Abstract
Bovine herpesvirus type 5 (BHV-5) is an alphaherpesvirus associated with meningoencephalitis, a disease highly prevalent in South America. In this study, we investigated the distribution of BHV-5 DNA in the brains of latently, experimentally infected calves by using a PCR for the glycoprotein B gene. Twelve calves inoculated intranasally with a Brazilian BHV-5 isolate were divided into two groups: group A calves (n = 4) were euthanized 55 days postinoculation (p.i.) for tissue collection; group B calves (n = 8) were submitted to dexamethasone administration at day 60 p.i. for reactivation of latent infection and were euthanized 50 days later. Latent infection was reactivated in all group B calves, as demonstrated by virus isolation from nasal secretions and/or seroconversion. Three calves developed neurological disease and died or were euthanized in extremis. For group A calves, viral DNA was consistently detected in the trigeminal ganglia (4/4), midbrain (4/4), thalamus (4/4), and olfactory cortex (4/4) and less frequently in the pons (3/4), cerebellum (3/4), anterior cerebral cortex (2/4), and olfactory bulb (2/4). For calves previously submitted to reactivation (group B), viral DNA was detected with roughly the same frequency in the same areas as for the group A calves. In addition, viral DNA was detected in the posterior (5/5) and dorso-lateral cortex (3/5). All DNA-positive tissues were negative for infectivity and viral antigens. These results demonstrated that latent BHV-5 DNA is present in several areas of the brain during latent infection and that virus reactivation may result in the establishment of latent infection in additional sites of the brain.
Bovine herpesvirus type 5 (BHV-5) is an alphaherpesvirus associated with usually fatal meningoencephalitis in cattle (29, 38). Severe outbreaks of neurological disease caused by BHV-5 have frequently been reported, mainly in central and southern Brazil and Argentina (7, 32, 39). The disease is characterized by tremors, nystagmus, teeth grinding, circling, ataxia, recumbency, paddling, and death (4, 7, 22, 25). Like other alphaherpesviruses, BHV-5 establishes a lifelong latent infection in the nerve sensory ganglia of animals that survive acute infection (6, 8, 22, 25, 34; F. S. F. Vogel, M. Lima, E. F. Flores, R. Weiblen, E. R. Winkelmann, S. V. Mayer, K. Mazzutti, and S. Arenhart, submitted for publication). Reactivation of latent infection may occur under certain natural or induced stimuli and provide adequate means for virus transmission and spread (6, 8, 22, 25, 28). Natural or artificially induced reactivation is frequently accompanied by recrudescence of neurological disease, both in the natural host and in a rabbit model (3, 4, 6, 25).
The major site of latent infection by human (i.e., herpes simplex virus 1 [HSV-1]) and animal alphaherpesviruses (BHV-5, BHV-1, and pseudorabies virus [PRV]) following ocular, nasal, or oral infection is the sensory nerve ganglia, mainly the trigeminal ganglia (1, 9, 11, 16, 17, 28, 31, 36). Nevertheless, other possible neural and nonneural sites of latent infection or virus persistence have also been described for these viruses (reviewed in references 11, 16, 17, 28, and 36). Latent HSV-1 DNA has been detected in human nodose ganglia, vagus nerve, and bone marrow and in some areas of the central nervous system (CNS) (5, 13, 14, 19). In latently infected mice, HSV-1 DNA has been detected in the cornea (15, 21), and it has been detected in nonneural sites in other animal models (11, 33, 36). The tonsils have been shown to harbor PRV DNA in latently infected pigs (9, 40). PRV DNA has also been detected in the olfactory bulbs, brain stem, pons, medulla oblongata, and cervical medulla (31, 40). The major site of latent infection by BHV-1 is also the sensory nerve ganglia, where the virus replicates lytically during acute infection (17, 27, 28). In addition, BHV-1 DNA has been detected in tonsils, in CD4+ T lymphocytes, and in peripheral blood mononuclear cells of latently infected animals (20, 42).
BHV-5 is very neuroinvasive and neurovirulent following natural and experimental infection in cattle (2, 3, 4, 8, 22, 25) and after experimental inoculation of sheep (34) and rabbits (3, 4, 6, 10, 18). During acute infection, infectious virus can be recovered from several regions of the brain in moderate to high titers (3, 4, 6, 10, 22). Although the ensuing neurological disease is often fatal, cases of mild infection followed by clinical recovery and even subclinical neurological infection have occasionally been reported (2, 3, 4, 6, 8, 25). In the rabbit model, animals previously immunized with BHV-5 frequently experience a mild, transient, and nonfatal neurological disease upon BHV-5 inoculation (3, 4). Dexamethasone (Dx)-induced BHV-5 reactivation in calves may also course with mild, transient neurological signs (3, 25).
These biological properties of BHV-5 prompted us to investigate potential areas of the brain in which the virus may remain latent after acute infection. As the virus is capable of reaching and replicating in deep areas of the brain during acute infection and not all neurological infections are fatal, the virus may remain latent in some of these areas. BHV-5 DNA has been consistently detected in the trigeminal ganglia (TG) of latently infected cattle (1, 8, 22) and was occasionally detected in some areas of the brain and trachea of an experimentally, latently infected calf (8, 22). Nevertheless, a systematic and detailed study of the distribution of BHV-5 DNA in the CNS of cattle has not yet been performed. In the present study, we report the presence of BHV-5 DNA in several areas of the CNSs of latently infected calves.
MATERIALS AND METHODS
Design of the experiment.
Twelve calves were inoculated intranasally with a BHV-5 isolate, and two calves remained as noninfected, negative controls. The animals were monitored clinically during the acute infection; virus replication was monitored by testing nasal swabs for infectivity. Fifty-five days after inoculation, four inoculated calves (group A) and one control were euthanized for tissue collection. The other eight inoculated calves were treated with Dx at day 60 postinoculation (p.i.) to reactivate the infection. Fifty days after Dx administration, these eight calves and a control calf were sacrificed for tissue collection. The distribution of latent viral DNA in group A and group B calves was investigated by submitting the different brain sections to PCR, using a set of primers to the glycoprotein B (gB) gene. The presence of infectious virus or viral proteins in PCR-positive tissues was investigated by inoculation of tissue homogenates into tissue cultures and by immunohistochemistry (IHC), respectively.
Cells and virus.
A bovine cell line named CRIB (12), derived from Madin-Darby bovine kidney cells (American Type Culture Collection no. CCL-22) was used for virus multiplication, quantitation, and isolation from nasal swabs and tissues. Cells were routinely maintained in Eagle's minimal essential medium (MEM; Cultilab, Campinas, São Paulo, Brazil) containing penicillin (1.6 mg/liter), streptomycin (0.4 mg/liter), and 5% fetal calf serum (Cultilab). The BHV-5 SV-507 strain was isolated from an outbreak of meningoencephalitis in southern Brazil and has been submitted to nucleotide sequencing of the entire DNA genome (23).
Calves, virus inoculation, and Dx treatment.
