Abstract
The neural basis of auditory fear conditioning (AFC) is almost universally believed to be the amygdala, where auditory fear memories are reputedly acquired and stored. This widely-accepted amygdala model holds that that the auditory conditioned stimulus (CS) and the nociceptive unconditioned stimulus (US) first converge in the lateral nucleus of the amygdala (AL), and are projected independently to it from the medial division of the medial geniculate nucleus (MGm) and the adjacent posterior intralaminar nucleus (PIN), which serve merely as sensory relays. However, the four criteria that are used to support the AL model, (a) CS–US convergence, (b) associative plasticity, (c) LTP and (d) lesion-induced learning impairment, are also met by the MGm/PIN. Synaptic and molecular approaches supporting the AL also implicate the MGm/PIN. As both the AL and its preceding MGm/PIN are critically involved, we propose that the latter be considered the “root” of AFC.
Keywords: Associative learning, Lateral amygdala, Medial division of medial geniculate
1. Introduction
This article is dedicated to the memory of Jeff Winer, a superlative auditory neuroanatomist, supreme scholar and close friend. Jeff published approximately eighty original research papers during his tragically shortened life. More than thirty of his primary source publications focused on the medial geniculate body. Among his numerous contributions, Jeff provided a thorough and incisive account of the structure and connections of the medial division of the medial geniculate nucleus (MGm). Winer's findings have directly applicability to the neural bases of auditory fear conditioning (AFC). Simply put, the auditory thalamus has been ignored in favor of the amygdala, where it is almost universally believed that memories of fearful sounds are acquired and permanently stored. The purpose of this paper is to redress this situation by summarizing the copious evidence that the criteria, which justify the lateral amygdala as the site of auditory fear memories, are all met by the MGm and its related posterior intralaminar nucleus (PIN).
2. Auditory fear conditioning
Auditory fear conditioning refers to a form of associative learning in which a sound (conditioned stimulus, CS) is followed by an aversive event (unconditioned stimulus, US, e.g., footshock in laboratory animals), and for which objective behavioral analysis indicates that the sound has acquired the ability to predict the US, produce behavioral signs of fear, and in appropriate circumstances enable behavior that avoids the US. Auditory fear conditioning is an example of Pavlovian (classical) conditioning. In circumstances that enable the learning of avoidance of a forthcoming US, by responding to the auditory CS, such learning is referred to as instrumental avoidance conditioning. Classical conditioning is generally viewed as a necessary first step before the learning of instrumental avoidance behavior. Although early scientific interpretations and current popular views of classical conditioning consider it to be “automatic” and “non-cognitive” (e.g., Pavlov's “drooling dogs”), a modern understanding of this form of learning shows it to be a ubiquitous type of learning about the “causal framework” of the environment (Rescorla, 1988a, 1988b).
Auditory fear conditioning is rapidly acquired but usually difficult to get rid of. As a prototypical exemplar of fear learning, it has proven to be a fruitful model in the understanding and development of therapies for human anxiety disorders (Mineka and Oehlberg, 2008). Auditory fear conditioning has been studied far more extensively than fear learning for other sensory modalities, and therefore understanding its neural bases has been of considerable interest.
3. The amygdala as the locus of auditory fear memories
3.1. Introduction
The belief that the amygdala is the locus of both the acquisition and storage of learned fear is so dominant that one can scarcely find any writings to the contrary. The view that the amygdala is the only subcortical structure critical to fear learning is so pervasive that in addition to being assumed in the Introductions of primary source papers (e.g., Boatman and Kim, 2006) and scientific reviews (e.g., Fanselow and Poulos, 2005; Rosen, 2004) that favor the exclusivity of the amygdala, this idea is routinely stated as fact in contemporary textbooks (e.g., Pinel, 2003), tertiary summaries and instructional material in allied fields (e.g., Chisholm, 1999; O'Brien et al., 2008) and popular books (e.g., Kandel, 2006).
Given that learned fear of sounds is a subclass of all learned fear, the literature scarcely makes a differentiation. However, for the purposes of this paper, the distinction is essential simply because it is not my intention to deal with the neural bases of all learned fear. Such a vast subject would be neither appropriate with reference to Jeff Winer, nor even conceivable in a single article. The presence focus on auditory fear learning, particularly to simple stimuli (e.g., tonal) and relatively simple associations (i.e., as develop in Pavlovian and instrumental conditioning) still covers a vast domain. Therefore, the present communication does not include an exhaustive review of the literature, but rather an illustrative compendium that is sufficient to preclude continued dismissal of the medial geniculate as a critical plastic associative component of the neural circuitry underlying auditory fear conditioning.
3.2. The “Amygdala Model” (AMYG) of auditory fear conditioning
The accepted model of auditory fear conditioning appears to have first been presented in diagrammatic form in 1992 (LeDoux, 1992) (Fig. 1). Its major features are that input from an acoustic CS and a nociceptive US first converge in the lateral nucleus of the amygdala (AL), where auditory fear memories are said to be acquired and stored. The auditory information is shown to be relayed from the medial geniculate body, specifically from the MGm and its subjacent PIN. The source of input about the nociceptive US was uncertain and not actually included for many years. (We will consider the US route in detail later.) Thus, we have the unusual situation of proposing that AFC depends upon convergence of the CS and the US in the lateral nucleus of the amygdala, without the latter input actually included either in descriptions or diagrams of the model.
Fig. 1.
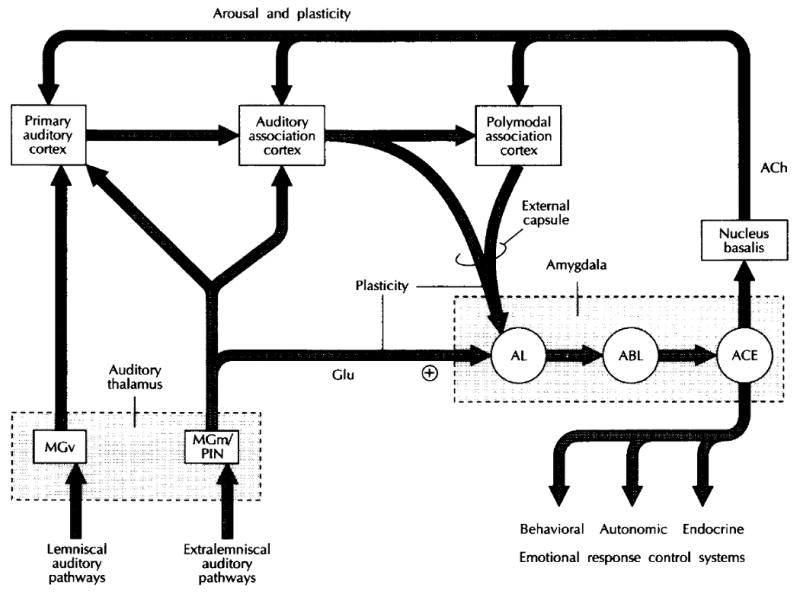
A diagram illustrating some of the pathways underlying emotional information processing and response control by the amygdala. Pathways through which auditory inputs are transmitted to the amygdala are shown but similar circuits also exist for other sensory systems. Tonotopically organized auditory signals are transmitted to the auditory thalamus over lemniscal pathways, which synapse in the ventral division of the medial geniculate body (MGv). Extralemniscal pathways transmit to other parts of the auditory thalamus, including the medial division of the medial geniculate body (MGm) and the posterior intralaminar nucleus (PIN). While MGv only projects to primary auditory cortex, MGm/PIN projects to both primary and association areas of auditory cortex, as well as to the lateral nucleus of the amygdala (AL). The thalamo-amygdala projection forms asymmetric, excitatory (+) contacts with AL, contains glutamate (Glu), and may use this excitatory substance as a neurotransmitter. Thalamo-amygdala projections have been implicated in emotional learning, and high-frequency stimulation of these projections produces long-term potentiation (LTP) in AL. Auditory and polymodal association areas relay auditory signals to AL by way of the external capsule. These pathways are also involved in emotional learning and exhibit LTP. AL projects to the basolateral nucleus of the amygdala (ABL), which projects widely to cortical areas (not shown) and to the central nucleus of the amygdala (ACE). ACE has extensive connectivity with brainstem areas involved in the control of emotional responses. It also projects to the nucleus basalis, which projects widely to cortical areas. The pathway from the nucleus basalis to cortex uses acetylcholine (ACh) as a neurotransmitter. Cholinergic transmission to the cortex from the nucleus basalis has been implicated in cortical arousal and plasticity. (LeDoux, 1992)
The AMYG model further holds that once the initial acquisition of AFC is made in the AL, the resultant plasticity is sent, via the basolateral nucleus (ABL), to the central nucleus of the amygdala (ACE). The latter is said to serve as a behavioral output which distributes information to several brain stem nuclei whose activation produces the behavioral, autonomic and endocrine expression of fear.
The AMYG model also includes auditory input to the auditory cortex, and thence to both auditory association cortex and polymodal association cortex, which project to the AL. This convergence of auditory information from the MGm/PIN and cortically-processed auditory information is a consistent feature of the AMYG model; the thalamic route is thought to provide “crude” information to the AL while the cortical route is thought to provide more precise information about the acoustic CS. The model also includes activation of the cholinergic nucleus basalis from the ACE, the former providing “arousal and plasticity” throughout the cortex.
The cortical parts of the AMYG model are beyond the scope of this report, which focuses on the role of the MGm/PIN in auditory fear conditioning. So, returning to Fig. 1, it is important to note that some of the connections are labeled “plasticity”. Most relevantly, the connection from the MGm/PIN to the AL receives this label. As noted in the figure legend, this reflects the ability of MGm/PIN stimulation to produce long-term potentiation (LTP) in the lateral amygdala. The MGm/PIN complex itself is considered not to be plastic. This follows from the belief that this region of the auditory thalamus serves only as an auditory “relay” to the LA. For example:
“… emotional conditioning depends upon the relay of information — presumably acoustic information — from the MGB …” (LeDoux, 1990, p. 19)
“Our working hypothesis, though, is that AL is the sensory interface of the amygdala and that lesions of this region interfere with aversive conditioning by blocking the synaptic transmission of the acoustic CS to the amygdala.” (LeDoux et al., 1990, p. 1068)
“First, the amygdala receives multiple inputs about the CS: inputs from auditory thalamus and various stages of auditory association cortex. … Second, the amygdala receives inputs from the US pathway, the origin of which is still unknown.” (LeDoux, 1993b, p. 75)
The assignment of a purely auditory relay function to the MGm/PIN has been maintained throughout the years:
“The neural pathways involved in fear conditioning are well characterized. When the CS is an acoustic stimulus, the pathways involve transmission to the lateral nucleus of the lateral amygdala (LA) from auditory processing area in the thalamus (MGm/PIN) …” (LeDoux, 2000, p. 163)
The input for the shock US was specified in 2005:
“The pathways mediating auditory fear conditioning in rats involve the convergence of the CS and US pathways onto single cells in the lateral nucleus of the amygdala (LA) from thalamic and cortical processing regions in the sensory systems that process the CS (auditory system) and US (somatosensory system).” (Phelps and LeDoux, 2005, p. 176)
The circuitry for AFC, which now included the US as well as the CS, is reproduced in Fig. 2 (Phelps and LeDoux, 2005). Note that the US somatosensory pathway “parallels” the auditory CS pathway, both of which are said to provide input to the lateral amygdala from their respective thalamic nuclei and cortical areas. No citations are provided for the US input to the LA, so at this point in the exposition of the AMYG model it is not clear which area of the somatosensory thalamus provides cutaneous nociceptive (shock) information to the amygdala. The same uncertainty applies to the somatosensory cortex. This may reflect the results of a previous study in which lesions of the auditory thalamus produced inconclusive results (Lanuza et al., 2004).
Fig. 2.
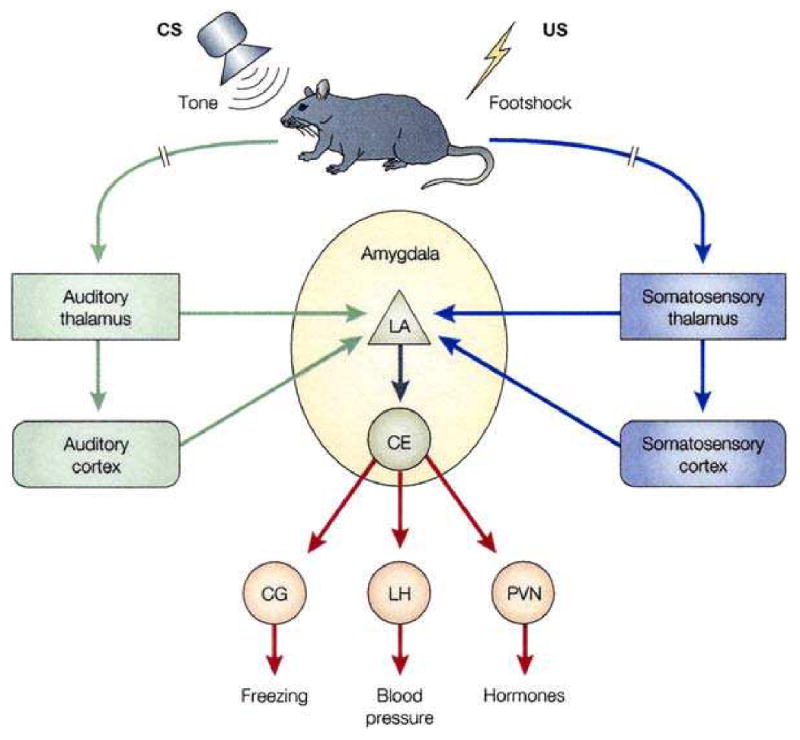
Neural pathways underlying fear conditioning. Fear conditioning is a procedure in which an emotionally neutral conditioned stimulus (CS) is presented in association with an aversive unconditioned stimulus (US). In studies of rats, the CS has typically been an auditory tone and the US an electric footshock. The pathways mediating auditory fear conditioning in rats involve the convergence of the CS and US pathways onto single cells in the lateral nucleus of the amygdala (LA) from thalamic and cortical processing regions in the sensory systems that process the CS (auditory system) and US (somatosensory system). The LA then connects with the CE both directly and by way of other amygdala regions (not shown). Outputs of the CE then control the expression of fear responses, including freezing behavior and related autonomic nervous system (e.g., blood pressure and heart rate) and endocrine (pituitary–adrenal hormones) responses. Lesion and imaging studies, described in the text, have confirmed that the human amygdala is also involved in fear conditioning, but the involvement of subregions of the amygdala is still poorly understood in humans. CG, central gray; LH, lateral hypothalamus; PVN, paraventricular hypothalamus. From Medina et al. (2002). (Phelps and LeDoux, 2005)
Subsequently, the nociceptive US source was assigned to both the MGm and the PIN on the basis of footshock-induced expression of c-Fos and reduction thereof by lesions of these nuclei. The MGm/PIN, originally considered to be purely an auditory relay, was now also considered to be somatosensory/nociceptive sensory relays to AL (Lanuza et al., 2008).
