Abstract
Hyperlipidemia, also known as high blood cholesterol, is a cardiovascular health risk that affects more than one third of adults in the United States. Statins are commonly prescribed and successful lipid-lowering medications that reduce the risks associated with cardiovascular disease. The side effects most commonly associated with statin use involve muscle cramping, soreness, fatigue, weakness, and, in rare cases, rapid muscle breakdown that can lead to death. Often, these side effects can become apparent during or after strenuous bouts of exercise. Although the mechanisms by which statins affect muscle performance are not entirely understood, recent research has identified some common causative factors. As musculoskeletal and exercise specialists, physical therapists have a unique opportunity to identify adverse effects related to statin use. The purposes of this perspective article are: (1) to review the metabolism and mechanisms of actions of statins, (2) to discuss the effects of statins on skeletal muscle function, (3) to detail the clinical presentation of statin-induced myopathies, (4) to outline the testing used to diagnose statin-induced myopathies, and (5) to introduce a role for the physical therapist for the screening and detection of suspected statin-induced skeletal muscle myopathy.
High blood cholesterol, or hyperlipidemia, is a common cardiovascular system problem. In the United States, 35.6% of adults have been told that they have hyperlipidemia.1 Coronary artery disease is the leading cause of morbidity and mortality in the United States, and hyperlipidemia is a major risk factor.2
The causes of hyperlipidemia are multifactorial. Blood cholesterol tends to rise with age and body mass index. However, women tend to have lower blood cholesterol before menopause and higher blood cholesterol after menopause.3 Alcohol intake,4 race, and education level5 have been associated with hyperlipidemia. There appears to be no one direct cause for hyperlipidemia, so the treatment also must be multifaceted.
Various treatments for hyperlipidemia are available. Medication, patient education, dietary changes, and exercise are all recommended treatments for hyperlipidemia.6 Hyperlipidemia represents an array of problems within the blood lipid profile that must be considered when determining a plan of care. In contrast to the other treatments, medications often have side effects. However, the effectiveness of medications in treating hyperlipidemia supports their use. Commonly known as statins, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors have been shown to be the most successful class of lipid-lowering drugs for reducing risks associated with cardiovascular disease, while having an acceptable risk-benefit ratio.7–9
Statins are widely used to manage hyperlipidemia. The most commonly prescribed and purchased medication in the United States during 2006 was a statin, atorvastatin, commonly known as Lipitor.*,10 Between 1988 and 1994, cholesterol-lowering medication use increased from 11.7% to 40.8% among people with hyperlipidemia.1 The widespread use of statins implies that physical therapists will likely evaluate and treat patients who are taking this commonly prescribed cholesterol-lowering medication.
Recently, there has been an increased interest regarding the side effects of statin use on patients being seen by physical therapists.11,12 Approximately 25 million Americans use statins,13 and 5% to 18% of these patients report some form of myalgia.14 Skeletal muscle side effects that are associated with statin use involve muscle cramping, soreness, fatigue, weakness, and, in rare cases, rapid muscle breakdown that can lead to death (ie, rhabdomyolysis).15,16 Side effects have been associated with all commonly used statins and are dose dependent.17,18 Advancing age, the presence of renal or hepatic disease, the use of concurrent medications, and being female are predisposing risk factors for statin-related myopathy.17,19 Although serious skeletal muscle side effects are rare,15 researchers and physicians with expertise in statin therapy have highlighted the importance of developing a reliable method for early diagnosis.20 However, because definitions of myopathy vary widely, it is difficult to draw reliable conclusions among studies and to determine accurate diagnostic critera.20,21
Using statins safely requires regular monitoring for side effects. However, it has recently been suggested that routine examinations for side effects may not be cost-effective.20,21 Armitage suggested that, currently the “best means of detecting myopathy clinically is awareness of the main risk factors”22(pp1784–1785) such as drug interactions, high-dose prescriptions, and being at high risk and that once “statin myopathy or rhabdomyolysis is detected, statin treatment should be immediately stopped.”22(p1785) Statin withdrawal, however, carries serious vascular risk,21,23–25 so the relative risk must be considered.
Physical therapists are well trained to evaluate abnormal muscle soreness. Muscle soreness after exercise that is caused by statin use may go undetected by patients.26 Physical therapists are in a unique position to differentiate between muscle soreness normally experienced after exercise and the side effects from statins. Although some clinicians may have extensive experience with identifying the side effects of commonly used medications, many physical therapists may not know the risk factors associated with statin use or the early warning signs of statin-related myopathy. The purposes of this perspective article are: (1) to review the metabolism and mechanisms of action of statin; (2) to discuss the effects of statins on skeletal muscle function; (3) to detail the role of exercise, genetics, and multiple medications on statin-induced myopathies; (4) to outline the testing used to diagnose statin-induced myopathies; and (5) to introduce a role for the physical therapist for the screening and detection of suspected statin-induced skeletal muscle myopathy. However, prior to presentation of the metabolism and mechanism of action of statins, we first need to present the current terminology related to statin-induced myopathies.
Current Terminology
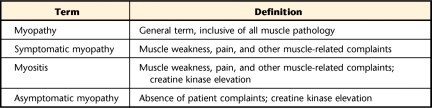
The National Lipids Association's (NLA) Muscle Expert Panel and other statin experts have emphasized the importance of standardizing terms related to myopathy to allow reliable comparisons among research studies and to improve care for statin users. Standard terminology also may encourage additional clinical trials investigating under-represented populations, such as people with mild increases in creatine kinase (CK) levels, people over the age of 75 years, or those with renal or hepatic comorbidities.27,28 Creatine kinase is an enzyme that acts with phosphocreatine to replenish the stores of ATP in skeletal muscle.29 Serum CK levels are commonly used determine the presence of skeletal muscle damage.30 Myopathy is the general term used to describe all muscle problems.15,28 More specifically, symptomatic myopathy is described as muscle pain, weakness, or other complaints that have a skeletal muscle origin.20 If these symptoms are accompanied by elevations in CK levels, the condition is known as myositis.12,21,28 Asymptomatic myopathy exists when CK levels are elevated, as can occur with vigorous exercise31,32 in the absence of other muscle symptoms (Tab. 1).20
Table 1.
Myopathy-Related Terminology
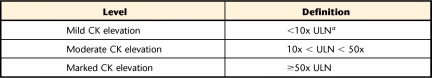
The most severe case of statin-induced myopathy is rhabdomyolysis,15,16,20,25 which includes the presence of muscle cell destruction or enzyme leakage, usually in combination with an increase in CK level.20 Clinically, rhabdomyolysis is associated with deterioration in renal function and death.16,33 Due to the unacceptably high incidence of severe muscle myopathies, cervastatin (Baycol†) was removed from the commercial market in 2001, and production ceased on a high-dose version of simvastatin in the mid-1990s.16 The NLA's Muscle Expert Panel has suggested using absolute CK levels in lieu of the broader term “rhabdomyolysis”20 to further standardize myopathic definitions (Tab. 2).20
Table 2.