Twelve calves seronegative for BHV-5 and BHV-1 were used for virus inoculation, and two calves were used as mock-infected controls. The inoculated and control groups were kept in separated areas. Calves were inoculated by the intranasal route with 2 ml of virus suspension in each nostril (total virus dose, 106.5 of the 50% tissue culture infective dose [TCID50]/animal). The control calves were inoculated with the same volume of MEM. Fifty-five days p.i., four calves (group A) were euthanized for tissue collection. The other eight inoculated calves (group B) were submitted to daily administrations of Dx (0.2 mg/kg of body weight/day for five days; Teresone, Intervet, São Paulo, Brazil), starting at day 60 p.i. (25). The Dx-treated calves were euthanized at day 55 post-Dx treatment (postreactivation [p.r.]). One control calf was euthanized with each group. All procedures for animal handling and experimentation were performed under veterinary supervision and according to recommendations by the Brazilian Committee on Animal Experimentation.
Animal monitoring and sample collection and processing.
After virus inoculation and Dx treatment, calves were monitored clinically on a daily basis. Nasal swabs for viral isolation were collected every day up to day 21 p.i. Thereafter, nasal swabs were obtained weekly until the first day of Dx administration. Then, nasal swabs were collected daily up to day 15 p.r. The swabs were collected in 1 ml of MEM containing 5× penicillin and stored at −70°C. At the time of analysis, the swabs were thawed and briefly vortexed and the contents were transferred to a microcentrifuge tube. The tubes were centrifuged at 10.000 × g for 1 min, and the supernatants were removed and transferred to a fresh microcentrifuge tube containing 5× penicillin and fungizone. The supernatants were cooled on ice for 30 min, and an aliquot of 0.2 ml was inoculated onto monolayers of CRIB cells grown in 24-well plates and submitted to three passages of 5 days each, with the cells being monitored for cytopathic effect. The infectivity of the nasal swabs that tested positive by virus isolation was subsequently quantitated by limiting dilution in CRIB cells grown in 96-well plates. The virus titers were calculated according to the method of Reed and Muench (26) and expressed as log10 TCID50/ml. Blood for serology was collected from all animals before virus inoculation, at day 21 p.i., at the day of the first Dx administration, and 15 days p.r. Serum samples were submitted to a standard microtiter virus-neutralizing (VN) assay using twofold dilutions of serum against a fixed dose of virus (100 to 200 TCID50 per well). At necropsy, different sections of the brain and peripheral nervous system were aseptically and individually collected for virus isolation and PCR.
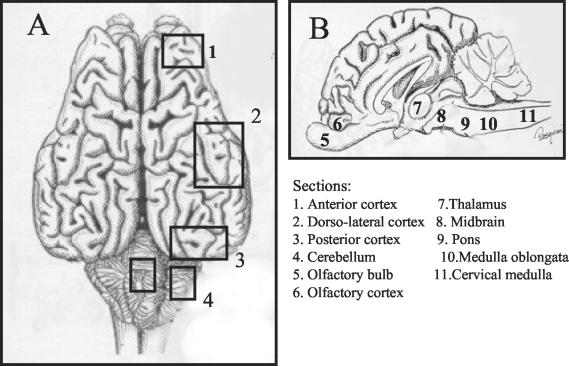
Tissue sections were also collected for histological examination and IHC. The following sections were collected individually: cerebral cortex (olfactory, anterior, posterior, and dorso-lateral), olfactory bulb, thalamus, midbrain, pons, medulla oblongata, cervical medulla, cerebellum, and TG. The location of the sections examined for the presence of viral DNA is depicted in Fig. 1. Tissue collection was performed bilaterally where applicable. Virus isolation and IHC were attempted only in sections that tested positive for viral DNA by PCR. For virus isolation, the tissue samples were processed by preparing a 10% (wt/vol) homogenized suspension, which was inoculated onto CRIB monolayers. Monitoring of virus replication was performed as described above.
FIG. 1.
Bovine brain. Localization of the sections examined for BHV-5 DNA is indicated. (A) Dorsal view; (B) sagital view.
DNA extraction from tissues.
Strict precautions were taken to avoid DNA carryover, including isolation of the pretarget and posttarget and amplification into separated facilities, the use of plastic disposable labware and positive-displacement pipettes in all steps of DNA manipulation, and the use of coverings for the operator's hair, clothing, and face. Total DNA for PCR was extracted from approximately 1 g of each section. Before digestion, the tissues were cleaned by removing fat and blood debris and were minced with a razor blade in petri dishes. Tissues for PCR analysis were transferred to a sterile plastic bag and digested overnight at 55°C, with shaking, in 5 ml of TEN buffer (0.01 M Tris, 0.001 M EDTA, 0.1 M NaCl, pH 7.4) containing 0.5% sodium dodecyl sulfate and 0.1 M proteinase K (Sigma) per ml per gram of tissue. Total cellular DNA was extracted with phenol-chloroform-isoamyl alcohol (25:24:1), precipitated with 10 M ammonium acetate (volume, 1/3) and cold 100% ethanol (twice the final volume). After being incubated for 2 h at −20°C, the nucleic acid solution was centrifuged in sterile plastic centrifuge tubes at 10.000 × g for 20 min. After centrifugation, the supernatant was discarded and the pellet was rinsed twice with cold 70% ethanol. The pellet was vacuum dried for 1 h and resuspended in 300 μl of Tris-EDTA buffer (0.01 M Tris and 0.001 M EDTA, pH 7.4). The DNA concentration was measured by UV absorbance at 260 nm. Alternatively, the Genome Star DNA isolation kit (ThermoHybaid, London, United Kingdom) was used for some samples for preparation of template DNA. For large areas (dorso-lateral, ventro-lateral, and cerebellum cortices, for example), representative fragments were collected bilaterally, pooled, and submitted to DNA extraction.
PCR.
PCR was performed by using a set of primers corresponding to positions 57143 and 57416 of the gB gene of BHV-5 strain SV-507 (23). The target region (273 bp) was amplified with the primers (forward) 5′-GTGGTGGCCTTTGACCGCGAC-3′ (position 57143) and (reverse) 5′-GCTCCGGCGAGTAGCTGGTGTG-3′ (position 57416). PCR was performed in a 25-μl volume of solution containing 1 μl of template DNA (containing approximately 1 μg of total DNA in Tris-EDTA buffer), 100 ng of each primer, 1 mM MgCl2, 10 mM (each) dNTPs, 10% DMSO, 1× reaction buffer, and 0.5 units of Taq polymerase (GIBCO BRL). The PCR conditions were initial denaturation (94°C for 10 min) followed by 40 cycles of 94°C for 1 min, 50°C for 30 s for primer annealing, 72°C for 30 s for primer extension, and a final extension of 7 min at 72°C at the end. PCR products were electrophoresed in a 1.5% agarose gel, stained with ethidium bromide, and visualized under UV light. To increase the sensitivity of detection, the PCR solutions of negative tissues (2 μl) from the first PCR were used as the template in a second PCR with the primers and conditions described above. DNA extracted from the brains of the control calves and a BHV-5-seronegative calf dying of unrelated causes was used as the negative control. DNA extracted from CRIB cells infected with BHV-5 SV-507 and the brain of an acutely BHV-5-infected calf was used as the positive control. The specificity of the PCR amplification product was confirmed by nucleotide sequencing of a 49-bp segment within the target region (nucleotides 1175 to 1223 of the gB gene) (30). The amplicon obtained from DNA extracted from the anterior cortex of calf 109 was chosen for sequencing. The obtained sequence was aligned and compared with the homologous region of the parental virus (SV-507) (23), BHV-5 strain N565 (GenBank accession number AF078726), BHV-1 strain K22 (GenBank accession number AF078725), and the complete BHV-1 genome (GenBank accession number AJ004801) (30) by using the BLAST and PSI-BLAST programs. To determine the sensitivity of the PCR, 10-fold dilutions of strain SV-507 DNA were prepared with DNA (1 μg/μl) extracted from the brain of a BHV-5-seronegative cow and used as templates for PCR. Based on the average size of the BHV-5 genome (137 kb), an estimate of the number of genome copies detected in the PCR was made.
