Abstract
Dimethyl sulfoxide (DMSO) has a variety of biological actions that suggest efficacy as a neuroprotectant. We (1) tested the neuroprotective potential of DMSO at different time windows on infarct size using 2,3,5-triphenyltetrazolium staining and (2) investigated the effects of DMSO on ischemia evolution using quantitative diffusion and perfusion imaging in a permanent middle cerebral artery occlusion (MCAO) model in rats. In experiment 1, DMSO treatment (1.5 g/kg intravenously over 3 h) reduced infarct volume 24 h after MCAO by 65% (P<0.00001) when initiated 20 h before MCAO, by 44% (P=0.0006) when initiated 1 h after MCAO, and by 17% (P=0.11) when started 2 h after MCAO. Significant infarct reduction was also observed after a 3-day survival in animals treated 1 h after MCAO (P=0.005). In experiment 2, treatment was initiated 1 h after MCAO and maps for cerebral blood flow (CBF) and apparent diffusion coefficient (ADC) were acquired before treatment and then every 30 mins up to 4 h. Cerebral blood flow characteristics and CBF-derived lesion volumes did not differ between treated and untreated animals, whereas the ADC-derived lesion volume essentially stopped progressing during DMSO treatment, resulting in a persistent diffusion/perfusion mismatch. This effect was mainly observed in the cortex. Our data suggest that DMSO represents an interesting candidate for acute stroke treatment.
Keywords: diffusion/perfusion mismatch, dimethyl sulfoxide, focal brain ischemia, neuroprotection
Introduction
Dimethyl sulfoxide (DMSO) is widely used as a solvent for a variety of drugs and has a broad spectrum of biological activities that suggest efficacy as a neuroprotectant. These activities include hydroxyl radical scavenging (Repine et al, 1981), anti-inflammatory (Wood and Wood, 1975) and anti-edema effects (Camp et al, 1981), impairment of platelet aggregation and adhesiveness (Dujovny et al, 1983; Schiffer et al, 1976), and prevention of glutamate-induced neuronal cell death (Lu and Mattson, 2001).
However, prior reports on the effect of DMSO on focal cerebral ischemia were contradictory. Some investigators reported substantial improvements in mortality, morbidity, and infarct volume (de la Torre and Surgeon, 1976; Laha et al, 1978; Shimizu et al, 1997), while others failed to confirm these findings (McGraw, 1977; Little et al, 1981). Thus, the beneficial effects of DMSO in the treatment of acute focal brain ischemia are not clearly delineated.
In circumstances where neuroprotective effects of DMSO have been reported, the mechanisms for the salubrious effects are poorly understood. Most of the prior studies on the neuroprotective effects of DMSO were snapshot measurements performed using classical histologic methods (de la Torre and Surgeon, 1976; McGraw, 1977; Laha et al, 1978; Shimizu et al, 1997). None of these studies investigated the dynamic in vivo effects of DMSO on the evolution of ischemic tissue injury; and none of these studies examined different time points of drug administration relative to ischemia onset in the same experimental setting. Furthermore, it remains unclear whether DMSO increases cerebral blood flow (CBF) to prevent injury in focal brain ischemia. Indeed, two studies claimed opposite effects of DMSO on CBF (Little et al, 1981; de la Torre, 1991).
Diffusion- (DWI) and perfusion-weighted (PWI) magnetic resonance imaging are powerful imaging modalities for early detection of cerebral ischemia (Hoehn-Berlage et al, 1995). The region of the ischemic brain with diminished perfusion but without altered diffusion characteristics (diffusion/perfusion mismatch) is thought to represent an approximation of the ischemic penumbra. Agents that favor the survival of the penumbra represent an attractive therapeutic approach to acute stroke (Schlaug et al, 1999).
The purpose of the present study was to investigate systematically the effects of intravenous DMSO in an established model of permanent ischemia in rats. Specifically, we (1) evaluated the neuroprotective potential of DMSO at different times relative to the onset of focal cerebral ischemia; (2) studied the effect of DMSO on the spatio-temporal evolution of the ischemic lesion using quantitative perfusion and diffusion imaging; and (3) investigated the influence of DMSO on CBF characteristics.
Methods
Animal Preparations
All procedures used in this study were performed in accordance with our institutional guidelines and all experiments were performed in a masked, randomized manner. Male Wistar-Kyoto rats (n=79, 300 to 350 g, Taconic Farms, Germantown, NY, USA) were used for all experiments, after an overnight fast. Anesthesia was induced with an intraperitoneal injection of chloral hydrate (400 mg/kg). PE-50 tubing was inserted into the right femoral vein for drug infusions. Another PE-50 tubing was inserted into the right femoral artery for monitoring of systemic arterial blood pressure and heart rate (Biopac, Santa Barbara, CA, USA), and for obtaining blood samples to measure pO2, pCO2, pH, and plasma glucose (I-STAT, East Windsor, NJ, USA). Blood sampling was performed before and 3 h after occlusion. Rectal temperature was monitored and kept at 37°C±0.5°C throughout the entire study using a feedback-controlled heating pad. Although brain temperature has been shown to be slightly different from core temperature, rectal temperature has been proposed to reflect the changes in brain temperature (Maier et al, 1998). Furthermore, the stable apparent diffusion coefficient (ADC) values in the normal left hemisphere in both groups (see results) indicated that the brain temperature was stable and did not differ between groups (Hasegawa et al, 1994).
Permanent focal cerebral ischemia was produced by intraluminal suture occlusion of the right middle cerebral artery (MCAO). Briefly, a 4-0 silicone-coated nylon filament with a thermically rounded tip was introduced into the distal common carotid artery, which was then advanced via the internal carotid artery until mild resistance indicated that the tip was lodged in the anterior cerebral artery, and thus blocked blood flow to the MCA.
Study Design
The study consisted of two different experiments:
Experiment 1 aimed to investigate the neuroprotective potential of DMSO when initiated at different times relative to the onset of ischemia. Infarct volume was defined at 24 h after MCAO using 2,3,5-triphenyltetrazolium chloride (TTC) staining. Dimethyl sulfoxide (Sigma-Aldrich, St Louis, MO, USA) was diluted to a 33% solution with saline. All treatment animals received a 3-h intravenous infusion at a total dose of 1.5 g/kg DMSO (total volume ~1.5 mL). Control animals were administered a 3-h infusion of 1.5mL saline. Animals were randomized to one of the following groups: start of infusion 20 h before induction of MCAO (groups DMSO−20 h (n=8) and saline−20 h (n=6)), start of infusion 1 h after MCAO (groups DMSO+1h (n=8) and saline+1h (n=8)), and start of infusion 2 h after MCAO (groups DMSO+2h (n=8) and saline+2h (n=6)).