Levels of Creatine Kinase (CK) Elevations
a ULN=upper limit of normal (the ULNs for CK levels are 532 u/L and 248 u/L for men and women, respectively78).
Metabolisms and Mechanisms of Action of Statins
Statins are primarily metabolized in the liver. In hepatic metabolism, a large number of diverse enzymes called cytochrome P450s can direct a drug's passage through the system or control its interaction with other enzymes.25,34 Cytochrome P450s are found most abundantly in the human liver, but also are seen in the gastrointestinal tract and kidneys.34 Lipophilic statins are metabolized by the enzymes of the CYP3A4 subfamily.34 With the exception of cervastatin, these statins also are metabolized significantly via first-pass metabolism, which occurs in the gastrointestinal tract and liver.34 If first-pass metabolism is inhibited by competing drug or food substances using the same pathway, statin toxicity can increase from 5% to 100%.34 Calcium channel blockers,34 fibrates,28 antifungals,16,28,35 and grapefruit juice34 are just a few of the known enzyme inhibitors of various statins by way of the CYP3A4 enzyme subfamily.
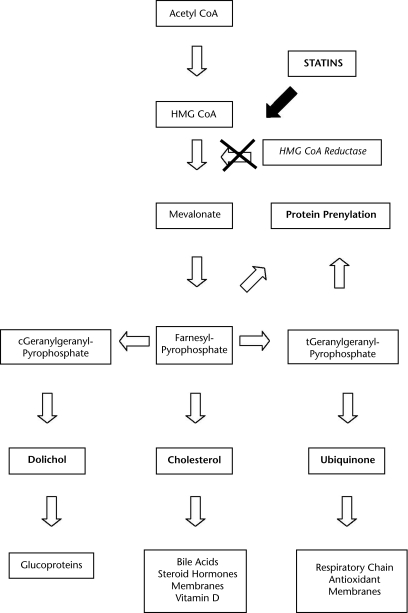
The production of cholesterol depends on the rate at which the enzyme HMG-CoA reductase reduces HMG-CoA to mevalonate (Figure).15,28,36 Statins as a group contain molecular side chains that allow them to be absorbed quickly into liver cells, and then bind to and inhibit HMG-CoA reductase.37 Statins reduce the production of endogenous cholesterol, and other products of the mevalonate pathway. Decreased hepatocyte low-density lipoprotein (LDL) levels cause an upregulation of LDL-specific cell receptors in the liver that work to pull LDL from the blood; this further reduces hyperlipidemia.38
Figure.
The role of statins in the metabolism of cholesterol. Adapted from Endres and Laufs.24
Effects of Statins on Skeletal Muscle Function
The mevalonate pathway also is important for protein regulation and skeletal muscle adaptation. Although cholesterol is the intended target for statins, dolichols, ubiquinone, and prenylated proteins production are reduced as well (Figure). Dolichols function to synthesize glycoproteins necessary for tissue growth.39 Ubiquinone (coenzyme Q10 [CoQ10]) acts within mitochondria to reduce metabolic equivalents in the tricarboxylic acid cycle.36 Prenylated proteins act to regulate cell growth, intracellular traffic with a specific function between the endoplasmic reticulum and the golgi apparatus,40 and gene transcription.41
The ubiquitin proteasome pathway has been shown to play a key role in the maintenance of skeletal muscle architecture.42 The ubiquitin proteasome pathway mediates protein turnover via several enzymes and, in the presence of catabolic disease states, upregulation of this pathway can lead to muscle atrophy.43,44 One of these enzymes, a ubiquitin protein ligase known as atrogin-1, is increased as a result of statins45 and is associated with muscle wasting secondary to disease or fasting.46,47
Statin-induced myopathy may be multifactorial, the result of impaired signal transduction, cell trafficking, gene transcription, structural protein formation and regulation, and oxidative phosphorylation.8,28 Also, a genetic tendency toward abnormal muscle and drug-induced mitochondrial dysfunction may be exacerbated by statins.25,34,48 Abnormal fat oxidation or mitochondrial dysfunction may be the primary mechanism underlying statin myopathy.44 Other possible mechanisms are reduced sarcolemmal cholesterol25 and isoprenoids involved in muscle fiber apoptosis.25,49 Cellular metabolism is negatively affected by statins alone through the inhibition of a cellular pathway (Figure).
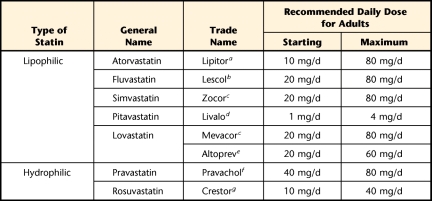
Commonly prescribed statins are identified by their lipophilic or hydrophilic nature49 and have different recommended dosages50 (Tab. 3). Lipophilic statins demonstrate a concentration-dependent adverse effect on muscle cell viability and promote cell disruption via proteolysis and apoptosis.15,49 In vitro studies confirm that lipophilic statins have greater myopathic effects than hydrophilic statins.15,28,49 Ease of diffusion via passive transport across the bilipid membrane layer by lipophilic statins has been associated with higher toxic effects.16,28 For example, cervastatin, which was a commonly prescribed lipophilic statin37,49 before its removal from the commercial market, has been shown to have the highest incidence of rhabdomyolysis and other myopathic complaints compared with other statins.9 Rosuvastatin is a hydrophilic statin that has demonstrated a propensity to reduce LDL levels more effectively than lipophilic atorvastatin, while having positive effects on high-density lipoprotein levels.51 It is worth noting, however, that pravastatin, another hydrophilic statin, can cause increases in plasma concentration 10 times greater than those of other lipophilic statins due to poor penetration into muscle cells.28
Table 3.
Recommended Doses of Currently Available Statins That Can Reduce Low-Density Lipoprotein Cholesterol by 30% to 40% (Adapted from Tomlinson and Mangione12 and Watson50)
a Parke-Davis, Div of Warner-Lambert Co LLC (a Pfizer company), 235 E 42nd St, New York, NY 10017-5755.
b Novartis Pharmaceuticals Corp, One Health Plaza, East Hanover, NJ 07936, and Reliant Pharmaceuticals, 110 Allen Rd, Liberty Corner, NJ 07938.
c Merck & Co Inc, Whitehouse Station, NJ 08889.
d Kowa Pharmaceuticals America, 530 Industrial Park Blvd, Montgomery, AL 36117.
e Andrx Pharmaceuticals 4955 Orange Dr, Davie, FL 33331.
f Bristol-Myers Squibb Co, PO Box 4500, Princeton, NJ 08543-4500.
g AstraZeneca Pharmaceuticals LP, 1800 Concord Pike, Wilmington, DE 19850-5437.