IHC.
Tissues collected at necropsy were fixed in 10% neutral buffered formalin, embedded in paraffin, and sectioned at 5 μm. The tissue sections were submitted to hematoxylin and eosin staining for histological examination and to IHC to determine BHV-5 antigens. For IHC, tissue sections were deparaffinized and rehydrated through successive xylol and ethanol series. The tissues were treated with proteinase K (25 μg/ml in Tris-EDTA, pH 8.0; Sigma) for 8 min at 37°C, and then endogenous peroxidase activity was blocked with H2O2 (10 volumes) for 30 min at room temperature (two incubations of 15 min each). The sections were then incubated with the monoclonal antibody 2F9 (I. Oldoni, E. F. Flores, R. Weiblen, and M. A. Winkelmann, submitted for publication) (1:1000 in phosphate-buffered saline, pH 7.2) overnight at 4°C. The presence of viral antigens was revealed by using a streptavidin-biotin-peroxidase kit (Dako LSAB Plus), followed by adding the substrate DAB (60 mg of diaminobenzidine in 100 ml of phosphate-buffered saline plus 1 ml of H2O2; Sigma). After that, the sections were dehydrated, stained with hematoxylin, and mounted for microscopic examination.
RESULTS
Acute infection.
The summary of acute- and latent-infection findings and the main viral DNA and antigen findings in brain sections of calves inoculated with a Brazilian BHV-5 isolate (SV-507) is presented in Table 1. Virus shedding in nasal secretions was detected up to day 21 in some animals. All inoculated calves shed virus continuously from day 2 through day 9. The mean viral titers (expressed as log10/ml) were day 2 p.i., 6.39; day 3 p.i., 6.96; day 4 p.i., 7.64; day 5 p.i., 5.78; day 6 p.i., 5.89; day 7 p.i., 6.22; day 8 p.i., 5.75; and day 9 p.i., 5.12. From days 10 to 21 p.i., virus shedding was intermittent and not detected in all animals. Seven calves shed virus until day 19. The mean virus titers between days 10 and 17 varied between 4.16 (day 13) and 3.0 (day 17). After day 17 p.i., the titers were below 101.8 TCID50/ml. Three animals were still shedding virus at day 21, when the collection was discontinued. Virus shedding was not detected at day 28 p.i. and in the subsequent weekly swab examinations. No significant increase in body temperature was observed in the inoculated calves during clinical monitoring. A mild serous nasal discharge was noticed in all inoculated calves, starting at day 3 and lasting from 2 to 5 days. No other clinical signs were reported during acute infection. All calves seroconverted to BHV-5 by day 30, developing VN titers from 16 to 64. Weekly examinations of nasal swabs for infectivity from day 28 p.i. to the day of the first Dx administration were negative. Nasal swabs collected on the day of Dx administration were also negative for the virus. The control calves remained healthy, did not shed virus in nasal secretions, and remained seronegative during the experiment.
TABLE 1.
Viral shedding during acute infection and after Dx administration and presence of viral antigens in brain sections of calves experimentally inoculated with a BHV-5 isolatea
| Group and animal | Virus shedding:
|
VN titer(s)
|
Presence ofc:
|
|||
|---|---|---|---|---|---|---|
| During Acute-infection (days p.i.) | After Dx (day[s] p.r.)a | 30 days p.i. | 30 days p.r.b | Neurological disease | Viral antigenf | |
| Group A | ||||||
| 101 | 3-20 | NR | 32 | NT | − | − |
| 103 | 3-21 | NR | 16 | NT | − | − |
| 111 | 3-21 | NR | 32 | NT | − | − |
| 116 | 2-21 | NR | 16 | NT | − | − |
| Group B | ||||||
| 109 | 3-20 | 8-13, 15 | 64 | 64 | − | − |
| 112 | 3-21 | 16 | 64 | − | − | |
| 117 | 3-21 | 10 | 16 | 64 | − | − |
| 122 | 3-20 | 16 | 16 | 16 | − | − |
| 124 | 3-19 | 4, 5, 8-16 | 64 | 256 | − | − |
| 118 | 3-20 | 7, 8 | 16 | NT | + (8c) | + |
| 121 | 3-21 | 16 | NT | + (7e) | + | |
| 123 | 2-21 | 32 | NT | + (8e) | + | |
| Controls | ||||||
| C1 | <2 | NT | − | − | ||
| C2 | <2 | <2 | − | − | ||
NR, Not submitted to reactivation.
NT, not tested.
+, positive for neurological disease or viral antigens; −, negative for neurological disease or viral antigens.
Day of onset of neurological signs.
Day died or were sacrificed in extremis.
IHC was performed during latent infection in the brains of calves from group A (55 days p.i.) and B (55 days, p.r.) to demonstrate the absence of viral replication or gene expression. In calves 118, 121, and 123, IHC was performed to demonstrate viral replication or gene expression associated with the neurological disease.
Latent infection.
After Dx administration, virus shedding in nasal secretions was detected in five out of eight calves (Table 1). Calf 109 shed virus continuously from day 8 to day 15 p.r. Calf 124 shed virus at days 4 and 5 and from day 8 to day 16 p.r. Virus shedding by calves 117 and 122 was detected in only one collection, at days 8 and 16 p.r, respectively. Calf 118 shed virus at days 7 and 8 p.r. In general, virus titers in nasal secretions collected after Dx treatment were much lower than those observed during acute infection. In most animals, the virus titers barely reached 103 TCID50/ml. No infectious virus was detected in the nasal secretions of the control calves. No virus shedding was detected after day 16 p.r. and up to the day of euthanasia for group B calves (day 50 p.r).
Although no infectious virus could be recovered from the nasal secretions of calves 112, 121, and 123 after Dx treatment, these calves had reactivated infections as well. Calf 112 seroconverted to BHV-5 after Dx treatment (with a VN titer of 16 at the day of Dx and 64 at day 30 p.r.). Calves 121 and 123 developed neurological signs and died or were euthanized after Dx administration. Virus shedding after Dx treatment in the other calves was observed after day 8 or 10 (121 and 123 died on days 7 and 8 p.r., respectively) and was detected only at day 16 in one animal. Thus, it is possible that calves 121 and 123 developed clinical signs and died before virus shedding was detectable in nasal secretions. Taken together, the virological, serological, clinical, and IHC data indicate that in all inoculated animals the infection was reactivated upon Dx administration.
