Abstract
PURPOSE:
Free radicals have been implicated in myonephropathic metabolic syndrome (MNMS), which not only damages muscles but also the kidneys and lungs. It was recently shown that the free radical scavenger edaravone suppressed reperfusion injury in rat extremities. The present study evaluated whether edaravone also protects against MNMS-induced tissue damage in the lungs and kidneys after reperfusion injury of rat extremities.
METHODS:
Ten male Lewis rats (mean [± SD] weight 508±33 g) were divided into two groups. The MNMS models were created by clamping the bilateral common femoral arteries for 5 h, followed by declamping. In another group, 3.0 mg/kg of edaravone was injected into the peritoneal cavity before clamping the bilateral common femoral arteries. Five hours after starting reperfusion, the kidneys and lungs were harvested from each rat for histological study (n=10). Kidney damage was expressed as the number of infiltrating cells in the glomeruli. Lung damage was expressed as the percentage area of the alveolar wall thickness with cellular infiltration, using computerized densitometry.
RESULTS:
Kidneys in the edaravone group showed less cellular infiltration than in the control group (62.2±2.4 cells versus 75.8±3.6 cells per glomerulus, respectively; P=0.002). Lungs in the edaravone group also showed a significantly lower percentage of damaged lung tissue area than in the control group (20.5±1.5% versus 63.6±2.8%, respectively; P<0.001).
CONCLUSION:
The results suggest that the free radical scavenger edaravone might protect against kidney and lung damage induced by MNMS after reperfusion injury of rat extremities.
Keywords: Edaravone, Free radical, Myonephropathic metabolic syndrome (MNMS)
Free radicals cause lower extremity reperfusion injury and myonephropathic metabolic syndrome (MNMS), which damages not only muscles but also the kidneys and lungs (1). Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one,C10H10N2O, molecular weight = 174.2; Radicut, Mitsubishi Tanabe Pharma Co, Japan) was the first free radical scavenger introduced for the treatment of acute cerebral infarction (2). We previously suggested that edaravone also suppresses reperfusion injury in rat extremities (3,4).
In the present study, we focused on other organs – the kidneys and lungs. We evaluated whether edaravone can protect against MNMS-induced tissue damage in the kidneys and lungs after reperfusion injury of rat extremities.
METHODS
MNMS model
All animal procedures complied with the Hyogo College of Medicine Animal Research Committee criteria (No B-09-010). Ten male Lewis rats (mean [± SD] weight 508±33 g) were divided into two groups, and treated with either edaravone (3.0 mg/kg; edaravone group) or the same volume of saline (control group).
MNMS was induced by clamping the bilateral common femoral arteries for 5 h, followed by removal of the clamp to allow reperfusion, as previously described (3,4). Complete general anesthesia was induced before MNMS by intraperitoneal administration of 30 mg/kg of sodium pentobarbital (Nembutal; Abbott Laboratories, USA). At the same time, 3.0 mg/kg of edaravone or the same volume of saline was injected into the peritoneal cavity preoperatively. Under the microscope (OME-J&N J73507R, Olympus, Japan) at original magnification ×30, both common femoral arteries were clamped for 5 h after ligating both the deep femoral arteries and epigastric arteries. The rat lower extremity reperfusion injuries were subsequently induced by removing the clamps from the bilateral common femoral arteries after 5 h.
Histological study
The kidneys and lungs were harvested bilaterally for histological study. Tissue sections (5 μm thick) of the kidney and lung were taken and immediately stained with hematoxylin and eosin.
Kidney damage was expressed as the number of infiltrating cells in the glomeruli. Each number of cells was counted in 10 glomeruli at original magnification ×400, in three different fields. The mean number of viable cells in each section was calculated and expressed per area (cells per glomerulus).
Lung damage was expressed as the percentage area of the alveolar wall thickness with cell infiltration using computerized densitometry (Image J, version 1.37, National Institutes of Health, USA; http://rsb.info.nih.gov/ij). On Image J densitometry, positive areas were defined as a number of dots between 125 and 250 over.
Statistical analysis
All results are expressed as mean ± SEM. The results were statistically analyzed using unpaired t tests (Stat View J-4.5 for Macintosh, Abacus Concept, USA). P<0.05 was considered to be statistically significant.
RESULTS
There were no operative deaths in either group.
Kidney damage
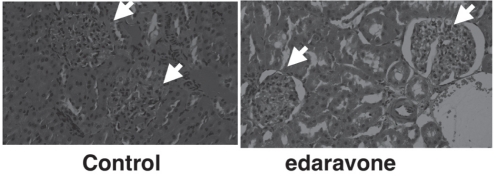
Representative kidney sections after reperfusion injury of rat lower extremities are shown in Figure 1. In the control group, there was extensive cellular infiltration of the glomeruli. However, in the edaravone group, there was less cellular infiltration in the glomeruli.
Figure 1).
Representative kidney sections of the control group and edaravone group (hematoxylin and eosin stain, original magnification ×200). Arrows indicate glomeruli
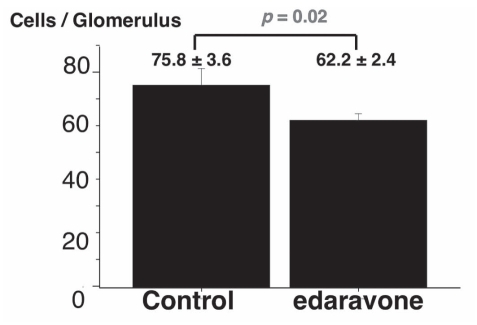
A statistical comparison of kidney cellular infiltration is shown in Figure 2. In the edaravone group, cellular infiltration was significantly lower than that in the control group (62.2±2.4 cells per glomerulus versus 75.8±3.6 cells per glomerulus, respectively; P=0.02).