“This supports previous ideas of the MGm/PIN complex being an important relay station on the transfer of somatosensory and nociceptive information to the LA …” (p. 966) [All quotations are exact; authors sometimes use “LA” rather than “AL” to refer to the lateral nucleus of the amygdala.]
3.3. Experimental support for the AMYG Model
The AMYG Model has been presented in numerous reviews (e.g., Fanselow and LeDoux, 1999; LeDoux, 1990, 1992, 1993a, 1994, 1995, 2000; LeDoux and Muller, 1997; Maren, 2001; Maren et al., 2004; Phelps and LeDoux, 2005; Rodrigues et al., 2009). The major findings on which the AMYG model is based are summarized here, conveniently taken from Blair et al., 2003. They are numbered, for ease of later reference.
“First, lesions or pharmacological inactivation of LA prevents the acquisition and expression of fear responses to an auditory CS, demonstrating that neural activity in LA during conditioning is required for this type of fear learning to take place.”
“Second, artificial stimulation of the thalamic and cortical synaptic inputs to LA can induce long-term potentiation (LTP) at these synapses, suggesting that sensory inputs to LA can indeed be strengthened as predicted by the associative hypothesis.”
“Third, neural responses evoked in LA by the auditory CS are enhanced when the CS is paired with US, supporting the prediction that pairing the CS and US leads to the strengthening of the auditory inputs to LA activated by the CS.”
“Fourth, afferents carrying information about the auditory CS and nociceptive US convergence in single neurons in LA, providing a substrate through which the US might modify processing of the CS.”
These commonly employed four criteria are not at issue. They are accepted for the purpose of comparing the AL and the MGm/PIN in auditory fear conditioning. For example, the requirement that LTP be demonstrated in a candidate structure, such as the AL, might be subject to question. On the other hand, Criterion #4 (CS and US convergence) and #3 (associative facilitation of responses to the CS) may be considered most important. But the issue before us is the extent to which the MGm/PIN meets the same criteria as does the AL.
Further, the detailed findings, which are the bases of these four criteria for the AMYG model, will not be reviewed here. They can be accepted for purposes of evaluating the Model's role for the MGm/PIN as a sensory relay, i.e., excluding these nuclei from plastic and associative functions in auditory fear conditioning. However, it should be noted that claims for the necessity of the amygdala have been questioned. For example, freezing is the behavior generally used to infer that amygdala lesions impair fear memory. However, if animals are allowed to show memory by other means, e.g., avoid the location where conditioning happened (Selden et al., 1991; Vazdarjanova and McGaugh, 1998) or learn to escape from the auditory CS (Amorapanth et al., 2000), then they can exhibit fear memory even with substantial lesions of the amygdala (see also Killcross et al., 1997; Lehmann et al., 2000; Tranel and Damasio, 1993).
An evaluation of the function of the MGm/PIN in auditory fear conditioning will take the form of applying the same four criteria used to conclude that the AL is the site of memory formation and storage, i.e., (1) lesion/inactivation impairs AFC, (2) LTP can be produced by stimulation of afferents, (3) responses to the CS are enhanced as a result of CS–US association and (4) CS and US afferents converge. If the MGm/PIN satisfies all four criteria, one may reasonably question why the AMYG model restricts their function as CS and US sensory relays. As part of this process, we begin with a very brief summary of the anatomy and physiology of the medial geniculate body.
4. Relevant anatomy and physiology of the medial geniculate body
Naturally, the most relevant medial geniculate structures are the medial division and the posterior intralaminar nucleus. The MGm has been more intensively studied and so will be emphasized. But to fully appreciate the role of the MGm/PIN in auditory fear learning, it will prove helpful to contrast it with the ventral division of the medial geniculate (MGv). Modern accounts of the structure and connections of the medial geniculate body (MGB) are plentiful and indicate that the major features of the MGv and the MGm appear to be conserved across mammals, including humans (e.g., Clerici and Coleman, 1990; Herkenham, 1980, 1986; Jordon, 1973; Kimura et al., 2003, 2005; Kudo et al., 1986; LeDoux et al., 1985; Linke and Schwegler, 2000; Morest, 1964, 1965a; Oliver and Hall, 1975, 1978; Rouiller and Welker, 1991; Scheel, 1988; Winer, 1984a, 1984b, 1984c, 1985; Winer and Morest, 1983a, 1983b; Winer and Wenstrup, 1994a, 1994b; Winer et al., 1999a, 1999b, 2005).
4.1. The ventral division (MGv)
The MGv is the lemniscal component of the medial geniculate body. The MGv's modality specificity is confined to acoustic stimulation (Aitkin and Webster, 1972; Lippe and Weinberger, 1973a, 1973b). It receives topographic projections from the central nucleus of the inferior colliculus, contains a tonotopic organization (Aitkin and Webster, 1972; Calford and Webster, 1981; Cetas et al., 2001; Imig and Morel, 1985), and its cells exhibit sharp tuning (Calford and Webster, 1981). The MGv is the major subcortical input to the primary auditory cortex (A1), where it projects topographically to layers III and IV, forming the substrate of its tonotopic map. The MGv also projects to other fields that contain a tonotopic organization, most studied in the cat: anterior auditory field (AAF), posterior auditory field (PAF), ventral field (Ve) and ventral posterior field (VP) (e.g., Andersen et al., 1980; Brandner and Redies, 1990; Huang and Winer, 2000; Imig and Morel, 1983; Merzenich et al., 1982; Raczkowski et al., 1976; Redies et al., 1989; Romanski and LeDoux, 1993; Winer and Larue, 1987; Winer et al., 1999b).
4.2. The medial division (MGm)
The MGm has several distinct features. Ascending projections to the MGm are remarkably diverse, suggesting multimodal response properties. In addition to auditory inputs from the central and external nuclei of the inferior colliculus (Kudo and Niimi, 1980; Linke, 1999a; Powell and Hatton, 1969; Wenstrup et al., 1994) and the ventral nucleus of the lateral lemniscus (Whitley and Henkel, 1984), the MGm receives efferents from the deep layers of the superior colliculus (Linke, 1999a; Morest and Winer, 1986), lateral tegmental system (Morest, 1965b), spinothalamic tract (Boivie, 1971; Whitlock and Perl, 1961; Zhang and Giesler, 2005), tooth pulp via the spinal trigeminal nucleus (Barnett et al., 1995), the vestibular nuclei (Roucoux-Hanus and Boisacq-Schepens, 1977) and the fastigial nuclear group of the cerebellum (Raffaele et al., 1969). Auditory (from the inferior colliculus) and somatosensory (from the spinal cord) projections overlap in the MGm (LeDoux et al., 1987).
Its cellular responses to tones are quite heterogeneous; some exhibit narrow tuning while others have exceptionally broad, sometimes multi-peaked, frequency tuning (Aitkin, 1973). Moreover, the MGm lacks tonotopic organization, as expected for such varied frequency response characteristics. Perhaps most importantly, and most strikingly different from the MGv, the MGm is highly multimodal, consistent with its diverse inputs. In addition to its major auditory character, cells in the MGm respond to tactile, thermal, nociceptive, vestibular and visceral stimulation (Blum et al., 1979; Brinkhus et al., 1979; Carstens and Yokota, 1980; Khorevin, 1980; Love and Scott, 1969; Wepsic, 1966). Single neurons in the MGm exhibit convergence of tone and footshock stimulation (Bordi and LeDoux, 1994a, 1994b).
As an interim summary, we should note that ascending convergence of auditory and somatosensory/nociceptive input to the MGm satisfies Criterion #4 for the lateral nucleus of the amygdala (above).
Efferents of the MGm exhibit a unique pattern. This nucleus projects to all auditory cortical fields, both tonotopic and non-tonotopic, as well as some adjacent non-auditory regions of the temporal cortex including agranular insular, ectorhinal, perirhinal and visceral cortices (Linke and Schwegler, 2000). Morover, in contradistinction to the lemniscal MGv, the MGm does not project to layers III and IV but rather to layers I and also VI (Huang and Winer, 2000; Linke and Schwegler, 2000; Niimi and Naito, 1974; Niimi et al., 1984; Ryugo and Killackey, 1974). Remarkably, the layer I projections within auditory cortical fields appear to be via giant axons. This suggests more rapid conduction to auditory cortex from the MGm than from the MGv (Huang and Winer, 2000). Its synapses on the apical dendrites of auditory cortical pyramidal cells provide for modulation of their excitability. Finally, the MGm projects to subcortical structures outside of the auditory system, principally the AL and basal ganglia (Clugnet et al., 1988, 1990; LeDoux et al., 1985; Ryugo and Killackey, 1974).
4.3. The posterior intralaminar nucleus
Like the MGm, the PIN is multimodal. Its major inputs are auditory (e.g., inferior colliculus, Linke, 1999a) but it also receives projections from the spinal cord and these overlap the auditory inputs (LeDoux et al., 1987). The PIN also receives inputs from the deep layers of the superior colliculus. Ascending projections of the PIN encompass layer I of auditory cortex, particularly the secondary field, and non-auditory regions such as ectorhinal, perirhinal, agranular insular and visceral cortex (Linke, 1999b; Linke and Schwegler, 2000). Of importance, the PIN, like the MGm, projects to the AL (Linke et al., 2000).
The PIN has been implicated in somatic pain (e.g., Peschanski et al., 1981). It responds to tone and footshock stimulation and single PIN neurons respond to both stimuli (Bordi and LeDoux, 1994a, 1994b). Thus, like the MGm, auditory and shock input to the PIN satisfy Criterion #4 of CS and US convergence.
4.4. Anatomical factors concerning MGm/PIN as sensory relay stations
The AMYG model holds that the MGm and PIN serve only to relay auditory and nociceptive information to the AL. This is consistent with the fact that these nuclei respond to such stimulation and project to the AL. However, it does not take into account that auditory and somatosensory/nociceptive input converge on the same neurons (above).
Moreover, given the extensive research on the anatomy of the medial geniculate, one ought to consider the implications of its structure for potential functions as a relay nucleus. That the MGv, as the lemniscal component of the MGB, “relays” precise auditory information to the primary auditory cortex is not at issue. How does the structure of the MGv subserve such a relay function? Fig. 3 presents the result of anatomical study which shows the types and organization of projecting cells in the medial geniculate body. For present purposes, we can focus on the MGv and compare it to the MGm.
Fig. 3.
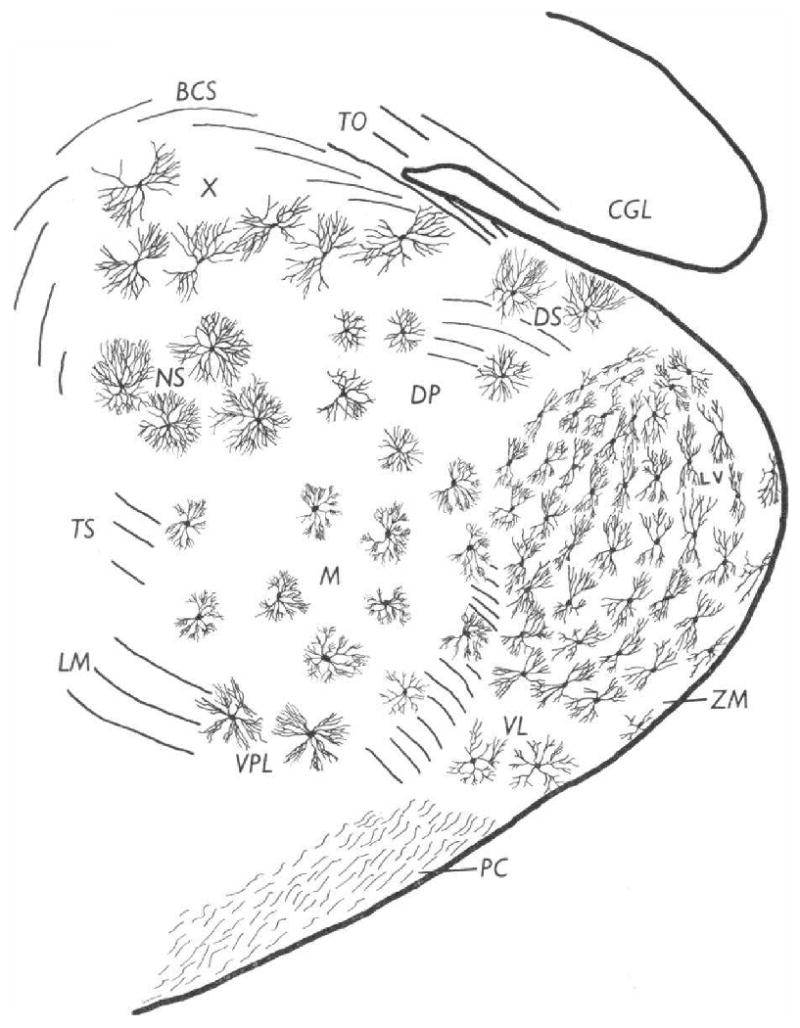
Typical distribution of principal neuron types at the junction of the anterior and middle thirds of the medial geniculate body. Transverse section, Golgi–Cox. 15-day-old cat. (Morest, 1964)
The differences are conspicuous. The cortical projection cells of the MGv have restricted “tufted” dendritic fields at opposite ends of the soma; these neurons may be said to resemble sheaves of wheat. The MGv projection neurons are organized in parallel lamina within the MGv, which contains a tonotopic map within this nucleus and is a basis for tonotopic auditory cortical maps. In contrast, the MGm is composed of a far more heterogeneous population of cells types, dominated by neurons with “radiate” dendritic fields that tend to surround the soma. A substantial population of cells in the MGm are of very large size, hence are called “magnocellular neurons”. This magnocellular type is highlighted in Fig. 3.
