelfand M. The biologic significance of cytologic atypia in progestogen-treated endometrial hyperplasia. Am J Obstet Gynecol. 1989;160:126–31. doi: 10.1016/0002-9378(89)90103-8. [DOI] [PubMed] [Google Scholar]
- 3.Lacey JV, Jr., Ioffe OB, Ronnett BM, Rush BB, Richesson DA, Chatterjee N, et al. Endometrial carcinoma risk among women diagnosed with endometrial hyperplasia: the 34-year experience in a large health plan. Br J Cancer. 2008;98:45–53. doi: 10.1038/sj.bjc.6604102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tavassoli FA, Devilee P. Pathology and Genetics: Tumours of the Breast and Female Genital Organs. IARC Press; Lyon, France: 2003. [Google Scholar]
- 5.Epplein M, Reed SD, Voigt LF, Newton KM, Holt VL, Weiss NS. Risk of complex and atypical endometrial hyperplasia in relation to anthropometric measures and reproductive history. Am J Epidemiol. 2008;168:563–70. doi: 10.1093/aje/kwn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epplein M, Reed SD, Voigt LF, Newton KM, Holt VL, Weiss NS. Endometrial hyperplasia risk in relation to recent use of oral contraceptives and hormone therapy. Ann Epidemiol. 2009;19:1–7. doi: 10.1016/j.annepidem.2008.08.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Writing Group for the PEPI Trial Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI Trial. JAMA. 1996;275:370–5. doi: 10.1001/jama.1996.03530290040035. [DOI] [PubMed] [Google Scholar]
- 8.Speroff L, Whitcomb RW, Kempfert NJ, Boyd RA, Paulissen JB, Rowan JP. Efficacy and local tolerance of a low-dose, 7-day matrix estradiol transdermal system in the treatment of menopausal vasomotor symptoms. Obstet Gynecol. 1996;88:587–92. doi: 10.1016/0029-7844(96)00272-4. [DOI] [PubMed] [Google Scholar]
- 9.Orbo A, Arnes M, Hancke C, Vereide AB, Pettersen I, Larsen K. Treatment results of endometrial hyperplasia after prospective D-score classification: a follow-up study comparing effect of LNG-IUD and oral progestins versus observation only. Gynecol Oncol. 2008;111:68–73. doi: 10.1016/j.ygyno.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Ramirez PT, Frumovitz M, Bodurka DC, Sun CC, Levenback C. Hormonal therapy for the management of grade 1 endometrial adenocarcinoma: a literature review. Gynecol Oncol. 2004;95:133–8. doi: 10.1016/j.ygyno.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 11.Randall TC, Kurman RJ. Progestin treatment of atypical hyperplasia and well-differentiated carcinoma of the endometrium in women under age 40. Obstet Gynecol. 1997;90:434–40. doi: 10.1016/s0029-7844(97)00297-4. [DOI] [PubMed] [Google Scholar]
- 12.Ushijima K, Yahata H, Yoshikawa H, Konishi I, Yasugi T, Saito T, et al. Multicenter phase II study of fertility-sparing treatment with medroxyprogesterone acetate for endometrial carcinoma and atypical hyperplasia in young women. J Clin Oncol. 2007;25:2798–803. doi: 10.1200/JCO.2006.08.8344. [DOI] [PubMed] [Google Scholar]
- 13.Clark TJ, Neelakantan D, Gupta JK. The management of endometrial hyperplasia: an evaluation of current practice. Eur J Obstet Gynecol Reprod Biol. 2006;125:259–64. doi: 10.1016/j.ejogrb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Trimble CL, Kauderer J, Zaino R, Silverberg S, Lim PC, Burke JJ, et al. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group study. Cancer. 2006;106:812–9. doi: 10.1002/cncr.21650. [DOI] [PubMed] [Google Scholar]
- 15.Zaino RJ, Kauderer J, Trimble CL, Silverberg SG, Curtin JP, Lim PC, et al. Reproducibility of the diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group study. Cancer. 2006;106:804–11. doi: 10.1002/cncr.21649. [DOI] [PubMed] [Google Scholar]
- 16.American College of Obstetricians and Gynecologists ACOG practice bulletin, clinical management guidelines for obstetrician-gynecologists, number 65, August 2005: management of endometrial cancer. Obstet Gynecol. 2005;106:413–25. doi: 10.1097/00006250-200508000-00050. [DOI] [PubMed] [Google Scholar]
- 17.Hammond R, Johnson J. Endometrial hyperplasia. Current Obstetrics and Gynaecology. 2004;14:99–103. [Google Scholar]
- 18.Zacur HA, Giuntoli RL, Jurema M. Endometrial Hyperplasia. Up to Date. http://www uptodateonline com/ 2008 [cited 2008 May 12];16.1(Last literature review version 16.1: January 2008)Available from: URL: http://www.uptodateonline.com/
