Abstract
This study investigated the functional MRI responses to graded hypoxia in awake/restrained and anesthetized animals by measuring cerebral blood flow (CBF) and blood oxygenation (BOLD) changes and estimating changes in cerebral metabolic rate of oxygen (CMRO2). Hypoxia in isoflurane anesthetized rats reduced blood pressure but did not change heart rate and respiration rate. In contrast, hypoxia in awake animals showed compensatory responses by sustaining blood pressure, increasing heart rate and respiration rate. Basal CBF was higher under isoflurane anesthesia than awake state because isoflurane is a vasodilator. Graded hypoxia decreased BOLD signals. Surprisingly, hypoxia also decreased CBF likely because hypoxia induced hypocapnia. Hypoxia-induced CBF and BOLD decreases were smaller in awake, relative to anesthetized, rats at low pO2, but similar at high pO2. CBF leveled off with decreasing hypoxia-induced pCO2 in awake rats, but monotonically decreased in anesthetized rats. CMRO2 estimated using a biophysical BOLD model did not change under mild hypoxia but was reduced under severe hypoxia relative to baseline. These results showed that isoflurane attenuated automomic responses to hypoxia, hypoxia-induced hypocapnia dominated CBF changes, tissues in awake conditions appeared better oxygenated, and severe hypoxia reduced oxygen metabolism. This study underscored the marked differences in BOLD and CBF MRI responses to hypoxia in vivo between awake and anesthetized conditions and has implications for functional MRI studies of hypoxia in anesthetized animal models.
Keywords: Awake fMRI, BOLD, CBF, CMRO2, Hypercapnia, Hyperoxia
1. Introduction
The mammalian brain depends on a continuous and adequate supply of oxygen to maintain its structural and functional integrity. If oxygen delivery is sufficiently compromised, loss of consciousness could occur within seconds, irreversible neuronal damage and severe brain dysfunction, within minutes (Siesjo, 1978). During hypoxia, there are systematic autonomic cerebrovascular responses, such as vasodilation and increases in respiration rate, heart rate, cerebral blood volume, and cerebral blood flow (CBF), to compensate for reduced inspired oxygen. These autonomic responses depend on the severity and duration of hypoxia, species, tissue type, and level and type of anesthetic (Siesjo, 1978).
Combined CBF and blood-oxygenation-level-dependent (BOLD; Ogawa et al., 1990) functional MRI provides a unique means to investigate the neurovascular coupling under graded hypoxia non-invasively. Stimulus-evoked CMRO2 changes can be estimated with combined BOLD and CBF measurements (Davis et al., 1998; Liu et al., 2004). While general anesthesia with mechanical ventilation is commonly used for immobilization in MRI studies of animal models, there has been increasing interest in imaging awake animals because of the important applications of awake models for mapping higher-order cognitive brain functions. The feasibility of performing fMRI under awake and restrained conditions has been demonstrated using the BOLD technique (Logothetis et al., 1999; Wyrwicz et al., 2000), MION (monocrystalline iron oxide nanoparticules) technique to detect stimulus-evoked changes in blood volume (Dubowitz et al., 2001; Vanduffel et al., 2001), and perfusion and perfusion-based functional MRI (Sicard et al., 2003). While fMRI studies of awake animals are generally challenging, they also offer many distinct advantages. First, the confounding effects of anesthesia on blood flow, metabolism and neuro-vascular coupling can be avoided. Second, general neural activity is not suppressed, which leads to more salient fMRI signal changes. Finally, sub-cortical and higher order cognitive functions can be studied in the awake model. Such studies would be very difficult if not impossible under anesthesia. The disadvantages of performing fMRI studies on awake animals include motion artifacts and restraint stress. Characterizing the differences and similarities in physiological and functional measures between awake and anesthetized conditions could help to better understand the fMRI signals and improve study designs.
The goal of this study was to investigate the cerebrovascular responses to graded hypoxia in awake and anesthetized (2% isoflurane) spontaneously breathing rats. CBF and BOLD fMRI signals were simultaneously measured using the continuous arterial spin-labeling technique with echo-planar-imaging acquisition. Cerebral metabolic rate of oxygen (CMRO2) was estimated using the biophysical BOLD model. MRI data were correlated with blood pressure, heart rate, respiration rate, and blood gases in the same animals.
2. Results
2.1. Physiological parameters
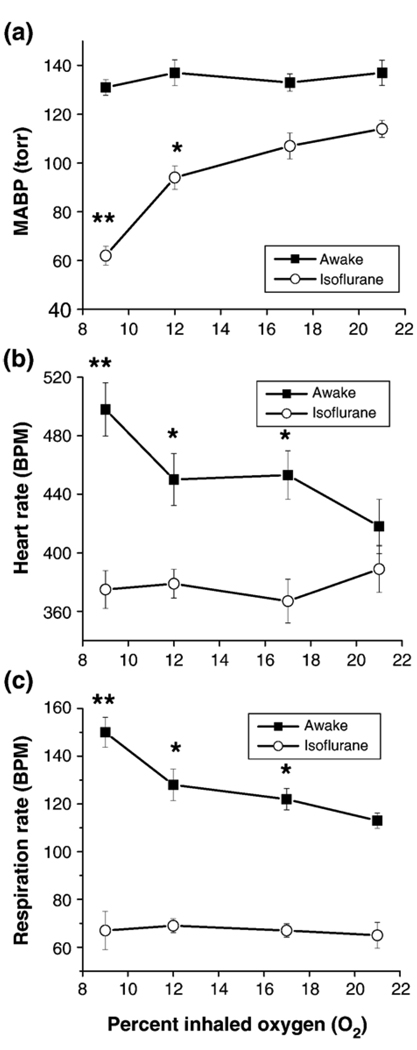
MABP, heart rate, and respiration rate under basal conditions and graded hypoxia are plotted in Fig. 1. Under awake basal (21% O2) conditions, the respiration rate, heart rate, and MABP were within normal physiological ranges (Sharp and LaRegina, 1998), consistent with previously reported values (Sicard et al., 2003). These parameters under anesthetized basal condition were clearly reduced (P<0.05) but remained within normal physiological ranges (Sharp and LaRegina, 1998). During graded hypoxia in the awake state, MABP did not decrease (P>0.05), and heart rate and respiration rate increased (P<0.05 for 9% O2 exposure). In contrast, in the anesthetized conditions, MABP decreased (P<0.05 for 12% and 9% O2 exposure), and heart rate and respiration rate remained unchanged (P>0.05). The differences in these parameters between the awake and anesthetized conditions generally grew larger as inspired oxygen tension decreased (P<0.05 for all hypoxic conditions).
