Abstract
While much progress has been made in developing drugs against a few prominent viruses such as HIV, few examples exist for emerging infectious agents. In some cases broad spectrum anti-viral drugs, such as ribavirin, are effective, but for some groups of viruses, these show little efficacy in animal models. Traditional methods focus on screening small molecule libraries to identify drugs that target virus factors, with the intention that side-effects to the host can be minimized. However, this greatly limits potential drug targets and virus genes can rapidly mutate to avoid drug action. Recent advances in siRNA gene targeting technologies have provided a powerful tool to specifically target and suppress the expression of cell genes. Since viruses are completely dependent upon host cell proteins for propagation, siRNA screening promises to reveal novel cell proteins and signaling pathways that may be viable targets for drug therapy regimens. Here we used an siRNA screening approach to identify gene products that play critical roles in Ebola virus infection. By gene cluster analysis, proteins in phosphatidylinositol-3-kinase and calcium/calmodulin kinase related networks were identified as important for Zaire Ebola virus infection and prioritized for further evaluation. Key roles of each were confirmed by testing available drugs specific for members of each pathway. Interestingly, both sets of proteins are also important in cancer and subject to intense investigation. Thus development of new drugs against these cancer targets may also prove useful in combating Ebola virus.
Keywords: Ebola virus, antiviral, PI3 kinase, CAMK2
Introduction
Over the past two decades we have seen a steady but slow growth in the identification and use of new antiviral compounds that began with members of the smallpox and herpes virus families [Bean 1992]. For both, virally encoded thymidine kinases that differed significantly in specificity from host enzymes were targeted. The viral enzymes promiscuously phosphorylated a number of toxic thymidine analogs which where then incorporated into the virus genome during replication, blocking further virus propagation. Since these successes, efforts to develop anti-viral drugs have primarily focused on similar virus proteins that are either unique to the virus or have differing properties from their mammalian counterparts. With HIV, this strategy has worked well, culminating in effective multi-drug cocktail therapies that target the viral RNA dependent DNA polymerase and the viral protease [Napravnik et al. 2007]. Broad spectrum anti-virals have also been developed that target viral RNA-dependent RNA polymerases, an enzyme found in all viruses that have RNA genomes (except retroviruses). Ribavirin is one such drug and is used in combination with interferon to treat Hepatitis C infections [Deutsch and Hadziyannis 2008]. However, for a number of viruses, including alphaviruses and filoviruses, ribavirin is ineffective at controlling virus replication and disease in animal studies [Canonico et al. 1984].
Aside from viral proteases and polymerases, few other viral proteins stand out as potential drug targets. Inhibitors of virus entry are one new promising class of drugs that show clinical efficacy. Inhibitors that block virus entry into cells, by targeting the virus envelope glycoproteins (GPs) have yielded a clinically approved compound for HIV [Chen et al. 2002] as well as a promising lead to treat respiratory syncytial virus infections [Cianci et al. 2005]. However, both of these compounds are unrelated and their development required substantial investments of time, effort and capital. Even for drugs that target virus specific components, patient tolerance still needs to be closely monitored as there is sufficient cross-reactivity with host proteins to cause adverse side-effects [Esser et al. 2007].
All viruses are obligate parasites needing access to cell proteins and constituents to establish an infection and produce new progeny. This aspect of the virus replication cycle offers many potential targets for therapy. By inhibiting specific cell proteins that are required for virus replication it should be possible to interrupt the virus life cycle. This approach is beginning to be exploited by the pharmaceutical industry and has led to the development of HIV infection inhibitors that block virus entry by interfering with co-receptor and virus interactions [Schurmann et al. 2007]. However, concerns about complex side effects of inhibiting cell functions exist. Recent work by our group and others has revealed that when multiple protein isoforms co-exist within a cell, with apparently overlapping function, a virus will have a greater dependency on one isoform over others [Kolokoltsov et al. 2007]. This implies that viruses are finely tuned to depend upon highly specialized cell pathways that co-exist in parallel with other pathways of seemingly redundant function. This finding indicates that for a drug to be active against a virus it need impact only one protein isoform and affect one sub-pathway. Then by specifically targeting this pathway it should be possible to inhibit virus infection with minimal impact on cell viability in general.
One advantage of targeting cellular proteins is that they are not subject to the rapid mutation rates seen for virus genomes. Virus replication is typically error prone due to polymerase function and other features of genome synthesis. Genomes of HIV have a mutation rate of 10−4 changes/base [Katz and Skalka 1990] and similar error rates are seen in other virus types [Freistadt et al. 2007]. Most of the 9 kb genomes in a population of HIV particles therefore contain at least one mutation. Viruses can then rapidly adapt to selective pressures applied by a drug, making emergence of drug resistance likely. Indeed, the major cause of HAART therapy failure against HIV is the development of multi-drug resistant viruses [Napravnik et al. 2007]. In contrast, cellular genes are maintained by high fidelity replication mechanisms that give mutation rates below 10−10/bp per replication cycle [Kunkel and Bebenek 2000]. This means that drug resistance is much less likely to occur as the result of mutation in the cellular gene target. Another advantage is that we already have a large repertoire of drugs that target cell proteins. Many of these have established histories of clinical use. Finding new uses for these drugs has a great potential for rapid, cheaper development of disease treatments [Carley 2005; Chong and Sullivan 2007].
In the present work we tested the potential of using siRNA screening to identify cell genes, that when suppressed, impact virus infection of cells. Zaire Ebola virus was used. It and other species of Ebola virus are emerging viruses, of serious medical and biodefense concern for which only limited information is available. The Zaire species is most often associated with outbreaks with very high mortality rates, on average between 80 to 90% [Peters 1996]. Apart from palliative measures, there is no effective treatment for the disease. Unfortunately, this virus has not commanded the necessary resources to develop drugs. By identifying cell proteins important for EBOV infection it may be possible to gain a greater understanding of the infection mechanisms of this virus and develop new treatment strategies. In our approach we screened for inhibitors of virus penetration into cells using a high-throughput virus entry assay based on pseudotyped virus, which can be performed at BSL2 conditions. Ranking of siRNA efficacy followed by gene cluster analysis was then used to prioritize hits for testing of candidate drugs targeting key members of candidate gene product networks. These drugs were then tested against wild type EBOV under BSL4 conditions. We show that this approach is useful and effective in identifying that EBOV has a novel and unexpected dependence on PI3 kinases and CAMK2 enzyme activities. Both enzymes are the subject of intense investigation as the root cause of other diseases, in particular cancer and numerous drugs are being tested in the clinic. This work therefore offers new prospects for treatment of EBOV infection.
