Abstract
Background
Phytochemicals are an important source of emerging preventive and therapeutic agents for cancer. Triptolide/PG490, an extract of the Chinese herb Tripterygium wilfordii Hook F, is a potent anti-inflammatory agent that also possesses anticancer activity. While its anti-proliferative effects are well-established, the potential anti-migratory effects of triptolide have not been characterized.
Material and Methods
Effects of triptolide on the proliferation and invasion of colon cancer cells and expression of cancer-related genes and proteins were assessed.
Results
Triptolide potently inhibited HT29 and HCT116 colon cancer cell growth and reduced basal and stimulated HCT116 migration through collagen by 65–80%. Triptolide inhibited mRNA expression of the positive cell cycle regulatory genes c-myc, and A, B, C, and D-type cyclins in multiple colon cancer cell lines. Additionally, we show that triptolide treatment decreased expression of VEGF and COX-2, which promote cancer progression and invasion, and inhibited the expression of multiple cytokine receptors potentially involved in cell migration and cancer metastasis, including the thrombin receptor, CXCR4, TNF receptors and TGF-β receptors.
Conclusions
Triptolide is a potent inhibitor of colon cancer proliferation and migration in vitro. The downregulation of multiple cytokine receptors, in combination with inhibition of COX-2 and VEGF and positive cell cycle regulators, may contribute to the anti-metastatic action of this herbal extract.
Keywords: triptolide, colorectal cancer, herbal extract, growth factor receptors, cell cycle, chemokine
INTRODUCTION
The link between chronic inflammation and tumor progression is now a well accepted concept (1, 2). A number of growth factors and cytokines, secreted by the tumor cells or by the surrounding local stroma, have important roles in tumor progression and metastasis (3). For example, the chemokine receptor CXCR4 is ubiquitously expressed in leukocytes but not in most normal tissues, and its ligand CXCL12 is constitutively expressed in many normal, nonmalignant tissues. CXCR4 expression has been described in many types of human cancers and is generally associated with metastasis to lymph nodes or other sites such as lung, liver, or bone (4). The inducible cyclooxygenase-2 (COX-2) enzyme is also involved in cross-talk with the inflammatory stroma in colorectal cancer (CRC), resulting in promotion of angiogenesis, cell survival, proliferation, migration, and invasion (5).
Natural plant-derived compounds are an attractive class of investigational agents for cancer prevention and treatment. Herbal extracts may modify biologic responses to classical chemotherapy agents and influence multiple signaling pathways; their actions likely include anti-inflammatory, antiproliferative, anti-angiogenic, pro-apoptotic, and/or anti-metastatic effects (6–8). Triptolide (TRP), also known as PG490, is the principal active compound from the roots of a vine native to southern China and Taiwan, Tripterygium wilfordii Hook. F (9). TRP, a diterpenoid triepoxide, has been used as an anti-inflammatory agent for diseases such as rheumatoid arthritis for centuries in Chinese natural medicine (9). A water-soluble form of TRP, PG490-88, entered Phase I clinical trials for therapy against solid tumors in 2002 (10). Gastrointestinal toxicity has been a limiting factor to the widespread use of this herb; however, synthetic analogues and novel formulations with reduced toxicity are currently being investigated (11). The anti-cancer mechanisms of TRP are only partially understood. Effects on apoptotic and cell cycle regulatory proteins have been shown in multiple cancer cell types. Inhibition of transcription factors NFAT and NF-κB by TRP has also been demonstrated, leading to reduced expression of a wide variety of genes regulated by these factors (9). Mechanisms by which TRP may prevent or treat metastasis of solid tumors have not specifically been examined.
We previously showed that curcumin, another herbal extract with anticancer properties, inhibits interleukin-8 secretion and migration of CRC cells (12). The purpose of our present study was to determine if TRP also possesses anti-migratory properties in addition to its established and potent anti-inflammatory and anti-proliferative actions. Here, we found that TRP inhibited CRC cell migration as well as proliferation. The expression of positive cell cycle regulators c-Myc and the cyclins were decreased in CRC cell lines. TRP also suppressed expression of the pro-invasive factors vascular endothelial growth factor (VEGF) and COX-2. Lastly, we found that TRP inhibited expression of multiple growth factor and cytokine receptors in CRC cells, including CXCR4, transforming growth factor-β (TGF-β), ) and thrombin receptors. Our results identify multiple molecular mechanisms to explain the anti-proliferative and anti-cancer effects of TRP.
MATERIALS AND METHODS
Materials
Neurotensin (NT), epidermal growth factor (EGF), DMSO, TRP, and anti-β-actin antibody were purchased from Sigma (St. Louis, MO). COX-2 and horseradish peroxidase-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Enhanced chemiluminescence reagents were from GE Healthcare (formerly Amersham Biosciences; Piscataway, NJ). Collagen type I was from BD Biosciences (Bedford, MA). Costar Transwell inserts were from Corning, Inc. (Lowell, MA). Supplies and reagents for gel electrophoresis were from Invitrogen (Carlsbad, CA). The RPA III kit for RNase protection assay (RPA) and MAXIscript kit for probe hybridization were from Ambion (Austin, TX). Ultraspec RNA isolation reagent was from Biotecx (Houston, TX). Mutli-probe template sets for RPA were from BD Pharmingen (San Diego, CA).
