Abstract
Hypertension is a leading cause of morbidity and mortality worldwide. Individuals with hypertension are at increased risk of stroke, heart disease and kidney failure. Both genetic and lifestyle factors, particularly diet, have been attributed an important role in the development of hypertension. Reducing dietary sugar and salt intake can help lower blood pressure; similarly, adequate protein intake may also attenuate hypertension. Observational, cross-sectional and longitudinal epidemiological studies, and controlled clinical trials, have documented significant inverse associations between protein intake and blood pressure. Human and animal studies have shown that specific amino acids within proteins may have antihypertensive effects. Cysteine, glutathione (a tripeptide), glutamate and arginine attenuate and prevent alterations that cause hypertension including insulin resistance, decreased nitric oxide bioavailability, altered renin angiotensin system function, increased oxidative stress and formation of advanced glycation end products. Leucine increases protein synthesis in skeletal muscle and improves insulin resistance by modulating hepatic gluconeogenesis. Taurine and tryptophan attenuate sympathetic nervous system activity. Soy protein helps lower blood pressure through its high arginine content and antioxidant activity exhibited by isoflavones. A diet containing an ample amount of protein may be a beneficial lifestyle choice for individuals with hypertension; one example is the Dietary Approaches to Stop Hypertension (DASH) diet, which is low in salt and saturated fat; includes whole grains, lean meat, poultry, fish and nuts; and is rich in vegetables, fruits and low-fat dairy products, which are good sources of antioxidant vitamins, minerals and fibre. Including an adequate supply of soy in the diet should also be encouraged.
Keywords: Advanced glycation end products, Amino acids, Hypertension, Insulin resistance, Nitric oxide, Oxidative stress, Protein
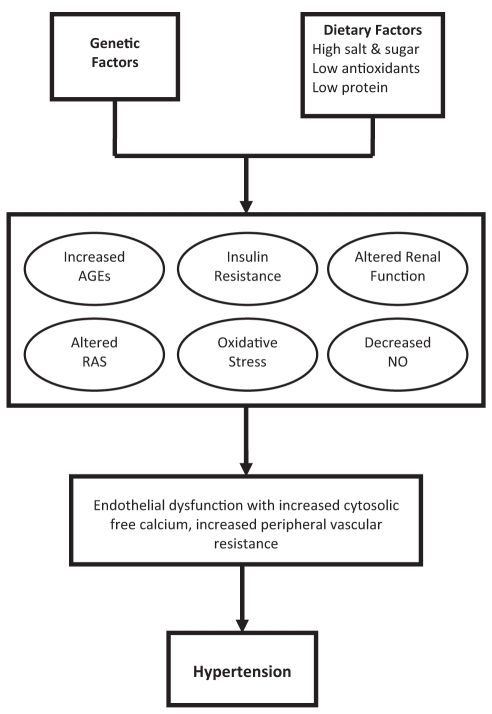
Approximately one-quarter of the world’s population is affected by hypertension – a disease that causes approximately 7.1 million deaths per year, or 13% of total deaths worldwide (1,2). The prevalence of hypertension is considered to be a major public health concern of epidemic proportions, especially because hypertension leads to an increased risk of both cardiovascular and renal diseases (2–4). Essential hypertension is caused by a combination of acquired and genetic metabolic defects involved in blood pressure regulation that interact with environmental factors such as diet and lifestyle (5). There are several metabolic alterations and downstream effects that increase blood pressure including insulin resistance, increased oxidative stress, increased formation of advanced glycation end products (AGEs), decreased nitric oxide (NO) bioavailability, altered renin angiotensin system (RAS) function and reduced renal sodium excretion (Figure 1). These alterations can lead to endothelial dysfunction, increased vascular cytosolic free calcium, peripheral vascular resistance and the development of hypertension.
Figure 1).
Mechanism of hypertension. Hypertension develops from a combination of genetic and lifestyle factors, such as diet. Diets high in salt and sugar, and low in antioxidants and protein, have been implicated in hypertension. Insulin resistance, altered glucose metabolism with an increase in advanced glycation end products (AGEs), increased oxidative stress, decreased bioavailability of nitric oxide (NO), alteration of renin-angiotensin system (RAS) function, altered renal function and endothelial dysfunction are all mechanisms that contribute to the development of hypertension
Diet is the lifestyle factor under the most scrutiny for its role in hypertension. To prevent hypertension, modifying various components of the diet, such as lowering salt and sugar intake, may be a key step in lowering high blood pressure (6). The Dietary Approaches to Stop Hypertension (DASH) study compared the DASH diet with a typical North American diet (7). The DASH diet is high in fruits, vegetables, whole cereal products and low-fat dairy products; low in salt and saturated fat; moderately high in protein; and includes whole grains, poultry, fish and nuts (7). It was found that the DASH diet lowered blood pressure more than the North American diet, even after modifications had been made so that both diets had lower and similar sodium contents. The DASH diet contains more protein than a typical North American diet (18% versus 15%, respectively) (8). The higher protein content may account for the effect of the DASH diet (7). Other studies such as the International Study of Salt and Blood Pressure (INTERSALT) (9), Multiple Risk Factor Intervention Trial (MRFIT) (10), Caerphilly Heart Study (11), Cardiovascular Diseases and Alimentary Comparison (CARDIAC) Study (12), Optimal Macronutrient Intake Trial to Prevent Heart Disease (OmniHeart) (13), and the International Study of Macro- and Micro-Nutrients and Blood Pressure (INTERMAP) (14) have demonstrated an inverse relationship between protein intake and blood pressure.
Studies have shown that vegetarians who consume more plant protein tend to have lower blood pressure than those who consume an omnivorous diet (15). As well, differences in dietary patterns among different cultures have identified relationships between protein intake and the prevalence of hypertension. Asian cultures, which receive the majority of their protein intake from plant (47%) and seafood (23%) sources, with only 18% of protein intake from red meat and poultry, tend to have lower blood pressure than cultures that receive the bulk of their protein intake from red meat and poultry (16). The United States population, which has relatively high blood pressure, consumes 36% of their protein intake from red meat and poultry, with only 6% from seafood and 33% from plants (17).
The present article reviews the antihypertensive effects of dietary protein, examines the evidence of its antihypertensive effects in animal and human studies, and discusses the potential mechanisms by which protein exerts its antihypertensive effects.
PROTEIN
In humans, proteins serve many functions including structural, kinetic, catalytic and signalling roles. They also act as carriers of vitamins, oxygen and carbon dioxide. Proteins also help maintain fluid balance, acid-base balance and form antibodies to help protect the body against disease (18,19).
The elemental composition of proteins is quite similar to those of lipids and carbohydrates because they all contain carbon, oxygen and hydrogen atoms. But unlike lipids and carbohydrates, proteins contain nitrogen, which creates the amino groups found in amino acids, nucleotides and hormones (19). There are 20 different amino acids, which are classified as non-essential or essential. Essential amino acids cannot be synthesized by the human body and must be obtained from dietary sources, whereas nonessential amino acids are synthesized by the body or may be obtained from food (19). Therefore, to achieve optimal body function, humans must consume a diet that contains an adequate amount of protein from a variety of sources to ensure that all amino acids required to perform normal body functions are acquired. Proteins can be obtained from a variety of food sources such as meat, fish, poultry, milk, eggs, legumes, grains, nuts and vegetables (19). According to the WHO, the safe acceptable intake for protein for adults is 0.83 g/kg/day, which is equivalent to 58.1 g/day for a 70 kg human (20).
ANTIHYPERTENSIVE EFFECT OF DIETARY PROTEIN
Protein studies
To date, the majority of studies conducted on hypertensive patients to determine whether dietary protein intake has an effect on blood pressure have suggested that a moderate increase in protein intake will indeed lower blood pressure (Table 1). For instance, a study conducted using the 10,020 participants from the INTERSALT study investigated the relationship of blood pressure to dietary protein. The results of this study showed an inverse relationship between blood pressure and dietary protein intake (9). Similar results were found during other population-based studies such as MRFIT (10), OmniHeart randomized trial (13), Caerphilly Heart Study (11) and the CARDIAC Study (12). Typically, human studies use data from 24 h dietary recall, food frequency questionnaires and common methods of blood pressure measurement to determine whether an inverse relationship between protein intake and blood pressure exists. However, human studies that used data from biochemical methods such as urinary markers of protein intake have also confirmed the existence of an inverse relationship between blood pressure and protein intake (21,22).
TABLE 1.
The effect of dietary protein on blood pressure (BP) in humans
| Reference | Human subjects | Dose and length of study | Effect on BP |
|---|---|---|---|
| Kihara et al, 1984 (24) | Japanese men and women | Protein intake assessed by measuring urinary markers of protein | Inverse relationship between animal protein intake and BP |
| Sacks et al, 1984 (129) | Vegetarian men and women | Subjects consumed either low- (63 g) or high- (119 g) protein diet for 6 weeks | No effect on mean SBP and DBP |
| Reed et al, 1985 (26) | Japanese men | Protein intake determined through 24 h recall; BP was measured | Inverse relationship between animal protein intake and BP |
| Margetts et al, 1986 (130) | Untreated, mildly hypertensive men and women | Subjects consumed omnivorous or ovolactovegetarian diet for 1 of 2 six-week periods | Vegetarian diet decreased SBP |
| Elliott et al, 1987 (11) | Normotensive men | BP measured at clinic, and weighed inventory conducted of all food and drink consumed over 7 days | Inverse relationship between protein intake and DBP |
| Prescott et al, 1987 (131) | Normotensive men and women | Subjects given meat or vegetable protein diet for 12 weeks; BP was measured | Neither meat nor vegetable protein diets had an effect on BP |
| Kestin et al, 1989 (132) | Normotensive men | Subjects assigned to high-fat, fat-modified lactovegetarian or lactovegetarian diet supplemented (15.2% to 16.9% total protein in diet) | Lactovegetarian diet lowered SBP; replacing plant with animal protein had no effect on lowering SBP |
| Havlik et al, 1990 (133) | Normotensive, male monozygotic twins | BP was measured and dietary intake determined using a food frequency questionnaire | Positive correlation between protein intake and DBP |
| Nara et al, 1990 (25) | Chinese normotensive men and women | Urinary markers of protein were measured | Inverse relationship between animal protein intake and BP |
| Zhou et al, 1994 (27) | Chinese normotensive men and women | 24 h recall of food intake and urinary amino acids measured | Inverse relationship between animal protein intake and BP |
| He et al, 1995 (134) | Chinese normotensive men | BP and dietary nutrient intake measured | Inverse relationship between protein intake and BP |
| Stamler et al, 1996 (9) | Men and women from INTERSALT study | Dietary protein intake assessed by urinary markers | Inverse relationship between protein intake and BP |
| Stamler et al, 1996 (10) | Men from MRFIT study | 24 h dietary recall | Inverse relationship between protein intake and BP |
| Appel et al, 1997 (135) | Normotensive men and women | Subjects fed control, fruit/vegetable or combination (high protein) diet for 8 weeks | Inverse relationship between protein intake and BP |
| Washburn et al, 1999 (30) | Normotensive women | 20 g complex carbohydrate or 20 g soy protein supplement once daily, or 10 g twice daily for 6 weeks | 10 g soy protein twice daily lowered DBP |
| Burke et al, 2001 (128) | Treated hypertensive men and women | Low- or high-protein (12.5% or 25%) diet and fibre (15 g/day or 27 g/day); 24 h ambulatory BP was measured | Protein and fibre have an additive effect in lowering BP |
| Hajjar et al, 2001 (136) | Men and women from NHANES III study | 24 h dietary recall | Positive correlation between protein intake and SBP |
| Teede et al, 2001 (32) | Normotensive men and postmenopausal women | Subjects received soy protein isolate (40 g soy protein, 118 mg isoflavone) or casein placebo for 3 months | Soy protein improved SBP, DBP and MBP |
| Cirillo et al, 2002 (21) | Normotensive men and women | Protein intake measured by urinary markers | Inverse relationship between BP and protein intake |
| Jenkins et al, 2002 (137) | Hyperlipidemic men and postmenopausal women | Fed soy protein diet (50 g to 52 g) with isoflavones | Decrease in SBP in men only |