To examine the long-term effects of DMSO, TTC-staining was performed after a 3-day survival period in two additional groups treated 1 h after ischemia (1.5 g/kg DMSO, n=6; saline, n=6).
Furthermore, the effects of DMSO on infarct size at 24 h after occlusion were evaluated when DMSO was administered at half dose (0.75 g/kg) starting 1 h after occlusion (n=8).
In experiment 2, the in vivo effects of DMSO on ADC and CBF characteristics and the spatio-temporal evolution of the ischemic lesion were evaluated. Immediately after occlusion, the animals were placed into the magnet, and anesthesia was switched to 1% isoflurane delivered in air at 1.5 L/min. Arterial blood pressure, heart rate, and rectal temperature were continuously monitored and temperature was maintained at 37.0°C±0.5°C using a feedback-controlled heating pad. Dimethyl sulfoxide (1.5 g/kg) was administrated intravenously over 3 h starting 1 h after MCAO (n=7, identical protocol to DMSO+1 h group in experiment 1). Control animals (n=8) received 1.5mL saline. Magnetic resonance imaging data were acquired initially at 45 mins (baseline), at 90 mins after MCAO, and then every 30 mins up to 4 h after occlusion. 2,3, 5,-Triphenyltetrazolium chloride-staining was performed 24 h after MCAO.
Magnetic Resonance Imaging Measurements
Magnetic resonance imaging was performed on a Bruker 4.7T/40cm horizontal magnet (Billerica, MA, USA), and a 20 G/cm magnetic field gradient insert. A surface coil was used for brain imaging and an actively decoupled neck coil was used for CBF labeling. Coil-to-coil electromagnetic interaction was actively decoupled.
The average apparent diffusion coefficient (ADCav) of water was obtained by averaging three ADC maps acquired separately with diffusion-sensitive gradients applied along the x, y, or z direction. Single-shot, spin-echo, and echo-planar images (EPI) were acquired with a 64×64 matrix, 20.3×20.3mm2 field of view (FOV), eight 1.5-mm slices, TR=2 secs, b=10 and 1270 secs/mm2, TE=39 ms, Δ=17.5 ms, δ=5.6 ms, and 16 averages (total acquisition time 2.5 mins).
Noninvasive quantitative CBF measurements were performed using the continuous arterial spin-labeling (ASL) technique (Duong et al, 2000). Paired images were acquired alternately, one with arterial spin-labeling and the other without spin-labeling preparation (control). Single-shot, gradient-echo EPI were acquired with a 64×64 matrix, 20.3×20.3mm2 FOV, eight 1.5-mm slices, TR=2 secs, TE=15 ms. The continuous arterial spin used a 1.7-sec square RF pulse, in the presence of a 1.0 G/cm gradient, applied to the neck labeling coil on alternate acquisitions. A total of 120 pairs of ASL scans were acquired in two separate sets of 60 pairs each, one set obtained before, and the other after the ADC measurements (total acquisition time 8.9 mins).
To provide anatomical localization, we acquired T2-weighted images using the fast spin-echo pulse sequence with a 128×128 matrix, 20.3×20.3mm2 FOV, eight 1.5-mm slices, TR=2 secs, effective TE=80 ms, echo train length 16, and 16 signal averages.
Neurobehavior and Postmortem Evaluation
Twenty-four hours after MCAO, the animals were scored neurologically according to the following 6-point scale modified from the original description by Menzies et al (1992): 0=no apparent deficit, 1=failure to extend left forepaw fully, 2=decreased grip of the left forelimb while tail pulled, 3=spontaneous movement in all directions, circling to the left only if pulled by tail, 4=spontaneous circling to the left or no spontaneous walking with reduced level of consciousness, 5=dead.
Animals were allowed to survive 24 h after ischemia (except in the 3-day survival experiment). Brains were quickly removed and cut into eight 1.5-mm-thick coronal slices starting 1mm from the frontal pole. Histologic staining was performed using TTC. The stained sections were then photographed and infarct volumes were determined using ImageJ software (Rasband, http://rsb.info.nih.gov/ij/). To compensate for the effects of brain edema, a corrected infarct volume was calculated, as previously described (Meng et al, 2004).
Data Analysis
MRI measurements were analyzed using the imaging processing programs Matlab (math-Works, Natick, MA, USA) and STIMULATE (Strupp, 1996). Quantitative ADCav and CBF maps were calculated, as previously described in detail (Shen et al, 2003).
Calculation of In Vitro Lesion Size
Apparent diffusion coefficient and CBF viability thresholds, previously established and validated in our lab (Shen et al, 2003; Meng et al, 2004), were used to identify all pixels with abnormal ADC or CBF characteristics on each of the eight imaged slices at each time point. The corresponding ADC and CBF lesion volumes were then calculated by summing the abnormal area and multiplying by the slice thickness. The viability thresholds were 0.53×10−3mm2/sec for ADC and 0.30 mL/g min for CBF (Meng et al, 2004).
To investigate whether the DMSO effects on ischemic lesion evolution may be different between cortical and subcortical regions, the lesion progression in these two regions was also analyzed separately. The two regions of interest, the cortex and the striatum with basal forebrain, were drawn on anatomical scans with reference to a rat brain atlas.
Quantitative Apparent Diffusion Coefficient and Cerebral Blood Flow Values
The time course of quantitative ADC and CBF values of the ischemic and the normal hemisphere were analyzed. The effects of DMSO on the temporal evolution of the CBF and ADC characteristics of the initial core and mismatch regions, defined at 45 mins before treatment, were also evaluated. On the initial ADC and CBF maps (45 mins), ischemic damage was classified into the core area (ADC and CBF<thresholds) and the mismatch area (ADC>threshold, CBF<threshold). Subsequent ADC and CBF values were then prospectively measured in each area at each time point.
Statistical Analysis
Data are presented as mean±standard deviation. Statistical analysis was performed with repeated-measures ANOVA for continuous variables. A two-tailed unpaired t-test was used to compare the parametric values. A two-tailed value of P<0.05 was considered significant.
