Abstract
Diffusion-weighted imaging (DWI) and perfusion-weighted imaging (PWI) can rapidly detect lesions in acute ischemic stroke patients. The PWI volume is typically substantially larger than the DWI volume shortly after onset, that is, a diffusion/perfusion mismatch. The aims of this study were to follow the evolution of the diffusion/perfusion mismatch in permanent and 60-minute temporary focal experimental ischemia models in Sprague-Dawley rats using the intraluminal middle cerebral artery occlusion (MCAO) method. DWI and arterial spin-labeled PWI were performed at 30, 60, 90, 120, and 180 minutes after occlusion and lesion volumes (mm3) calculated At 24 hours after MCAO, and infarct volume was determined using triphenyltetrazolium chloride staining. In the permanent MCAO group, the lesion volume on the ADC maps was significantly smaller than that on the cerebral blood flow maps through the first 60 minutes after MCAO; but not after 90 minutes of occlusion. With 60 minutes of transient ischemia, the diffusion/perfusion mismatch was similar, but after reperfusion, the lesion volumes on ADC and cerebral blood flow maps became much smaller. There was a significant difference in 24-hour infarct volumes between the permanent and temporary occlusion groups.
Diffusion-weighted imaging (DWI) is widely used to investigate hyperacute cerebral ischemia both in experimental stroke models and in patients with ischemic stroke, detecting early ischemic abnormalities related to reduction of the apparent diffusion coefficient (ADC) of brain water.1–8 Perfusion-weighted imaging (PWI) provides information about the hemodynamic status of brain tissue and detects regions with impaired cerebral perfusion.9,10 Clinical reports have demonstrated that the impaired perfusion region is typically larger than the lesion detected by DWI early after stroke onset.11–13 The difference between the PWI and DWI abnormalities was termed the diffusion/perfusion mismatch, and the DWI lesion usually enlarges over time until it coincides with the perfusion deficit.11–13 The mismatch region may represent potentially salvageable brain tissue with timely and appropriate therapy.14 The diffusion/perfusion mismatch evolution has not been well characterized during the first few hours in individual patients, nor in animal models. The aims of this study were to delineate the temporal evolution of the diffusion/perfusion mismatch volume in a rat permanent and temporary focal ischemia model and to confirm that the mismatch region identifies salvageable tissue if subjected to early reperfusion.
Materials and Methods
Animal Preparation
All procedures used in this study were in accordance with our institutional guidelines. Seventeen male Sprague-Dawley rats weighing 300 to 350gm were used. Animals were initially anesthetized intraperitoneally with 400mg/kg chloral hydrate. PE-50 polyethylene tubing was inserted into the left femoral artery for continuous blood pressure monitoring and for measuring pH, PaCO2, and PaO2, before occlusion and 60 minutes after middle cerebral artery occlusion (MCAO). Temperature was continuously monitored with a rectal probe and maintained at 37.0°C during the surgical procedure with a heating pad.
Focal Brain Ischemia
This study consisted of three experimental groups. Two groups underwent permanent MCAO, Group 1 for establishing the ADC and cerebral blood flow (CBF) thresholds (n = 5) and a second validation group (Group 2, n = 6). In Group 3 (n = 6), the rats were mechanically reperfused by withdrawing the occluder at 60 minutes after MCAO while the animal was in the magnet.
Focal brain ischemia was induced with the intraluminal suture MCAO method under chloral hydrate anesthesia (400 mg/kg, IP) as originally described by Koizumi and colleagues.15 After MCAO, the animals were quickly placed into the magnet and anesthesia was switched to 1% isoflurane delivered in air at 1.0L/min. Temperature was monitored using a rectal probe and maintained at 37.0°C using a thermostatically regulated heating pad.
Magnetic Resonance Imaging Measurements
Magnetic resonance imaging (MRI) experiments were performed on a 4.7T/40cm horizontal magnet equipped with a Biospec Bruker console (Billerica, MA), and a 20 Gauss/cm magnetic field gradient insert (ID = 12cm). The animals were imaged initially at 30 minutes after MCAO and then at 60, 90, 120, and 180 minutes after MCAO. A surface coil (2.3cm ID) was used for brain imaging and an actively decoupled neck coil was used for CBF labeling.