| Liu et al, 2002 (12) | Normotensive men and women | Animal protein intake measured by urinary markers | Inverse relationship between animal protein intake and BP |
| Rivas et al, 2002 (123) | Mildly to moderately hypertensive men and women | Consumed 500 mL soy milk (18 g/L protein) twice daily for 3 months | Decrease in SBP, DBP and MBP |
| Stamler et al, 2002 (138) | Normotensive men | Dietary intake assessed by interviews | Inverse relationship between vegetable protein intake and SBP and DBP |
| Iseki et al, 2003 (22) | Normotensive men and women | Examined relationship of BP and dietary protein using daily urinary excretion of urea nitrogen | Inverse relationship between higher daily protein intake (≥1.0 g/kg/day) and BP in men only |
| Sagara et al, 2004 (126) | Middle-aged Scottish men | ≥20 g of soy protein diet with 80 mg of isoflavones for 5 weeks | Decrease in SBP and DBP |
| Appel et al, 2005 (13) | Prehypertensive or stage 1 hypertensive men and women | Subjects fed carbohydrate, protein or unsaturated fat diet for 6 weeks | Inverse relationship between SBP and protein intake |
| He et al, 2005 (29) | Chinese normotensive men and women | 40 g soybean protein supplements per day or complex carbohydrates for 12 weeks | Inverse relationship between soybean protein intake and SBP and DBP |
| Yang et al, 2005 (125) | Normotensive women from Shanghai Women’s Health Study | Soy protein intake was assessed by food frequency questionnaire | Inverse relationship between soy protein intake and BP |
| Elliott et al, 2006 (14) | Normotensive men and women | 24 h dietary recall conducted and BP measured at 4 visits | Inverse relationship between vegetable protein intake and BP |
| Hodgson et al, 2006 (139) | Hypertensive men and women | Regular diet (18.6% protein) or lean red meat diet (23.8% protein) for 8 weeks | Inverse relationship between protein intake and SBP |
| Teede et al, 2006 (124) | Hypertensive men and women | Received soy cereal (40 g soy protein, 118 mg isoflavones) for 3 months | Increased soy protein intake had no effect on BP |
| Muzio et al, 2007 (140) | Obese men and women with metabolic syndrome | 13% or 19% protein diet for 5 months | High-protein diet lowered SBP |
| Welty et al, 2007 (31) | Normotensive, postmenopausal women | 25 g soy protein from soy nuts for 8 weeks | Soy protein diet lowered SBP and DBP |
| Umesawa et al, 2009 (23) | Japanese men and women | Protein intake measured by 24 h dietary recall | Inverse relationship between protein intake and SBP and DBP |
DBP Diastolic BP; INTERSALT International Study of Salt and Blood Pressure; MBP Mean BP; MRFIT Multiple Risk Factor Intervention Trial; NHANES III National Health and Nutrition Examination Survey III; SBP Systolic BP
Many human studies have found that the antihypertensive effect of protein is related not only to the amount of protein in the diet, but also to the source of protein. Some researchers have investigated how the source of protein and its amino acid content play a role in lowering blood pressure. In doing so, many studies have compared omnivorous and vegetarian diets. For instance, the INTERMAP study (14) was conducted using 4680 subjects whose nutrient intake was determined using 24 h dietary recall and blood pressure measured at four different meetings. It was found that protein intake from vegetable sources was inversely related to blood pressure, with no significant blood pressure-lowering effect found for animal protein, suggesting that the amino acids found in plant proteins may have a greater impact on blood pressure than the amino acids found in animal proteins. However, studies conducted in both Japan and China have found an inverse relationship between animal protein intake and blood pressure after analyzing the nutrient intake and blood pressure measurements of over 19,000 Asian inhabitants (23–27). Based on the results of these studies, it appears that specific amino acids within the protein, and increased amount of protein, are important for attenuating high blood pressure.
Soy protein has been linked to the differences in blood pressure observed among those living in western versus Asian cultures. Soy protein is a representative vegetable protein with a higher arginine, cysteine and glycine content than other proteins (28). Studies that supplemented 20 g to 40 g of soy protein in the diet of normotensive human subjects found that soy protein caused a greater decrease in blood pressure than nonsoy proteins (29–31). The findings of these studies suggested that the amino acid content of soy-based products may account for the differences in the observed blood pressure. In addition, the isoflavones associated with soy protein may help account for the difference observed in blood pressure in these studies. Isoflavones tend to act as phytoestrogens in mammals and, therefore, may influence endothelial function of blood vessels through biological estrogenic mechanisms (30–32).
There are few studies that focus solely on the antihypertensive effects of dietary protein in animal models of hypertension (Table 2). Studies (33,34) conducted using spontaneously hypertensive rats (SHRs) have shown that fish protein was more effective in lowering blood pressure than casein after the rats were fed a 20% casein (milk protein) or highly purified fish protein diet for two months. Another study (35) using SHRs showed that soy protein also had a greater blood pressure-lowering effect than casein after rats were fed 20 g of soy protein or casein for five weeks. The substitution of soybean protein hydrolysate in the diet (0.5% or 1.0%) has also been shown to be effective in lowering blood pressure in SHRs (36).
TABLE 2.
The effect of dietary protein on blood pressure (BP) in animal models of hypertension
| Reference | Animal model | Dose and length of study | Effect on BP |
|---|---|---|---|
| Nevala et al, 2000 (35) | SHRs | 20 g of casein or soy protein in rat chow for 5 weeks | Only soy protein lowered BP |
| Ait-Yahia et al, 2003 (34) | SHRs | 20% casein or 20% fish protein for 2 months | Fish protein had a significant BP lowering effect |
| Yang et al, 2004 (36) | SHRs | Received 0%, 0.5% or 1% soybean protein hydrolysate in diet for 12 weeks | Soybean protein hydrolysate lowered SBP and mean BP |
| Ait Yahia et al, 2005 (33) | SHRs | 20% casein or highly purified fish protein in diet for 2 months | Fish protein diet lowered BP |
| Mattson et al, 2005 (141) | DSS rats | Casein or gluten protein (18% to 20%) diet | Gluten protein reduces BP in both low- and high-salt diets |
DSS Dahl salt-sensitive; SBP Systolic BP; SHRs Spontaneously hypertensive rats
In summary, an extensive and generally consistent body of evidence from observational studies, and cross-sectional and longitudinal epidemiological studies, as well as controlled clinical trials, has documented significant inverse associations between protein intake and blood pressure. Studies have also suggested that the source of protein and its amino acid composition are also important for its antihypertensive effects (37).
Amino acid studies
Rather than investigating the effects of whole dietary protein on hypertension, most animal studies investigated the effects of a specific amino acid such as cysteine, glutamate, arginine, taurine or tryptophan (Table 3). Most animal studies that investigated the effect of cysteine on blood pressure used N-acetylcysteine (NAC), a cysteine analogue. Cysteine is rapidly oxidized in the air, so it cannot be added in its natural form to the diet or drinking water. Therefore, NAC is most often used as a dietary supplement. In the human body, NAC is deacylated, mostly in the kidneys, to form cysteine. In previous studies conducted at our laboratory (38), it was found that a 760 mg/kg body weight/day oral dose of NAC given to SHRs lowered systolic blood pressure (SBP). It was also found that the inclusion of 664 mg NAC/kg body weight/day (or 1.5% NAC) for 11 weeks in the chow diet of fructose-induced hypertensive Wistar-Kyoto rats (4% fructose in drinking water) prevented an increase in SBP (39). Studies (40,41) that gave 1.5 g/kg/day of NAC to both SHRs and Sprague-Dawley (SD) rats found increased insulin sensitivity and lower blood pressure in these animals. These results have also been replicated in studies (42,43) in which Dahl salt-sensitive (DSS) rats and SHRs were fed diets supplemented with 4 g/kg/day of NAC, and a decrease in mean arterial pressure was observed.
TABLE 3.
The effect of dietary amino acids on blood pressure (BP) in animal models of hypertension
| Reference | Animal model | Intervention | Effect on BP |
|---|---|---|---|
| Chen et al, 1993 (142) | Dahl/Rapp salt-sensitive rats | 1.25 g/L L-arginine in drinking water for 4 weeks | Arginine prevented increase in BP |
| He et al, 1997 (143) | Dahl-Iwai salt-sensitive rats | 1.5% L-arginine in drinking water | Arginine prevented increase in mean BP |
| Ono et al, 1999 (144) | SHRs | 2 g/L L-arginine in drinking water for 3 weeks | Arginine had no effect on MAP |
| Artigues et al, 2000 (145) | DSS rats | 1.25 g/L L-arginine in drinking water for 4 weeks | Arginine decreased SBP |
| Özçelikay et al, 2000 (146) | Streptozotocin-diabetic Wistar rats | 1.25 mg/mL L-arginine in drinking water for 4 weeks | Arginine decreased SBP |
| Zhou et al, 2001 (44) | DSS rats | 20 g/L L-arginine in drinking water for 4 weeks | Arginine decreased SBP |
| Tay et al, 2002 (45) | Fructose-fed SD rats | 1 g/L L-arginine in drinking water for 8 weeks | Arginine decreased SBP |
| Fujii et al, 2003 (46) | DSS rats | 20 mg/mL L-arginine in drinking water for 4 weeks | Arginine lowered SAP |
| Vasdev et al, 1996 (38) | SHRs | 760 mg/kg body weight/day oral dose of NAC | NAC lowered SBP |
| Vasdev et al, 1998 (147) | Methylglyoxal-treated hypertensive WKY rats | 1.5% NAC in diet and methylglyoxal in drinking water for 18 weeks | NAC prevented increase in SBP |
| Girouard et al, 2003 (42) | SHRs | 4 g NAC/kg body weight/day in drinking water for 4 weeks | NAC decreased MAP |
| Song et al, 2005 (41) | Normotensive SD rats | 1.5 g/kg/day NAC in drinking water for 12 weeks | NAC decreased BP |
| Pechánová et al, 2006 (40) | SHRs | 1.5 g NAC/kg body weight/day in drinking water for 8 weeks | NAC prevented rise in MAP, SBP and DBP in young SHRs; no effect in adult SHRs |
| Tian et al, 2006 (43) | DSS rats | 4 g/kg/day NAC in diet for 5 weeks | NAC prevented increase in MAP |
| Vasdev et al, 2010 (148) | Fructose-treated WKY rats | 1.5% NAC and 1.5% L-arginine in drinking water for 6 weeks | NAC and arginine prevented increase in SBP |
| Fujita and Sato, 1986 (117) | DOCA salt-sensitive SD rats | 1% or 3% taurine solution for 28 days | Taurine prevented increase in SBP |
| Fujita and Sato, 1988 (47) | DOCA salt-sensitive SD rats | 1% taurine in drinking water for 4 weeks | Taurine prevented increase in SBP |
| Inoue et al, 1988 (116) | DOCA salt-sensitive SD rats | 2% taurine in drinking water for 4 weeks | Taurine prevented increase in SBP |
| Trachtman et al, 1989 (118) | SHRs | 1% taurine in drinking water for 16 weeks | Taurine attenuated increase in SBP |
| Ideishi et al, 1994 (149) | DSS rats | 4% NaCl in diet and 3% taurine in drinking water for 4 weeks | Taurine prevented increase in SBP |
| Anuradha and Balakrishnan, 1999 (120) | Fructose-treated Wistar rats | 2% taurine in drinking water for 6 weeks | Taurine prevented increase in SBP |
| Harada et al, 2004 (150) | Fructose-treated SD rats | 1% taurine in drinking water for 4 weeks | Taurine prevented increase in SBP |
| Fregly et al, 1987 (49) | DOCA salt-sensitive rats | Fed diet containing 25 g/kg or 50 g/kg tryptophan in food for 7 weeks | Tryptophan prevented increase in SBP |
| Fregly et al, 1989 (50) | SHRs | Fed 2.5% and 5.0% tryptophan by weight for 15 weeks | Tryptophan reduced SBP |
| Lark et al, 1990 (48) | DSS rats | 2.5% tryptophan in diet for 5 weeks | Tryptophan prevented the development of hypertension |
DBP Diastolic BP; DOCA Deoxycorticosterone acetate; DSS Dahl salt-sensitive; MAP Mean arterial pressure; NAC N-acetylcysteine; SAP Systolic arterial pressure; SBP Systolic BP; SD Sprague-Dawley; SHRs Spontaneously hypertensive rats; WKY Wistar-Kyoto
Arginine supplementation in the diet has also been shown to have an antihypertensive effect when given to animal models of hypertension (Table 3). When L-arginine supplementation (20 g/L in drinking water for four weeks) was given to DSS rats on an 8% NaCl diet, arginine reduced blood pressure and prevented hypertension in these animals (44). Arginine also decreased SBP in DSS rats and fructose-fed hypertensive SD rats when given in drinking water (45,46).