Materials and Methods
Chemicals
All chemicals were Ultragrade from Sigma (St. Louis, MO) unless stated otherwise. Dharmafect Cell Culture Reagent (DCCR) and DharmaFECT 1 transfection reagent used for siRNA transfection were from Dharmacon (Lafayette, CO).
Cell lines and culture conditions
Cells were maintained in a humidified air-5% CO2 atmosphere incubator at 37°C. 293 FT cells (Invitrogen, Carlsblad, CA) were used to express envelope glycoproteins by plasmid transfection. Screens were performed using HEK293 cells and were grown in DMEM (Invitrogen, Carlsblad, CA) supplemented with 10% fetal bovine serum (Gemini Bioproducts, West Sacramento, CA). Vero-E6 cells were used for verification of drug activities on wild type virus and were cultured as for HEK293 cells.
Virus stocks
Cultivation of wild type EBOV and determination of virus titer
EBOV, Zaire strain, was cultivated on Vero-E6 cells by infection at an MOI of 0.1. Culture supernatants were collected after 10 d and clarified by centrifugation at 2000 × g for 15 min. Virus concentration was determined by titration on Vero-E6 cells. Cells were incubated with virus for 1 h and then overlaid with 0.8% tragacanth gum in DMEM/4% FBS. 10 d post-infection cells were fixed with 2% formalin and stained with crystal violet to count plaques. All experiments with EBOV were performed under biosafety level 4 conditions in the Robert E. Shope BSL-4 Laboratory, UTMB.
Production of envelope glycoprotein pseudotyped lentiviruses
Pseudotyped lentiviruses were produced following previously published methods [Kolokoltsov et al. 2005]. Virus GP pseudotyped lentiviruses (LV) were prepared for use in siRNA screens and as controls in drug treatment experiments. Pseudotypes were made bearing the GPs of Zaire Ebola virus. For screening purposes, the LV pseudotype encoded firefly luciferase as the reporter of infection. Pseudotyped virus was assembled by transfecting 293 FT cells (Invitrogen) with plasmids encoding HIV LV core structural proteins (pLP1), HIV Rev (pLP2) together with plasmids encoding the firefly luciferase reporter gene (pLENTI6-fluc) and the GP of the Zaire strain of EBOV (pEBOVenv). The EBOV envelope expression construct was supplied by Dr. Sanchez (CDC). The firefly luciferase reporter construct, pLENTI6-fluc, was made by inserting a codon optimized firefly luciferase gene between BamHI and XhoI sites of the pLENTI6 plasmid (Invitrogen). Transfection was done by calcium phosphate [Chen and Okayama 1987]. After 2 d, culture supernatants were collected, filtered through a 0.45 μm filter to remove cell debris, and the filtered virus was used immediately or stored frozen at −80°C.
The titer of the pseudotyped virus was determined by limiting dilution on HEK293 cells. The concentration of virus was then optimized to yield 20–50,000 cps per 10 μl of virus culture supernatant added per well of a 96-well plate. The expression of luciferase was detected 36 h post-infection using a Veritas 96-well plate reading luminometer. Assays were performed in white walled 96-well Costar tissue culture plates (Corning, Lowell, MA). From previous work, approximately 100 cps corresponded to 1 colony forming units of virus. This set the MOI for infection to 0.05 per well.
Production of envelope glycoprotein pseudotyped vesicular stomatitis viruses
Vesicular stomatitis virus core pseudotyped virus was made according to previously described methods [Matsuura et al. 2001]. HEK293 cells were transfected with plasmids encoding EBOV (described above) or VEEV GP [Kolokoltsov et al. 2005]. After 24 h, the cells were infected with a GFP-encoding VSV parent stock virus that was defective for VSV-G expression (provipded by Dr. M. Whitt, University of Tennessee, Memphis, TN) and progeny pseudotyped virus harvested after a further 24 h. The titer of the pseudotyped virus was determined by counting GFP-expressing infected cells, 18 h post infection, using an epifluorescence microscope.
siRNA library screening and analysis
The siRNA library used was a subset of the druggable genome library (Ambion, Austin, TX) comprising siRNA targeting kinase and phosphorylase genes. A total of 720 distinct genes were targeted using 3 independent siRNA for each gene. Each siRNA was tested independently in separate wells.
The method used was described previously [Kolokoltsov et al. 2007]. All transfections were performed in 96-well format. The siRNA was dissolved in 25 μl of 1.6% (v/v) stock of DharmaFECT 1 transfection reagent in DCCR (Dharmacon, CA) and incubated for 30 min. Then 0.5 × 104 HEK293 cells in 100 μl medium were added and incubated for 24 h. The final concentration of pooled siRNA per well was 40 nM. This amount was sufficient to reduce the expression of recombinant firefly luciferase by 95% [Kolokoltsov et al. 2007]. Also, this amount was shown to effectively reduce expression of numerous endogenous genes by at least 5-fold, as determined by Western blot [Kolokoltsov et al. 2007].
For screening purposes, 48 h after transfection with siRNA, pseudotyped virus was added to HEK293 cells at an MOI of 0.05. Firefly luciferase expression was measured at 36 h post-infection using Dual-glo reagent (Promega, Madison, WI) in a Veritas plate reader (Turner Biosystems, CA). Controls used in the assays were use of transfection reagent alone, and two non-targeting siRNA (Ambion, TX). Two other siRNA served as positive controls and indicators of transfection efficacy and knockdown efficacy. One was against firefly luciferase (Ambion, TX) which suppressed expression of pseudotyped virus-encoded firefly luciferase. The second was against kif11 (Ambion, TX) which when suppressed, is cytotoxic, suppressing productive infection. In all cases, cell viability was checked by visual inspection using phase contrast microscopy.