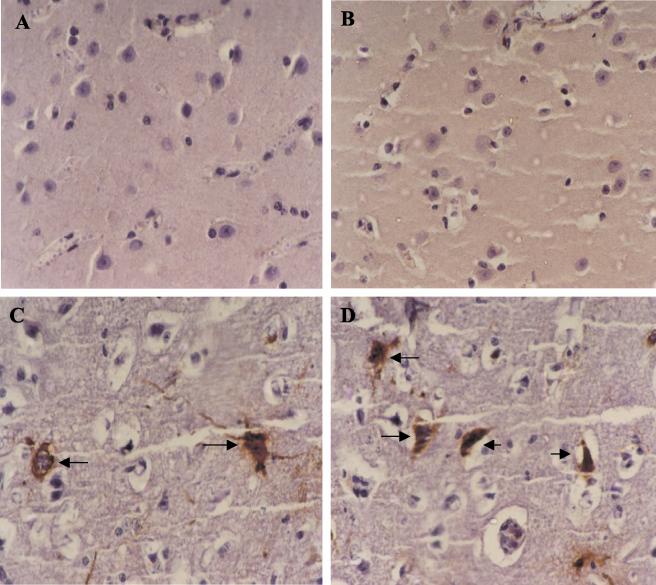
Three calves (118, 121, and 123) developed severe neurological signs by days 7 to 8 p.r. and died or were euthanized in extremis. The neurological disease was characterized by tremors, bruxism, circling, protusion of the tongue, salivation, recumbency, and paddling. These findings are in agreement with previous observations with cattle (3, 25) and rabbits (3, 6), in which reactivation of latent BHV-5 infection was frequently accompanied by clinical recrudescence. Histological examination of the brains of these calves revealed a mild to moderate nonsuppurative meningoencephalitis characterized by mononuclear cell perivascular cuffing and gliosis. No infectious virus was recovered from tissue homogenates. This was not a surprising finding, since we and others (3, 6, 25) have previously reported difficulty in isolating virus from the brain tissues of calves and rabbits upon BHV-5 infection, mainly in animals undergoing clinical recrudescence after Dx-induced reactivation. In spite of the lack of detection of infectious virus, viral antigens were demonstrated by IHC in neurons and other cells in several areas of the brains of these animals (Table 1 and Fig. 2C and D). The other five calves remained healthy until the end of the experiment.
FIG. 2.
Immunoperoxidase staining of brain sections of calves. (A) Dorso-lateral cortex of control calf C1; (B) dorso-lateral cortex of calf 109; (C) dorso-lateral cortex of calf 123; (D) dorso-lateral cortex of calf 121. BHV-5 antigens can be observed in neurons (indicated by arrows). An avidin-biotin-peroxidase detection system, using monoclonal antibody 2F9, and hematoxylin counterstaining were used. Magnification, ×400.
Specificity and sensitivity of the PCR.
The specificity of the PCR amplification was determined by nucleotide sequencing of 49 bp (nucleotides 1175 to 1223) within the target region and comparing them with the sequence of the parental virus (23) and other available BHV-1 and BHV-5 sequences (Fig. 3D). The PCR product obtained by amplification of the target region from total DNA from the anterior cortex of calf 109 was used for DNA sequencing. Alignment of the obtained sequence revealed 100% homology with the sequence of the parental virus (BHV-5 strain SV-507) (23) and of another BHV-5 strain (strain N565 (30) and 97% homology with the equivalent sequence of BHV-1 K22 and the complete BHV-1 sequence (30). These results confirmed the specificity of the PCR amplification. Sensitivity of the PCR was determined as described in Materials and Methods and estimated to be around 5 to 20 genome copies per reaction (1 μg of total DNA). To enhance sensitivity, all products that were negative in the first reaction were used as templates in a second PCR. Only a few tissues were detected as positive by this second round of PCR, most being positive in the first reaction.
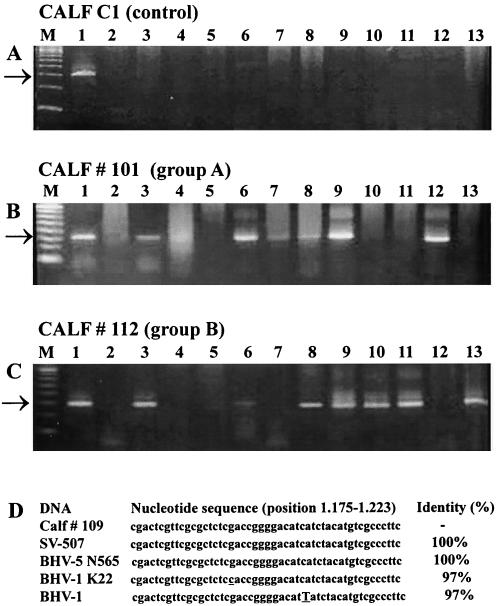
FIG. 3.
Specificity of the PCR for a gB gene sequence used in the present study. PCR products were electrophoresed in a 1.5% agarose gel, stained with ethidium bromide, and visualized under UV light. Shown are brain sections of the control calf (C1) (A), brain sections of calf 101 (group A) (B), brain sections of calf 112 (group B) (C), and the nucleotide sequence of a 49-nucleotide fragment within the amplified target sequence (D). The lanes of panels A through C show molecular weight markers (lane M) and DNA from the brain of a calf acutely infected with BHV-5 (lane 1), the brain of a BHV-5-seronegative calf dying of unrelated causes (lane 2), the trigeminal ganglia (lane 3), the olfactory bulb (lane 4), the pons (lane 5), the thalamus (lane 6), the olfactory cortex (lane 7), the cerebellum (lane 8), the anterior cortex (lane 9), the dorso-lateral cortex (lane 10), the posterior cortex (lane 11), the midbrain (lane 12), and the medulla oblongata (lane 13). The size of the amplified products (273 bp) is indicated by arrows. Shown in panel D are a sequence obtained from the PCR product amplified from total DNA extracted from the anterior cortex of calf 109, a sequence from the parental SV-507 isolate, sequences from BHV-5 N565 (3) and BHV-1 K22 (4), and the complete BHV-1 sequence (5). The divergent nucleotides are underlined.
Distribution of viral DNA in neural tissues.
The frequency of detection of viral DNA in brain tissues of group A and B calves is presented in Table 1. In general, the animals previously submitted to Dx treatment (group B) presented a higher frequency of PCR-positive tissues than group A calves (the exception was calf 103) (Tables 2 and 3). Among these, there was an apparent association between the length of virus shedding after reactivation and the frequency of DNA-positive tissues. The animals presenting the highest frequency of positive sections as determined by PCR (animal 109, 11/12, and animal 124, 9/12) were those experiencing the longest periods of virus shedding after Dx (8 and 13 days, respectively).