To provide anatomical localization, we acquired T2-weighted images using the fast spin-echo pulse sequence with TR = 2 seconds (90-degree flip angle), effective TE = 80 milliseconds, data matrix = 256 × 256 or 128 × 128, echo train length = 16, field of view = 2.5cm × 1.9cm, six 1.5-mm slices, and four signal averages. A directionally averaged ADC (ADCav) map was obtained by averaging three ADC maps acquired separately with diffusion-sensitive gradients applied along the x, y, or z direction.16 Single-shot, spin-echo, echo-planar images (EPIs) were acquired over 2 minutes with TR = 2 seconds (90-degree flip angle), TE = 45 milliseconds, data matrix = 64 × 64, field of view = 2.5 × 1.9cm, six 1.5mm slices, b = 10, and 1,504 sec/mm2, Δ= 20 milliseconds, δ= 6.5 milliseconds, and eight signal averages.
Quantitative CBF was measured using the continuous arterial spin-labeling technique17,18 with single-shot, gradient-echo EPI. One hundred paired images (for signal averaging) were acquired over 6.7 minutes, alternately, one with arterial spin labeling and the other (control) without spin-labeling preparation. The MRI parameters were similar to ADC measurements except TE = 15 milliseconds. Arterial spin labeling utilized a 1.78-second, square radiofrequency pulse in the presence of 1.0 Gauss/cm gradient along the flow direction. The sign of the frequency offset was switched for nonlabeled images.
Data Analysis for In Vivo Lesion Size Calculation
Quantitative ADCav maps, in units of square millimeters per second, were calculated using the Stejskal–Tanner equation.16 Quantitative CBF maps, in units of milliliters per gram of tissue per min (ml/g/min) were calculated using the water brain–blood partition coefficient λ of 0.9, tissue T1 of 1.5 seconds, and spin-labeling efficiency α of 0.75.17,18
In an initial group of permanently occluded animals (n = 5), ADC and CBF thresholds of abnormality were derived by adjusting the respective threshold values so that the ADC-and CBF-derived lesion volumes at 3 hours were equal to the 2,3,4-triphenyltetrazolium chloride (TTC) infarct volume at 24 hours. Earlier experimental studies have shown that the DWI-defined lesion volume is maximized by 2 to 3 hours after permanent MCAO and demonstrates high correlation and correspondence to infarct volumes determined by postmortem histology.19–21 The ADC and CBF thresholds derived from this training data set then were used prospectively to calculate the lesion volumes for all time points in this group, in the second permanent occlusion group (n = 6) and the temporary occlusion group (n = 6). All the pixels comprising the abnormal area on the ADC and CBF maps were identified using these thresholds on the six imaged slices from each animal at each time point. The ADC- and CBF-derived lesion volumes then were calculated by multiplying the abnormal areas by the slice thickness, 1.5mm, and summing the volumes from each slice. The ADC- and CBF-derived lesion volumes at 3 hours after MCAO then were independently correlated with TTC-derived infarct volumes at 24 hours for experimental Groups 2 and 3.
Neurological and Postmortem Evaluation
Twenty-four hours after MCAO, the animals underwent neurological scoring using the Zea-Longa scale as previously described and were killed with an overdose of chloral hydrate (600mg/kg of body weight) and decapitated.19 The brains were quickly removed and sectioned coronally into eight, 1.5mm-thick slices. The first and last slices were not included in the calculation of infarct volumes, because these slices were not evaluated in the MRI data sets. The brain slices were incubated for 30 minutes in a 2% solution of TTC at 37°C and fixed by immersion in a 10% of buffered formalin solution and infarct volumes (with edema correction) were determined as previously described.19,20 To correct for the effects of brain edema, a corrected infarct volume was calculated by the following formula: corrected infarct volume = left hemisphere volume – (right hemisphere volume – infarct volume).