Results
Experiment 1
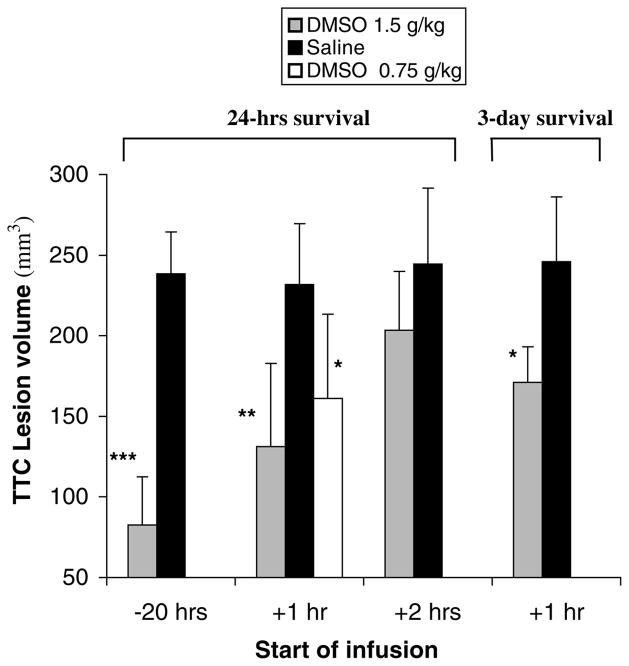
Physiologic variables are shown in Table 1. All data were within the normal range and did not vary significantly among different groups or at different time points (P>0.05). The corrected TTC-defined infarct volumes 24 h after MCAO for each group are illustrated in Figure 1. DMSO (1.5 g/kg) resulted in a highly significant reduction in infarct size when the infusion was initiated 20 h before (65% reduction; P<0.00001) or 1 h after (44% reduction; P=0.0006) occlusion. The infarct volume was also smaller in rats treated at 2 h after MCAO, but this was not significant (P=0.11).
Table 1.
Physiologic data before occlusion (baseline) and 3 or 4 h after MCAO
| Experiment 1 |
Experiment 2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| DMSO−20 | Saline−20 | DMSO+1 | Saline+1 | DMSO+2 | Saline+2 | DMSO+1 | Saline+1 | |
| Baseline | ||||||||
| MABP | 95±7 | 89±12 | 88±10 | 96±9 | 94±7 | 90±11 | 89±10 | 92±13 |
| Temp | 36.8±0.3 | 36.7±0.03 | 36.6±0.04 | 36.9±0.02 | 37.0±0.03 | 36.7±0.03 | 36.6±0.04 | 36.7±0.04 |
| pO2 | 84±9 | 91±10 | 88±9 | 85±5 | 86±7 | 90±8 | 87±8 | 89±6 |
| pCO2 | 42±6 | 41±8 | 42±5 | 39±6 | 43±6 | 41±5 | 38±6 | 42±5 |
| pH | 7.43±0.04 | 7.4±0.03 | 7.41±0.03 | 7.42±0.04 | 7.4±0.05 | 7.42±0.03 | 7.38±0.04 | 7.41±0.03 |
| Gluc | 169±31 | 180±34 | 153±28 | 167±37 | 187±35 | 161±37 | 175±28 | 159±38 |
| 3 or 4 h | ||||||||
| MABP | 93±11 | 91±12 | 93±8 | 97±11 | 98±12 | 93±7 | 95±10 | 90±10 |
| Temp | 36.9±0.02 | 36.8±0.03 | 36.8±0.04 | 37.2±0.03 | 37.1±0.03 | 36.8±0.03 | 36.9±0.03 | 37.1±0.04 |
| pO2 | 87±8 | 86±7 | 92±9 | 86±8 | 86±6 | 93±9 | 85±9 | 86±6 |
| pCO2 | 39±5 | 36±8 | 40±5 | 43±6 | 37±6 | 38±6 | 36±5 | 37±6 |
| pH | 7.39±0.05 | 7.41±0.03 | 7.42±0.03 | 7.38±0.04 | 7.37±0.05 | 7.41±0.04 | 7.37±0.03 | 7.38±0.05 |
| Gluc | 147±28 | 164±21 | 141±30 | 136±33 | 159±27 | 151±31 | 147±31 | 138±33 |
DMSO=dimethyl sulfoxide; MABP=mean arterial blood pressure (mm Hg); Temp=rectal body temperature (°C); pO2 and pCO2 in mm Hg; Gluc=plasma glucose (mg/dL). In experiments 1 and 2, physiologic data were acquired at 3 and 4 h after MCAO, respectively.
Figure 1.
2,3,5-Triphenyltetrazolium chloride (TTC) -defined corrected infarct volume 24 or 72 h after permanent middle cerebral artery occlusion (MCAO). Data are expressed as mean ±s.d. *P<0.01; **P<0.001; ***P<0.00001.
Dimethyl sulfoxide at a dose of 0.75 g/kg initiated 1 h after occlusion also resulted in a significant infarct reduction (31% reduction; P=0.008), but the extent of neuroprotection was less robust compared with the higher dose of 1.5 g/kg (P=0.24).
In the 3-day survival group (1.5 g/kg DMSO starting 1 h after MCAO), 50% of the untreated animals died prematurely between 48 and 72 h after MCAO, whereas no rat died prematurely in the DMSO group. 2,3,5-Triphenyltetrazolium chloride staining in rats that died prematurely was performed within 3 h after death. Infarct volume was significantly reduced in the DMSO-treated animals (30% reduction, P=0.005, Figure 1). There was a trend towards an increase in infarct size after 3 days in treated animals compared with the 24-h survival DMSO group (P>0.05).
The neurologic scores 24 h after MCAO were significantly improved in the 20 h preocclusion treatment group (2.0±0.7 versus 3.5±0.6; P=0.0008), the 1 h postocclusion treatment group (2.3±0.7 versus 3.3±0.8; P=0.02), and the 1 h postocclusion treatment group that received half of the dose (2.5±0.5 versus 3.3±0.8; P=0.04). No significant difference in neurologic status was observed with the infusion starting 2 h after occlusion (2.7±0.8 versus 3.3±0.7; P=0.17).
Experiment 2
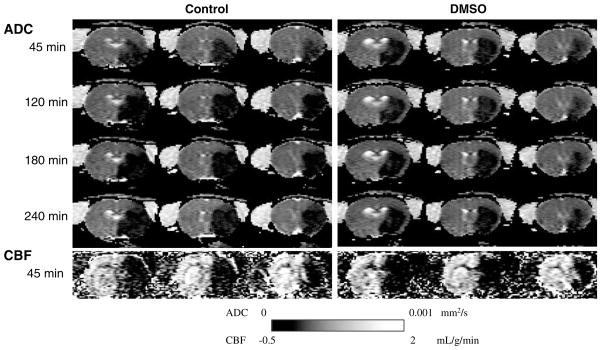
Mean arterial blood pressure, heart rate, and body temperature were comparable between the two groups and within the normal range throughout the study period (see also Table 1). Figure 2 shows the representative ADC and CBF maps from one control and one DMSO treated animal subjected to permanent MCAO.
Figure 2.
Representative apparent diffusion coefficient (ADC) and cerebral blood flow (CBF) maps from one control and one dimethyl sulfoxide (DMSO)-treated animal subjected to permanent middle cerebral artery occlusion (MCAO). Three of eight maps are shown at 45 mins (baseline), 120, 180, and 240 mins after occlusion for ADC maps, and at 45 mins for CBF maps. Dimethyl sulfoxide treatment was initiated 1 h after occlusion.
