Abstract
Background
Previous studies comparing low-carbohydrate and low-fat diets have not included a comprehensive behavioral treatment, resulting in suboptimal weight loss.
Objective
To evaluate the effects of 2-year treatment with a low-carbohydrate or low-fat diet, each of which was combined with a comprehensive lifestyle modification program.
Design
Randomized parallel-group trial. (ClinicalTrials.gov registration number: NCT00143936)
Setting
3 academic medical centers.
Patients
307 participants with a mean age of 45.5 years (SD, 9.7 years) and mean body mass index of 36.1 kg/m2 (SD, 3.5 kg/m2).
Intervention
A low-carbohydrate diet, which consisted of limited carbohydrate intake (20 g/d for 3 months) in the form of low–glycemic index vegetables with unrestricted consumption of fat and protein. After 3 months, participants in the low-carbohydrate diet group increased their carbohydrate intake (5 g/d per wk) until a stable and desired weight was achieved. A low-fat diet consisted of limited energy intake (1200 to 1800 kcal/d; ≤30% calories from fat). Both diets were combined with comprehensive behavioral treatment.
Measurements
Weight at 2 years was the primary outcome. Secondary measures included weight at 3, 6, and 12 months and serum lipid concentrations, blood pressure, urinary ketones, symptoms, bone mineral density, and body composition throughout the study.
Results
Weight loss was approximately 11 kg (11%) at 1 year and 7 kg (7%) at 2 years. There were no differences in weight, body composition, or bone mineral density between the groups at any time point. During the first 6 months, the low-carbohydrate diet group had greater reductions in diastolic blood pressure, triglyceride levels, and very-low-density lipoprotein cholesterol levels, lesser reductions in low-density lipoprotein cholesterol levels, and more adverse symptoms than did the low-fat diet group. The low-carbohydrate diet group had greater increases in high-density lipoprotein cholesterol levels at all time points, approximating a 23% increase at 2 years.
Limitation
Intensive behavioral treatment was provided, patients with dyslipidemia and diabetes were excluded, and attrition at 2 years was high.
Conclusion
Successful weight loss can be achieved with either a low-fat or low-carbohydrate diet when coupled with behavioral treatment. A low-carbohydrate diet is associated with favorable changes in cardiovascular disease risk factors at 2 years.
Primary Funding Source
National Institutes of Health.
Data from several randomized trials over the past 6 years have demonstrated that low-carbohydrate diets produced greater short-term (6 months) weight loss than low-fat, calorie-restricted diets (1-5). The longer-term (1 to 2 years) results are mixed. Some studies found greater weight loss with low-carbohydrate diets than with low-fat diets (5, 6), whereas others found no difference (1, 7-9). However, weight loss with either diet was usually minimal (10-12), presumably because of the modest dose of behavioral treatment provided in these studies (1, 6). The only 2-year randomized, controlled trial of a low-carbohydrate diet to date found greater 2-year weight loss with a low-carbohydrate than a low-fat diet (6). The Israel-based study used visual prompts in a cafeteria setting to guide the selection of the main meal (lunch). Whether the results would be similar in different settings and cultures is unknown. In addition, few previous studies have evaluated the effect of low-carbohydrate diets on symptoms or bone, and the assessments have been limited to 6 months (3, 4).
The purpose of our randomized, 3-center trial was to evaluate the effects of long-term (2-year) treatment with either a low-carbohydrate or low-fat, calorie-restricted diet on key clinical end points, namely body weight, cardiovascular risk factors, bone mineral density, and general symptoms. The primary outcome was weight loss at 2 years. All participants received comprehensive behavioral treatment (13, 14) to enhance weight loss associated with both diets. We hypothesized that a low-carbohydrate diet would produce greater weight loss at 2 years than a low-calorie, low-fat diet.
Context
Previous studies comparing low-carbohydrate with low-fat diets focused on short-term outcomes and did not uniformly include interventions to change physical activity and other aspects of lifestyle.
Contribution
This randomized trial compared outcomes of a behavioral intervention combined with either a low-carbohydrate or low-fat diet and found that after 2 years, participants in both groups lost about 7% of body weight. Greater improvement in high-density lipoprotein cholesterol levels was observed with a low-carbohydrate diet, but other metabolic measures were similar in both groups.
Implication
Overweight persons can achieve substantial weight loss at 2 years if they participate in a behavioral intervention combined with a low-fat or a low-carbohydrate diet.
—The Editors
Methods
Design
Our study was a randomized, controlled trial conducted over 2 years with outcome assessments at baseline, 3, 6, 12, and 24 months.
Setting
Recruitment and data collection were completed at the University of Colorado Denver, Denver, Colorado; Washington University, St. Louis, Missouri; and the University of Pennsylvania, Philadelphia, Pennsylvania.
Participants
The primary inclusion criteria were age 18 to 65 years, body mass index of 30 to 40 kg/m2, and body weight less than 136 kg. A total of 307 adults (208 women and 99 men) with a mean age of 45.5 years (SD, 9.7 years) and a mean body mass index of 36.1 kg/m2 (SD, 3.5 kg/m2) participated in this study. Most (74.9%) participants were white; 22.1% were African American, and 3% were of other race or ethnicity. There were no statistically significant differences between the 2 diet groups in any baseline variables (Table 1).
Table 1.
Baseline Participant Characteristics*
| Characteristic | Low-Fat Diet Group (n = 154) | Low-Carbohydrate Diet Group (n = 153) |
|---|---|---|
| Sex, n (%) | ||
| Male | 49 (32) | 50 (33) |
| Female | 105 (68) | 103 (67) |
| Race (non-Hispanic or Latino), n (%) | ||
| White | 111 (72) | 106 (69) |
| Black | 33 (21) | 35 (23) |
| Asian | 2 (1) | 0 (0) |
| American Indian or Alaska Native | 1 (<1) | 0 (0) |
| >1 race | 1 (<1) | 1 (<1) |
| Race (Hispanic or Latino), n (%) | ||
| American Indian or Alaska Native | 0 (0) | 3 (2) |
| Black | 0 (0) | 1 (<1) |
| White | 6 (4) | 7 (5) |
| Mean age (SD), y | 44.9 (10.2) | 46.2 (9.2) |
| Mean body mass index (SD), kg/m2 | 36.1 (3.46) | 36.1 (3.59) |
| Mean weight (SD), kg | 103.5 (14.4) | 103.3 (15.5) |
| Mean systolic blood pressure (SD), mm Hg | 124.6 (15.8) | 124.3 (14.1) |
| Mean diastolic blood pressure (SD), mm Hg | 76 (9.7) | 73.9 (9.4) |
| Mean triglyceride level (SD) | ||
| mmol/L | 1.40 (0.83) | 1.28 (0.62) |
| mg/dL | 124 (73.5) | 113.3 (54.6) |
| Mean total cholesterol level (SD) | ||
| mmol/L | 4.98 (0.85) | 4.88 (0.78) |
| mg/dL | 192.4 (32.9) | 188.6 (30.2) |
| Mean LDL cholesterol level (SD) | ||
| mmol/L | 3.21 (0.76) | 3.11 (0.67) |
| mg/dL | 124 (29.2) | 120.2 (25.7) |
| Mean HDL cholesterol level (SD) | ||
| mmol/L | 1.18 (0.30) | 1.20 (0.35) |
| mg/dL | 45.4 (11.7) | 46.2 (13.5) |
| Mean VLDL cholesterol level (SD) | ||
| mmol/L | 0.60 (0.42) | 0.58 (0.36) |
| mg/dL | 23 (16.1) | 22.4 (14) |
| Mean total cholesterol level (SD) | ||
| mmol/L | 0.12 (0.03) | 0.11 (0.04) |
| mg/dL | 4.5 (1.3) | 4.4 (1.7) |
| Mean non-HDL cholesterol level (SD) | ||
| mmol/L | 3.80 (0.82) | 3.68 (0.75) |
| mg/dL | 147 (31.7) | 142.2 (29.1) |
| Mean hip BMD (SD), g/cm2 | 1.1 (0.12) | 1.1 (0.14) |
| Mean spine BMD (SD), g/cm2 | 1.1 (0.13) | 1.1 (0.14) |
| Mean lean mass (SD), kg | 61.3 (12.2) | 61.3 (13.0) |
| Mean fat mass (SD), kg | 40.4 (7.8) | 40.0 (7.6) |
BMD = bone mineral density; HDL = high-density lipoprotein; LDL = low-density lipoprotein; VLDL = very-low-density lipoprotein.
There were no significant differences between the 2 groups.
All participants completed a comprehensive medical examination and routine blood tests. We excluded study applicants if they had serious medical illnesses, such as type 2 diabetes; took lipid-lowering medications; were pregnant or lactating; or took medications that affect body weight, including antiobesity agents. Participants with blood pressures of 140/90 mm Hg or more were excluded regardless of whether they were treated. We recruited, enrolled, and followed participants from March 2003 to June 2007. Recruitment methods were consistent across sites and included newspaper advertisements, flyers in the university or hospital setting, physician referral, and self-referral. After a scripted phone screening, eligible patients attended an in-person screening during which the study’s purpose and requirements were fully discussed, eligibility was confirmed, and written informed consent was obtained. The institutional review boards of each of the 3 participating institutions approved the study.
Randomization and Interventions
Using a random-number generator, we randomly assigned participants within each site to treatment with either a low-carbohydrate or low-fat, calorie-restricted diet for 2 years (Figure 1).
Figure 1. Study flow diagram.
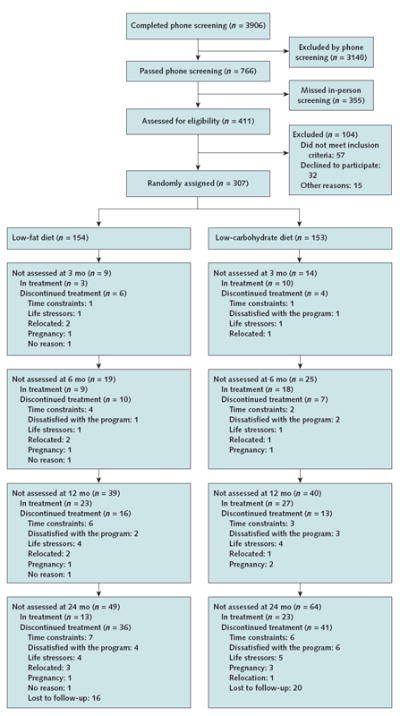
“In treatment” refers to the participants who were still in treatment but did not complete the assessment. “Discontinued treatment” refers to the participants who formally withdrew from the study or could not be contacted (that is, lost to follow-up).