Analysis of screening results was performed in two phases. The first phase used a recently reported algorithm, redundant siRNA activity analysis (RSA), that was specifically designed to analyze data from siRNA screens [Konig et al. 2007]. The algorithm takes into account the fact that siRNA targeting the same gene may not be equally effective at inducing a knockdown effect. The importance of a gene in assay outcome is determined by assigning probability values that define the statistical significance of the impact of a set of siRNA targeting one gene that yielded a significant effect on the assay being used. This approach has been shown to be more effective at identifying active siRNA, with higher confirmation rates, in high throughput screens than standard ranking methods [Konig et al. 2007]. For this, the activity of the luciferase infection reporter, in cells transfected with library siRNA, was normalized to the mean of the signals obtained for the negative control siRNA. The negative control siRNA had been performed at least 39 times each for the assay and were distributed throughout the sets of plates. The normalized data was then analyzed using the RSA algorithm implemented in the Perl programming language. Two sets of data were generated. One set analyzed reductions in activity of the infection reporter and the other analyzed increases in the infection signal. Values less than or equal to 0.58, or greater than or equal to 1.72 times the mean of the control siRNA were used as the cut-off thresholds respectively. This corresponded to 3 times the average standard deviation of the signals from the control sets.
In the second phase of analysis, the assigned probability values generated by the RSA algorithm and associated gene identifiers were submitted for analysis using the Ingenuity Pathways Analysis software package (Ingenuity Systems, www.ingenuity.com). Each gene identifier was mapped to known networks in the Ingenuity Pathways Knowledge Base. A cutoff value of less than or equal to a probability score of 0.2 was set as the threshold for selection of genes of interest. For canonical pathways, p-values were calculated by the Ingenuity software using Fischer’s exact test, which is a measure of the probability that the selected genes are associated with a pathway by chance alone.
Drug treatments
To confirm involvement of siRNA identified signaling proteins in EBOV entry and infection, cells were challenged with virus in the presence of specific inhibitor drugs. KN-93 inhibits calmodulin kinase 2 (CAMK2). KN-92 is a derivative of KN-93 that has weak affinity for CAMK2 and served as a negative control for off-target activities. LY294002 was used to target phosphatidylinositol-3-kinase (PI3K) and is widely accepted to be specific for PI3K with no appreciable off-target effects at the concentration used. In each case the drugs were dissolved in DMSO to give a 100× concentrated stock solution and were diluted in culture medium immediately before use.
For testing of drugs, both pseudotyped virus and wild type EBOV were used. Cells were prepared similarly for each assay. Cells were seeded to 50% confluency, which is approximately 104 cells for each well of a 96-well plate or 5 × 105 cells for each well of a 6-well plate. The plate was incubated at 37°C for 4 h to allow for cell attachment. For pseudotyped virus, HEK293 cells were used in 96-well plates while Vero cells in 6-well plates were used for wild type EBOV. Each drug was added to the cells 1 h before addition of virus. The medium was then replaced with fresh medium containing drug and virus. Typical infection dosage was at an MOI of 0.005 for wild type EBOV or 0.05 for pseudotyped virus.
For the pseudotyped lentivirus, assays were read after 40 h. The medium was removed and Dual-glo luciferase substrate added (Promega, Madison, WI), following the manufacturer’s recommendations. The plate was then read using a Veritas luminometer (Turner Biosystems, Sunnyvale, CA). For wild type EBOV, cell monolayers were treated as described for determination of virus titer and plaques were counted. For the pseudotyped VSV particles, assays were read at 18 h post infection by counting GFP-expressing cells using an epifluorescence microscope.
Statistical analysis, curve fitting and calculation of IC50 values was performed using Graphpad Prism software (GraphPad Prism version 4.00 for Windows, GraphPad Software, San Diego California USA, www.graphpad.com). Data were compared by one way ANOVA with the Tukey-Kramer post test.
Results
To identify new potential leads for developing antiviral therapies against EBOV we screened an siRNA library targeting cell kinases and phosphatases, often referred to as the kinome. Both sets of enzymes are important drug targets, as they are active in many aspects of cell function that could impact virus infection. These include receptor-ligand mediated signaling pathways, gene regulation and cell cycle control. They are also often found dysregulated in many chronic diseases such as cancers [Parsons et al. 2005].
An EBOV GP pseudotyped virus was used for screening. A viral pseudotype is a virus particle that bears the GPs of a virus of interest over the core of another virus, in this case a lentivirus. The pseudotyped virus particle typically adopts the receptor specificity, cell tropism and entry characteristics dictated by the foreign GP. Retroviruses are often used to make pseudotypes as they readily adopt new GPs which can function to give infection by the retrovirus core. Retroviral pseudotypes are also advantageous as they can be made in the absence of the natural GPs and with replication incompetent genomes that are modified to express marker proteins upon infection [Sanders 2002]. We and others have demonstrated that such pseudotyped viruses recapitulate the entry characteristics of the GP donor [Kolokoltsov et al. 2006a; Kolokoltsov et al. 2006b] and EBOV retrovirus pseudotypes have been used extensively to study EBOV infection mechanisms [Simmons et al. 2003; Watson et al. 2002; Wool-Lewis and Bates 1998]. Most importantly, pseudotyped viruses offer the ability to study the features of a highly pathogenic virus at lower biocontainment and are more amenable to high-throughput screening platforms. Nonetheless, it is still important to validate all findings attained with pseudotyped viruses by testing live virus as well.