Temporal evolution of apparent diffusion coefficient- and cerebral blood flow-derived lesion volumes
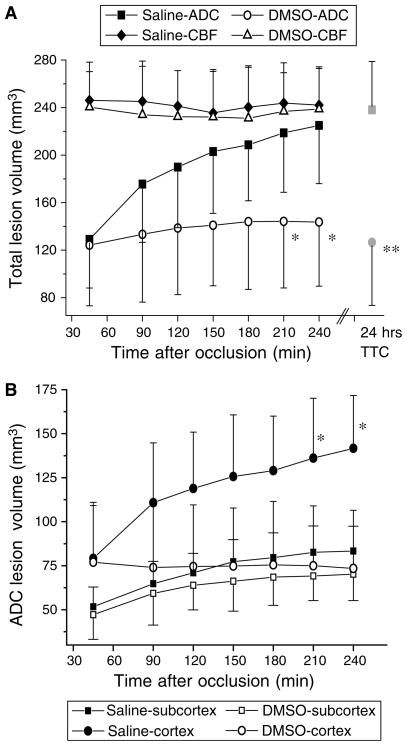
Figure 3A shows the temporal evolution of lesion volumes over the 4-h imaging time period for both the treatment and the control groups. Cerebral blood flow-derived lesion volumes remained relatively constant in both groups without significant changes across time. No significant differences were observed between groups at any time point (P>0.05). In contrast, the temporal evolution of the ADC-derived lesion volumes differed significantly between the two groups. The initial ADC lesion volumes were essentially identical (129±41 versus 124±51mm3, P=0.8). While the ADC lesions continued to enlarge gradually over time in the untreated animals, lesion volumes in the DMSO-treated group increased only slightly at 90 mins and then largely stopped evolving. The ADC lesion at 4 h was 225±51mm3 in the control group, whereas it was 143±64mm3 in the DMSO group (P<0.05 for the group differences at 210 and 240 mins).
Figure 3.
(A) Temporal evolution of total apparent diffusion coefficient (ADC)- and cerebral blood flow (CBF)-derived lesion volumes (mean±s.d.) by using previously validated viability thresholds in permanently occluded rats. Dimethyl sulfoxide (DMSO) or saline infusion was initiated 1 h after occlusion. Saline group: CBF-lesion=closed diamonds, ADC-lesion= closed squares, 2,3,5-triphenyltetrazolium chloride (TTC)-lesion=gray square; DMSO group: CBF-lesion=open triangles, ADC-lesion=open circles, TTC-lesion=gray circle. (B) Temporal evolution of cortical and subcortical ADC lesion volumes (mean±s.d.). Saline lesion: cortex=closed circles, subcortex =closed squares; DMSO lesion: cortex=open circles, subcortex=open squares. *P<0.05 for differences in ADC lesion volumes at same time points. **P=0.002 for difference in TTC lesion volume at 24 h after occlusion.
As shown in Figure 3B, the treatment effect was maximal in the cerebral cortex as the cortical ADC-derived lesion volume completely stopped progressing during DMSO treatment. No significant differences in the evolution of the subcortical ADC lesions were observed.
The diffusion/perfusion mismatch region identified as the difference between the abnormal perfusion and diffusion regions was virtually identical between groups at baseline. During the infusion, the mismatch volume in the DMSO group was similar at all time points (ranging from 41% to 46% of the CBF deficit) and the abnormal perfusion volume was significantly larger than the abnormal diffusion volume at all time points (P<0.02 each). In the control group, the difference between the abnormal perfusion and diffusion regions was only significant at 45 and 90 mins after MCAO (P<0.0001 and P<0.01, respectively), and gradually decreased over time. By 4 h after occlusion, the abnormal diffusion volume was 94% of the abnormal perfusion volume.
Consistent with the results in experiment 1, the mean TTC-defined infarct volume 24 h after occlusion was significantly smaller in the DMSO-treated group (P=0.002, Figure 3A). Compared with the MRI lesion volumes at 4 h after MCAO, the TTC infarct volume in the control group was slightly larger than the ADC-derived lesion volume and essentially identical to the CBF-derived lesion. In the DMSO-treated animals, however, the TTC-defined infarct size 24 h after occlusion was virtually identical to the baseline ADC lesion before treatment (Figure 3A).
Quantitative apparent diffusion coefficient and cerebral blood flow values
In the nonischemic left hemisphere (LH), the mean CBF and ADC values remained constant in both groups without significant differences between groups or different time points (P>0.05). The average CBF and ADC were 1.07±0.19 mL/g min and 0.77±0.03mm2/sec in the untreated animals, and 1.04±0.16 mL/g min and 0.78±0.03mm2/sec in the DMSO-treated group, respectively.
In the ischemic right hemisphere (RH), the CBF was constantly reduced to an average of 36%±2% of the LH in the control animals (P<0.0001) and to an average of 37%±3% of the LH in the treated animals (P<0.0001). The ADC in the RH gradually decreased over time in the control group, whereas the decline in ADC was less pronounced in the DMSO group and stopped decreasing by 180 mins after MCAO.
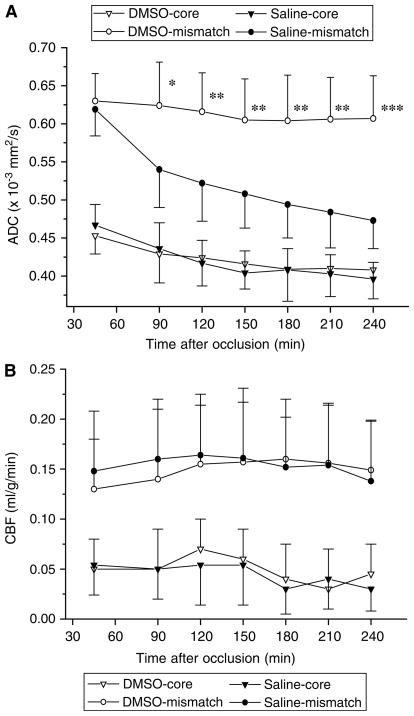
The temporal evolution of ADC and CBF values in the core and mismatch regions defined before treatment were prospectively quantified (Figures 4A and 4B). At baseline, the ADC and CBF characteristics of the core and mismatch regions were comparable between the two groups (P>0.05). In the mismatch region, the mean ADC values in the control group continuously decreased over time. In the DMSO group, the ADC values in the mismatch area declined only slightly at 90 and 150 mins and then remained constant up to 4 h after occlusion. The difference in mean ADC values between the DMSO and the control group was significant at all time points during treatment (P<0.05 each). In the core region, no significant differences in the evolution of ADC values were observed between untreated and treated animals (Figure 4A).
Figure 4.