Low-Carbohydrate Diet
Approximately half of the participants (n = 153) were assigned to a low-carbohydrate diet, which limited carbohydrate intake but allowed unrestricted consumption of fat and protein. During the first 12 weeks of treatment, participants were instructed to limit carbohydrate intake to 20 g/d in the form of low–glycemic index vegetables. After the first 12 weeks, participants gradually increased carbohydrate intake (5 g/d per week) by consuming more vegetables, a limited amount of fruits, and eventually small quantities of whole grains and dairy products, until a stable and desired weight was achieved. They followed guidelines described in Dr. Atkins’ New Diet Revolution (15) but were not provided with a copy of the book. Participants were instructed to focus on limiting carbohydrate intake and to eat foods rich in fat and protein until they were satisfied. The primary behavioral target was to limit carbohydrate intake.
Low-Fat Diet
The remaining 154 participants were assigned to consume a low-fat diet, which consisted of limiting energy intake to 1200 to 1500 kcal/d for women and 1500 to 1800 kcal/d for men, with approximately 55% of calories from carbohydrate, 30% from fat, and 15% from protein. Participants were instructed to limit calorie intake, with a focus on decreasing fat intake. However, limiting overall energy intake (kcal/d) was the primary behavioral target.
Common Instructions
All participants received comprehensive, in-person group behavioral treatment (13, 14) weekly for 20 weeks, every other week for 20 weeks, and then every other month for the remainder of the 2-year study period. Each treatment session lasted 75 to 90 minutes. The Appendix (available at www.annals.org) provides details of the treatment. Topics included self-monitoring, stimulus control, and relapse management. All participants were prescribed the same level of physical activity (principally walking), beginning at week 4, with 4 sessions of 20 minutes each and progressing by week 19 to 4 sessions of 50 minutes each. Group sessions reviewed participants’ completion of their eating and activity records, as well as other skill builders. Participants in both groups were instructed to take a daily multivitamin supplement (provided by the study). The lifestyle intervention is described in greater detail in the Appendix.
Outcomes and Measurements
Weight
Body weight was measured at each treatment visit on calibrated scales while participants wore light clothing and no shoes. Height was measured by a stadiometer at baseline. The primary outcome was weight at 2 years.
The following measurements were collected at baseline and at 3, 6, 12 and 24 months.
Serum Lipoproteins
We obtained blood samples after participants fasted overnight (12 hours). Plasma lipid levels were analyzed (16) in a lipid laboratory that participates continuously in the Centers for Disease Control and Prevention Lipid Standardization Program. We measured plasma high-density lipoprotein (HDL) cholesterol and triglyceride levels enzymatically on a Hitachi autoanalyzer by using Sigma reagents (Sigma Chemical Company, St. Louis, Missouri). Very-low-density lipoprotein (VLDL) cholesterol and low-density lipoprotein (LDL) cholesterol concentrations were directly measured by β-quantification after ultracentrifugation at a density of 1.006 g/mL to separate VLDL.
Blood Pressure
We assessed blood pressure by using automated instruments (Dinamap, GE Health Care, Milwaukee, Wisconsin) with cuff sizes based on measured arm circumference. After participants were sitting quietly for 5 minutes, 2 readings of blood pressure were obtained, separated by a 1-minute rest period. The average of the 2 readings was used to determine blood pressure.
Urine Ketones
Dipsticks (Bayer Ketostix 2880, Elkhart, Indiana) were used to measure fasting urinary ketones and were characterized as negative (0 mg/dL) or positive (trace, 5 mg/dL; small, 15 mg/dL; moderate, 40 mg/dL; or large, 80 to 160 mg/dL).
Symptoms
We assessed general symptoms with a symptom checklist used in previous weight-loss studies (17). The checklist contains 26 symptoms rated as none, mild, moderate, or severe. Symptoms were categorized as either absent (none) or present (mild, moderate, or severe) because the symptom data were not normally distributed (most symptoms were listed as none or mild).
Bone Mineral Density and Body Composition
We assessed bone mineral density and body composition (percentage of body fat) by using dual-energy x-ray absorptiometry at baseline and at 6, 12, and 24 months. All sites used a Hologic (Bedford, Massachusetts) Delphi or Discovery model bone densitometer. Whole-body, posteroanterior lumbar spine (L1 to L4), and left proximal femur scans were acquired according to manufacturer guidelines for participant positioning. We cross-calibrated scanners by using the same Hologic anthropomorphic spine and whole-body phantom set before data collection. Long-term calibration was monitored at each site with a spine phantom scanned daily and a whole-body phantom scanned 3 times a week. Based on these phantoms, the long-term precision was less than 1% for spine bone mineral density and less than 2% for percentage of body fat. A single technician analyzed all scans centrally by using Hologic software, version 11.2, and one investigator independently reviewed for scan and analysis quality. We excluded poor-quality scans (movement artifacts and improper position) from the analysis (0.7% for spine; 3.9% for hip; and 3.1% for whole body).
Follow-up Procedures
All randomly assigned participants, regardless of whether they were actively attending treatment, were contacted by phone, mail, and e-mail to schedule a follow-up assessment.
Statistical Analysis
Sample Size
To detect a 3% (SD, 5%) difference between the groups in the primary outcome—body weight at 24 months—with 90% power and an α value of 0.05, we needed 85 participants per treatment group. To detect a 10% (SD, 20%) difference in LDL cholesterol level and other secondary outcomes, 119 participants per group were required. We aimed to enroll 150 participants per group to account for attrition and to provide power for secondary outcomes.
We used a random-effects linear model that was fitted to all observed data for each variable on each of the 307 participants for the primary analysis. Each random-effects model consisted of a random intercept and slope to adjust for individual participant variability due to within-participant correlations among the observed longitudinal data. These models also contained the following fixed effects: main effects for each follow-up visit, group assignment, interactions between each follow-up visit and group indicator variables, and baseline value as a covariate. We estimated with maximum likelihood by using the PROC MIXED procedure in SAS, version 9 (SAS Institute, Cary, North Carolina). A parallel longitudinal model structure based on main effects for visit, treatment group, and baseline value and visit-treatment interactions was implemented with logistic regression for binary outcomes. We did estimates by using generalized estimating equations under the logistic regression model for correlated longitudinal binary outcomes implemented in the GENMOD procedure in SAS, version 9. Predicted values for each treatment and visit combination at the mean level of the baseline outcome, with corresponding lower and upper confidence bounds, were produced under each model for the figures.
The previously mentioned longitudinal models preclude the use of less robust approaches, such as fixed-imputation methods (for example, last observation carried forward or the analysis of participants with complete data [that is, complete case analyses]). These alternative approaches assume that missing data are unrelated to previously observed outcomes or baseline covariates, including treatment (that is, missing completely at random). The longitudinal models implemented for this study relax this missing-completely-at-random assumption in different ways. The generalized estimating equation–based longitudinal logistic models assume that missing data are unrelated to previously observed outcomes but can be related to the treatment because it is a covariate in the model. (that is, covariate-dependent missing completely at random) (18). The likelihood-based mixed-effects models further relax the covariate-dependent missing-completely-at-random assumption by allowing missing data to be dependent on previously observed outcomes and treatment (that is, missing at random). To assess departures from the missing-at-random assumption under informative withdrawal—that is, the missing weights are informative for which patients chose to withdraw or continue to participate in the study—we present sensitivity analyses. As such, we assume that all participants who withdraw would follow first the maximum and then minimum patient trajectory of weight under the random intercept model.
The α value was set at 5% for weight loss at 24 months and 1% for all other outcomes to account for comparisons at 3, 6, 12, and 24 months (or whatever the pair-wise comparisons are). Adding site to the above models revealed no site effects for weight loss or attrition at 3, 6, 12, or 24 months, so the entire sample (n = 307) was collapsed and analyzed together. Triglyceride values were not normally distributed, so analyses were done on the log-transformed values.
Attrition
There were no statistically significant differences between the 2 groups in attrition, defined as not undergoing an assessment at a specific time point, independent of the reason. Attrition included participants who withdrew and intermittent missingness at each time point. In the low-fat group, 6%, 12%, 25%, and 32% of participants did not participate in assessments at 3, 6, 12, and 24 months, respectively. Values for the low-carbohydrate participants were 9%, 16%, 26%, and 42%, respectively (Figure 1). Under the sensitivity analysis based on imputing missing outcomes with the highest (13.795) and lowest (−18.355) random-effects slopes (that is, change in weight per month) under the mixed-effects model for weight, our qualitative findings were not sensitive to either imputation approach.
Role of the Funding Source
The National Institutes of Health funded this study. The funding source had no role in the design, conduct, or reporting of the study.
Results
Body Weight
Participants in both groups lost approximately 11% of initial weight at 6 and 12 months, with subsequent weight regain to a 7% weight loss at 2 years (Table 2 and Figure 2). We found no statistically significant differences in weight loss at any time point between the low-carbohydrate and low-fat diet groups, although there was a strong trend (P = 0.019) for greater weight loss in the low-carbohydrate group at 3 months.
Table 2.
