Abstract
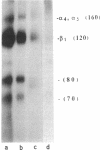
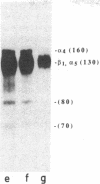
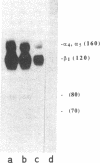
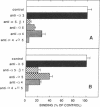
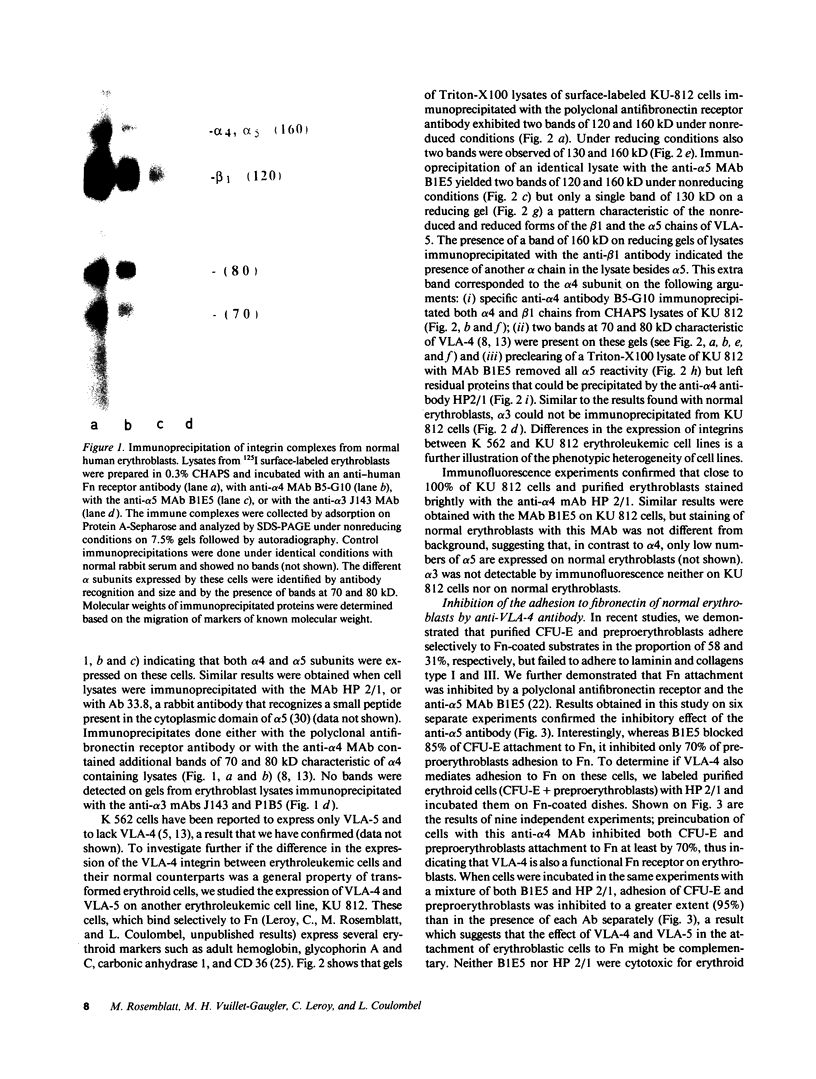
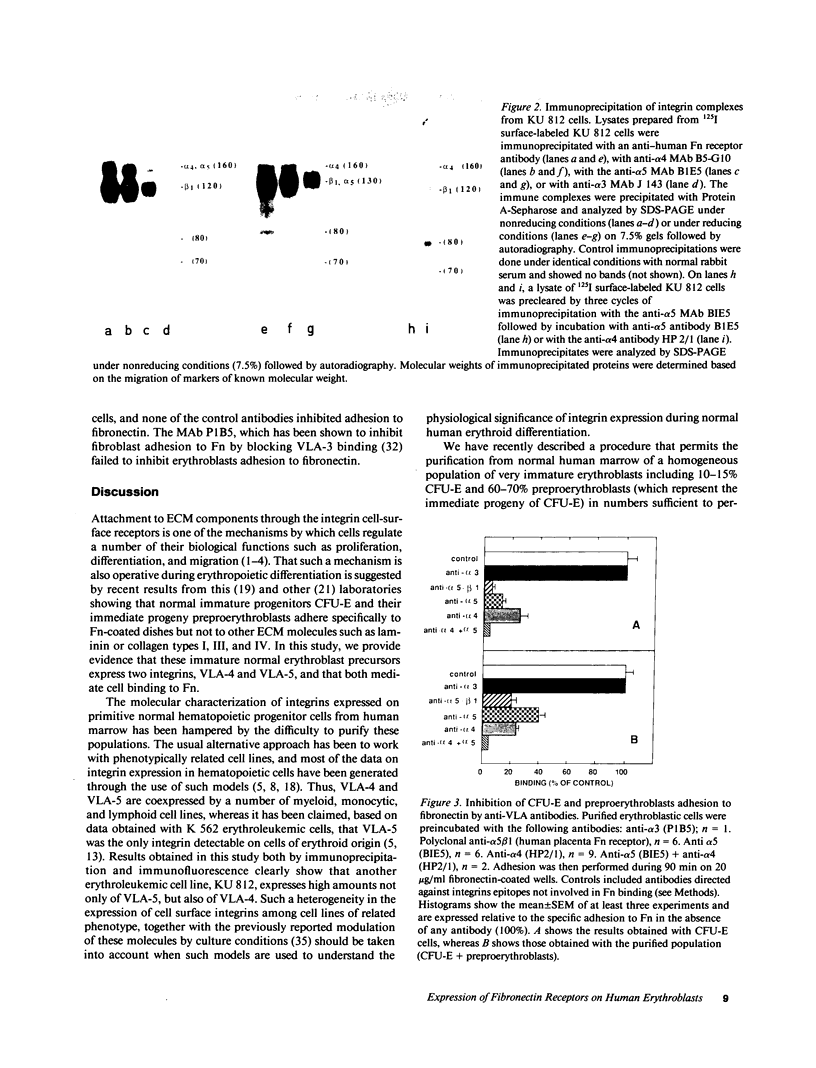
Human erythroblastic precursor cells adhere to fibronectin (Fn) but the exact nature of the receptors mediating this interaction has not been characterized. In this study, we report data showing that immature human erythroblasts express the integrins VLA-4 and VLA-5 and that both these molecules act as fibronectin receptors on these cells. We have recently demonstrated that adhesion to Fn of purified human CFU-E and their immediate progeny preproerythroblasts was inhibited by antibodies directed against the human fibronectin receptor (VLA-5). Here we have extended those results and characterized by immunoprecipitation with specific antibodies the integrins expressed on surface-labeled normal human immature erythroblasts. A polyclonal antibody recognizing the common VLA beta 1 subunit yielded two polypeptides of 120 and 160 kD. Our data further demonstrate that the polypeptide of 160 kD contains alpha subunits corresponding to both alpha 4 and alpha 5. Thus, erythroblast lysates prepared in 0.3% CHAPS and immunoprecipitated with antibodies which specifically recognize the alpha 4 subunit showed a heterodimer with peptides of 120 (beta 1) and 160 kD (alpha 4) and the additional peptides of 70 and 80 kD which usually coprecipitate with the alpha 4 chain. On the other hand, specific anti-alpha 5 antibodies immunoprecipitated an alpha 5/beta 1 complex with peptides of 120 and 160 kD which under reducing conditions migrated as a single band of 130 kD. Similar experiments performed with an erythroleukemic cell line (KU 812) showed that these cells also coexpress both the VLA-4 and VLA-5 members of the integrin family. Furthermore, monoclonal antibodies recognizing the VLA alpha 4 chain blocked the adhesion of immature erythroblasts to Fn-coated surfaces, thus demonstrating that, as VLA-5, VLA-4 is also a functional Fn receptor on these cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bednarczyk J. L., McIntyre B. W. A monoclonal antibody to VLA-4 alpha-chain (CDw49d) induces homotypic lymphocyte aggregation. J Immunol. 1990 Feb 1;144(3):777–784. [PubMed] [Google Scholar]
- Boucaut J. C., Darribère T., Poole T. J., Aoyama H., Yamada K. M., Thiery J. P. Biologically active synthetic peptides as probes of embryonic development: a competitive peptide inhibitor of fibronectin function inhibits gastrulation in amphibian embryos and neural crest cell migration in avian embryos. J Cell Biol. 1984 Nov;99(5):1822–1830. doi: 10.1083/jcb.99.5.