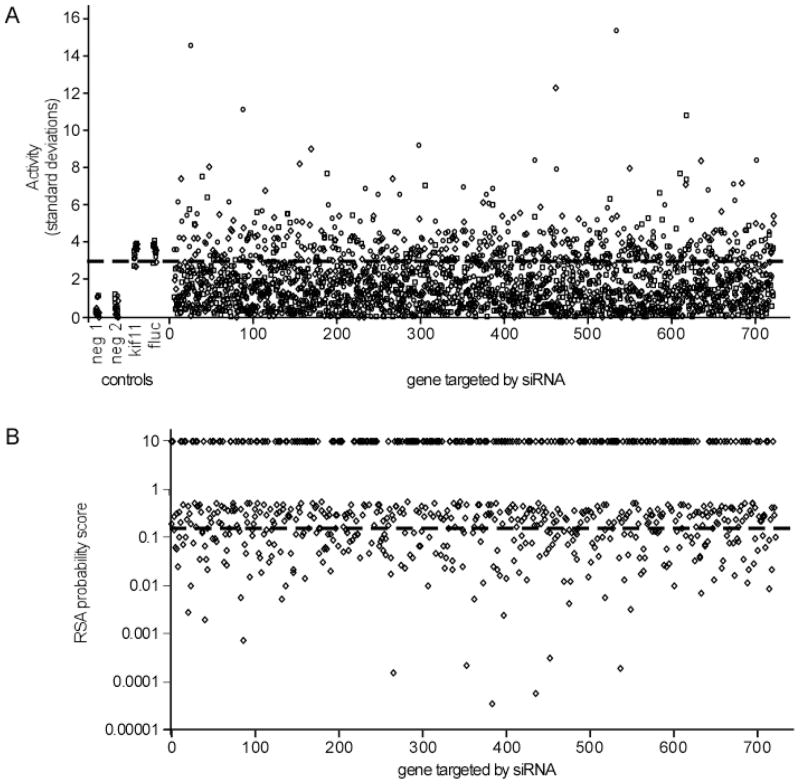
Since three distinct siRNA were screened per gene target, it was important to rank the importance of targets based on the overall impact of each set of siRNA on infection. This is relevant because not all siRNA perform equally well. Simple ranking of siRNA effectiveness based on a single strong effect of one siRNA or the average effect of each does not take this into account. For this, redundant siRNA activity (RSA) analysis was used. This algorithm assigns a probability score (p-value) that indicates the statistical relevance for each gene being important for screening outcome, based on the extent of impact on the assay and the performance of each siRNA targeting the same gene [Konig et al. 2007]. The cut-off threshold used in this analysis was set to 3 times the standard deviation in signals seen in the negative and positive controls (one standard deviation was 14%). Therefore a change of or less than or equal to 0.58 or greater than or equal to 1.72 times the average infection rate seen for controls was used as the threshold for significance. One quarter (26.2%) of the siRNA were defined as potential hits, having a significant impact on pseudotyped virus infection. These siRNA not only inhibited infection but many (45% of the active siRNA) actually increased the infection signal (Fig. 1). The latter may indicate that these siRNA inhibited expression of cell proteins that misdirect virus into non-productive pathways or are part of anti-viral host defense mechanisms.
Fig. 1.
Distribution of effects of siRNA on infection of EBOV pseudotyped viruses. A) The impact of each single siRNA was plotted for the 720 targeted genes as a function of fold-increase in standard deviation over that seen for the negative control non-targeting siRNA (neg 1 and neg 2). Positive controls targeting kif11 and firefly luciferase (fluc) are also indicated. The fluc siRNA suppressed the infection reporter gene encoded by the pseudotyped virus. 15 replicates of each control are shown. For the siRNA that were part of the screen, circles, squares and diamonds indicate distinct siRNA targeting each gene. The cut-off of significance is indicated by the dashed line and corresponds to three times the standard deviation of the control signals. B) RSA ranking of siRNA hits. All siRNA that suppressed or elevated infection of EBOV GP pseudotyped virus were evaluated using the RSA algorithm that is specifically designed to analyze siRNA screening data from screens using multiple siRNA targeting each gene. Probability scores assigned for each gene are shown. Scores of 10 indicate the gene product was not classified as a potential hit. The threshold used for the network analysis is indicated by the dashed line. All scores below this line were used.
To gain further insight into potential roles that these genes and gene products played in infection, all hits were analyzed using Ingenuity Pathway Analysis software. While this software is primarily designed to identify changes in gene expression patterns using genechips, it was recently used to identify related genes important for intracellular growth of Salmonella bacteria [Kuijl et al. 2007]. In the current study, instead of supplying infection assay data, the probability scores from the RSA analysis were used to prioritize network assignments. This provides an additional filter to remove false positive signals and permit detection for genes that would otherwise be missed using a simple ranking system. A total of 9 networks containing more than 15 genes each were indicated as important for infection by the EBOV pseudotyped virus. Two of the top four networks were related and shared MAP kinases. These gene products are key signaling proteins involved in control of cell growth and proliferation [Roberts and Der 2007]. The remaining networks did not share more than one gene in common. Of the remaining networks, one containing PI3 kinases and another containing calcium/calmodulin dependent kinases were ranked in the top four (Fig. 2). PI3 kinases modulate signaling from membrane bound receptors to promote cell growth, survival and movement [Marone et al. 2008]. Calcium/calmodulin kinases are key for nerve function and memory but also play important roles in signal transduction to the nucleus through CREB in non-neuronal cells [Wu and McMurray 2001].
Fig. 2.
Identified gene product networks impacting infection by the EBOV pseudotyped virus. Three distinct kinase network types were identified using the Ingenuity Pathway Analysis software. These were MAP kinase (MAPK, top panel), PI3 kinase (PI3K, middle panel) and calcium/calmodulin dependent kinase (Ca2+/CALM, lower panel) dependent networks. Genes or gene products are represented by inverted triangles and circles. Lines connecting symbols represent known interactions in the Ingenuity database. Shaded symbols indicate siRNA that were indicated as hits using the RSA algorithm. Numbers below each symbol indicate assigned RSA probability values.
A set of canonical, (well characterized) signaling pathways were also indicated as important (Table 1). These contained each of the gene products from the networks discussed above, except for the calcium/calmodulin kinase-containing network. For each of the canonical pathways identified, either MAP kinase or PI3 kinase related gene products were prominent components. Fisher’s exact test yielded scores showing a non-random association of each set of hits from the screen with the indicated pathways (Table 1).
Table 1.