Predicted Mean Changes in Body Weight, Cardiovascular Disease Risk Factors, Bone Mineral Density, and Body Composition Over 2 Years
| Variable | Absolute Change From Baseline (95% CI) | P Value* | |
|---|---|---|---|
| Low-Fat Diet | Low-Carbohydrate Diet | ||
| Weight, kg | |||
| 3 mo | −8.37 (−9.04 to −7.71) | −9.49 (−10.1 to −8.85) | 0.019 |
| 6 mo | −11.34 (−12.4 to −10.3) | −12.18 (−13.1 to −11.2) | 0.25 |
| 12 mo | −10.81 (−12.4 to −9.28) | −10.87 (−12.1 to −9.67) | 0.95 |
| 24 mo | −7.37 (−9.10 to −5.63) | −6.34 (−8.06 to −4.63) | 0.41 |
| Overall | 0.30 | ||
| Triglyceride level, mg/dL† | |||
| 3 mo | −17.99 (−24.6 to −11.4) | −40.08 (−45.2 to −34.9) | <0.001 |
| 6 mo | −24.30 (−31.2 to −17.4) | −40.06 (−45.7 to −34.4) | <0.001 |
| 12 mo | −17.92 (−28.3 to −7.58) | −31.52 (−39.5 to −23.6) | 0.039 |
| 24 mo | −14.58 (−25.8 to −3.40) | −12.19 (−22.9 to −1.49) | 0.76 |
| Overall | 0.26 | ||
| VLDL cholesterol level, mg/dL† | |||
| 3 mo | −3.25 (−5.03 to −1.47) | −8.91 (−10.3 to −7.49) | <0.001 |
| 6 mo | −4.79 (−6.40 to −3.18) | −8.88 (−10.4 to −7.40) | <0.001 |
| 12 mo | −3.60 (−6.34 to −0.87) | −8.18 (−10.2 to −6.11) | 0.009 |
| 24 mo | −2.18 (−4.53 to 0.16) | −2.18 (−4.63 to 0.26) | 0.99 |
| Overall | 0.027 | ||
| LDL cholesterol level, mg/dL† | |||
| 3 mo | −6.36 (−9.81 to −2.91) | 7.20 (2.38 to 12.02) | <0.001 |
| 6 mo | −9.52 (−12.9 to −6.15) | 0.54 (−3.25 to 4.33) | <0.001 |
| 12 mo | −8.66 (−12.7 to −4.56) | −8.57 (−12.9 to −4.26) | 0.98 |
| 24 mo | −8.01 (−11.4 to −4.62) | −4.78 (−9.17 to −0.39) | 0.25 |
| Overall | 0.0009 | ||
| HDL cholesterol level, mg/dL† | |||
| 3 mo | −0.47 (−1.42 to 0.48) | 2.30 (1.04 to 3.55) | <0.001 |
| 6 mo | 0.89 (−0.24 to 2.02) | 6.21 (4.74 to 7.67) | <0.001 |
| 12 mo | 3.94 (2.52 to 5.36) | 7.96 (6.33 to 9.59) | <0.001 |
| 24 mo | 4.64 (3.17 to 6.10) | 7.75 (6.00 to 9.49) | 0.008 |
| Overall | 0.0058 | ||
| Total cholesterol/HDL cholesterol level, mg/dL† | |||
| 3 mo | −0.28 (−0.41 to −0.16) | −0.31 (−0.48 to −0.15) | 0.79 |
| 6 mo | −0.48 (−0.59 to −0.37) | −0.68 (−0.82 to −0.53) | 0.035 |
| 12 mo | −0.61 (−0.75 to −0.46) | −0.87 (−1.02 to −0.71) | 0.016 |
| 24 mo | −0.61 (−0.73 to −0.49) | −0.67 (−0.82 to −0.51) | 0.56 |
| Overall | 0.030 | ||
| Systolic blood pressure, mm Hg | |||
| 3 mo | −5.20 (−7.09 to −3.31) | −7.74 (−9.59 to −5.89) | 0.06 |
| 6 mo | −6.97 (−8.89 to −5.05) | −7.36 (−9.26 to −5.47) | 0.78 |
| 12 mo | −4.06 (−6.07 to −2.05) | −5.64 (−7.62 to −3.67) | 0.27 |
| 24 mo | −2.59 (−5.07 to −0.12) | −2.68 (−5.08 to −0.27) | 0.96 |
| Overall | 0.40 | ||
| Diastolic blood pressure, mm Hg | |||
| 3 mo | −3.05 (−4.29 to −1.81) | −5.53 (−6.70 to −4.36) | 0.004 |
| 6 mo | −2.50 (−3.76 to −1.25) | −5.15 (−6.49 to −3.82) | 0.005 |
| 12 mo | −2.19 (−3.58 to −0.79) | −3.25 (−4.74 to −1.76) | 0.31 |
| 24 mo | −0.50 (−2.13 to 1.13) | −3.19 (−4.66 to −1.73) | 0.016 |
| Overall | 0.36 | ||
| Hip bone mineral density, g/cm2 | |||
| 6 mo | −0.01 (−0.01 to −0.00) | −0.01 (−0.02 to −0.00) | 0.34 |
| 12 mo | −0.02 (−0.02 to −0.01) | −0.01 (−0.02 to −0.01) | 0.83 |
| 24 mo | −0.01 (−0.02 to −0.01) | −0.02 (−0.03 to −0.01) | 0.64 |
| Overall | 0.41 | ||
| Spine bone mineral density, g/cm2 | |||
| 6 mo | 0.01 (0.00 to 0.01) | 0.01 (0.01 to 0.02) | 0.67 |
| 12 mo | 0.01 (0.00 to 0.01) | 0.01 (0.00 to 0.01) | 0.59 |
| 24 mo | 0.00 (−0.01 to 0.01) | 0.00 (−0.00 to 0.01) | 0.79 |
| Overall | 0.48 | ||
| Lean mass, kg | |||
| 6 mo | −3.18 (−3.40 to −2.55) | −3.53 (−3.66 to −2.81) | 0.39 |
| 12 mo | −2.74 (−3.19 to −2.29) | −3.04 (−3.21 to −2.31) | 0.95 |
| 24 mo | −2.14 (−2.68 to −1.59) | −2.35(−3.07 to −1.80) | 0.48 |
| Overall | 0.49 | ||
| Fat mass, kg | |||
| 6 mo | −8.16 (−8.45 to −6.62) | −8.65 (−8.75 to −7.20) | 0.47 |
| 12 mo | −7.29 (−8.55 to −6.03) | −7.83 (−7.89 to −6.14) | 0.72 |
| 24 mo | −3.84 (−5.03 to −2.64) | −3.99 (−5.50 to −2.79) | 0.74 |
| Overall | 0.18 | ||
HDL = high-density lipoprotein; LDL = low-density lipoprotein; VLDL = very-low–density lipoprotein.
P values are for the differences between the 2 groups at each time point.
To convert values for triglycerides to mmol/L, multiply by 0.01129. To convert values for cholesterol to mmol/L, multiply by 0.02586.
Figure 2. Predicted absolute mean change in body weight for participants in the low-fat and low-carbohydrate diet groups, based on a random-effects linear model.
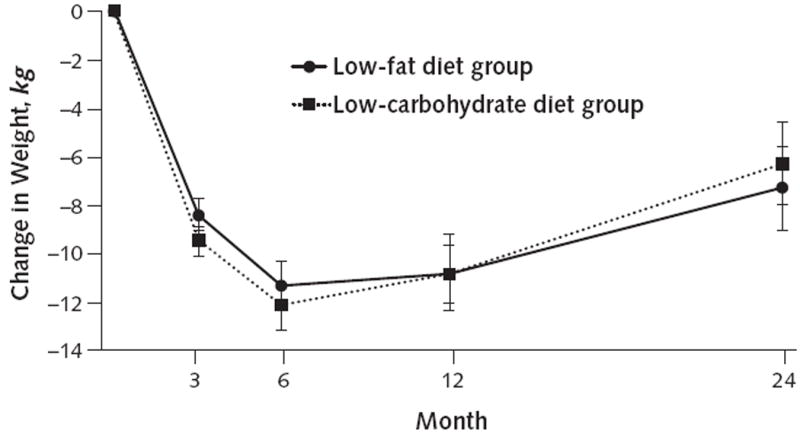
Error bars represent 95% CIs.
Urinary Ketones
The percentage of participants who had positive test results for urinary ketones was greater in the low-carbohydrate than in the low-fat group at 3 months (63% vs. 20%; P < 0.001) and 6 months (28% vs. 9%; P < 0.01). We found no statistically significant differences between groups after 6 months. The decrease from 3 to 24 months is consistent with liberalization of carbohydrate intake over time, as part of the study protocol.
Blood Pressure
Systolic blood pressure decreased with weight loss in both diet groups relative to baseline, but systolic blood pressure did not significantly differ between groups at any time. However, reductions in diastolic pressure were significantly greater (2 to 3 mm Hg) in the low-carbohydrate than in the low-fat group at 3 and 6 months with a strong trend (P = 0.016) at 24 months (Table 2).
Plasma Lipid Concentrations
The macronutrient content of the 2 diets influenced the effect of weight loss on plasma lipid concentrations. Most of the differences in plasma lipid concentrations between groups were observed during the first 6 months of the diets (Table 2, Figure 3, and Appendix Table, available at www.annals.org). We found a significantly greater decrease in LDL cholesterol levels at 3 and 6 months in the low-fat group than in the low-carbohydrate group, but this difference did not persist at 12 or 24 months. Decreases in triglyceride levels were greater in the low-carbohydrate than in the low-fat group at 3 and 6 months but not at 12 or 24 months. Decreases in VLDL cholesterol levels were significantly greater in the low-carbohydrate than in the low-fat group at 3, 6, and 12 months but not at 24 months. Increases in HDL cholesterol levels were significantly greater in the low-carbohydrate than in the low-fat group at 3, 6, 12 and 24 months. The ratio of total-cholesterol to HDL cholesterol levels decreased significantly in both groups through 24 months but did not significantly differ between groups at any time. There was a trend for greater reductions in the low-carbohydrate group at 6 months (P = 0.035) and 12 months (P = 0.016) (Table 2). Therefore, the only effect on plasma lipid concentrations that persisted at 2 years was the significantly greater increases in HDL cholesterol levels among low-carbohydrate participants.
Figure 3. Predicted absolute mean change in serum triglyceride, VLDL cholesterol, LDL cholesterol, and HDL cholesterol concentrations in the low-fat and low-carbohydrate diet groups, based on a random-effects linear model.