A study conducted by Fujita and Sato (47) found that taurine supplementation prevented increases in blood pressure when a 1% taurine supplement was added to the drinking water of deoxycorticosterone acetate (DOCA) salt-sensitive rats. Finally, tryptophan has been shown to have a blood pressure-lowering effect in animal models of hypertension. Lark et al (48) found that including 2.5% tryptophan in the diet of DSS rats for five weeks prevented the development of hypertension. Similar results were also found in DOCA salt-sensitive hypertensive rats (49). Fregly et al (50) also found that feeding tryptophan 2.5% and 5.0% by weight to SHRs for 15 weeks decreased SBP.
There are only limited studies that investigated the effect of dietary amino acids in humans (Table 4). NAC and arginine have been shown to lower blood pressure when administered at doses of 1200 mg/day each for six months to hypertensive men with type 2 diabetes (51). Taurine has also been shown to have a blood pressure-lowering effect when 6 g/day of taurine was given to borderline hypertensive men for seven days (52). Finally, it was also shown that an inverse relationship between glutamic acid intake and blood pressure exists, based on dietary intake data obtained from normotensive men and women (53).
TABLE 4.
The effect of dietary amino acids on blood pressure (BP) in humans
| Reference | Human subjects | Dose and length of study | Effect on BP |
|---|---|---|---|
| Fujita et al, 1987 (52) | Borderline hypertensive men | Oral administration of 6 g of taurine for 7 days | Taurine intake decreased BP |
| Suárez et al, 1995 (151) | Hypertensive subjects receiving ACE inhibitor lisinopril | 1.2 g/day oral NAC for 1 week | Decreased SBP and DBP |
| Pezza et al, 1998 (152) | Hypertensive men and women receiving enalapril and hydrochlorothiazide | 6 g/day oral L-arginine for 6 weeks | Decreased SBP and DBP |
| Barrios et al, 2002 (153) | Hypertensive subjects who smoked and were receiving ACE inhibitors captopril or enalapril | 1.8 g/day oral NAC for 3 weeks | Decreased 24 h ambulatory and daytime SBP and DBP |
| Martina et al, 2008 (51) | Hypertensive male subjects with type 2 diabetes | Received 600 mg of NAC twice daily, and 1200 mg of arginine once daily for 6 months | NAC and arginine lowered SBP, DBP and mean BP |
| Stamler et al, 2009 (53) | Normotensive men and women | Dietary data obtained from multipass 24 h dietary recall and 24 h urine collections. BP measured at examinations | Dietary glutamic acid was inversely related to BP |
ACE Angiotensin-converting enzyme; DBP Diastolic BP; NAC N-acetylcysteine; SBP Systolic BP
POTENTIAL ANTIHYPERTENSIVE MECHANISM OF DIETARY PROTEIN
It is suggested that the antihypertensive effect of dietary protein is due to various amino acids in the protein. More specifically, cysteine, glutamate, glutathione (GSH) (a tripeptide), arginine, leucine, taurine and tryptophan have been suggested to have a blood pressure-lowering effect. In the following section, the potential mechanisms by which protein and amino acids exhibit antihypertensive effects will be discussed.
Cysteine, glutamate and GSH
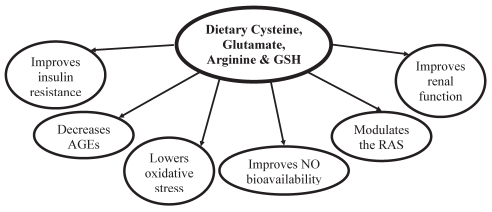
Cysteine, a nonessential amino acid, is found in foods such as meats, fish, whole grains, soybeans and legumes, and can be formed endogenously via metabolism of its precursor, the essential amino acid methionine (6,54). GSH is found in moderate to high amounts in fruits, vegetables and meats (55). The antihypertensive effects of cysteine, glutamate and GSH are interrelated in the processes by which they regulate blood pressure (Figure 2). GSH is a tripeptide that consists of glycine, cysteine and glutamate (54). The free sulfhydryl (SH) group of the cysteine molecule within GSH accounts for many of its functional capabilities because the SH group of cysteine allows GSH to form disulphide linkages with other molecules. Cysteine is also able to form disulphide bonds with other cysteine molecules to form cystine, which maintains the integrity of the vascular structure (6). The redox pairs cysteine/cystine and GSH/oxidized GSH are powerful antioxidants found in cells of the body, and are directly involved in neutralizing reactive oxygen species (ROS) and increasing antioxidant capacity (56–58).
Figure 2).
Antihypertensive mechanism of cysteine, arginine, glutamate and glutathione (GSH). These amino acids from dietary sources help to lower blood pressure by improving insulin resistance, decreasing oxidative stress, decreasing advanced glycation end product (AGE) formation, improving nitric oxide (NO) bioavailability and modulating the renin-angiotensin system (RAS)
Oxidative stress has been shown to reduce the bioavailability of the vasodilator NO and alters the activity of enzymes involved in blood pressure regulation (59). The membrane enzyme NADPH oxidase, found in vascular tissue, is a major source of superoxide (O2−), which can give rise to hydrogen peroxide and hydroxyl radicals. These reactive compounds are controlled by participating in a reaction with superoxide dismutase, GSH peroxidase and GSH reductase to yield less reactive molecules (6,54,60). GSH is an essential participant in these enzymatic reactions.
Cysteine is found at the active site of several enzymes including the vascular enzyme endothelial NO synthase (eNOS) and it regulates catalytic activity (6). It has been shown that oxidative stress can limit NO formation by uncoupling eNOS to form O2−. Because eNOS has a free cysteine SH group at its catalytic site, it is more susceptible to inactivation by aldehydes and ROS (61–63). NO is a reactive molecule that can form reactive nitrogen species such as peroxynitrite and peroxynitrous acid, which are unstable molecules that can result in the production of hydroxyl radicals, thus decreasing NO bioavailability (6). It has been suggested that cysteine and GSH help preserve NO synthesis and bioavailability by preventing the degradation of NO, as well as protecting NO synthase activity and signalling pathways from oxidative stress, thus preventing hypertension (6).
In essential hypertension, altered glucose metabolism due to insulin resistance results in increased formation of the aldehyde methylglyoxal (64,65). Also, there is increased formation of other aldehydes due to increased oxidative stress in hypertension (64). These aldehydes react with free amino and SH groups of proteins to form AGEs. This results in altered structure and function of these proteins (6,64). Cysteine has been shown to help increase insulin sensitivity through neutralizing the actions of ROS in hypertension. When SHRs were fed a diet supplemented with NAC, the progression of insulin resistance was attenuated. NAC also reduces oxidative stress by suppressing alpha-1 adrenoceptor-mediated vasoconstriction, increasing insulin sensitivity and preventing an increase in blood pressure (41).
When NAC was administered with water at a dose of 10 mg/kg/day, insulin resistance and increased methylglyoxal levels were reversed due to the methylglyoxal scavenger activity of NAC (66). An additional experiment conducted during this study showed that by treating 3T3-L1 adipocytes with NAC (600 μM), a methylglyoxal impairment in insulin signalling was relieved, as shown by decreased insulin-induced insulin receptor substrate-1 tyrosine phosphorylation and decreased activity of phosphatidylinositol 3-kinase (66). These results show the ability of cysteine, or NAC, to reverse and prevent the effects caused by methylglyoxal in the development of insulin resistance and hypertension (66).
The GSH-dependent glyoxalase system normally catabolizes methylglyoxal and glyoxal to maintain a low level of these aldehydes. Increased consumption of cysteine in the diet would help alleviate the effects of aldehydes and AGEs because cysteine binds these molecules so they are excreted in the bile and urine (67). Cysteine may also help boost the activity of the GSH-dependent catabolism and excretion of reactive aldehydes, which would prevent the formation of AGEs and their detrimental effects (6). A study (68) conducted using 3T3-L1 adipocytes showed that decreased glucose uptake results from increased oxidative stress caused by the interaction between AGEs and their receptors. This effect was reversed by treating cells with NAC. Increases in oxidative stress and angiotensin II production caused by AGEs binding have also been shown to be prevented in rat mesangial cells by the addition of NAC (69).
Because the RAS regulates vasoconstriction, alterations in this system may contribute to hypertension, and amino acids may have a potential antihypertensive role. Studies have shown that when the kidney, heart and brain tissues are subjected to oxidizing agents in vitro, there is an increase in angiotensin-converting enzyme (ACE) activity. However, this activity is inhibited when NAC is applied to the kidney cortex (70,71). NAC also prevented the actions of angiotensin II due to the interaction between AGEs and their receptors in mesangial cells when treated in vitro (69). Angiotensin II may also influence insulin resistance because angiotensin II has been shown to inhibit insulin signalling in aortic vascular smooth muscle cells by decreasing insulin receptor substrate-1 protein levels. However, this decrease was prevented by treating the cells with NAC (72,73). Cysteine and GSH may exert control over the RAS through their antioxidant properties by modulating vasoconstriction caused by ROS-stimulated ACE activity and the ROS that act as secondary messengers in many of the angiotensin II-mediated pathways (70,74). These effects may be due to a direct reaction of NAC with the disulphide bonds of the angiotensin II type I receptor.
Glutamate – or glutamic acid – is the most common dietary amino acid, especially in vegetable protein (53). Several mechanisms have been suggested for the antihypertensive effect of glutamate. Glutamate is a part of the GSH molecule, and is also a substrate for arginine synthesis (53). Glutamate also enhances kidney function (53). A study conducted using 4680 normotensive subjects from the INTERMAP study found an inverse relationship between dietary glutamic acid and blood pressure (53).
Together, cysteine, glutamate and GSH may attenuate hypertension by improving insulin resistance, reducing oxidative stress, preventing and reducing AGE formation, improving NO bioavailability and modulating the activity of the RAS.
Arginine
Arginine is a semiessential amino acid found in dietary sources such as meat, fish, soy, beans, lentils, nuts and whole grains (75). Arginine can also be produced in the kidney, or by the urea cycle in the liver (76,77). Dietary arginine can affect blood pressure control in a number of ways (Figure 2). First, arginine acts as a substrate for the formation of NO. Second, arginine can promote the release of insulin from the pancreas (78,79). Insulin can then stimulate cellular uptake of arginine by decreasing the plasma concentration of asymmetrical dimethyl arginine (ADMA), an arginine analogue (80–84). Similar to cysteine and GSH, arginine can also modulate the RAS by inhibiting ACE activity, causing a decrease in angiotensin II and its harmful effects (85). Finally, arginine may help to regulate redox-sensitive proteins and lower blood pressure by acting as an antioxidant (86,87).
There are few studies available that demonstrate the effects of dietary arginine supplementation on insulin resistance. A study in which SD rats with fructose-induced hypertension were given 1 g/L arginine in their drinking water for eight weeks found that arginine supplementation prevented a decrease in insulin sensitivity, as well as increases in glucose and insulin concentrations in response to fructose-induced hypertension (45). Oral arginine (9 g/day for one month) supplementation has been shown to improve hepatic and peripheral insulin sensitivity in type 2 diabetes (88). It has been suggested that arginine improves insulin resistance by increasing NO synthesis, and improves endothelial function as demonstrated in one study (88) that showed that oral arginine supplementation normalized plasma cyclic guanosine monophosphate and increased blood flow in the forearm. In normotensive subjects, arginine supplementation has been shown to increase the ratio of arginine to ADMA, which helps improve insulin-mediated glucose utilization (89).
