The National Institute for Health and Clinical Excellence (NICE) is a decision-maker. Where NICE reaches a positive conclusion about the use of a particular health technology (such as a pharmaceutical product) in the National Health Service (NHS), there is a legal requirement for the service to make it available if a patient's physician considers it clinically appropriate [1]. Although this legal obligation does not apply to technologies recommended in NICE's clinical guidelines, there is still a reasonable expectation by the Care Quality Commission for NHS healthcare professionals to use NICE's clinical guidelines as the basis, where appropriate, for their clinical practice.
NICE's independent advisory bodies (the Appraisal Committees, the Public Health Advisory Committee and Guideline Development Groups) are required to take account of both clinical and cost-effectiveness in reaching their conclusions about the use of health technologies generally and of pharmaceuticals in particular [2–4]. These advisory bodies must fully assess the available evidence on benefits and costs in order to come to a decision as to whether the use of a technology is considered a cost-effective use of resources.
However, the scientific evidence underpinning any decision about the use of a particular health technology is never perfect. Nor is it all-embracing. Advisory bodies therefore need to use their experience to make judgements beyond the existing evidence.
These judgements are of two types [5]:
Scientific value judgements are ones about what can be inferred from the available evidence base; and the extent to which imperfections in the evidence base should influence decisions.
Social value judgements are concerned with what is appropriate and acceptable for society in delivering healthcare across the NHS.
Scientific value judgements
Health technologies, including pharmaceuticals, can be considered to be clinically effective if, in normal clinical practice, they confer an overall health benefit (taking account of any harmful effects) when compared with relevant alternative treatments [6]. This is the central concern of patients for whom the treatment might be indicated. Technologies can be considered to be cost-effective if their health benefits are greater than the opportunity costs of services, in the NHS, that may be displaced were they to be adopted [3]. This is the central concern of all other NHS patients whose treatments might be displaced by prioritized spending on the new technology.
Clinical effectiveness
The evidence for clinical effectiveness may be derived from experimental or observational studies [7]. These are usually pooled in a systematic review that often includes one or more meta-analyses.
NICE as a decision-maker, however, needs to consider:
-
The strengths and limitations of the systematic evidence review.
Although systematic reviewing is based on well-accepted principles, judgment is required in deciding which studies should be included as well as in the interpretation of the results. Thus different inclusion and exclusion criteria used for the review, as well as for the meta-analyses, will yield different results.
-
The absence of direct comparisons.
Many randomized controlled trials for new pharmaceutical products are compared with placebo rather than with the active comparators used in current clinical care. The use of indirect or mixed treatment comparisons adds an additional layer of complexity to the analysis [8].
-
The end-points used in the relevant studies.
Only a minority of trials measure health-related quality of life together with reliable estimates of the period for which it is ‘enjoyed’. Frequently the data for effectiveness rely heavily (or exclusively) on surrogate outcomes or other biomarkers.
-
The time scale of the studies.
Randomized controlled trials rarely last more than 6–12 months, but chronic diseases and their treatments often persist throughout life.
-
External validity (generalizability) of the available data.
Despite the advantages of randomized controlled trials, in respect of their internal validity, their small scale and often homogeneous patient populations can render the real impact of treatment uncertain [4]. Observational evidence, which could assist generalizability, is likely to be limited for new products.
All of these considerations, and more, demand judgements on the part of the particular advisory body. These judgements are informed by the science, but are nevertheless judgements.
Cost-effectiveness
NICE's preferred measure of cost-effectiveness is the incremental cost-effectiveness ratio (ICER) [6]. This relates the increased marginal gain in health, expressed as the quality-adjusted life year (QALY), to the increased (or decreased) marginal costs less the savings attributable to the use of the product. This almost invariably requires the use of an economic model that has normally been developed specifically for the particular decision problem.