Statistical Analysis
Data are presented as mean ± standard deviation. Statistical analysis of the physiological variables was performed using a repeated-measures analysis of variance. Two-tailed, paired or unpaired, Student's t tests were used to compare the parametric variables. A linear-regression analysis was used to correlate the ADC- and CBF-derived lesion volumes with TTC-derived infarct volumes. A p value less than 0.05 was considered significant.
Results
Physiological variables such as body temperature, mean arterial blood pressure, pH, PaCO2, and PaO2 were within the reference range throughout the experiment and were not significantly different between the groups (data not shown). The neurological deficits 24 hours after MCAO did not differ in the permanent and temporary ischemia groups, 2.6 ± 1.2 and 2.3 ± 0.5.
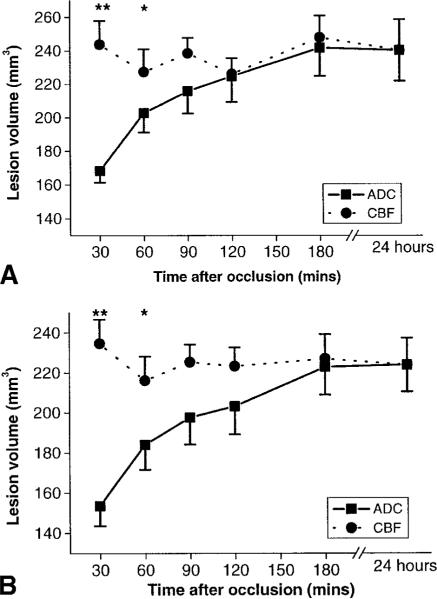
The abnormal thresholds values derived at 3 hours after MCAO from Group 1 (training set data of permanently occluded animals) were, for ADC, 0.53 ± 0.03 × 10−3 mm2/sec, and CBF, 0.30 ± 0.09ml/gm/min, a 30 ± 2% and 57 ± 11% reduction, respectively, as compared with the mean normal hemisphere values. These thresholds were applied to evaluate lesion volumes in a second group of permanently occluded animals, Group 2. The CBF-derived lesion volume remained relatively constant over the 180-minute imaging time period, and the lesion volume at each time point was significantly correlated with the infarct volume at 24 hours except for the 90- and 120-minute time points in Group 2 when analyzed alone (Table). The diffusion/perfusion mismatch the data from Group 2 initially was evaluated independently. Then the data from Groups 1 and 2 of permanently occluded animals were combined to improve estimates of the ADC and CBF lesion volume evolution over time. The mismatch region was identified as the difference between the abnormal perfusion and diffusion regions (as defined using the ADC and CBF thresholds given above). For Group 2 (Fig 1A), the abnormal perfusion volume was significantly larger than the abnormal diffusion volume at both 30 and 60 minutes after MCAO. This also was the case when the data from both Groups 1 and 2 were combined (see Fig 1B). At 90 minutes after occlusion, the mean abnormal perfusion volume was 23mm3 larger than the mean abnormal diffusion volume for Group 2 (p = 0.13) and 25mm3 larger than the diffusion volume for Groups 1 and 2 (p = 0.06). By 180 minutes, the ADC- and CBF-defined volumes were almost identical. The ADC-defined lesion at 3 hours was highly correlated with the 24-hour TTC-derived infarct volume for both Group 2 (r = 0.92, p = 0.008) and the combined data from Groups 1 and 2 (r = 0.93, p = 0.00002).
Table.