Cell culture
Human CRC cell lines HCT116, HT29, and SW620 were from American Type Culture Collection (Manassas, VA). HCT116 and HT29 cells were maintained in McCoy’s 5A medium supplemented with 10% fetal bovine serum (FBS), 1000 U/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin B. SW620 cells were maintained in a 50:50 mixture of Liebovitz L-15 and DMEM with 10% FBS. The human CRC cell line KM20 was obtained from Dr. Isaiah Fidler (M. D. Anderson Cancer Center, Houston, TX) and grown in MEM supplemented with MEM essential vitamin mixture, MEM non-essential amino acids, 100 mM sodium pyruvate, and 10% FBS. All cells were maintained at 37°C with 5% CO2 mixed with air. Subconfluent cells were serum-starved overnight before all experiments unless indicated otherwise.
Cell proliferation assays
A cell proliferation assay measuring total DNA content was performed using crystal violet dye. Briefly, HCT116 and HT29 cells (1×105) were plated in 12-well plates and allowed to adhere overnight. Cells were treated with TRP or DMSO in serum-free media containing 0.5% bovine serum albumin (BSA) and, after 24, 48, and 72 h, the media was removed and wells for each condition, in triplicate, were fixed and stained. A staining solution containing 20% methanol and 0.5% crystal violet was added to each well, incubated for 30 min at room temperature, and rinsed thoroughly. After air-drying, a constant volume of 15% acetic acid solution was added to each well to solubilize the dye. The plates were placed on an orbital shaker until all dye was completely dissolved. Equal aliquots from each well were transferred to a 96-well plate and their absorbance was read at 570 nm.
Collagen invasion assays
Modified Boyden chamber assays were performed using Transwell inserts (8 μm pore size, polycarbonate membrane, 6.5 mm diameter). Both sides of each Transwell membrane were coated with collagen type I at a concentration of 15 μg/mL for 30 min at 37 °C, then nonspecific binding sites were blocked by incubation in 2% BSA for 30 min at 37°C. HCT116 cells (1×105 in serum-free media) were seeded into the top chamber with drug treatments and chemoattractant in serum-free media was added to the well below the chamber. Cells were treated with TRP (25, 50, and 100 nM), and EGF (20 ng/mL) or NT (100 nM) was used as a chemoattractant. Chambers were incubated 6–12 h and then the cells inside the chamber were removed from the upper side of the membrane with a cotton-tipped swab. The membranes were fixed with 100% methanol, stained with 0.5% crystal violet, and a minimum of 4 high-power fields per membrane were counted under a microscope. Transwells were plated in triplicate and each experiment was repeated on three separate occasions with similar results.
RNA isolation and RPAs
HCT116, KM20, HT29, or SW480 cells were treated with TRP for 16–24 h and RNA was then isolated using Ultraspec RNA reagent according to the manufacturer’s protocol. [α-32P]UTP-labeled antisense RNA probe was prepared using the multiprobe template sets Angio-1, h-myc, hcyc-1, hcR-4, and hcc-2 and MAXI-script SP6/T7 in vitro transcription kit. RPAs were performed using the RPA III RNase protection kit according to the manufacturer’s recommendations and as we have previously described (13, 14). Finally, samples were analyzed by electrophoresis on 5% denaturing polyacrylamide gels and detected by autoradiography.
Western blotting
Whole-cell lysates were prepared using cell lysis buffer supplemented with protease inhibitor cocktail and 1 mM PMSF. Protein concentrations were measured using the Bradford assay (15) and protein (50 μg) was resolved by polyacrylamide gel electrophoresis as we have previously described (16). Briefly, after electrotransfer to polyvinylidene difluoride membranes, nonspecific binding sites were blocked with 5% nonfat milk in TBST for 1 h at room temperature. Membranes were incubated with primary antibodies overnight at 4°C, washed with TBST, and then incubated with the appropriate HRP-conjugated secondary antibody for 1 h at room temperature. Immune complexes were visualized using enhanced chemiluminescence. Consistent loading and transfer were confirmed by probing the same membrane with anti-β-actin. Data are representative of three independent experiments with similar results.
Statistical analysis
The dose effects of TRP on HCT116 and HT29 cell growth were analyzed, separately for each time point (24, 48 or 72 h) using one-way classification analysis of variance (ANOVA). The factor was TRP dose (0, 25, 50 or 100 nM). The effect of TRP removal on HT29 cell growth was also analyzed using ANOVA separately for each time point; the factor was time of drug removal (0, 24 or 72 h). The effect of TRP on migration was analyzed using ANOVA for a two-factor experiment (for one dose in multiple conditions), with the two factors being TRP and chemoattractant. The dose effect of TRP was analyzed using a one-way ANOVA. The factor was TRP dose (0, 25, 50 or 100 nM). The factors were assessed at the 0.05 level of significance. Multiple comparisons were conducted using a t statistic with the standard error computed from the residual mean square in the analysis of variance and the comparisonwise error rate with Bonferroni adjustment for the number of comparisons. Statistical computations were carried out using PROC GLM in SAS®, Release9.1 (17).