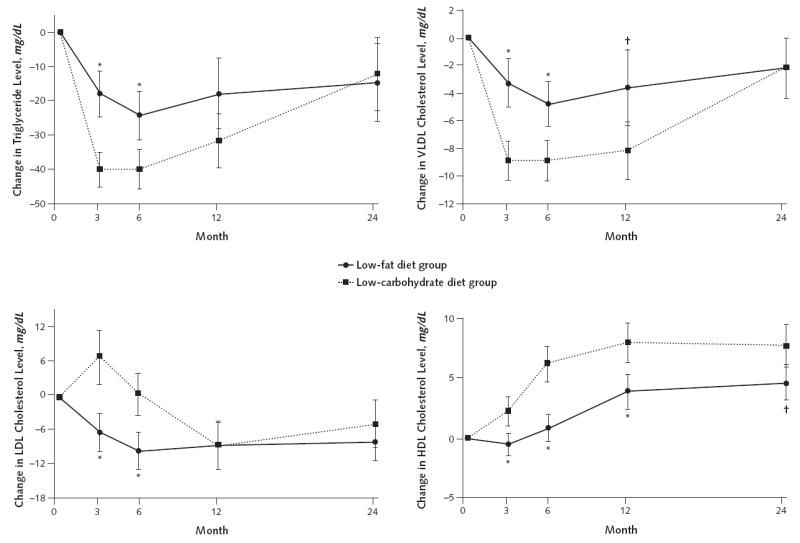
Error bars represent 95% CIs. To convert triglycerides to mmol/L, multiply by 0.0113. To convert HDL, LDL, and VLDL cholesterol to mmol/L, multiply by 0.0259. HDL= high-density lipoprotein cholesterol; LDL= low-density lipoprotein cholesterol; VLDL = very-low-density lipoprotein cholesterol.
* P < 0.001.
† P < 0.01 for between-group differences.
Bone Mineral Density and Body Composition
We found no differences between groups in changes in bone mineral density or body composition over 2 years (Table 2). For both hip and spine bone mineral density, the change from baseline was 1.5% or less at 6, 12, and 24 months, and we found no significant differences between groups. For body composition, both groups experienced similar reductions in lean mass (approximately 5%) and fat mass (11% to 20%), and we found no differences between groups at anytime during the study (Table 2). Finally, the groups did not differ in the percentage of weight lost from fat or lean mass.
Symptoms
A significantly greater percentage of participants who consumed the low-carbohydrate than the low-fat diet reported bad breath, hair loss, constipation, and dry mouth (Table 3). Except for constipation, all of these differences were limited to the first 6 months of treatment. No serious cardiovascular events (for example, stroke, myocardial infarction) were reported. The Appendix includes all serious adverse events (type, time, and attribution to diet).
Table 3.
Significant Differences in Symptom Reporting
| Symptom | Patients (95% CI), % | P Value* | |
|---|---|---|---|
| Low-Fat Diet Group | Low-Carbohydrate Diet Group | ||
| Bad breath | |||
| 3 mo | 37 (28–46) | 64 (54–72) | <0.001 |
| 6 mo | 19 (12–29) | 38 (28–49) | 0.007 |
| 12 mo | 30 (21–41) | 42 (31–53) | 0.147 |
| 24 mo | 30 (20–43) | 35 (25–47) | 0.56 |
| Overall | 0.102 | ||
| Hair loss | |||
| 3 mo | 18 (11–29) | 21 (12–34) | 0.67 |
| 6 mo | 21 (12–33) | 45 (31–60) | 0.006 |
| 12 mo | 19 (11–31) | 29 (17–44) | 0.24 |
| 24 mo | 15 (7–28) | 23 (12–39) | 0.32 |
| Overall | 0.27 | ||
| Constipation | |||
| 3 mo | 39 (30–48) | 63 (53–71) | <0.001 |
| 6 mo | 36 (26–46) | 43 (33–54) | 0.31 |
| 12 mo | 33 (23–44) | 53 (41–64) | 0.010 |
| 24 mo | 17 (11–26) | 39 (28–52) | 0.002 |
| Overall | 0.195 | ||
| Dry mouth | |||
| 3 mo | 25 (18–35) | 48 (39–58) | <0.001 |
| 6 mo | 16 (10–26) | 32 (23–43) | 0.017 |
| 12 mo | 21 (14–32) | 38 (27–50) | 0.028 |
| 24 mo | 23 (14–34) | 32 (22–44) | 0.22 |
| Overall | 0.070 | ||
P values are for the difference between the 2 groups for each time point.
Discussion
Our study has 2 main findings. First, neither dietary fat nor carbohydrate intake influenced weight loss when combined with a comprehensive lifestyle intervention. Second, because both diet groups achieved nearly identical weight loss, we were able to determine that a low-carbohydrate diet has greater beneficial long-term effects on HDL cholesterol concentrations than a low-fat diet.
Our participants had similar and clinically significant weight losses with either a low-carbohydrate or low-fat diet at 1 year (11%) and 2 years (7%), demonstrating that either diet can be used to achieve successful long-term weight loss if coupled with behavioral treatment. The weight losses are similar to those obtained with the best available pharmacotherapy for obesity (19, 20). Data from the most previous studies found greater weight loss among low-carbohydrate than low-fat dieters (1-4, 6), presumably because short-term adherence to a low-carbohydrate diet was easier than complying with a low-fat diet. We found a strong trend for greater short-term (3 month) weight loss among the low-carbohydrate participants, but the difference was small (1.3%) and not clinically significant. Our data suggest that the difference in adherence may be overcome by behavioral treatment, although a 2 × 2 analysis (both diets with and without behavioral treatment) would be required to rigorously test this hypothesis. The similar weight losses observed with low-carbohydrate and low-fat diets demonstrate that the comprehensive lifestyle intervention produced the same energy deficit in both groups, despite marked differences in their behavioral targets (carbohydrates vs. calories and fat). This long-term finding in an outpatient setting is consistent with data from short-term metabolic ward studies showing that macronutrient composition did not influence weight loss when energy content was fixed (21-23).
The nearly identical weight loss in the 2 diet groups during our study provided a unique opportunity to assess the relative effects of the macronutrient content of the 2 diets on cardiovascular disease risk factors. The results demonstrate that dietary macronutrient composition had differential effects on plasma lipid concentrations. At 3 and 6 months, LDL cholesterol concentrations increased in the low-carbohydrate group but decreased in the low-fat group, such that the differences between groups were statistically significant. These differences cannot be explained by differences in weight loss and are probably due to the increase in total fat intake in participants who consumed the carbohydrate-restricted diet. Over the long-term, however, plasma LDL cholesterol concentration in the low-carbohydrate diet group was similar to baseline values, and changes in LDL cholesterol concentrations did not statistically differ between groups. Therefore, the short-term increases in plasma LDL cholesterol concentration in the low-carbohydrate diet group are unlikely to be of clinical importance. Moreover, assessment of LDL cholesterol concentration without information on LDL particle size has limitations as an indicator of coronary heart disease risk because small, dense LDL particles are more atherogenic than large LDL particles (24). Data from carefully controlled studies demonstrated that isocaloric replacement of dietary carbohydrate with fat increases plasma LDL cholesterol concentration but shifts LDL particle size from smaller to larger and less atherogenic LDL (25). Nonetheless, weight loss with the low-carbohydrate diet was not associated with the decrease in LDL cholesterol observed in the low-fat diet group and usually observed with weight reduction (26, 27).
The low-carbohydrate diet caused a decrease in plasma triglyceride concentration that was more than double the reduction observed with a low-fat diet at 3, 6, and 12 months. However, at 2 years, plasma triglyceride concentration returned toward baseline in the low-carbohydrate group to values that did not differ from those in the low-fat group. Similarly, the decline in directly measured VLDL cholesterol concentration was also greater in the low-carbohydrate than in the low-fat group at 3, 6, and 12 months. However, as with triglyceride levels, at 2 years we found no significant differences between groups. The close relationship and tracking between fasting plasma triglyceride concentrations (which are primarily contained within VLDL) and VLDL cholesterol concentrations supports a model in which the low-carbohydrate diet decreased hepatic VLDL secretion, enhanced VLDL clearance, or both compared with the low-fat diet during the first year of the study.
The low-carbohydrate diet produced a much greater increase in plasma HDL cholesterol concentration than did the low-fat diet at all assessments during the 2-year study. Plasma HDL cholesterol concentration increased by approximately 20% at 6 months in the low-carbohydrate diet group, which persisted throughout the study and was more than twice the increase observed in the low-fat diet group. The magnitude of the changes observed in the low-carbohydrate group approximates that obtained with the maximal doses of nicotinic acid (niacin), the most effective HDL-raising pharmacologic intervention currently available (28). The fact that the HDL cholesterol levels remained substantially elevated at 24 months, when the plasma triglyceride levels had returned to baseline in the low-carbohydrate group, argues against the conventional explanation that the increase in plasma HDL cholesterol concentration is solely secondary to a reduction in plasma triglyceride levels. The increased HDL cholesterol during a low-carbohydrate diet could result, at least in part, from the increased intake of dietary fat (29). Although weight loss and increased physical activity undoubtedly contributed to the elevation of HDL cholesterol in both groups, the marked difference in HDL cholesterol between the 2 groups, despite similar weight loss, demonstrates that macronutrient composition has independent effects on HDL. The mechanism responsible for the robust and sustained increase in HDL cholesterol levels among low-carbohydrate participants is unknown and will require additional mechanistic studies. The clinical implications of this increase in HDL cholesterol, which is conventionally believed to be beneficial, are uncertain and will probably depend on the mechanism responsible for this effect.
Weight loss caused a decrease in bone mineral density, which was within the range reported in previous weight-loss studies (30). The changes in bone mineral density did not differ between diet groups, suggesting the hypothetical concerns that weight loss induced by a low-carbohydrate diet causes greater bone loss than weight loss induced by a low-fat diet (31) are unfounded. In addition, the decrease in body fat mass and fat-free mass were within the range reported in previous weight-loss studies, and no differences were found between diet groups.