Temporal evolution of apparent diffusion coefficient (ADC) (A) and cerebral blood flow (CBF) (B) in the core and mismatch regions defined before treatment (45 mins) in permanently occluded rats. Dimethyl sulfoxide (DMSO) or saline was administered 1 h after occlusion. Dimethyl sulfoxide group: core=open triangle, mismatch=open circles. Saline group: core=closed triangles, mismatch=closed circles. *P<0.05; **P<0.01; ***P<0.001.
Region-specific CBF characteristics did not differ significantly between the two groups at any time point (P>0.05, Figure 4B). In both groups, a similar gradient of CBF reduction from the ischemic periphery towards the ischemic core was observed.
Discussion
The present study demonstrated robust neuroprotection with DMSO in a widely used and established model of permanent focal cerebral ischemia. This effect was shown in vivo by diffusion and perfusion imaging, on neurologic scoring, and at postmortem by TTC staining at concentrations near those shown to be tolerated in humans.
Because immediate treatment is not clinically feasible in acute sporadic stroke, it is important to determine the therapeutic window. Dimethyl sulfoxide treatment initiated 1 h after occlusion resulted in a highly significant reduction of infarct size, whereas postischemic therapy after a 2-h delay showed only a trend towards neuroprotection. This agreed with the MRI findings, as the mismatch region in the untreated animals was significant up to 90 mins after MCAO but not at 2 h, implying that treatment initiated at 2 h is unlikely to show a significant reduction in infarct size. Moreover, the TTC infarct volume at 24 h in the non-MRI DMSO+ 2 h group was comparable with the ADC-derived lesion volume observed at 2 h in the MRI experiment control group, suggesting DMSO stopped further lesion growth and a rapid onset of action even with therapy initiated at 2 h. It is possible that higher concentrations of DMSO would provide a wider therapeutic window.
A long-lasting therapeutic effect was observed in the 3-day survival experiment when DMSO was administered 1 h after ischemia onset. Interestingly, the effect was less pronounced compared with the 24 h survival group. This suggests that part of the acute neuroprotection was only temporary. It is possible that repeated doses of intravenous DMSO would more effectively maintain neuroprotection. Nevertheless, DMSO therapy resulted in 100% survival for 3 days, whereas mortality rate in untreated animals was 50%. A 3-day period as an indicator for long-term effects was chosen because earlier studies demonstrated that there is no further increase in infarct volume 2 to 3 days after onset of ischemia in this stroke model (Rudin et al, 2001; van Lookeren Campagne et al, 1999). Furthermore, this permanent ischemia model is not ideal for examining longer survival periods because of the high mortality rate even in treated animals.
Previous studies on the effects of DMSO on focal brain ischemia have demonstrated contradictory results. In a permanent MCAO model in cats, de la Torre (1991) observed significant cell protection with 0.9 mg/kg intravenous DMSO administered 1 h after ischemia, whereas Little et al (1981) found no beneficial effects although treatment was initiated earlier (at occlusion) at an even higher dose (2.5 mg/kg intravenous). In rhesus monkeys, DMSO administered 4 h after MCAO reduced neurologic deficits and histopathologic changes (de la Torre and Surgeon, 1976), while treatment starting 30 mins after occlusion did not influence neurologic status or infarct size in baboons (Little et al, 1983). Interestingly, in dogs subjected to 6 h of embolic MCA occlusion, DMSO treatment (2 g/kg) beginning at the time of occlusion completely prevented cerebral infarction (Laha et al, 1978). The reasons for these discrepancies between different studies remain unclear, but differences in route of administration, timing, dosage, and stroke models used may be possible explanations. It will be important in future studies to use the paradigm outlined herein in a non-primate model of ischemia.
In the same permanent MCAO model as used in this study, Shimizu et al (1997) evaluated the effects of different DMSO doses when administered intraperitoneally 30 mins before occlusion. A significant reduction in infarct size, of approximately 36%, was achieved with doses ranging between 0.09 and 3 g/kg. Interestingly, this degree of neuroprotection is comparable with our findings in the animals where treatment was initiated 1 h after occlusion.
The initial pretreatment ADC lesion volume remained essentially constant in the DMSO-treated animals, while it progressively increased in the control group. This effect of ‘ischemic lesion freezing’ occurred mainly in the cortex. Because the CBF-derived lesion volumes remained unchanged, DMSO treatment resulted in a persistent diffusion/perfusion mismatch. Consistent with the persistent mismatch, the ADC in the mismatch zone remained relatively stable without a further decrease in DMSO-treated rats, whereas in the control group, the ADC in the mismatch area shifted towards the core zone values as ischemia progressed. In contrast, DMSO had no effect on the ADC characteristics in the core region, typically located in the striatum. These findings indicated that DMSO essentially exhibited its neuroprotective effects on tissue with still normal diffusion characteristics (mismatch tissue), and such tissues were primarily in the cortex, as confirmed by a complete cessation of lesion growth in the cortex.
In isolated dog brains subjected to complete ischemia, DMSO administration during reoxygenation resulted in a substantial increase in ATP associated with significantly lower lactate levels and incomplete metabolic recovery (Gilboe et al, 1991). Dimethyl sulfoxide has been reported to suppress neuronal synaptic activity and may consequently reduce cellular energy consumption (Nakahiro et al, 1992; Sawada and Sato, 1975). Dimethyl sulfoxide has also been shown to increase the latency of anoxic depolarization of hippocampal neurons after acute ischemia (Hulsmann et al, 1999). Peri-infarct depolarizations in the penumbral tissue, however, are accompanied by disruption of ion homeostasis and the need for metabolic energy to restore the ionic gradients, eventually leading to ATP depletion and irreversible cell damage (Back et al, 1995). These findings—an increase in ATP combined with reduced energy consumption—suggest that the protective effects of DMSO on tissue that has not yet advanced to bioenergetic failure (mismatch) may reflect favorable changes in the balance of energy supply and demand. These changes would maintain ion-pump function and membrane potential as well as preventing a further increase in lactate and tissue acidosis.
Two previous studies evaluated the effect of DMSO on the CBF in permanent MCAO in cats. Little et al (1981) found no effect of DMSO on CBF, accompanied with no effects on histopathology. However, de la Torre (1991) observed a sustained increase in CBF in the ischemic cortex by 27%, associated with cortical cell protection. Based on these findings, the authors concluded that the protective effects of DMSO may result, at least partially, from its ability to increase CBF in brain ischemia. Our data do not support this hypothesis. Using combined perfusion and diffusion imaging, we demonstrated that the neuroprotective effect of DMSO was not associated with an increase in CBF, neither hemispheric nor region-specific, and that changes in CBF are unlikely to be a major mechanism of neuroprotection in the acute phase of ischemia in this stroke model.
