Abstract
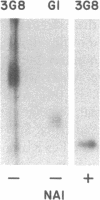
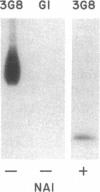
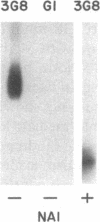
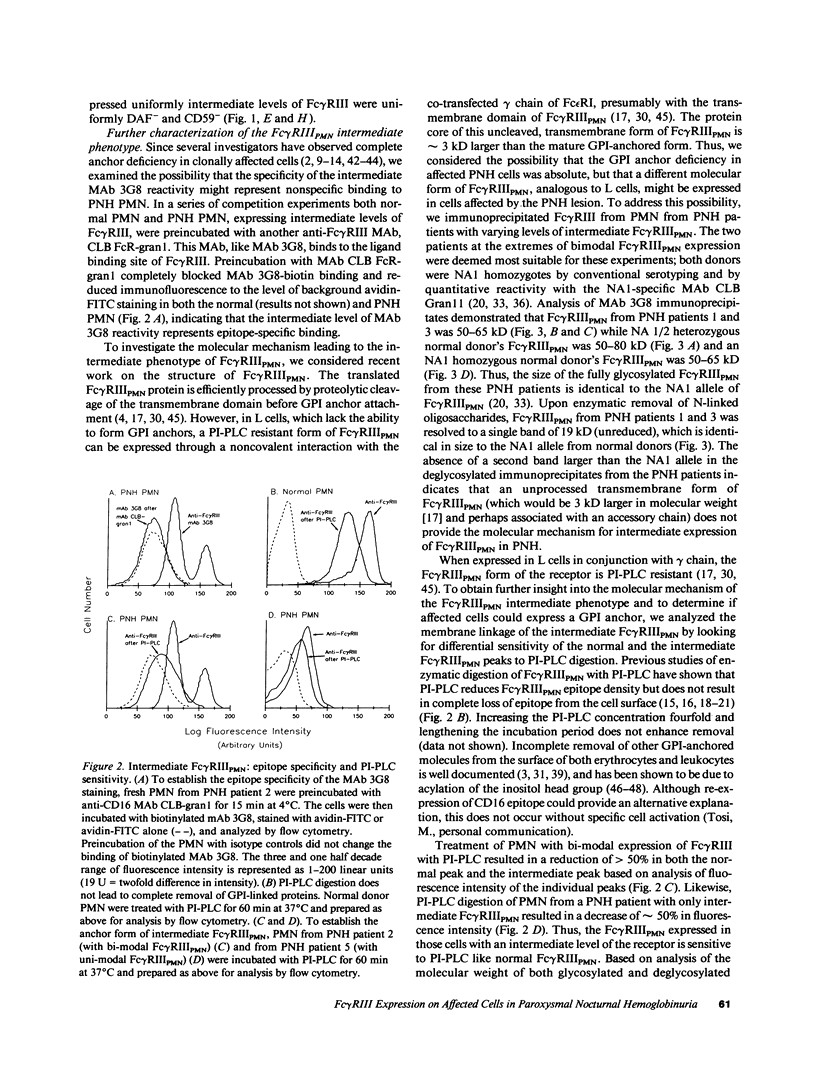
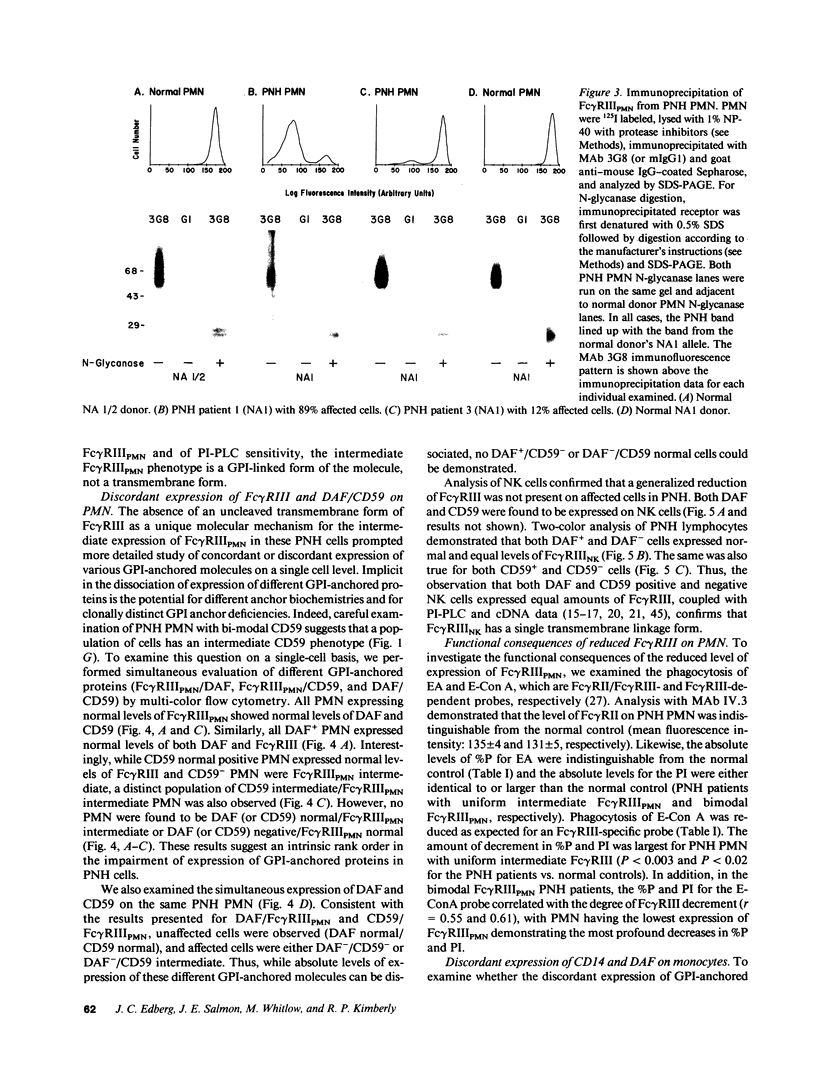
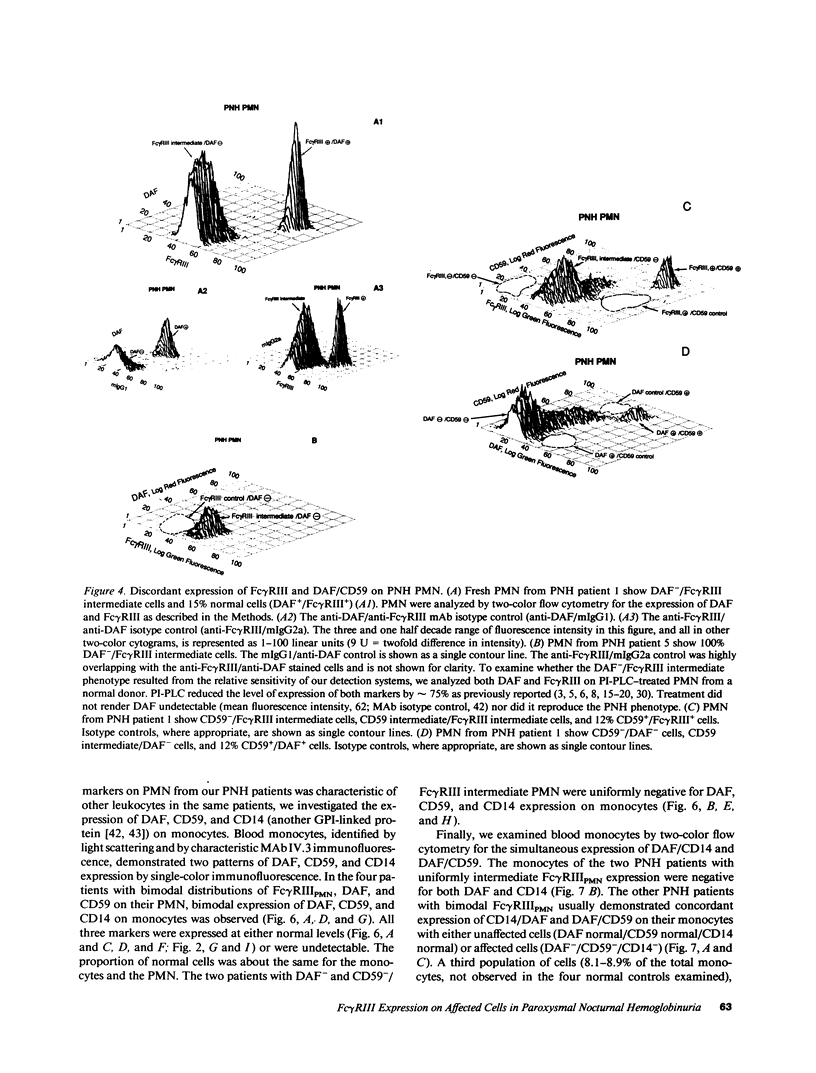
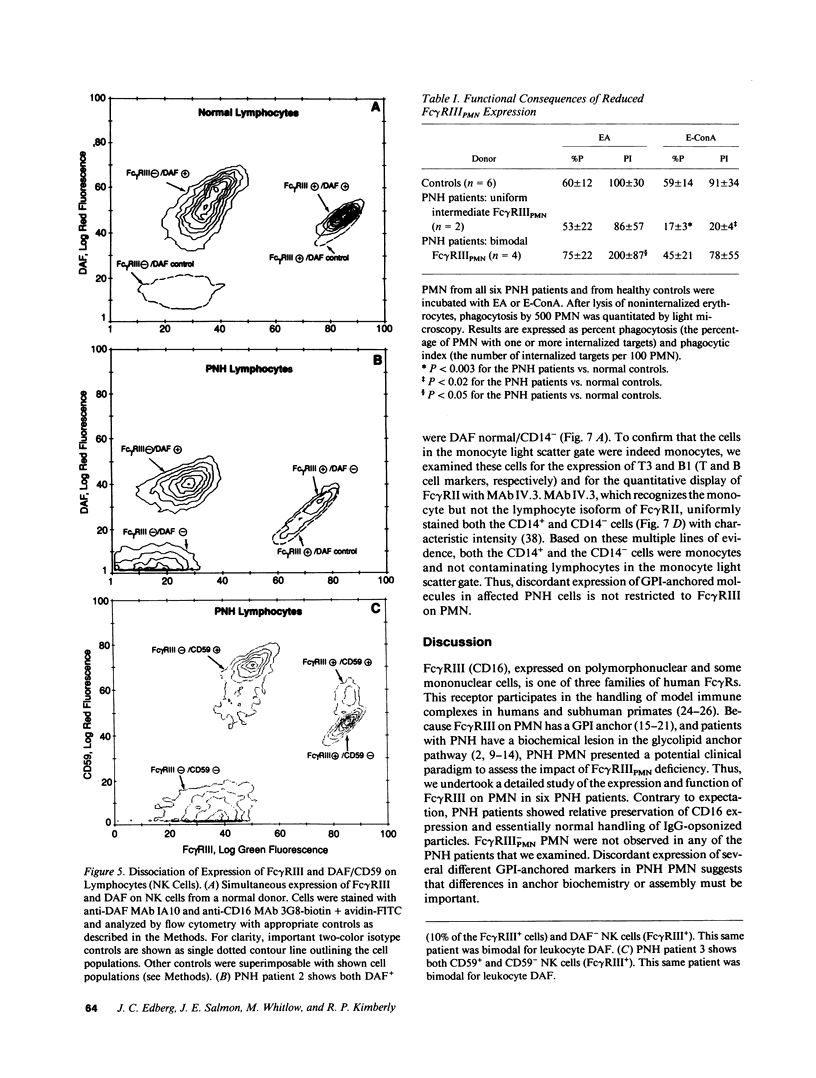
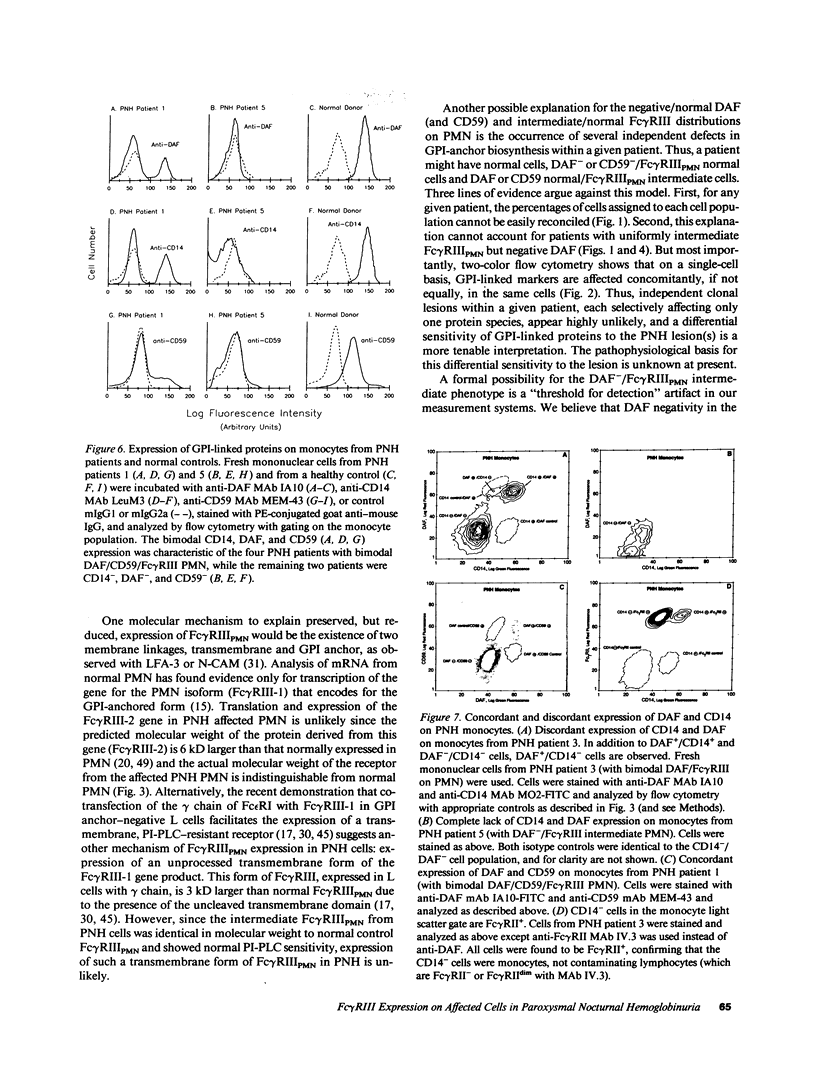
The isoform of Fc gamma RIII (CD16) expressed on PMN has a GPI membrane anchor, and in paroxysmal nocturnal hemoglobinuria (PNH) there is a deficiency in Fc gamma RIII expression on PMN. Contrary to expectation, however, CD16 expression is preserved (albeit at reduced levels) in all affected PNH PMN that completely lack the GPI-anchored proteins DAF (CD55) and CD59. Fc gamma RIII negative PMN are not observed in any of the six PNH patients examined in this study. Analysis of the molecular weight of both glycosylated and deglycosylated Fc gamma RIII from PMN with reduced Fc gamma RIII expression indicates no variations in size relative to normal donor Fc gamma RIIIPMN. Indeed, the Fc gamma RIII expressed at intermediate levels is phosphatidylinositol-specific phospholipase C (PI-PLC)-sensitive. Thus, there is no evidence suggestive of expression of a transmembrane isoform and all data indicate that Fc gamma RIIIPMN on affected cells in PNH is a GPI-linked isoform. With Fc gamma RIIIPMN expression preserved at reduced levels on affected cells in PNH, PMN from PNH patients retain the capacity to internalize the Fc gamma RIIIPMN-specific probe E-ConA (at reduced levels) as well as IgG-opsonized erythrocytes. Reduced expression of GPI-anchored molecules on PNH PMN is not restricted to Fc gamma RIIIPMN since intermediate levels of CD59 were observed in the PNH PMN that were decay-accelerating factor (DAF)-negative and Fc gamma RIIIPMN intermediate. In addition, discordant expression of GPI-linked molecules in individual cells is not restricted to PMN since DAF+/CD14- monocytes were observed in one PNH patient. These data suggest that, when analyzed on an individual cell level, the GPI anchor defect in PNH is not absolute and must involve either a hierarchy of access of different protein molecules to available GPI anchors, distinct anchor biochemistries for the different proteins, or differential regulation of protein-anchor assembly.