RESULTS
Triptolide inhibits CRC cell growth
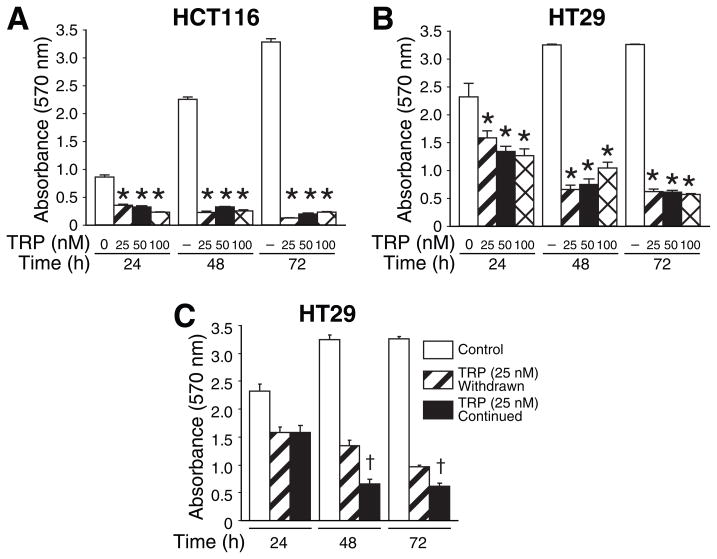
TRP has been shown to inhibit proliferation, induce apoptosis and sensitize other cancer cell types to chemotherapeutic agents in vitro and in vivo (18–22). In our current study, we examined the effects of TRP on human CRC cells. HCT116 cells were plated in triplicate wells of 24-well plates and treated with TRP 25, 50, and 100 nM in serum-free media containing 0.5% BSA. Cell quantity by crystal violet DNA staining was assessed at 24, 48, and 72 h. Cell growth was inhibited by ~60% after 24 h and 95% after 72 h by all doses of TRP; the antiproliferative effect of TRP (25–100 nM) was significant compared to the vehicle treatment at each time point (Fig. 1A). The same experiment was performed in HT29 cells (Fig. 1B) and KM20 cells (data not shown). The antiproliferative effect of TRP appears less pronounced in HT29 than in HCT116 cells; however, this is most likely due to an increased DNA content in HT29 cells, which results in higher crystal violet absorbance values at baseline.
Figure 1. Antiproliferative effect of TRP on colorectal cancer cells.
HCT116 (A) or HT29 (B) cells were treated with 25, 50, or 100 nM TRP over a 72 h time course. Crystal violet assays were performed as described in the Methods section (* = p<0.01 vs. control at each time point). C) After 24 h of exposure of HT29 cells to TRP (25 nM), the medium was replaced with fresh serum-free medium without TRP. The assay was continued for 72 h to compare the effects of TRP withdrawal with those of continuous TRP exposure. († = p<0.05 vs. TRP withdrawn)
We further questioned whether or not the growth arrest induced by TRP would persist despite withdrawal of the drug. We treated HT29 cells with TRP (25 nM) for 24 h and subsequently half of the TRP-treated wells were changed to serum-free (control) media for the remainder of the experiment. The growth inhibition effect persisted even after withdrawal of TRP, as demonstrated by decreasing crystal violet absorbance at 48 and 72h (Fig. 1C). However, the cells with continued TRP treatment demonstrated significantly less cell viability than the treatment withdrawal group at 48 and 72 h.
Triptolide suppresses cell cycle regulatory proteins
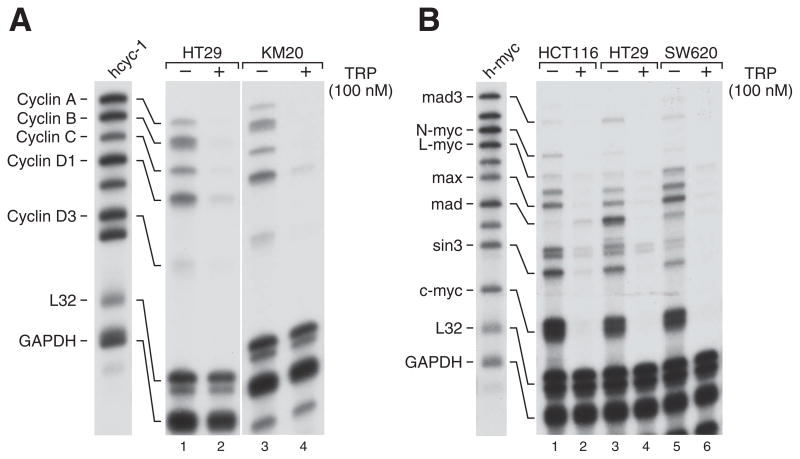
We next sought to examine the mechanisms contributing to the antiproliferative effects of TRP in CRC cells. One mechanism for promoting apoptosis in cancer cells is growth arrest by inhibition of positive cell cycle regulators (23). The kinase cyclin B controls the G2-M transition of the cell cycle, while cyclins A and D are necessary for the G1-S progression to occur. We used RPA to simultaneously study expression of multiple cell cycle control genes in CRC cells (Fig. 2). Expression of cyclins A, B, C, D1, and D3 were all markedly suppressed in HT29 and KM20 cells after overnight treatment with TRP. The levels of the housekeeping genes L32 and GAPDH were constant, demonstrating equal RNA loading. The inhibition of expression of multiple cyclins by TRP is likely to contribute to cell cycle arrest and subsequent apoptosis of CRC cells.
Figure 2. TRP potently suppresses mRNA expression of cyclins A, B, C and D1 and c-myc.
HCT116, HT29, KM20, and SW620 cells were treated with TRP at the indicated doses for 24 h and the extracted RNA subjected to RPA as described in the Methods section. The hcyc-1 (A), and h-myc (B) probes were used to examine multiple related gene products with L32 and GAPDH used as loading controls.
Additional RPA analyses using the h-myc probe which contains cDNAs for the proto-oncogenes c-Myc, l-Myc, n-Myc and other related transcription factors including Max and Mad demonstrate that c-Myc is the most highly expressed mRNA among this group in HCT116, HT29, and SW620 cells (Fig. 2B). Expression of c-Myc was completely inhibited by overnight treatment with TRP. L-Myc was inhibited in these CRC cells as well, though its baseline expression was much lower than c-Myc. N-myc was detected only in HCT116 cells, but its expression was also suppressed by TRP. The myc oncogenes act to drive cellular proliferation, so their decreased expression probably contributes to the antiproliferative effects of TRP. The levels of Max, Mad, and sin3 were also decreased by TRP treatment, however the specific roles of these myc-related transcription factors in CRC have not yet been elucidated.