TABLE 2.
Detection of viral DNA by PCR during latent infection in brain sections of group A calves inoculated with BHV-5
| Tissue section | Result for animala:
|
No. of animals with positive result/total no. of animals | |||
|---|---|---|---|---|---|
| 101 | 103 | 111 | 116 | ||
| Olfactory bulb | − | + | − | + | 2/4 |
| Olfactory cortex | + | + | + | + | 4/4 |
| Anterior cortex | + | + | − | − | 2/4 |
| Dorso-lateral cortex | − | − | − | − | 0/4 |
| Posterior cortex | − | − | − | − | 0/4 |
| Cerebellum | + | + | + | − | 3/4 |
| Thalamus | + | + | + | + | 4/4 |
| Midbrain | + | + | + | + | 4/4 |
| Pons | + | + | + | − | 3/4 |
| Medulla oblongata | − | − | − | + | 1/4 |
| Cervical medulla | − | + | − | − | 1/4 |
| Trigeminal ganglia | + | + | + | + | 4/4 |
+, positive for viral DNA; −, negative for viral DNA.
TABLE 3.
Detection of viral DNA by PCR during latent infection in brain sections of group B calves inoculated with BHV-5
| Tissue section | Result for animala:
|
No. of animals with positive result/total no. of animals | ||||
|---|---|---|---|---|---|---|
| 109 | 112 | 117 | 122 | 124 | ||
| Olfactory bulb | + | − | − | − | − | 1/5 |
| Olfactory cortex | + | − | + | + | + | 4/5 |
| Anterior cortex | + | + | − | + | + | 4/5 |
| Dorso-lateral cortex | + | + | + | + | + | 5/5 |
| Posterior cortex | − | + | − | + | + | 3/5 |
| Cerebellum | + | + | − | − | − | 2/5 |
| Thalamus | + | + | − | + | − | 3/5 |
| Midbrain | + | − | + | − | + | 3/5 |
| Pons | + | − | + | + | + | 4/5 |
| Medulla oblongata | + | + | − | − | + | 3/5 |
| Cervical medulla | + | + | − | − | + | 3/5 |
| Trigeminal ganglia | + | + | + | + | + | 5/5 |
+, positive for viral DNA; −, negative for viral DNA.
Details of the distribution of BHV-5 DNA in different CNS sections is presented in Tables 2 and 3 for groups A and B, respectively. The PCR products obtained by amplification of DNA from different brain sections of calf C1 (control), calf 101 (group A), and calf 112 (group B) are presented in Fig. 3A, B, and C, respectively. In addition to the TG, which was positive in all animals, BHV-5 DNA was also frequently detected in the olfactory cortex, pons, midbrain, and thalamus. The overall distribution of DNA in most sections was similar among the animals and between the groups, yet the frequency of positivity varied, and particular patterns of distribution can be observed. In a comparison of group A and B calves, the major differences in the distribution and frequency of DNA detection were observed in the dorso-lateral and posterior cortices. These sections were negative in all group A calves and were consistently positive in group B calves (5/5 and 3/5, respectively). Likewise, the anterior cortex (2/4 to 4/5 positive calves in group A and B, respectively), medulla oblongata, and cervical medulla (1/4 to 3/5 positive calves) were also more consistently positive in the calves previously submitted to Dx treatment. The possible reasons for these differences, in which a higher number of brain sections became positive for viral DNA in latently infected animals previously submitted to virus reactivation, will be discussed elsewhere in this article.
The distribution of viral DNA in the brains of the calves who developed neurological disease upon reactivation was roughly similar to that of the rest of the animals in group B. These calves were not considered in the analysis of DNA distribution during latent infection because they developed neurological signs upon reactivation, likely as a result of virus reactivation and replication in the CNS (as demonstrated by detection of viral antigens by IHC). Calf 118 presented BHV-5 DNA in the anterior and posterior cortices, cerebellum, pons, medulla oblongata, cervical medulla, and TG. Calf 121 was positive for viral DNA in the anterior and dorso-lateral cortices, pons, medulla oblongata, cervical medulla, and TG. The olfactory bulbs, olfactory cortex, anterior-posterior and dorso-lateral cortices, cerebellum, thalamus, cervical medulla, and TG of calf 123 were positive for BHV-5 DNA. In particular, these animals frequently harbored viral DNA in the pons medulla and cervical medulla (2/3 and 3/3). It is tempting to speculate that the presence of viral DNA in these areas is possibly due to reactivated virus coming from a nearby site of latent infection (i.e., the TG).
Virus isolation and IHC.
All tissue sections from calves of groups A and B determined to be positive for viral DNA by PCR were tested for infectivity. Tissue homogenates were inoculated onto cell cultures and monitored for cytopathic effects during three passages of 5 days each. The attempts to isolate virus from DNA-positive tissues were conducted to demonstrate that viral DNA was present in these tissues without actively replicating virus, i.e., to fulfill the biological criteria used to define latent infection (28, 36). Viral isolation attempts were performed twice for each tissue section, and no infectious virus was detected in any of the tissues. A brain section of a cow acutely infected with BHV-5 was used as an internal positive control for this virus isolation system.
The limit of detection of BHV-1 and BHV-5 in this virus isolation system was previously established to be around 1 to 5 TCID50. However, as no infectious virus was detected in the brain tissues of animals developing neurological disease upon reactivation (calves 118, 121, and 123), a situation similar to that reported previously (3, 6, 25), IHC was employed to ensure that no acute virus replication was taking place in those tissues that tested positive by PCR at the time of tissue collection.
In this assay, a recently produced and characterized monoclonal antibody (2F9) to the same BHV-5 isolate used in the present study (Oldoni et al., submitted) was used in a highly sensitive avidin-biotin detection system. Three nonconsecutive histological sections of each DNA-positive tissue were processed by IHC. A brain section of a BHV-5 naturally infected calf was used as a positive control. No viral antigens were detected in the brain tissues of the mock-infected control (Fig. 2A) or in brain sections of latently infected calves of groups A and B that were positive for DNA (Fig. 2B). Taken together with the negative results of virus isolation, the results of IHC clearly indicate that the presence of viral DNA in the brains of these calves was not accompanied by detectable viral gene expression and/or productive virus replication.
The opportunity for using a highly sensitive antigen-detection assay prompted us to investigate the presence of antigens in the brains of calves 118, 121, and 123, which developed neurological signs after Dx administration. We tested a series of histological sections from the brain tissues of these animals (both DNA-positive and -negative) and detected viral antigens in abundant neuronal and glial cells in several brain sections (Fig. 2C and D). A detailed distribution of viral antigens in the brains of these calves, however, was not performed. The results of IHC not only corroborated the negative results for virus isolation in the brains of latently infected calves but also indicated that the neurological disease developed by calves 118, 121, and 123 was likely associated with neurological infection due to virus reactivation.
In summary, IHC for BHV-5 antigens was performed for two different purposes: (i) to demonstrate the absence of viral gene expression (favoring latency rather than viral persistence) in the brains of calves during latent infection and (ii) to demonstrate that the neurological disease was associated with viral replication in the brains of calves which developed neurological signs upon reactivation.