Although consistent with many established techniques, the accuracy of perfusion measurements by arterial spin labeling could be subject to errors from the magnetization-transfer (Silva et al, 1995), transit-time (Zhou et al, 2001), and water-exchange (Zhou et al, 2001; Parkes and Tofts, 2002) effects. The magnetization-transfer effect was not an issue with the actively decoupled two-coil system (Duong et al, 2000). Transit-time effect in small animals and water-exchange effect are small (Zhou et al, 2001; Parkes and Tofts, 2002) and unlikely to alter the conclusions of this study. Many of these effects are more severe at low CBF value. Despite these limitations, CBF imaging remains a valuable noninvasive tool for imaging strokes.
It has been postulated that there is a critical CBF threshold(s) below which tissues are destined to become infarcted. Crockard et al (1987) used a hydrogen-clearance technique and derived a CBF threshold of 0.2 mL/g min that correlated with the loss of high-energy phosphates in gerbils. Two studies using 14C-iodoantipyrine autoradiography reported a critical CBF threshold of 0.18 and 0.19mL/g min that corresponded to energy failure at 2 h after permanent MCAO in rats (Hoehn-Berlage et al, 1995; Kohno et al, 1995). The CBF threshold that approximated lactate acidosis was shown to be 0.31±0.11 mL/g min (Hoehn-Berlage et al, 1995), and diffusion disturbance was observed at CBF values below 0.34 to 0.41 mL/g min depending on the duration of ischemia (Kohno et al, 1995). Belayev et al (1997) and Zhao et al (1997) used 14C-iodoantipyrine autoradiography and derived upper CBF limits for the ischemic core and the penumbra of 0.24 and 0.47 mL/g min, respectively; the average penumbral CBF threshold was reported to be 0.3 mL/g min. These findings suggest that different critical thresholds exist for different biological events after ischemia and that these thresholds are dependent on animal models, duration and/or severity of ischemic injury, and the accuracy of cross-modality comparison. While these critical thresholds derived using different postmortem correlation techniques are expected to be slightly different from each other, our TTC-derived thresholds were most consistent with the onset of reduced glucose metabolism and lactate acidosis but before energy failure (Hoehn-Berlage et al, 1995; Kohno et al, 1995), and the penumbral CBF threshold (Belayev et al, 1997, Zhao et al, 1997).
Other properties of DMSO which may confer neuroprotection include antiinflammatory effects (Wood and Wood, 1975), impairment of platelets aggregation and adhesiveness (Dujovny et al, 1983; Schiffer et al, 1976), prevention of glutamate-induced excitotoxic death in hippocampal neurons (Lu and Mattson, 2001), Na+-channel blocking activity (Hulsmann et al, 1999), and free radical scavenging (Repine et al, 1981). These data show that DMSO is capable of modifying a variety of subsystems believed to be involved in the pathophysiologic cascade of ischemia. It is not yet known which effect is the most prominent and responsible for its neuroprotective properties. It seems more likely that the benefits are the result of a more complex interaction of several mechanisms and the actions on different subsystems deliver additive or synergistic effects.
The most robust effect we observed was when DMSO was administered 20 h before ischemia. The reason for this greater degree of neuroprotection is unclear. Dimethyl sulfoxide has a biologic half-life of 10 to 12 h, and after a single intravenous injection in rats, approximately 80% is eliminated after 24 h (Kolb et al, 1967; Denko et al, 1967). Because a low dose of even 0.09 mg/kg DMSO (6% of the dose used in this study) has been shown by others to provide substantial neuroprotection (Shimizu et al, 1997), the efficacy of a 20 h pretreatment may be the result of residual DMSO in the brain tissue. Alternatively, indirect mechanisms initiated by DMSO pretreatment, such as induction of gene expression (Rifkind et al, 1983), may also contribute to the observed effects.
Our data suggest DMSO should be revisited as a candidate for stroke therapy. It was effective in a reasonable time window in this permanent occlusion model and showed long-lasting neuroprotection. Dimethyl sulfoxide exerted its effect by preservation of the diffusion/perfusion mismatch, an approximation of the tissue at risk with clearly limited lifespan, and the main target for rtPA thrombolysis (Schlaug et al, 1999). However, complete recanalization within the first hours after rtPA treatment has been reported to occur in only about one third of the patients (Christou et al, 2000; Chalela et al, 2004; Rother et al, 2002). Thus, a drug capable of protecting the mismatch tissue for several hours, such as DMSO, could extend the time window for eventual recanalization. Dimethyl sulfoxide may also offer an option as a preventative measure in patients undergoing procedures with an increased risk of developing per interventional brain ischemia, such as carotid stenting or coronary artery bypass surgery. Dimethyl sulfoxide treatment has been shown to be feasible and safe over several days in case series of acute stroke patients (Karaca et al, 2002) and patients with increased ICP (Karaca et al, 1991; Waller et al, 1983), at doses comparable with those used in this study (1.12 g/kg/day and 1 to 1.12 g/kg every 6 h, respectively). Adverse side effects including hypernatremia, fluid overload, and hemolysis have mainly been reported at high rates of infusion or highly concentrated solutions (Waller et al, 1983; Marshall et al, 1984).