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. L., Phillips D. R., Damsky C. H., Charo I. F. Synthesis and expression of the fibroblast fibronectin receptor in human monocytes. J Clin Invest. 1989 Jul;84(1):366–370. doi: 10.1172/JCI114166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardarelli P. M., Pierschbacher M. D. Identification of fibronectin receptors on T lymphocytes. J Cell Biol. 1987 Jul;105(1):499–506. doi: 10.1083/jcb.105.1.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombel L., Vuillet M. H., Leroy C., Tchernia G. Lineage- and stage-specific adhesion of human hematopoietic progenitor cells to extracellular matrices from marrow fibroblasts. Blood. 1988 Feb;71(2):329–334. [PubMed] [Google Scholar]
- Edelman P., Vinci G., Villeval J. L., Vainchenker W., Henri A., Miglierina R., Rouger P., Reviron J., Breton-Gorius J., Sureau C. A monoclonal antibody against an erythrocyte ontogenic antigen identifies fetal and adult erythroid progenitors. Blood. 1986 Jan;67(1):56–63. [PubMed] [Google Scholar]
- Elices M. J., Osborn L., Takada Y., Crouse C., Luhowskyj S., Hemler M. E., Lobb R. R. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990 Feb 23;60(4):577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- Fingerman E., Hemler M. E. Regulation of proteins in the VLA cell substrate adhesion family: influence of cell growth conditions on VLA-1, VLA-2, and VLA-3 expression. Exp Cell Res. 1988 Jul;177(1):132–142. doi: 10.1016/0014-4827(88)90031-6. [DOI] [PubMed] [Google Scholar]
- Guan J. L., Hynes R. O. Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor alpha 4 beta 1. Cell. 1990 Jan 12;60(1):53–61. doi: 10.1016/0092-8674(90)90715-q. [DOI] [PubMed] [Google Scholar]
- Hall D. E., Reichardt L. F., Crowley E., Holley B., Moezzi H., Sonnenberg A., Damsky C. H. The alpha 1/beta 1 and alpha 6/beta 1 integrin heterodimers mediate cell attachment to distinct sites on laminin. J Cell Biol. 1990 Jun;110(6):2175–2184. doi: 10.1083/jcb.110.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler M. E. Adhesive protein receptors on hematopoietic cells. Immunol Today. 1988 Apr;9(4):109–113. doi: 10.1016/0167-5699(88)91280-7. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Huang C., Schwarz L. The VLA protein family. Characterization of five distinct cell surface heterodimers each with a common 130,000 molecular weight beta subunit. J Biol Chem. 1987 Mar 5;262(7):3300–3309. [PubMed] [Google Scholar]
- Hemler M. E., Huang C., Schwarz L. The VLA protein family. Characterization of five distinct cell surface heterodimers each with a common 130,000 molecular weight beta subunit. J Biol Chem. 1987 Mar 5;262(7):3300–3309. [PubMed] [Google Scholar]
- Hemler M. E., Huang C., Takada Y., Schwarz L., Strominger J. L., Clabby M. L. Characterization of the cell surface heterodimer VLA-4 and related peptides. J Biol Chem. 1987 Aug 25;262(24):11478–11485. [PubMed] [Google Scholar]