Economic modelling requires judgements to be made by both modellers and decision-makers. The inputs to the model will be all those necessary to derive both the QALY and cost differences. The QALY differences include overall survival, the time in several different health states, and the valuation of those states on a ‘utility’ scale. The cost differences include not only the acquisition costs of the product but administrative costs (such as the requirements for hospital admission, the input from nursing or other healthcare staff) as well as additional costs of monitoring the response and the costs of treating adverse effects. These inputs are subject to considerable uncertainty both qualitatively and quantitatively. For example, what is currently considered to be standard UK clinical practice on the management of febrile neutropenia with a new anticancer drug? NICE takes into account the views of clinical experts and patients in order to decide the best estimates of the various inputs that yield, in turn, the most plausible estimate (or estimates) of the ICER. But the judgements of NICE's own advisory bodies are critical.
Social value judgements
Once NICE's advisory bodies have reached a conclusion about the most plausible modelled ICER for a product's particular indication, they must then decide whether the increased benefits are worth the added costs. In this they are influenced by the principles of distributive justice, but they ultimately decide on a case-by-case approach.
Distributive justice
Distributive justice is the term used by moral and political philosophers in discussing what is just, or right, in allocating goods within a society. Two moral theories of distributive justice – utilitarianism and egalitarianism – have a particular place in allocating healthcare resources in the NHS [9]. Utilitarians consider distributive justice to be best served by seeking to maximize the health of the population as a whole [9]. Utilitarianism is often expressed, in shorthand, as ‘the greatest good for the greatest number’. Egalitarians want healthcare to be distributed, in so far as is possible, so that each person receives a fair share of the opportunities available in society [9]. NICE favours an approach based on maximizing benefit per unit cost, but recognizes that this can conflict with the considered moral convictions of many people (including the members of its advisory bodies). Consequently, NICE uses a flexible approach that treats decisions on a case-by-case basis.
The case-by-case approach
NICE is careful to avoid the use of an absolute threshold to distinguish cost-effective from cost-ineffective technologies. The reasons are fourfold:
To set a threshold would imply that efficiency has an absolute priority over other objectives (such as fairness).
The empirical basis for deciding the value at which a threshold might be set is still very weak.
Many health technology suppliers are monopolists and a threshold could be taken to imply a definite price that could discourage price competition.
Rigid adherence to a cost-effectiveness threshold would create the impression that NICE's advisory bodies accept all the calculations that have gone into estimating a technology's cost-effectiveness. It would therefore remove their discretion to assess costs and benefits appropriately when modelling has reached its limits.
NICE's case-by-case approach is shown stylistically in Figure 1. As the ICER increases, the likelihood of rejection on grounds of cost ineffectiveness rises in a dose–response manner [5]. The critical issues are the values of the ICERs at inflections A and B. NICE and its advisory bodies have made the judgement that inflection A corresponds to an ICER of around £20 000 per QALY and inflection B at around £30 000 per QALY. Consequently, NICE's advisory bodies would be unlikely to reject, as cost ineffective, an intervention <£20 000 per QALY; and increasingly likely to reject, as cost ineffective, interventions >£20 000 per QALY.
Figure 1.
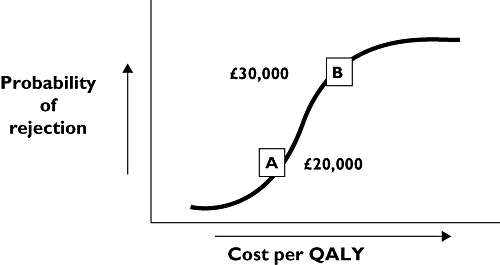
Relation between the likelihood of a technology being considered as cost ineffective plotted against the log of the incremental cost-effectiveness ratio
The Institute thus provides its advisory bodies with some flexibility in deciding whether to commend technologies as cost-effective interventions costing <£20 000 per QALY or >£20 000 per QALY [6]. It does so for two reasons. First, NICE appreciates that the usual tools (such as the EQ-5D) used to calculate the QALY may not invariably capture the totality of an intervention's benefits. The EQ-5D is, for example, relatively insensitive to the quality of life decrement attributable to sensory loss and to cognitive impairment. Second, there are various legal obligations that are placed on the Institute, as a public body, that it is required to uphold. These include promoting equality, eliminating unlawful discrimination, and actively considering the implications for human rights legislation [6]. Third, there are special circumstances, based on social value judgements, that reflect societal preferences in the allocation of resources. These preferences are, predominantly, based on the views of the Institute's Citizens Council and reflected in the guidance given to the Institute's advisory bodies [10].