References
- Back T, Zhao W, Ginsberg MD. Three-dimensional image analysis of brain glucose metabolism–blood flow uncoupling and its electrophysiological correlates in the acute ischemic penumbra following middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1995;15:566–77. doi: 10.1038/jcbfm.1995.70. [DOI] [PubMed] [Google Scholar]
- Belayev L, Zhao W, Busto R, Ginsberg MD. Transient middle cerebral artery occlusion by intraluminal suture: I. Three-dimensional autoradiographic image-analysis of local cerebral glucose metabolism-blood flow interrelationships during ischemia and early recirculation. J Cereb Blood Flow Metab. 1997;17:1266–80. doi: 10.1097/00004647-199712000-00002. [DOI] [PubMed] [Google Scholar]
- Camp PE, James HE, Werner R. Acute dimethyl sulfoxide therapy in experimental brain edema: Part I. Effects on intracranial pressure, blood pressure, central venous pressure, and brain water and electrolyte content. Neurosurgery. 1981;9:28–33. doi: 10.1227/00006123-198107000-00005. [DOI] [PubMed] [Google Scholar]
- Chalela JA, Kang DW, Luby M, Ezzeddine M, Latour LL, Todd JW, Dunn B, Warach S. Early magnetic resonance imaging findings in patients receiving tissue plasminogen activator predict outcome: Insights into the pathophysiology of acute stroke in the thrombolysis era. Ann Neurol. 2004;55:105–12. doi: 10.1002/ana.10781. [DOI] [PubMed] [Google Scholar]
- Christou I, Alexandrov AV, Burgin WS, Wojner AW, Felberg RA, Malkoff M, Grotta JC. Timing of recanalization after tissue plasminogen activator therapy determined by transcranial doppler correlates with clinical recovery from ischemic stroke. Stroke. 2000;31:1812–6. doi: 10.1161/01.str.31.8.1812. [DOI] [PubMed] [Google Scholar]
- Crockard HA, Gadian DG, Frackowiak RS, Proctor E, Allen K, Williams SR, Russell RW. Acute cerebral ischemia: concurrent changes in cerebral blood flow, energy metabolites, pH, and lactate measured with hydrogen clearance and 31P and 1H nuclear magnetic resonance spectroscopy. II. Changes during ischemia. J Cereb Blood Flow Metab. 1987;7:394–402. doi: 10.1038/jcbfm.1987.82. [DOI] [PubMed] [Google Scholar]
- de la Torre JC, Surgeon JW. Dexamethasone and DMSO in experimental transorbital cerebral infarction. Stroke. 1976;7:577–83. doi: 10.1161/01.str.7.6.577. [DOI] [PubMed] [Google Scholar]
- de la Torre JC. Synergic activity of combined prostacyclin: dimethyl sulfoxide in experimental brain ischemia. Can J Physiol Pharmacol. 1991;69:191–8. doi: 10.1139/y91-028. [DOI] [PubMed] [Google Scholar]
- Denko CW, Goodman RM, Miller R, Donovan T. Distribution of dimethyl sulfoxide-35S in the rat. Ann NY Acad Sci. 1967;141:77–84. doi: 10.1111/j.1749-6632.1967.tb34868.x. [DOI] [PubMed] [Google Scholar]
- Dujovny M, Rozario R, Kossovsky N, Diaz FG, Segal R. Antiplatelet effect of dimethyl sulfoxide, barbiturates, and methyl prednisolone. Ann NY Acad Sci. 1983;411:234–44. doi: 10.1111/j.1749-6632.1983.tb47304.x. [DOI] [PubMed] [Google Scholar]
- Duong TQ, Silva AC, Lee SP, Kim SG. Functional MRI of calcium-dependent synaptic activity: cross correlation with CBF and BOLD measurements. Magn Reson Med. 2000;43:383–92. doi: 10.1002/(sici)1522-2594(200003)43:3<383::aid-mrm10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Gilboe DD, Kintner D, Fitzpatrick JH, Emoto SE, Esanu A, Braquet PG, Bazan NG. Recovery of postischemic brain metabolism and function following treatment with a free radical scavenger and platelet-activating factor antagonists. J Neurochem. 1991;56:311–9. doi: 10.1111/j.1471-4159.1991.tb02597.x. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Latour LL, Sotak CH, Dardzinski BJ, Fisher M. Temperature dependent change of apparent diffusion coefficient of water in normal and ischemic brain of rats. J Cereb Blood Flow Metab. 1994;14:383–90. doi: 10.1038/jcbfm.1994.49. [DOI] [PubMed] [Google Scholar]
- Hoehn-Berlage M, Norris DG, Kohno K, Mies G, Leibfritz D, Hossmann KA. Evolution of regional changes in apparent diffusion coefficient during focal ischemia of rat brain: the relationship of quantitative diffusion NMR imaging to reduction in cerebral blood flow and metabolic disturbances. J Cereb Blood Flow Metab. 1995;15:1002–11. doi: 10.1038/jcbfm.1995.126. [DOI] [PubMed] [Google Scholar]
- Hulsmann S, Greiner C, Kohling R, Wolfer J, Moskopp D, Riemann B, Lucke AH, Speckmann EJ. Dimethyl sulfoxide increases latency of anoxic terminal negativity in hippocampal slices of guinea pig in vitro. Neurosci Lett. 1999;261:1–4. doi: 10.1016/s0304-3940(98)00999-9. [DOI] [PubMed] [Google Scholar]
- Karaca M, Bilgin UY, Akar M, de la Torre JC. Dimethyl sulphoxide lowers ICP after closed head trauma. Eur J Clin Pharmacol. 1991;40:113–4. doi: 10.1007/BF00315149. [DOI] [PubMed] [Google Scholar]
- Karaca M, Kilic E, Yazici B, Demir S, de la Torre JC. Ischemic stroke in elderly patients treated with a free radical scavenger-glycolytic intermediate solution: a preliminary pilot trial. Neurol Res. 2002;24:73–80. doi: 10.1179/016164102101199567. [DOI] [PubMed] [Google Scholar]
- Kohno K, Hoeh-Berlage M, Mies G, Back T, Hossmann KA. Relationship between diffusion-weighted MR images, cerebral blood flow, and energy state in experimental brain infarction. Magn Reson Imag. 1995;13:73–80. doi: 10.1016/0730-725x(94)00080-m. [DOI] [PubMed] [Google Scholar]
- Kolb KH, Jaenicke G, Kramer M, Schulze PE. Absorption, distribution and elimination of labeled dimethyl sulfoxide in man and animals. Ann NY Acad Sci. 1967;141:85–95. doi: 10.1111/j.1749-6632.1967.tb34869.x. [DOI] [PubMed] [Google Scholar]
- Laha RK, Dujovny M, Barrionuevo PJ, DeCastro SC, Hellstrom HR, Maroon JC. Protective effects of methyl prednisolone and dimethyl sulfoxide in experimental middle cerebral artery embolectomy. J Neurosurg. 1978;49:508–16. doi: 10.3171/jns.1978.49.4.0508. [DOI] [PubMed] [Google Scholar]
- Little JR, Cook A, Lesser RP. Treatment of acute focal cerebral ischemia with dimethyl sulfoxide. Neurosurgery. 1981;9:34–9. doi: 10.1227/00006123-198107000-00006. [DOI] [PubMed] [Google Scholar]
- Little JR, Spetzler RF, Roski RA, Selman WR, Zabramski J, Lesser RP. Ineffectiveness of DMSO in treating experimental brain ischemia. Ann NY Acad Sci. 1983;411:269–77. doi: 10.1111/j.1749-6632.1983.tb47308.x. [DOI] [PubMed] [Google Scholar]