Each statin can be further classified into its lactone or acid forms.15 Both forms, for all statins, interconvert to achieve equilibrium in vivo.15 Researchers in Norway15 evaluated the potency on muscle cells of the lactone forms versus the more active acid forms of several commonly prescribed statins. Morphological analysis revealed that both lactone and acid forms of statins reduced the number of living muscle cells.15 Although myopathy was found to be time and concentration dependent, based on the number of remaining viable cells, lactone forms consistently showed more extensive myopathic effects compared with their respective acid forms.15 The mechanism of the higher myopathic potential of the lactone forms of statins is unclear,15 but researchers surmise that the lipophilic nature of the lactone versions may enhance their passive transport across the muscle membrane, yielding a greater propensity for damage.16
Although cholesterol is the target of statins, CoQ10 also is affected. Coenzyme Q10 is produced within the mevalonate pathway and is involved in the electron transport during oxidative phosphorylation in mitochondria.36,41 Coenzyme Q10 acts as a mobile component of the respiratory chain in mitochondria.28 A reduction in CoQ10 levels has been suggested to mediate statin myopathy as a result of its proposed ill effect on mitochondrial function.25,52 It is suggested that depleted serum levels of CoQ10 play a more integral role as a predisposing factor, rather than a primary role, in statin-induced myopathies.28 Statin treatment may impair mitochondrial oxidative metabolism and alter resting substrate utilization, shifting the balance toward carbohydrate metabolism.39 Statins have been associated with a decrease in CoQ10 synthesis that leads to impaired oxidative phosphorylation and impaired energy production.28 Although there have been inconsistent findings of altered mitochondrial dysfunction in the presence of reduced CoQ10, there is a lack of evidence supporting the mitigating results of myopathy with CoQ10 supplementation.25,28 Depleted CoQ10 as a result of statin use, therefore, may affect mitochondrial oxidative metabolism and impair energy production during exercise. Skeletal muscle impairments related to the combined effects of exercise and statin use are not thoroughly understood and are currently under investigation.
Role of Exercise, Genetics, and Multiple Medications on Statin-Induced Myopathies
Statins and Exercise
Statin treatments have been shown to exacerbate exercise-induced skeletal muscle injury.53 The extent of statin-influenced myopathies during exercise is still under investigation. Thus, because statins are a commonly prescribed cholesterol-lowering intervention, it is likely that a clinician or physical therapist may come across signs and symptoms of an adverse side effect during treatment.
Exercise-induced muscle damage and increased serum levels of CK increase as a result of damage to the muscle tissue following intense, prolonged exercise, especially exercises that are weight bearing and include eccentric muscular contractions.18,31,32 Myocytes that are affected by exercise-induced muscle damage release intracellular substances, such as CK, into the blood; therefore, CK levels are a commonly used marker to determine skeletal muscle damage.30 Individual CK level responses vary and may be influenced by multiple factors, such as genetics, level of athletic performance, and types of exercises being performed.18,30 Individuals with significant CK level increases as a response to exercise have been labeled as “high responders,” but there is no clear clinical definition of a high responder, and the phenomenon is not well understood.30 Exertional rhabdomyolysis is a clinical syndrome that may occur as a result of severe skeletal muscle destruction in response to exercise.30
Thompson et al53 compared the CK response to downhill treadmill walking and bicep curl exercises in young men who were healthy taking lovastatin or a placebo. These men were physically inactive, exercising less than once per week during the preceding 6 months, consumed fewer than 2 alcoholic drinks per day, and had lipid-lowering medications, probuchol, and supplemental vitamins discontinued 6, 12, and 4 weeks, respectively, prior to the beginning of the study. Thompson et al53 found that the mean CK concentrations were 62% and 77% higher 24 and 48 hours after treadmill exercise in subjects who received lovastatin versus those who received a placebo, respectively. Thompson et al53 also found elevated CK levels in the subjects who received lovastatin versus those who received a placebo following the completion of a biceps curl exercise protocol, although these differences were not significant. Although baseline CK levels were different between the randomized groups, statistical analyses accounted for these differences, and the authors concluded that statins and eccentric exercise together exacerbated muscle injury more than exercise alone.
Adverse effects of statins on the skeletal muscle during exercise are widely accepted by many professionals and investigators to be dose dependent.37 Kearns et al32 examined the contrasts in the effects of different statin doses (atorvastatin, 10 mg versus 80 mg) on skeletal muscle during eccentric exercise. Seventy-nine men completed both the 5-week treatment and a downhill treadmill protocol during the fifth week of treatment. Using inclusion criteria similar to those of Thompson et al,53 Kearns and colleagues randomly assigned 42 men to the 10-mg atorvastatin group and 37 men to the 80-mg atorvastatin group. Baseline values were used as the control. Plasma CK levels were significantly elevated from baseline 24, 48, and 72 hours after the completion of the exercise protocol in both treatment groups. There was no significant difference in the postexercise CK levels or muscle soreness between the 2 dose-dependent treatment groups.32 Interestingly, these findings contradict other literature that suggests the side effects of statins during exercise are dose dependent. Pretreatment CK values were slightly, but significantly, higher in the 10-mg atorvastatin group than in the 80-mg atorvastatin group. This finding may have obscured any effects due to the higher dosage, as baseline CK levels are used as a marker for the predisposition to statin toxicity or muscle injury.32 However, the results from this study showed an increase in CK values during exercise with statin use occurred in both treatment groups, regardless of dosage.53
The exact mechanisms or pathways through which statins adversely affect the skeletal muscles during exercise are not fully understood.8,32,53 The ubiquitin proteasome pathway is one possible mechanism for statin-induced muscle myopathy during exercise. Upregulation of the ubiquitin proteasome pathway during eccentric exercise has been shown to be associated with increased muscle injury, decreased muscle strength, and a decrease in myofibrillar protein.54 Atrogin-1 is a ubiquitin protein ligase strongly induced by any stimulus that induces muscle atrophy and may be a key effector in muscle degradation during catabolic states.47 Atrogin-1 may be referred to as FBX32, because it is classified as a muscle F-box family protein. Increases in FBX03, another F-box family protein, has been observed after statin treatment and exercise; this finding suggests that the ubiquitin ligase machinery may be altered by exercise with statin treatments, which, in turn, may affect protein degradation and repair.8
Changes in ubiquitin proteasome pathway gene expression in skeletal muscle as a result of exercise and statin use were investigated by Urso et al.8 In that study, subjects performed 300 eccentric exercises with one leg before and after the completion of an 80-mg atorvastatin or placebo treatment. The unexercised leg was used as a control. With the statin treatment alone, only 5 genes were differentially expressed compared with the unexercised leg or the exercised leg of the placebo group, indicating that the use of statins has little effect on skeletal muscle gene expression. Furthermore, eccentric exercise alone presented 80 genes that were differentially expressed compared with the nonexercised leg of the placebo group. According to the gene ontology classification database used in the study, the authors concluded that the genes that were differentially expressed due to eccentric exercise alone often were involved in cell functions. They found that eccentric exercise along with statin treatment had the greatest effect on transcription factors and genes involved in the ubiquitin proteasome pathway when compared with eccentric exercise or statin use alone. These results indicate that altered gene expression resulting from a combination of statins and exercise may be one explanation for observed side effects on skeletal muscle with statin treatments.