There have been to date six special circumstances to which the Institute's advisory bodies have given special weighting when making judgements about cost-effectiveness. Some of the conditions, and the associated treatments that reflect these circumstances, are shown in Table 1.
Table 1.
Application of ‘special circumstances’ in the appraisal of some products with incremental cost-effectiveness above £30 000 per quality adjusted life year
| Topic | ICER ('000s) | Severity | End of life* | Stakeholder persuasion | Significant innovation | Disadvantaged population | Children |
|---|---|---|---|---|---|---|---|
| Riluzole (motor neurone disease) | 38–42 | ✓ | ✓ | ✓ | |||
| Trastuzumab (advanced breast cancer) | 37.5 | ✓ | ✓ | ||||
| Imatinib (chronic myeloid leukaemia) | 36–65 | ✓ | ✓ | ||||
| Imatinib (gastrointestinal stromal tumour) | ✓ | ✓ | ✓ | ||||
| Pemetrexed (malignant mesothelioma) | 34.5 | ✓ | ✓ | ✓ | |||
| Ranizumab (age-related macular degeneration) | ≫30 | ✓ | ✓ | ||||
| Omalizumab (severe asthma) | >30 | ✓ | ✓ | ✓ | |||
| Sunitinib (advanced renal cancer) | 50 | ✓ | ✓ | ✓ | ✓ | ||
| Lenalidomide (multiple myeloma) | 43 | ✓ | ✓ | ✓ | |||
| Somatotropin (growth hormone deficiency) | n/a | ✓ | ✓ | ✓ | |||
| Chronic subcutaneous insulin infusion (childhood Type 1 diabetes) | n/a | ✓ | ✓ |
End-of-life considerations have only been explicitly taken into account since January 2009 on the basis of supplementary advice from the Institute to the Appraisals Committee. ICER, incremental cost-effectiveness ratio (£ per quality-adjusted life year).
Severity of the underlying illness. Although the relatively inexpensive relief of a mild discomfort may be calculated to give an equivalent ICER to the expensive relief of a very serious condition, society would give priority to the latter. NICE's advisory bodies have therefore often given more generous consideration to the acceptability of an ICER in serious conditions.
End-of-life treatments. The Institute recognizes that the public, generally, places special value on treatments that prolong life – even for a few months – at the end of life, as long as that extension of life is of reasonable quality (at least pain-free if not disability-free). NICE has therefore provided its advisory bodies with supplementary advice about the circumstances under which they should consider advising, as cost-effective, treatments costing >£30 000 per QALY.
Stakeholder persuasion. Patients and their advocates play an important role in shaping the views of NICE's advisory committees. Most particularly, they can explain where, in their experience, the symptomatology of their condition is poorly reflected in either the clinical trials (because the most severely affected were not distinguished or even included), or inadequately reflected in the measure of health-related quality of life that has been used (because the instrument was too insensitive).
Significant innovation. The Institute considers an innovative technology as one where the use of the product produces a demonstrable and distinct benefit, of a substantial nature, that may not have been adequately captured in the quality of life measure used.
Disadvantaged populations. The NHS gives special priority to improving the health of the most disadvantaged members of the population. This particularly applies to poorer people and ethnic minorities.
Children. The Institute recognizes that compilation of the evidence, and assessment of improvements in the quality of life in children are methodologically challenging. It understands that society would generally favour ‘the benefit of the doubt’ being afforded to sick children.