Our study has several important strengths, including a long duration, a large sample that contained both men and women, and the first long-term assessment of bone and adverse symptoms. Our study also has several limitations. First, the comprehensive behavioral therapy program used in this study makes it difficult to extrapolate our results to general weight management in the community. However, the clinically significant weight losses achieved at 24 months underscore the need for providing patients with long-term behavioral support, whether by registered dietitians or other allied health professionals (32, 33). Our protocol was based on an Atkins version of a low-carbohydrate plan, which prescribes an increase in carbohydrate intake over time; thus, the effects of longer than 12 weeks of severe (20 g/d) carbohydrate restriction could not be assessed. Finally, our findings should not be generalized to obese persons who have obesity-related diseases that were excluded from our study population, such as diabetes and hypercholesterolemia.
In conclusion, this 2-year, multicenter study of more than 300 participants revealed that neither dietary fat nor carbohydrate intake influenced weight loss when combined with a comprehensive lifestyle intervention. Both diet groups achieved clinically significant and nearly identical weight loss (11% at 6 months and 7% at 24 months), and persons who received the low-carbohydrate diet had greater 24-month increases in HDL-cholesterol concentrations than persons who received the low-fat diet. We found no differences between the groups for changes in bone or body composition. These long-term data suggest that a low-carbohydrate approach is a viable option for obesity treatment for obese adults.
Acknowledgments
The authors thank Brooke Bailer, Eva Greenberg, Eileen Ford, Joan Heins, Jennifer Lundgren, Jennifer McCrea, Donna Paulhamus, Gary Skolnick, Emily Smith, Philippe Szapary, Adam Tsai, and Leslie Womble and for their assistance in conducting this study and the study participants for their participation.
Grant Support: By the National Institutes of Health (NIH) grant R01 AT1103 to Temple University; NIH grant UL1RR024134 to University of Pennsylvania; NIH grant UL1 RR000051 to University of Colorado; and NIH grant UL1 RR024992 and DK 56341 to Washington University.
APPENDIX
The group treatment sessions were 75 to 90 minutes and were held weekly from weeks 1 to 20, every other week from weeks 21 to 40, and every 8 weeks from weeks 41 to 104. Groups included 8 to 12 participants and only contained persons assigned to the same diet condition (low-carbohydrate or low-fat). Once the group sessions began, no additional members were added, and participants could not attend other group sessions. There was 1 brief (15 minute) individual session at week 30 that focused on assessing progress and goal setting for the future.
During weeks 1 to 20, participants were instructed in traditional behavioral methods of weight control, such as self-monitoring, stimulus control, slowed eating, shaping, and reasonable goal setting. During weeks 21 to 104, there was a focus on skills to maintain weight loss, such as continuing to record food intake regularly, measuring and recording body weight regularly, consuming a low-carbohydrate or a high-carbohydrate diet, identifying high-risk situations, differentiating lapse from relapse, responding effectively to overeating episodes, and learning to reverse small weight gains as they occur. Group sessions varied between the 2 treatment conditions only in the type of diet plan that was prescribed. Sample group leader protocols (week 2) for each treatment condition are included under “week 2” of the section “Low Carbohydrate.”
Groups were conducted by a registered dietitian or psychologist with experience in weight control. Group leaders attended an initial, 2-day, in-person training in Philadelphia, and all group leaders attended biweekly calls throughout the study. The calls were led by a psychologist with extensive experience in behavioral methods of weight control. The calls focused on any clarifications of the protocol and the discussion of nonadherent participants.
Low Carbohydrate
Week 2
-
Welcome (5 minutes)
Begin with reintroduction (names only). If new members, include reasons for weight loss as in week 1 but keep abbreviated and limit your comments.
Ask for volunteers to recall as many names as possible.
Address any questions left from last week.
Briefly review tonight’s agenda. This week we will focus on making changes in eating habits.
-
SAFE (Handout) (10 minutes)
Indicate that we want to provide a way for members to check in briefly at the beginning of each group. For the next few weeks, everyone will check in but over time (depending on the number of persons in the group, guest lecturers, etc), participants may take turns. Remind about the need to avoid spending too much time on any one individual.
-
SAFE was chosen to remind us that we want this to be a safe place to discuss eating and exercise habits. (Remind about confidentiality). It also reminds us about the key things to concentrate on each week.
S~self care—Important to view weight loss as self-care rather than as punitive. It’s something to do for yourself rather than some punishment that is imposed. Also important to develop non-food alternatives to nurture self. Each week participants to report things they did to take care of themselves that did not include food. Should be things focused on the participant rather than her/his family, job, etc. (e.g., massage, going to movie that they have been wanting to see, pedicure, manicure, small “gift”; being inaccessible to others for brief times; going home on time). See “Self-Care” handout for more examples. Part of long-term success is being nice to yourself. Complete “Self-Care” handout and pick at least one thing each week.
A~adherence—How were you able to achieve your goals this week? This includes skill development each week (slow eating, limiting times, etc) as well as individualized goals (special situations, behaviors from goal worksheet). Review particular successes or difficulties. This is a way to get individual attention as well as help the group sharpen its problem-solving skills.
F~food records—Review progress with keeping records of food and other activities. This is the primary tool of weekly assessment.
E~exercise—The physical activity that you performed this week (type, duration, frequency).
-
Note that W~(weight) is not included in the weekly review. Review reasons why weight is a poor short-term measure of success (Brownell, pp. 48–49).
salt intake
water shifts, menstrual cycle, humidity
no relation between weight and weekly behavior
Focus on SAFE and weight loss will follow.
Next week, we will use SAFE to check in.
-
Skill Review (15 minutes)
Ask participants to describe rationale for self-monitoring from week 1 (Brownell, pp. 14–15).
-
Ask about participants’ experiences with recording.
Was it helpful?
What patterns emerged?
What were the barriers to recording?
What were participants’ experiences with recording in previous programs?
Was it difficult to record overeating episodes?
Did friends or family members comment about record keeping?
It is especially important that participants believe in the utility of keeping records, so be sure to assess this before suggesting ways to record better. Focus on any barriers (time, size of record booklets, embarrassment, forgetting) with specific suggestions. Use group to come up with benefits and suggestions. Emphasize that this is a skill that is critical for individualized treatment.
Review the weekly food records and how to complete them (time, amount, type and description of food, add carbohydrates this week). Stress importance of recording ASAP after eating or it will be difficult to recall. Tally carbohydrate later if necessary. Recommend that they subtotal carbohydrate throughout the day. They can calculate carbohydrate using the carbohydrate counter we will distribute tonight. Briefly review how the book is organized.
Indicate that you will collect food record booklets each week and make brief comments about any patterns you observe. This review should be brief (2 minutes) and include positive comments. Emphasize that these records are for the participants’ benefit not yours. You are trying to provide a structure to make record keeping easier.
-
Goal Setting (15 minutes) (Brownell, pp. 61–62)
-
Weight
Ask participants to think about how much weight they expect to lose over the next 20 weeks. Record them on the board. Ask several participants to describe how they arrived at their numbers. Point out that they are probably making assumptions about the benefits (e.g., losing 40 pounds will make me feel/look twice as good as losing 20 pounds) as well as the costs (e.g., losing the second 20 pounds will be similar to losing the first 20 pounds). Are these assumptions about additional weight loss correct? Review faulty assumptions briefly. Avoid getting into a contest of wills about how much weight people can or should lose. Ultimately, the decision is the participant’s.
Compare participants’ goals on the board to what can be reasonably expected (1–2 lb per week) (see Brownell, p. 38). Use Brownell diagram (pp. 100–101) to illustrate that when outcomes (what is achieved) do not match goals (what is expected) there are typically negative effects on self-evaluation. How would participants feel if they did not reach their desired weight goals? Probably tend to blame self rather than program or unrealistic goals. Use examples (based on their weight goals) of how same outcomes can be viewed differently based on what was expected.
Actual weight loss will vary due to differences in weight, metabolism and genetics (we will review causes of overweight next week). Typical weight loss is 1–2 pounds per week (see Atkins, p. 177). Rather than setting a final weight goal now, we recommend that participants focus on behavior change and observe what weight loss is accomplished. Weight loss after week 12 will probably be representative of monthly weight loss during the program.
We recommend an initial goal of a 10% reduction because it is associated with improvements in medical conditions and most persons can achieve it with modest changes in eating and exercise. When 10% is reached, another goal can be set based on costs/benefits. Remind participants that body composition will be measured at week 26 so they can make an informed decision about further weight loss. It is impossible and imprudent to set a long-term weight goal now because of the lack of information about costs/benefits.
-
Behavior
-
Have participants think about one change in their eating that they would like to make (over the next 4 weeks) that would lead to weight loss. Use several examples to discuss the following characteristics of effective goal setting (see handout).
specific—define precisely what is to be accomplished. Specific goals such as “walk two times this week after work on Tuesday and Thursday in the park are more likely to be accomplished that general ones such as, “walk more this week.” Similarly, “eat 20 grams of carbohydrate per day” is more likely to be accomplished than “eat less carbohydrate this week.”
reasonable—make small changes. If you’re not walking at all, do not try to walk every day. The smaller the difference between your current behavior and your goal behavior the greater the chances you will accomplish it. Small successes lead to big successes.
active—define your goals in terms of what to do rather than what not to do. For example, “eat every four hours” instead of “stop going all day without eating” or “walk after dinner” rather than “stop lying on the couch after dinner.”
short-term—assess your goals over short intervals (no more than a week). Sometimes, even shorter intervals are helpful (day by day). Reviewing your progress after short periods will enable you to review your accomplishments and troubleshoot any difficulties.
limited—select no more than two goals per week. Selecting more will decrease your focus and make adherence more difficult. Once your goals have been accomplished and maintained, you can select new goals.
record—it is helpful to keep a written record of your goals and progress each week. It will increase awareness of your goal and provide an accurate record of your progress. The simplest and easiest records work best. Do what works for you.
Instruct each participant to select one behavioral goal for the next week (using the handout as a guide) and have them record it in the front of their weekly record. There will not be time in group to review each goal. They will discuss this goal under “A” of SAFE next week.
-
-
-
Weight Loss: Short-Term Dieting Versus Long-Term Behavior Change (10 minutes)
-
Before establishing a carbohydrate prescription for weight loss, let’s review how this approach to long-term weight control differs from dieting. (Brownell, pp. 6–7, 12–13).