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burroughs S. F., Devine D. V., Browne G., Kaplan M. E. The population of paroxysmal nocturnal hemoglobinuria neutrophils deficient in decay-accelerating factor is also deficient in alkaline phosphatase. Blood. 1988 Apr;71(4):1086–1089. [PubMed] [Google Scholar]
- CROSBY W. H. Paroxysmal nocturnal hemoglobinuria: relation of the clinical manifestations to underlying pathogenic mechanisms. Blood. 1953 Sep;8(9):769–812. [PubMed] [Google Scholar]
- Carothers D. J., Hazra S. V., Andreson S. W., Medof M. E. Synthesis of aberrant decay-accelerating factor proteins by affected paroxysmal nocturnal hemoglobinuria leukocytes. J Clin Invest. 1990 Jan;85(1):47–54. doi: 10.1172/JCI114432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow F. L., Telen M. J., Rosse W. F. The acetylcholinesterase defect in paroxysmal nocturnal hemoglobinuria: evidence that the enzyme is absent from the cell membrane. Blood. 1985 Oct;66(4):940–945. [PubMed] [Google Scholar]
- Clarkson S. B., Kimberly R. P., Valinsky J. E., Witmer M. D., Bussel J. B., Nachman R. L., Unkeless J. C. Blockade of clearance of immune complexes by an anti-Fc gamma receptor monoclonal antibody. J Exp Med. 1986 Aug 1;164(2):474–489. doi: 10.1084/jem.164.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross G. A. Eukaryotic protein modification and membrane attachment via phosphatidylinositol. Cell. 1987 Jan 30;48(2):179–181. doi: 10.1016/0092-8674(87)90419-3. [DOI] [PubMed] [Google Scholar]
- Davitz M. A., Low M. G., Nussenzweig V. Release of decay-accelerating factor (DAF) from the cell membrane by phosphatidylinositol-specific phospholipase C (PIPLC). Selective modification of a complement regulatory protein. J Exp Med. 1986 May 1;163(5):1150–1161. doi: 10.1084/jem.163.5.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edberg J. C., Barinsky M., Redecha P. B., Salmon J. E., Kimberly R. P. Fc gamma RIII expressed on cultured monocytes is a N-glycosylated transmembrane protein distinct from Fc gamma RIII expressed on natural killer cells. J Immunol. 1990 Jun 15;144(12):4729–4734. [PubMed] [Google Scholar]