Although a detailed account of the morphological features of the MGv and MGm is far beyond the scope of this paper, functional interpretations have been offered. For example, with respect to the MGv, Morest states:
“… the structure of the ventral nucleus is suitable for relay of a highly differentiated sensory input or for differentiation in its own right, of a relatively homogeneous, viz. auditory, modality.” (Morest, 1965b, p. 625)
Concerning the anatomical implications for the function of the medial division, we find:
“The main finding of this study is the structural diversity of the neurons and axons in the medial division of the medial geniculate body. This contrasts with the more limited stereotypy of the ventral and the dorsal division. These divisions must differ fundamentally from the medial one in organization and function.” (Winer and Morest, 1983a, pp. 2641, 2643)
“The medial division thus acts as a polymodal hub for the redistribution of thalamcortical and thalamolimbic influence. Since medial division neurons show enduring associative changes in learning paradigms …, they could exert a polysynaptic influence on the responses of cortical, subcortical and brain stem neurons to acoustic and non-acoustic stimuli.” (Winer et al., 1999a, p. 36)
Thus, the anatomical characteristics of the medial division, particularly when compared to the ventral division, do not favor the former as a purely sensory relay station to the amygdala or any other structures.
4.5. Summary and précis
A review of the anatomy and physiology of the medial division of the medial geniculate and the posterior intralaminar nuclei reveal that they receive and exhibit convergent input from ascending auditory and somatosensory/nociceptive sources. Moreover, a comparison of the major anatomical characteristics of the medial division with the lemniscal ventral division strongly suggests that the former is not suited for the role of “sensory relay”. The criterion for convergence of the CS and the US used to justify the lateral nucleus of the amygdala in the AMYG model, i.e., Criterion #4, is met by the MGm/PIN. Therefore, it is now appropriate to evaluate the remaining three criteria. This will be done by brief reviews of (a) the effects of stimulation of the MGm/PIN and of afferents to the MGm, (b) the effects of lesioning these nuclei and (c) neurophysiological studies of the formation and specificity of plasticity in the MGm. Synaptic and molecular approaches, which are not part of the four criteria, will also receive consideration.
5. Stimulation of the MGm and PIN and of afferents to the MGm
Stimulation of the MGm itself induces heterosynaptic LTP of auditory responses in A1. Pairing a click with a succeeding brief train of stimulation increases the amplitude of click-elicited local field potentials over hours, indicating its powerful ability to modulate auditory processing in the primary auditory field (Weinberger et al., 1995) (Fig. 4).
Fig. 4.
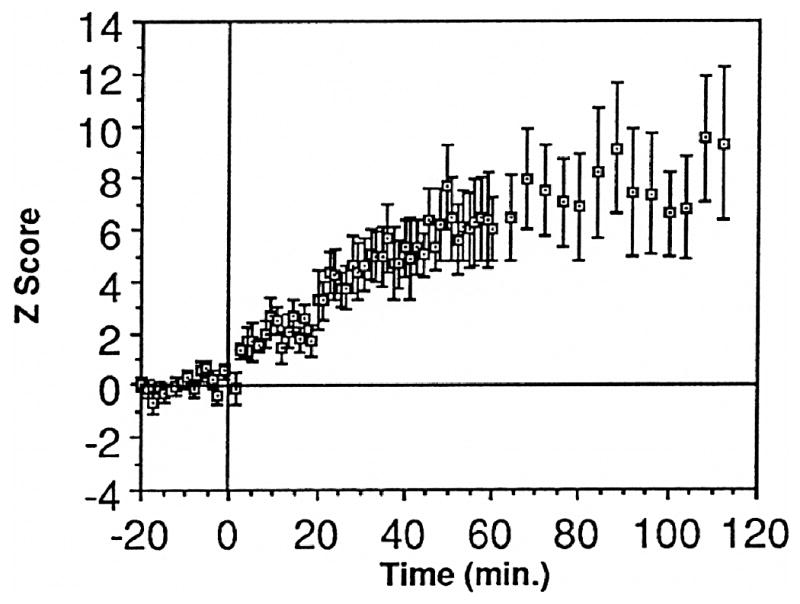
Heterosynaptic long term potentiation (LTP) of auditory stimuli by the MGm. Stimulation of the MGm (200 Hz, 50 ms) paired with a preceding click produces LTP of click evoked local field potentials (LFPs) in the primary auditory cortex. Group mean (± SE) before and for 2 h after 30 click–stimulation pairings (n = 9). Probe clicks were given at 0.2 Hz before and for 60 min after pairing, and less often thereafter. Note the large magnitude of potentiation (ordinate Z-scores above pre-stimulation). Controls exhibited no facilitation. (Weinberger et al., 1995)
Stimulation of the MGm/PIN can also be used to determine the source of nociceptive unconditioned stimulus input to the lateral amygdala. It will be recalled that the source of the US was reported to be both the MGm and the PIN because lesions of these nuclei reduced footshock-induced c-Fos induction in the AL (Lanuza et al., 2008). Previously, several nuclei in the medial geniculate had been stimulated as a proxy for the nociceptive US, to determine which, if any, could support auditory fear conditioning. A tone was paired with stimulation of the MGm, PIN and also the MGv and the dorsal division (MGd), in different groups. Cardiac conditioned responses in waking animals were used as the index of learning as conditioned bradycardia previously had been well established as a sensitive and reliable measure of conditioned fear (e.g., Fehr and Stern, 1965; Gallagher et al., 1980; Obrist, 1968; Powell and Kazis, 1976). Only stimulation of the PIN proved to be able to serve as an effective US; there was no fundamental difference between cardiac conditioning established with footshock and with PIN stimulation. Sensitization control groups established that the effects of PIN stimulation were associative (Cruikshank et al., 1992) (Fig. 5).
Fig. 5.
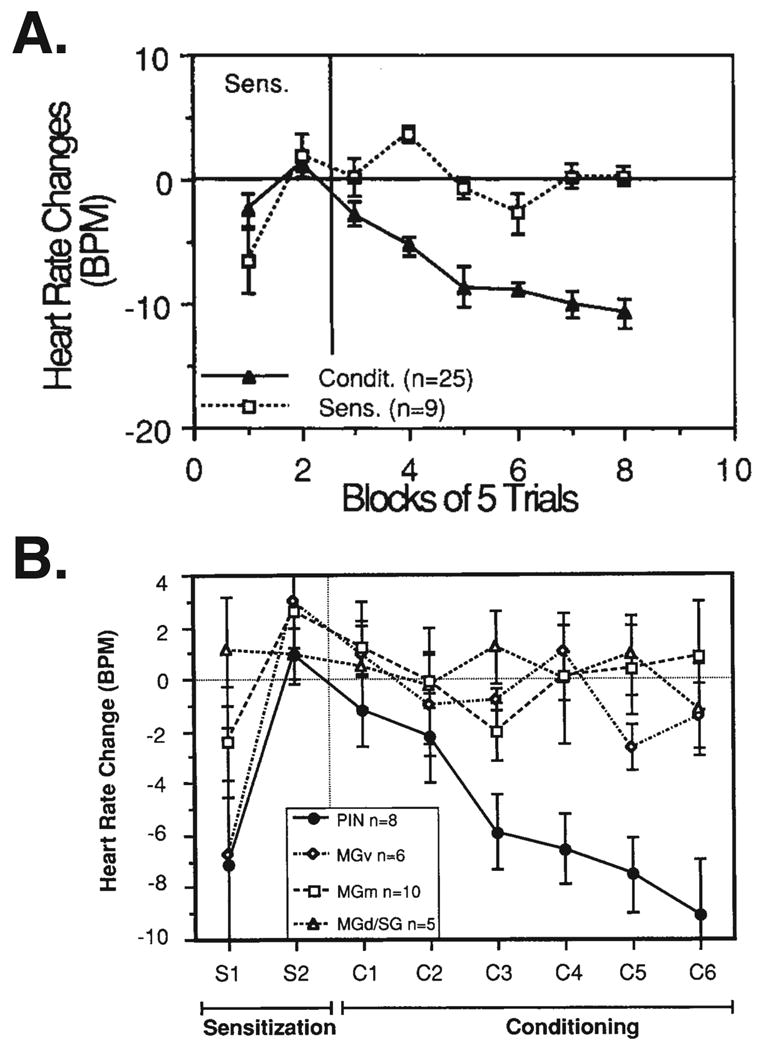
Stimulation of the posterior intralaminar nucleus (PIN), but not other medial geniculate nuclei, serves as an effective unconditioned stimulus (US) in auditory fear conditioning. (A) Behavioral conditioned bradycardia (“Condit”, n = 25) or lack thereof in a sensitization control group (“Sens.”, n = 9) to tone and shock. Guinea pigs received 10 trials of the CS and US unpaired followed by 30 trials of CS-US paired in the Conditioning group, while there was no change in protocol for the Sensitization control group. Note elicitation of novelty-induced bradycardia during the first five trials, which rapidly habituated by the end of the second block of unpaired presentation (block 2) for both groups. However, when pairing was initiated, the Conditioning group rapidly developed CS-elicited bradycardia during the first block of five trials, which continued to increase in magnitude during training (Edeline and Weinberger, 1992). (B) Substitution of stimulation of the PIN for a shock US produces conditioned bradycardia. However, stimulation of the ventral division (MGv), medial division (MGm) and dorsal/suprageniculate nuclei (MGd/SG, data combined) was ineffective and did not differ significantly from the Sensitization control group that received tone and shock unpaired (see [A] above). The ordinate is the magnitude of change in heart rate during the CS relative to pre-trial baseline. (Cruikshank et al., 1992)
This level of specificity of behavioral effects is notable. These findings indicate that the PIN, but not the MGm, is the source of behaviorally effective nociceptive US input to the AL and/or elsewhere. Thus, the induction of c-Fos by thalamic stimulation appears to be insufficient to determine the source of the effective US input to the amygdala in AFC. A more direct behaviorally-validated approach seems preferable. The nociceptive input to the MGm (e.g., Boivie, 1971) may be necessary for associative plasticity to develop in this nucleus, and for the MGm's effects on the auditory cortex (below), but apparently is not itself an effective US that is relayed to the AL or elsewhere.
More relevant to the issue of Criterion #2 (i.e., the induction of LTP) are studies of stimulation of afferents to the MGm/PIN. Brief, high frequency stimulation of the brachium of the inferior colliculus (BIC), a major input to the MGm, does induce LTP in this nucleus (Fig. 6). Antidromic responses in the inferior colliculus were unchanged, indicating that this potentiation is endemic to the MGm rather than due to changes in the conduction properties of BIC axons (Gerren and Weinberger, 1983). These findings satisfy Criterion #2.
Fig. 6.