Triptolide inhibits NT and EGF-stimulated migration of HCT116 colon cancer cells
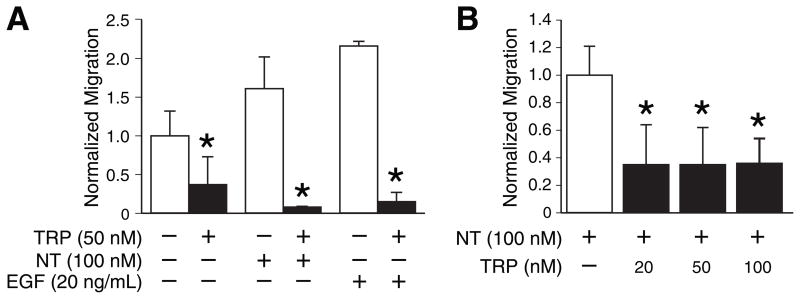
Next, we sought to determine whether TRP inhibited cancer cell migration or invasion, as we recently showed for curcumin, another anti-inflammatory herbal extract (12). NT and EGF were used as chemoattractants for in vitro migration assays since they are both mitogenic for colon cancer cells but act through distinct classes of receptors. TRP significantly inhibited NT- and EGF-stimulated invasion through collagen-coated membranes by HCT116 cells at a dose of 50 nM in overnight chamber assays (Fig. 3A). A shorter assay (6 h incubation) was performed to ensure that the suppression of migration was not due to the antiproliferative effects; TRP still inhibited migration by approximately 70% in this shorter assay (Fig. 3B).
Figure 3. TRP inhibits NT- and EGF-stimulated collagen invasion by HCT116 cells in chamber-based assays.
Cells were treated with TRP for 6 h prior to seeding into chambers in serum-free media and then allowed to migrate overnight (A) or for 6 h (B) toward the specified chemoattractant with the indicated dose of TRP. The number of cells per high-power field (HPF) for the control condition was arbitrarily assigned a value of 1.0 and cell counts for all conditions were normalized to control conditions. (Each bar is an average of 2 or 3 wells; similar results were obtained on three separate occasions; * = p<0.05 vs. no TRP). NT, neurotensin; EGF, epidermal growth factor.
Triptolide decreases expression of the pro-inflammatory and pro-invasive gene COX-2
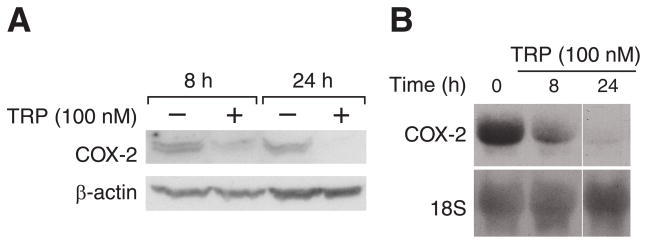
We next examined potential mechanisms by which TRP decreases migration of CRC cells, possibly contributing to reduced tumor invasion. COX-2 is over-expressed in up to 85% of colon cancers and is associated with advanced clinical stage and distant metastasis of human colon cancers (24, 25). Therefore, we investigated the effects of TRP on COX-2 expression in HT29 and HCT116 cells by Western and Northern blotting. A decrease in COX-2 protein levels after 8 and 24 h TRP treatment in HT29 cells is shown in Fig. 4A, with β-actin probed on the same membrane to demonstrate equal loading. Similar inhibition of COX-2 expression was noted in HCT116 cells (data not shown). We also noted a decrease in COX-2 mRNA expression at 8 and 24 h in HT29 cells, with the 28S ribosomal subunit RNA shown as a loading control (Fig. 4B). The reduction in COX-2 expression at both the RNA and protein level by TRP is likely to contribute to the reduced invasive capacity of CRC cells.
Figure 4. TRP suppresses COX-2 protein expression.
A) HT29 cells were treated with TRP for 8 to 24 h and Western blots probed for COX-2; blots were reprobed with β-actin as a loading control. B) RNA extracted from HT29 cells after 8 to 24 h of TRP treatment was subjected to Northern blot analysis for COX-2 expression; expression of ribosomal subunit 28S was utilized as a loading control.
Triptolide decreases expression of cytokine receptors and VEGF in CRC cells
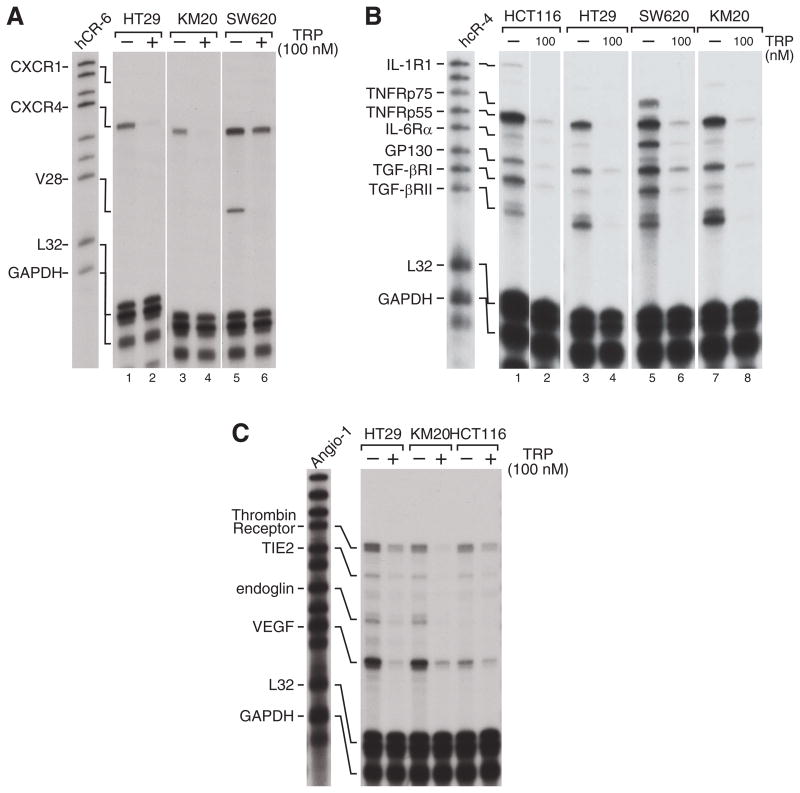
We examined TRP-induced changes in expression of angiogenesis-related genes and pro-invasive cytokine receptors using RPA. CXCR4 is a chemokine receptor that plays a critical role in metastasis of many types of cancer, including CRC (26, 27). We found that CXCR4 mRNA expression was reduced in HT29, KM20, and SW620 cells after 24 h of TRP treatment (Fig. 5A). The CX3CR1 receptor was expressed only in SW620 cells, but its expression was also inhibited by TRP.