Diets are all-or-none. For many people a diet implies short-term dietary change. You’re either on the diet or off the diet; you’ve had either a good day or a bad day. There is no middle ground (Brownell, pp. 220– 221).
Long-term weight control is based on a regular pattern of eating that avoids extremes and deprivation. It is important to note that the Induction stage of the program is only a temporary period designed to initiate the process of consuming a low carbohydrate diet. Subsequent stages of the program incorporate a larger variety of foods. Long-term weight control emphasizes changes that last. It is based on choosing foods that you enjoy while making healthy carbohydrate choices. The basic theme of any good nutritional approach is adaptability. Adding new carbohydrate containing foods slowly and carefully will help you learn good eating habits. You will be less prone to feeling hungry, irritated, and unhappy. These are feelings that lead to overeating.
-
Some days will be better than others; it is not realistic to assume that you should eat the same amount every day. The goal is to consume a variety of acceptable foods that you enjoy. The goal is not perfection. Eating is not a moral issue. It is inaccurate an ineffective to make self-evaluations based on eating and exercise behavior.
-
VI
Induction (15 minutes) (Atkins’ New Diet Revolution, pp. 121–144)
-
Review principles of Induction:
To switch from a high carbohydrate eating plan to a controlled carbohydrate eating plan.
To lose weight while eating palatable foods.
To realize that the Induction phase is not going to be your lifelong way of eating.
-
In order for Induction to work, it must be followed precisely; therefore, we suggest that participants follow these guidelines (see Induction Guidelines handout, also on pages 122–124 in Atkins’ New Diet Revolution):
Eat 3 regular-size meals a day or 4–5 smaller meals and do not go for more than 4 waking hours without eating.
Eat liberal amounts of fat and protein foods (i.e., poultry, fish, shellfish, red meat). When you consume fat, use butter, mayonnaise, olive oil, safflower oil, sunflower oil and other vegetable oils rather than margarine. See pamphlet for rules regarding egg and shellfish consumption.
Eat no more than 20 grams of carbohydrate per day (primarily in the form of salad greens or other permitted vegetables).
Do not eat any fruit, bread, pasta, grains, starchy vegetables, dairy product (other than cheese, cream, or butter), or protein/carbohydrate foods (legumes).
Only eat acceptable foods (group leaders, see Atkins, pp. 124–129) listed in the Instruction for Induction pamphlet.
Adjust quantities of non-carbohydrate containing foods to suit your appetite (amount that makes you feel satisfied, not stuffed).
Read food labels and check carbohydrate content (see Be a Carbohydrate Detective handout).
Be aware for hidden carbohydrates in gravies, sauces, and dressings when eating out. For example, gravy is often made with flour or cornstarch and sugar is sometimes added to salad dressings.
Drink at least eight 8-oz glasses of water per day (for hydration, to avoid constipation).
-
Alcohol is not a source of nutritive carbohydrate and shouldn’t be consumed in place of food (Atkins for Life, p. 46). Alcoholic beverages should be avoided during Induction for a variety of reasons:
acts as alternate fuel source
decreases hydration
decreases self-control
Do not try to do a low-fat version of the program as it will disrupt weight loss (Atkins, p. 127).
In addition to these rules, we ask you to also take a multivitamin each day.
This approach counts carbohydrates rather than calories. Although you will not be counting calories, calories do matter. Gaining weight results from eating more calories than you burn, so eat until satisfied and do not gorge (Atkins, p. 143).
-
-
VII
Carbohydrate Counting (10 minutes)
-
Explain the general concept of carbohydrate counting using a household budget or bank account as a model. Review the basic principles of carbohydrate counting.
You receive a 20-gram carbohydrate deposit each day, which you can spend according to your own personal preferences. You decide how to spend your carbohydrates. This will require you to consider how much you enjoy a particular food versus what it costs. You can have 4 cups of salad vegetables per day. However, if you would like to add vegetables that contain slightly higher carbohydrate contents (limited to 1 cup per day), you must reduce your intake of salad vegetables from 4 to 3 cups per day. Emphasize that participants cannot simply eat the maximum amount of servings from each food group listed in the pamphlet because they will likely go over the 20-gram limit. The sample menu handout provides some meal ideas during Induction.
Using your carbohydrate counter and food labels, record the number of carbohydrates that you spend each day in your weekly record.
Using the handout, briefly review key aspects of the food label and review how to calculate net carbohydrate.
Tally your carbohydrate consumption. The key factor is to consume no more than 20 grams of carbohydrate per day. Point out that grams of carbohydrate are based on serving size, so measuring utensils and scale (distributed during baseline food intake measurement) should be used to accurately determine the amount consumed. Need to weigh and measure foods in the short-term (2 weeks) to become accustomed to actual portions. Review guidelines for estimating portion sizes when measuring utensils are not available (see weekly record). Over time, can perform occasional checks or weigh novel foods.
Review two principal benefits of carbohydrate counting.
You can lose weight while eating high protein and/or fat foods. Fish, shellfish, fowl, meat, and butter are unrestricted.
Does not involve self-deprivation or hunger. This eating plan consists of a variety of foods that are palatable, pleasant, and filling (Atkins, pg. 5, 19, 32).
-
Tips for consuming a reduced carbohydrate diet.
Eliminate unnecessary, hidden carbohydrates, which you do not need such as sugar in sodas and coffee, and choose lower carbohydrate alternatives such as saccharin or sucralose. Limit sweeteners to 3 packets a day (Atkins, p. 144).
Plan ahead. Examine your schedule and prime your environment. Stock up on low carbohydrate snacks and eliminate high carbohydrate temptations. Some examples of low carbohydrate snacks are turkey and cheese roll, single serving can of tuna, hard boiled eggs, Laughing Cow cheese or string cheese, seeds, nuts or nut butter on a celery stick, and sugar free Jello. Carbohydrate controlled foods are generally found around the periphery of the grocery store.
Avoid deprivation. Eat regularly (every 4 hours) to prevent hunger. Have a small carbohydrate controlled snack high in fat or protein if you are hungry between meals (Atkins, p. 153).
Eat primarily unprocessed foods but when you eat packaged foods (i.e., cheese), read the food labels carefully. Generally, “low fat” means “high carbohydrate.”
-
NOTE: Although ATKINS Ready to Drink Shakes (up to 1 per day), ATKINS Shake Mix (up to 2 scoops per day), and ATKINS ADVANTAGE BARS (up to 1 per day) can be consumed in place of whole foods during Induction, this option should only be initiated when it has been determined that the individual cannot incorporate whole foods into his/her eating plan (like during crunch times). At this point it would be premature to offer this as an option. ATKINS Endulge products cannot be consumed during Induction.
-
F
Inform participants that they may experience some undesirable symptoms (i.e., headaches, constipation) after the second day of Induction (see back of Instructions for Induction sheet). Call participants after the third day of Induction and ask about their progress and whether they are experiencing any problems. Do not specifically ask about symptoms. Example: “I am calling to see how you are doing on your new eating plan and to find out whether you have any questions or are experiencing any problems so far.”
-
VIII
Skill Building (Handout) (5 minutes)
Follow Induction diet.
Take one multivitamin each day.
Record all food (time, amount, type and description of food, carbohydrates).
Use the carbohydrate counter and food labels to determine carbohydrate intake. Key thing is to eat a wide variety of acceptable foods.
Record one personal goal for this week in the beginning of the weekly record and assess progress as appropriate.
-
IX
Handouts
SAFE Handout
Self-Care Handout
Effective Goal Setting Handout
Induction Guidelines Handout
Instructions for Induction Pamphlet
Sample Menus
Be a Carbohydrate Detective Handout
Carbohydrate Gram Counter Handout
Skill Builder
Weekly Record
Low Fat
Week 2
-
Welcome (5 minutes)
Begin with reintroduction (names only). If new members, include reasons for weight loss as in week 1, but keep abbreviated and limit your comments.
Ask for volunteers to recall as many names as possible.
Address any questions left from last week.
Briefly review tonight’s agenda. This week we will focus on making changes in eating habits.
-
SAFE (Handout) (10 minutes)
Indicate that we want to provide a way for members to check in briefly at the beginning of each group. For the next few weeks, everyone will check in but over time (depending on the number of persons in the group, guest lecturers, etc), participants may take turns. Remind about the need to avoid spending too much time on any one individual.
-
SAFE was chosen to remind us that we want this to be a safe place to discuss eating and exercise habits. (Remind about confidentiality). It also reminds us about the key things to concentrate on each week.
S~self care—Important to view weight loss as selfcare rather than as punitive. It’s something to do for yourself rather than some punishment that is imposed. Also important to develop non-food alternatives to nurture self. Each week participants to report things they did to take care of themselves that did not include food. Should be things focused on the participant rather than her/his family, job, etc. (e.g., massage, going to movie that they have been wanting to see, pedicure, manicure, small “gift”; being inaccessible to others for brief times; going home on time). See “Self-Care” handout for more examples. Part of long-term success is being nice to yourself. Complete “Self-Care” handout and pick at least one thing each week.
A~adherence—How were you able to achieve your goals this week? This includes skill development each week (slow eating, limiting times, etc) as well as individualized goals (special situations, behaviors from goal worksheet). Review particular successes or difficulties. This is a way to get individual attention as well as help the group sharpen its problem-solving skills.
F~food records—Review progress with keeping records of food, exercise and other activities. This is the primary tool of weekly assessment.
E~exercise—The physical activity that you performed this week (type, duration, frequency).
-
Note that W~(Weight) is not included in the weekly review. Review reasons why weight is a poor short-term measure of success (Brownell, pp. 48–49).
salt intake
water shifts, menstrual cycle, humidity
no relation between weight and weekly behavior
Focus on SAFE and weight loss will follow.
Next week, we will use SAFE to check in.
-
Skill Review (10 minutes)
Ask participants to describe rationale for self-monitoring from week 1 (Brownell, pp. 14–15).
-
Ask about participants’ experiences with recording.
Was it helpful?
What patterns emerged?
What were the barriers to recording?
Did they have difficulty estimating portions?
What were participants’ experiences with recording in previous programs?
Was it difficult to record overeating episodes?
Did friends or family members comment about record keeping?