Exercise provides a well-defined stimulus for skeletal muscle oxidative metabolism.55 Respiratory exchange ratio (RER) is the ratio of carbon dioxide production to oxygen consumption (RER=CO2 production/O2 consumption)56 and indicates the types of fuel sources used for energy production. The relative contributions from fatty acids versus carbohydrates to oxidative energy production can be measured under controlled conditions using ventilatory gas exchange.56 Impaired fat oxidation at rest, represented by a higher RER value, may indicate increased carbohydrate use, which would be a limiting factor during exercise, leading to lower levels of exercise tolerance, increasing fatigue, and possibly causing other symptoms during exercise.57 Phillips et al58 found statin-induced increases in fasting RER at rest. Patients postmyositis, who were off statins, had a significantly increased RER compared with a control group. Interestingly, Phillips et al58 also found that a 6-week statin treatment increased the fasting RER within the control group. These findings may be explained by a reduction in mitochondrial utilization of fatty acids. Contrary to the results of other investigations, Chung et al55 did not see any changes in resting energy expenditure or resting RER in 13 subjects who were administered 40 mg per day of atorvastatin. Eight weeks of a 40-mg atorvastatin treatment did not have an effect on steady-state RER, fatty acid oxidation, or oxygen consumption. Based on these results, Chung et al55 concluded that atorvastatin at 40 mg per day does not impair fatty acid oxidation or muscle mitochondrial capacity in people who are healthy.
Statins and Related Genetic Factors
Genetic predisposition testing may become an integral component of personalized medicine and may influence the efficacy and safety in which medications are selected.41 Muscle toxicity related to statin use may be influenced by genetic factors or the presence or absence of specific genotypes. Genetic risk factors contributing to the development of statin-induced myopathies that are of interest relate either to drug metabolism or to muscle metabolism.41 According to Vladutiu,41 the SEARCH collaborative group found that more than 60% of 85 patients with myopathic symptoms had variations of the C allele of the SLCO1B1 gene. Encoded polypeptides within the SLCO1B1 gene are involved in the regulation of the hepatic uptake of statins.59
Genomic variations or reductions of specific coenzymes, such as CoQ10, may be another possible mechanism for statin-induced myopathy. Oh et al48 compared 133 subjects who were statin intolerant with 158 matched controls who were statin tolerant and found that genomic variation in CoQ10 was associated with an increased risk of statin intolerance, defined through muscle symptomatology. Coenzyme Q2 is the second enzyme in the CoQ10 biosynthetic pathway.25 These findings reinforce the concept that adverse muscle symptoms or statin intolerance may be associated with a genomic variation in the CoQ10 pathway.
Effects of Multiple Medications
The occurrence of drug interactions between statins and other medications have been well documented.7,25,34,60 Either the medication itself or its metabolic products can pose the largest threat of harmful effects to the user.34 Recently, mechanisms underlying the effects of multiple medications have gained attention due to the effects severe myopathies have on some statin users.7,37
For many patients, additional lipid-lowering medication often is needed to reduce their risk of adverse cardiovascular events.61 Niacin, bile acid sequestrants, and plant stanols are supplemental lipid-lowering agents often used in combination with statins to maximize efficacious management of high LDL cholesterol levels.60,61 Stein et al60 found that atorvastatin combined with ezetimibe, which inhibits cholesterol absorption in the intestine, is more effective at reducing LDL cholesterol levels compared with double doses of atorvastatin. Although the safety and tolerability of both treatments were found to be similar in this study,60 other researchers have shown that myopathic effects are dose dependent.37
In contrast, there is evidence that statins will interact in a negative way with other medications that are broken down through the same metabolic process in the liver.7,62 Many patients who are taking statins for hyperlipidemia also may be taking other medications that could interact.63 Varying degrees of myopathy, including rhadomyolysis, have occurred with the concomitant use of statins with other medications, including colchicine,64 digitoxin and amiodarone,65 nefazodone,66 and others.67–69 Obtaining a detailed medical history that includes current medications is imperative for physical therapists to facilitate the management of skeletal muscle dysfunction in statin users.
Fibrates are another class of medication used to mitigate high cholesterol and, when used with statins, can effectively modulate complex hyperlipidemias.28 This combination therapy, however, increases the incidence of statin-induced myopathies.9,28,33,58 McClure et al9 reported the increased risk of myositis (moderate CK levels as per the NLA's Muscle Expert Panel) with statins, fibrates, and other patient-related variables. These researchers showed that the average time to the onset of myositis was ≤2 years with both statin-fibrate combination therapy and statin use alone.9 Statins, fibrates, pre-existing renal disease, and hepatic disease are all significantly associated with myositis.9 Molokhia et al7 reported a significant increase in the risk of myopathies and myalgias with prolonged statin exposure at both 26 and 52 weeks and that the risk for myopathy for all statins and fibrates increases significantly after 12 months of use.
Despite recent research regarding increased risk with the combined use of statins and fibrates, fibrates do not inhibit the P-450s during hepatic metabolism. A process known as glucuronidation has been suggested not only as a means for statin metabolism but also as a possible mechanism for statin toxicity in the presence of multiple medications.16,20 When statins are administered as stable, lactone forms, they are quickly converted to more active acid forms. Glucuronidation is the statin-metabolizing process by which these active acid forms become unstable glucuronides that, in turn, quickly become inactive lactone forms.16 Fibrates, like gemfibrozil, are proposed to inhibit this glucuronidation pathway, thus increasing the concentration of the acid forms of some statins, and potentially, the risk of myopathy.28
Testing Used to Diagnose Statin-Induced Myopathies
Creatine kinase levels, liver function testing, and myoglobin levels.
Historically, CK levels have been used to assist in the diagnosis of statin-related myopathy.20 Utilizing CK levels as the sole marker to identify the presence of myopathy may be misleading.7,16,20,45 Elevated CK levels can occur without myopathic effects and often are seen as a result of exercise.31,32 Clarkson et al31 monitored several indicators of muscle damage, including CK, myoglobin, LDH, and other measures of renal function, following an eccentric exercise protocol. No subjects with moderate to marked increases in CK levels had signs or symptoms of renal failure. The results of this study confirm that large increases in CK and myoglobin as a result of exercise in individuals who are healthy are not sufficient to induce renal damage.31 In the presence of statin therapy, therefore, clinicians using a strength program for their patients should exercise caution when evaluating the presence of elevated CK levels.31,32 Clarkson and colleagues31,32 also showed that simply maintaining adequate hydration in the presence of these elevated serum concentrations is adequate in preventing renal compromise. Because the onset of myopathy can be multifactorial, the need for more judicious monitoring of patients using statins, as well as more nontraditional screening methods, is indicated.