- Edberg J. C., Redecha P. B., Salmon J. E., Kimberly R. P. Human Fc gamma RIII (CD16). Isoforms with distinct allelic expression, extracellular domains, and membrane linkages on polymorphonuclear and natural killer cells. J Immunol. 1989 Sep 1;143(5):1642–1649. [PubMed] [Google Scholar]
- Ferguson M. A., Williams A. F. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- Fleit H. B., Wright S. D., Unkeless J. C. Human neutrophil Fc gamma receptor distribution and structure. Proc Natl Acad Sci U S A. 1982 May;79(10):3275–3279. doi: 10.1073/pnas.79.10.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R., Cooper R. A. Concanavalin A mediated attachment and ingestion of red blood cells by macrophages. Exp Cell Res. 1975 Oct 1;95(1):223–231. doi: 10.1016/0014-4827(75)90627-8. [DOI] [PubMed] [Google Scholar]
- Haziot A., Chen S., Ferrero E., Low M. G., Silber R., Goyert S. M. The monocyte differentiation antigen, CD14, is anchored to the cell membrane by a phosphatidylinositol linkage. J Immunol. 1988 Jul 15;141(2):547–552. [PubMed] [Google Scholar]
- Hibbs M. L., Selvaraj P., Carpén O., Springer T. A., Kuster H., Jouvin M. H., Kinet J. P. Mechanisms for regulating expression of membrane isoforms of Fc gamma RIII (CD16). Science. 1989 Dec 22;246(4937):1608–1611. doi: 10.1126/science.2531918. [DOI] [PubMed] [Google Scholar]
- Huizinga T. W., van der Schoot C. E., Jost C., Klaassen R., Kleijer M., von dem Borne A. E., Roos D., Tetteroo P. A. The PI-linked receptor FcRIII is released on stimulation of neutrophils. Nature. 1988 Jun 16;333(6174):667–669. doi: 10.1038/333667a0. [DOI] [PubMed] [Google Scholar]
- Hänsch G. M., Schönermark S., Roelcke D. Paroxysmal nocturnal hemoglobinuria type III. Lack of an erythrocyte membrane protein restricting the lysis by C5b-9. J Clin Invest. 1987 Jul;80(1):7–12. doi: 10.1172/JCI113065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberly R. P., Edberg J. C., Merriam L. T., Clarkson S. B., Unkeless J. C., Taylor R. P. In vivo handling of soluble complement fixing Ab/dsDNA immune complexes in chimpanzees. J Clin Invest. 