It is especially important that participants believe in the utility of keeping records, so be sure to assess this before suggesting ways to record better. Focus on any barriers (time, size of record booklets, embarrassment, forgetting) with specific suggestions. Use group to come up with benefits and suggestions. Emphasize that this is a skill that is critical for individualized treatment.
Review the new food record booklets and how to complete them (time, amount, type and description, add calories this week). Stress importance of recording ASAP after eating or it will be difficult to recall. Tally calories later if necessary. Recommend that they subtotal calories throughout the day. They can calculate calories using the calorie counter we will distribute tonight. Briefly review how the book is organized.
Indicate that you will collect food record booklets each week and make brief comments about any patterns you observe. This review should be brief (2 minutes) and include positive comments. Emphasize that these records are for the participants’ benefit not yours. You are trying to provide a structure to make record keeping easier.
-
Goal Setting (Brownell, pp. 61–62) (15 minutes)
-
Weight
Ask participants to think about how much weight they expect to lose over the next 20 weeks. Record them on the board. Ask several participants to describe how they arrived at their numbers. Point out that they are probably making assumptions about the benefits (e.g., losing 40 pounds will make me feel/ look twice as good as losing 20 pounds) as well as the costs (e.g., losing the second 20 pounds will be similar to losing the first 20 pounds). Are these assumptions about additional weight loss correct? Review faulty assumptions briefly. Avoid getting into a contest of wills about how much weight people can or should lose. Ultimately, the decision is the participant’s.
Compare participants’ goals on the board to what can be reasonably expected (1–2 lb per week) (see Brownell p. 38). Use Brownell diagram (pp. 100–101) to illustrate that when outcomes (what is achieved) do not match goals (what is expected) there are typically negative effects on self-evaluation. How would participants feel if they did not reach their desired weight goals? Probably tend to blame self rather than program or unrealistic goals. Use examples (based on their weight goals) of how same outcomes can be viewed differently based on what was expected.
Actual weight loss will vary due to differences in weight, metabolism and genetics (we will review causes of overweight next week). Typical weight loss is 1–2 pounds per week (see Brownell p. 38). Rather than setting a final weight goal now, we recommend that participants focus on behavior change and observe what weight loss is accomplished. Weight loss after week 12 will probably be representative of monthly weight loss during the program.
We recommend an initial goal of a 10% reduction because it is associated with improvements in medical conditions and most persons can achieve it with modest changes in eating and exercise. When 10% is reached, another goal can be set based on costs/benefits. Remind participants that body composition will be measured at week 26 so they can make an informed decision about further weight loss. It is impossible and imprudent to set a long-term weight goal now because of the lack of information about costs/benefits.
-
Behavior
-
Have participants think about one change in their eating that they would like to make (over the next 4 weeks) that would lead to weight loss. Use several examples to discuss the following characteristics of effective goal setting (see handout).
specific—define precisely what is to be accomplished. Specific goals such as “walk two times this week after work on Tuesday and Thursday in the park are more likely to be accomplished that general ones such as, “walk more this week.” Similarly, “eat 1200–1400 calories per day” is more likely to be accomplished than “eat less this week.”
reasonable—make small changes. If you’re not walking at all, do not try to walk every day. If you’re eating 10 candy bars each week, do not attempt to eat only 2. The smaller the difference between your current behavior and your goal behavior the greater the chances you will accomplish it. Small successes lead to big successes.
active—define your goals in terms of what to do rather than what not to do. For example, “eat every four hours” instead of “stop going all day without eating” or “walk after dinner” rather than “stop lying on the couch after dinner.”
short-term—assess your goals over short intervals (no more than a week). Sometimes, even shorter intervals are helpful (day by day). Reviewing your progress after short periods will enable you to review your accomplishments and troubleshoot any difficulties.
limited—select no more than two goals per week. Selecting more will decrease your focus and make adherence more difficult. Once your goals have been accomplished and maintained, you can select new goals.
record—it is helpful to keep a written record of your goals and progress each week. It will increase awareness of your goal and provide an accurate record of your progress. The simplest and easiest records work best. Do what works for you.
Instruct each participant to select one behavioral goal for the next week (using the handout as a guide) and have them record it in the front of their weekly record. There will not be time in group to review each goal. They will discuss this goal under “A” of SAFE next week.
-
-
-
Weight Loss: Short-Term Dieting Versus Long-Term Behavior Change (10 minutes)
-
Before establishing a caloric prescription for weight loss, let’s review how our approach to long-term weight control differs from dieting (Brownell, pp. 6–7, 12–13).
Diets are all-or-none. For many people a diet implies short-term dietary change. You’re either on the diet or off the diet; you’ve had either a good day or a bad day. There is no middle ground (Brownell, pp. 220–221). Furthermore, many diets are based on fads, extremes, and severe deprivation. As such, they are only successful in the short-term. Can only make dramatic changes for a short time.
Long-term weight control is based on a regular pattern of eating that avoids extremes and deprivation. It emphasizes small changes that last. It is based on choosing foods that you enjoy while staying within the boundaries of daily caloric allowances. There are no forbidden foods. Allow yourself to have some special foods or treats and work them into daily/weekly/monthly allowances. You will be less prone to feeling deprived, irritated, unhappy. These are feelings that lead to overeating.
There are no absolutes (never, always, must) in successful weight control. Some days will be better than others; it is not realistic to assume that you should eat the same amount every day. The goal is to consume a variety of foods that you can enjoy and sustain balanced eating over the long haul. The goal is not perfection. Eating is not a moral issue. It is inaccurate and ineffective to make self-evaluations based on eating and exercise behavior.
-
-
Picking a Calorie Range (10 minutes) (Brownell, pp. 44–46)
-
Review principles of energy balance:
Intake > Output = Weight Gain
Intake < Output = Weight Loss
Intake = Output = Weight Maintenance
3500 calories = 1 pound. To lose one pound/week, you’ll need to eat 3500 calories less than you burn. Easier to decrease intake than to increase output (i.e., easier to eat 500 calories less per day than it is to exercise 500 calories more per day). Give examples. That’s why exercise alone is not the best method for weight loss. Regular physical activity, however, is the best predictor of maintaining weight loss.
We are recommending a calorie range (1200–1500 calories/d for women and 1500–1800 calories/d for men); participants will decide how to “spend” those calories using the principles below. As above, need to observe weight loss over 1-month period.
-
-
A Calorie Account (10 minutes)
-
Explain the general concept of a calorie account using a household budget or bank account as a model. Review the basic principles of using a calorie account. The sample menu handouts provide some meal ideas.
You receive a 1200–1500 (women) or 1500–1800 (men) calorie deposit each day, which you can spend according to your own personal preferences. You decide how to spend your calories. This will require you to consider how much you enjoy a particular food versus what it costs calorically.
Using your fat and calorie counter, record the number of calories that you spend each day in your weekly record. Point out that calories are based on serving size, so measuring utensils and scale (distributed during baseline food intake measurement) should be used to accurately determine the amount consumed. Need to weigh and measure foods in the short-term (2 weeks) to become accustomed to actual portions. Review guidelines for estimating portion sizes when measuring utensils are not available (see weekly record). Over time, can perform occasional checks or weigh novel foods. Briefly review some basic components (e.g. serving size, calories) of the food label using the “Be a Calorie Detective” handout.
Tally your calorie account as expenditures are made. Although you are allotted a certain number or calories each day, you will not spend this amount every day. You can save calories for special occasions, just as you save money. For example, a person could save 100 calories per day, Monday through Friday, and spend the extra 500 calories over the weekend. You may also spend less to adjust for an unusually large expenditure.
The key factor is that the calorie ledger must balance (i.e., average 1200–1500 calories/day or 1500–1800 calories/day) in order for your rate of weight loss to remain constant. It is best to review your ledger for a weekly balance (8400 –10,500 calories per week).
-
Review two principal benefits of keeping a calorie account.
Allows for flexibility and variety.
No single overeating episode is paramount, since you can balance your calorie account with adjustments.
-
Tips for reducing caloric intake.
Reduce or eliminate unnecessary, hidden calories which you do not need or really enjoy (e.g., eliminate butter, sugar in sodas and coffee, reduce use of cream, choose lower calorie alternatives if similar taste).
Plan ahead. Examine your schedule and prime your environment. Stock up on low calorie snacks and eliminate high fat and calorie temptations. Be conscious of the caloric cost of food choices. Are the calories worth it? If they are, fine; if not, skip it or choose an alternative. Examples of low calorie snacks can be found on the handout. Encourage participants to consume a variety of foods.
-
NOTE: Although meal replacement shakes and bars (e.g., Slim-Fast) can be consumed in place of whole foods, this option should only be initiated when it has been determined that the individual cannot incorporate whole foods into his/her eating plan (like during crunch times). At this point it would be premature to offer this as an option.
-
3
Avoid deprivation. It’s a short-term solution to a long-term problem. Do not totally eliminate foods that you really enjoy. Find a way to work them in. Make changes that you can live with.
-
4
Eat regularly (every 4 hours) to prevent hunger. This will be addressed in greater detail next week when we discuss developing an eating schedule.
-
D
Remind participants to take a multivitamin each day.
-
E
Inform participants that they may experience some undesirable symptoms. Call participants within the first three days to ask about their progress and whether they are experiencing any problems. Do not specifically ask about symptoms. Example: “I am calling to see how you are doing on your new eating plan and to find out whether you have any questions or are experiencing any problems so far.”
-
VIII
Skill Building (Handout) (5 minutes)
Eat a diet that is consistent with your calorie goal (1200–1500 calories per day for women, 1500–1800 calories per day for men).
Record all food (time, amount, type and description, calories).
Use the calorie counter and food labels to determine calorie intake. Key thing is to eat a wide variety of foods.
Record one personal goal for this week in beginning of the food record and assess progress as appropriate.
-
IX
Handouts
SAFE Handout
Self-Care Handout
Effective Goal Setting Handout
Tip the Calorie Balance Handout
Be a Calorie Detective Handout
Enjoy the Variety-Healthy Food Choices
Sample Menus
Calorie King
Skill Builder
Weekly Record
Appendix Table.