Correlations between Mean CBF-Derived Lesion Volumes (mm3) at Various Time Points and 24-Hour TTC Infarct Volume in the Permanent Occlusion Groups and the CBF Volumes over Time in the Temporary Group That Could Not Be Correlated to Infarct Volume because of the Reperfusion
| Group | 30 min | 60 min | 90 min | 120 min | 180 min | 24 hr (TTC) |
|---|---|---|---|---|---|---|
| 1 + 2 | ||||||
| LV | 235 | 216 | 224 | 223 | 227 | 224 |
| r | 0.77 | 0.73 | 0.62 | 0.60 | 0.81 | |
| p | 0.005 | 0.01 | 0.04 | 0.05 | 0.002 | |
| 2 | ||||||
| LV | 244 | 227 | 239 | 226 | 248 | 245 |
| r | 0.85 | 0.91 | 0.77 | 0.80 | 0.92 | |
| p | 0.03 | 0.01 | 0.07 | 0.06 | 0.009 | |
| 3 | ||||||
| 247 | 238 | 101 | 131 | 115 | 140 |
n = 11 in Group 1 + 2, n = 6 in Group 2, p < 0.05 was considered significant. 24-hour lesion volume represents the TTC infarct volume (mm3).
CBF = cerebral blood flow; TTC = 2,3,4-triphenyltetrazolium chloride; LV = lesion volume.
Fig 1.
(A) Temporal evolution of apparent diffusion coefficient (ADC)– and cerebral blood flow (CBF)–derived ischemic lesion volumes (mm3) in Group 2 (n = 6) subjected to permanent suture middle cerebral artery occlusion (MCAO) based on ADC and CBF reduction thresholds of 30 ± 2% and 57 ± 11%, respectively. The ADC- and CBF-derived lesion volumes are compared with the 2,3,4-triphenyltetrazolium chloride (TTC)–derived infarct volume at 24 hours. The error bars are standard error of the mean. *p < 0.01, **p < 0.005. (B) Temporal evolution of ADC- and CBF-derived ischemic lesion volumes (mm3) in Groups 1 and 2 (n = 11) subjected to permanent suture MCAO based on ADC and CBF reduction thresholds of 30 ± 2% and 57 ± 11%, respectively. The ADC- and CBF-derived lesion volumes are compared with the TTC-derived infarct volume at 24 hours. The error bars are standard error of the mean. *p < 0.01; **p < 0.001.
For the 60-minute reperfusion cohort (Group 3), there was also a statistically significant perfusion/diffusion mismatch at 30 and 60 minutes after MCAO (but before reperfusion; Fig 2). After mechanical reperfusion at 60 minutes, the CBF-defined lesion volume decreased significantly (see Table). However, the CBF-defined lesion volumes at the 90-, 120-, and 180-minute time points did not differ significantly. The ADC-defined lesion volume also declined upon reperfusion, decreasing by 62mm3 between the 60- and 90-minute time points and then remained statistically similar to the 90-minute value at the 120- and 180-minute time points. The TTC-derived lesion volume at 24 hours was somewhat larger than the 3-hour, ADC-derived lesion volume, but this was not a statistically significant difference (p = 0.35). The corrected infarct volume at 24 hours, 140 ± 32mm3 was, however, significantly smaller in the temporary occlusion group than in Group 2, 245 ± 45 (p < 0.01), or the combined permanent occlusion groups, 224 ± 46 (p < 0.001).
Fig 2.
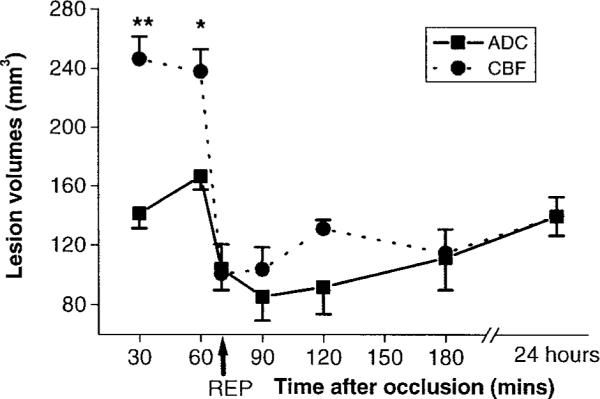
Temporal evolution of apparent diffusion coefficient (ADC)– and cerebral blood flow (CBF)–derived ischemic lesion volumes (mm3) in Group 3 (n = 6) subjected to 60 minutes of suture MCAO based on ADC and CBF reduction thresholds of 30 ± 2% and 57 ± 11%, respectively. The ADC- and CBF-derived lesion volumes are compared with the 2,3,4-triphenyltetrazolium chloride–derived infarct volume at 24 hours. The error bars are standard error of the mean. Arrow indicates reperfusion (REP). *p < 0.005; **p <0.001.