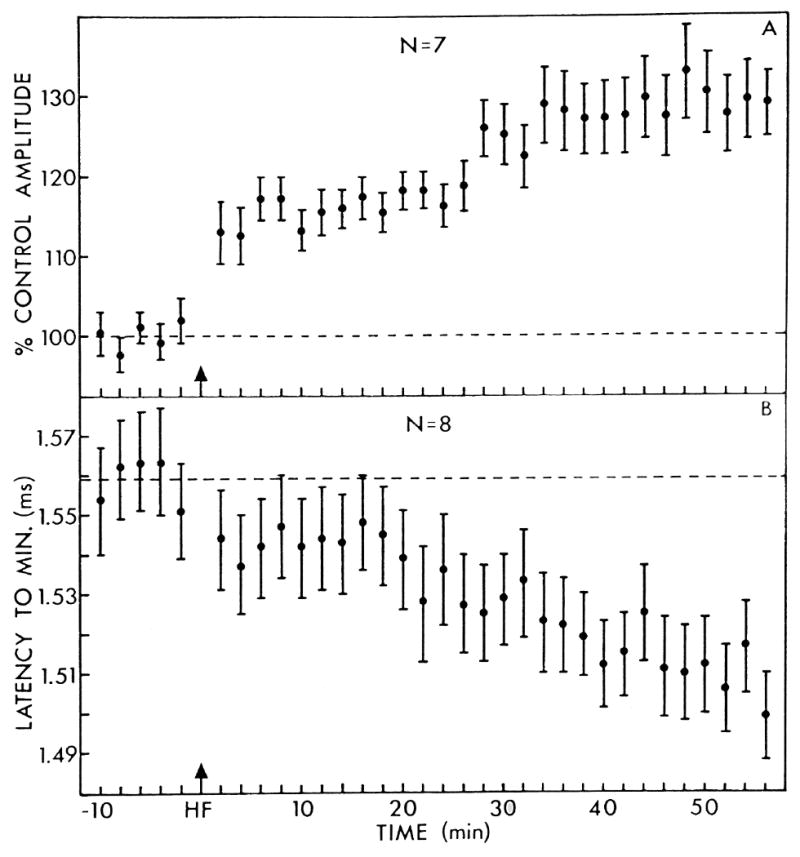
Long term potentiation (LTP) of responses (local field potentials) of the MGm to brief high frequency (100 Hz, 285 ms) stimulation of its afferents from the brachium of the inferior colliculus (BIC). (A) The average amplitude of the MGm response, with respect to the baseline (n = 7). Note the growth in amplitude over the 1 h post-stimulation recording period. (B) The mean latency to peak of the local field potential. Note the decrease in latency over time. Vertical bars represent ± 1 standard error of the mean. (Gerren and Weinberger, 1983)
6. Lesions of the auditory thalamus
Lesions of the entire medial geniculate, or only the MGm/PIN region, impair or prevent AFC (e.g., Campeau and Davis, 1995; LeDoux et al., 1984, 1986; McCabe et al., 1993). Lesions of the MGm also interfere both with the acquisition (Jarrell et al., 1986a, 1986b) and retention of two-tone cardiac discrimination learning. The latter impairment is not a deficit in the formation of conditioned responses but in the failure to discriminate between the CS+ and CS– frequencies, as evident in continued responses to the CS– (Jarrell et al., 1987). Medial geniculate lesions that could encompass all of its nuclei disrupt learning-induced plasticity in the basolateral nucleus of the amygdala (BLA), the anterior and posterior cingulate cortex and the anterior– ventral and medial–dorsal thalamic nuclei. They also retard behavioral avoidance fear conditioning. Importantly, within the MGB, only the size of lesions of the MGm/PIN (the MGm and PIN lesions were not assessed separately) was correlated with the severity of behavioral impairment (Poremba and Gabriel, 1997). It appears that two parallel auditory pathways can mediate fear conditioning as indexed by freezing: MGm/PIN to the AL, and MGv to primary auditory cortex to non-primary auditory cortex to the AL. The cortical route is thought to subserve complex acoustic stimuli or discriminations while the thalamic route is considered the basic circuit for simple sounds (Romanski and LeDoux, 1992).
That lesions of the MGm/PIN impair AFC satisfies Criterion #1 for concluding that the AL is the site of storage of fear memories. Therefore, the MGm/PIN cannot be discounted on the basis of this criterion.
However, it should be understood that if a “sensory” structure (such as the MGm/PIN) also has associative functions, then lesions could not differentiate between these two functions. Thus, by claiming that lesions of the MGm/PIN merely interrupt the relay of sensory information to the amygdala, the proponents of the AMYG model would seem to hold that the MGm/PIN has no such associative function. We can now turn to this issue.
7. Associative plasticity in the MGm/PIN
7.1. Plasticity during fear conditioning
Initial studies of the medial geniculate in auditory fear conditioning detected increased amplitude of evoked potentials and increased neuronal discharges to acoustic conditioned stimuli (Buchwald et al., 1966; Halas et al., 1970; Sommer-Smith and Morocutti, 1970; Weinberger et al., 1972). While critical in establishing the involvement of the auditory thalamus in AFC, they failed to localize the sites of plasticity within the MGB. Implication of the medial division began with Gabriel and co-workers in studies of instrumental avoidance learning in the rabbit (Gabriel et al., 1975, 1976). Such avoidance learning necessarily implies prior association of sound and shock. This finding was followed by a comparison of unit discharges in the MGv, MGd and MGm. Associative plasticity, i.e., pairing-dependent increase of responses to an acoustic CS was found only in the MGm (Ryugo and Weinberger, 1978). Since that time, there have been numerous replications and extensions of associative plasticity in the MGm. Table 1 lists more than twenty-five reports of this effect.
Table 1.
Electrophysiological studies of the MGm/PIN complex during behavioral conditioning
| Reference | Subject | Associative Plasticity? |
|---|---|---|
| Gabriel et al. 1975 | Rabbit | Yes |
| Gabriel et al. 1976 | Rabbit | Yes |
| Disterhoft and Stuart, 1976 | Rat | Yes |
| Ryugo and Weinberger, 1978 | Cat | Yes |
| Birt et al., 1979 | Rat | Yes |
| Birt and Olds, 1981 | Rat | Yes |
| Weinberger, 1982 | Cat | Yes |
| Jarrell et al., 1986a | Rabbit | Yes |
| LeDoux, 1986 | Rat | Yes |
| Edeline et al., 1988 | Rat | Yes |
| Supple and Kapp, 1989 | Rabbit | Yes |
| Edeline et al., 1990 | Rat | Yes |
| Edeline, 1990 | Rat | Yes |
| Edeline and Weinberger, 1992 | Guinea pig | Yes |
| Hennevin et al., 1992 | Rat | Yes |
| Lennartz and Weinberger, 1992 | Guinea pig | Yes |
| Hennevin et al., 1993 | Rat | Yes |
| McEchron et al., 1995 | Rabbit | Yes |
| McEchron et al., 1996 | Rabbit | Yes |
| O'Connor et al., 1997 | Rabbit | Yes |
| Hennevin et al., 1998 | Rat | Yes |
| Maho and Hennevin, 2002 | Rat | Yes |
| Poremba and Gabriel, 2001 | Rabbit | Yes |
| Maren et al., 2001 | Rat | Yes |
| Talk et al., 2004 | Rabbit | Yes |
| Hennevin and Maho, 2005 | Rat | Yes |
| Morris et al., 1998 | Human | Yes * |
PET neuroimaging, subnucleus of MG not identifiable
This MGm plasticity is robust because, while induced in the waking state, it can be expressed during REM (“paradoxical”) sleep (Hennevin et al., 1993, 1998), slow wave sleep (Hennevin and Maho, 2005) and even under general anesthesia (Lennartz and Weinberger, 1992). This plasticity even can be induced with subjects under light Ketamine general anesthesia (Edeline, 1990). The MGm also has been implicated in both short-term and long-term memory. For example, MGm plasticity has been specifically identified as a substrate for short-term memory in trace conditioning. That is, when there is a silent gap between the offset of the CS tone and the onset of the shock US, associative increases in response to the CS are maintained (O'Connor et al., 1997). MGm plasticity also exhibits long-term retention, plasticity being retained for at least 45 days, the longest period tested (Edeline et al., 1990). Classical conditioning in the human induces associative plasticity in the medial geniculate, although the precise location within the auditory thalamus could not be determined with the available imaging methods (Morris et al., 1998).
7.2. CS-specific tuning shifts in the MGm
In addition to the development of plasticity during AFC, its degree of frequency specificity can be determined by comparing frequency receptive fields (RF) before and after training (Bakin and Weinberger, 1990). Neurons in the MGm develop CS-specific tuning plasticity; responses to the conditioned stimulus develop the largest response increases while most other frequencies change less or decrease in response (Figs. 7A1 and 7A2). Specific plasticity is often sufficiently great to shift tuning so that the CS frequency becomes the new best frequency (Figs. 7A3 and 7A4). Tuning plasticity is maintained for at least an hour (the longest period tested). Moreover, it is highly specific: frequencies 0.1 octave from the CS frequency exhibit only small increases and those 0.2 or more octaves away generally develop no change or decrease responses (Edeline and Weinberger, 1992) (Fig. 7B). CS specific receptive field plasticity in the MGm is associative because it requires CS–US pairing; sensitization control subjects never developed CS-specific plasticity. Thus, although tuning curves are generally broad and may be multi-peaked, the MGm develops highly-specific associative receptive field plasticity. That is, more spikes are elicited by the CS frequency relative to other frequencies, and these are presumably sent on to the AL, the cortex and any other targets of MGm projections.
Fig. 7.
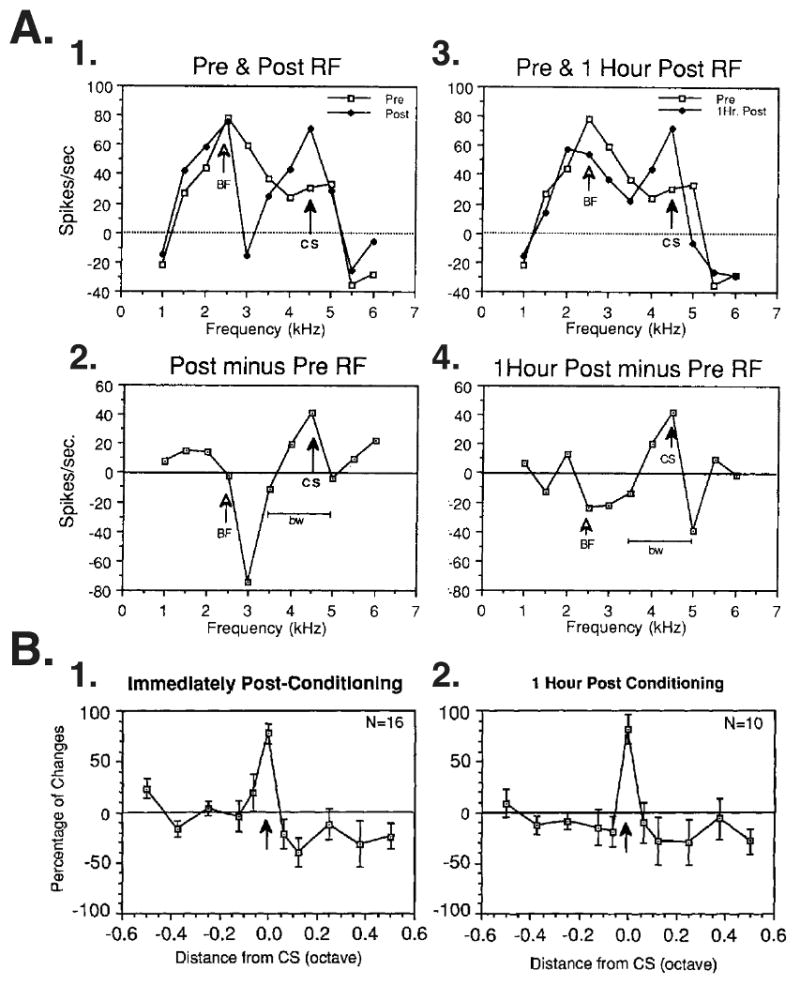
CS-specific receptive field plasticity in the medial division of the medial geniculate body in auditory fear conditioning. Guinea pigs received 30 trials of tone–shock pairing and developed behavior signs of AFC, i.e., conditioned bradycardia to the CS tone. (A) An example of frequency receptive fields before (“Pre”) and both immediately and 1 hour after (“Post”) the single conditioning session. (A1) Before conditioning, the best frequency was 2.5 kHz; the CS was selected to be 4.5 kHz. Immediately post-conditioning, there were increased responses at 4.0 and 4.5 kHz and some lower frequencies. (A2) RF difference function, i.e., the pre RF was subtracted from the post-training RF. Note that the maximum increase is at 4.5 kHz, the CS frequency. There was no change in response of the pre-training best frequency of 2.5 kHz. (A3) Comparable data obtained after a retention period of 1 h, i.e., with no additional training, shows continued development (“neural consolidation”). Compared to the pre-training RF, responses to some non-CS frequencies have declined, producing a shift in tuning so that the CS frequency became the new BF. (A4) The RF difference function shows that the largest increase in response is maintained at the CS frequency. In this case, the bandwidth (bw) of CS-related facilitation of response has been maintained as well. (B) MGm group data shown as normalized mean group RF difference functions for CS-specific plasticity. Graphs show percent change as a function of octave distance from the CS frequency. (B1) Immediately after conditioning (n = 16), the CS frequency showed a marked increase while frequencies as close as 0.1 octaves showed no change or decreased response. (B2) One hour later, the magnitude and specificity of CS-specific RF plasticity were maintained for cells still recorded (n = 10). CS-specific facilitation and CS-directed tuning shifts were never found in sensitization control subjects. (Edeline and Weinberger, 1992)
7.3. Synaptic and molecular findings
Synaptic and molecular findings are also used to justify the acquisition and storage of auditory fear memories in the AL (e.g., Apergis-Schoute et al., 2005; Maren et al., 2003). However, these approaches also support the MGm. For example, fear conditioning produces increased presynaptic transmitter release from MGm terminals within the lateral amygdala (McKernan and Shinnick-Gallagher, 1997). This could result from pre-synaptic processes within the AL, increased intracellular activity endemic to the MGm or both. Other synaptic findings directly implicate the MGm. Thus, fear conditioning to tone induces specific increased synaptic strength of responses to auditory afferents from the inferior colliculus to the MGm but not responses to other non-auditory afferents that are not involved in auditory fear conditioning (McEchron et al., 1996).
Recent findings have demonstrated the necessity of the synthesis of new protein and mRNA in the MGm for auditory fear conditioning (Parsons et al., 2006). Moreover, gain-of-function within the MGm/PIN facilitates AFC. First, auditory fear conditioning was found to specifically increase the levels of activated CREB in these nuclei. Second, by the use of viral vectors to increase CREB, the authors found facilitation specifically of AFC, but not contextual conditioning or auditory processing itself (Han et al., 2008).
