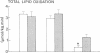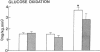Abstract
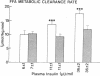
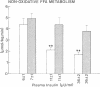
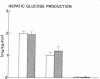
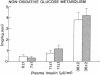
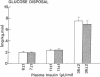
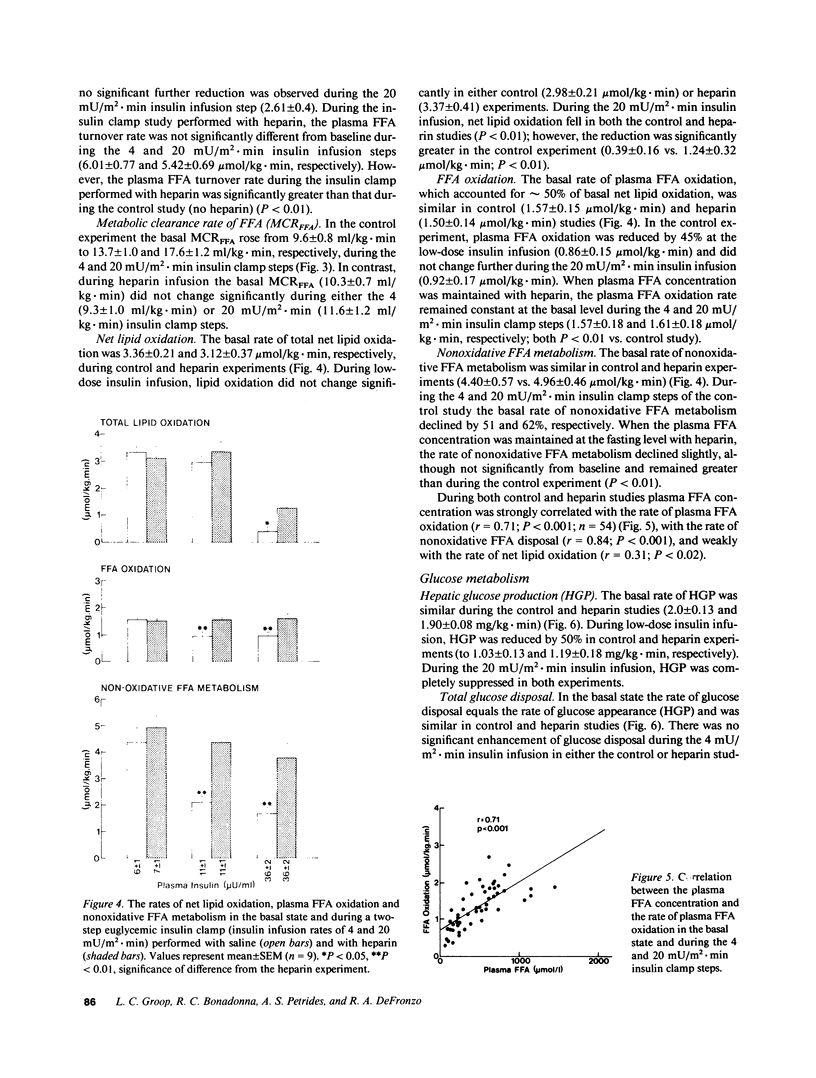
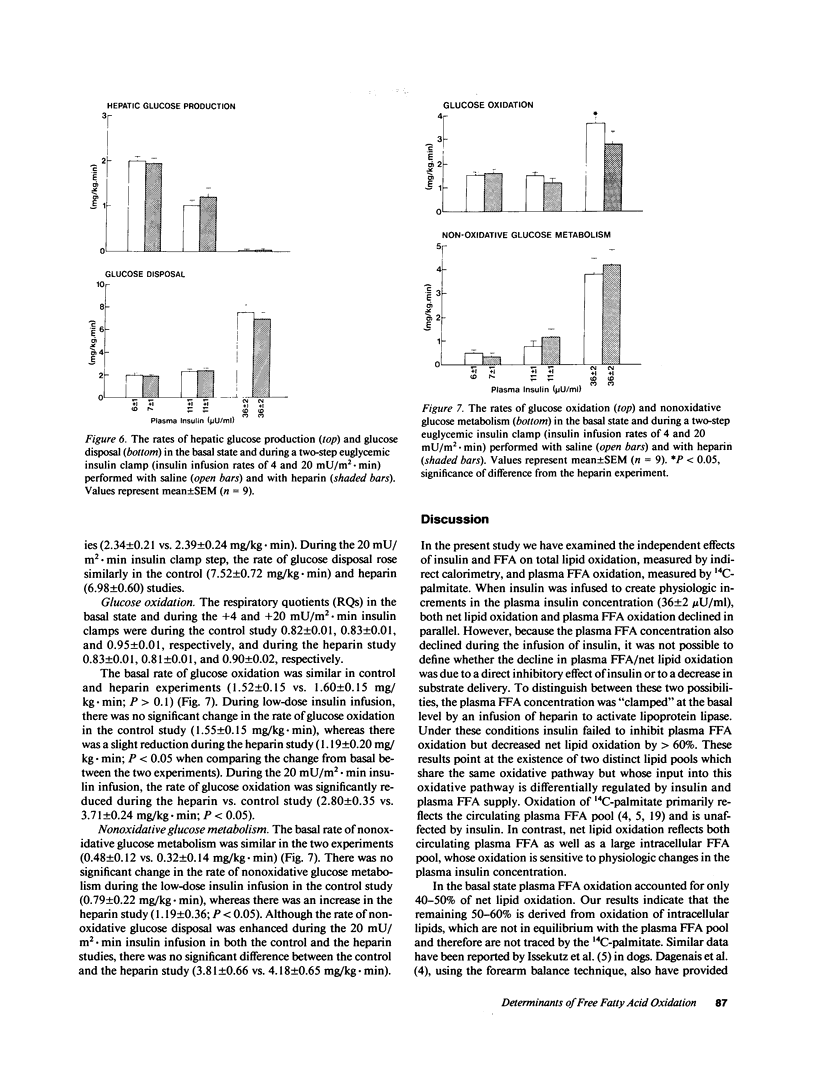
Plasma FFA oxidation (measured by infusion of 14C-palmitate) and net lipid oxidation (indirect calorimetry) are both inhibited by insulin. The present study was designed to examine whether these insulin-mediated effects on lipid metabolism resulted from a decline in circulating FFA levels or from a direct action of the hormone on FFA/lipid oxidation. Nine subjects participated in two euglycemic insulin clamps, performed with and without heparin. During each insulin clamp study insulin was infused at two rates, 4 and 20 mU/m2.min for 120 min. The studies were performed with indirect calorimetry and 3-3H-glucose and 14C-palmitate infusion. During the control study plasma FFA fell from 610 +/- 46 to 232 +/- 42 to 154 +/- 27 mumol/liter, respectively. When heparin was infused basal plasma FFA concentration remained constant. During the control study, FFA/lipid oxidation rates decreased in parallel with the fall in the plasma FFA concentration. During the insulin/heparin study, plasma 14C-FFA oxidation remained unchanged while net lipid oxidation decreased. In conclusion, when the plasma FFA concentration is maintained unchanged by heparin infusion, insulin has no direct effect on FFA turnover and disposal. These results thus suggest that plasma FFA oxidation is primarily determined by the plasma FFA concentration, while net lipid oxidation is regulated by both the plasma FFA and the insulin level.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG D. T., STEELE R., ALTSZULER N., DUNN A., BISHOP J. S., DE BODO R. C. Regulation of plasma free fatty acid turnover. Am J Physiol. 1961 Jul;201:9–15. doi: 10.1152/ajplegacy.1961.201.1.9. [DOI] [PubMed] [Google Scholar]
- Altszuler N., Barkai A., Bjerknes C., Gottlieb B., Steele R. Glucose turnover values in the dog obtained with various species of labeled glucose. Am J Physiol. 1975 Dec;229(6):1662–1667. doi: 10.1152/ajplegacy.1975.229.6.1662. [DOI] [PubMed] [Google Scholar]
- Baker N., Rostami H. Effect of glucose feeding on net transport of plasma free fatty acids. J Lipid Res. 1969 Jan;10(1):83–90. [PubMed] [Google Scholar]