Figure 5. TRP inhibits mRNA expression of cytokine receptors.
The indicated CRC cell lines were treated overnight with TRP 100 nM and RNA extracts were subjected to RPA as described in the Methods section. Multi-probe templates hcR-6 (A), hcR-4 (B), and Angio-1 (C) were used to examine expression of CXCR genes, cytokine receptors, and angiogenesis-related genes, respectively. L32 and GAPDH expression was utilized as a loading control for all assays.
We next investigated the expression of interleukin, TNF-α and TGF-β family receptors (Fig. 5B). The TNF receptor p55 subunit (TNFRp55) was highly expressed in all 4 CRC cell lines tested, and its expression universally inhibited by TRP. The IL-6 receptor α subunit (IL-6Rα) was minimally expressed except in the SW620 cells, where its strong expression was inhibited by TRP treatment. GP130, a co-receptor for the IL-6 family cytokines, was expressed and inhibited by TRP in all 4 cell lines. Both TGF-β receptor subunits (TGF βRI and TGFβRII) were also universally expressed in CRC cells and inhibited by TRP.
Lastly, the Angio-1 RPA probe was used to examine angiogenesis-related genes that contribute to CRC invasive capacity. We found that VEGF and the thrombin receptor was expressed in HT29, KM20, and HCT116 cells and their mRNA expression was inhibited by 24h treatment with TRP (Fig. 5C). The decrease in VEGF levels after TRP treatment should contribute to its anticancer efficacy through blockade of neoangiogenesis, whereas the reduced thrombin receptor levels may impede migration of cancer cells toward sources of the potent chemokine and coagulant protein, thrombin.
DISCUSSION
Naturally-occuring, plant-derived compounds are increasingly being investigated as potential therapies at a time when new preventive or therapeutic agents for cancer are desperately needed (28). Phytochemicals that influence multiple signaling pathways often enhance the activity of conventional chemotherapy and radiation therapy and may be used in lower doses when such synergistic effects are present (6). In our current study, we show that TRP inhibits expression of the cyclin genes, c-myc, COX-2, and VEGF in CRC cells. Additionally, we found that TRP inhibited expression of multiple cytokine receptors, including thrombin, CXCR4, TGF-β, TNF-α, and IL-6. The combination of these cellular effects likely contributes to the potent anti-cancer actions of TRP.
TRP has been shown to induce apoptosis in certain tumor cells, although its precise cellular target(s) remain unclear (29). In our current study, we show that TRP has anti-proliferative effects in CRC cells, inhibiting cell growth by 60–95% in a time-dependent fashion. These results are similar to findings in other cancer cells, including the CRC cell line SW114 (30), human fibrosarcoma and cervical carcinoma cells (31). We additionally demonstrated inhibition of cyclins A, B, C, D1, and D3 by TRP, which leads to cell cycle arrest during G1 (cyclin D1 and D3), at the G1/S transition (cyclin A) or the G2/M checkpoint (cyclin B). The inhibition of expression of cyclin B1 in T-cells (9), and cyclins A, B1, and D1 in breast cancer cells (32) and bronchial epithelial cells (33) by TRP was previously demonstrated, but our study is the first report of cyclin inhibition by TRP in CRC cells. Consistent with our results, it was previously demonstrated in leukemia and CRC cells that TRP treatment results in cell cycle arrest at either the G1/S or G2/M checkpoint, depending on the duration and concentration of treatment (34, 35).
Metastasis, the main cause of CRC mortality, requires multiple sequential and integrated cellular processes (36). Migration of cancer cells toward blood or lymphatic vessels, invasion of basement membranes and endothelial cell layers, and proliferation at a distant location are necessary for successful metastasis. A number of cytokines and chemokines are believed to promote tumor cell migration toward vessels and adhesion at distant sites of metastasis (4, 37). For example, the chemokine CXCL12 (SDF-1α) is widely expressed in non-malignant human tissues, and it is the sole ligand for the chemokine receptor CXCR4. CXCR4 is selectively expressed in lymph and hematopoetic tissues, but is also expressed in a wide variety of human cancers; evidence in multiple tumor models supports its involvement in homing of metastatic cancer cells to distant sites (37). We show that CXCR4 expression is downregulated by TRP in three distinct CRC cell lines. It was also recently described that TRP inhibited the expression of CXCR4 in primary lymphoma cells (38). This was demonstrated by flow cytometry after in vitro treatment of freshly isolated cells by TRP, and by migration assays toward recombinant CXCL12 and cultured lymph node stromal cells. The inhibition of CXCR4 expression is likely to be an important mechanism of this herbal extract’s anti-cancer activity.