- Lu C, Mattson MP. Dimethyl sulfoxide suppresses NMDA- and AMPA-induced ion currents and calcium influx and protects against excitotoxic death in hippocampal neurons. Exp Neurol. 2001;170:180–5. doi: 10.1006/exnr.2001.7686. [DOI] [PubMed] [Google Scholar]
- Maier CM, Ahern K, Cheng ML, Lee JE, Yenari MA, Steinberg GK. Optimal depth and duration of mild hypothermia in a focal model of transient cerebral ischemia: effects on neurologic outcome, infarct size, apoptosis, and inflammation. Stroke. 1998;29:2171–80. doi: 10.1161/01.str.29.10.2171. [DOI] [PubMed] [Google Scholar]
- Marshall LF, Camp PE, Bowers SA. Dimethyl sulfoxide for the treatment of intracranial hypertension: a preliminary trial. Neurosurgery. 1984;14:659–63. doi: 10.1227/00006123-198406000-00002. [DOI] [PubMed] [Google Scholar]
- McGraw CP. The effect of dimethyl sulfoxide (DMSO) on cerebral infarction in the Mongolian gerbil. Acta Neurol Scand. 1977;64(Suppl):160–1. [PubMed] [Google Scholar]
- Meng X, Fisher M, Shen Q, Sotak CH, Duong TQ. Characterizing the diffusion/perfusion mismatch in experimental focal cerebral ischemia. Ann Neurol. 2004;55:207–12. doi: 10.1002/ana.10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies SA, Hoff JT, Betz AL. Middle cerebral artery occlusion in rats: a neurological and pathological evaluation of a reproducible model. Neurosurgery. 1992;31:100–6. doi: 10.1227/00006123-199207000-00014. [DOI] [PubMed] [Google Scholar]
- Nakahiro M, Arakawa O, Narahashi T, Ukai S, Kato Y, Nishinuma K, Nishimura T. Dimethyl sulfoxide (DMSO) blocks GABA-induced current in rat dorsal root ganglion neurons. Neurosci Lett. 1992;138:5–8. doi: 10.1016/0304-3940(92)90459-k. [DOI] [PubMed] [Google Scholar]
- Parkes LM, Tofts PS. Improved accuracy of human cerebral blood perfusion measurements using arterial spin labeling: accounting for capillary water permeability. Magn Reson Med. 2002;48:27–41. doi: 10.1002/mrm.10180. [DOI] [PubMed] [Google Scholar]
- Repine JE, Pfenninger OW, Talmage DW, Berger EM, Pettijohn DE. Dimethyl sulfoxide prevents DNA nicking mediated by ionizing radiation or iron/hydrogen peroxide-generated hydroxyl radical. Proc Natl Acad Sci USA. 1981;78:1001–3. doi: 10.1073/pnas.78.2.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkind RA, Sheffery M, Profous-Juchelka HR, Reuben RC, Marks PA. Induction of globin gene expression during erythroid cell differentiation. Ann NY Acad Sci. 1983;411:141–9. doi: 10.1111/j.1749-6632.1983.tb47296.x. [DOI] [PubMed] [Google Scholar]
- Rother J, Schellinger PD, Gass A, Siebler M, Villringer A, Fiebach JB, Fiehler J, Jansen O, Kucinski T, Schoder V, Szabo K, Junge-Hulsing GJ, Hennerici M, Zeumer H, Sartor K, Weiller C, Hacke W Kompetenznetzwerk Schlaganfall Study Group. Effect of intravenous thrombolysis on MRI parameters and functional outcome in acute stroke <6 hours. Stroke. 2002;33:2438–45. doi: 10.1161/01.str.0000030109.12281.23. [DOI] [PubMed] [Google Scholar]
- Rudin M, Baumann D, Ekatodramis D, Stirnimann R, McAllister KH, Sauter A. MRI analysis of the changes in apparent water diffusion coefficient, T(2) relaxation time, and cerebral blood flow and volume in the temporal evolution of cerebral infarction following permanent middle cerebral artery occlusion in rats. Exp Neurol. 2001;169:56–63. doi: 10.1006/exnr.2001.7650. [DOI] [PubMed] [Google Scholar]
- Sawada M, Sato M. The effect of dimethyl sulfoxide on the neuronal excitability and cholinergic transmission in Aplysia ganglion cells. Ann NY Acad Sci. 1975;243:337–57. doi: 10.1111/j.1749-6632.1975.tb25375.x. [DOI] [PubMed] [Google Scholar]
- Schiffer CA, Whitaker CL, Schmukler M, Aisner J, Hilbert SL. The effect of dimethyl sulfoxide on in vitro platelet function. Thromb Haemost. 1976;36:221–9. [PubMed] [Google Scholar]
- Schlaug G, Benfield A, Baird AE, Siewert B, Lovblad KO, Parker RA, Edelman RR, Warach S. The ischemic penumbra: operationally defined by diffusion and perfusion MRI. Neurology. 1999;53:1528–37. doi: 10.1212/wnl.53.7.1528. [DOI] [PubMed] [Google Scholar]
- Shen Q, Meng X, Fisher M, Sotak CH, Duong TQ. Pixel-by-pixel spatiotemporal progression of focal ischemia derived using quantitative perfusion and diffusion imaging. J Cereb Blood Flow Metab. 2003;23:1479–1488. doi: 10.1097/01.WCB.0000100064.36077.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Simon RP, Graham SH. Dimethylsulfoxide (DMSO) treatment reduces infarction volume after permanent focal cerebral ischemia in rats. Neurosci Lett. 1997;239:125–7. doi: 10.1016/s0304-3940(97)00915-4. [DOI] [PubMed] [Google Scholar]
- Silva AC, Zhang W, Williams DS, Koretsky AP. Multi-slice MRI of rat brain perfusion during amphetamine stimulation using arterial spin labeling. Magn Reson Med. 1995;33:209–14. doi: 10.1002/mrm.1910330210. [DOI] [PubMed] [Google Scholar]
- Strupp JP. Stimulate: A GUI based fMRI analysis software package. Neuroimage. 1996;3:S607. [Google Scholar]
- van Lookeren Campagne M, Thomas GR, Thibodeaux H, Palmer JT, Williams SP, Lowe DG, van Bruggen N. Secondary reduction in the apparent diffusion coefficient of water, increase in cerebral blood volume, and delayed neuronal death after middle cerebral artery occlusion and early reperfusion in the rat. J Cereb Blood Flow Metab. 1999;19:1354–64. doi: 10.1097/00004647-199912000-00009. [DOI] [PubMed] [Google Scholar]
- Waller FT, Tanabe CT, Paxton HD. Treatment of elevated intracranial pressure with dimethyl sulfoxide. Ann NY Acad Sci. 1983;411:286–92. doi: 10.1111/j.1749-6632.1983.tb47310.x. [DOI] [PubMed] [Google Scholar]
- Wood DC, Wood J. Pharmacologic and biochemical considerations of dimethyl sulfoxide. Ann NY Acad Sci. 1975;243:7–19. doi: 10.1111/j.1749-6632.1975.tb25339.x. [DOI] [PubMed] [Google Scholar]
- Zhao W, Belayev L, Ginsberg MD. Transient middle cerebral artery occlusion by intraluminal suture: II. Neurological deficits, and pixel-based correlation of histopathology with local blood flow and glucose utilization. J Cereb Blood Flow Metab. 1997;17:1281–90. doi: 10.1097/00004647-199712000-00003. [DOI] [PubMed] [Google Scholar]
- Zhou J, Wilson DA, Ulatowski JA, Traystman RJ, van Zijl PC. Two-compartment exchange model for perfusion quantification using arterial spin tagging. J Cereb Blood Flow Metab. 2001;21:440–55. doi: 10.1097/00004647-200104000-00013. [DOI] [PubMed] [Google Scholar]