Discussion
This study demonstrated a substantial mismatch between ADC- and CBF-derived lesion volumes after the acute the onset of focal ischemia in the rat suture permanent MCAO model. The volume of diffusion/perfusion mismatch was significant at 30 and 60 minutes after occlusion and approached statistical significance at the 90-minute time point. By 180 minutes after MCAO, the ADC- and CBF-derived lesion volumes were essentially identical. Because the CBF-derived lesion volumes remain essentially constant during the first 3 hours after permanent MCAO (see Fig 1), the initial diffusion/perfusion mismatch arose almost entirely from the smaller ADC-derived lesion volume. The magnitude of the statistical differences between the ADC- and CBF-derived lesion volumes will depend on the choice of the ADC threshold used to delineate the ischemic lesion. For example, we have demonstrated previously that lower ADC thresholds can be derived at early time points that give ADC-derived lesion volumes that also show high correlation and correspondence with the histologically derived infarct volume.21 However, as pointed out by Olah and colleagues,22 there is a minimum ADC threshold that is associated with the metabolic energy failure (ATP depletion) that subsequently causes infarction. Olah and colleagues found that an ADC reduction threshold of 23% (derived from comparisons with postmortem bioluminescence maps of ATP depletion) was a good estimate at all time points during the MCAO period and early after reperfusion. The validity of using the 3-hour time point for deriving the CBF reduction threshold was justified by the observation that the perfusion lesion volume based on the 57 ± 11% reduction threshold remained relatively stable over the 3-hour imaging time period and was correlated with the postmortem infarct volume in the combined data set from both permanent occlusion groups, providing additional confirmation that the 3-hour CBF reduction threshold is a reliable indicator of a perfusion abnormality related to histologically confirmed infarction (see Table).
Upon mechanical reperfusion in this suture occlusion model, we observed a substantial reduction in the hypoperfused tissue volume. Approximately 40% of the initially hypoperfused territory did not reperfuse, consistent with the results obtained by other groups using the same model.22,23 Despite suture withdrawal, persistent microvascular sludging and endothelial injury may contribute to persistent perfusion deficits after reperfusion.24 It is noteworthy (see Fig 2) that the CBF-derived lesion volumes in the first hour postreperfusion are somewhat smaller than those at the subsequent 120- and 180-minute time points. This may be related to reactive hyperemia, observed by several groups initially after reflow following transient focal cerebral ischemia.22,25,26
Within 30 minutes of reperfusion, the mean ADC-derived lesion volume declined by more than 35%. These results are consistent with previous animal studies and in a few human studies after intraarterial and intravenous thrombolysis, demonstrating full/partial reversibility of diffusion abnormalities with early reperfusion.22,27–30 The ADC reversal observed after relatively short periods of transient focal ischemia does not necessarily portend tissue salvage, because secondary energy failure related to mitochondrial dysfunction from calcium overload, free radical formation, and lactic acidosis potentially could lead to subsequent increases in ADC-derived lesion volume over time.22,27,31 In the reperfusion group, the ADC-defined lesion volume at 3 hours tended to underestimate the 24-hour, TTC-derived lesion volume. We attribute this observation to the likely occurrence of secondary, reperfusion injury that evolves over many hours,22,27,32 resulting in concomitantly larger ischemic lesion volumes at postmortem.