We also found that the mRNA expression of both TGF-β receptor subunits (I and II) was suppressed by TRP. There are no previous reports of TGF superfamily receptor modulation by TRP in cancer cells, although inhibition of TGF-β cytokine expression has been studied in models of chronic allograft rejection and post-lung transplant obliterative airway disease (39, 40). TGF-β is thought to act as a tumor suppressor by inhibiting epithelial cell growth. However, advanced tumors are often resistant to TGF-β growth inhibitory signals due to acquired mutations or aberrant cell cycle regulation, in which case this cytokine promotes invasion, angiogenesis, and metastasis (41). For example, TGF-β produced by human CRC cells increased their migration in a paracrine/autocrine fashion, and blockade of cellular TGF-β production suppressed the pro-migratory activity (5). A chemical inhibitor of TGF-β receptor I kinase (SD-208) has been shown to reduce growth and invasion or metastasis of human glioma cells, pancreatic cancer cells, multiple myeloma, and mouse mammary carcinoma (42–45). Therefore, inhibiting the expression of TGF-β receptors using TRP should contribute to its anticancer activity in late stage tumors.
Another cytokine receptor inhibited by TRP in our study was the TNF-α receptor p55 (TNFR1). The p55 receptor is constitutively expressed by most human tissues, whereas expression of the p75 (TNFR2) type receptor is generally limited to cells of the immune system (46). Like TGF-β, the actions of the cytokine TNF-α are numerous and depend on the cell type and environment (46). TNF-α can contribute to epithelial-mesenchymal transition in CRC, the progression of cancer cells toward a motile, metastatic phenotype (47). Importantly, expression of the TNFRp55 was shown to enhance CRC liver metastasis in a preclinical TNFRp55 knockout model (48). There are no previous reports of TNF-α receptor modulation by TRP in immune or cancer cells, although TRP and TNF-α are synergistic in their cytotoxicity toward multiple human cells lines in vitro (21, 49).
In this age of targeted therapy, single-receptor targeted drugs for cancer have largely proven to be problematic with respect to cost, adverse effects, and efficacy (28). Therefore, targeting multiple cytokine receptors with a plant-derived agent could be a more effective strategy alone or in combination with conventional chemotherapy. We have shown that TRP inhibits cytokines and growth factor receptors related to multiple distinct pathways including angiogenesis (VEGF), migration (thrombin receptor, CXCR4, TGF-βR, TNFRs, IL-6R), invasion (COX-2) and proliferation (c-myc). TRP is still under intense investigation in preclinical studies. While a variety of cellular and molecular effects have been reported, the specific structural mechanisms of TRP have not been identified. A recent report by McCallum et. al.(50) demonstrated that TRP binds irreversibly and specifically to an unidentified 90-kD nuclear protein. Another study reported the binding of TRP to a 110-kD calcium channel protein involved in polycystic kidney disease, PC2 or polycystin-2 (51). A better understanding of the actions of this herbal extract will facilitate its use in more specific clinical trials, may allow for synthetic derivatives to be engineered (equally effective but less toxic), and potentially offer insight into additional therapeutic uses for TRP.
In conclusion, we show that TRP modulates expression of cell cycle regulators and multiple cytokine receptors in CRC cells. These novel properties likely contribute to the anti-metastatic potential of TRP and provide new insights into the mechanisms of action of TRP.
Acknowledgments
The authors thank Tatsuo Uchida for statistical analysis, Karen Martin for manuscript preparation, and Andrea Ramirez for technical assistance. This work was supported by grants R01 DK48498, R01 CA104748, T32 DK07639 and P01 DK35408 from the National Institutes of Health and a Jeanne B. Kempner post-doctoral scholarship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schottenfeld D, Beebe-Dimmer J. Chronic inflammation: a common and important factor in the pathogenesis of neoplasia. CA: a cancer journal for clinicians. 2006;56:69. doi: 10.3322/canjclin.56.2.69. [DOI] [PubMed] [Google Scholar]
- 2.Cendan JC, Behrns KE. Associated neoplastic disease in inflammatory bowel disease. The Surgical clinics of North America. 2007;87:659. doi: 10.1016/j.suc.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka T, Bai Z, Srinoulprasert Y, et al. Chemokines in tumor progression and metastasis. Cancer Sci. 2005;96:317. doi: 10.1111/j.1349-7006.2005.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruffini PA, Morandi P, Cabioglu N, et al. Manipulating the chemokine-chemokine receptor network to treat cancer. Cancer. 2007;109:2392. doi: 10.1002/cncr.22706. [DOI] [PubMed] [Google Scholar]
- 5.Liu G, Ding W, Liu X, et al. c-Fos is required for TGFbeta1 production and the associated paracrine migratory effects of human colon carcinoma cells. Mol Carcinog. 2006;45:582. doi: 10.1002/mc.20189. [DOI] [PubMed] [Google Scholar]
- 6.HemaIswarya S, Doble M. Potential synergism of natural products in the treatment of cancer. Phytother Res. 2006;20:239. doi: 10.1002/ptr.1841. [DOI] [PubMed] [Google Scholar]