1989 Sep;84(3):962–970. doi: 10.1172/JCI114259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberly R. P., Tappe N. J., Merriam L. T., Redecha P. B., Edberg J. C., Schwartzman S., Valinsky J. E. Carbohydrates on human Fc gamma receptors. Interdependence of the classical IgG and nonclassical lectin-binding sites on human Fc gamma RIII expressed on neutrophils. J Immunol. 1989 Jun 1;142(11):3923–3930. [PubMed] [Google Scholar]
- Kinoshita T., Medof M. E., Silber R., Nussenzweig V. Distribution of decay-accelerating factor in the peripheral blood of normal individuals and patients with paroxysmal nocturnal hemoglobinuria. J Exp Med. 1985 Jul 1;162(1):75–92. doi: 10.1084/jem.162.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T., Rosenfeld S. I., Nussenzweig V. A high m.w. form of decay-accelerating factor (DAF-2) exhibits size abnormalities in paroxysmal nocturnal hemoglobinuria erythrocytes. J Immunol. 1987 May 1;138(9):2994–2998. [PubMed] [Google Scholar]
- Klaassen R. J., Ouwehand W. H., Huizinga T. W., Engelfriet C. P., von dem Borne A. E. The Fc-receptor III of cultured human monocytes. Structural similarity with FcRIII of natural killer cells and role in the extracellular lysis of sensitized erythrocytes. J Immunol. 1990 Jan 15;144(2):599–606. [PubMed] [Google Scholar]
- Kurosaki T., Ravetch J. V. A single amino acid in the glycosyl phosphatidylinositol attachment domain determines the membrane topology of Fc gamma RIII. Nature. 1989 Dec 14;342(6251):805–807. doi: 10.1038/342805a0. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Cwirla S., Yu G., Testi R., Phillips J. H. Membrane anchoring of a human IgG Fc receptor (CD16) determined by a single amino acid. Science. 1989 Dec 22;246(4937):1611–1613. doi: 10.1126/science.2531919. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Ruitenberg J. J., Phillips J. H. Functional and biochemical analysis of CD16 antigen on natural killer cells and granulocytes. J Immunol. 1988 Nov 15;141(10):3478–3485. [PubMed] [Google Scholar]