The efficacy and cost-effectiveness of liver function testing for patients using statins has been discussed by several experts.20,21 Elevated transaminases, as regularly seen with statin use, in the absence of muscle symptoms and increased bilirubin are not indicative of serious risk to the patient.21,34 It has been suggested that transaminase elevations may be a normal and transient pharmacological effect of the reduction of cholesterol within the hepatocytes and that the costs of screening and monitoring would be staggering.21 Elevated myoglobin is yet another marker used to identify damage to myocytes and often accompanies elevated circulatory CK levels.16,31 The release of myoglobin from damaged cells can instigate renal failure via accumulation in the renal tubules.31 Researchers have shown that despite elevations in myoglobin and CK levels after an eccentric exercise protocol, evidence of renal compromise was not evident.31
Phosphodiesters.
Several procedures previously suggested as efficacious testing measures to identify the presence of statin-related myopathy have been re-evaluated.15,20,21,45 Glycerophosphocholine, the primary phosphodiester in skeletal muscle, is a key factor in cell membrane turnover as a result of lipid layer breakdown. Evaluation of this metabolite can give valuable information to researchers exploring the energetic mechanisms of physiological stresses.70 Elevation of phosphodiesters has been reported in other muscle disorders, including muscular dystrophies.70 It is thought that the elevation of this metabolite and associated myopathy are present with statin use due to accelerated myocyte membrane turnover or reduction in cholesterol synthesis.70 These researchers also found that even in the presence of statin-induced elevated levels of phosphodiesters, muscle symptoms were absent.70 Testing of phosphodiesters may assist physicians in identifying those patients who may have adverse effects due to statin use.
Skeletal muscle.
Evaluation of skeletal muscle composition45 and function71 has been used to assess the presence of myopathy in statin users. Muscle biopsies are an invasive procedure that may be used in research to assess histochemical and morphological changes but are not clinical tests for muscle myopathy. Phillips et al45 used muscle biopsies in a small sample of statin users to confirm the presence of myopathy in the absence of elevated CK levels. Four of the initial 21 patients were able to identify statin therapy versus placebo treatment based on the presence or absence of their reported muscle symptoms.45 Although different statins were used by each subject, biopsies showed myopathic effects, including diffuse lipid droplet accumulation vacuoles, cytochrome oxidase-negative myofibers, and an increased number of ragged red fibers.45 These findings were verified as myopathic effects by absence of carnitine deficiency and thyroid dysfunction.45
In addition to muscle biopsies, investigators have measured muscle performance as an alternative method of identifying the presence of myopathy.11,71 Dobkin71 found that functional lower-extremity weakness of the hip flexors and abductors was related to myopathy and could be independent of CK level. Dobkin evaluated several patients, all of whom were on statin therapy and sought outpatient care several months after neurological insult due to unsteadiness during walking. Proximal upper- and lower-extremity muscle weakness were identified and, in one third of the cohort, affected the neurologically intact side. Difficulty standing from a chair, functional hip abductor weakness, and altered gait were observed even after completion of a 6-week strengthening protocol.71 Interestingly, no evidence of myopathy was seen with CK testing. Three months after cessation of statin therapy, subjects' complaints of weakness resolved, and all subjects regained 5/5 manual muscle testing strength of their proximal musculature.71 Recently, Chatham and colleagues11 found that the inhibited inspiratory muscle performance of one statin user resolved and even improved after cessation of statin use and in conjunction with high-intensity inspiratory muscle training. These researchers demonstrated that noninvasive and inexpensive muscle performance testing can be effective measures to identify and track muscle function and recovery after statin-induced myopathy.11,71
Role of the Physical Therapist in the Screening and Detection of Suspected Statin-Induced Skeletal Muscle Myopathy
Several research groups have concluded that regular screening and monitoring of CK levels and completion of liver function tests are not cost-effective or efficacious methods by which to identify the presence of myopathy.20,21,45 The overwhelming evidence against regular testing to detect myopathies or other system dysfunction has great clinical implications for physical therapists who treat patient populations on statin therapy. Regular interaction with these patients provides physical therapists the opportunity and responsibility to identify changes in the patients' status that may indicate more- serious myopathy.
Currently, there is no paradigm for physical therapists to follow when attempting to identify the presence of statin-related myopathy. Qualitative and quantitative information gathered during a patient's physical therapy sessions may be extremely valuable to the physician. Reports of muscle weakness can be a common complaint of statin users.45 Complaints of muscle-specific soreness or fatigue as a result of strength training intervention should resolve within 48 to 72 hours.72 Additionally, strength testing would not likely demonstrate muscle weakness in individuals who are experiencing postexercise muscle soreness. Therefore, persistent muscle weakness that affects several muscle groups not involved in strength training in a patient taking statins suggests a problem that would warrant additional testing and possibly referral to the patient's physician. This self-reported fatigue or weakness should be validated with functional tests and other quantitative measures. The patient's perceived decline in strength may be experienced during activities of daily living, such as rising from a chair or climbing stairs, or for very active patients, during their recreational fitness and sport activities. For example, if a patient who is generally fit and is receiving physical therapy for postoperative shoulder pain complains of recent fatigue with stair climbing, the physical therapist should be alerted to the potential presence of statin-related side effects. Administering functional tests, such as the Stair Climbing Test73 and the Six-Minute Walk Test,74 may identify performance levels below those of age-matched norms or unexpected declines in the patient's functional status.
The relationship between quantitatively measured muscle weakness and more-invasive forms of testing when diagnosing the presence of myopathy has not been extensively investigated.45 Physical therapy intervention often includes aggressive strengthening routines that may cause benign elevations in levels of CK and myoglobin.31 Elevated CK levels are seen with both statin-related myopathies and exercise-induced muscle cell damage.18,31,32,53 Therefore, physical therapists should use clinical tests and measures to differentiate exercise-related muscle fatigue and soreness from statin-related symptoms. Exercise-induced muscle fatigue and soreness should be limited to the muscles previously exercised and should resolve within several days. Statin-related weakness may involve muscles not recently exercised and may progress or fail to show signs of improvement even after several days of rest. Patients should be educated regarding the signs and symptoms that may indicate an adverse effect of their statin medication, including unusual exertion in performing their daily activities and discoloration of their urine.28 Physical therapists have the responsibility of documenting and reporting patient reports, changes in functional performance (eg, weakness in response to a strengthening program), and other clinical symptoms. This information may be crucial in the physician's evaluation when combined with the results of muscle biopsies, liver function tests, and serum tests and may be used to avoid the unnecessary withdrawal of statin therapy and subsequent adverse cardiovascular events.21,25
Development and implementation of a validated diagnostic paradigm for statin users, administered by physical therapists, is indicated. As musculoskeletal and exercise specialists, physical therapists have the education and training to effectively screen for and identify statin intolerance. There are several measures that physical therapists could use to identify meaningful changes in a patient's functional status. Research has shown that changes in manual muscle testing performance can be a clinical indicator of statin intolerance.71 We propose that the use of dynamometers, which have demonstrated greater validity and reliability in strength testing compared with manual muscle testing,75 would be a viable clinical diagnostic tool for this patient population. The Guide to Physical Therapist Practice76 supports the use of strength testing via manual muscle testing and dynamometry techniques, as well as timed functional testing, as appropriate for people with impaired neuromotor systems. Routine assessment of strength changes in proximal musculature such as the quadriceps or scapular stabilizers could help capture the early onset of weakness in statin users and allow for prompt, appropriate medical intervention. Strength testing, when combined with a thorough patient history and performance on the Stair-Climbing Test and Six-Minute Walk Test, could prove to be an adequate battery of tests to detect meaningful declines in patient status. As with any test or measure, baseline levels must be established in order to identify relative change from initial values, as well as for comparison with normal values. The validity of these tests in detecting statin intolerance warrants investigation.