- Hemler M. E., Sanchez-Madrid F., Flotte T. J., Krensky A. M., Burakoff S. J., Bhan A. K., Springer T. A., Strominger J. L. Glycoproteins of 210,000 and 130,000 m.w. on activated T cells: cell distribution and antigenic relation to components on resting cells and T cell lines. J Immunol. 1984 Jun;132(6):3011–3018. [PubMed] [Google Scholar]
- Holzmann B., McIntyre B. W., Weissman I. L. Identification of a murine Peyer's patch--specific lymphocyte homing receptor as an integrin molecule with an alpha chain homologous to human VLA-4 alpha. Cell. 1989 Jan 13;56(1):37–46. doi: 10.1016/0092-8674(89)90981-1. [DOI] [PubMed] [Google Scholar]
- Humphries M. J., Komoriya A., Akiyama S. K., Olden K., Yamada K. M. Identification of two distinct regions of the type III connecting segment of human plasma fibronectin that promote cell type-specific adhesion. J Biol Chem. 1987 May 15;262(14):6886–6892. [PubMed] [Google Scholar]
- Kantor R. R., Mattes M. J., Lloyd K. O., Old L. J., Albino A. P. Biochemical analysis of two cell surface glycoprotein complexes, very common antigen 1 and very common antigen 2. Relationship to very late activation T cell antigens. J Biol Chem. 1987 Nov 5;262(31):15158–15165. [PubMed] [Google Scholar]
- Kaufmann R., Frösch D., Westphal C., Weber L., Klein C. E. Integrin VLA-3: ultrastructural localization at cell-cell contact sites of human cell cultures. J Cell Biol. 1989 Oct;109(4 Pt 1):1807–1815. doi: 10.1083/jcb.109.4.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer N., Bettaieb A., Legrand C., Coulombel L., Vainchenker W., Edelman L., Breton-Gorius J. Developmentally regulated expression of a 78 kDa erythroblast membrane glycoprotein immunologically related to the platelet thrombospondin receptor. Biochem J. 1989 Sep 15;262(3):835–842. doi: 10.1042/bj2620835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi K. A new leukemia cell line with Philadelphia chromosome characterized as basophil precursors. Leuk Res. 1985;9(3):381–390. doi: 10.1016/0145-2126(85)90060-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Menko A. S., Boettiger D. Occupation of the extracellular matrix receptor, integrin, is a control point for myogenic differentiation. Cell. 1987 Oct 9;51(1):51–57. doi: 10.1016/0092-8674(87)90009-2. [DOI] [PubMed] [Google Scholar]
- Mould A. P., Wheldon L. A., Komoriya A., Wayner E. A., Yamada K. M., Humphries M. J. Affinity chromatographic isolation of the melanoma adhesion receptor for the IIICS region of fibronectin and its identification as the integrin alpha 4 beta 1. J Biol Chem. 1990 Mar 5;265(7):4020–4024. [PubMed] [Google Scholar]