- Cobelli C., Mari A., Ferrannini E. Non-steady state: error analysis of Steele's model and developments for glucose kinetics. Am J Physiol. 1987 May;252(5 Pt 1):E679–E689. doi: 10.1152/ajpendo.1987.252.5.E679. [DOI] [PubMed] [Google Scholar]
- Dagenais G. R., Tancredi R. G., Zierler K. L. Free fatty acid oxidation by forearm muscle at rest, and evidence for an intramuscular lipid pool in the human forearm. J Clin Invest. 1976 Aug;58(2):421–431. doi: 10.1172/JCI108486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton R. P., Berman M., Steinberg D. Kinetic studies of plasma free fatty acid and triglyceride metabolism in man. J Clin Invest. 1969 Aug;48(8):1560–1579. doi: 10.1172/JCI106122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREDRICKSON D. S., GORDON R. S., Jr The metabolism of albumin-bound C14-labeled unesterified fatty acids in normal human subjects. J Clin Invest. 1958 Nov;37(11):1504–1515. doi: 10.1172/JCI103742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRITZ I. B. Factors influencing the rates of long-chain fatty acid oxidation and synthesis in mammalian systems. Physiol Rev. 1961 Jan;41:52–129. doi: 10.1152/physrev.1961.41.1.52. [DOI] [PubMed] [Google Scholar]
- Felber J. P., Ferrannini E., Golay A., Meyer H. U., Theibaud D., Curchod B., Maeder E., Jequier E., DeFronzo R. A. Role of lipid oxidation in pathogenesis of insulin resistance of obesity and type II diabetes. Diabetes. 1987 Nov;36(11):1341–1350. doi: 10.2337/diab.36.11.1341. [DOI] [PubMed] [Google Scholar]
- Ferrannini E., Barrett E. J., Bevilacqua S., DeFronzo R. A. Effect of fatty acids on glucose production and utilization in man. J Clin Invest. 1983 Nov;72(5):1737–1747. doi: 10.1172/JCI111133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrannini E. The theoretical bases of indirect calorimetry: a review. Metabolism. 1988 Mar;37(3):287–301. doi: 10.1016/0026-0495(88)90110-2. [DOI] [PubMed] [Google Scholar]
- GEORGE J. C., NAIK R. M. Relative distribution and chemical nature of the fuel store of the two types of fibres in the pectoralis major muscle of the pigeon. Nature. 1958 Mar 8;181(4610):709–711. doi: 10.1038/181709b0. [DOI] [PubMed] [Google Scholar]
- Groop L. C., Bonadonna R. C., DelPrato S., Ratheiser K., Zyck K., Ferrannini E., DeFronzo R. A. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest. 1989 Jul;84(1):205–213. doi: 10.1172/JCI114142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenfeldt L., Wahren J., Pernow B., Räf L. Uptake of individual free fatty acids by skeletal muscle and liver in man. J Clin Invest. 1972 Sep;51(9):2324–2330. doi: 10.1172/JCI107043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerr R. A., Yu Y. M., Wagner D. A., Burke J. F., Young V. R. Recovery of 13C in breath from NaH13CO3 infused by gut and vein: effect of feeding. Am J Physiol. 1989 Sep;257(3 Pt 1):E426–E438. doi: 10.1152/ajpendo.1989.257.3.E426. [DOI] [PubMed] [Google Scholar]