Fig. 1.
(a) Mean arterial blood pressure (MABP), (b) heart rate, (c) respiration rate under basal conditions and graded hypoxia. *P<0.05, **P<0.001 relative to baseline condition (21% O2).
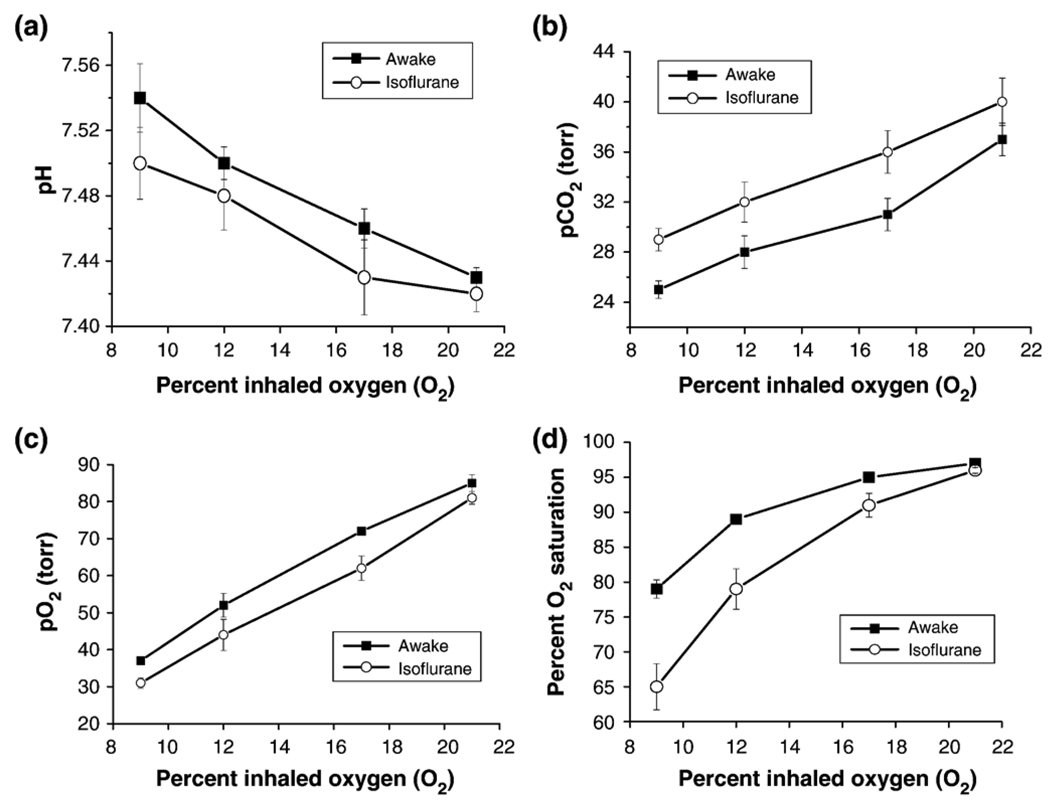
Blood gases under basal conditions and graded hypoxia are shown in Fig. 2. Basal blood-gas pH, pCO2, pO2, and oxygenation saturation in both the awake and the anesthetized condition were within normal physiological ranges (Sharp and LaRegina, 1998) but there were systematic differences between awake and anesthetized animals. During graded hypoxia, pH increased, and pCO2, pO2, and O2 saturation decreased under both conditions (P<0.05 for all hypoxic conditions). The differences in O2 saturation between the awake and anesthetized conditions grew larger as inspired oxygen tension decreased (P<0.05).
Fig. 2.
Blood gases (pH, pCO2, pO2, O2 saturation) under basal conditions and graded hypoxia. Basal blood gases were not statistically different between awake and anesthetized rats (P>0.05). During graded hypoxia, pH increased, and pCO2, pO2, and O2 saturation decreased under both awake and anesthetized conditions (P<0.05).
2.2. CBF and BOLD signal responses to hypoxia
CBF and BOLD responses to hypoxia were initially analyzed for five different brain regions (cortex, hippocampus, thalamus, corpus callosum, and caudoputamen). The data trends were similar and thus we chose to perform whole-brain ROI analysis for high signal-to-noise ratios.
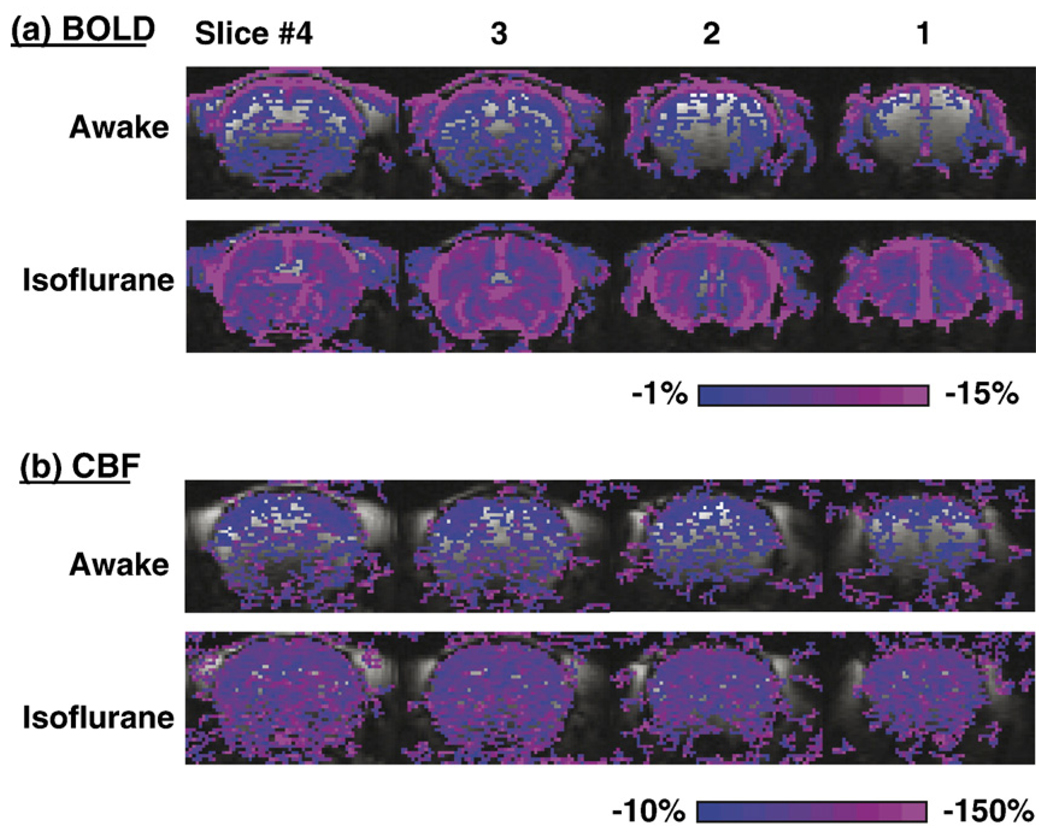
Representative CBF and BOLD responses to graded hypoxia from a single animal in the awake and anesthetized conditions are shown in Fig. 3. BOLD and CBF image time courses in anesthetized and awake rats generally showed no ostensible movement artifacts. Surprisingly, no hypoxia-induced CBF increase was observed. Both CBF and BOLD decreased with graded hypoxia. However, hypoxia-induced CBF and BOLD percent decreases in the awake state were smaller than those in the anesthetized state.