- 7.Sagar SM, Yance D, Wong RK. Natural health products that inhibit angiogenesis: a potential source for investigational new agents to treat cancer-Part 1. Current oncology (Toronto, Ont. 2006;13:14. [PMC free article] [PubMed] [Google Scholar]
- 8.Treasure J. Herbal medicine and cancer: an introductory overview. Seminars in oncology nursing. 2005;21:177. doi: 10.1016/j.soncn.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Qiu D, Kao PN. Immunosuppressive and anti-inflammatory mechanisms of triptolide, the principal active diterpenoid from the Chinese medicinal herb Tripterygium wilfordii Hook. f. Drugs R D. 2003;4:1. doi: 10.2165/00126839-200304010-00001. [DOI] [PubMed] [Google Scholar]
- 10.Fidler JM, Li K, Chung C, et al. PG490-88, a derivative of triptolide, causes tumor regression and sensitizes tumors to chemotherapy. Molecular cancer therapeutics. 2003;2:855. [PubMed] [Google Scholar]
- 11.Mei Z, Li X, Wu Q, et al. The research on the anti-inflammatory activity and hepatotoxicity of triptolide-loaded solid lipid nanoparticle. Pharmacol Res. 2005;51:345. doi: 10.1016/j.phrs.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Wang Q, Ives KL, et al. Curcumin inhibits neurotensin-mediated interleukin-8 production and migration of HCT116 human colon cancer cells. Clin Cancer Res. 2006;12:5346. doi: 10.1158/1078-0432.CCR-06-0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evers BM, Zhou Z, Celano P, et al. The neurotensin gene is a downstream target for Ras activation. The Journal of clinical investigation. 1995;95:2822. doi: 10.1172/JCI117987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Q, Wang X, Hernandez A, et al. Regulation of TRAIL expression by the phosphatidylinositol 3-kinase/Akt/GSK-3 pathway in human colon cancer cells. The Journal of biological chemistry. 2002;277:36602. doi: 10.1074/jbc.M206306200. [DOI] [PubMed] [Google Scholar]
- 15.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical biochemistry. 1976;72:248. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 16.Kim S, Domon-Dell C, Kang J, et al. Down-regulation of the tumor suppressor PTEN by the tumor necrosis factor-alpha/nuclear factor-kappaB (NF-kappaB)- inducing kinase/NF-kappaB pathway is linked to a default IkappaB-alpha autoregulatory loop. The Journal of biological chemistry. 2004;279:4285. doi: 10.1074/jbc.M308383200. [DOI] [PubMed] [Google Scholar]
- 17.Clark V, editor. SAS/STAT® 9.1 User’s Guide. Cary, NC: SAS Institute Inc; 2004. [Google Scholar]
- 18.Chang WT, Kang JJ, Lee KY, et al. Triptolide and chemotherapy cooperate in tumor cell apoptosis. A role for the p53 pathway. The Journal of biological chemistry. 2001;276:2221. doi: 10.1074/jbc.M009713200. [DOI] [PubMed] [Google Scholar]
- 19.Carter BZ, Mak DH, Schober WD, et al. Triptolide induces caspase-dependent cell death mediated via the mitochondrial pathway in leukemic cells. Blood. 2006;108:630. doi: 10.1182/blood-2005-09-3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiviharju TM, Lecane PS, Sellers RG, et al. Antiproliferative and proapoptotic activities of triptolide (PG490), a natural product entering clinical trials, on primary cultures of human prostatic epithelial cells. Clin Cancer Res. 2002;8:2666. [PubMed] [Google Scholar]
- 21.Lee KY, Chang W, Qiu D, et al. PG490 (triptolide) cooperates with tumor necrosis factor-alpha to induce apoptosis in tumor cells. The Journal of biological chemistry. 1999;274:13451. doi: 10.1074/jbc.274.19.13451. [DOI] [PubMed] [Google Scholar]
- 22.Lee KY, Park JS, Jee YK, et al. Triptolide sensitizes lung cancer cells to TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by inhibition of NF-kappaB activation. Experimental & molecular medicine. 2002;34:462. doi: 10.1038/emm.2002.64. [DOI] [PubMed] [Google Scholar]
- 23.Maddika S, Ande SR, Panigrahi S, et al. Cell survival, cell death and cell cycle pathways are interconnected: implications for cancer therapy. Drug Resist Updat. 2007;10:13. doi: 10.1016/j.drup.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Eberhart CE, Coffey RJ, Radhika A, et al. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 25.Sinicrope FA. Targeting cyclooxygenase-2 for prevention and therapy of colorectal cancer. Mol Carcinog. 2006;45:447. doi: 10.1002/mc.20232. [DOI] [PubMed] [Google Scholar]
- 26.Kim J, Takeuchi H, Lam ST, et al. Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol. 2005;23:2744. doi: 10.1200/JCO.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 27.Scala S, Ottaiano A, Ascierto PA, et al. Expression of CXCR4 predicts poor prognosis in patients with malignant melanoma. Clin Cancer Res. 2005;11:1835. doi: 10.1158/1078-0432.CCR-04-1887. [DOI] [PubMed] [Google Scholar]
- 28.Aggarwal BB, Sethi G, Baladandayuthapani V, et al. Targeting cell signaling pathways for drug discovery: An old lock needs a new key. J Cell Biochem. 2007;102:580. doi: 10.1002/jcb.21500. [DOI] [PubMed] [Google Scholar]
- 29.Corson TW, Crews CM. Molecular understanding and modern application of traditional medicines: triumphs and trials. Cell. 2007;130:769. doi: 10.1016/j.cell.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tong X, Zheng S, Jin J, et al. Triptolide inhibits cyclooxygenase-2 and inducible nitric oxide synthase expression in human colon cancer and leukemia cells. Acta biochimica et biophysica Sinica. 2007;39:89. doi: 10.1111/j.1745-7270.2007.00254.x. [DOI] [PubMed] [Google Scholar]