List of canonical pathways and gene product networks identified important for infection by pseudotyped EBOV particles. Infection data from the siRNA screen was first analyzed by the RSA algorithm and probability values assigned. These values and associated gene identifiers were then submitted for network analysis using the Ingenuity Pathways Analysis suite. Of the top 4 ranked networks, 3 were associated with the 5 canonical pathways indicated. The calcium/calmodulin dependent network was the second highest ranking network but was not part of an identified canonical pathway
| Canonical pathway | Number of siRNA targeting/total genes in network | * p-value |
|---|---|---|
| SAPK/JNK Signaling | 32/147 | 4.1 × 10−25 |
| PI3K/AKT Signaling | 30/176 | 2.0 × 10−21 |
| Inositol Phosphate metabolism | 26/173 | 6.7 × 10−20 |
| Ephrin Receptor Signaling | 32/232 | 9.1 × 10−20 |
| ERK/MAPK Signaling | 30/226 | 1.6 × 10−17 |
| Other networks | ||
| Calcium/calmodulin signaling | 24/34 | * N.A. |
p-values were calculated by the Ingenuity Pathways Analysis package for canonical pathways using Fisher’s exact test as described in the methods.
The siRNA profiling coupled with RSA and network analysis predicted the involvement of signaling pathways in EBOV infection. We tested the usefulness of this prediction by treatment of cells with inhibitors of components for two of the identified networks. Drugs that target the catalytic subunit of phosphatidylinositol-3-kinase (PI3K) and calcium/calmodulin kinase 2 (CAMK2) were used. LY294002 inhibits the kinase activity of PI3K [Vlahos et al. 1994] and KN-93 prevents association of CAMK2 with calmodulin which is required for its kinase activity [Sumi et al. 1991]. These targets were chosen as they are integral parts of two distinct networks and blocking either of these was predicted to inhibit the function of the entire network for which they are part. Each drug is also highly specific for its substrate, is cell membrane permeable and share low micromolar affinities for their enzyme targets.
Each drug was tested for inhibition of infection of the pseudotyped virus using a range of dosages and dose response curves were fitted to the data by non-linear regression (Fig. 3a). LY294002 was most active with a calculated IC50 value for infection of 6.5 ± 1.1 μM (R2=0.94), while KN-93 had an IC50 of 21.4 ± 1.1 μM (R2=0.94). The KN-93 derivative, KN-92, which has much weaker activity against CAMK2, was also tested and was found to be less effective at blocking infection by the pseudotyped virus, only approaching 50% reduction in infection at the highest dose tested (100 μM). These findings suggested that the siRNA profiling had successfully identified two important cell signaling pathways for which activity is required for infection by the pseudotyped virus. However, since a pseudotyped virus was used, the siRNA and drugs may have impacted the expression of the infection marker that was controlled by the lentivirus core.
Fig. 3.
Affects of drug inhibitors of PI3 Kinase and CAMK2 proteins on EBOV pseudotyped virus infection. Cells were treated with LY294002, a specific inhibitor of the PI3K catalytic subunit or KN-93, an inhibitor of CAMK2 or its inactive derivative KN-92. A) EBOV pseudotyped lentivirus was added in the presence of each drug and then incubated with virus for 6 h. Both drug and unbound virus were then removed and cells were assayed for infection 40 h later by measuring expression of a reporter gene (firefly luciferase). The average of 4 replicates ± st. dev. is shown. B) To demonstrate that drug activity was not against the lentivirus core, a vesicular stomatitis virus core was used to make additional pseudotyped viruses. Aside from EBOV pseudotyped particles (open bars), an additional pseudotype was made, using the GPs of VEEV (solid bars). Each drug was tested at 100 μM. The average of two replicates ± st. dev. is shown.
To demonstrate that the drugs were specific for the function of the EBOV GPs and not the lentivirus core, an additional pseudotyped virus was generated using a vesicular stomatitis virus (VSV) core. As with the lentivirus core, infection of the VSV core pseudotyped virus was significantly impacted (P<0.001) by either LY294002 or KN-93 but not KN-92 (P>0.05). Since each pseudotype shared a common GP, the activity of the drugs most likely act at an EBOV GP-dependent step in infection. As a final test, the EBOV GP was replaced with that of Venezuelan equine encephalitis virus (VEEV). In contrast to what was seen with the EBOV pseudotyped virus, the VEEV pseudotyped particles were not significantly affected by any of the drugs (P>0.05, Fig. 3b). This finding confirmed that the drugs were specifically affecting the function of the EBOV GPs and not the activity of the pseudotyped virus core.
To prove useful as new drug leads against EBOV infection and to further validate our approach, it was important to show that live EBOV infection was inhibited by each of the drugs. Unlike the pseudotyped virus which is a BSL2 agent, working with live EBOV requires the highest level biocontainment, BSL4, and is difficult and cumbersome. Evaluation of EBOV infectivity involved counting plaques formed after 10 d on VeroE6 cells. Cells were pretreated with each drug at 50 μM. Drug remained on cells for 6 h and then was removed. It was found that each drug severely impacted infection, dropping virus titers by 65% and >95% for LY294002 and KN-93 respectively (P<0.001, Fig. 4). DMSO, the vehicle used to dissolve each drug or KN-92 did not significantly affect infection. We conclude that the drugs identified by siRNA pathway profiling are effective against wild type EBOV and each represents a potentially useful lead for development of effective therapies against infection by this virus.
Fig. 4.
Affects of PI3K and CAMK2 inhibitors on wild type EBOV. The effects of a 50 μM dose of LY294002, KN-93 or KN-92 was tested using wild type EBOV. Vero-E6 cells were preincubated for 1 h with drug and then virus was added for 6 h, after which medium was replaced with fresh medium. Cells were incubated for 10 d, fixed and then plaques counted. All work was performed under BSL4 conditions. The average of 3 replicates ± st. dev. is shown.
Discussion
The development and availability of commercial siRNA libraries has provided a valuable resource for the identification of potential drug targets that can be used to combat disease. Screens have been used to identify potential cancer drug targets [Morgan-Lappe et al. 2007] and to understand the role of specific proteins in cell function [Pelkmans 2005]. Previously, we used a small library to identify the unexpected role of clathrin-mediated endocytosis for infection by Respiratory syncytial virus [Kolokoltsov et al. 2007] and others used similar limited libraries to identify proteins that suppress HIV replication [Kameoka et al. 2007]. Recently two groups used larger “druggable genome” targeting libraries to try to identify cell proteins important for replication of Hepatitis C [Ng et al. 2007] and a kinome-targeting library to identify a role for Akt in intracellular growth of a bateria, Salmonella typhimurium [Kuijl et al. 2007]. The latter study was the first to utilize network profiling to identify useful drugs that could be used to inhibit bacterial growth in cells. The heavy dependence of viruses on cellular machinery for infection and replication means that the use of such libraries will continue to be useful in identifying proteins that are required for infection and replication of viruses and to identify effective new drugs useful in antiviral therapy. The work shown here is the first example of confirmed drug leads against a virus being identified using siRNA profiling coupled with network analysis. It is also the first to report that EBOV infection can be suppressed by treating cells with inhibitors of PI3K and CAMK2 proteins.