DISCUSSION
The results presented herein demonstrate the presence of BHV-5 DNA in several areas of the brains of latently infected calves in addition to the TG, which is the most common site of latent infection by human and animal alphaherpesviruses (1, 11, 17, 28, 31, 40). BHV-5 DNA was frequently detected in the cerebral cortex, thalamus, midbrain, pons medulla, and cerebellum and less frequently found in the olfactory bulb. The brains of calves previously submitted to Dx treatment showed a wider distribution of viral DNA than the brains of calves from group A. In calves from group B, viral DNA was consistently detected in the posterior and dorso-lateral cerebral cortices, sites that were negative in calves not submitted to prior virus reactivation. At the time of tissue collection, calves from both groups (A and B) met the biological criteria traditionally used to define latent infection: the presence of viral DNA in the absence of productive viral replication (17, 28). The lack of detection of infectious virus and viral antigens in DNA-positive tissues favors the hypothesis of latency rather than viral persistence.
Although the TG is the major site of latency for human and animal alphaherpesviruses following ocular, oral, or nasal infection, several studies have demonstrated persistence or latency in other neural and nonneural sites as well. Latent HSV-1 DNA has been detected in human nodose ganglia and vagus nerve (14, 19) and bone marrow (5) and occasionally in some areas of the CNS (11, 13, 37). HSV-1 DNA has also been detected in mouse cornea (15) and in nonneural sites in other animal models (16, 21, 33, 37). Besides the TG, PRV DNA has been detected in the tonsils, olfactory bulbs, brain stem, pons medulla, and cervical medulla (9, 31, 40). The major site of latent infection for BHV-1 is also the sensory nerve ganglia, where the virus replicates lytically during acute infection (17, 24, 28). Acute BHV-1 replication has also been demonstrated in tonsils (42), in CD4+ T lymphocytes (20, 41), and in peripheral blood mononuclear cells (20), sites which have been shown to harbor viral DNA in latently infected animals (20, 42).
BHV-5 is highly neuroinvasive following natural and experimental infection of cattle. During acute infection, infectious virus can be recovered from several regions of the brain in moderate to high titers (3, 4, 8, 22, 25). Although the ensuing neurological disease is often fatal, cases of mild disease or subclinical neurological infections have been reported as well (2, 3, 4, 8, 25). Therefore, it is conceivable that latent BHV-5 infection may be established in the areas of the CNS where the virus replicates during acute infection. In fact, recent studies have detected BHV-5 DNA in some areas of the CNS in latently infected calves (8, 22). The data presented herein further support this hypothesis. The SV-507 strain, which is highly neuroinvasive during acute infection, was capable of persisting in several areas of the brain of latently infected animals. Moreover, the overall distribution of viral DNA in the brain of latently infected calves correlates well with the distribution of infectious virus in the brain of acutely, experimentally infected cattle (3, 22). Nevertheless, as marked differences in neuroinvasiveness and neurovirulence have been reported among BHV-5 isolates (3, 4, 6), different patterns of distribution of viral DNA in the brain would certainly be expected for different viral strains. In contrast, BHV-1 is far less neuroinvasive and replication during acute infection is rarely detected in neural sites beyond the TG (1, 17, 28).
The biological significance of the presence of viral DNA in several areas of the brain during latent infection and the possible implications for the pathogenesis of BHV-5 infection are unclear at this point. Upon reactivation, alphaherpesviruses replicate acutely in the site of latency and travel back to the sites of primary infection (11, 17, 28, 36). In rabbits infected experimentally with BHV-5 and BHV-1, infectious virus and viral nucleic acids were demonstrated in the TG following spontaneous and Dx-induced reactivation (6, 27). HSV-1 has occasionally been associated with human meningoencephalitis, yet the source of the virus for the neurological infection is controversial. It has been suggested that encephalitis is mainly due to HSV-1 reactivation from the TG, but neurological infection due to primary infection is also possible (36). Although reactivation of latent infection by animal alphaherpesviruses has been largely accepted to be predominantly subclinical, we and others have demonstrated that both natural and Dx-induced BHV-5 reactivation is frequently accompanied by neurological disease in cattle and rabbits (3, 4, 6, 25). These observations were confirmed in the present study; three calves developed neurological signs and died or were euthanized after virus reactivation. Based on this finding, we hypothesize that the viral DNA present in deep areas of the brain during latent infection, in addition to virus reactivating from the TG, may serve as a source of virus for neurological infection during reactivation. In this case, the broad distribution of latent viral DNA in these areas may facilitate the spread and neuroinvasion that occur during viral reactivation, thus contributing to the severity of neurological infection upon reactivation. In favor of this hypothesis, it was recently demonstrated that the timing, kinetics, and distribution of histological changes in the CNS after BHV-5 reactivation differed from those observed during acute infection (25). Furthermore, we and others have observed that the time that elapsed between Dx administration and the onset of neurological signs was significantly shorter than the incubation period during acute infection in cattle and rabbits (3, 6, 25). Detection of additional sites of latent DNA in the group B calves is also consistent with this hypothesis. It is possible that these additional areas were infected during reactivation by virus reactivated from nearby tissues or from brain areas innervating the newly affected regions. Nevertheless, our findings do not discard the possibility that the virus reactivated from the TG may be the source of virus for the CNS infection observed upon reactivation.
In animals experimentally infected with HSV-1, viral DNA can be detected in several areas of the brain, yet attempts to reactivate the virus from brain tissue by explant cultures have failed (36, 37). Attempts to reactivate HSV-1 from human brain tissue have also been unsuccessful (36). Recently, BHV-1 DNA has been demonstrated in peripheral blood mononuclear cells of latently infected animals, yet attempts to reactivate the virus by cocultivation have been fruitless (20). The type and levels of expression of latency-associated transcripts (LAT) and unknown host and CNS-specific factors that may inhibit viral reactivation are among the reasons proposed to explain the inability to reactivate HSV-1 from brain tissue (36). Thus, whether latent BHV-5 DNA can be reactivated from brain tissue and the origin of the virus (brain tissue versus TG) causing neurological infection upon reactivation remain open questions that will need further investigation. In other words, demonstration that BHV-5 DNA in CNS sites is biologically active, i.e., that it can be reactivated as the DNA in the TG, for example, is necessary to support this concept.