Because physical therapists possess a unique skill set by which to identify adverse effects of statin use and now that direct access laws for physical therapy have been implemented in 42 states,77 an increasingly important role for physical therapists is to identify detrimental functional changes and refer patients to their treating physician. Notifying the physician of unexpected changes in functional status, after all other possible explanations are ruled out, is imperative in managing the potential risks associated with statin use.
Multidisciplinary management of patients who use statins to control hyperlipidemia is paramount when considering efficacious treatments. If medical examination confirms statin-related myopathy, both the treating physician and the physical therapist should devise a plan of care suitable for the patient's recovery from the side effects, as well as appropriate for the patient's orthopedic rehabilitation. Thus, as millions of Americans continue to use statins to reduce their high cholesterol levels, physical therapists will play an increasingly important role in detecting the presence of statin-induced side effects and reducing the likelihood of serious disability.
Future Directions
Despite the growing body of literature investigating the mechanisms by which statins affect skeletal muscle, little is known about the most effective means of diagnosing and managing statin-related myopathies. Their ability to identify and manage different musculoskeletal pathologies gives physical therapists an important role in the recognition and management of statin-related myopathies. Currently, this unique role is unrecognized, and physical therapists are underutilized as professionals who can aid in the identification and conservative management of these myopathies. Side effects or symptoms of statin-related myopathies vary from patient to patient, and modifications to a patient's exercise routine need to be adapted based upon the severity of these myopathies. Physical therapists are trained to identify adverse responses to exercise and make appropriate adjustments. In addition to individualized modification of patients' exercise routines (resistance, repetitions, and frequency of activity), we propose that regular reassessment of functional measures and patient reports are indicated.
Future research should evaluate the efficacy of selected functional tests and measures, as administered by physical therapists, in detecting the presence of statin-related myopathy. This information then can be used to devise protocols for the clinical diagnosis of statin-related myopathy, thereby greatly improving physical therapists' ability to quickly identify and assist in the management of myopathic side effects. Furthermore, developing treatment and monitoring guidelines for physical therapists working with patients with diagnosed statin-related myopathy may facilitate the patients' full functional recovery.
Footnotes
Each author contributed to the concept and development of this perspective article. Ms Di Stasi, Mr MacLeod, and Mr Winters participated equally in the manuscript writing and review. Dr Binder-Macleod provided regular consultation and review of the manuscript before submission.
This work was supported National Institutes of Nursing Research RO1 grant NR010786 awarded to Dr Binder-Macleod and a Foundation for Physical Therapy PODS I scholarship awarded to Ms Di Stasi.
Parke-Davis, Div of Warner-Lambert Co LLC (a Pfizer company), 235 E 42nd St, New York, NY 10017-5755.
Voluntarily withdrawn from the market by manufacturer (Bayer Pharmaceuticals Corp, 400 Morgan Ln, West Haven, CT 06515).
References
- 1.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics, 2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146 [DOI] [PubMed] [Google Scholar]
- 2.Karalis DG. Intensive lowering of low-density lipoprotein cholesterol levels for primary prevention of coronary artery disease. Mayo Clin Proc. 2009;84:345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaefer EJ, Lamon-Fava S, Cohn SD, et al. Effects of age, gender, and menopausal status on plasma low density lipoprotein cholesterol and apolipoprotein B levels in the Framingham Offspring Study. J Lipid Res. 1994;35:779–792 [PubMed] [Google Scholar]
- 4.Schaefer EJ, Lamon-Fava S, Ordovas JM, et al. Factors associated with low and elevated plasma high density lipoprotein cholesterol and apolipoprotein A-I levels in the Framingham Offspring Study. J Lipid Res. 1994;35:871–882 [PubMed] [Google Scholar]
- 5.Gardner CD, Winkleby MA, Fortmann SP. Population frequency distribution of non-high-density lipoprotein cholesterol (Third National Health and Nutrition Examination Survey [NHANES III], 1988–1994). Am J Cardiol. 2000;86:299–304 [DOI] [PubMed] [Google Scholar]
- 6.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497 [DOI] [PubMed] [Google Scholar]
- 7.Molokhia M, McKeigue P, Curcin V, Majeed A. Statin induced myopathy and myalgia: time trend analysis and comparison of risk associated with statin class from 1991–2006. PLoS One. 2008;3:e2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urso ML, Clarkson PM, Hittel D, et al. Changes in ubiquitin proteasome pathway gene expression in skeletal muscle with exercise and statins. Arterioscler Thromb Vasc Biol. 2005;25:2560–2566 [DOI] [PubMed] [Google Scholar]
- 9.McClure DL, Valuck RJ, Glanz M, et al. Statin and statin-fibrate use was significantly associated with increased myositis risk in a managed care population. J Clin Epidemiol. 2007;60:812–818 [DOI] [PubMed] [Google Scholar]
- 10.Lamb E. Top 200 prescription drugs of 2006. Pharmacy Times. 2007:34–41 Available at: http://www.pharmacytimes.com/issue/pharmacy/2007/2007-05/2007-05-6472 Accessed December 1, 2008
- 11.Chatham K, Gelder CM, Lines TA, Cahalin LP. Suspected statin-induced respiratory muscle myopathy during long-term inspiratory muscle training in a patient with diaphragmatic paralysis. Phys Ther. 2009;89:257–266 [DOI] [PubMed] [Google Scholar]