EBOV is a dangerous pathogen and work must be performed in biosafety level 4 facilities. Traditionally, infection required counting of plaques in cell monolayers but more recently, recombinant viruses expressing green fluorescent protein have been produced [Towner et al. 2005]. While such modified viruses increase throughput, they still require high level containment. The pseudotyped virus used in the current work offers the ability to quantitatively measure the penetration of virus particles into cells at biosafety level 2. Retrovirus pseudotypes have been well developed through interests in gene therapy and many different envelope protein variants exist. They also have been extensively modified to be replication incompetent but to express sensitive markers of infection. The marker used in this study was firefly luciferase which is ideal for screening purposes as it is readily detected using a simple assay system that is commercially available. The enzyme also has a high turnover, meaning that it does not accumulate in cells and detection systems remain linear over 6-orders of magnitude [Gould and Subramani 1988]. The common criticism of pseudotyped viruses is that they are not the same as the wild type virus. This may be true on the basis of morphological criteria but in terms of functionality of the adopted GPs we and others have shown that they closely mimic the parental virus in aspects of binding and penetration into cells [Kolokoltsov et al. 2006a; Kolokoltsov et al. 2006b; Wool-Lewis and Bates 1998].
The screen produced a large array of data that needed to be analyzed to prioritize specific hits for further evaluation. The key goal for this study was to transition hits to drug leads as quickly as possible. This was performed by first analyzing the data using a probability-based method, previously shown to be more effective at eliminating false-positive signals and identifying genes important for assay outcome. The method scores genes based on the effectiveness of a set of siRNA targeting the same gene. This means that multiple moderately performing siRNA will be scored similarly to a single high performing siRNA. This method is more accurate than simple ranking criteria and identifies genes that would otherwise be omitted from further analysis [Konig et al. 2007]. By using the statistical scores as the input into the network profiling software, networks were then weighted on the basis of overall performance for each set of siRNA targeting members of the network. Again this provides further rigor to the analysis, further eliminating false positive hits by identifying groups of proteins that act similarly within one network.
Of the top 4 networks identified, 2 shared MAP kinases, one contained phosphatidylinositol-3-kinases and the other calcium/calmodulin dependent kinases. This suggests that at least 3 distinct signaling cascades were required to be activated for infection by EBOV. It is unclear if each is required to operate in series or in parallel and what the downstream effectors may be. A similar approach to that taken here was used to identify a role for Akt in intracellular growth of Salmonella and Mycobacterium species [Kuijl et al. 2007] and indicated that PI3 kinases may also be required. Indeed, Akt-1 is known to be a downstream effector of PI3K and each can work in concert to regulate cell growth, motility and survival [Harvey and Lonial 2007]. However, involvement of PI3K was not verified in that study. While Akt-1 was not identified in the siRNA screen, a closely related gene product, Akt-3 was indicated as important, it will now be of interest to determine if drugs that target this protein can equally impact EBOV infection. It will also be important to determine if molecules upstream of CAMK2, such as calmodulin, are important for EBOV infection as predicted by this analysis. Indeed, calmodulin (CALM2) targeting siRNA were identified in the screen. This protein is widely expressed in a variety of tissues and acts to trigger CAMK2 in a calcium dependent manner.
The potential drug targets identified through the siRNA screen were verified using specific drugs that inhibited PI3K and CAMK2 activities. Each specifically inhibited infection by two different pseudotyped virus types, as well as the wild type virus. This finding supports the premise that pseudotyped viruses can provide useful information that can be applied for drug discovery and development [Kolokoltsov et al. 2006b]. The use of an alternative pseudotype core, from VSV, and showing that the drugs still affected its infection, indicated that the drug action was against a function of the EBOV GP. This was further confirmed by the finding that replacement of the EBOV GP with that from VEEV rendered the pseudotyped virus resistant to drug action. Virus GPs function to bind receptor and mediate membrane fusion to release the virus core containing the genome into the cell cytoplasm. This strongly suggests that the drugs act on related or distinct components of receptor utilization, trafficking of the virus into the cell or the mechanism by which membrane fusion is triggered. At least PI3K is known to affect steps important for membrane turnover by affecting the cell cytoskeleton [Zhao et al. 2007]. However, it is involved in other aspects of cell function such as metabolism and cell cycle. In contrast, CAMK2 signaling is important for activation of gene transcription through CREB [Sato et al. 2006] suggesting a requirement for expression of other proteins that aid in EBOV entry into cells. To determine which aspect of PI3K and CAMK2 function and what parts of the infection process are affected by each will require a more detailed and extensive analysis of the mechanism of entry of EBOV and this will be the focus of future work.
Overall, this approach identified two lead compounds that may prove useful in treatment of EBOV infection. Inhibitors of PI3K, such as stabilized derivatives of LY294002 have and are being used in clinical trials against cancer [Garlich et al. 2008]. CAMK2 inhibitors have not been tested in the clinic but if proteins required for activation of CAMK2, such as calmodulin, are important, then these provide additional targets for intervention. Likewise, determining if calcium flux, which activates calmodulin, is important for EBOV infection, will be important for not only identifying potential new drugs to treat infection but to understand and treat the disease state. Exploiting such dependencies opens up new possibilities for development of new antiviral therapies. We feel the current approach may be particularly useful in quickly identifying treatments for other emerging pathogens that often fail to command sufficient resources for conventional drug development.
Acknowledgments
This work was supported in part by funding provided by NIH/NIAID, 5R01AI063513, U54AI057156 and DOD HDTRA1-07-C-0083 4
Funding support. This work was supported in part by grants from NIAID to R.A.D. 1R01AI063513-01A2 and through the Western Regional Center of Excellence for Biodefense and Emerging Infectious Diseases, U54 AI057156 as well as DOD HDTRA1-07-Cs0083 4.