- Looney R. J., Ryan D. H., Takahashi K., Fleit H. B., Cohen H. J., Abraham G. N., Anderson C. L. Identification of a second class of IgG Fc receptors on human neutrophils. A 40 kilodalton molecule also found on eosinophils. J Exp Med. 1986 Apr 1;163(4):826–836. doi: 10.1084/jem.163.4.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G. Biochemistry of the glycosyl-phosphatidylinositol membrane protein anchors. Biochem J. 1987 May 15;244(1):1–13. doi: 10.1042/bj2440001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G., Saltiel A. R. Structural and functional roles of glycosyl-phosphatidylinositol in membranes. Science. 1988 Jan 15;239(4837):268–275. doi: 10.1126/science.3276003. [DOI] [PubMed] [Google Scholar]
- Lublin D. M., Atkinson J. P. Decay-accelerating factor: biochemistry, molecular biology, and function. Annu Rev Immunol. 1989;7:35–58. doi: 10.1146/annurev.iy.07.040189.000343. [DOI] [PubMed] [Google Scholar]
- Medof M. E., Gottlieb A., Kinoshita T., Hall S., Silber R., Nussenzweig V., Rosse W. F. Relationship between decay accelerating factor deficiency, diminished acetylcholinesterase activity, and defective terminal complement pathway restriction in paroxysmal nocturnal hemoglobinuria erythrocytes. J Clin Invest. 1987 Jul;80(1):165–174. doi: 10.1172/JCI113043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Walter E. I., Roberts W. L., Haas R., Rosenberry T. L. Decay accelerating factor of complement is anchored to cells by a C-terminal glycolipid. Biochemistry. 1986 Nov 4;25(22):6740–6747. doi: 10.1021/bi00370a003. [DOI] [PubMed] [Google Scholar]
- Micklem K. J., Stross W. P., Willis A. C., Cordell J. L., Jones M., Mason D. Y. Different isoforms of human FcRII distinguished by CDw32 antibodies. J Immunol. 1990 Mar 15;144(6):2295–2303. [PubMed] [Google Scholar]
- Nagler A., Lanier L. L., Cwirla S., Phillips J. H. Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol. 1989 Nov 15;143(10):3183–3191. [PubMed] [Google Scholar]
- Nicholson-Weller A., Burge J., Fearon D. T., Weller P. F., Austen K. F. Isolation of a human erythrocyte membrane glycoprotein with decay-accelerating activity for C3 convertases of the complement system. J Immunol. 1982 Jul;129(1):184–189. [PubMed] [Google Scholar]