Arginine may both directly and indirectly affect the production of AGEs and their subsequent effects on blood pressure. Indirectly, arginine may act to limit the formation of AGEs by improving insulin resistance and reducing oxidative stress, which helps to decrease the production of methylglyoxal and other aldehydes (90). In a more direct manner, arginine binds to aldehydes to form free glycation adducts, which are excreted in urine (90–92). In diabetic mice, 50 mg/kg body weight/day oral doses of arginine reduced AGE formation in glomerular basement membrane collagen (93).
NO is synthesized in the vascular endothelium from L-arginine by eNOS, and influences blood pressure through its impact on vascular tone (94). eNOS requires the cofactor tetrahydrobiopterin (BH4) to maintain its stability (95). In the periphery, nonadrenergic and noncholinergic nerves operate using a NO-mediated mechanism to cause vasodilation of the blood vessels (94). When the concentration of L-arginine or BH4 is low, or BH4 becomes oxidized under conditions of oxidative stress, there is increased endothelial dysfunction because vasoconstriction increases and vasodilation decreases, contributing to increased pressure within the vessels (91). Increased arginine will prevent these alterations by acting as both an antioxidant and a substrate for NO. Studies have shown that in humans, intravenous injections of arginine (30 g per 30 min) can cause a 72-fold increase in the plasma levels of arginine (100 μmol/L to 7200 μmol/L), accompanied by a 15% decrease in blood pressure (28).
The activity of ACE has been shown to be inhibited by arginine supplementation, which decreases the production of angiotensin II and its cascade of effects (85). Insulin may also mediate the effect of arginine on the RAS because insulin has been shown to lower angiotensinogen and angiotensin II type I receptor expression in endothelial cells (96,97). In hypertension, the increased effects of angiotensin II may be the result of a functional imbalance due to a decrease in the effects of both insulin and NO. Vascular homeostasis may be restored if arginine can improve the balance between NO production and insulin resistance (98).
Similar to cysteine and GSH, arginine may also have antioxidant properties (87). Arginine may help to minimize the damage caused by radicals by helping to restore normal functioning of the NO pathway and the RAS. As well, arginine may react with hydrogen peroxide nonenzymatically to form NO in vitro (99). This action has two benefits – it increases the amount of vasodilator present, and also decreases ROS. Arginine has been shown to protect NO from degradation by diminishing O2− generation in vascular endothelial cells in vitro (87).
Arginine may also help to regulate redox-sensitive proteins. For instance, by preventing the oxidation of dimethylarginine dimethylaminohydrolase by ROS, ADMA breakdown can be increased, which reduces the competitive inhibition of arginine uptake (100). As well, it has been suggested that normal kidney function may be regulated by the balance of O2− and NO (101). If poor NO bioavailability in the kidney results from an O2−-favoured imbalance, the antioxidant properties of arginine may partly be the cause of its antihypertensive effects. Arginine has been shown to decrease glomerulosclerosis and improve renal hemodynamics and morphology in a rat hypertension model (102).
Leucine
Leucine is a branched-chain essential amino acid that, along with the other branched-chain amino acids, isoleucine and valine, accounts for 15% to 25% of the total protein intake (103,104). Branched-chain amino acids are particularly abundant in dairy products (104). Unlike other amino acids, branched-chain amino acids are metabolized in the peripheral tissues, particularly muscle, rather than in the liver, which functions to regulate the amount of amino acids in circulation (105). The role of leucine in the human body is associated with the absence of the branched-chain aminotransferase enzyme in the liver, which results in the large supply of branched-chain amino acids appearing in the muscle (106).
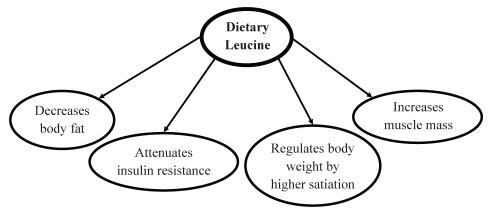
Leucine has been suggested to have an indirect effect on lowering blood pressure through its influence on a number of metabolic processes, including modulating phosphatidylinositol 3-kinase in insulin signalling, regulating translation initiation for protein synthesis, as well as a nitrogen donor for alanine and glutamine production in skeletal muscle (Figure 3) (106). The activation of signalling pathways is considered to be an important nonprotein function of amino acids. On a daily basis, after the need for leucine for protein synthesis has been met, leucine is available to contribute to alanine or glutamine production, or to have an impact on the insulin signalling pathway (104). The impact of leucine on these processes, and thus its effect on hypertension, is dependent on dietary intake of leucine and the increase in its concentration in skeletal muscle. It has been estimated that metabolic use of leucine ranges from 7 g to 12 g per day (104).
Figure 3).
Antihypertensive mechanism of dietary leucine. Leucine lowers blood pressure by helping to decrease body fat, attenuating insulin resistance and maintaining muscle mass by increasing protein synthesis
To help attenuate insulin resistance, leucine stimulates cellular response to insulin by acting on mammalian target rapamycin (mTOR), a kinase within the insulin signalling cascade (106). By increasing dietary protein, more leucine is available to contribute to the de novo synthesis of glucose through gluconeogenesis; it also increases the recycling of glucose carbon through the glucose-alanine cycle. It has been reported that 1 g of dietary protein can contribute 0.6 g to 0.7 g of glucose through gluconeogenesis (106). The substitution of dietary proteins for simple carbohydrates in the diet will help to control postprandial glucose levels because proteins take a longer route via gluconeogenesis to form glucose. In subjects with type 2 diabetes or obesity, diets high in protein have been reported to reduce postprandial glucose and insulin, and stabilize blood glucose levels in these subjects. This may, in part, be due to the fact that amino acids are absorbed more slowly in the gut than carbohydrates, before being metabolized in the body via another slow process. As well, amino acids cause a lower insulin response than carbohydrates, which are usually absorbed within 2 h of consumption and require a greater increase in insulin to maintain blood glucose levels within a normal physiological range (107).
In addition, it has been reported that protein has a higher satiation effect on hunger than glucose (108). Therefore, if hypertensive subjects consume a diet that is adequate in protein and low in carbohydrates, the protein will be able to maintain normal blood glucose over a longer period of time than the carbohydrates; subjects would consume less food, contributing to a decrease in body fat because the body would break down glycogen stored in adipose tissue to obtain glucose and maintain muscle mass. As well, by consuming fewer carbohydrates, less insulin is released, which would otherwise inhibit gluconeogenesis and gluconeogenic enzymes. For example, human weight loss trials conducted using women who were given a diet that contained 3.5 g/kg per day carbohydrates and 1.5 g/kg per day protein, including a daily leucine intake of approximately 5 g (or 8% of dietary protein), showed that subjects fed a diet that contained an increased amount of protein, including leucine, tended to lose more weight than those fed a diet that contained less protein. The results also suggest that diets that contain ample amounts of protein and reduced amounts of carbohydrates lead to weight loss due to the loss of body fat. Protein loss is spared (103,109–111).
To help suppress food intake, it has been suggested that leucine may act via mTOR in the hypothalamus in the central nervous system (105). mTOR acts as a cellular fuel sensor that regulates energy intake through its hypothalamic activity, rather than just through an amino acid sensor (112). A study by Cota et al (112) used antibodies to localize mTOR and showed that mTOR signalling plays a role in mechanisms in the brain that are associated with nutrient availability – the administration of leucine increased hypothalamic mTOR signalling and decreased food intake and body weight in the rats. The results of this experiment also indicated that the mTOR pathway has the ability to modify the activity of hormones, such as leptin, which helps control body weight by regulating hunger and food consumption, as well as lipolysis, energy consumption and body temperature (113). It was suggested that by increasing leucine intake, more leptin will be secreted, suppressing food intake, which leads to weight loss, improved insulin sensitivity and decreases in blood pressure.
During periods of fasting, reduced energy intake or insulin resistance, protein from muscle is often used as a source of energy. Amino acids are converted to glucose through gluconeogenesis, causing a decrease in muscle mass and creating an imbalance favouring muscle breakdown over synthesis. By increasing protein intake, the additional amino acids in circulation, including leucine, can stimulate muscle protein synthesis by acting on translation mechanisms. More specifically, leucine is able to exert control over translation initiation factors, mainly eIF4, in the skeletal muscle (97). By stimulating protein synthesis, leucine contributes to the production of lean muscle tissue, which in turn increases the metabolic rate and energy requirement of muscle. This also helps to increase the loss of body fat, which may lower blood pressure (107).
Taurine
Taurine is the most abundant intracellular amino acid distributed in many human tissues, but it is not incorporated into proteins (114,115). In humans, taurine is a conditionally essential amino acid. It can be synthesized from methionine and cysteine in the presence of vitamin B6 in the brain and liver, in a reaction catalyzed by cysteine sulphinic acid decarboxylase (114,115). On average, humans synthesize 0.4 mmol to 1.0 mmol (50 mg to 125 mg) of taurine daily (115). However, for humans, diet is the main source of taurine. Taurine is found in high concentrations in foods from animal sources, and is undetectable in vegetables (115). Food sources of taurine include meats such as beef, pork and poultry; seafood such as tuna, mussels, white fish, oysters, cod and clams; and dairy products such as pasteurized milk, cheddar cheese, low-fat plain yogourt and vanilla ice cream (115).
There is strong experimental data to support the efficacy of taurine in lowering blood pressure. Studies (116,117) in DOCA salt-sensitive rats who were given 1% to 3% taurine in their drinking water for four weeks showed that taurine prevented an increase in SBP. In DSS hypertensive rats, 1% taurine supplementation prevented increased sympathetic nervous system activity and blood pressure (47). As well, in SHRs, taurine supplementation led to decreases in blood pressure (118).
It has been suggested that orally administered taurine is absorbed from the gastrointestinal tract and, after passing through the blood-brain barrier, it accumulates in the brain and increases the taurine content of the hypothalamus (116). In the hypothalamus, taurine suppresses the activity of the sympathetic nervous system, which is elevated in hypertension. Taurine modulates the activity of the sympathetic nervous system by modulating the secretion of arginine-vasopressin, which results in a decrease in blood pressure (52,119). This is supported by the experiments in which taurine was infused into brain ventricles, which led to decreases in blood pressure and modulated the release of arginine-vasopressin in hypothalamic neurons (23). It has also been suggested that taurine supplementation may attenuate the activity of the sympathetic nervous system using its natriuretic effects through osmoregulation in the kidney, and helping to improve renal ion handling (120). Taurine supplementation has also been shown to improve insulin sensitivity, with decreases in blood pressure in fructose-induced models of hypertension (120). In both in vivo and in vitro studies, taurine attenuated the formation of glycated protein, glycated hemoglobin and fructosamine (121). It has been proposed that taurine may also act on the RAS in the brain to help reduce blood pressure (119).
Tryptophan
Tryptophan is a neutral, branched-chain amino acid. It is also an essential amino acid for humans. Food sources rich in tryptophan include soybeans, salami, pork, mollusks, game meat, tofu, beef and pumpkin seeds (122). The mechanism by which tryptophan exerts its blood pressure-lowering effect is not certain. A study by Fregly et al (50) showed that treating SHRs with 5.0% tryptophan resulted in a significant reduction in the urinary output of adrenaline and noradrenaline, and an increase in dopamine. It is suggested that increased dietary intake of tryptophan would affect the enzymes dopamine-beta-hydroxylase and phenylethanolamine N-methyltransferase in the adrenal medulla and the peripheral sympathetic nervous system (50). The inhibition of these enzymes would reduce the conversion of dopamine to adrenaline and noradrenaline, decreasing their effects on blood pressure (50).