In clinical MRI studies, the diffusion/perfusion mismatch typically is identified by visual inspection of the DWI or ADC maps for diffusion MRI and mean-transit-time or time-to-peak maps for perfusion MRI.6,11 Using this approach, several groups have evaluated the presence of a diffusion/perfusion mismatch in stroke patients and observed substantial volumes of mismatch in many patients imaged within 6 hours or even longer after stroke onset, substantially longer than our animal model.11,33 The mismatch concept also has been used to determine if such patients are likely to respond more favorably to thrombolytic therapy than patients treated at a similar time point after stroke who do not demonstrate a mismatch.34,35
An important advantage of identifying the diffusion/perfusion mismatch in an animal stroke model is that information about the temporal evolution of potentially salvageable ischemic tissue can be investigated under carefully controlled conditions. The diffusion/perfusion mismatch provides a volumetric estimate of the putative ischemic penumbra and the duration of its temporal existence, information that could be useful for defining an effective therapeutic window. For example, in our laboratory, using the same suture-occlusion model in this study, we have never observed significant treatment effects with any neuroprotective or mechanical-reperfusion therapy beyond 60 minutes after the onset of permanent ischemia.36 The MRI results from this study suggest that treatment initiated at 90 minutes or later would be of limited success because the volume of diffusion/perfusion mismatch (and hence potentially salvageable tissue) is not significant beyond the 60-minute time point in this model. It is likely that different animal stroke models will have different diffusion/perfusion-mismatch characteristics as compared with the suture permanent MCAO model, and thus the window for potential therapeutic interventions will likely vary between models.
In conclusion, this study demonstrates the presence of a substantial diffusion/perfusion mismatch up to 60 minutes after MCAO in our rat suture MCAO model. With mechanical reperfusion at 60 minutes, the infarct volume was significantly smaller than in permanently occluded animals at the same time points, supporting the possibility of a treatment effect with early reperfusion in this model.
References
- 1.Minematsu K, Li L, Fisher M, et al. Diffusion-weighted magnetic resonance imaging: rapid and quantitative detection of focal brain ischemia. Neurology. 1992;42:235–240. doi: 10.1212/wnl.42.1.235. [DOI] [PubMed] [Google Scholar]
- 2.Moseley ME, Cohen Y, Mintorovitch J, et al. Early detection of regional cerebral ischemia in cats: comparison of diffusion- and T2-weighted MRI and spectroscopy. Magn Res Med. 1990;14:330–346. doi: 10.1002/mrm.1910140218. [DOI] [PubMed] [Google Scholar]
- 3.Fisher M, Albers GW. Applications of diffusion-perfusion magnetic resonance imaging in acute ischemic stroke. Neurology. 1999;52:1750–1756. doi: 10.1212/wnl.52.9.1750. [DOI] [PubMed] [Google Scholar]
- 4.Fisher M, Prichard JW, Warach S. New magnetic resonance techniques for acute ischemic stroke. JAMA. 1995;274:908–911. [PubMed] [Google Scholar]
- 5.Kidwell CS, Saver JL, Mattiello J, et al. Thrombolytic reversal of acute human cerebral ischemic injury shown by diffusion/perfusion magnetic resonance imaging. Ann Neurol. 2000;47:462–469. [PubMed] [Google Scholar]
- 6.Barber PA, Darby DG, Desmond PM, et al. Identification of major ischemic change. Diffusion-weighted imaging versus computed tomography. Stroke. 1999;30:2059–2065. doi: 10.1161/01.str.30.10.2059. [DOI] [PubMed] [Google Scholar]