Bibliography
- Bean B. Antiviral therapy: current concepts and practices. Clin Microbiol Rev. 1992;5(2):146–82. doi: 10.1128/cmr.5.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canonico PG, Kende M, Luscri BJ, Huggins JW. In-vivo activity of antivirals against exotic RNA viral infections. J Antimicrob Chemother. 1984;14(Suppl A):27–41. doi: 10.1093/jac/14.suppl_a.27. [DOI] [PubMed] [Google Scholar]
- Carley DW. Drug repurposing: identify, develop and commercialize new uses for existing or abandoned drugs. Part I. IDrugs. 2005;8(4):306–9. [PubMed] [Google Scholar]
- Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7(8):2745–52. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RY, Kilby JM, Saag MS. Enfuvirtide. Expert Opin Investig Drugs. 2002;11(12):1837–43. doi: 10.1517/13543784.11.12.1837. [DOI] [PubMed] [Google Scholar]
- Chong CR, Sullivan DJ., Jr New uses for old drugs. Nature. 2007;448(7154):645–6. doi: 10.1038/448645a. [DOI] [PubMed] [Google Scholar]
- Cianci C, Meanwell N, Krystal M. Antiviral activity and molecular mechanism of an orally active respiratory syncytial virus fusion inhibitor. J Antimicrob Chemother. 2005;55(3):289–92. doi: 10.1093/jac/dkh558. [DOI] [PubMed] [Google Scholar]
- Deutsch M, Hadziyannis SJ. Old and emerging therapies in chronic hepatitis C: an update. J Viral Hepat. 2008;15(1):2–11. doi: 10.1111/j.1365-2893.2007.00887.x. [DOI] [PubMed] [Google Scholar]
- Esser S, Helbig D, Hillen U, Dissemond J, Grabbe S. Side effects of HIV therapy. J Dtsch Dermatol Ges. 2007;5(9):745–54. doi: 10.1111/j.1610-0387.2007.06322.x. [DOI] [PubMed] [Google Scholar]
- Freistadt MS, Vaccaro JA, Eberle KE. Biochemical characterization of the fidelity of poliovirus RNA-dependent RNA polymerase. Virol J. 2007;4:44. doi: 10.1186/1743-422X-4-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlich JR, De P, Dey N, Su JD, Peng X, Miller A, Murali R, Lu Y, Mills GB, Kundra V, et al. A vascular targeted pan phosphoinositide 3-kinase inhibitor prodrug, SF1126, with antitumor and antiangiogenic activity. Cancer Res. 2008;68(1):206–15. doi: 10.1158/0008-5472.CAN-07-0669. [DOI] [PubMed] [Google Scholar]
- Gould SJ, Subramani S. Firefly luciferase as a tool in molecular and cell biology. Anal Biochem. 1988;175(1):5–13. doi: 10.1016/0003-2697(88)90353-3. [DOI] [PubMed] [Google Scholar]
- Harvey RD, Lonial S. PI3 kinase/AKT pathway as a therapeutic target in multiple myeloma. Future Oncol. 2007;3(6):639–47. doi: 10.2217/14796694.3.6.639. [DOI] [PubMed] [Google Scholar]
- Kameoka M, Kitagawa Y, Utachee P, Jinnopat P, Dhepakson P, Isarangkura-na-ayuthaya P, Tokunaga K, Sato H, Komano J, Yamamoto N, et al. Identification of the suppressive factors for human immunodeficiency virus type-1 replication using the siRNA mini-library directed against host cellular genes. Biochem Biophys Res Commun. 2007;359(3):729–34. doi: 10.1016/j.bbrc.2007.05.173. [DOI] [PubMed] [Google Scholar]
- Katz RA, Skalka AM. Generation of diversity in retroviruses. Annu Rev Genet. 1990;24:409–45. doi: 10.1146/annurev.ge.24.120190.002205. [DOI] [PubMed] [Google Scholar]
- Kolokoltsov AA, Deniger D, Fleming EH, Roberts NJ, Jr, Karpilow JM, Davey RA. Small interfering RNA profiling reveals key role of clathrin-mediated endocytosis and early endosome formation for infection by respiratory syncytial virus. J Virol. 2007;81(14):7786–800. doi: 10.1128/JVI.02780-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolokoltsov AA, Fleming EH, Davey RA. Venezuelan equine encephalitis virus entry mechanism requires late endosome formation and resists cell membrane cholesterol depletion. Virology. 2006a;347(2):333–42. doi: 10.1016/j.virol.2005.11.051. [DOI] [PubMed] [Google Scholar]
- Kolokoltsov AA, Wang E, Colpitts TM, Weaver SC, Davey RA. Pseudotyped viruses permit rapid detection of neutralizing antibodies in human and equine serum against Venezuelan equine encephalitis virus. Am J Trop Med Hyg. 2006b;75(4):702–9. [PubMed] [Google Scholar]
- Kolokoltsov AA, Weaver SC, Davey RA. Efficient functional pseudotyping of oncoretroviral and lentiviral vectors by Venezuelan equine encephalitis virus envelope proteins. J Virol. 2005;79(2):756–63. doi: 10.1128/JVI.79.2.756-763.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig R, Chiang CY, Tu BP, Yan SF, DeJesus PD, Romero A, Bergauer T, Orth A, Krueger U, Zhou Y, et al. A probability-based approach for the analysis of large-scale RNAi screens. Nat Methods. 2007;4(10):847–9. doi: 10.1038/nmeth1089. [DOI] [PubMed] [Google Scholar]
- Kuijl C, Savage ND, Marsman M, Tuin AW, Janssen L, Egan DA, Ketema M, van den Nieuwendijk R, van den Eeden SJ, Geluk A, et al. Intracellular bacterial growth is controlled by a kinase network around PKB/AKT1. Nature. 2007;450(7170):725–30. doi: 10.1038/nature06345. [DOI] [PubMed] [Google Scholar]
- Kunkel TA, Bebenek K. DNA replication fidelity. Annu Rev Biochem. 2000;69:497–529. doi: 10.1146/annurev.biochem.69.1.497. [DOI] [PubMed] [Google Scholar]