Soy protein and isoflavones influence blood pressure
In recent years, soy and soy-based products have increased in popularity. Studies conducted in humans that investigated the effects of soy proteins on blood pressure have credited various components of soy protein as having antihypertensive effects. Some research credits the high arginine content of soy protein, whereas other studies suggest that the benefits of consuming soy protein are due to the antioxidant activity exhibited by isoflavones. Soy proteins are also considered to be a representative vegetable protein, with a higher content of arginine, cysteine and glycine than other vegetable proteins (28).
Studies (29,31,123–125) have shown that humans who consume a large amount of soy protein in their diet have a lower risk of cardiovascular disease due to having lower blood pressure. For instance, the Shanghai Women’s Health Study (125) followed more than 45,000 Chinese women for a period of two to three years. It was found that women who consumed at least 25 g/day of soy protein in their diet had lower SBP and diastolic blood pressure (DBP). A study (29) conducted in 302 subjects from China investigated the effect of soybean protein supplementation on blood pressure. The subjects were given 40 g of isolated soybean protein supplements per day or a complex carbohydrate control for 12 weeks (29). The results of this study showed that soybean protein supplementation lowered both SBP and DBP, suggesting that soy protein has potential as a treatment for high blood pressure (29).
In addition to containing higher amounts of important amino acids, soy proteins also contain isoflavones, which allow soy proteins to act as phytoestrogens. Phytoestrogens have been shown to reduce blood pressure by having a direct effect on blood vessel walls through biological estrogenic mechanisms (32). Isoflavones also decrease oxidative stress (both in vivo and in vitro), stimulate NO production, improve systemic arterial compliance, and favourably affect salt and water balance, all of which contribute to a protective role against the development of hypertension (125). In particular, genistein, a soy isoflavone, has been shown to stimulate NO production (125). A study (123) that compared the effects of soy milk with cow’s milk in 40 mildly to moderately hypertensive men and women for three months found that soy milk caused a significant decrease in SBP, DBP and mean blood pressure. It was also found that urinary genistein levels were strongly correlated with decreases in blood pressure (123). A study by Sagara et al (126) investigated the effects of soy protein and isoflavones on blood pressure in 61 high-risk (SBP of 130 mmHg or greater) middle-aged Scottish men. The men were given diets that contained at least 20 g of soy protein and 80 mg of isoflavones, or a placebo diet for five weeks. They found significant decreases in both SBP and DBP for men who consumed the experimental diet compared with those who were given the placebo diet (126). Soy protein also contains ACE inhibitory peptides, which have been shown to alter sodium and water balance and, therefore, reduce blood pressure (127). It is important to note, however, that it is unlikely that the isoflavone content of soy protein alone accounts for blood pressure reduction (128).
CONCLUSION
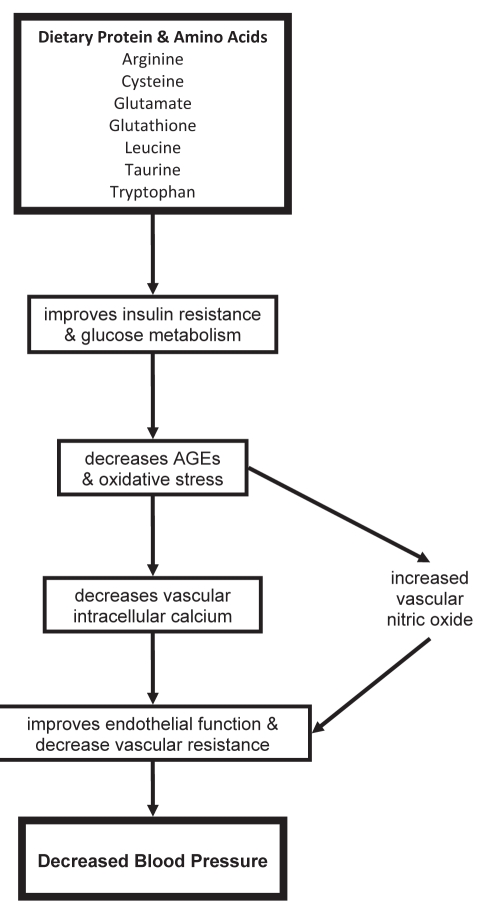
Essential hypertension develops due to an interaction of genetic and lifestyle factors, such as diet. Increased dietary salt is one lifestyle factor that causes an increase in blood pressure. A moderate increase in protein intake, along with low salt and sugar intake, has been identified as a possible lifestyle change that can help lower high blood pressure. An extensive and generally consistent body of evidence has documented an inverse relationship between protein intake and blood pressure. Several amino acids in dietary protein, including cysteine, arginine, taurine and tryptophan, have been shown to have antihypertensive effects in humans and animals. These amino acids attenuate the metabolic reactions associated with hypertension including insulin resistance, increased AGEs, increased oxidative stress, altered renal function, decreased NO bioavailability and altered RAS (Figure 4). Soy proteins have high arginine and isoflavone contents, which contribute to their antihypertensive effects. To ensure that hypertensive patients receive an adequate protein intake, it is recommended that patients adopt a diet that contains ample amounts of protein, such as the DASH diet, which is rich in vegetables, fruit and low-fat dairy products; low in salt and saturated fat; and includes whole grains, lean meat, poultry, fish and nuts. In addition, hypertensive patients should include an adequate supply of soy in their diet. By implementing these dietary habits, individuals with hypertension can obtain an appropriate and beneficial protein intake in a safe and realistic manner.
Figure 4).
The antihypertensive mechanism of dietary protein and amino acids. The blood pressure-lowering effect of protein is performed by its amino acids. Arginine, cysteine, glutamate, glutathi-one, leucine, taurine and tryptophan all help to attenuate insulin resistance and improve glucose metabolism. This in turn helps to decrease advanced glycation end products (AGEs), improve oxidative stress, decrease vascular intracellular calcium and increase nitric oxide production. This all contributes to improving endothelial function and decreasing peripheral vascular resistance, resulting in decreased blood pressure
Acknowledgments
The authors thank the Canadian Institutes of Health Research Regional Partnership Program and the Discipline of Medicine, Memorial University, St John’s, Newfoundland and Labrador, for financial support.
REFERENCES
- 1.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: Analysis of worldwide data. Lancet. 2005;365:217–23. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . World Health Organization The World Health Report [chapter 4] Geneva: World Health Organization; 2002. Quantifying selected major risks to health; pp. 1–13. [Google Scholar]
- 3.He J, Whelton PK. Epidemiology and prevention of hypertension. Med Clinics North Am. 1997;81:1077–97. doi: 10.1016/s0025-7125(05)70568-x. [DOI] [PubMed] [Google Scholar]
- 4.Klag MJ, Whelton PK, Randall BL, et al. Blood pressure and end-stage renal disease in men. N Engl J Med. 1996;334:13–8. doi: 10.1056/NEJM199601043340103. [DOI] [PubMed] [Google Scholar]
- 5.Deshmukh R, Smith A, Lilly LS. Hypertension. In: Lilly LS, editor. Pathophysiology of Heart Disease. 2nd edn. Philadelphia: Lippincott Williams & Wilkins; 1998. pp. 267–88. [Google Scholar]
- 6.Vasdev S, Singal P, Gill V. The antihypertensive effect of cysteine. Int J Angiol. 2009;18:7–21. doi: 10.1055/s-0031-1278316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sacks FM, Obarzanek E, Windhauser MM, et al. Rationale and design of the dietary approaches to stop hypertension trial (DASH). A multicenter controlled-feeding study of dietary patterns to lower blood pressure. Ann Epidemiol. 1995;5:108–18. doi: 10.1016/1047-2797(94)00055-x. [DOI] [PubMed] [Google Scholar]
- 8.Sacks FM, Svetkey LP, Vollmer WM, et al. Effect on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 9.Stamler J, Elliott P, Kesteloot H, et al. Inverse relation of dietary protein markers with blood pressure. Findings for 10,020 men and women in the INTERSALT study. Circulation. 1996;94:1629–34. doi: 10.1161/01.cir.94.7.1629. [DOI] [PubMed] [Google Scholar]
- 10.Stamler J, Caggiula A, Grandits GA, Kjelsberg M, Cutler JA. Relationship to blood pressure of combinations of dietary macronutrients. Findings of the multiple risk factor intervention trial (MRFIT) Circulation. 1996;94:2417–23. doi: 10.1161/01.cir.94.10.2417. [DOI] [PubMed] [Google Scholar]
- 11.Elliott P, Fehily AM, Sweetnam PM, Yarnell JW. Diet, alcohol, body mass, and social factors in relation to blood pressure: The Caerphilly heart study. J Epidemiol Community Health. 1987;41:37–43. doi: 10.1136/jech.41.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu L, Ikeda K, Yamori Y. Inverse relationship between urinary markers of animal protein intake and blood pressure in Chinese: Results from the WHO cardiovascular diseases and alimentary comparison (CARDIAC) study. Int J Epidemiol. 2002;31:227–33. doi: 10.1093/ije/31.1.227. [DOI] [PubMed] [Google Scholar]
- 13.Appel LJ, Frank MS, Vincent JC, Obarzanek E, Janis FS, Edgar RM. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: Results of the OmniHeart randomized trial. JAMA. 2005;294:2455–64. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 14.Elliott P, Stamler J, Dyer AR, et al. Association between protein intake and blood pressure: The INTERMAP study. Arch Intern Med. 2006;166:79–87. doi: 10.1001/archinte.166.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rouse IL, Beilin LJ, Armstrong BK, Vandongen R. Blood pressure-lowering effect of a vegetarian diet: Controlled trial in normotensive subjects. Lancet. 1983;1:5–10. doi: 10.1016/s0140-6736(83)91557-x. [DOI] [PubMed] [Google Scholar]
- 16.Ministry of Health, Labour and Welfare . Kokumin Kennko-Eiyo Chousa. [The National Nutrition Survey in Japan, 2003.] Tokyo: Daiichi Shuppan; 2006. [Google Scholar]
- 17.Smit E, Nieto FJ, Crespo CJ, Mitchell P. Estimates of animal and plant protein intake in US adults: Results from the Third National Health and Nutrition Examination Survey, 1988–1991. J Am Diet Assoc. 1999;99:813–20. doi: 10.1016/S0002-8223(99)00193-5. [DOI] [PubMed] [Google Scholar]
- 18.Rodwell VW. Proteins: Structure & function. In: Murray RK, Granner DK, Mayes PA, Rodwell VW, editors. Harper’s Biochemistry. 34th edn. Stamford: Appleton & Lange; 1996. pp. 41–52. [Google Scholar]
- 19.Whitney E, Rolfes SR. Understanding Nutrition. 10th edn. Belmont: Thomson Wadsworth; 2005. [Google Scholar]
- 20.World Health Organization . WHO technical report series – protein and amino acid requirements in human nutrition. Geneva: World Health Organization; 2007. [PubMed] [Google Scholar]