BHV-1 and BHV-5 have been shown to establish and reactivate latent infections in cattle (2, 8, 22, 25; Vogel et al., submitted) and in other animal models (6, 27, 34). Recent nucleotide sequencing of the entire BHV-5 genome revealed important differences with BHV-1, mainly in the region of the LRT (23). Products of this gene are supposedly involved in the establishment and/or reactivation of latent infection by BHV-1 and other alphaherpesviruses (17, 27, 28). Thus, it has been speculated that the divergence observed in the BHV-5 LRT gene might result in a distinct ability of this virus to establish and/or reactivate latent infection. In the present study, the seroconversion observed after Dx treatment was weaker and less consistent than previously reported for BHV-1, suggesting that BHV-5 indeed reactivates the latent infection less efficiently than BHV-1. However, virus shedding was detected in nasal secretions of animals which did not seroconvert (109 and 122), indicating that they did reactivate the infection as well. Low magnitude seroconversion after Dx treatment—though accompanied by virus shedding and occasionally by neurological disease—has been observed in calves (25; Vogel et al., submitted), sheep (34), and rabbits (6) inoculated with BHV-5. These findings indicate that seroconversion should not be taken as the only indicator of reactivation, since virus reactivation (and shedding) may take place without a corresponding rise in titers of neutralizing antibodies. In order to specifically address this issue, we recently performed an experiment to compare the efficiency of BHV-1 and BHV-5 reactivation in calves (Vogel et al., submitted). The magnitude of seroconversion was lower in the BHV-5 group than among BHV-1-infected calves, yet both groups of calves reactivated the infection at similar levels, as judged by the percentage of animals reactivating the infection (8/8 [100%] for each group) and the magnitude and duration of virus shedding upon Dx administration (Vogel et al., submitted). Thus, that BHV-5 reactivates latent infections less efficiently than BHV-1 is still hypothetic, based on scattered and circumstantial data, and confirmation will require further experimentation and evidence.
Another interesting result was the long period of BHV-5 shedding in nasal secretions during acute infection (up to days 19 and 21) observed in the present study. We and others (3, 25, 35; Vogel et al., submitted) have already noticed that BHV-5 may be excreted for longer periods than BHV-1 upon experimental inoculation. Whether these findings were circumstantial (derived from different experimental conditions, animal age and genetic background, and/or different virus strains) or whether they reflect an inherent property of BHV-5 that distinguishes it from BHV-1 is still unclear.
The demonstration that BHV-5 DNA is frequently present in the brains of latently infected cattle may also have an important implication for diagnosis. In regions where BHV-5 infection is enzootic, the use of a BHV-5-specific PCR to diagnose neurological disease in cattle should be carefully considered, because animals latently infected with BHV-5 and suffering from any other neurological disorder may be misdiagnosed as suffering from acute BHV-5 infection. In other words, the detection of BHV-5 DNA in the brains of cattle with neurological signs and without concomitant virus isolation and/or viral antigen detection would not necessarily indicate acute BHV-5 infection.
In summary, our results demonstrate that, in the absence of detectable viral proteins and/or infectious virus, BHV-5 DNA is frequently present in several areas of the brains of latently infected cattle. After reactivation, the distribution of DNA changed slightly; i.e., additional areas became positive for viral DNA. The biological significance of these findings and the potential implications for the pathogenesis of BHV-5 infection are merely speculative at this point and will be the subject of future investigations in our lab.
Acknowledgments
We thank Mauro Pires Moraes (UFV, Viçosa, Minais Gerais) for performing the DNA sequencing of the PCR products and critically reviewing the manuscript and Luciane T. Lovato for reviewing the manuscript and for helpful discussions. Help in animal handling and care by the student workers in our lab is greatly appreciated.
This work was supported by an MCT/CNPq/CAPES/Finep grant (PRONEX em Virologia Veterinária 215/96).
REFERENCES
- 1.Ashbaugh, S. E., K. E. Thompson, E. B. Belknap, P. C. Schulteiss, S. Chowdhury, and J. K. Collins. 1997. Specific detection of shedding and latency of bovine herpesvirus 1 and 5 using a nested polymerase chain reaction. J. Vet. Diagn. Investig. 9:387-394. [DOI] [PubMed] [Google Scholar]
- 2.Belknap, E. B., J. K. Collins, V. K. Ayers, and P. Schultheiss. 1994. Experimental infection of neonatal calves with neurovirulent bovine herpesvirus type 1.3. Vet. Pathol. 31:358-365. [DOI] [PubMed] [Google Scholar]
- 3.Beltrão, N. 2000. Herpesvírus bovino tipo 5 (BHV-5): infecção experimental de bovinos e utilização de coelhos como modelo. M.S. dissertation. Universidade Federal de Santa Maria, Santa Maria, Rio Grande do Sul, Brazil.
- 4.Beltrão, N., E. F. Flores, R. Weiblen, A. M. Silva, P. M. Roehe, and L. F. Irigoyen. 2000. Acute infection and neurological disease by bovine herpesvirus type 5 (BHV-5): rabbits as experimental model. Pesqui. Vet. Bras. 20:144-150. [Google Scholar]
- 5.Cantin, E., J. Chen, L. Gaidulis, Z. Valo, and E. MacLaughlin-Taylor. 1994. Detection of herpes simplex virus DNA sequences in human blood and bone marrow cells. J. Med. Virol. 42:279-286. [DOI] [PubMed] [Google Scholar]
- 6.Caron, L., E. F. Flores, C. F. C. Scherer, R. Weiblen, L. F. Irigoyen, P. M. Roehe, A. Odeon, and J. H. Sur. 2002. Latent infection by bovine herpesvirus type-5 in experimentally infected rabbits: virus reactivation, shedding and recrudescence of neurological disease. Vet. Microbiol. 4:285-295. [DOI] [PubMed] [Google Scholar]
- 7.Carrillo, B. J., A. Ambrogi, A. A. Schudel, M. Vasquez, E. Dahme, and A. Pospischil. 1983. Meningoencephalitis caused by IBR virus in calves in Argentina. Zentbl. Vet. Med. B. 30:327-332. [DOI] [PubMed] [Google Scholar]
- 8.Cascio, K. E., E. B. Belknap, P. C. Schultheiss, A. D. Ames, and J. K. Collins. 1999. Encephalitis induced by bovine herpesvirus 5 and protection by prior vaccination or infection with bovine herpesvirus 1. J. Vet. Diagn. Investig. 11:134-139. [DOI] [PubMed] [Google Scholar]
- 9.Cheung, A. K. 1995. Investigation of pseudorabies virus DNA and RNA in trigeminal ganglia and tonsil tissues of latently infected swine. Am. J. Vet. Res. 56:45-50. [PubMed] [Google Scholar]
- 10.Chowdhury, S. I., B. J. Lee, D. Mosier, J.-H. Sur, F. A. Osorio, G. Kennedy, and M. L. Weiss. 1997. Neuropathology of bovine herpesvirus type 5 (BHV-5) meningo-encephalitis in a rabbit seizure model. J. Comp. Pathol. 117:295-310. [DOI] [PubMed] [Google Scholar]
- 11.Croen, K. D. 1991. Latency of human herpesvirus. Annu. Rev. Med. 42:61-67. [DOI] [PubMed] [Google Scholar]
- 12.Flores, E. F., and R. O. Donis. 1995. Isolation and characterization of a bovine cell line resistant to infection with the pestivirus bovine viral diarrhea virus (BVDV). Virology 208:565-575. [DOI] [PubMed] [Google Scholar]
- 13.Fraser, N. W., N. C. Lawrence, Z. Wroblewska, D. H. Gilden, and H. Koprowsky. 1991. Herpes simplex virus type 1 DNA in human brain tissue. Proc. Natl. Acad. Sci. USA 78:6461-6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gesser, R. M., and S. C. Koo. 1997. Latent herpes simplex virus type 1 gene expression in ganglia innervating the human gastrointestinal tract. J. Virol. 71:4103-4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon, J. Y., E. Romanowski, T. Araullo-Cruz and J. L. McKnight. 1991. HSV-1 corneal latency. Investig. Ophthalmol. Vis. Sci. 32:663-665. [PubMed] [Google Scholar]
- 16.Johnson, R. T. 1982. Viral infections of the nervous system, p. 129-157. Raven Press, New York, N.Y.