In taking account of these factors, when reaching conclusions about cost-effectiveness NICE does not typically expect them to be used to ‘weight’, quantitatively, the QALYs attributable to an intervention. First, the methodology for developing and applying ‘equity weightings’ (as they are sometimes called) is largely untried and untested. Second, there is no methodology available to take account of more than one ‘weight’ when more than one special factor exists (as in many instances in Table 1). Should they, for example, be additive, multiplicative, or something else? Third, there would be much less scope for advisory bodies to use their own judgement. Appraisal Committees frequently meet new circumstances, or unique combinations of circumstances, where quasi-scientific weighting would have far less merit than the collective judgement of a balanced group of experienced people. NICE's Appraisal Committee considered that, in the case of pemetrexed for malignant mesothelioma, there was a combination of factors: a disadvantaged population; an urgent but time-limited problem; and also, arguably, a corporate responsibility to provide treatment for an occupational hazard that at the time workers were exposed to asbestos was unrecognized.
Rather than apply ‘equity weightings’, the Institute expects the members of its advisory bodies to exercise their collective judgement in the application of these special considerations to conclusions about cost-effectiveness when the ICER exceeds £20 000–30 000 per QALY.
Conclusions
Decision-makers have to make judgements when deciding whether interventions are clinically and cost-effective. There is a need for a level playing field for different technologies across different diseases. NICE uses a standard approach to calculating costs and benefits in the form of QALYs that satisfies this requirement for broad consistency. It is essential, though, that the approach is used as a tool, not a rule. Recommendations need to be based on scientific judgements about clinical and cost-effectiveness, but also to take into account societal preferences as expressed through social value judgments.
Competing interests
MDR is Chairman of NICE (1999–present). DBB was Chairman of NICE's Appraisal Committee (1999–2009). AS is a Chairman of NICE's Appraisal Committee (2002–present).
REFERENCES
- 1.Secretary of State for Health. Directions to Primary Care Trusts and NHS trusts in England concerning Arrangements for the Funding of Technology Appraisal Guidance from the National Institute for Clinical Excellence (NICE) London: Department of Health; 2003. [Google Scholar]
- 2.National Institute for Health and Clinical Excellence. Developing NICE Technology Appraisal Guidance. London & Manchester: National Institute for Health and Clinical Excellence; 2008. Available at http://www.nice.org.uk/aboutnice/howwework/devnicetech/developing_nice_technology_appraisals.jsp. [Google Scholar]
- 3.National Institute for Health and Clinical Excellence. Developing NICE Clinical Guidelines. London & Manchester: National Institute for Health and Clinical Excellence; 2008. Available at http://www.nice.org.uk/aboutnice/howwework/developingniceclinicalguidelines/developing_nice_clinical_guidelines.jsp. [Google Scholar]
- 4.National Institute for Health and Clinical Excellence. Developing NICE Public Health Guidance. London & Manchester: National Institute for Health and Clinical Excellence; 2008. Available at http://www.nice.org.uk/aboutnice/howwework/developingnicepublichealthguidance/developing_nice_public_health_guidance.jsp. [Google Scholar]
- 5.Rawlins MD, Culyer AJ. National Institute for Clinical Excellence and its value judgements. BMJ. 2004;329:224–7. doi: 10.1136/bmj.329.7459.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institute for Health and Clinical Excellence. Guide to the Methods of Technology Appraisal. London: National Institute for Health and Clinical Excellence; 2008. [PubMed] [Google Scholar]
- 7.Rawlins MD. De Testimonio. On the Evidence for Decisions about the Use of Therapeutic Interventions. London: Royal College of Physicians; 2008. [DOI] [PubMed] [Google Scholar]
- 8.Sutton A, Ades A, Abrams K, Cooper N. Briefing Paper for Methods Review Workshop on Evidence Synthesis (Indirect and Mixed Treatment Comparisons) London: National Institute for Health and Clinical Excellence; 2008. Available at http://www.nice.org.uk/media/4A6/2F/EvidenceSynthesisBriefingPaper.pdf. [Google Scholar]
- 9.Beauchamp TL, Childress JF. Principles of Biomedical Ethics. Oxford and New York: Oxford University Press; 2001. [Google Scholar]
- 10.National Institute for Health and Clinical Excellence. Social Value Judgements: Principles for the Development of NICE's Guidance. London: National Institute for Health and Clinical Excellence; 2005. [PubMed] [Google Scholar]