- 21.Cirillo M, Lombardi C, Laurenzi M, De Santo NG. Relation of urinary urea to blood pressure: Interaction with urinary sodium. J Hum Hypertens. 2002;16:205–12. doi: 10.1038/sj.jhh.1001323. [DOI] [PubMed] [Google Scholar]
- 22.Iseki K, Iseki C, Itoh K, et al. Estimated protein intake and blood pressure in a screened cohort in Okinawa, Japan. Hypertens Res. 2003;26:289–94. doi: 10.1291/hypres.26.289. [DOI] [PubMed] [Google Scholar]
- 23.Umesawa M, Sato S, Imano H, et al. Relations between protein intake and blood pressure in Japanese men and women: The circulatory risk in communities study (CIRCS) Am J Clin Nutr. 2009;90:377–84. doi: 10.3945/ajcn.2008.27109. [DOI] [PubMed] [Google Scholar]
- 24.Kihara M, Fujikawa J, Ohtaka M, et al. Interrelationships between blood pressure, sodium, potassium, serum cholesterol, and protein intake in Japanese. Hypertension. 1984;6:736–42. doi: 10.1161/01.hyp.6.5.736. [DOI] [PubMed] [Google Scholar]
- 25.Nara Y, Zhao GS, Huang ZD, et al. Relationship between dietary factors and blood pressure in China. The Sino-Japan CARDIAC cooperative research group. J Cardiovasc Pharmacol. 1990;16(Suppl 8):S40–2. [PubMed] [Google Scholar]
- 26.Reed D, McGee D, Yano K, Hankin J. Diet, blood pressure, and multicollinearity. Hypertension. 1985;7:405–10. [PubMed] [Google Scholar]
- 27.Zhou B, Zhang X, Zhu A, et al. The relationship of dietary animal protein and electrolytes to blood pressure: A study on three Chinese populations. Int J Epidemiol. 1994;23:716–22. doi: 10.1093/ije/23.4.716. [DOI] [PubMed] [Google Scholar]
- 28.Obarzanek E, Velletri PA, Cutler JA. Dietary protein and blood pressure. JAMA. 1996;275:1598–603. doi: 10.1001/jama.1996.03530440078040. [DOI] [PubMed] [Google Scholar]
- 29.He J, Gu D, Wu X, et al. Effect of soybean protein on blood pressure: A randomized, controlled trial. Ann Intern Med. 2005;143:1–9. doi: 10.7326/0003-4819-143-1-200507050-00004. [DOI] [PubMed] [Google Scholar]
- 30.Washburn S, Burke GL, Morgan T, Anthony M. Effect of soy protein supplementation on serum lipoproteins, blood pressure, and menopausal symptoms in perimenopausal women. Menopause. 1999;6:7–13. [PubMed] [Google Scholar]
- 31.Welty FK, Lee KS, Lew NS, Zhou JR. Effect of soy nuts on blood pressure and lipid levels in hypertensive, prehypertensive, and normotensive postmenopausal women. Arch Intern Med. 2007;167:1060–7. doi: 10.1001/archinte.167.10.1060. [DOI] [PubMed] [Google Scholar]
- 32.Teede HJ, Dalais FS, Kotsopoulos D, Liang YL, Davis S, McGrath BP. Dietary soy has both beneficial and potentially adverse cardiovascular effects: A placebo-controlled study in men and postmenopausal women. J Clin Endocrinol Metab. 2001;86:3053–60. doi: 10.1210/jcem.86.7.7645. [DOI] [PubMed] [Google Scholar]
- 33.Ait Yahia D, Madani S, Prost J, Bouchenak M, Belleville J. Fish protein improves blood pressure but alters HDL2 and HDL3 composition and tissue lipoprotein lipase activities in spontaneously hypertensive rats. Eur J Nutr. 2005;44:10–7. doi: 10.1007/s00394-004-0486-y. [DOI] [PubMed] [Google Scholar]
- 34.Ait-Yahia D, Madani S, Savelli JL, Prost J, Bouchenak M, Belleville J. Dietary fish protein lowers blood pressure and alters tissue polyunsaturated fatty acid composition in spontaneously hypertensive rats. Nutrition. 2003;19:342–6. doi: 10.1016/s0899-9007(02)00858-4. [DOI] [PubMed] [Google Scholar]
- 35.Nevala R, Vaskonen T, Vehniäinen J, Korpela R, Vapaatalo H. Soy based diet attenuates the development of hypertension when compared to casein based diet in spontaneously hypertensive rat. Life Sci. 2000;66:115–24. doi: 10.1016/s0024-3205(99)00569-x. [DOI] [PubMed] [Google Scholar]
- 36.Yang HY, Yang SC, Chen JR, Tzeng YH, Han BC. Soyabean protein hydrolysate prevents the development of hypertension in spontaneously hypertensive rats. Br J Nutr. 2004;92:507–12. doi: 10.1079/bjn20041218. [DOI] [PubMed] [Google Scholar]
- 37.Blumenthal JA, Babyak MA, Hinderliter A, et al. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: The ENCORE study. Arch Intern Med. 2010;170:126–35. doi: 10.1001/archinternmed.2009.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vasdev S, Mian T, Ford CA, Longerich L, Parai S. Role of endogenous aldehydes in spontaneously hypertensive and disulfiram-induced hypertensive rats. Nutr Metab Cardiovasc Dis. 1996;6:130–40. [Google Scholar]
- 39.Vasdev S, Ford CA, Longerich L, Gadag V, Wadhawan S. Role of aldehydes in fructose induced hypertension. Mol Cell Biochem. 1998;181:1–9. doi: 10.1023/a:1006844222963. [DOI] [PubMed] [Google Scholar]
- 40.Pechánová O, Zicha J, Kojsová S, Dobesová Z, Jendeková L, Kunes J. Effect of chronic N-acetylcysteine treatment on the development of spontaneous hypertension. Clin Sci (Lond) 2006;110:235–42. doi: 10.1042/CS20050227. [DOI] [PubMed] [Google Scholar]
- 41.Song D, Hutchings S, Pang CC. Chronic N-acetylcysteine prevents fructose-induced insulin resistance and hypertension in rats. Eur J Pharmacol. 2005;508:205–10. doi: 10.1016/j.ejphar.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 42.Girouard H, Chulak C, Wu L, Lejossec M, de Champlain J. N-acetylcysteine improves nitric oxide and alpha-adrenergic pathways in mesenteric beds of spontaneously hypertensive rats. Am J Hypertens. 2003;16:577–84. doi: 10.1016/s0895-7061(03)00863-x. [DOI] [PubMed] [Google Scholar]
- 43.Tian N, Rose RA, Jordan S, Dwyer TM, Hughson MD, Manning RD., Jr N-acetylcysteine improves renal dysfunction, ameliorates kidney damage and decreases blood pressure in salt-sensitive hypertension. J Hypertens. 2006;24:2263–70. doi: 10.1097/01.hjh.0000249705.42230.73. [DOI] [PubMed] [Google Scholar]
- 44.Zhou MS, Kosaka H, Tian RX, et al. L-arginine improves endothelial function in renal artery of hypertensive dahl rats. J Hypertens. 2001;19:421–9. doi: 10.1097/00004872-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 45.Tay A, Ozçelikay AT, Altan VM. Effects of L-arginine on blood pressure and metabolic changes in fructose-hypertensive rats. Am J Hypertens. 2002;15:72–7. doi: 10.1016/s0895-7061(01)02231-2. [DOI] [PubMed] [Google Scholar]
- 46.Fujii S, Zhang L, Igarashi J, Kosaka H. L-arginine reverses p47phox and gp91phox expression induced by high salt in Dahl rats. Hypertension. 2003;42:1014–20. doi: 10.1161/01.HYP.0000094557.36656.D0. [DOI] [PubMed] [Google Scholar]
- 47.Fujita T, Sato Y. Hypotensive effect of taurine. Possible involvement of the sympathetic nervous system and endogenous opiates. J Clin Invest. 1988;82:993–7. doi: 10.1172/JCI113709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lark LA, Becker KB, Park RE, Weyhenmeyer JA. Prevention of Dahl salt-induced hypertension by chronic dietary tryptophan. Can J Physiol Pharmacol. 1990;68:1432–6. doi: 10.1139/y90-217. [DOI] [PubMed] [Google Scholar]
- 49.Fregly MJ, Lockley OE, van der Voort J, Sumners C, Henley WN. Chronic dietary administration of tryptophan prevents the development of deoxycorticosterone acetate salt induced hypertension in rats. Can J Physiol Pharmacol. 1987;65:753–64. doi: 10.1139/y87-122. [DOI] [PubMed] [Google Scholar]
- 50.Fregly MJ, Sumners C, Cade JR. Effect of chronic dietary treatment with L-tryptophan on the maintenance of hypertension in spontaneously hypertensive rats. Can J Physiol Pharmacol. 1989;67:656–62. doi: 10.1139/y89-105. [DOI] [PubMed] [Google Scholar]
- 51.Martina V, Masha A, Gigliardi VR, et al. Long-term N-acetylcysteine and L-arginine administration reduces endothelial activation and systolic blood pressure in hypertensive patients with type 2 diabetes. Diabetes Care. 2008;31:940–4. doi: 10.2337/dc07-2251. [DOI] [PubMed] [Google Scholar]
- 52.Fujita T, Ando K, Noda H, Ito Y, Sato Y. Effects of increased adrenomedullary activity and taurine in young patients with borderline hypertension. Circulation. 1987;75:525–32. doi: 10.1161/01.cir.75.3.525. [DOI] [PubMed] [Google Scholar]
- 53.Stamler J, Brown IJ, Daviglus ML, et al. Glutamic acid, the main dietary amino acid, and blood pressure: The INTERMAP Duty (International Collaborative Study of Macronutrients, Micronutrients and Blood Pressure) Circulation. 2009;120:221–8. doi: 10.1161/CIRCULATIONAHA.108.839241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Champe PC, Harvey RA. Lippincott’s Illustrated Reviews Biochemistry. 2nd edn. Philadelphia: JB Lippincott Company; 1994. [Google Scholar]
- 55.Jones DP, Coates RJ, Flagg EW, et al. Glutathione in foods listed in the National Cancer Institute’s Health Habits and History Food Frequency Questionnaire. Nutr Cancer. 1992;17:57–75. doi: 10.1080/01635589209514173. [DOI] [PubMed] [Google Scholar]
- 56.May JM, Qu ZC, Neel DR, Li X. Recycling of vitamin C from its oxidized forms by human endothelial cells. Biochem Biophys Acta. 2003;1640:153–61. doi: 10.1016/s0167-4889(03)00043-0. [DOI] [PubMed] [Google Scholar]
- 57.May JM, Qu Z, Li X. Requirement for GSH in recycling of ascorbic acid in endothelial cells. Biochem Pharmacol. 2001;62:873–81. doi: 10.1016/s0006-2952(01)00736-5. [DOI] [PubMed] [Google Scholar]
- 58.Ho CT, Chan AC. Regeneration of vitamin E in rat polymorphonuclear leucocytes. FEBS Lett. 1992;306:269–72. doi: 10.1016/0014-5793(92)81015-e. [DOI] [PubMed] [Google Scholar]
- 59.Vasdev S, Gill VD, Singal PK. Modulation of oxidative stress-induced changes in hypertension and atherosclerosis by antioxidants. Exp Clin Cardiol. 2006;11:206–16. [PMC free article] [PubMed] [Google Scholar]
- 60.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am J Physiol. 1996;271:C1424–37. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 61.Chen PF, Tsai AL, Wu KK. Cysteine 184 of endothelial nitric oxide synthase is involved in heme coordination and catalytic activity. J Biol Chem. 1994;269:25062–6. [PubMed] [Google Scholar]
- 62.Landmesser U, Dikalov S, Price SR, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–9. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xia Y, Tsai AL, Berka V, Zweier JL. Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J Biol Chem. 1998;273:25804–8. doi: 10.1074/jbc.273.40.25804. [DOI] [PubMed] [Google Scholar]
- 64.Wu L. Is methylglyoxal a causative factor for hypertension development? Can J Physiol Pharmacol. 2006;84:129–39. doi: 10.1139/Y05-137. [DOI] [PubMed] [Google Scholar]