Fig. 3.
Representative BOLD and CBF responses to hypoxia (9% O2) under anesthetized and awake condition from one animal. BOLD and CBF percent-change maps were calculated and color-coded. The threshold was set the same to allow comparison between awake and anesthetized conditions. Percent changes in text employed the same whole-brain ROI without statistical threshold.
The group-averaged quantitative CBF values were evaluated as a function of percent inhaled oxygen under awake and anesthetized conditions (Fig. 4a). Basal (21% O2) CBF in the anesthetized condition (1.13±0.12 ml/g/min) was greater than that in the awake condition (0.93±0.10 ml/g/min). However, this was reversed under 9% O2 hypoxia—that is basal CBF in the awake condition was greater than that in the anesthetized condition. Fig. 4b re-plots the quantitative CBF as a function of arterial blood pO2. For each hypoxic gas condition, pO2 was slightly higher in the awake relative to the anesthetized state, resulting in a shift between the two curves. CBF under isoflurane decreased markedly with decreasing arterial blood pO2. In contrast, CBF in the awake condition decreased more gradually and appeared to level off.
Fig. 4.
(a) Quantitative CBF values as a function of percent inhaled oxygen under awake and anesthetized conditions. (b) CBF and (c) BOLD percent changes as a function of arterial blood pO2.
BOLD percent changes were re-plotted as a function of blood pO2 (Figs. 4c). CBF and BOLD percent decreases were more negative in the anesthetized condition than in the awake state at low arterial pO2 (<50 mm Hg), whereas there were no statistical differences at high arterial pO2 (>50 mm Hg).
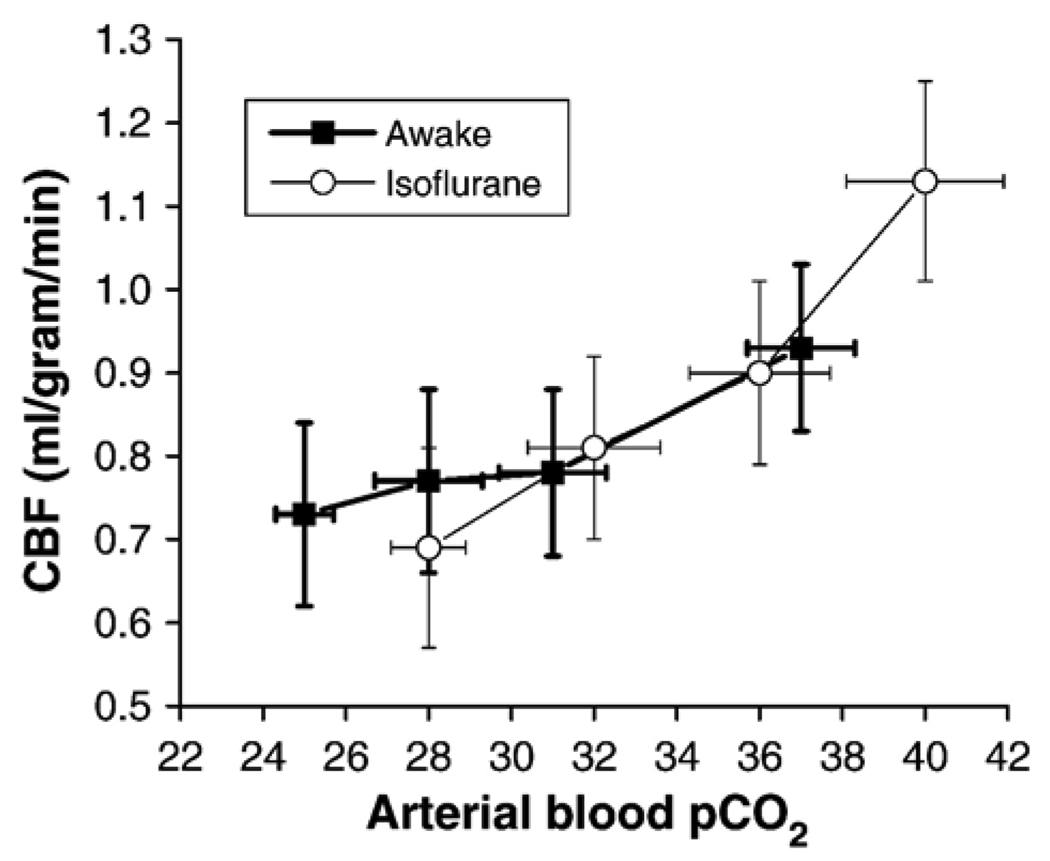
The correlation between CBF and arterial pCO2 is shown in Fig. 5. At high pCO2 (non-hypoxic condition), the difference in CBF under graded hypoxia between awake and anesthetized conditions could be explained by the differences in pCO2. At low pCO2 (hypoxic condition), CBF leveled off slightly with decreasing arterial pCO2 in the awake condition, whereas CBF decreased monotonically with decreasing pCO2 in the anesthetized condition.
Fig. 5.
Cerebral blood flow (CBF) as a function of arterial blood pCO2 during graded hypoxia under awake and anesthetized conditions. CBF leveled off with decreasing arterial pCO2 under awake condition, whereas CBF decreased monotonically with decreasing arterial pCO2 under anesthetized condition.