- ISSEKUTZ B., Jr, MILLER H. I., PAUL P., RODAHL K. SOURCE OF FAT OXIDATION IN EXERCISING DOGS. Am J Physiol. 1964 Sep;207:583–589. doi: 10.1152/ajplegacy.1964.207.3.583. [DOI] [PubMed] [Google Scholar]
- Miles J., Glasscock R., Aikens J., Gerich J., Haymond M. A microfluorometric method for the determination of free fatty acids in plasma. J Lipid Res. 1983 Jan;24(1):96–99. [PubMed] [Google Scholar]
- Miller H. I., Bortz W. M., Durham B. C. The rate of appearance of FFA in plasma triglyceride of normal and obese subjects. Metabolism. 1968 Jun;17(6):515–521. doi: 10.1016/0026-0495(68)90043-7. [DOI] [PubMed] [Google Scholar]
- Nestel P. J. Relationship between FFA flux and TGFA influx in plasma before and during the infusion of insulin. Metabolism. 1967 Dec;16(12):1123–1132. doi: 10.1016/0026-0495(67)90058-3. [DOI] [PubMed] [Google Scholar]
- RANDLE P. J., GARLAND P. B., HALES C. N., NEWSHOLME E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963 Apr 13;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Radziuk J., Norwich K. H., Vranic M. Measurement and validation of nonsteady turnover rates with applications to the inulin and glucose systems. Fed Proc. 1974 Jul;33(7):1855–1864. [PubMed] [Google Scholar]
- Robin A. P., Jeevanandam M., Elwyn D. H., Askanazi J., Kinney J. M. Measurement of fatty acid oxidation: validation of isotopic equilibrium extrapolation. Metabolism. 1989 Jan;38(1):67–72. doi: 10.1016/0026-0495(89)90182-0. [DOI] [PubMed] [Google Scholar]
- Robin A. P., Nordenström J., Askanazi J., Carpentier Y. A., Elwyn D. H., Kinney J. M. Influence of parenteral carbohydrate on fat oxidation in surgical patients. Surgery. 1984 May;95(5):608–618. [PubMed] [Google Scholar]
- Thiebaud D., Jacot E., DeFronzo R. A., Maeder E., Jequier E., Felber J. P. The effect of graded doses of insulin on total glucose uptake, glucose oxidation, and glucose storage in man. Diabetes. 1982 Nov;31(11):957–963. doi: 10.2337/diacare.31.11.957. [DOI] [PubMed] [Google Scholar]
- Waterhouse C., Baker N., Rostami H. Effect of glucose ingestion on the metabolism of free fatty acids in human subjects. J Lipid Res. 1969 Sep;10(5):487–494. [PubMed] [Google Scholar]