8. Summary
The previous section summarized findings that the MGm develops plasticity during AFC. Specifically, responses to conditioned auditory stimuli are increased due to the association of a sound with an aversive stimulus. Beyond simply developing increased responses to an acoustic CS, the MGm develops CS-specific frequency tuning shifts, an effect that has not been demonstrated for the AL, which in any event responds mainly to high frequencies, not to the full acoustic spectrum to which the MGm responds and to which animals can be conditioned (Bordi and LeDoux, 1992; Bordi et al., 1993). Associative plasticity in the MGm fulfills Criterion #3. Thus, overall, plasticity in the MGm satisfies the same four criteria that justify the conclusion that the AL is the site of the storage of memories for auditory fear conditioning.
That the MGm fulfills the same criteria used to justify auditory fear memory storage in the AL does not imply that the AL contributes nothing new beyond the capabilities of the MGm. However, this implication of MGm/PIN associative plasticity, which is excluded from the AMYG model, has been enunciated:
“This ubiquitous plasticity in the conditioning circuitry would be trivial if plasticity in all levels of the pathway reflects learning by some early station (such as the auditory thalamus [italics added]).” (LeDoux, 1995, p. 217).
However, there are alternatives. For example, as the initial stage of CS–US convergence, the MGm/PIN could be the “root” of the circuitry underlying AFC while “downstream” structures, including the amygdala, could make their own, special contributions. This alternative is consistent with the finding that while the MGm projects to the auditory cortex, the latter exhibits specific characteristics of learning-induced plasticity that are not present in the MGm (Weinberger, 2007).
Clearly, the issue is not which is more important, the AL or the MGm/PIN. Rather, we need to understand the respective roles of the AL and the MGm/PIN in auditory fear conditioning. Thus, an important question is “What is the influence of ‘upstream’ MGm plasticity on the amygdala?” A closely related second question is “What are the functions of associative plasticity in the AL and in the MGm?” Detailed consideration of these issues is beyond the scope of this paper. But insofar as the AMYG model is widely assumed to be correct, i.e., that the role of the MGm/PIN is simply to relay CS and US information to the AL, the matter is generally considered to be closed. In short, there has been no sign in the literature that either question has been seriously addressed. We may then ask why, despite fulfilling the same criteria as the AL, has the MGm been virtually ignored as a locus of auditory fear memory storage?
8.1. Concerning exclusion of MGm/PIN associative plasticity from the AMYG model
It is a canon of science that all evidence should be considered, provided that it is trustworthy. The validity of the findings relevant to the issue of the role of the MGm/PIN in auditory fear conditioning has not been seriously questioned. In fact, CS–US convergence, associative plasticity and LTP in the auditory thalamus were included in discussions of AFC during early phases of attempts to determine the involved circuitry. For example:
“The extent to which afferent projections to the thalamus from the inferior colliculus and spinal cord converge is thus graded in the caudorostral plane, with the greatest overlap occurring at the level of the rostral third of the MGB. These observations identify potential areas of acoustic and somesthetic integration and may account for observations of neuronal plasticity in the thalamus in response to the pairing of acoustic and somesthetic inputs.” (LeDoux et al., 1987, p. 123)
“… a number of studies have shown that unit activity in MG is modified during auditory conditioning …” (LeDoux et al., 1984, p. 696)
“The fact that LTP has been demonstrated in both the medial MGB and AL, the origin and termination of the thalamoamygdala fear conditioning pathway, respectively, suggests the possibility of an ‘LTP cascade’ in fear conditioning.” (Clugnet and LeDoux, 1990, p. 2823)
Rather, it seems that by the time that the AMYG model was formally introduced in 1992 (LeDoux, 1992), a shift in focus to the amygdala had eliminated the auditory thalamus from inclusion as an associative component of the circuitry. I am grateful to Dr. LeDoux for providing the following explanation:
“I was thinking that aud thal [sic] and cortex would be involved in plasticity that enhances the processing within a particular modality while the amygdala would be involved in modality independent plasticity for multiple modalities. So plasticity MGm/PIN [sic] might enhance the detection of auditory stimuli and enhance activation of LA by such stimuli.” (J.E. LeDoux, personal communication, December 2009)
While this elucidation does not fully explain assigning limited sensory relay roles to the MGm/PIN, it does endorse their status as developing plasticity that would then enhance processing of auditory fear information in the lateral amygdala. The emphasis on the lateral amygdala, as thought to be common to all sensory modalities in fear learning, is understandable. However, it has been at the cost of the promulgation of an incomplete and potentially misleading, by omission, explanation of auditory fear conditioning.
9. Additional developments
Four additional developments require consideration: (a) a formal revision of the AMYG model, (b) potential additional routes of CS and US input to the amygdala, (c) the effects of amygdala lesions on associative plasticity in the MGm/PIN, and (d) a recent novel model of auditory fear conditioning.
9.1. Inclusion of the central nucleus of the amygdala
The AMYG model has been amended recently. The original model holds that the function of the central nucleus (ACE) is the expression of fear conditioning that has been acquired and stored in the AL. However, on the basis of functional inactivation (muscimol) and presumptive inhibition of protein synthesis (anisomycin) applied to the ACE, this nucleus is now considered also to be a site of acquisition and storage. Accordingly, the revised AMYG model holds that the auditory CS and nociceptive US are relayed not only to the AL but also to the ACE (Wilensky et al., 2006). However, insofar as the revised model continues to ignore the associative plasticity and other features of the MGm/PIN, it has no new implications for the current discourse.
9.2. Potential additional routes of CS and US input to the amygdala
Recently, it has been noted that, in addition to projections from the MGm and PIN, there are other potential routes of CS and US input to the amygdala. On the CS side, these include the possibility that the inferior colliculus, dorsal nucleus of the lateral lemniscus and the nucleus of the brachium of the inferior colliculus might send auditory information via the posterior nucleus of the thalamus (PO) to the amygdala. As for nociceptive US input, the spinothalamic tract is known also to project to PO and the parabrachial nucleus projects both to PO and to the amygdala (Pape and Paré, 2010).
The realization of the complexity of connectivity and multiplicity of potential subcortical CS and US inputs to the amygdala is a welcome development. As the amygdala (particularly the BLA) has extremely powerful modulatory influences on memory consolidation (e.g., McGaugh, 2004), it is not surprising that it would receive information from a large variety of sources. The PO is affiliated with the somatosensory system and may serve for that system similar functions that the MGm/PIN serves for the auditory system. The functional importance of these additional routes to the amygdala needs to be determined. At present, of all potential subcortical inputs to the amygdala, only the MGm/PIN is known to satisfy the criteria required to qualify as a locus of either or both acquisition and memory storage in AFC. The existence of potential alternative CS and US inputs to the amygdala is not antithetical to the established properties of the MGm/PIN. Clearly, much more research is needed before the entire circuitry for AFC is adequately determined.
9.3. Amygdala lesions and MGm plasticity
Three studies have reported that muscimol inactivation of the amygdala impairs the formation of learning-induced plasticity in the MGm: Maren et al., 2001; Poremba and Gabriel, 2001; Talk et al., 2004. The route of such influence is unknown as the amygdala does not project to the medial geniculate, in distinction to the efferents of the MGm to the amygdala. One possible explanation is the spread of muscimol to the MGm. At the volumes employed in these studies (0.5 μl), radiolabeling studies have shown the spread of muscimol to be millimeters farther than has been assumed (Edeline et al., 2002). Another, yet unexplored, possibility is that the amygdala influences the auditory cortex, which in turn modulates tuning plasticity in the MGm/PIN via established corticofugal connections (Winer, 2006).
However, even if MGm associative plasticity were dependent on the amygdala via some indirect route, MGm plasticity still would be projected to the AL during learning. While one might argue that the AL develops plasticity earlier than the MGm during AFC, so that the MGm's plasticity would have minimal or no effect on the AL, two observations oppose this scenario. First, the AL does not develop plasticity before the MGm; they both develop plasticity within five trials. Moreover, the latency of associative increase in response to the CS is shorter in the MGm (Hennevin et al., 1998; McEchron et al., 1995). Second, MGm plasticity can be expressed in the absence of plasticity in the AL (Maho and Hennevin, 2002). As this latter study applies to appetitive conditioning, it should be repeated for auditory fear conditioning. Finally, if plasticity in the MGm depends upon the amygdala, then a positive feedback loop should result because the MGm would then send plasticity to the AL. An alternative would be that the MGm would send only auditory, but not associative, effects to the AL while the MGm develops amygdala-dependent plasticity. However, there is no known physiological mechanism by which the additional spikes elicited in the MGm due to association would not be “passed on” to the AL, while those spikes that reflect the original response to tone before training would be the only ones to make that passage during and after training. While this issue is currently unsettled, at the very least Occam's Razor should be kept in mind.
9.4. Recent cortical model of auditory fear conditioning
Recently, a model of AFC has been proposed in which CS–US convergence first occurs in “associated cortex” (sic), which then projects it to the amygdala (Suga, 2008). As this model completely ignores the established associative plasticity in the MGm, as well as CS–US convergence, etc. it is not further considered here. (For a more detailed discussion of this model see Weinberger, 2008.)
10. Conclusions and future directions
What can be concluded from the foregoing? Most clearly, the current AMYG model of auditory fear conditioning cannot be accurate because it treats the MGm/PIN as sensory relays for auditory and nociceptive input to the lateral amygdala. But the MGm/PIN complex satisfies the same four criteria used to conclude that the AL is the locus of auditory fear memories. Therefore, logic dictates that the MGm/PIN would then also have to be a locus of auditory fear memories. Consequently, the MGm/PIN cannot be excluded, unless the AL is also excluded, from associative and mnemonic functions in auditory fear conditioning.
The past twenty years have seen a sea change in the conceptual foundations of thalamo-cortical levels of the auditory system. Once regarded as purely an acoustic analyzer, studies in behaving, learning subjects have revealed quite another picture, one of a highly dynamic system that can rapidly develop experience-dependent neuronal plasticity, which presumably serves not only to analyze sounds but also to comprehend them and use sound information in the service of immediate and long-term adaptive behavior. While most recent attention has focused on the primary and other fields of the auditory cortex, the first study of the medial thalamus by Gabriel and colleagues in the mid-1970s (Gabriel et al., 1975) was actually complementary to the seminal finding that CS-evoked responses in A1 are increased during auditory fear conditioning (Galambos et al., 1956). The intervening years witnessed increasing, but generally sporadic, efforts to fully understand the implications of these findings (e.g., reviewed in Weinberger and Diamond, 1987). But since the early 1990s, the amount of research has increased greatly, showing no sign of abatement, but rather an expansion of the domains of auditory inquiry so that the primary cortical field can be considered no less “cognitive” than other cortical regions (e.g., Scheich et al., 2007).
It is somewhat ironic that the discovery of associative plasticity in the auditory system by Galambos and colleagues was made possible by their study of auditory fear conditioning, yet the AMYG model of AFC actually opposes the extension of their findings to the auditory thalamus. There is little doubt that Jeff Winer would find this state of affairs both amusing and exasperating.
Where does the future lie? It is time to change the questions that have and are currently being addressed. As pointed out above, a recent revision of the AMYG model seeks to be more inclusive of various nuclei with the amygdala, such as the central nucleus (Wilensky et al., 2006). While perhaps a modest step forward, focus on the amygdala to the exclusion of the auditory thalamus will not get the job done. Instead of asking, “How does the amygdala produce auditory fear learning”, investigators need to shift to questions such as, “What are the functions of the MGm/PIN and of the amygdala in auditory fear conditioning.” They are bound to be different, yet complementary. One might hypothesize that the MGm/PIN forms the initial association while the lateral (and perhaps other regions of the) amygdala “adds emotional tone”.
Additionally, given that both the AL and the MGm/PIN appear to be critically involved in auditory fear conditioning, and that the latter sends the effects of its associative plasticity to the former, it seems reasonable to consider the MGm/PIN complex as the “root” of the auditory fear conditioning circuit.
Further, insofar as the basolateral amygdala is recognized to subserve memory modulation of information stored elsewhere in the brain (McGaugh et al., 2002), an adequate understanding of AFC will require a synthesis of the conditioning and memory modulation findings.
Finally, for the present and as reviewed above, we must bear in mind that the MGm/PIN complex has strong projections to numerous auditory and adjacent cortical fields. Given their projections to Layer I, and likely influence on the apical dendrites of pyramidal cells, their influence is probably both extensive and powerful. The MGm/PIN is in a position to promote the storage of information in the auditory and related cortical fields. Any account of even so basic a process as auditory fear conditioning is likely to resist solution unless future models and research efforts are inclusive.