- 12.Tomlinson SS, Mangione KK. Potential adverse effects of statins on muscle. Phys Ther. 2005;85:459–465 [PubMed] [Google Scholar]
- 13.Mann D, Reynolds K, Smith D, Muntner P. Trends in statin use and low-density lipoprotein cholesterol levels among US adults: impact of the 2001 National Cholesterol Education Program guidelines. Ann Pharmacother. 2008;42:1208–1215 [DOI] [PubMed] [Google Scholar]
- 14.Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients: the PRIMO study. Cardiovasc Drugs Ther. 2005;19:403–414 [DOI] [PubMed] [Google Scholar]
- 15.Skottheim IB, Gedde-Dahl A, Hejazifar S, et al. Statin induced myotoxicity: the lactone forms are more potent than the acid forms in human skeletal muscle cells in vitro. Eur J Pharm Sci. 2008;33:317–325 [DOI] [PubMed] [Google Scholar]
- 16.Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289:1681–1690 [DOI] [PubMed] [Google Scholar]
- 17.Antons KA, Williams CD, Baker SK, Phillips PS. Clinical perspectives of statin-induced rhabdomyolysis. Am J Med. 2006;119:400–409 [DOI] [PubMed] [Google Scholar]
- 18.Brancaccio P, Maffulli N, Limongelli FM. Creatine kinase monitoring in sport medicine. Br Med Bull. 2007;81–82:209–230 [DOI] [PubMed] [Google Scholar]
- 19.Arora R, Liebo M, Maldonado F. Statin-induced myopathy: the two faces of Janus. J Cardiovasc Pharmacol Ther. 2006;11:105–112 [DOI] [PubMed] [Google Scholar]
- 20.Thompson PD, Clarkson PM, Rosenson RS, National Lipid Association Statin Safety Task Force Muscle Safety Expert Panel An assessment of statin safety by muscle experts. Am J Cardiol. 2006;97:69C–76C [DOI] [PubMed] [Google Scholar]
- 21.Sniderman AD. Is there value in liver function test and creatine phosphokinase monitoring with statin use?. Am J Cardiol. 2004;94:30F–34F [DOI] [PubMed] [Google Scholar]
- 22.Armitage J. The safety of statins in clinical practice. Lancet. 2007;370:1781–1790 [DOI] [PubMed] [Google Scholar]
- 23.Endres M, Laufs U. Effects of statins on endothelium and signaling mechanisms. Stroke. 2004;35(11 suppl 1):2708–2711 [DOI] [PubMed] [Google Scholar]
- 24.Endres M, Laufs U. Discontinuation of statin treatment in stroke patients. Stroke. 2006;37:2640–2643 [DOI] [PubMed] [Google Scholar]
- 25.Klopstock T. Drug-induced myopathies. Curr Opin Neurol. 2008;21:590–595 [DOI] [PubMed] [Google Scholar]
- 26.Dirks AJ, Jones KM. Statin-induced apoptosis and skeletal myopathy. Am J Physiol. 2006;291:C1208–C1212 [DOI] [PubMed] [Google Scholar]
- 27.Sewright KA, Clarkson PM, Thompson PD. Statin myopathy: incidence, risk factors, and pathophysiology. Curr Atheroscler Rep. 2007;9:389–396 [DOI] [PubMed] [Google Scholar]
- 28.Vaklavas C, Chatzizisis YS, Ziakas A, et al. Molecular basis of statin-associated myopathy. Atherosclerosis. 2009:202:18–28[Epub ahead of print May 21 2008] [DOI] [PubMed] [Google Scholar]
- 29.MacIntosh BR, Gardiner PF, McComas AJ. Skeletal Muscle: Form and Function. 2nd ed.Champaign, IL: Human Kinetics; 2006 [Google Scholar]
- 30.Heled Y, Bloom MS, Wu TJ, et al. CK-MM and ACE genotypes and physiological prediction of the creatine kinase response to exercise. J Appl Physiol. 2007;103:504–510 [DOI] [PubMed] [Google Scholar]
- 31.Clarkson PM, Kearns AK, Rouzier P, et al. Serum creatine kinase levels and renal function measures in exertional muscle damage. Med Sci Sports Exerc. 2006;38:623–627 [DOI] [PubMed] [Google Scholar]
- 32.Kearns AK, Bilbie CL, Clarkson PM, et al. The creatine kinase response to eccentric exercise with atorvastatin 10 mg or 80 mg. Atherosclerosis. 2008;200:121–125 [DOI] [PubMed] [Google Scholar]
- 33.Bruno-Joyce J, Dugas JM, MacCausland OE. Cerivastatin and gemfibrozil-associated rhabdomyolysis. Ann Pharmacother. 2001;35:1016–1019 [DOI] [PubMed] [Google Scholar]
- 34.Herman RJ. Drug interactions and the statins. CMAJ. 1999;161:1281–1286 [PMC free article] [PubMed] [Google Scholar]
- 35.Omar MA, Wilson JP, Cox TS. Rhabdomyolysis and HMG-CoA reductase inhibitors[erratum in Ann Pharmacother. 2001;35:1296]. Ann Pharmacother. 2001;35:1096–1107 [DOI] [PubMed] [Google Scholar]
- 36.Marks DB, Marks AD, Smith CM. Basic Medical Biochemistry: A Clinical Approach. Baltimore, MD: Lippincott, Williams & Wilkins; 1996 [Google Scholar]
- 37.Schachter M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol. 2005;19:117–125 [DOI] [PubMed] [Google Scholar]
- 38.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430 [DOI] [PubMed] [Google Scholar]
- 39.Carroll KK, Guthrie N, Ravi K. Dolichol: function, metabolism, and accumulation in human tissues. Biochem Cell Biol. 1992;70:382–384 [DOI] [PubMed] [Google Scholar]
- 40.Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci U S A. 2006;103:11821–11827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vladutiu GD. Genetic predisposition to statin myopathy. Curr Opin Rheumatol. 2008;20:648–655 [DOI] [PubMed] [Google Scholar]
- 42.Taillandier D, Combaret L, Pouch MN, et al. The role of ubiquitin-proteasome-dependent proteolysis in the remodelling of skeletal muscle. Proc Nutr Soc. 2004;63:357–361 [DOI] [PubMed] [Google Scholar]
- 43.Haas KF, Woodruff E, III, Broadie K. Proteasome function is required to maintain muscle cellular architecture. Biol Cell. 2007;99:615–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phillips PS, Haas RH. Statin myopathy as a metabolic muscle disease. Expert Rev Cardiovasc Ther. 2008;6:971–978 [DOI] [PubMed] [Google Scholar]
- 45.Phillips PS, Haas RH, Bannykh S, et al. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med. 2002;137:581–585 [DOI] [PubMed] [Google Scholar]
- 46.Gomes MD, Lecker SH, Jagoe RT, et al. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci U S A. 2001;98:14440–14445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoffman EP, Nader GA. Balancing muscle hypertrophy and atrophy. Nat Med. 2004;10:584–585 [DOI] [PubMed] [Google Scholar]