- 19.Reed SD, Newton KM, Clinton WL, Epplein M, Garcia R, Allison K, et al. Incidence of endometrial hyperplasia. Am J Obstet Gynecol. 2009;200:678–86. doi: 10.1016/j.ajog.2009.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heckbert SR, Weiss NS, Koepsell TD, Lemaitre RN, Smith NL, Siscovick DS, et al. Duration of estrogen replacement therapy in relation to the risk of incident myocardial infarction in postmenopausal women. Arch Intern Med. 1997;157:1330–6. [PubMed] [Google Scholar]
- 21.Pennant S, Manek S, Kehoe S. Endometrial atypical hyperplasia and subsequent diagnosis of endometrial cancer: a retrospective audit and literature review. J Obstet Gynaecol. 2008;28:632–3. doi: 10.1080/01443610802355817. [DOI] [PubMed] [Google Scholar]
- 22.Horn LC, Schnurrbusch U, Bilek K, Hentschel B, Einenkel J. Risk of progression in complex and atypical endometrial hyperplasia: clinicopathologic analysis in cases with and without progestogen treatment. Int J Gynecol Cancer. 2004;14:348–53. doi: 10.1111/j.1048-891x.2004.014220.x. [DOI] [PubMed] [Google Scholar]
- 23.Lindahl B, Willen R. Spontaneous endometrial hyperplasia. A 5 year follow-up of 82 patients after high-dose gestagen treatment. Anticancer Res. 1994;14:2831–4. [PubMed] [Google Scholar]
- 24.Lindahl B, Willen R. Spontaneous endometrial hyperplasia. A prospective, 5 year follow-up of 246 patients after abrasio only, including 380 patients followed-up for 2 years. Anticancer Res. 1994;14:2141–6. [PubMed] [Google Scholar]
- 25.Orbo A, Kaino T, Arnes M, Kopp M, Eklo K. Genetic derangements in the tumor suppressor gene PTEN in endometrial precancers as prognostic markers for cancer development: a population-based study from northern Norway with long-term follow-up. Gynecol Oncol. 2004;95:82–8. doi: 10.1016/j.ygyno.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 26.Janicek MF, Rosenshein NB. Invasive endometrial cancer in uteri resected for atypical endometrial hyperplasia. Gynecol Oncol. 1994;52:373–8. doi: 10.1006/gyno.1994.1064. [DOI] [PubMed] [Google Scholar]
- 27.Kurman RJ, Norris HJ. Evaluation of criteria for distinguishing atypical endometrial hyperplasia from well-differentiated carcinoma. Cancer. 1982;49:2547–59. doi: 10.1002/1097-0142(19820615)49:12<2547::aid-cncr2820491224>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 28.Shutter J, Wright TC., Jr Prevalence of underlying adenocarcinoma in women with atypical endometrial hyperplasia. Int J Gynecol Pathol. 2005;24:313–8. doi: 10.1097/01.pgp.0000164598.26969.c3. [DOI] [PubMed] [Google Scholar]
- 29.Silverberg SG. Problems in the differential diagnosis of endometrial hyperplasia and carcinoma. Mod Pathol. 2000;13:309–27. doi: 10.1038/modpathol.3880053. [DOI] [PubMed] [Google Scholar]
- 30.Tavassoli F, Kraus FT. Endometrial lesions in uteri resected for atypical endometrial hyperplasia. Am J Clin Pathol. 1978;70:770–9. doi: 10.1093/ajcp/70.5.770. [DOI] [PubMed] [Google Scholar]
- 31.Valenzuela P, Sanz JM, Keller J. Atypical endometrial hyperplasia: grounds for possible misdiagnosis of endometrial adenocarcinoma. Gynecol Obstet Invest. 2003;56:163–7. doi: 10.1159/000073950. [DOI] [PubMed] [Google Scholar]
- 32.Widra EA, Dunton CJ, McHugh M, Palazzo JP. Endometrial hyperplasia and the risk of carcinoma. Int J Gynecol Cancer. 1995;5:233–5. doi: 10.1046/j.1525-1438.1995.05030233.x. [DOI] [PubMed] [Google Scholar]
- 33.Allison KH, Reed SD, Voigt LF, Jordan CD, Newton KM, Garcia RL. Diagnosing endoemtrial hyperplasia: Why is it so difficult to agree? Am J Surg Pathol. 2008;32:691–8. doi: 10.1097/PAS.0b013e318159a2a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergeron C, Nogales FF, Masseroli M, Abeler V, Duvillard P, Muller-Holzner E, et al. A multicentric European study testing the reproducibility of the WHO classification of endometrial hyperplasia with a proposal of a simplified working classification for biopsy and curettage specimens. Am J Surg Pathol. 1999;23:1102–8. doi: 10.1097/00000478-199909000-00014. [DOI] [PubMed] [Google Scholar]
- 35.Kendall BS, Ronnett BM, Isacson C, Cho KR, Hedrick L, Diener-West M, et al. Reproducibility of the diagnosis of endometrial hyperplasia, atypical hyperplasia, and well-differentiated carcinoma. Am J Surg Pathol. 1998;22:1012–9. doi: 10.1097/00000478-199808000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Skov BG, Broholm H, Engel U, Franzmann MB, Nielsen AL, Lauritzen AF, et al. Comparison of the reproducibility of the WHO classifications of 1975 and 1994 of endometrial hyperplasia. Int J Gynecol Pathol. 1997;16:33–7. doi: 10.1097/00004347-199701000-00006. [DOI] [PubMed] [Google Scholar]