Acknowledgments
I thank Gabriel K. Hui and Jacquie Weinberger for assistance and Gabriel A. Elias for illuminating conversations. This research was supported by research grants from the National Institutes of Health/National Institute on Deafness and Other Communication Disorders (NIDCD), DC-02938, DC-05592 and DC-010013.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aitkin LM. Medial geniculate body of the cat: Responses to tonal stimuli of neurons in medial division. J Neurophysiol. 1973;36(2):275–283. doi: 10.1152/jn.1973.36.2.275. [DOI] [PubMed] [Google Scholar]
- Aitkin LM, Webster WR. Medial geniculate body of the cat: Organization and responses to tonal stimuli of neurons in ventral division. J Neurophysiol. 1972;35(3):365–380. doi: 10.1152/jn.1972.35.3.365. [DOI] [PubMed] [Google Scholar]
- Amorapanth P, LeDoux JE, Nader K. Different lateral amygdala outputs mediate reactions and actions elicited by a fear-arousing stimulus. Nat Neurosci. 2000;3(1):74–79. doi: 10.1038/71145. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Knight PL, Merzenich MM. The thalamocortical and corticothalamic connections of AI, AII, and the anterior auditory field (AAF) in the cat: Evidence for two largely segregated systems of connections. J Comp Neurol. 1980;194(3):663–701. doi: 10.1002/cne.901940312. [DOI] [PubMed] [Google Scholar]
- Apergis-Schoute AM, Debiec J, Doyère V, LeDoux JE, Schafe GE. Auditory fear conditioning and long-term potentiation in the lateral amygdala require ERK/MAP kinase signaling in the auditory thalamus: A role for presynaptic plasticity in the fear system. J Neurosci. 2005;25(24):5730–5739. doi: 10.1523/JNEUROSCI.0096-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Classical conditioning induces CS-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Res. 1990;536(1–2):271–286. doi: 10.1016/0006-8993(90)90035-a. [DOI] [PubMed] [Google Scholar]
- Barnett EM, Evans GD, Sun N, Perlman S, Cassell MD. Anterograde tracing of trigeminal afferent pathways from the murine tooth pulp to cortex using herpes simplex virus type 1. J Neurosci. 1995;15(4):2972–2984. doi: 10.1523/JNEUROSCI.15-04-02972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birt D, Nienhuis R, Olds M. Separation of associative from non-associative short latency changes in medial geniculate and inferior colliculus during differential conditioning and reversal in rats. Brain Res. 1979;167(1):129–138. doi: 10.1016/0006-8993(79)90268-3. [DOI] [PubMed] [Google Scholar]
- Birt D, Olds M. Associative response changes in lateral midbrain tegmentum and medial geniculate during differential appetitive conditioning. J Neurophysiol. 1981;46(5):1039–1055. doi: 10.1152/jn.1981.46.5.1039. [DOI] [PubMed] [Google Scholar]
- Blair HT, Tinkelman A, Moita MA, LeDoux JE. Associative plasticity in neurons of the lateral amygdala during auditory fear conditioning. Ann NY Acad Sci. 2003;985:485–487. doi: 10.1111/j.1749-6632.2003.tb07106.x. [DOI] [PubMed] [Google Scholar]
- Blum PS, Abraham LD, Gilman S. Vestibular, auditory, and somatic input to the posterior thalamus of the cat. Exp Brain Res. 1979;34(1):1–9. doi: 10.1007/BF00238337. [DOI] [PubMed] [Google Scholar]
- Boatman JA, Kim JJ. A thalamo-cortico-amygdala pathway mediates auditory fear conditioning in the intact brain. Eur J Neurosci. 2006;24(3):894–900. doi: 10.1111/j.1460-9568.2006.04965.x. [DOI] [PubMed] [Google Scholar]
- Boivie J. The termination of the spinothalamic tract in the cat: An experimental study with silver impregnation methods. Exp Brain Res. 1971;112(4):331–353. doi: 10.1007/BF00234489. [DOI] [PubMed] [Google Scholar]
- Bordi F, LeDoux J. Sensory tuning beyond the sensory system: An initial analysis of auditory response properties of neurons in the lateral amygdaloid nucleus and overlying areas of the striatum. J Neurosci. 1992;12(7):2493–2503. doi: 10.1523/JNEUROSCI.12-07-02493.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordi F, LeDoux JE. Response properties of single units in areas of rat auditory thalamus that project to the amygdala: I. Acoustic discharge patterns and frequency receptive fields. Exp Brain Res. 1994a;98(2):261–274. doi: 10.1007/BF00228414. [DOI] [PubMed] [Google Scholar]
- Bordi F, LeDoux JE. Response properties of single units in areas of rat auditory thalamus that project to the amygdala: II. Cells receiving convergent auditory and somatosensory inputs and cells antidromically activated by amygdala stimulation. Exp Brain Res. 1994b;98(2):275–286. doi: 10.1007/BF00228415. [DOI] [PubMed] [Google Scholar]
- Bordi F, LeDoux J, Clugnet MC, Pavlides C. Single-unit activity in the lateral nucleus of the amygdala and overlying areas of the striatum in freely behaving rats: Rates, discharge patterns, and responses to acoustic stimuli. Behav Neurosci. 1993;107(5):757–769. doi: 10.1037/0735-7044.107.5.757. [DOI] [PubMed] [Google Scholar]
- Brandner S, Redies H. The projection from medial geniculate to field AI in cat: Organization in the isofrequency dimension. J Neurosci. 1990;10(1):50–61. doi: 10.1523/JNEUROSCI.10-01-00050.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkhus HB, Carstens E, Zimmermann M. Encoding of graded noxious skin heating by neurons in posterior thalamus and adjacent areas in the cat. Neurosci Lett. 1979;15(1):37–42. doi: 10.1016/0304-3940(79)91526-x. [DOI] [PubMed] [Google Scholar]
- Buchwald JS, Halas ES, Schramm S. Changes in cortical and subcortical unit activity during behavioral conditioning. Physiol Behav. 1966;1(1):11–22. [Google Scholar]
- Calford MB, Webster WR. Auditory representation within principal division of cat medial geniculate body: An electrophysiology study. J Neurophysiol. 1981;45(6):1013–1028. doi: 10.1152/jn.1981.45.6.1013. [DOI] [PubMed] [Google Scholar]
- Campeau S, Davis M. Involvement of subcortical and cortical afferents to the lateral nucleus of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci. 1995;15(3 Pt 2):2312–2327. doi: 10.1523/JNEUROSCI.15-03-02312.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstens E, Yokota T. Viscerosomatic convergence and responses to intestinal distension of neurons at the junction of midbrain and posterior thalamus in the cat. Exp Neurol. 1980;70(2):392–402. doi: 10.1016/0014-4886(80)90036-9. [DOI] [PubMed] [Google Scholar]
- Cetas JS, Price RO, Velenovsky DS, Sinex DG, McMullen NT. Frequency organization and cellular lamination in the medial geniculate body of the rabbit. Hear Res. 2001;155(1–2):113–123. doi: 10.1016/s0378-5955(01)00257-x. [DOI] [PubMed] [Google Scholar]
- Chisholm JS. Death, Hope, and Sex: Steps To an Evolutionary Ecology of Mind and Morality. Cambridge University Press; New York: 1999. p. 89. [Google Scholar]
- Clerici WJ, Coleman JR. Anatomy of the rat medial geniculate body: I. Cytoarchitecture, myeloarchitecture, and neocortical connectivity. J Comp Neurol. 1990;297(1):14–31. doi: 10.1002/cne.902970103. [DOI] [PubMed] [Google Scholar]
- Clugnet MC, LeDoux JE. Synaptic plasticity in fear conditioning circuits: Induction of LTP in the lateral nucleus of the amygdala by stimulation of the medial geniculate body. J Neurosci. 1990;10(8):2818–2824. doi: 10.1523/JNEUROSCI.10-08-02818.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clugnet MC, LeDoux JE, Morrison SF. Unit responses evoked in the amygdala and striatum by electrical stimulation of the medial geniculate body. J Neurosci. 1990;10(4):1055–1061. doi: 10.1523/JNEUROSCI.10-04-01055.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clugnet C, LeDoux JE, Morrison SF, Reis DJ. Short latency orthodromic action potential evoked in amygdala and caudate–putamen by stimulation of the medial geniculate body. Soc Neurosci Abst. 1988;14(2):1227. [Google Scholar]
- Cruikshank SJ, Edeline JM, Weinberger NM. Stimulation at a site of auditory–somatosensory convergence in the medial geniculate nucleus is an effective unconditioned stimulus for fear conditioning. Behav Neurosci. 1992;106(3):471–483. doi: 10.1037//0735-7044.106.3.471. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Stuart DK. Trial sequence of changed unit activity in auditory system of alert rat during conditioned response acquisition and extinction. J Neurophysiol. 1976;39(2):266–281. doi: 10.1152/jn.1976.39.2.266. [DOI] [PubMed] [Google Scholar]
- Edeline JM. Frequency-specific plasticity of single unit discharges in the rat medial geniculate body. Brain Res. 1990;529(1–2):109–119. doi: 10.1016/0006-8993(90)90817-u. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Dutrieux G, Neuenschwander-el Massioui N. Multiunit changes in hippocampus and medial geniculate body in free-behaving rats during acquisition and retention of a conditioned response to a tone. Behav Neural Biol. 1988;50(1):61–79. doi: 10.1016/s0163-1047(88)90780-7. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Hars B, Hennevin E, Cotillon N. Muscimol diffusion after intracerebral microinjections: A reevaluation based on electrophysiological and autoradiographic quantifications. Neurobiol Learn Mem. 2002;78(1):100–124. doi: 10.1006/nlme.2001.4035. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Neuenschwander-el Massioui N, Dutrieux G. Discriminative long-term retention of rapidly induced multiunit changes in the hippocampus, medial geniculate and auditory cortex. Behav Brain Res. 1990;39(2):145–155. doi: 10.1016/0166-4328(90)90101-j. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Weinberger NM. Associative retuning in the thalamic source of input to the amygdala and auditory cortex: Receptive field plasticity in the medial division of the medial geniculate body. Behav Neurosci. 1992;106(1):81–105. doi: 10.1037//0735-7044.106.1.81. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23(2):229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annu Rev Psychol. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Fehr FS, Stern JA. Heart rate conditioning in the rat. J Psychoso Res. 1965;8:441–453. doi: 10.1016/0022-3999(65)90086-3. [DOI] [PubMed] [Google Scholar]
- Gabriel M, Miller JD, Saltwick SE. Multiple-unit activity of the rabbit medial geniculate nucleus in conditioning, extinction, and reversal. Physiol Psychol. 1976;4(2):124–134. [Google Scholar]
- Gabriel M, Saltwick SE, Miller JD. Conditioning and reversal of short-latency multiple-unit responses in the rabbit medial geniculate nucleus. Science. 1975;189(4208):1108–1109. doi: 10.1126/science.1162365. [DOI] [PubMed] [Google Scholar]
- Galambos R, Sheatz G, Vernier VG. Electrophysiological correlates of a conditioned response in cats. Science. 1956;123(3192):376–377. doi: 10.1126/science.123.3192.376. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Kapp BS, Frysinger RC, Rapp PR. β-adrenergic manipulation in amygdala central n. alters rabbit heart rate conditioning. Pharmacol Biochem Behav. 1980;12(3):419–426. doi: 10.1016/0091-3057(80)90047-7. [DOI] [PubMed] [Google Scholar]
- Gerren RA, Weinberger NM. Long term potentiation in the magnocellular medial geniculate nucleus of the anesthetized cat. Brain Res. 1983;265(1):138–142. doi: 10.1016/0006-8993(83)91344-6. [DOI] [PubMed] [Google Scholar]
- Han JH, Yiu AP, Cole CJ, Hsiang HL, Neve RL, Josselyn SA. Increasing CREB in the auditory thalamus enhances memory and generalization of auditory conditioned fear. Learn Mem. 2008;15(6):443–453. doi: 10.1101/lm.993608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halas ES, Beardsley JV, Sandlie ME. Conditioned neuronal responses at various levels in conditioning paradigms. Electroencephalogr Clin Neurophysiol. 1970;28(5):468–477. doi: 10.1016/0013-4694(70)90272-5. [DOI] [PubMed] [Google Scholar]
- Hennevin E, Maho C. Fear conditioning-induced plasticity in auditory thalamus and cortex: To what extent is it expressed during slow-wave sleep? Behav Neurosci. 2005;119(5):1277–1289. doi: 10.1037/0735-7044.119.5.1277. [DOI] [PubMed] [Google Scholar]
- Hennevin E, Maho C, Hars B. Learning-induced increase of tone-evoked response in the auditory thalamus during paradoxical sleep. Soc Neurosci Abst. 1992;18(2):1064. [Google Scholar]
- Hennevin E, Maho C, Hars B. Neuronal plasticity induced by fear conditioning is expressed during paradoxical sleep: Evidence from simultaneous recordings in the lateral amygdala and the medial geniculate in rats. Behav Neurosci. 1998;112(4):839–862. doi: 10.1037//0735-7044.112.4.839. [DOI] [PubMed] [Google Scholar]
- Hennevin E, Maho C, Hars B, Dutrieux G. Learning-induced plasticity in the medial geniculate nucleus is expressed during paradoxical sleep. Behav Neurosci. 1993;107(6):1018–1030. doi: 10.1037//0735-7044.107.6.1018. [DOI] [PubMed] [Google Scholar]
- Herkenham M. Laminar organization of thalamic projections to the rat neocortex. Science. 1980;207(4430):532–535. doi: 10.1126/science.7352263. [DOI] [PubMed] [Google Scholar]
- Herkenham M. New perspectives on the organization and evolution of nonspecific thalamocortical projections. In: Jones EG, Peters A, editors. Cerebral Cortex: Sensory–Motor Areas and Aspects of Cortical Connectivity. Vol. 5. Plenum Press; New York: 1986. pp. 403–445. [Google Scholar]