Serious Adverse Events Among 307 Participants Over 2 Years
| Week | Study Group | Event | Related |
|---|---|---|---|
| 48 | Low-fat diet | Right and left knee replacement | No |
| 43 | Low-fat diet | Severe allergic reaction to trimethoprim–sulfamethoxazole | No |
| 80 | Low-fat diet | Cellulitis from dog bite | No |
| 7 | Low-carbohydrate diet | Ovarian mass | No |
| 20 | Low-carbohydrate diet | Renal stones or diverticulitis | Possibly, but not likely; weight loss was 1.09 kg at 20 wk |
| 39 | Low-fat diet | Umbilical hernia repaired | No |
| 56 | Low-carbohydrate diet | Torn left meniscus | Possibly due to prescribed exercise program |
Footnotes
Author Contributions: Conception and design: G.D. Foster, H.R. Wyatt, J.O. Hill, A.P. Makris, C. Brill, D.J. Rader, T.A. Wadden, S. Klein.
Analysis and interpretation of the data: G.D. Foster, H.R. Wyatt, J.O. Hill, D.J. Rader, B. Zemel, T. Tenhave, C.W. Newcomb, S. Klein.
Drafting of the article: G.D. Foster, H.R. Wyatt, J.O. Hill, B. Zemel, T. Tenhave, S. Klein.
Critical revision of the article for important intellectual content: G.D. Foster, H.R. Wyatt, J.O. Hill, A.P. Makris, D.L. Rosenbaum, R.I. Stein, B.S. Mohammed, B. Miller, D.J. Rader, T.A. Wadden, S. Klein.
Final approval of the article: G.D. Foster, H.R. Wyatt, J.O. Hill, C. Brill, R.I. Stein, B.S. Mohammed, B. Miller, D.J. Rader, T.A. Wadden, T. Tenhave, C.W. Newcomb, S. Klein.
Provision of study materials or patients: G.D. Foster, H.R. Wyatt, J.O. Hill, B.S. Mohammed, B. Miller, T.A. Wadden.
Statistical expertise: T. Tenhave, C.W. Newcomb.
Obtaining of funding: G.D. Foster, H.R. Wyatt, J.O. Hill, T. Tenhave, S. Klein.
Administrative, technical, or logistic support: G.D. Foster, J.O. Hill, D.L. Rosenbaum, C. Brill, R.I. Stein, B.S. Mohammed, B. Miller, B. Zemel, T. Tenhave, S. Klein.
Collection and assembly of data: G.D. Foster, H.R. Wyatt, J.O. Hill, A.P. Makris, D.L. Rosenbaum, C. Brill, R.I. Stein, B.S. Mohammed, B. Miller, D.J. Rader, B. Zemel, T.A. Wadden, S. Klein.
Note: Dr. Foster had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Potential Conflicts of Interest: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M09-1901.
Reproducible Research Statement: Study protocol: Available from Dr. Foster (gfoster@temple.edu). Statistical code: Available from Dr. Tenhave (ttenhave@upenn.edu). Data set: Available from Dr. Foster (gfoster@temple.edu), subject to study group approval and National Institutes of Health policy.
References
- 1.Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348:2082–90. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- 2.Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;348:2074–81. doi: 10.1056/NEJMoa022637. [DOI] [PubMed] [Google Scholar]
- 3.Brehm BJ, Seeley RJ, Daniels SR, D’Alessio DA. A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J Clin Endocrinol Metab. 2003;88:1617–23. doi: 10.1210/jc.2002-021480. [DOI] [PubMed] [Google Scholar]
- 4.Yancy WS, Jr, Olsen MK, Guyton JR, Bakst RP, Westman EC. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med. 2004;140:769–77. doi: 10.7326/0003-4819-140-10-200405180-00006. [DOI] [PubMed] [Google Scholar]
- 5.Gardner CD, Kiazand A, Alhassan S, Kim S, Stafford RS, Balise RR, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297:969–77. doi: 10.1001/jama.297.9.969. [DOI] [PubMed] [Google Scholar]
- 6.Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, et al. Dietary Intervention Randomized Controlled Trial (DIRECT) Group. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359:229–41. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 7.Stern L, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, et al. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: one-year follow-up of a randomized trial. Ann Intern Med. 2004;140:778–85. doi: 10.7326/0003-4819-140-10-200405180-00007. [DOI] [PubMed] [Google Scholar]
- 8.Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293:43–53. doi: 10.1001/jama.293.1.43. [DOI] [PubMed] [Google Scholar]
- 9.Yancy WS, Jr, Westman EC, McDuffie JR, Grambow SC, Jeffreys AS, Bolton J, et al. A randomized trial of a low-carbohydrate diet vs orlistat plus a low-fat diet for weight loss. Arch Intern Med. 2010;170:136–45. doi: 10.1001/archinternmed.2009.492. [DOI] [PubMed] [Google Scholar]
- 10.Malik VS, Hu FB. Popular weight-loss diets: from evidence to practice. Nat Clin Pract Cardiovasc Med. 2007;4:34–41. doi: 10.1038/ncpcardio0726. [DOI] [PubMed] [Google Scholar]
- 11.Makris AP, Foster GD, Astrup A. Diet composition and weight loss. In: Bray GA, Bouchard C, editors. Handbook of Obesity. Clinical Applications. 3. New York: Informa Healthcare USA; 2008. pp. 269–90. [Google Scholar]
- 12.Nordmann AJ, Nordmann A, Briel M, Keller U, Yancy WS, Jr, Brehm BJ. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:285–93. doi: 10.1001/archinte.166.3.285. [DOI] [PubMed] [Google Scholar]
- 13.Foster GD, Makris AP, Bailer BA. Behavioral treatment of obesity. Am J Clin Nutr. 2005;82:230S–235S. doi: 10.1093/ajcn/82.1.230S. [DOI] [PubMed] [Google Scholar]
- 14.Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology. 2007;132:2226–38. doi: 10.1053/j.gastro.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 15.Atkins RC. Dr. Atkins’ New Diet Revolution. New York: Avon Books; 1998. [Google Scholar]
- 16.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–5. [PubMed] [Google Scholar]
- 17.Wadden TA, Stunkard AJ, Day SC, Gould RA, Rubin CJ. Less food, less hunger: reports of appetite and symptoms in a controlled study of a protein-sparing modified fast. Int J Obes. 1987;11:239–49. [PubMed] [Google Scholar]
- 18.Little RJ. Modeling the drop-out mechanism in repeated-measures studies. J Am Stat Assoc. 1995;90:1112–21. [Google Scholar]
- 19.James WP, Astrup A, Finer N, Hilsted J, Kopelman P, Rössner S, et al. Effect of sibutramine on weight maintenance after weight loss: a randomised trial. STORM Study Group. Sibutramine Trial of Obesity Reduction and Maintenance. Lancet. 2000;356:2119–25. doi: 10.1016/s0140-6736(00)03491-7. [DOI] [PubMed] [Google Scholar]
- 20.Sjöström L, Rissanen A, Andersen T, Boldrin M, Golay A, Koppeschaar HP, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet. 1998;352:167–72. doi: 10.1016/s0140-6736(97)11509-4. [DOI] [PubMed] [Google Scholar]
- 21.Yang MU, Van Itallie TB. Composition of weight lost during short-term weight reduction. Metabolic responses of obese subjects to starvation and low-calorie ketogenic and nonketogenic diets. J Clin Invest. 1976;58:722–30. doi: 10.1172/JCI108519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golay A, Allaz AF, Morel Y, de Tonnac N, Tankova S, Reaven G. Similar weight loss with low- or high-carbohydrate diets. Am J Clin Nutr. 1996;63:174–8. doi: 10.1093/ajcn/63.2.174. [DOI] [PubMed] [Google Scholar]
- 23.Boden G, Sargrad K, Homko C, Mozzoli M, Stein TP. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann Intern Med. 2005;142:403–11. doi: 10.7326/0003-4819-142-6-200503150-00006. [DOI] [PubMed] [Google Scholar]
- 24.Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res. 2002;43:1363–79. doi: 10.1194/jlr.r200004-jlr200. [DOI] [PubMed] [Google Scholar]
- 25.Krauss RM, Blanche PJ, Rawlings RS, Fernstrom HS, Williams PT. Separate effects of reduced carbohydrate intake and weight loss on atherogenic dyslipidemia. Am J Clin Nutr. 2006;83:1025–31. doi: 10.1093/ajcn/83.5.1025. [DOI] [PubMed] [Google Scholar]
- 26.Dattilo DJ, Ige JT, Nwana EJ. Intraoral lipoma of the tongue and submandibular space: report of a case. J Oral Maxillofac Surg. 1996;54:915–7. doi: 10.1016/s0278-2391(96)90549-2. [DOI] [PubMed] [Google Scholar]
- 27.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 28.Szapary PO, Rader DJ. Pharmacological management of high triglycerides and low high-density lipoprotein cholesterol. Curr Opin Pharmacol. 2001;1:113–20. doi: 10.1016/s1471-4892(01)00028-5. [DOI] [PubMed] [Google Scholar]
- 29.Kris-Etherton PM, Etherton TD, Yu S. Efficacy of multiple dietary therapies in reducing cardiovascular disease risk factors [Editorial] Am J Clin Nutr. 1997;65:560–1. doi: 10.1093/ajcn/65.2.560. [DOI] [PubMed] [Google Scholar]
- 30.Villareal DT, Fontana L, Weiss EP, Racette SB, Steger-May K, Schechtman KB, et al. Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss: a randomized controlled trial. Arch Intern Med. 2006;166:2502–10. doi: 10.1001/archinte.166.22.2502. [DOI] [PubMed] [Google Scholar]
- 31.Adam-Perrot A, Clifton P, Brouns F. Low-carbohydrate diets: nutritional and physiological aspects. Obes Rev. 2006;7:49–58. doi: 10.1111/j.1467-789X.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- 32.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. N Engl J Med. 2006;355:1563–71. doi: 10.1056/NEJMoa061883. [DOI] [PubMed] [Google Scholar]
- 33.Svetkey LP, Stevens VJ, Brantley PJ, Appel LJ, Hollis JF, Loria CM, et al. Weight Loss Maintenance Collaborative Research Group. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299:1139–48. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]