- Nicholson-Weller A., March J. P., Rosenfeld S. I., Austen K. F. Affected erythrocytes of patients with paroxysmal nocturnal hemoglobinuria are deficient in the complement regulatory protein, decay accelerating factor. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5066–5070. doi: 10.1073/pnas.80.16.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson-Weller A., Russian D. A., Austen K. F. Natural killer cells are deficient in the surface expression of the complement regulatory protein, decay accelerating factor (DAF). J Immunol. 1986 Aug 15;137(4):1275–1279. [PubMed] [Google Scholar]
- Nicholson-Weller A., Spicer D. B., Austen K. F. Deficiency of the complement regulatory protein, "decay-accelerating factor," on membranes of granulocytes, monocytes, and platelets in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1985 Apr 25;312(17):1091–1097. doi: 10.1056/NEJM198504253121704. [DOI] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Deficiency of an erythrocyte membrane protein with complement regulatory activity in paroxysmal nocturnal hemoglobinuria. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5430–5434. doi: 10.1073/pnas.80.17.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch J. V., Perussia B. Alternative membrane forms of Fc gamma RIII(CD16) on human natural killer cells and neutrophils. Cell type-specific expression of two genes that differ in single nucleotide substitutions. J Exp Med. 1989 Aug 1;170(2):481–497. doi: 10.1084/jem.170.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. L., Myher J. J., Kuksis A., Low M. G., Rosenberry T. L. Lipid analysis of the glycoinositol phospholipid membrane anchor of human erythrocyte acetylcholinesterase. Palmitoylation of inositol results in resistance to phosphatidylinositol-specific phospholipase C. J Biol Chem. 1988 Dec 15;263(35):18766–18775. [PubMed] [Google Scholar]
- Roberts W. L., Santikarn S., Reinhold V. N., Rosenberry T. L. Structural characterization of the glycoinositol phospholipid membrane anchor of human erythrocyte acetylcholinesterase by fast atom bombardment mass spectrometry. J Biol Chem. 1988 Dec 15;263(35):18776–18784. [PubMed] [Google Scholar]