- Huang CL, Winer JA. Auditory thalamocortical projections in the cat: Laminar and areal patterns of input. J Comp Neurol. 2000;427(2):302–331. doi: 10.1002/1096-9861(20001113)427:2<302::aid-cne10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Imig TJ, Morel A. Organization of the thalamocortical auditory system in the cat. Annu Rev Neurosci. 1983;6:95–120. doi: 10.1146/annurev.ne.06.030183.000523. [DOI] [PubMed] [Google Scholar]
- Imig TJ, Morel A. Tonotopic organization in ventral nucleus of medial geniculate body in the cat. J Neurophysiol. 1985;53(1):309–340. doi: 10.1152/jn.1985.53.1.309. [DOI] [PubMed] [Google Scholar]
- Jarrell TW, Gentile CG, McCabe PM, Schneiderman N. The role of the medial geniculate region in differential Pavlovian conditioning of bradycardia in rabbits. Brain Res. 1986a;374(1):126–136. doi: 10.1016/0006-8993(86)90401-4. [DOI] [PubMed] [Google Scholar]
- Jarrell TW, Gentile CG, Romanski LM, McCabe PM, Schneiderman N. Involvement of cortical and thalamic auditory regions in retention of differential bradycardiac conditioning to acoustic conditioned stimuli in rabbits. Brain Res. 1987;412(2):285–294. doi: 10.1016/0006-8993(87)91135-8. [DOI] [PubMed] [Google Scholar]
- Jarrell TW, Romanski LM, Gentile CG, McCabe PM, Schneiderman N. Ibotenic acid lesions in the medial geniculate region prevent the acquisition of differential Pavlovian conditioning of bradycardia to acoustic stimuli in rabbits. Brain Res. 1986b;382(1):199–203. doi: 10.1016/0006-8993(86)90133-2. [DOI] [PubMed] [Google Scholar]
- Jordan H. The structure of the medial geniculate nucleus (MGN): A cyto- and myeloarchitectonic study in the squirrel monkey. J Comp Neurol. 1973;148(4):469–479. doi: 10.1002/cne.901480405. [DOI] [PubMed] [Google Scholar]
- Kandel ER. Search of Memory: The Emergence of a New Science of Mind. W.W. Norton & Company; New York: 2006. p. 343. [Google Scholar]
- Khorevin VI. Interaction between responses evoked by acoustic and somatosensory stimuli in neurons of the magnocellular part of the medial geniculate body. Neurophysiology. 1980;12(4):241–245. [PubMed] [Google Scholar]
- Killcross S, Robbins TW, Everitt BJ. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature. 1997;388(6640):377–380. doi: 10.1038/41097. [DOI] [PubMed] [Google Scholar]
- Kimura A, Donishi T, Okamoto K, Tamai Y. Topography of projections from the primary and non-primary auditory cortical areas to the medial geniculate body and thalamic reticular nucleus in the rat. Neuroscience. 2005;135(4):1325–1342. doi: 10.1016/j.neuroscience.2005.06.089. [DOI] [PubMed] [Google Scholar]
- Kimura A, Donishi T, Sakoda T, Hazama M, Tamai Y. Auditory thalamic nuclei projections to the temporal cortex in the rat. Neuroscience. 2003;117(4):1003–1016. doi: 10.1016/s0306-4522(02)00949-1. [DOI] [PubMed] [Google Scholar]
- Kudo M, Glendenning KK, Frost SB, Masterton RB. Origin of mammalian thalamocortical projections: I. Telencephalic projections of the medial geniculate body in the opossum (Didelphis virginiana) J Comp Neurol. 1986;245(2):176–197. doi: 10.1002/cne.902450205. [DOI] [PubMed] [Google Scholar]
- Kudo M, Niimi K. Ascending projections of the inferior colliculus in the cat: An autoradiographic study. J Comp Neurol. 1980;191(4):545–556. doi: 10.1002/cne.901910403. [DOI] [PubMed] [Google Scholar]
- Lanuza E, Moncho-Bogani J, LeDoux JE. Unconditioned stimulus pathways to the amygdala: Effects of lesions of the posterior intralaminar thalamus on foot-shock-induced c-Fos expression in the subdivisions of the lateral amygdala. Neuroscience. 2008;155(3):959–968. doi: 10.1016/j.neuroscience.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanuza E, Nader K, LeDoux JE. Unconditioned stimulus pathways to the amygdala: Effects of posterior thalamic and cortical lesions on fear conditioning. Neuroscience. 2004;125(2):305–315. doi: 10.1016/j.neuroscience.2003.12.034. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Sensory systems and emotion: A model of affective processing. Integr Psychiatry. 1986;4(4):237–248. [Google Scholar]
- LeDoux JE. Information flow from sensation to emotion: Plasticity in the neural computation of stimulus value. In: Gabriel M, Moore J, editors. Learning and Computation in Neuroscience. MIT Press; Cambridge, MA: 1990. pp. 2–51. [Google Scholar]
- LeDoux JE. Brain mechanisms of emotion and emotional learning. Curr Opin Neurobiol. 1992;2(2):191–197. doi: 10.1016/0959-4388(92)90011-9. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotional memory: In search of systems and synapses. Ann NY Acad Sci. 1993a;702:149–157. doi: 10.1111/j.1749-6632.1993.tb17246.x. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotional memory systems in the brain. Behav Brain Res. 1993b;58(1–2):69–79. doi: 10.1016/0166-4328(93)90091-4. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion, memory and the brain. Sci Am. 1994;270(6):50–57. doi: 10.1038/scientificamerican0694-50. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion: Clues from the brain. Annu Rev Psychol. 1995;46:209–235. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: Sensory interface of the amygdala in fear conditioning. J Neurosci. 1990;10(4):1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Muller J. Emotional memory and psychopathology. Philos Trans R Soc Lond B Biol Sci. 1997;352(1362):1719–1726. doi: 10.1098/rstb.1997.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Ruggiero DA, Forest R, Stornetta R, Reis DJ. Topographic organization of convergent projections to the thalamus from the inferior colliculus and spinal cord in the rat. J Comp Neurol. 1987;264(1):123–146. doi: 10.1002/cne.902640110. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Ruggiero DA, Reis DJ. Projections to the subcortical forebrain from anatomically defined regions of the medial geniculate body in the rat. J Comp Neurol. 1985;242(2):182–213. doi: 10.1002/cne.902420204. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Sakaguchi A, Iwata J, Reis DJ. Interruption of projections from the medial geniculate body to an archi-neostriatal field disrupts the classical conditioning of emotional responses to acoustic stimuli. Neuroscience. 1986;17(3):615–627. doi: 10.1016/0306-4522(86)90034-5. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Sakaguchi A, Reis DJ. Subcortical efferent projections of the medial geniculate nucleus mediate emotional responses conditioned to acoustic stimuli. J Neurosci. 1984;4(3):683–698. doi: 10.1523/JNEUROSCI.04-03-00683.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann H, Treit D, Parent MB. Amygdala lesions do not impair shock-probe avoidance retention performance. Behav Neurosci. 2000;114(1):107–116. doi: 10.1037//0735-7044.114.1.107. [DOI] [PubMed] [Google Scholar]
- Lennartz RC, Weinberger NM. Frequency-specific receptive field plasticity in the medial geniculate body induced by Pavlovian fear conditioning is expressed in the anesthetized brain. Behav Neurosci. 1992;106(3):484–497. doi: 10.1037//0735-7044.106.3.484. [DOI] [PubMed] [Google Scholar]
- Linke R. Differential projection patterns of superior and inferior collicular neurons onto posterior paralaminar nuclei of the thalamus surrounding the medial geniculate body in the rat. Eur J Neurosci. 1999a;11(1):187–203. doi: 10.1046/j.1460-9568.1999.00422.x. [DOI] [PubMed] [Google Scholar]
- Linke R. Organization of projections to temporal cortex originating in the thalamic posterior intralaminar nucleus of the rat. Exp Brain Res. 1999b;127(3):314–320. doi: 10.1007/s002210050801. [DOI] [PubMed] [Google Scholar]
- Linke R, Braune G, Schwegler H. Differential projection of the posterior paralaminar thalamic nuclei to the amygdaloid complex in the rat. Exp Brain Res. 2000;134(4):520–532. doi: 10.1007/s002210000475. [DOI] [PubMed] [Google Scholar]
- Linke R, Schwegler H. Convergent and complementary projections of the caudal paralaminar thalamic nuclei to rat temporal and insular cortex. Cereb Cortex. 2000;10(8):753–771. doi: 10.1093/cercor/10.8.753. [DOI] [PubMed] [Google Scholar]
- Lippe WR, Weinberger NM. The distribution of click-evoked activity within the medical geniculate body of the anesthetized cat. Exp Neurol. 1973a;39(3):507–523. doi: 10.1016/0014-4886(73)90035-6. [DOI] [PubMed] [Google Scholar]
- Lippe WR, Weinberger NM. The distribution of sensory evoked activity within the medial geniculate body of the unanesthetized cat. Exp Neurol. 1973b;40(2):431–444. doi: 10.1016/0014-4886(73)90085-x. [DOI] [PubMed] [Google Scholar]
- Love JA, Scott JW. Some response characteristics of cells of the magnocellular division of the medial geniculate body of the cat. Can J Physiol Pharmacol. 1969;47(10):881–888. doi: 10.1139/y69-145. [DOI] [PubMed] [Google Scholar]
- Maho C, Hennevin E. Appetitive conditioning-induced plasticity is expressed during paradoxical sleep in the medial geniculate, but not in the lateral amygdala. Behav Neurosci. 2002;116(5):807–823. doi: 10.1037//0735-7044.116.5.807. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S, Ferrario CR, Corcoran KA, Desmond TJ, Frey KA. Protein synthesis in the amygdala, but not the auditory thalamus, is required for consolidation of Pavlovian fear conditioning in rats. Eur J Neurosci. 2003;18(11):3080–3088. doi: 10.1111/j.1460-9568.2003.03063.x. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5(11):844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Maren S, Yap SA, Goosens KA. The amygdala is essential for the development of neuronal plasticity in the medial geniculate nucleus during auditory fear conditioning in rats. J Neurosci. 2001;21(6):RC135. doi: 10.1523/JNEUROSCI.21-06-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe PM, McEchron MD, Green EJ, Schneiderman N. Electrolytic and ibotenic acid lesions of the medial subnucleus of the medial geniculate prevent the acquisition of classically conditioned heart rate to a single acoustic stimulus in rabbits. Brain Res. 1993;619(1–2):291–298. doi: 10.1016/0006-8993(93)91623-z. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Green EJ, Winters RW, Nolen TG, Schneiderman N, McCabe PM. Changes of synaptic efficacy in the medial geniculate nucleus as a result of auditory classical conditioning. J Neurosci. 1996;16(3):1273–1283. doi: 10.1523/JNEUROSCI.16-03-01273.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEchron MD, McCabe PM, Green EJ, Llabre MM, Schneiderman N. Simultaneous single unit recording in the medial nucleus of the medial geniculate nucleus and amygdaloid central nucleus throughout habituation, acquisition, and extinction of the rabbit's classically conditioned heart rate. Brain Res. 1995;682(1–2):157–166. doi: 10.1016/0006-8993(95)00331-j. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, McIntyre CK, Power AE. Amygdala modulation of memory consolidation: Interaction with other brain systems. Neurobiol Learn Mem. 2002;78(3):539–552. doi: 10.1006/nlme.2002.4082. [DOI] [PubMed] [Google Scholar]
- McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390(6660):607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- Medina JF, Repa JC, Mauk MD, LeDoux JE. Parallels between cerebellum- and amygdala-dependent conditioning. Nat Rev Neurosci. 2002;3(2):122–131. doi: 10.1038/nrn728. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Colwell SA, Andersen RA. Auditory forebrain organization: Thalamocortical and corticothalamic connections in the cat. In: Woolsey CN, editor. Cortical Sensory Organization. Humana Press; Clifton, NJ: 1982. pp. 43–57. [Google Scholar]
- Mineka S, Oehlberg K. The relevance of recent developments in classical conditioning to understanding the etiology and maintenance of anxiety disorders. Acta Psychol. 2008;127(3):567–580. doi: 10.1016/j.actpsy.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Morest DK. The neuronal architecture of the medial geniculate body of the cat. J Anat. 1964;98:611–630. [PMC free article] [PubMed] [Google Scholar]
- Morest DK. The laminar structure of the medial geniculate body of the cat. J Anat. 1965a;99:143–160. [PMC free article] [PubMed] [Google Scholar]
- Morest DK. The lateral tegmental system of the midbrain and the medial geniculate body: Study with Golgi and Nauta methods in cat. J Anat. 1965b;99(Pt 3):611–634. [PMC free article] [PubMed] [Google Scholar]
- Morest DK, Winer JA. The comparative anatomy of neurons: Homologous neurons in the medial geniculate body of the opossum and the cat. Adv Anat Embryol Cell Biol. 1986;97:1–94. doi: 10.1007/978-3-642-70652-3. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Dolan RJ. Experience-dependent modulation of tonotopic neural responses in human auditory cortex. Proc R Soc Lond B Biol Sci. 1998;265(1397):649–657. doi: 10.1098/rspb.1998.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimi K, Naito F. Cortical projections of the medial geniculate body in the cat. Exp Brain Res. 1974;19(3):326–342. doi: 10.1007/BF00233238. [DOI] [PubMed] [Google Scholar]
- Niimi K, Ono K, Kusunose M. Projections of the medial geniculate nucleus to layer 1 of the auditory cortex in the cat traced with horseradish peroxidase. Neurosci Lett. 1984;45(2):223–228. doi: 10.1016/0304-3940(84)90103-4. [DOI] [PubMed] [Google Scholar]