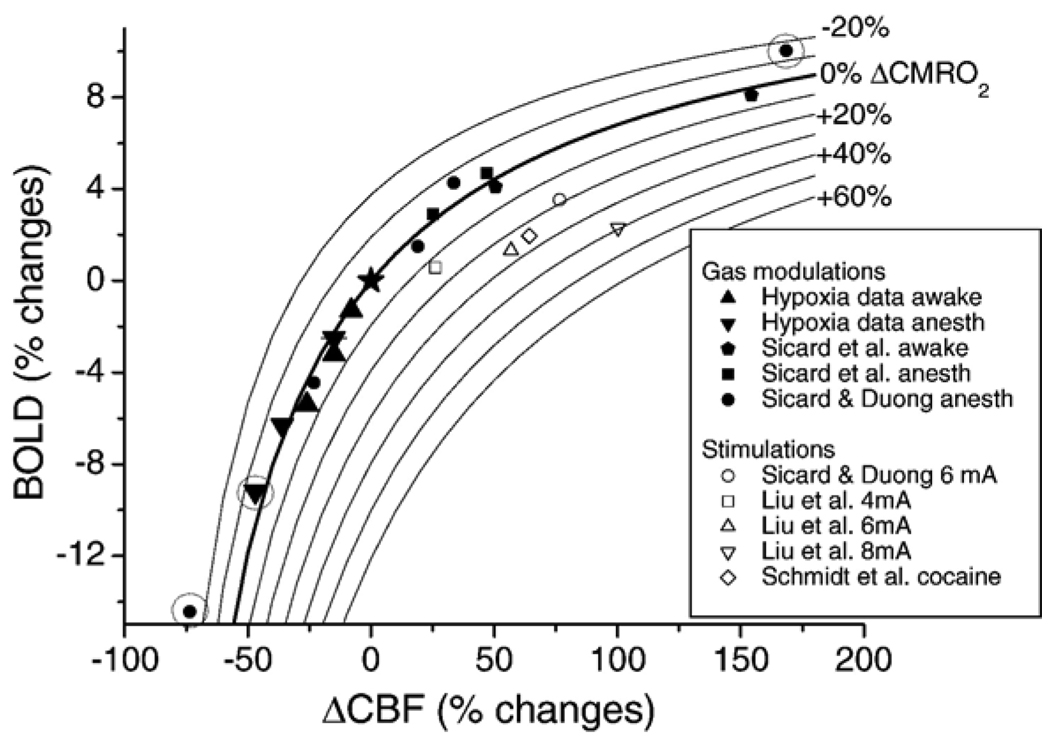
Fig. 6 shows the CBF and BOLD hypoxia data superimposed on iso-CMRO2 contour plots. The CBF and BOLD hypoxia data herein (▲ and ▼ symbols) mostly fell on or near the 0% iso-CMRO2 line as expected because mild hypoxia does not alter CMRO2. Similarly, previously published data (Sicard et al., 2003; Liu et al., 2004; Sicard and Duong, 2005; Schmidt et al., 2006) obtained under mild hypoxia and hypercapnia also fell on or near 0% iso-CMRO2 line. Three data points from physiological perturbation (encircled in Fig. 6), however, deviated significantly from the 0% iso-CMRO2 line and they are those under severe hypoxia (9% O2) and severe hypercapnia (10% CO2) for anesthetized conditions. Forepaw and pharmacological stimulation data are also superimposed on the iso-CMRO2 contour plots and these data showed positive increase in CMRO2 as expected.
Fig. 6.
Iso-CMRO2 contour lines from −20% to 60% in steps of 10% are overlaid on the plot of BOLD versus CBF percent changes under different physiological modulations and functional stimulations. CMRO2 contour lines were calculated using M=0.16, α of 0.4, and β of 1.2. The open data points were functional (forepaw or cocaine stimuli) stimulations and the solid data points were physiological modulations (normal conditions, hypercapnia, hyperoxia or hypoxia). ▲ and ▼ are awake and anesthetized hypoxia data obtained from this study; ⬟ and ■ are CO2 challenges data under awake and anesthetized conditions, respectively, obtained from (Sicard et al., 2003); ● and ○ are CO2 challenges and 6 mA forepaw stimulation data, respectively, obtained from (Sicard and Duong, 2005);□, △, and ▽ are 4, 6, and 8 mA forepaw stimulation data, respectively, obtained from Liu et al. (2004); ◊ is cocaine data (1 mg/kg) from Schmidt et al. (2006). Three data points that are encircled show deviation from the 0% CMRO2 iso-contour line: ▼ at ~50% ΔCBF and ● at ~70% ΔCBF were 9% O2 hypoxia under anesthesia, and ● at 170% ΔCBF was 10% CO2 hypercapnia under anesthesia. ★ indicates the point of origin.
3. Discussion
Relative to normoxia, hypoxia (i.e., 9% O2) in 2%-isoflurane anesthetized rats reduces in blood pressure and does not change heart and respiration rate. In contrast, hypoxia in awake animals shows compensatory responses by sustaining blood pressure, and increasing heart rate and respiration rate. Hypoxia induces hypocapnia. Basal CBF under anesthesia is higher than under the awake state because isoflurane is a potent vasodilator. Graded hypoxia decreases BOLD signals. Surprisingly, hypoxia decreases CBF because hypoxia induces hypocapnia. However, hypoxia-induced CBF and BOLD reductions are smaller in awake relative to anesthetized rats at low pO2, but similar at high pO2. CBF levels off with decreasing hypoxia-induced pCO2 in awake rats, but monotonically decreases in anesthetized rats. CMRO2 does not change under mild hypoxia but appears to decrease under severe hypoxia and severe hypercapnia. These results further support the feasibility of awake fMRI studies, show that isoflurane attenuates automomic responses to hypoxia, hypoxia-induced hypocapnia dominates CBF changes, tissues in awake conditions appear better oxygenated under identical hypoxic conditions, and severe hypoxia appears to reduce cerebral oxygen metabolism. These findings have strong implications for functional MRI studies of awake animals and hypoxia studies in anesthetized animals.
3.1. Potential issues
MRI offers a non-invasive tool to obtain quantitative CBF images at reasonably high spatial and temporal resolution at the level of the capillary (Duong et al., 2001b). Although consistent with many established invasive CBF techniques, the continuous arterial spin-labeling CBF technique could yield CBF data that are subject to errors. These errors could arise from magnetization transfer, transit time, and water exchange effects. With the actively decoupled two-coil system for CBF measurements used herein, there was no error arising from magnetization transfer effect on the quantitative CBF values (Silva et al., 1999; Duong et al., 2000). Transit time (Zhou and van Zijl, 1999) and water-exchange effect (Zhou et al., 2001) are likely to be small (Zhou et al., 2001) and are not expected to alter the overall conclusions of this study.