- 17.Jones, C. 1998. Alphaherpesvirus latency: its role in disease and survival of the virus in nature. Adv. Virus Res. 51:47-99. [DOI] [PubMed] [Google Scholar]
- 18.Lee, B. J., M. L. Weiss, B. J. Mosier, and S. I. Chowdhury. 1999. Spread of bovine herpesvirus type 5 (BHV-5) in rabbit brain after intranasal inoculation. J. Neurovirol. 5:474-484. [DOI] [PubMed] [Google Scholar]
- 19.Lohr, J. M., J. A. Nelson, and M. B. Oldstone. 1990. Is herpes simplex virus associated with peptic ulcer? J. Virol. 64:2168-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lovato, L. T., M. T. Winkler, M. Stone-Inman, A. Doster, and C. Jones. 2000. Detection of bovine herpesvirus type 1 (BHV-1) viral DNA in peripheral blood mononuclear cells (PBMC), p. 129. In Proceedings of the 81st Annual Meeting of the Conference of Research Workers in Animal Disease. Iowa University Press, Ames, Iowa.
- 21.Maggs, D. J., E. Chang, M. P. Nasisse, and W. J. Mitchell. 1998. Persistence of herpes simplex virus type 1 DNA in chronic conjunctival and eyelid lesions of mice. J. Virol. 72:9166-9172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer, G., M. Lemaire, C. Ros, K. Belak, A. Gabriel, D. Cassart, E. Coignoul, S. Belak, and E. Thiry. 2001. Comparative pathogenesis of acute and latent infections of calves with bovine herpesvirus types 1 and 5. Arch. Virol. 146:633-652. [DOI] [PubMed] [Google Scholar]
- 23.Moraes, M. P., Z. Lu, C. L. Afonso, E. F. Flores, R. Weiblen, D. Rock, and G. F. Kutish. 2001. The genome of bovine herpesvirus type 5: comparison with bovine herpesvirus type 1. Virus Rev. Res. 6:119. [Google Scholar]
- 24.Narita, M., S. Inui, K. Namba, and Y. Shimizu. 1976. Trigeminal ganglionitis and encephalitis in calves intranasally inoculated with infectious bovine rhinotracheitis virus. J. Comp. Pathol. 86:93-100. [DOI] [PubMed] [Google Scholar]
- 25.Perez, S. E., M. R. Bretschneider, F. A. Leunda, E. A. Osorio, E. F. Flores, and A. C. Odeon. 2002. Primary infection, latency and reactivation of bovine herpesvirus type 5 in the bovine nervous system. Vet. Pathol. 39:437-444. [DOI] [PubMed] [Google Scholar]
- 26.Reed, L., and D. E. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 27.Rock, D. L., J. Lokensgard, T. Lewis, and G. Kutish. 1992. Characterization of dexamethasone-induced reactivation of latent bovine herpesvirus 1. J. Virol. 66:2484-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rock, D. L. 1994. Latent infection with bovine herpesvirus type-1. Sem. Virol. 5:233-240. [Google Scholar]
- 29.Roizman, B. 1992. The family Herpesviridae: an update. Arch. Virol. 123:432-445. [DOI] [PubMed] [Google Scholar]
- 30.Ros, C., and S. Belak. 1999. Studies of genetic relationships between bovine, caprine, cervine, and rangiferine alphaherpesviruses and improved molecular methods for virus detection and identification. J. Clin. Microbiol. 37:1247-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rziha, J. H., T. C. Mettenleiter, V. Ohlinger, and G. Wittmann. 1986. Herpesvirus (pseudorabies virus) latency in swine: occurrence and physical state of viral DNA in neural tissues. Virology 155:600-613. [DOI] [PubMed] [Google Scholar]
- 32.Salvador, S. C., R. A. A. Lemos, F. Riet-Correa, P. M. Roehe, and A. L. A. R. Osório. 1998. Meningoencefalite em bovinos causada por herpesvírus no Mato Grosso do Sul e São Paulo. Pesqui. Vet. Bras. 18:76-83. [Google Scholar]
- 33.Scriba, M. 1997. Extraneural localization of herpes simplex virus in latently infected guinea pigs. Nature 267:529-531. [DOI] [PubMed] [Google Scholar]
- 34.Silva, A. M., R. Weiblen, L. F. Irigoyen, P. M. Roehe, H.-J. Sur, F. A. Osorio, and E. F. Flores. 1999. Experimental infection of sheep with bovine herpesvirus type-5 (BHV-5). Vet. Microbiol. 66:89-99. [DOI] [PubMed] [Google Scholar]
- 35.Spilki, F. R., P. A. Esteves, A. C. Franco, M. Lima, C. L. Holz, H. B. R. Batista, D. Driemeier, E. F. Flores, R. Weiblen, and P. M. Roehe. 2002. Neurovirulência e neuroinvasividade de herpesvírus bovinos tipos 1 e 5 em coelhos. Pesqui. Vet. Bras. 22:58-63. [Google Scholar]
- 36.Steiner, I., and P. G. Kennedy. 1995. Herpes simplex virus latent infection in the nervous system. J. Neurovirol. 1:19-29. [DOI] [PubMed] [Google Scholar]
- 37.Stevens, J. G., and M. L. Cook. 1971. Latent herpes simplex virus in spinal ganglia of mice. Science 173:842-845. [DOI] [PubMed] [Google Scholar]
- 38.Studdert, M. J. 1989. Bovine encephalitis herpesvirus. Vet. Rec. 125:584. [PubMed] [Google Scholar]
- 39.Weiblen, R., C. S. Lombardo de Barros, T. F. Canabarro, and I. E. Flores. 1989. Bovine meningo-encephalitis from IBR virus. Vet. Rec. 124:666-667. [DOI] [PubMed] [Google Scholar]
- 40.Wheeler, J. G., and F. A. Osorio. 1991. Investigation of sites of pseudorabies virus latency, using polymerase chain reaction. Am. J. Vet. Res. 52:1799-1803. [PubMed] [Google Scholar]
- 41.Winkler, M. T., A. Doster, and C. Jones. 1999. Bovine herpesvirus 1 can infect CD4+ T lymphocytes and induce programmed cell death during acute infection of cattle. J. Virol. 73:8657-8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winkler, M. T., A. Doster, and C. Jones. 2000. Persistence and reactivation of bovine herpesvirus 1 in the tonsils of infected calves. J. Virol. 74:5337-5346. [DOI] [PMC free article] [PubMed] [Google Scholar]