- 7.Moseley ME, Kucharczyk J, Mintorovitch J, et al. Diffusion-weighted MR imaging of acute stroke: correlation with T2-weighted and magnetic susceptibility-enhanced MR imaging in cats. Am J Neuroradiol. 1990;11:423–429. [PMC free article] [PubMed] [Google Scholar]
- 8.Mintorovitch J, Moseley ME, Chileuitt L, et al. Comparison of diffusion- and T2-weighted MRI for the early detection of cerebral ischemia and reperfusion in rats. Magn Res Med. 1991;18:39–50. doi: 10.1002/mrm.1910180106. [DOI] [PubMed] [Google Scholar]
- 9.Wittlich F, Kohno K, Mies G, et al. Quantitative measurement of regional blood flow with gadolinium diethylenetriaminepentaacetate bolus track NMR imaging in cerebral infarcts in rats: validation with the iodo[14C]antipyrine technique. Proc Natl Acad Sci USA. 1995;92:1846–1850. doi: 10.1073/pnas.92.6.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamberg LM, Macfarlane R, Tasdemiroglu E, et al. Measurement of cerebrovascular changes in cats after transient ischemia using dynamic magnetic resonance imaging. Stroke. 1993;24:444–451. doi: 10.1161/01.str.24.3.444. [DOI] [PubMed] [Google Scholar]
- 11.Neumann HT, Wittsack HJ, Wenserski F, et al. Diffusion- and perfusion-weighted MRI. The DWI/PWI mismatch region in acute stroke. Stroke. 1999;30:1591–1597. doi: 10.1161/01.str.30.8.1591. [DOI] [PubMed] [Google Scholar]
- 12.Karonen JO, Vanninen RL, Liu Y, et al. Combined diffusion and perfusion MRI with correlation to single-photon emission CT in acute ischemic stroke. Ischemic penumbra predicts infarct growth. Stroke. 1999;30:1583–1590. doi: 10.1161/01.str.30.8.1583. [DOI] [PubMed] [Google Scholar]
- 13.Baird AE, Benfield A, Schlaug G, et al. Enlargement of human cerebral ischemic lesion volumes measured by diffusion-weighted magnetic resonance imaging. Ann Neurol. 1997;41:581–589. doi: 10.1002/ana.410410506. [DOI] [PubMed] [Google Scholar]
- 14.Albers GW. Expanding the window for thrombolytic therapy in acute stroke. The potential role of acute MRI for patient selection. Stroke. 1999;30:2230–2237. doi: 10.1161/01.str.30.10.2230. [DOI] [PubMed] [Google Scholar]
- 15.Koizumi J, Yoshida Y, Nakazawa T, Ooneda G. Experimental studies of ischemic brain edema. 1. New experimental model of cerebral embolism rats in which recirculation can be introduced in the ischemic area. Jpn J Stroke. 1986;8:1–8. [Google Scholar]
- 16.Stejskal EO, Tanner JE. Spin diffusion measurements: spin echoes in the presence of a time-dependent field gradient. J Chem Phys. 1965;42:288–292. [Google Scholar]
- 17.Silva A, Lee S-P, Yang C, et al. Simultaneous BOLD and perfusion functional MRI during forepaw stimulation in rats. J Cereb Blood Flow Metab. 1999;19:871–879. doi: 10.1097/00004647-199908000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Duong TQ, Silva AC, Lee S-P, Kim S-G. Functional MRI of calcium-dependent synaptic activity: cross correlation with CBF and BOLD measurements. Magn Reson Med. 2000;43:338–392. doi: 10.1002/(sici)1522-2594(200003)43:3<383::aid-mrm10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 19.Tatlisumak T, Carano RAD, Takano K, et al. Broad-spectrum cation channel inhibition by LOE 908 MS reduces infarct volume in vivo and postmortem in focal cerebral ischemia in the rat. J Neurol Sci. 2000;178:107–113. doi: 10.1016/s0022-510x(00)00380-4. [DOI] [PubMed] [Google Scholar]
- 20.Reith W, Hasegawa Y, Latour LL, et al. Multislice diffusion mapping for 3-D evolution of cerebral ischemia in a rat stroke model. Neurology. 1995;45:172–177. doi: 10.1212/wnl.45.1.172. [DOI] [PubMed] [Google Scholar]
- 21.Li F, Carano RAD, Irie K, et al. Temporal evolution of average apparent diffusion coefficient threshold to define ischemic abnormalities in a rat permanent occlusion model. J Stroke Cerebrovasc Disease. 2000;9:1–7. [Google Scholar]