- 48.Oh J, Ban MR, Miskie BA, et al. Genetic determinants of statin intolerance. Lipids Health Dis. 2007;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kobayashi M, Chisaki I, Narumi K, et al. Association between risk of myopathy and cholesterol-lowering effect: a comparison of all statins. Life Sci. 2008;82:969–975 [DOI] [PubMed] [Google Scholar]
- 50.Watson KE. Cardiovascular risk reduction among African Americans: a call to action. J Natl Med Assoc. 2008;100:18–26 [DOI] [PubMed] [Google Scholar]
- 51.Ai M, Otokozawa S, Asztalos BF, et al. Effects of maximal doses of atorvastatin versus rosuvastatin on small dense low-density lipoprotein cholesterol levels. Am J Cardiol. 2008;101:315–318 [DOI] [PubMed] [Google Scholar]
- 52.Marcoff L, Thompson PD. The role of coenzyme Q10 in statin-associated myopathy: a systematic review. J Am Coll Cardiol. 2007;49:2231–2237 [DOI] [PubMed] [Google Scholar]
- 53.Thompson PD, Zmuda JM, Domalik LJ, et al. Lovastatin increases exercise-induced skeletal muscle injury. Metabolism. 1997;46:1206–1210 [DOI] [PubMed] [Google Scholar]
- 54.Willoughby DS, Taylor M, Taylor L. Glucocorticoid receptor and ubiquitin expression after repeated eccentric exercise. Med Sci Sports Exerc. 2003;35:2023–2031 [DOI] [PubMed] [Google Scholar]
- 55.Chung J, Brass EP, Ulrich RG, Hiatt WR. Effect of atorvastatin on energy expenditure and skeletal muscle oxidative metabolism at rest and during exercise. Clin Pharmacol Ther. 2008;83:243–250 [DOI] [PubMed] [Google Scholar]
- 56.Bergman BC, Brooks GA. Respiratory gas-exchange ratios during graded exercise in fed and fasted trained and untrained men. J Appl Physiol. 1999;86:479–487 [DOI] [PubMed] [Google Scholar]
- 57.Fisher NM, Meksawan K, Limprasertkul A, et al. Statin therapy depresses total body fat oxidation in the absence of genetic limitations to fat oxidation. J Inherit Metab Dis. 2007;30:388–399 [DOI] [PubMed] [Google Scholar]
- 58.Phillips PS, Phillips CT, Sullivan MJ, et al. Statin myotoxicity is associated with changes in the cardiopulmonary function. Atherosclerosis. 2004;177:183–188 [DOI] [PubMed] [Google Scholar]
- 59.Pasanen MK, Fredrikson H, Neuvonen PJ, Niemi M. Different effects of SLCO1B1 polymorphism on the pharmacokinetics of atorvastatin and rosuvastatin. Clin Pharmacol Ther. 2007;82:726–733 [DOI] [PubMed] [Google Scholar]
- 60.Stein E, Stender S, Mata P, et al. Achieving lipoprotein goals in patients at high risk with severe hypercholesterolemia: efficacy and safety of ezetimibe co-administered with atorvastatin. Am Heart J. 2004;148:447–455 [DOI] [PubMed] [Google Scholar]
- 61.Stein EA. Management of dyslipidemia in the high-risk patient. Am Heart J. 2002;144(6 suppl):S43–S50 [DOI] [PubMed] [Google Scholar]
- 62.Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97:52C–60C [DOI] [PubMed] [Google Scholar]
- 63.Mukhtar RY, Reckless JP. Statin-induced myositis: a commonly encountered or rare side effect? Curr Opin Lipidol. 2005;16:640–647 [DOI] [PubMed] [Google Scholar]
- 64.van der Velden W, Huussen J, Ter Laak H, de Sevaux R. Colchicine-induced neuromyopathy in a patient with chronic renal failure: the role of clarithromycin. Neth J Med. 2008;66:204–206 [PubMed] [Google Scholar]
- 65.Nagele H, Behrens S, Hashagen S, Azizi M. Rhabdomyolysis after addition of digitoxin to chronic simvastatin and amiodarone therapy. Drug Metabolism and Drug Interactions. 2007;22:195–200 [DOI] [PubMed] [Google Scholar]
- 66.Skrabal MZ, Stading JA, Monaghan MS. Rhabdomyolysis associated with simvastatin-nefazodone therapy. South Med J. 2003;96:1034–1035 [DOI] [PubMed] [Google Scholar]
- 67.Boger RH. Drug interactions of the statins and consequences for drug selection. Int J Clin Pharmacol Ther. 2001;39:369–382 [DOI] [PubMed] [Google Scholar]
- 68.Bielecki JW, Schraner C, Briner V, Kuhn M. Rhabdomyolysis and cholestatic hepatitis under treatment with simvastatin and chlorzoxazone [in German]. Schweiz Med Wochenschr. 1999;129:514–518 [PubMed] [Google Scholar]
- 69.van Puijenbroek EP, Du Buf-Vereijken PW, Spooren PF, van Doormaal JJ. Possible increased risk of rhabdomyolysis during concomitant use of simvastatin and gemfibrozil. J Intern Med. 1996;240:403–404 [DOI] [PubMed] [Google Scholar]
- 70.Slade JM, Delano MC, Meyer RA. Elevated skeletal muscle phosphodiesters in adults using statin medications. Muscle Nerve. 2006;34:782–784 [DOI] [PubMed] [Google Scholar]
- 71.Dobkin BH. Underappreciated statin-induced myopathic weakness causes disability. Neurorehabil Neural Repair. 2005;19:259–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tiidus PM. Skeletal Muscle Damage and Repair. Champaign, IL: Human Kinetics; 2008 [Google Scholar]
- 73.Kennedy DM, Stratford PW, Wessel J, et al. Assessing stability and change of four performance measures: a longitudinal study evaluating outcome following total hip and knee arthroplasty. BMC Musculoskelet Disord. 2005;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parent E, Moffet H. Comparative responsiveness of locomotor tests and questionnaires used to follow early recovery after total knee arthroplasty. Arch Phys Med Rehabil. 2002;83:70–80 [DOI] [PubMed] [Google Scholar]
- 75.Kelln BM, McKeon PO, Gontkof LM, Hertel J. Hand-held dynamometry: reliability of lower extremity muscle testing in healthy, physically active, young adults. J Sport Rehabil. 2008;17:160–170 [DOI] [PubMed] [Google Scholar]
- 76.Guide to Physical Therapist Practice. 2nd ed.Phys Ther. 2001;81:9–746 [PubMed] [Google Scholar]
- 77.Direct Access to Physical Therapy Services. Alexandria, VA: American Physical Therapy Association; 2009. Available at: http://www.apta.org/AM/Template.cfm?Section=Home&TEMPLATE=/CM/ContentDisplay.cfm&CONTENTID=31402 Accessed November 17, 2009 [Google Scholar]
- 78.Lev EI, Tur-Kaspa I, Ashkenazy I, et al. Distribution of serum creatine kinase activity in young healthy persons. Clin Chim Acta. 1999;279:107–115 [DOI] [PubMed] [Google Scholar]