Perfusion imaging is particularly sensitive to movement artifacts because it has relatively low signal-to-noise ratio and it involves paired images acquired at two different time points between which movement could occur. The use of a proper restrainer provided BOLD and quantitative perfusion images free of movement artifacts. Echo-planar-imaging technique was also helpful because EPI samples the entire k-space in a single shot acquisition within ~20 ms. Therefore, the approach taken herein offers a means to quantitatively evaluate regional CBF in fully conscious rats, allowing direct comparison of quantitative CBF in graded hypoxia under awake and anesthetized conditions in the same animals.
Restraint could have a substantial effect on animal physiology which could complicate the comparison between awake and anesthetized conditions. A detailed investigation of acclimation and restraint effects has been described elsewhere (King et al., 2005). In this study, blood gases, heart rate, respiration rate, and mean arterial blood pressure in the awake animals were within normal physiological ranges, and the number of struggling movements markedly decreased after the first hour of being awake in the restrainer. Taken together, these observations suggest that the animals had at least partially acclimated to the restrainer during the imaging session. Nonetheless, regional differences in CBF as well as the CBF and BOLD responses to hypoxia could be influenced in part by various stress factors which include restraint stress. Similarly, hypoxia could also activate brain regions related to hypoxia exposure differently between awake and anesthetized conditions. These changes, however, are unlikely to alter the conclusions where the whole-brain signals were analyzed. Furthermore, only isoflurane was used for comparison in this study. Other anesthetics could have different effects on autonomic responses under hypoxia and may show different BOLD and CBF responses to hypoxia. The advantages and disadvantages of isoflurane anesthesia under physiological and stimulation studies have been extensively discussed (Sicard et al., 2003; Liu et al., 2004).
3.2. CBF in anesthetized conditions
Oxygen delivery to the brain during hypoxia involves many complicated physiological factors. Hypoxia induced respiration and heart rate increases were observed in awake but not in isoflurane-anesthetized animals (Fig. 1). MABP was maintained in awake rats under graded hypoxia but was markedly reduced in anesthetized rats. These observations suggest that hypoxia-induced compensatory responses were attenuated under 2% isoflurane anesthesia. Increased respiration in awake rats is expected to be helpful in compensating for reduced inspired oxygen tension. Ironically, hyperventilation greatly reduces CBF and thus tissue oxygen delivery because hyperventilation-induced hypocapnia leads to vascular constriction (Fig. 2), although reduced CBF increases oxygen extraction to some extent. Furthermore, the hypoxia-related changes in pCO2 and pH also affect the oxygen unloading from oxyhemoglobin via the Bohr effect; this also has an effect on oxygen delivery.
In the anesthetized and spontaneously breathing animals, no hypoxia-induced CBF increase was observed (Figs. 3 and 4). Instead, CBF decreased from 1.13±0.12ml/g/min during 21% O2 (arterial pO2=81±4 mm Hg, pCO2=40±3 mm Hg) to 0.69±0.12 ml/g/min during 9% O2 exposure (arterial pO2=31±3 mm Hg, pCO2=29±2 mm Hg). In a previous study in which the animals were anesthetized with 2% isoflurane but mechanically ventilated (Duong et al., 2001a), CBF decreased from1.3 ml/g/min under 21% O2 (arterial pO2~100 mm Hg, pCO2=30–40 mm Hg) to 0.5 ml/g/min under 10–12% O2 (arterial pO2~30 mm Hg, pCO2=30–40 mm Hg). These two studies are qualitatively similar. Both studies reported hypoxia-induced CBF decreases, likely due to hypoxia-induced hypocapnia and the suppression of the autonomic responses to hypoxia by isoflurane. The latter is also supported by the monotonic decrease of CBF with decreasing pCO2 in the anesthetized (but not awake) condition (Fig. 5). There are, however, some notable differences between the results of the two studies. In the mechanically ventilated rats under anesthesia, animals: (1) had lower arterial pO2, (2) were less hypocapnic, and (3) showed a larger CBF decrease for similar hypoxic exposure, relative to spontaneously breathing rats under anesthesia. These quantitative differences could be explained by the differences in animal physiology arising from “spontaneous breathing” versus “mechanically ventilation”. Mechanical ventilation prevented the anesthetized animals from changing their respiration rate and volume, resulting in a lower pO2 and a higher pCO2 compared to the spontaneously breathing, anesthetized animals. Despite being less hypocapnic under mechanical ventilation (Duong et al., 2001a), CBF under mechanically ventilated anesthetized animals was lower compared to the spontaneously breathing and anesthetized animals herein, potentially due to the confounding effects of mechanical ventilation and anesthesia.
Interestingly, other studies have reported hypoxia-induced CBF increases in anesthetized animals. A hypoxia-induced CBF increase of ~80% (measured using butanol indicator-fractionation method) was reported in choral hydrate-anesthetized rats with an arterial pO2 of 49 mm Hg (Shockley and LaManna, 1988). A hypoxia-induced CBF increase was reported in mechanically ventilated animals that were anesthetized with thiopental (Julien-Dolber et al., 2002). These differences in findings could be due to the use of different anesthetics, the duration of hypoxic exposure, or other methodological details. There is evidence that isoflurane interferes with cerebral autoregulation during hypoxia in a dose-dependent manner (Hoffman et al., 1991; Lee et al., 1994). This could partially account for the discrepancy across some laboratories.
3.3. CBF in awake conditions
In the awake conditions, CBF reduction was smaller under the awake state compared to the anesthetized state at low arterial pO2 (<50 mm Hg) (Fig. 4c) although the awake animals were more hypocapnic (Fig. 2). These comparisons suggest that a hypoxia-induced CBF increase was present but masked by the hypoxia-induced hypocapnia. This notion is also supported by the observation that the CBF leveled off with decreasing arterial pCO2 in awake but not in anesthetized animals (Fig. 5). The hypoxia-induced hypocapnia has been reported previously (Marshall and Metcalfe, 1989; Bao et al., 1997; Poulin et al., 2002). Bao et al. (1997) reported a marked hypoxia-induced hypocapnia although they did not measure CBF. Poulin et al. (2002) reported hypoxia-induced hypocapnia and a decrease in CBF as measured by transcranial Doppler ultrasound. Interestingly, when CO2 was added to the inspired gas to maintain physiological blood pCO2, the CBF decrease was abolished (Poulin et al., 2002).