- 65.Vasdev S, Gill V, Longerich L. Role of methylglyoxal in essential hypertension. In: Gupta SK, Singal PK, Agrawal SS, editors. Pharmacotherapy of Heart Failure. New Delhi: Anamaya Publishers; 2005. pp. 72–88. [Google Scholar]
- 66.Jia X, Wu L. Accumulation of endogenous methylglyoxal impaired insulin signalling in adipose tissue of fructose-fed rats. Mol Cell Biochem. 2007;306:133–9. doi: 10.1007/s11010-007-9563-x. [DOI] [PubMed] [Google Scholar]
- 67.Thornalley PJ. The glyoxalase system in health and disease. Mol Aspects Med. 1993;14:287–371. doi: 10.1016/0098-2997(93)90002-u. [DOI] [PubMed] [Google Scholar]
- 68.Unoki H, Bujo H, Yamagishi S, Takeuchi M, Imaizumi T, Saito Y. Advanced glycation end products attenuate cellular insulin sensitivity by increasing the generation of intracellular reactive oxygen species in adipocytes. Diabetes Res Clin Pract. 2007;76:236–44. doi: 10.1016/j.diabres.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 69.Fukami K, Ueda S, Yamagishi S, et al. AGEs activate mesangial TGF-beta-Smad signalling via an angiotensin II type I receptor interaction. Kidney Int. 2004;66:2137–47. doi: 10.1111/j.1523-1755.2004.66004.x. [DOI] [PubMed] [Google Scholar]
- 70.Ikemoto F, Song G, Tominaga M, Yamamoto K. Oxidation-induced increase in activity of angiotensin converting enzyme in the rat kidney. Biochem Biophys Res Commun. 1988;153:1032–7. doi: 10.1016/s0006-291x(88)81332-9. [DOI] [PubMed] [Google Scholar]
- 71.Tominaga M, Song GB, Ikemoto F, Yamamoto K. Effect of oxidation on the activity of angiotensin converting enzyme in the rat kidney, heart and brain. Clin Exp Hypertens A. 1988;10:1271–8. doi: 10.1080/07300077.1988.11878917. [DOI] [PubMed] [Google Scholar]
- 72.Folli F, Kahn CR, Hansen H, Bouchie JL, Feener EP. Angiotensin II inhibits insulin signalling in aortic smooth muscle cells at multiple levels. A potential role for serine phosphorylation in insulin/angiotensin II crosstalk. J Clin Invest. 1997;100:2158–69. doi: 10.1172/JCI119752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taniyama Y, Hitomi H, Shah A, Alexander RW, Griendling KK. Mechanisms of reactive oxygen species-dependent downregulation of insulin receptor substrate-1 by angiotensin II. Arterioscler Thromb Vasc Biol. 2005;25:1142–7. doi: 10.1161/01.ATV.0000164313.17167.df. [DOI] [PubMed] [Google Scholar]
- 74.Touyz RM, Tabet F, Schiffrin EL. Redox-dependent signalling by angiotensin II and vascular remodelling in hypertension. Clin Exp Pharmacol Physiol. 2003;30:860–6. doi: 10.1046/j.1440-1681.2003.03930.x. [DOI] [PubMed] [Google Scholar]
- 75.Pennington J. Supplementary tables – amino acids. In: Allen A, editor. Bowes & Church’s Food Values of Portions Commonly Used. 16th edn. Philadelphia: JB Lippincott Company; 1994. pp. A325–77. [Google Scholar]
- 76.Brosnan ME, Brosnan JT. Renal arginine metabolism. J Nutr. 2004;134:2791S–5S. doi: 10.1093/jn/134.10.2791S. [DOI] [PubMed] [Google Scholar]
- 77.Tong BC, Barbul A. Cellular and physiological effects of arginine. Mini Rev Med Chem. 2004;4:823–32. doi: 10.2174/1389557043403305. [DOI] [PubMed] [Google Scholar]
- 78.Fajans SS, Floyd JC, Jr, Knopf RF, Conn FW. Effect of amino acids and proteins on insulin secretion in man. Recent Prog Horm Res. 1967;23:617–62. doi: 10.1016/b978-1-4831-9826-2.50017-9. [DOI] [PubMed] [Google Scholar]
- 79.Sener A, Best LC, Yates AP, et al. Stimulus-secretion coupling of arginine-induced insulin release: Comparison between the cationic amino acid and its methyl ester. Endocrine. 2000;13:329–40. doi: 10.1385/ENDO:13:3:329. [DOI] [PubMed] [Google Scholar]
- 80.Brunini T, Moss M, Siqueira M, et al. Inhibition of l-arginine transport in platelets by asymmetric dimethylarginine and N-monomethyl-l-arginine: Effects of arterial hypertension. Clin Exp Pharmacol Physiol. 2004;31:738–40. doi: 10.1111/j.1440-1681.2004.04067.x. [DOI] [PubMed] [Google Scholar]
- 81.Closs EI, Basha FZ, Habermeier A, Förstermann U. Interference of L-arginine analogues with L-arginine transport mediated by the y+ carrier hCAT-2B. Nitric Oxide. 1997;1:65–73. doi: 10.1006/niox.1996.0106. [DOI] [PubMed] [Google Scholar]
- 82.Eid HM, Reims H, Arnesen H, Kjeldsen SE, Lyberg T, Seljeflot I. Decreased levels of asymmetric dimethylarginine during acute hyperinsulinemia. Metabolism. 2007;56:464–9. doi: 10.1016/j.metabol.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 83.Mann GE, Yudilevich DL, Sobrevia L. Regulation of amino acid and glucose transporters in endothelial and smooth muscle cells. Physiol Rev. 2003;83:183–252. doi: 10.1152/physrev.00022.2002. [DOI] [PubMed] [Google Scholar]
- 84.Sobrevia L, Nadal A, Yudilevich DL, Mann GE. Activation of L-arginine transport (system y+) and nitric oxide synthase by elevated glucose and insulin in human endothelial cells. J Physiol. 1996;490:775–81. doi: 10.1113/jphysiol.1996.sp021185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Higashi Y, Oshima T, Ono N, et al. Intravenous administration of L-arginine inhibits angiotensin-converting enzyme in humans. J Clin Endocrinol Metab. 1995;80:2198–202. doi: 10.1210/jcem.80.7.7608279. [DOI] [PubMed] [Google Scholar]
- 86.Lubec B, Hayn M, Kitzmüller E, Vierhapper H, Lubec G. L-arginine reduces lipid peroxidation in patients with diabetes mellitus. Free Radic Biol Med. 1997;22:355–7. doi: 10.1016/s0891-5849(96)00386-3. [DOI] [PubMed] [Google Scholar]
- 87.Wascher TC, Posch K, Wallner S, Hermetter A, Kostner GM, Graier WF. Vascular effects of L-arginine: Anything beyond a substrate for the NO-synthase? Biochem Biophys Res Commun. 1997;234:35–8. doi: 10.1006/bbrc.1997.9994. [DOI] [PubMed] [Google Scholar]
- 88.Piatti PM, Monti LD, Valsecchi G, et al. Long-term oral L-arginine administration improves peripheral and hepatic insulin sensitivity in type 2 diabetic patients. Diabetes Care. 2001;24:875–80. doi: 10.2337/diacare.24.5.875. [DOI] [PubMed] [Google Scholar]
- 89.Stühlinger MC, Abbasi F, Chu JW, et al. Relationship between insulin resistance and an endogenous nitric oxide synthase inhibitor. JAMA. 2002;287:1420–6. doi: 10.1001/jama.287.11.1420. [DOI] [PubMed] [Google Scholar]
- 90.Ahmed N, Thornalley PJ. Advanced glycation endproducts: What is their relevance to diabetic complications? Diabetes Obes Metab. 2007;9:233–45. doi: 10.1111/j.1463-1326.2006.00595.x. [DOI] [PubMed] [Google Scholar]
- 91.Selwood T, Thornalley PJ. Binding of methylglyoxal to albumin and formation of fluorescent adducts. Inhibition by arginine, N-alpha-acetylarginine and aminoguanidine. Biochem Soc Trans. 1993;21:170S. doi: 10.1042/bst021170s. [DOI] [PubMed] [Google Scholar]
- 92.Thornalley PJ, Battah S, Ahmed N, et al. Quantitative screening of advanced glycation endproducts in cellular and extracellular proteins by tandem mass spectrometry. Biochem J. 2003;375:581–92. doi: 10.1042/BJ20030763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weninger M, Xi Z, Lubec B, Szalay S, Höger H, Lubec G. L-arginine reduces glomerular basement membrane collagen N epsilon-carboxymethyl lysine in the diabetic db/db mouse. Nephron. 1992;62:80–3. doi: 10.1159/000187000. [DOI] [PubMed] [Google Scholar]
- 94.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–12. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 95.Yung LM, Leung FP, Yao X, Chen ZY, Huang Y. Reactive oxygen species in vascular wall. Cardiovasc Hematol Disord Drug Targets. 2006;6:1–19. doi: 10.2174/187152906776092659. [DOI] [PubMed] [Google Scholar]
- 96.Hodroj W, Legedz L, Foudi N, et al. Increased insulin-stimulated expression of arterial angiotensinogen and angiotensin type 1 receptor in patients with type 2 diabetes mellitus and atheroma. Arterioscler Thromb Vasc Biol. 2007;27:525–31. doi: 10.1161/01.ATV.0000254814.63768.3b. [DOI] [PubMed] [Google Scholar]
- 97.Kamide K, Rakugi H, Nagai M, et al. Insulin-mediated regulation of the endothelial renin-angiotensin system and vascular cell growth. J Hypertens. 2004;22:121–7. doi: 10.1097/00004872-200401000-00021. [DOI] [PubMed] [Google Scholar]
- 98.Vasdev S, Gill V. The antihypertensive effect of arginine. Int J Angiol. 2008;17:7–21. doi: 10.1055/s-0031-1278274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nagase S, Takemura K, Ueda A, et al. A novel nonenzymatic pathway for the generation of nitric oxide by the reaction of hydrogen peroxide and D- or L-arginine. Biochem Biophys Res Commun. 1997;233:150–3. doi: 10.1006/bbrc.1997.6428. [DOI] [PubMed] [Google Scholar]
- 100.Jia SJ, Jiang DJ, Hu CP, Zhang XH, Deng HW, Li YJ. Lysophosphatidylcholine-induced elevation of asymmetric dimethylarginine level by the NADPH oxidase pathway in endothelial cells. Vascul Pharmacol. 2006;44:143–8. doi: 10.1016/j.vph.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 101.Majid DS, Kopkan L. Nitric oxide and superoxide interactions in the kidney and their implication in the development of salt-sensitive hypertension. Clin Exp Pharmacol Physiol. 2007;34:946–52. doi: 10.1111/j.1440-1681.2007.04642.x. [DOI] [PubMed] [Google Scholar]
- 102.Trolliet MR, Rudd MA, Loscalzo J. Oxidative stress and renal dysfunction in salt-sensitive hypertension. Kidney Blood Press Res. 2001;24:116–23. doi: 10.1159/000054217. [DOI] [PubMed] [Google Scholar]
- 103.Layman DK. Role of leucine in protein metabolism during exercise and recovery. Can J Appl Physiol. 2002;27:646–63. doi: 10.1139/h02-038. [DOI] [PubMed] [Google Scholar]
- 104.Layman DK. The role of leucine in weight loss diets and glucose homeostasis. J Nutr. 2003;133:261S–7S. doi: 10.1093/jn/133.1.261S. [DOI] [PubMed] [Google Scholar]
- 105.Fried SK, Watford M. Leucing weight with a futile cycle. Cell Metab. 2007;6:155–6. doi: 10.1016/j.cmet.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 106.Layman DK, Walker DA. Potential importance of leucine in treatment of obesity and the metabolic syndrome. J Nutr. 2006;136:319S–23S. doi: 10.1093/jn/136.1.319S. [DOI] [PubMed] [Google Scholar]
- 107.Wilson JM. The effects of leucine and fat metabolism. < http://www.abcbodybuilding.com/leucine5.pdf> (Accessed on December 10, 2009).
- 108.Wilson G. An investigation of the satiety mechanism: A research initiative. < http://www.abcbodybuilding.com/investigation.pdf> (Accessed on December 10, 2009).