- Rosse W. F., Parker C. J. Paroxysmal nocturnal haemoglobinuria. Clin Haematol. 1985 Feb;14(1):105–125. [PubMed] [Google Scholar]
- Salmon J. E., Edberg J. C., Kimberly R. P. Fc gamma receptor III on human neutrophils. Allelic variants have functionally distinct capacities. J Clin Invest. 1990 Apr;85(4):1287–1295. doi: 10.1172/JCI114566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon J. E., Kapur S., Kimberly R. P. Opsonin-independent ligation of Fc gamma receptors. The 3G8-bearing receptors on neutrophils mediate the phagocytosis of concanavalin A-treated erythrocytes and nonopsonized Escherichia coli. J Exp Med. 1987 Dec 1;166(6):1798–1813. doi: 10.1084/jem.166.6.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon J. E., Kimberly R. P. Phagocytosis of concanavalin A-treated erythrocytes is mediated by the Fc gamma receptor. J Immunol. 1986 Jul 15;137(2):456–462. [PubMed] [Google Scholar]
- Scallon B. J., Scigliano E., Freedman V. H., Miedel M. C., Pan Y. C., Unkeless J. C., Kochan J. P. A human immunoglobulin G receptor exists in both polypeptide-anchored and phosphatidylinositol-glycan-anchored forms. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5079–5083. doi: 10.1073/pnas.86.13.5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj P., Dustin M. L., Silber R., Low M. G., Springer T. A. Deficiency of lymphocyte function-associated antigen 3 (LFA-3) in paroxysmal nocturnal hemoglobinuria. Functional correlates and evidence for a phosphatidylinositol membrane anchor. J Exp Med. 1987 Oct 1;166(4):1011–1025. doi: 10.1084/jem.166.4.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj P., Rosse W. F., Silber R., Springer T. A. The major Fc receptor in blood has a phosphatidylinositol anchor and is deficient in paroxysmal nocturnal haemoglobinuria. Nature. 1988 Jun 9;333(6173):565–567. doi: 10.1038/333565a0. [DOI] [PubMed] [Google Scholar]
- Simmons D. L., Tan S., Tenen D. G., Nicholson-Weller A., Seed B. Monocyte antigen CD14 is a phospholipid anchored membrane protein. Blood. 1989 Jan;73(1):284–289. [PubMed] [Google Scholar]