Hypoxia-induced CBF increases have also been reported in awake animals. CBF measured using (14C)iodoantipyrine technique increased 25–90% in awake rats exposed to 10% oxygen (Bereczki et al., 1993), and CBF measured using radiolabeled microsphere increased 200–250% in awake sheep with an arterial pO2 of 40 mm Hg while the arterial pCO2 was allowed to vary (Iwamoto et al., 1991). These results contradict our findings as well as those of others (Bao et al., 1997; Poulin et al., 2002). One possible explanation could be the different physiological conditions under which the animals were prepared. For example, basal inspired oxygen tension prior to hypoxic challenge affects the hypoxic response (Kannurpatti et al., 2002). When the basal inspired oxygen tension before apnea was 21% in mechanically ventilated and anesthetized rats, MABP decreased and CBF, as measured by Laser Doppler technique, markedly increased. When the basal inspired oxygen tension before apnea was 100%, MABP remained constant and CBF increased slightly. BOLD signal responses decreased when the basal inspired pO2 before apnea was 21% but increased when the basal inspired pO2 was 100% before apnea. These comparisons underscore the importance of specifying the experimental conditions as well as carefully monitoring physiological parameters.
3.4. BOLD responses
BOLD signals decreased under hypoxia for both awake and anesthetized conditions as expected. BOLD changes largely paralleled CBF changes. There are, however, some differences in the BOLD responses to hypoxia between awake and anesthetized conditions. The BOLD signals under anesthetized conditions decreased more significantly at the same hypoxic conditions even after adjusting for blood gas pO2 values (Fig 4c). Quantitative interpretation of BOLD signals is, however, complicated because of (1) the potential loss of cerebral autoregulation under anesthesia, (2) strong perturbation of vascular coupling leading to non-linearity of the BOLD signals under hypoxia, and (3) alkaline-induced changes in the affinity of hemoglobin for oxygen.
3.5. CMRO2 changes
CMRO2 is an important physiological parameter. Davis’ biophysical model uses CBF and BOLD measurements to derive CMRO2 (Davis et al., 1998). This derivation relies on the constants α and β which are pixel-specific. However, Davis et al. showed that CMRO2 is weakly dependent on α and β. The calculated CMRO2 changes herein were relatively independent for β ranging from 1 to 1.5 (data not shown). Further improvements in Davis’ CMRO2 MRI model can be made by validating the assumptions in the CMRO2 formalism, demonstrating the reproducibility of the CMRO2 maps, and cross-validating with PET-CMRO2 measurements. Nonetheless, CBF and BOLD hypoxia data superimposed on the iso-CMRO2 contour plots provided another perspective to analyze the hypoxia data. Our analyses suggest mild hypercapnia and hypoxia did not significantly change CMRO2. Severe hypoxia (9% O2) and severe hypercapnia (10% CO2), however, decreased CMRO2 under anesthetized conditions but not under awake conditions, suggesting that isoflurane anesthesia could be a confounding factor for energy metabolism under hypoxia. An alternative explanation is that strong physiological perturbations could render the CMRO2 biophysical model inaccurate. Cross-validation studies are needed.
In summary, BOLD and CBF responses to identical hypoxic exposure are remarkably different between awake and anesthetized rats. In contrast to the anesthetized condition, compensatory mechanisms in the awake state allow blood pressure to be maintained, heart rate and respiration rate increase. CBF and tissue oxygenation in awake animals decrease to a lesser extent and appear to level off during hypoxia whereas CBF and tissue oxygenation in anesthetized animals decrease monotonically. CMRO2 appears to decrease under severe, but not mild, hypoxia, although alternative explanation is possible. This study also further supports the feasibility of BOLD and CBF fMRI of conscious and restrained animals in a serial and non-invasive manner, and underscores the marked differences in BOLD and CBF responses to hypoxia between awake and anesthetized models for fMRI studies.
4. Experimental procedures
4.1. Animal preparation
These studies were approved and closely monitored by Institutional Animal Care and Use Committee (IACUC). Seven Sprague–Daley rats (250–300 g) were imaged under awake and anesthetized (2% isoflurane) conditions in the same animals. Rats were initially anesthetized with 2% isoflurane. A femoral artery was catheterized for monitoring the mean arterial blood pressure (MABP), heart rate (HR), and for sampling blood gases. Topical anesthetics (2% lidocaine) was applied to the surgical site which was sutured close. Respiration rates (RR) and waveforms were continuously monitored via a force transducer. MABP, HR, and RR were recorded onto a PC using the BIOPAC system (MP100 model, Santa Barbara, CA). The rectal temperature was monitored and maintained at 37±1 °C via a feedback-regulated, circulating-water pad. Rats were immobilized in a rat restrainer with ear-, nose-, tooth-, and shoulder-bars, and a body restraining tube (Sicard et al., 2003). All rats respired spontaneously without mechanically ventilation.
4.2. Hypoxia
For each animal, 17%, 12%, and 9% O2, (balance N2) with air (21% O2) as the baseline in a random order were studied under awake and anesthetized conditions (randomized). The randomized acquisition minimized potential habituation effects related to restraint and/or repeated hypoxia. In a typical trial, the animal was exposed to 1:00 min air, 1:30 min hypoxia, 2:00 min air, 1:30 min hypoxia, and 0:45 min air. At least 15 min elapsed between different hypoxia exposures and between awake and anesthetized conditions.
4.3. MR experiments
All MRI experiments were performed on a 4.7-T/40-cm horizontal magnet a 20-G/cm magnetic field gradient insert (Biospec Bruker, Billerica, MA). Animals were placed in a restrainer (Sicard et al., 2003) that had a built-in, actively decoupled brain imaging surface coil (2.3-cm ID) and a neck coil for CBF labeling. Coil-to-coil electromagnetic interaction was actively decoupled. Anatomical images were acquired using the fast spin-echo pulse sequence (RARE) with TR=2 s, effective TE=65 ms, matrix=128 × 128, FOV=2 cm × 2 cm, six 1.5-mm slices, and 4 averages.
Simultaneous CBF and BOLD measurements were made using the two-coil continuous arterial spin-labeling method (Silva et al., 1999; Duong et al., 2000) with single-shot, gradient-echo, echo-planar-imaging (EPI) acquisition. Paired images were acquired alternately—one with arterial spin labeling and the other without spin labeling. The MR parameters were: data matrix=64 × 64, FOV=2 cm × 2 cm, six 1.5-mm slices, TE=17 ms, and TR=2 s. For each trial, 100 pairs of images (~6 min 45 s) were acquired. The entire study took ~3 h. The EPI image has a SNR of at least 30 (without signal averaging).