- O'Brien PG, Kennedy WZ, Ballard KA. Psychiatric Mental Health Nursing: An Introduction to Theory and Practice. Jones and Bartlett Publishers; Sudbury, MA: 2008. p. 70. [Google Scholar]
- Obrist PA. Heart rate and somatic-motor coupling during classical aversive conditioning in humans. J Exp Psychol. 1968;77(2):180–193. doi: 10.1037/h0025814. [DOI] [PubMed] [Google Scholar]
- O'Connor KN, Allison TL, Rosenfield ME, Moore JW. Neural activity in the medial geniculate nucleus during auditory trace conditioning. Exp Brain Res. 1997;113(3):534–556. doi: 10.1007/pl00005605. [DOI] [PubMed] [Google Scholar]
- Oliver DL, Hall WC. Subdivisions of the medial geniculate body in the tree shrew (Tupaia glis) Brain Res. 1975;86(2):217–227. doi: 10.1016/0006-8993(75)90698-8. [DOI] [PubMed] [Google Scholar]
- Oliver DL, Hall WC. The medial geniculate body of the tree shrew, Tupaia glis: I. Cytoarchitecture and midbrain connections. J Comp Neurol. 1978;182(3):423–458. doi: 10.1002/cne.901820305. [DOI] [PubMed] [Google Scholar]
- Pape HC, Paré D. Plastic synaptic networks of the amygdala regulating acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010 doi: 10.1152/physrev.00037.2009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons RG, Riedner BA, Gafford GM, Helmstetter FJ. The formation of auditory fear memory requires the synthesis of protein and mRNA in the auditory thalamus. Neuroscience. 2006;141(3):1163–1170. doi: 10.1016/j.neuroscience.2006.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschanski M, Guilbaud G, Gautron M. Posterior intralaminar region in rat: Neuronal responses to noxious and nonnoxious cutaneous stimuli. Exp Neurol. 1981;72(1):226–238. doi: 10.1016/0014-4886(81)90140-0. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pinel JPJ. Biopsychology. 5th. Allyn and Bacon; Boston, MA: 2003. p. 454. [Google Scholar]
- Poremba A, Gabriel M. Medial geniculate lesions block amygdalar and cingulothalamic learning-related neuronal activity. J Neurosci. 1997;17(21):8645–8655. doi: 10.1523/JNEUROSCI.17-21-08645.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poremba A, Gabriel M. Amygdalar efferents initiate auditory thalamic discriminative training-induced neuronal activity. J Neurosci. 2001;21(1):270–278. doi: 10.1523/JNEUROSCI.21-01-00270.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell DA, Kazis E. Blood pressure and heart rate changes accompanying classical eyeblink conditioning in the rabbit (Oryctolagus cuniculus) Psychophysiology. 1976;13(5):441–448. doi: 10.1111/j.1469-8986.1976.tb00858.x. [DOI] [PubMed] [Google Scholar]
- Powell EW, Hatton JB. Projections of the inferior colliculus in cat. J Comp Neurol. 1969;136(2):183–192. doi: 10.1002/cne.901360205. [DOI] [PubMed] [Google Scholar]
- Raczkowski D, Diamond IT, Winer J. Organization of thalamocortical auditory system in the cat studied with horseradish peroxidase. Brain Res. 1976;101(2):345–354. doi: 10.1016/0006-8993(76)90275-4. [DOI] [PubMed] [Google Scholar]
- Raffaele R, Sapienza S, Urbano A. Fastigial projections to the magnocellular portion of the medial geniculate body of the cat. Arch Sci Biol. 1969;53:149–161. [Google Scholar]
- Redies H, Brandner S, Creutzfeldt OD. Anatomy of the auditory thalamocortical system of the guinea pig. J Comp Neurol. 1989;282(4):489–511. doi: 10.1002/cne.902820403. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Behavioral studies of Pavlovian conditioning. Annu Rev Neurosci. 1988a;11:329–352. doi: 10.1146/annurev.ne.11.030188.001553. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Pavlovian conditioning. It's not what you think it is. Am Psychol. 1988b;43(3):151–160. doi: 10.1037//0003-066x.43.3.151. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- Romanski LM, LeDoux JE. Equipotentiality of thalamo-amygdala and thalamo-cortico-amygdala circuits in auditory fear conditioning. J Neurosci. 1992;12(11):4501–4509. doi: 10.1523/JNEUROSCI.12-11-04501.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, LeDoux JE. Organization of rodent auditory cortex: Anterograde transport of PHA–L from MGv to temporal neocortex. Cereb Cortex. 1993;3(6):499–514. doi: 10.1093/cercor/3.6.499. [DOI] [PubMed] [Google Scholar]
- Rosen JB. The neurobiology of conditioned and unconditioned fear: A neurobehavioral system analysis of the amygdala. Behav Cogn Neurosci Rev. 2004;3(1):23–41. doi: 10.1177/1534582304265945. [DOI] [PubMed] [Google Scholar]
- Roucoux-Hanus M, Boisacq-Schepens N. Ascending vestibular projections: Further results at cortical and thalamic levels in the cat. Exp Brain Res. 1977;29(2):283–292. doi: 10.1007/BF00237048. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Welker E. Morphology of corticothalamic terminals arising from the auditory cortex of the rat: A Phaseolus vulgaris–leucoagglutinin (PHA–L) tracing study. Hear Res. 1991;56(1–2):179–190. doi: 10.1016/0378-5955(91)90168-9. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Killackey HP. Differential telencephalic projections of the medial and ventral divisions of the medial geniculate body of the rat. Brain Res. 1974;82(1):173–177. doi: 10.1016/0006-8993(74)90903-2. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Weinberger NM. Differential plasticity of morphologically distinct neuron populations in the medical geniculate body of the cat during classical conditioning. Behav Biol. 1978;22(3):275–301. doi: 10.1016/s0091-6773(78)92351-9. [DOI] [PubMed] [Google Scholar]
- Scheel M. Topographic organization of the auditory thalamocortical system in the albino rat. Anat Embryol. 1988;179(2):181–190. doi: 10.1007/BF00304700. [DOI] [PubMed] [Google Scholar]
- Scheich H, Brechmann A, Brosch M, Budinger E, Ohl FW. The cognitive auditory cortex: Task-specificity of stimulus representations. Hear Res. 2007;229(1–2):213–224. doi: 10.1016/j.heares.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Selden NR, Everitt BJ, Jarrard LE, Robbins TW. Complementary roles for the amygdala and hippocampus in aversive conditioning to explicit and contextual cues. Neuroscience. 1991;42(2):335–350. doi: 10.1016/0306-4522(91)90379-3. [DOI] [PubMed] [Google Scholar]
- Sommer-Smith JA, Morocutti G. Cortical and subcortical evoked potentials during conditioning. Electroencephalogr Clin Neurophysiol. 1970;29(4):383–391. [PubMed] [Google Scholar]
- Suga N. The neural circuit for tone-specific plasticity in the auditory system elicited by conditioning. Learn Mem. 2008;15(4):198–201. doi: 10.1101/lm.791408. [DOI] [PubMed] [Google Scholar]
- Supple WF, Jr, Kapp BS. Response characteristics of neurons in the medial component of the medial geniculate nucleus during Pavlovian differential fear conditioning in rabbits. Behav Neurosci. 1989;103(6):1276–1286. doi: 10.1037//0735-7044.103.6.1276. [DOI] [PubMed] [Google Scholar]
- Talk A, Kashef A, Gabriel M. Effects of conditioning during amygdalar inactivation on training-induced neuronal plasticity in the medial geniculate nucleus and cingulated cortex in rabbits (Oryctolagus cuniculus) Behav Neurosci. 2004;118(5):944–955. doi: 10.1037/0735-7044.118.5.944. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio AR. The covert learning of affective valence does not require structures in hippocampal system or amygdala. J Cogn Neurosci. 1993;5(1):79–88. doi: 10.1162/jocn.1993.5.1.79. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, McGaugh JL. Basolateral amygdala is not critical for cognitive memory of contextual fear conditioning. Proc Natl Acad Sci USA. 1998;95(25):15003–15007. doi: 10.1073/pnas.95.25.15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. Sensory plasticity and learning: The magnocellular medial geniculate nucleus of the auditory system. In: Woody CD, editor. Conditioning: Representation of Involved Neural Functions (Advances in Behavioral Biology, Vol 26) Plenum Press; New York: 1982. pp. 697–710. [Google Scholar]
- Weinberger NM. Associative representational plasticity in the auditory cortex: A synthesis of two disciplines. Learn Mem. 2007;14(1–2):1–16. doi: 10.1101/lm.421807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. Retuning the brain by learning, literature, and logic: Reply to Suga. Learn Mem. 2008;15(4):202–207. [Google Scholar]
- Weinberger NM, Diamond DM. Physiological plasticity in auditory cortex: Rapid induction by learning. Prog Neurobiol. 1987;29(1):1–55. doi: 10.1016/0301-0082(87)90014-1. [DOI] [PubMed] [Google Scholar]
- Weinberger NM, Imig TJ, Lippe WR. Modification of unit discharges in the medial geniculate nucleus by click–shock pairing. Exp Neurol. 1972;36(1):46–58. doi: 10.1016/0014-4886(72)90135-5. [DOI] [PubMed] [Google Scholar]
- Weinberger NM, Javid R, Lepan B. Heterosynaptic long-term facilitation of sensory-evoked responses in the auditory cortex by stimulation of the magnocellular medial geniculate body in guinea pigs. Behav Neurosci. 1995;109(1):10–17. doi: 10.1037//0735-7044.109.1.10. [DOI] [PubMed] [Google Scholar]
- Wenstrup JJ, Larue DT, Winer JA. Projections of physiologically defined subdivisions of the inferior colliculus in the mustached bat: Targets in the medial geniculate body and extrathalamic nuclei. J Comp Neurol. 1994;346(2):207–236. doi: 10.1002/cne.903460204. [DOI] [PubMed] [Google Scholar]
- Wepsic JG. Multimodal sensory activation of cells in the magnocellular medial geniculate nucleus. Exp Neurol. 1966;15(3):299–318. doi: 10.1016/0014-4886(66)90053-7. [DOI] [PubMed] [Google Scholar]
- Whitley JM, Henkel CK. Topographical organization of the inferior collicular projection and other connections of the ventral nucleus of the lateral lemniscus in the cat. J Comp Neurol. 1984;229(2):257–270. doi: 10.1002/cne.902290210. [DOI] [PubMed] [Google Scholar]
- Whitlock DG, Perl ER. Thalamic projections of spinothalamic pathways in monkey. Exp Neurol. 1961;3:240–255. doi: 10.1016/0014-4886(61)90015-2. [DOI] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: The central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26(48):12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JA. Identification and structure of neurons in the medial geniculate body projecting to primary auditory cortex (AI) in the cat. Neuroscience. 1984a;13(2):395–413. doi: 10.1016/0306-4522(84)90239-2. [DOI] [PubMed] [Google Scholar]
- Winer JA. Advances in Anatomy Embryology and Cell Biology. Springer; Berlin: 1984b. The anatomical organization of the medial geniculate body of the cat: Cytoarchitecture, neuronal architecture, 2nd cortical connections; pp. 1–98. [Google Scholar]
- Winer JA. The human medial geniculate body. Hear Res. 1984c;15(3):225–247. doi: 10.1016/0378-5955(84)90031-5. [DOI] [PubMed] [Google Scholar]
- Winer JA. The medial geniculate body of the cat. Adv Anat Embryol Cell Biol. 1985;86:1–97. [PubMed] [Google Scholar]
- Winer JA. Decoding the auditory corticofugal systems. Hear Res. 2006;212(1–2):1–8. doi: 10.1016/j.heares.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Winer JA, Kelly JB, Larue DT. Neural architecture of the rat medial geniculate body. Hear Res. 1999a;130(1–2):19–41. doi: 10.1016/s0378-5955(98)00216-0. [DOI] [PubMed] [Google Scholar]
- Winer JA, Larue DT. Patterns of reciprocity in auditory thalamocortical and corticothalamic connections: Study with horseradish peroxidase and autoradiographic methods in the rat medial geniculate body. J Comp Neurol. 1987;257(2):282–315. doi: 10.1002/cne.902570212. [DOI] [PubMed] [Google Scholar]
- Winer JA, Miller LM, Lee CC, Schreiner CE. Auditory thalamocortical transformation: Structure and function. Trends Neurosci. 2005;28(5):255–263. doi: 10.1016/j.tins.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Winer JA, Morest DK. The medial division of the medial geniculate body of the cat: Implications for thalamic organization. J Neurosci. 1983a;3(12):2629–2651. doi: 10.1523/JNEUROSCI.03-12-02629.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JA, Morest DK. The neuronal architecture of the dorsal division of the medial geniculate body of the cat. A study with the rapid Golgi method. J Comp Neurol. 1983b;221(1):1–30. doi: 10.1002/cne.902210102. [DOI] [PubMed] [Google Scholar]
- Winer JA, Sally SL, Larue DT, Kelly JB. Origins of medial geniculate body projections to physiologically defined zones of rat primary auditory cortex. Hear Res. 1999b;130(1–2):42–61. doi: 10.1016/s0378-5955(98)00217-2. [DOI] [PubMed] [Google Scholar]
- Winer JA, Wenstrup JJ. Cytoarchitecture of the medial geniculate body in the mustached bat (Pteronotus parnellii) J Comp Neurol. 1994a;346(2):161–182. doi: 10.1002/cne.903460202. [DOI] [PubMed] [Google Scholar]
- Winer JA, Wenstrup JJ. The neurons of the medial geniculate body in the mustached bat (Pteronotus parnellii) J Comp Neurol. 1994b;346(2):183–206. doi: 10.1002/cne.903460203. [DOI] [PubMed] [Google Scholar]
- Zhang X, Giesler GJ., Jr Response characterstics of spinothalamic tract neurons that project to the posterior thalamus in rats. J Neurophysiol. 2005;93(5):2552–2564. doi: 10.1152/jn.01237.2004. [DOI] [PubMed] [Google Scholar]