- 22.Olah L, Wecker S, Hoehn M. Relation of apparent diffusion coefficient changes and metabolic disturbances after 1 hour of focal cerebral ischemia and at different reperfusion phases in rats. J Cereb Blood Flow Metab. 2001;21:430–439. doi: 10.1097/00004647-200104000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Beaulieu C, Busch E, Rother J, et al. Polynitoxyl albumin reduces infarct size in transient cerebral ischemia in the rat: potential mechanisms studied by magnetic resonance imaging. J Cereb Blood Flow Metab. 1998;18:1022–1031. doi: 10.1097/00004647-199809000-00012. [DOI] [PubMed] [Google Scholar]
- 24.del Zoppo G. Microvascular changes during cerebral ischemia and reperfusion. Cerebrovasc Brain Metab Rev. 1994;6:47–95. [PubMed] [Google Scholar]
- 25.De Crespigny AJ, Wendland MF, Derugin N, et al. Real-time observation of transient focal ischemia and hyperemia in cat brain. Magn Reson Med. 1992;27:391–397. doi: 10.1002/mrm.1910270220. [DOI] [PubMed] [Google Scholar]
- 26.Hamberg LM, Boccalini P, Stranjalis G, et al. Continuous assessment of relative cerebral blood volume in transient ischemia using steady-state susceptibility-contrast MRI. Magn Reson Med. 1996;35:168–173. doi: 10.1002/mrm.1910350207. [DOI] [PubMed] [Google Scholar]
- 27.Li F, Liu KF, Silva, et al. Secondary decline in apparent diffusion coefficient and neurological outcome after a short period of focal brain ischemia in rats. Ann Neurol. 2000;48:236–244. [PubMed] [Google Scholar]
- 28.Kidwell CS, Saver JL, Starkman S, et al. Late secondary ischemic injury in patients receiving intraarterial thrombolysis. Ann Neurol. 2002;52:698–703. doi: 10.1002/ana.10380. [DOI] [PubMed] [Google Scholar]
- 29.Carano RAD, Li F, Irie K, et al. Multispectral analysis of the temporal evolution of cerebral ischemia in the rat brain. J Magn Reson Imaging. 2000;12:842–858. doi: 10.1002/1522-2586(200012)12:6<842::aid-jmri7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 30.Fiehler J, Foth M, Kucinski T, et al. Severe ADC decreases do not predict irreversible tissue damage in humans. Stroke. 2002;33:79–86. doi: 10.1161/hs0102.100884. [DOI] [PubMed] [Google Scholar]
- 31.Murphy AN, Fiskum G, Beal MF. Mitochondria in neurodegeneration: bioenergetic function in cell life and death. J Cereb Blood Flow Metab. 1999;19:231–245. doi: 10.1097/00004647-199903000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Neumann-Haefelin T, Kastrup A, de Crespigny A, et al. Serial MRI after transient focal cerebral ischemia in rats: dynamics of tissue injury, blood-brain barrier damage and edema formation. Stroke. 2000;31:1965–1973. doi: 10.1161/01.str.31.8.1965. [DOI] [PubMed] [Google Scholar]
- 33.Beaulieu C, de Crespigny A, Tong DC, et al. Longitudinal magnetic resonance imaging study of perfusion and diffusion in stroke: evolution of lesion volume and correlation with clinical outcome. Ann Neurol. 1999;46:568–578. doi: 10.1002/1531-8249(199910)46:4<568::aid-ana4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 34.Parsons MW, Barber PA, Chalk J, et al. Diffusion- and perfusion-weighted MRI response to thrombolysis in stroke. Ann Neurol. 2002;51:28–37. doi: 10.1002/ana.10067. [DOI] [PubMed] [Google Scholar]
- 35.Rother J, Schellinger PD, Gass A, et al. Effect of intravenous thrombolysis on MRI parameters and functional outcome in acute stroke < 6 hours. Stroke. 2002;33:2438–2445. doi: 10.1161/01.str.0000030109.12281.23. [DOI] [PubMed] [Google Scholar]
- 36.Meadows ME, Fisher M, Minematsu K. Delayed treatment with a non-competitive NMDA antagonist, CNS-1102, reduces infarct size in rats. Cerebrovasc Dis. 1994;4:26–31. [Google Scholar]