4.4. Data analysis
Image analysis employed codes written in Matlab (MathWorks Inc, Natick, MA) and STIMULATE software (Univ. of Minnesota). BOLD image was obtained from the control images of the CBF measurement. CBF image (SCBF) with intensity in unit of ml/g/min was calculated pixel-by-pixel using (Silva et al., 1999; Duong et al., 2000), SCBF=λ/T1 * (Sc−SL)/[SL+(2α−1)Sc], where SC and SL are signal intensities of the control and labeled images, respectively. λ is the water brain–blood partition coefficient, T1 is the water spin-lattice relaxation time of tissue, and α is the arterial spin-labeling efficiency. λ was taken to be 0.9 (Herscovitch and Raichle, 1985). T1 and α were measured to be 1.5 s and 0.75, respectively.
BOLD and CBF percent-change maps were also calculated. BOLD and CBF data were correlated with physiological measurements. Quantitative CBF and relative CBF and BOLD changes were analyzed with whole-brain region of interests (ROI), avoiding surface vessels, without statistical thresholds to avoid bias. The same ROIs were used for awake and anesthetized conditions in the same animal. All statistical tests employed the two-tail paired t-test and all reported values are inmean±SD. Error bars on graphs are standard errors of the means.
To evaluate the possible CMRO2 changes associated graded hypoxia, the awake and anesthetized hypoxia data were overlaid onto iso-CMRO2 contour plots for comparison. The iso-CMRO2 contour plots were calculated using (Davis et al., 1998; Hoge et al., 1999),
| (1) |
where M is the proportionality constant and parameters with subscript zero indicate baseline values. α is the Grubb’s factor in CBV=CBF and a value of 0.4 was used (Eichling et al., 1974; Grubb et al., 1974; Mandeville et al., 1999). β is a proportionality constant relating deoxyhemoglobin concentration to the BOLD signals and a value of 1.2 was used (Boxerman et al., 1995; Davis et al., 1998). The proportionality constant M relating BOLD and CBF changes reflects the maximum BOLD response that can be expected from a given region. An average M value of 0.16 was used (Liu et al., 2004). Published data from hypercapnia and hypoxia and functional stimulation experiments obtained under essentially identical experimental protocols (Sicard et al., 2003; Liu et al., 2004; Sicard and Duong, 2005; Schmidt et al., 2006) were also overlaid for comparison.
Temporal dynamics of the fMRI responses were not analyzed because the stimulus onsets and flow rates of the gas challenges were manually controlled. The temporal dynamics between awake and anesthetized conditions was addressed using somatosensory stimulation previously and we found that the onset times of the awake conditions were markedly shorter (Sicard et al., 2003), consistent with isoflurane being a potent vasodilator.
Acknowledgments
This work was supported in part by the NIH (NINDS, R01-NS45879), the American Heart Association (SDG-0430020N), and the Whitaker Foundation (RG-02-0005).
REFERENCES
- Bao G, Randhawa PM, Fletcher EC. Acute blood pressure elevation during repetitive hypocapnic and eucapnic hypoxia in rats. J. Appl. Physiol. 1997;82:1071–1078. doi: 10.1152/jappl.1997.82.4.1071. [DOI] [PubMed] [Google Scholar]
- Bereczki D, Wei L, Otsuka T, Acuff V, Pettigrew K, Patlak C, Fenstermacher J. Hypoxia increases velocity of blood flow through parenchymal microvascular systems in rat brain. J. Cereb. Blood Flow Metab. 1993;13:475–486. doi: 10.1038/jcbfm.1993.62. [DOI] [PubMed] [Google Scholar]
- Boxerman JL, Hamberg LM, Rosen BR, Weisskoff RM. MR contrast due to intravascular magnetic susceptibility perturbations. Magn. Reson. Med. 1995;34:555–566. doi: 10.1002/mrm.1910340412. [DOI] [PubMed] [Google Scholar]
- Davis TL, Kwong KK, Weisskoff RM, Rosen BR. Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc. Natl. Acad. Sci. U. S. A. 1998;95:1834–1839. doi: 10.1073/pnas.95.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubowitz DJ, Bernheim KA, Chen DY, Bradley JWG, Andersen RA. Enhancing fMRI contrast in awake-behaving primates using intravascular magnetite dextran nanoparticles. NeuroReport. 2001;12:2335–2340. doi: 10.1097/00001756-200108080-00011. [DOI] [PubMed] [Google Scholar]
- Duong TQ, Silva AC, Lee S-P, Kim S-G. Functional MRI of calcium-dependent synaptic activity: cross correlation with CBF and BOLD measurements. Magn. Reson. Med. 2000;43:383–392. doi: 10.1002/(sici)1522-2594(200003)43:3<383::aid-mrm10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Duong TQ, Iadacola C, Kim S-G. Effect of hyperoxia, hypercapnia and hypoxia on cerebral interstitial oxygen tension and cerebral blood flow in the rat brain: an 19F/1H study. Magn. Reson. Med. 2001a;45:61–70. doi: 10.1002/1522-2594(200101)45:1<61::aid-mrm1010>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Duong Q, Kim DS, Ugurbil K, Kim S-G. Localized blood flow response at sub-millimeter columnar resolution. Proc. Natl. Acad. Sci. U. S. A. 2001b;98:10904–10909. doi: 10.1073/pnas.191101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichling JO, Raichle ME, Grubb RL, Jr, Ter-Pogossian MM. Evidence of the limitations of water as a freely diffusible tracer in brain of the Rhesus monkey. Circ. Res. 1974;35:358–364. doi: 10.1161/01.res.35.3.358. [DOI] [PubMed] [Google Scholar]
- Grubb RL, Raichle ME, Eichling JO, Ter-Pogossian MM. The effects of changes in PaCO2 on cerebral blood volume, blood flow, and vascular mean transit time. Stroke. 1974;5:630–639. doi: 10.1161/01.str.5.5.630. [DOI] [PubMed] [Google Scholar]
- Herscovitch P, Raichle ME. What is the correct value for the brain–blood partition coefficient for water? J. Cereb. Blood Flow Metab. 1985;5:65–69. doi: 10.1038/jcbfm.1985.9. [DOI] [PubMed] [Google Scholar]