- Marone R, Cmiljanovic V, Giese B, Wymann MP. Targeting phosphoinositide 3-kinase-Moving towards therapy. Biochim Biophys Acta. 2008;1784(1):159–85. doi: 10.1016/j.bbapap.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Matsuura Y, Tani H, Suzuki K, Someya T, Suzuki R, Aizaki H, Ishii K, Moriishi K, Robison C, Whitt MA, et al. Characterization of pseudotype VSV possessing HCV envelope proteins. Virology. 2001 doi: 10.1006/viro.2001.0971. In press. [DOI] [PubMed] [Google Scholar]
- Morgan-Lappe SE, Tucker LA, Huang X, Zhang Q, Sarthy AV, Zakula D, Vernetti L, Schurdak M, Wang J, Fesik SW. Identification of Ras-related nuclear protein, targeting protein for xenopus kinesin-like protein 2, and stearoyl-CoA desaturase 1 as promising cancer targets from an RNAi-based screen. Cancer Res. 2007;67(9):4390–8. doi: 10.1158/0008-5472.CAN-06-4132. [DOI] [PubMed] [Google Scholar]
- Napravnik S, Keys JR, Quinlivan EB, Wohl DA, Mikeal OV, Eron JJ., Jr Triple-class antiretroviral drug resistance: risk and predictors among HIV-1-infected patients. Aids. 2007;21(7):825–34. doi: 10.1097/QAD.0b013e32805e8764. [DOI] [PubMed] [Google Scholar]
- Ng TI, Mo H, Pilot-Matias T, He Y, Koev G, Krishnan P, Mondal R, Pithawalla R, He W, Dekhtyar T, et al. Identification of host genes involved in hepatitis C virus replication by small interfering RNA technology. Hepatology. 2007;45(6):1413–21. doi: 10.1002/hep.21608. [DOI] [PubMed] [Google Scholar]
- Parsons DW, Wang TL, Samuels Y, Bardelli A, Cummins JM, DeLong L, Silliman N, Ptak J, Szabo S, Willson JK, et al. Colorectal cancer: mutations in a signalling pathway. Nature. 2005;436(7052):792. doi: 10.1038/436792a. [DOI] [PubMed] [Google Scholar]
- Pelkmans L. Viruses as probes for systems analysis of cellular signalling, cytoskeleton reorganization and endocytosis. Curr Opin Microbiol. 2005;8(3):331–7. doi: 10.1016/j.mib.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Peters CJ. Emerging infections--Ebola and other filoviruses. West J Med. 1996;164(1):36–8. [PMC free article] [PubMed] [Google Scholar]
- Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26(22):3291–310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- Sanders DA. No false start for novel pseudotyped vectors. Curr Opin Biotechnol. 2002;13(5):437–42. doi: 10.1016/s0958-1669(02)00374-9. [DOI] [PubMed] [Google Scholar]
- Sato K, Suematsu A, Nakashima T, Takemoto-Kimura S, Aoki K, Morishita Y, Asahara H, Ohya K, Yamaguchi A, Takai T, et al. Regulation of osteoclast differentiation and function by the CaMK-CREB pathway. Nat Med. 2006;12(12):1410–6. doi: 10.1038/nm1515. [DOI] [PubMed] [Google Scholar]
- Schurmann D, Fatkenheuer G, Reynes J, Michelet C, Raffi F, van Lier J, Caceres M, Keung A, Sansone-Parsons A, Dunkle LM, et al. Antiviral activity, pharmacokinetics and safety of vicriviroc, an oral CCR5 antagonist, during 14-day monotherapy in HIV-infected adults. Aids. 2007;21(10):1293–9. doi: 10.1097/QAD.0b013e3280f00f9f. [DOI] [PubMed] [Google Scholar]
- Simmons G, Rennekamp AJ, Chai N, Vandenberghe LH, Riley JL, Bates P. Folate receptor alpha and caveolae are not required for Ebola virus glycoprotein-mediated viral infection. J Virol. 2003;77(24):13433–8. doi: 10.1128/JVI.77.24.13433-13438.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumi M, Kiuchi K, Ishikawa T, Ishii A, Hagiwara M, Nagatsu T, Hidaka H. The newly synthesized selective Ca2+/calmodulin dependent protein kinase II inhibitor KN-93 reduces dopamine contents in PC12h cells. Biochem Biophys Res Commun. 1991;181(3):968–75. doi: 10.1016/0006-291x(91)92031-e. [DOI] [PubMed] [Google Scholar]
- Towner JS, Paragas J, Dover JE, Gupta M, Goldsmith CS, Huggins JW, Nichol ST. Generation of eGFP expressing recombinant Zaire ebolavirus for analysis of early pathogenesis events and high-throughput antiviral drug screening. Virology. 2005;332(1):20–7. doi: 10.1016/j.virol.2004.10.048. [DOI] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269(7):5241–8. [PubMed] [Google Scholar]
- Watson DJ, Kobinger GP, Passini MA, Wilson JM, Wolfe JH. Targeted transduction patterns in the mouse brain by lentivirus vectors pseudotyped with VSV, Ebola, Mokola, LCMV, or MuLV envelope proteins. Mol Ther. 2002;5(5 Pt 1):528–37. doi: 10.1006/mthe.2002.0584. [DOI] [PubMed] [Google Scholar]
- Wool-Lewis RJ, Bates P. Characterization of Ebola virus entry by using pseudotyped viruses: identification of receptor-deficient cell lines. J Virol. 1998;72(4):3155–60. doi: 10.1128/jvi.72.4.3155-3160.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, McMurray CT. Calmodulin kinase II attenuation of gene transcription by preventing cAMP response element-binding protein (CREB) dimerization and binding of the CREB-binding protein. J Biol Chem. 2001;276(3):1735–41. doi: 10.1074/jbc.M006727200. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Gaidarov I, Keen JH. Phosphoinositide 3-kinase C2alpha links clathrin to microtubule-dependent movement. J Biol Chem. 2007;282(2):1249–56. doi: 10.1074/jbc.M606998200. [DOI] [PubMed] [Google Scholar]