- 109.Layman DK, Boileau R, Painter J, Erickson D, Shiue H, Sather C. Carbohydrates versus protein in diets for mid-life women. FASEB J. 2000;14:A564. [Google Scholar]
- 110.Layman DK, Boileau RA, Erickson DJ, et al. A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. J Nutr. 2003;133:411–7. doi: 10.1093/jn/133.2.411. [DOI] [PubMed] [Google Scholar]
- 111.Layman DK, Shiue H, Sather C, Erickson DJ, Baum J. Increased dietary protein modifies glucose and insulin homeostasis in adult women during weight loss. J Nutr. 2003;133:405–10. doi: 10.1093/jn/133.2.405. [DOI] [PubMed] [Google Scholar]
- 112.Cota D, Proulx K, Smith KA, et al. Hypothalamic mTOR signalling regulates food intake. Science. 2006;312:927–30. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 113.Lynch CJ, Gern B, Lloyd C, Hutson SM, Eicher R, Vary TC. Leucine in food mediates some of the postprandial rise in plasma leptin concentrations. Am J Physiol Endocrinol Metab. 2006;291:E621–30. doi: 10.1152/ajpendo.00462.2005. [DOI] [PubMed] [Google Scholar]
- 114.O’Flarherty L, Stapleton PP, Redmond HP, Bouchier-Hayes DJ. Intestinal taurine transport: A review. Eur J Clin Invest. 1997;27:873–80. doi: 10.1046/j.1365-2362.1997.2000747.x. [DOI] [PubMed] [Google Scholar]
- 115.Lourenço R, Camilo ME. Taurine: A conditionally essential amino acid in humans? An overview in health and disease. Nutr Hosp. 2002;17:262–70. [PubMed] [Google Scholar]
- 116.Inoue A, Takahashi H, Lee LC, et al. Retardation of the development of hypertension in DOCA salt rats by taurine supplement. Cardiovasc Res. 1988;22:351–8. doi: 10.1093/cvr/22.5.351. [DOI] [PubMed] [Google Scholar]
- 117.Fujita T, Sato Y. Changes in blood pressure and extracellular fluid with taurine in DOCA-salt rats. Am J Physiol. 1986;250:R1014–20. doi: 10.1152/ajpregu.1986.250.6.R1014. [DOI] [PubMed] [Google Scholar]
- 118.Trachtman H, Del Pizzo R, Rao P, Rujikarn N, Sturman JA. Taurine lowers blood pressure in the spontaneously hypertensive rat by a catecholamine independent mechanism. Am J Hypertens. 1989;2:909–12. doi: 10.1093/ajh/2.12.909. [DOI] [PubMed] [Google Scholar]
- 119.Militante JD, Lombardini JB. Treatment of hypertension with oral taurine: Experimental and clinical studies. Amino Acids. 2002;23:381–93. doi: 10.1007/s00726-002-0212-0. [DOI] [PubMed] [Google Scholar]
- 120.Anuradha CV, Balakrishnan SD. Taurine attenuates hypertension and improves insulin sensitivity in the fructose-fed rat, an animal model of insulin resistance. Can J Physiol Pharmacol. 1999;77:749–54. [PubMed] [Google Scholar]
- 121.Nandhini AT, Thirunavukkarasu V, Anuradha CV. Stimulation of glucose utilization and inhibition of protein glycation and AGE products by taurine. Acta Physiol Scand. 2004;181:297–303. doi: 10.1111/j.1365-201X.2004.01287.x. [DOI] [PubMed] [Google Scholar]
- 122.Health Canada Canadian nutrient file (tryptophan) < http://webprod.hc-sc.gc.ca/cnf-fce/nutrientSearch-rechercheAliment.do?lang=eng> (Accessed on January 26, 2010).
- 123.Rivas M, Garay RP, Escanero JF, Cia P, Jr, Cia P, Alda JO. Soy milk lowers blood pressure in men and women with mild to moderate essential hypertension. J Nutr. 2002;132:1900–2. doi: 10.1093/jn/132.7.1900. [DOI] [PubMed] [Google Scholar]
- 124.Teede HJ, Giannopoulos D, Dalais FS, Hodgson J, McGrath BP. Randomised, controlled, cross-over trial of soy protein with isoflavones on blood pressure and arterial function in hypertensive subjects. J Am Coll Nutr. 2006;25:533–40. doi: 10.1080/07315724.2006.10719569. [DOI] [PubMed] [Google Scholar]
- 125.Yang G, Shu XO, Jin F, et al. Longitudinal study of soy food intake and blood pressure among middle-aged and elderly Chinese women. Am J Clin Nutr. 2005;81:1012–7. doi: 10.1093/ajcn/81.5.1012. [DOI] [PubMed] [Google Scholar]
- 126.Sagara M, Kanda T, NJelekera M, et al. Effects of dietary intake of soy protein and isoflavones on cardiovascular disease risk factors in high risk, middle-aged men in Scotland. J Am Coll Nutr. 2004;23:85–91. doi: 10.1080/07315724.2004.10719347. [DOI] [PubMed] [Google Scholar]
- 127.Hecker KD. Effects of dietary animal and soy protein on cardiovascular disease risk factors. Curr Atheroscler Rep. 2001;3:471–8. doi: 10.1007/s11883-001-0037-4. [DOI] [PubMed] [Google Scholar]
- 128.Burke V, Hodgson JM, Beilin LJ, Giangiulioi N, Rogers P, Puddey IB. Dietary protein and soluble fiber reduce ambulatory blood pressure in treated hypertensives. Hypertension. 2001;38:821–6. doi: 10.1161/hy1001.092614. [DOI] [PubMed] [Google Scholar]
- 129.Sacks FM, Wood PG, Kass EH. Stability of blood pressure in vegetarians receiving dietary protein supplements. Hypertension. 1984;6:199–201. [PubMed] [Google Scholar]
- 130.Margetts BM, Beilin LJ, Vandongen R, Armstrong BK. Vegetarian diet in mild hypertension: A randomised controlled trial. Br Med J (Clin Res Ed) 1986;293:1468–71. doi: 10.1136/bmj.293.6560.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Prescott SL, Jenner DA, Beilin LJ, Margetts BM, Vandongen R. Controlled study of the effects of dietary protein on blood pressure in normotensive humans. Clin Exp Pharmacol Physiol. 1987;14:159–62. doi: 10.1111/j.1440-1681.1987.tb00368.x. [DOI] [PubMed] [Google Scholar]
- 132.Kestin M, Rouse IL, Correll RA, Nestel PJ. Cardiovascular disease risk factors in free-living men: Comparison of two prudent diets, one based on lactoovovegetarianism and the other allowing lean meat. Am J Clin Nutr. 1989;50:280–7. doi: 10.1093/ajcn/50.2.280. [DOI] [PubMed] [Google Scholar]
- 133.Havlik RJ, Fabsitz RR, Kalousdian S, Borhani NO, Christian JC. Dietary protein and blood pressure in monozygotic twins. Prev Med. 1990;19:31–9. doi: 10.1016/0091-7435(90)90004-4. [DOI] [PubMed] [Google Scholar]
- 134.He J, Klag MJ, Whelton PK, Chen JY, Qian MC, He GQ. Dietary macronutrients and blood pressure in southwestern China. J Hypertens. 1995;13:1267–74. doi: 10.1097/00004872-199511000-00008. [DOI] [PubMed] [Google Scholar]
- 135.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH collaborative research group. N Engl J Med. 1997;336:1117–24. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 136.Hajjar IM, Grim CE, George V, Kotchen TA. Impact of diet on blood pressure and age-related changes in blood pressure in the US population: Analysis of NHANES III. Arch Intern Med. 2001;161:589–93. doi: 10.1001/archinte.161.4.589. [DOI] [PubMed] [Google Scholar]
- 137.Jenkins DJ, Kendall CW, Jackson CJ, et al. Effects of high- and low-isoflavone soyfoods on blood lipids, oxidized LDL, homocysteine, and blood pressure in hyperlipidemic men and women. Am J Clin Nutr. 2002;76:365–72. doi: 10.1093/ajcn/76.2.365. [DOI] [PubMed] [Google Scholar]
- 138.Stamler J, Liu K, Ruth KJ, Pryer J, Greenland P. Eight-year blood pressure change in middle-aged men: Relationship to multiple nutrients. Hypertension. 2002;39:1000–6. doi: 10.1161/01.hyp.0000016178.80811.d9. [DOI] [PubMed] [Google Scholar]
- 139.Hodgson JM, Burke V, Beilin LJ, Puddey IB. Partial substitution of carbohydrate intake with protein intake from lean red meat lowers blood pressure in hypertensive persons. Am J Clin Nutr. 2006;83:780–7. doi: 10.1093/ajcn/83.4.780. [DOI] [PubMed] [Google Scholar]
- 140.Muzio F, Mondazzi L, Harris WS, Sommariva D, Branchi A. Effects of moderate variations in the macronutrient content of the diet on cardiovascular disease risk factors in obese patients with the metabolic syndrome. Am J Clin Nutr. 2007;86:946–51. doi: 10.1093/ajcn/86.4.946. [DOI] [PubMed] [Google Scholar]
- 141.Mattson DL, Meister CJ, Marcelle ML. Dietary protein source determines the degree of hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension. 2005;45:736–41. doi: 10.1161/01.HYP.0000153318.74544.cc. [DOI] [PubMed] [Google Scholar]
- 142.Chen PY, St John PL, Kirk KA, Abrahamson DR, Sanders PW. Hypertensive nephrosclerosis in the Dahl/Rapp rat. Initial sites of injury and effect of dietary L-arginine supplementation. Lab Invest. 1993;68:174–84. [PubMed] [Google Scholar]
- 143.He H, Kimura S, Fujisawa Y, et al. Dietary L-arginine supplementation normalizes regional blood flow in Dahl-Iwai salt-sensitive rats. Am J Hypertens. 1997;10:89S–93S. [PubMed] [Google Scholar]
- 144.Ono H, Ono Y, Frohlich ED. L-arginine reverses severe nephrosclerosis in aged spontaneously hypertensive rats. J Hypertens. 1999;17:121–8. doi: 10.1097/00004872-199917010-00018. [DOI] [PubMed] [Google Scholar]
- 145.Artigues C, Richard V, Roussel C, Lallemand F, Henry JP, Thuillez C. Increased endothelium-monocyte interactions in salt-sensitive hypertension: Effect of L-arginine. J Cardiovasc Pharmacol. 2000;35:468–73. doi: 10.1097/00005344-200003000-00018. [DOI] [PubMed] [Google Scholar]
- 146.Özçelikay AT, Tay A, Güner S, et al. Reversal effects of L-arginine treatment on blood pressure and vascular responsiveness of streptozotocin-diabetic rats. Pharmacol Res. 2000;41:201–9. doi: 10.1006/phrs.1999.0576. [DOI] [PubMed] [Google Scholar]
- 147.Vasdev S, Ford CA, Longerich L, Parai S, Gadag V, Wadhawan S. Aldehyde induced hypertension in rats: Prevention by N-acetyl cysteine. Artery. 1998;23:10–36. [PubMed] [Google Scholar]
- 148.Vasdev S, Gill VD, Randell E, Han Y, Gadag V. Fructose and moderately high dietary salt-induced hypertension: Prevention by a combination of N-acetylcysteine and L-arginine. Mol Cell Biochem. 2010;337:9–16. doi: 10.1007/s11010-009-0281-4. [DOI] [PubMed] [Google Scholar]
- 149.Ideishi M, Miura S, Sakai T, Sasaguri M, Misumi Y, Arakawa K. Taurine amplifies renal kallikrein and prevents salt-induced hypertension in Dahl rats. J Hypertens. 1994;12:653–61. [PubMed] [Google Scholar]
- 150.Harada H, Tsujino T, Watari Y, Nonaka H, Emoto N, Yokoyama M. Oral taurine supplementation prevents fructose-induced hypertension in rats. Heart Vessels. 2004;19:132–6. doi: 10.1007/s00380-003-0757-1. [DOI] [PubMed] [Google Scholar]
- 151.Suárez C, del Arco C, Lahera V, Ruilope LM. N-acetylcysteine potentiates the antihypertensive effect of angiotensin converting enzyme inhibitors. Am J Hypertens. 1995;8:859. doi: 10.1016/0895-7061(95)00153-G. [DOI] [PubMed] [Google Scholar]
- 152.Pezza V, Bernardini F, Pezza E, Pezza B, Curione M. Study of supplemental oral l-arginine in hypertensives treated with enalapril + hydrochlorothiazide. Am J Hypertens. 1998;11:1267–70. doi: 10.1016/s0895-7061(98)00153-8. [DOI] [PubMed] [Google Scholar]
- 153.Barrios V, Calderón A, Navarro-Cid J, Lahera V, Ruilope LM. N-acetylcysteine potentiates the antihypertensive effect of ACE inhibitors in hypertensive patients. Blood Press. 2002;11:235–9. doi: 10.1080/08037050213760. [DOI] [PubMed] [Google Scholar]