- Nakazawa M., Mitjavila M. T., Debili N., Casadevall N., Mayeux P., Rouyer-Fessard P., Dubart A., Roméo P. H., Beuzard Y., Kishi K. KU 812: a pluripotent human cell line with spontaneous erythroid terminal maturation. Blood. 1989 May 15;73(7):2003–2013. [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984 May 3;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Plantefaber L. C., Hynes R. O. Changes in integrin receptors on oncogenically transformed cells. Cell. 1989 Jan 27;56(2):281–290. doi: 10.1016/0092-8674(89)90902-1. [DOI] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985 Jan;40(1):191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Roman J., LaChance R. M., Broekelmann T. J., Kennedy C. J., Wayner E. A., Carter W. G., McDonald J. A. The fibronectin receptor is organized by extracellular matrix fibronectin: implications for oncogenic transformation and for cell recognition of fibronectin matrices. J Cell Biol. 1989 Jun;108(6):2529–2543. doi: 10.1083/jcb.108.6.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Takada Y., Wayner E. A., Carter W. G., Hemler M. E. Extracellular matrix receptors, ECMRII and ECMRI, for collagen and fibronectin correspond to VLA-2 and VLA-3 in the VLA family of heterodimers. J Cell Biochem. 1988 Aug;37(4):385–393. doi: 10.1002/jcb.240370406. [DOI] [PubMed] [Google Scholar]
- Tsai S., Patel V., Beaumont E., Lodish H. F., Nathan D. G., Sieff C. A. Differential binding of erythroid and myeloid progenitors to fibroblasts and fibronectin. Blood. 1987 Jun;69(6):1587–1594. [PubMed] [Google Scholar]
- Vuillet-Gaugler M. H., Breton-Gorius J., Vainchenker W., Guichard J., Leroy C., Tchernia G., Coulombel L. Loss of attachment to fibronectin with terminal human erythroid differentiation. Blood. 1990 Feb 15;75(4):865–873. [PubMed] [Google Scholar]
- Wayner E. A., Carter W. G., Piotrowicz R. S., Kunicki T. J. The function of multiple extracellular matrix receptors in mediating cell adhesion to extracellular matrix: preparation of monoclonal antibodies to the fibronectin receptor that specifically inhibit cell adhesion to fibronectin and react with platelet glycoproteins Ic-IIa. J Cell Biol. 1988 Nov;107(5):1881–1891. doi: 10.1083/jcb.107.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Garcia-Pardo A., Humphries M. J., McDonald J. A., Carter W. G. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol. 1989 Sep;109(3):1321–1330. doi: 10.1083/jcb.109.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Garcia-Pardo A., Humphries M. J., McDonald J. A., Carter W. G. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol. 1989 Sep;109(3):1321–1330. doi: 10.1083/jcb.109.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Tremble P. M., Behrendtsen O., Crowley E., Damsky C. H. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989 Aug;109(2):877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]