- 31.Miyata Y, Sato T, Ito A. Triptolide, a diterpenoid triepoxide, induces antitumor proliferation via activation of c-Jun NH2-terminal kinase 1 by decreasing phosphatidylinositol 3-kinase activity in human tumor cells. Biochem Biophys Res Commun. 2005;336:1081. doi: 10.1016/j.bbrc.2005.08.247. [DOI] [PubMed] [Google Scholar]
- 32.Yang S, Chen J, Guo Z, et al. Triptolide inhibits the growth and metastasis of solid tumors. Molecular cancer therapeutics. 2003;2:65. [PubMed] [Google Scholar]
- 33.Zhao G, Vaszar LT, Qiu D, et al. Anti-inflammatory effects of triptolide in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2000;279:L958. doi: 10.1152/ajplung.2000.279.5.L958. [DOI] [PubMed] [Google Scholar]
- 34.Ko JK, Leung WC, Ho WK, et al. Herbal diterpenoids induce growth arrest and apoptosis in colon cancer cells with increased expression of the nonsteroidal anti-inflammatory drug-activated gene. Eur J Pharmacol. 2007;559:1. doi: 10.1016/j.ejphar.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Yinjun L, Jie J, Yungui W. Triptolide inhibits transcription factor NF-kappaB and induces apoptosis of multiple myeloma cells. Leukemia research. 2005;29:99. doi: 10.1016/j.leukres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 36.Mehlen P, Puisieux A. Metastasis: a question of life or death. Nature reviews. 2006;6:449. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- 37.Zlotnik A. Involvement of chemokine receptors in organ-specific metastasis. Contrib Microbiol. 2006;13:191. doi: 10.1159/000092973. [DOI] [PubMed] [Google Scholar]
- 38.Zhang C, Cui GH, Liu F, et al. Inhibitory effect of triptolide on lymph node metastasis in patients with non-Hodgkin lymphoma by regulating SDF-1/CXCR4 axis in vitro. Acta pharmacologica Sinica. 2006;27:1438. doi: 10.1111/j.1745-7254.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 39.Leonard CT, Soccal PM, Berry GJ, et al. PG490-88, a derivative of triptolide, attenuates obliterative airway disease in a mouse heterotopic tracheal allograft model. J Heart Lung Transplant. 2002;21:1314. doi: 10.1016/s1053-2498(02)00449-7. [DOI] [PubMed] [Google Scholar]
- 40.Leuenroth SJ, Crews CM. Studies on calcium dependence reveal multiple modes of action for triptolide. Chemistry & biology. 2005;12:1259. doi: 10.1016/j.chembiol.2005.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jakowlew SB. Transforming growth factor-beta in cancer and metastasis. Cancer Metastasis Rev. 2006;25:435. doi: 10.1007/s10555-006-9006-2. [DOI] [PubMed] [Google Scholar]
- 42.Uhl M, Aulwurm S, Wischhusen J, et al. SD-208, a novel transforming growth factor beta receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo. Cancer Res. 2004;64:7954. doi: 10.1158/0008-5472.CAN-04-1013. [DOI] [PubMed] [Google Scholar]
- 43.Gaspar NJ, Li L, Kapoun AM, et al. Inhibition of transforming growth factor beta signaling reduces pancreatic adenocarcinoma growth and invasiveness. Molecular pharmacology. 2007;72:152. doi: 10.1124/mol.106.029025. [DOI] [PubMed] [Google Scholar]
- 44.Hayashi T, Hideshima T, Nguyen AN, et al. Transforming growth factor beta receptor I kinase inhibitor down-regulates cytokine secretion and multiple myeloma cell growth in the bone marrow microenvironment. Clin Cancer Res. 2004;10:7540. doi: 10.1158/1078-0432.CCR-04-0632. [DOI] [PubMed] [Google Scholar]
- 45.Ge R, Rajeev V, Ray P, et al. Inhibition of growth and metastasis of mouse mammary carcinoma by selective inhibitor of transforming growth factor-beta type I receptor kinase in vivo. Clin Cancer Res. 2006;12:4315. doi: 10.1158/1078-0432.CCR-06-0162. [DOI] [PubMed] [Google Scholar]
- 46.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 47.Bates RC, DeLeo MJ, 3rd, Mercurio AM. The epithelial-mesenchymal transition of colon carcinoma involves expression of IL-8 and CXCR-1-mediated chemotaxis. Experimental cell research. 2004;299:315. doi: 10.1016/j.yexcr.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 48.Kitakata H, Nemoto-Sasaki Y, Takahashi Y, et al. Essential roles of tumor necrosis factor receptor p55 in liver metastasis of intrasplenic administration of colon 26 cells. Cancer Res. 2002;62:6682. [PubMed] [Google Scholar]
- 49.Panichakul T, Wanun T, Reutrakul V, et al. Synergistic cytotoxicity and apoptosis induced in human cholangiocarcinoma cell lines by a combined treatment with tumor necrosis factor-alpha (TNF-alpha) and triptolide. Asian Pacific journal of allergy and immunology/launched by the Allergy and Immunology Society of Thailand. 2002;20:167. [PubMed] [Google Scholar]
- 50.McCallum C, Kwon S, Leavitt P, et al. Triptolide binds covalently to a 90 kDa nuclear protein. Role of epoxides in binding and activity. Immunobiology. 2007;212:549. doi: 10.1016/j.imbio.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 51.Leuenroth SJ, Okuhara D, Shotwell JD, et al. Triptolide is a traditional Chinese medicine-derived inhibitor of polycystic kidney disease. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:4389. doi: 10.1073/pnas.0700499104. [DOI] [PMC free article] [PubMed] [Google Scholar]