- Stafford H. A., Tykocinski M. L., Lublin D. M., Holers V. M., Rosse W. F., Atkinson J. P., Medof M. E. Normal polymorphic variations and transcription of the decay accelerating factor gene in paroxysmal nocturnal hemoglobinuria cells. Proc Natl Acad Sci U S A. 1988 Feb;85(3):880–884. doi: 10.1073/pnas.85.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanová I., Hilgert I., Kristofová H., Brown R., Low M. G., Horejsí V. Characterization of a broadly expressed human leucocyte surface antigen MEM-43 anchored in membrane through phosphatidylinositol. Mol Immunol. 1989 Feb;26(2):153–161. doi: 10.1016/0161-5890(89)90097-7. [DOI] [PubMed] [Google Scholar]
- Ueda E., Kinoshita T., Nojima J., Inoue K., Kitani T. Different membrane anchors of Fc gamma RIII (CD16) on K/NK-lymphocytes and neutrophils. Protein- vs lipid-anchor. J Immunol. 1989 Aug 15;143(4):1274–1277. [PubMed] [Google Scholar]
- Ueda E., Kinoshita T., Terasawa T., Shichishima T., Yawata Y., Inoue K., Kitani T. Acetylcholinesterase and lymphocyte function-associated antigen 3 found on decay-accelerating factor-negative erythrocytes from some patients with paroxysmal nocturnal hemoglobinuria are lost during erythrocyte aging. Blood. 1990 Feb 1;75(3):762–769. [PubMed] [Google Scholar]
- Unkeless J. C., Scigliano E., Freedman V. H. Structure and function of human and murine receptors for IgG. Annu Rev Immunol. 1988;6:251–281. doi: 10.1146/annurev.iy.06.040188.001343. [DOI] [PubMed] [Google Scholar]
- Villaescusa R., Santos M. N., García Y., Trujillo Y., Bernal B., Ballester J. M., Hernández P. Circulating immune complexes in paroxysmal nocturnal hemoglobinuria. Acta Haematol. 1982;68(2):136–141. doi: 10.1159/000206965. [DOI] [PubMed] [Google Scholar]
- Walter E. I., Roberts W. L., Rosenberry T. L., Ratnoff W. D., Medof M. E. Structural basis for variations in the sensitivity of human decay accelerating factor to phosphatidylinositol-specific phospholipase C cleavage. J Immunol. 1990 Feb 1;144(3):1030–1036. [PubMed] [Google Scholar]
- Zalman L. S., Wood L. M., Frank M. M., Müller-Eberhard H. J. Deficiency of the homologous restriction factor in paroxysmal nocturnal hemoglobinuria. J Exp Med. 1987 Feb 1;165(2):572–577. doi: 10.1084/jem.165.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]