- Hoffman WE, Edelman G, Kochs E, Werner C, Segil L, Albrecht RF. Cerebral autoregulation in awake versus isoflurane-anesthetized rats. Anesth. Analg. 1991;73:753–757. doi: 10.1213/00000539-199112000-00013. [DOI] [PubMed] [Google Scholar]
- Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc. Natl. Acad. Sci. 1999;96:9403–9408. doi: 10.1073/pnas.96.16.9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto J, Curran-Everett DC, Krasney E, Krasney JA. Cerebral metabolic and pressure-flow responses during sustained hypoxia in awake sheep. J. Appl. Physiol. 1991;71:1447–1453. doi: 10.1152/jappl.1991.71.4.1447. [DOI] [PubMed] [Google Scholar]
- Julien-Dolber C, Tropres I, Montigon O, Reutenauer H, Ziegler A, Decorps M, Payen JF. Regional response of cerebral blood volume to graded hypoxic hypoxia in rat brain. Br. J. Anaesth. 2002;89:287–293. doi: 10.1093/bja/aef182. [DOI] [PubMed] [Google Scholar]
- Kannurpatti SS, Biswal BB, Hudetz AG. Differential fMRI-BOLD signal response to apnea in humans and anesthetized rats. Magn. Reson. Med. 2002;47:864–870. doi: 10.1002/mrm.10131. [DOI] [PubMed] [Google Scholar]
- King JA, Brevard ME, Messenger TL, Shen Q, Duong TQ, Ferris CF. Procedures for minimizing stress for fMRI studies in conscious rats. J. Neurosci. Methods. 2005;148:154–160. doi: 10.1016/j.jneumeth.2005.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JG, Hudetz AG, Smith JJ, Hillard CJ, Bosnjak ZJ, Kampine JP. The effects of halothane and isoflurane on cerebrocortical microcirculation and autoregulation as assessed by laser-Doppler flowmetry. Anesth. Analg. 1994;79:58–65. [PubMed] [Google Scholar]
- Liu ZM, Schmidt KF, Sicard KM, Duong TQ. Imaging oxygen consumption in forepaw somatosensory stimulation in rats under isoflurane anesthesia. Magn. Reson. Med. 2004;52:277–285. doi: 10.1002/mrm.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Guggenberger H, Peled S, Pauls J. Functional imaging of the monkey brain. Nat. Neurosci. 1999;2:555–562. doi: 10.1038/9210. [DOI] [PubMed] [Google Scholar]
- Mandeville JB, Marota JJ, Ayata C, Moskowitz MA, Weisskoff RM, Rosen BR. MRI measurement of the temporal evolution of relative CMRO2 during rat forepaw stimulation. Magn. Reson. Med. 1999;42:944–951. doi: 10.1002/(sici)1522-2594(199911)42:5<944::aid-mrm15>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Marshall JM, Metcalfe JD. Influences on the cardiovascular response to graded levels of systemic hypoxia of the accompanying hypocapnia in the rat. J. Physiol. 1989;410:381–394. doi: 10.1113/jphysiol.1989.sp017539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. U. S. A. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin MJ, Fatemian M, Tansley JG, O’Connor DF, Robbins PA. Changes in cerebral blood flow during and after 48 h of both isocapnic and poikilocapnic hypoxia in humans. Exp. Physiol. 2002;87:633–642. doi: 10.1113/eph8702437. [DOI] [PubMed] [Google Scholar]
- Schmidt KF, Febo M, Shen Q, Ferris CF, Stein E, Duong TQ. Hemodynamic and metabolic changes induced by cocaine in anesthetized rat observed with multimodal functional. Psychopharmacology. 2006;185:479–486. doi: 10.1007/s00213-006-0319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P, LaRegina M. Animal Pocket Reference Series. Washington, DC: CRC Press; 1998. [Google Scholar]
- Shockley RP, LaManna JC. Determination of rat cortical blood volume changes by capillary mean transit time analysis during hypoxia, hypercapnia and hyperventilation. Brain Res. 1988;454:170–178. doi: 10.1016/0006-8993(88)90816-5. [DOI] [PubMed] [Google Scholar]
- Sicard KM, Duong TQ. Effects of hypoxia, hyperoxia and hypercapnia on baseline and stimulus-evoked BOLD, CBF and CMRO2 in spontaneously breathing animals. NeuroImage. 2005;25:850–858. doi: 10.1016/j.neuroimage.2004.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard K, Shen Q, Brevard ME, Sullivan R, Ferris CF, King JA, Duong TQ. Regional cerebral blood flow and BOLD responses in conscious and anesthetized rats under basal and hypercapnic conditions: implications for functional MRI studies. J. Cereb. Blood Flow Metab. 2003;23:472–481. doi: 10.1097/01.WCB.0000054755.93668.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siesjo B. Brain Energy Metabolism. New York: Wiley; 1978. [Google Scholar]
- Silva AC, Lee S-P, Yang C, Iadecola C, Kim S-G. Simultaneous blood oxygenation level-dependent and cerebral blood flow functional magnetic resonance imaging during forepaw stimulation in the rat. J. Cereb. Blood Flow Metab. 1999;19:871–879. doi: 10.1097/00004647-199908000-00006. [DOI] [PubMed] [Google Scholar]
- Vanduffel W, Fize D, Mandeville JB, Nelissen K, Van Hecke P, Rosen BR, Tootell RB, Orban GA. Visual motion processing investigated using contrast agent-enhanced fMRI in awake behaving monkeys. Neuron. 2001;32:565–577. doi: 10.1016/s0896-6273(01)00502-5. [DOI] [PubMed] [Google Scholar]
- Wyrwicz AM, Chen N, Li L, Weiss C, Disterhoft JF. fMRI of visual system activation in the conscious rabbit. Magn. Reson. Med. 2000;44:474–478. doi: 10.1002/1522-2594(200009)44:3<474::aid-mrm19>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Zhou J, van Zijl PC. Effect of transit times on quantification of cerebral blood flow by the FAIR T1-difference approach. Magn. Reson. Med. 1999;42:890–894. doi: 10.1002/(sici)1522-2594(199911)42:5<890::aid-mrm8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Zhou J, Wilson DA, Ulatowski JA, Traystman RJ, vanZijl PC. Two-compartment exchange model for perfusion quantification using arterial spin tagging. J. Cereb. Blood Flow Metab. 2001;21:440–455. doi: 10.1097/00004647-200104000-00013. [DOI] [PubMed] [Google Scholar]