Figure 2).
Comparison of the number of infiltrating cells per glomerulus in the control group and edaravone group. Results are expressed as mean ± SEM
Lung damage
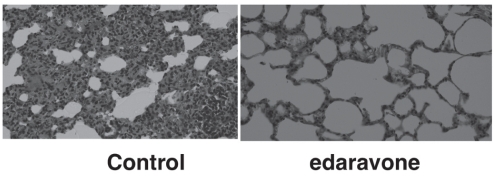
The lungs from the saline-treated control group had extensive swelling. In contrast, the lungs from the edaravone group were filled with air, similar to normal lungs.
Representative lung sections after reperfusion injury are shown in Figure 3. In the control group, there was extensive cellular infiltration in the alveolar wall and a small amount in the lung cavity. The alveolar wall was very thick in the control group. In contrast, in the edaravone group there was less cellular infiltration and the lung cavity appeared nearly normal. Furthermore, the alveolar walls were very thin.
Figure 3).
Representative lung sections of the control group and edaravone group (hematoxylin and eosin stain, original magnification ×200)
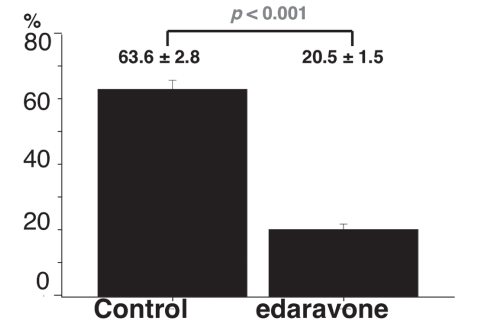
The percentage of the alveolar wall thickness (lung damage) is shown in Figure 4. In the edaravone group, the mean percentage of alveolar wall thickness was significantly lower than that in the control group (20.5±1.5% versus 63.6±2.8%, respectively; P<0.001).
Figure 4).
Comparison of percentage area of the alveolar wall thickness (damaged area of lung tissue) in the control group and edaravone group. Results are expressed as mean ± SEM
DISCUSSION
Free radicals are responsible for MNMS induced by lower extremity reperfusion injury (1). In our previous studies (3,4), we showed that edaravone suppressed reperfusion injury in a rat model. In another in vivo rat study (5), we showed that edaravone also suppressed endothelial cell injury, resulting in postoperative intimal hyperplasia of the vein graft.
In the present study, we evaluated the effect of edaravone on tissue damage in the kidneys and lungs. We found that both kidneys and lungs in the edaravone group showed significantly less cellular infiltration after reperfusion injury. This may offer a clinical advantage because edaravone is effective not only in muscles, but it also protects against tissue damage in kidneys and lungs caused by lower extremity ischemia. Therefore, results of the present study suggest that edaravone has a protective effect against tissue damage resulting from MNMS after reperfusion injury in rat extremities.
The in vitro mechanism of edaravone as a peroxyl radical scavenger was first reported in 1996 (6). The mechanism of edaravone after acute cerebral infarction is believed to involve the scavenging effect of peroxyl free radicals (7). Moreover, edaravone reduced mitochondrial damage in homogenate made from ischemic incubated rat brain (8). Mitochondria are a rich source of free radicals; however, they also contain a free radical scavenger (superoxide dismutase). Our recent immunohistological study (4) showed that edaravone can maintain high levels of mitochondrial activity after reperfusion injury in rat lower extremities. Recently, using an electron microscope, we observed well-characterized mitochondrial swelling in the lower extremities of rats after reperfusion injury (unpublished data). Therefore, we believe that the mechanism of edaravone is protection from mitochondrial reperfusion damage to the lower extremities, which may lead to MNMS in the lungs and kidneys.
As the next step in the investigation of the clinical application of edaravone, we are identifiying the types of cells infiltrating the kidneys and lungs. We suspect that macrophages and monocytes might be infiltrating the kidneys and lungs because these cells have chemoattractant activity. However, ED-1 staining, which is specific for rat macrophages and monocytes, demonstrated only a few cells (unpublished data). Thus, more studies are needed to determine the precise effects of edavarone before it is used clinically.
CONCLUSION
We provide evidence that the free radical scavenger edaravone might protect against tissue damage induced by MNMS in the lungs and kidneys after reperfusion injury in rat lower extremities.
Acknowledgments
The authors thank Ryoji Hariyama MT (Department of Surgical Pathology, Hyogo College of Medicine, Hyogo, Japan), and Hirotsugu Kubo (Common Research Laboratory, Hyogo College of Medicine) for their technical support. The authors also thank Mitsubishi Tanabe Pharma Co, Japan, for kindly donating edaravone.
Footnotes
FUNDING: This study is supported by a Grant-in-Aid for Researchers, Hyogo College of Medicine 2008–2009.
REFERENCES
- 1.Haimovici H. Metabolic complications of acute arterial occlusions and related conditions: Role of free radicals (myonephropathic-metabolic syndrome) In: Haimovici H, editor. Haimovici’s Vascular Surgery. 3rd edn. Norwalk: Appleton & Lange; 1989. pp. 386–408. [Google Scholar]
- 2.Watanabe T, Morita I, Nishi H, Murota S. Preventive effect of MCI-186 on 15-HPETE induced vascular endothelial cell injury in vitro. Prostaglandins Leukot Essent Fatty Acids. 1988;33:81–7. doi: 10.1016/0952-3278(88)90127-5. [DOI] [PubMed] [Google Scholar]
- 3.Yamamura M, Miyamoto Y, Mitsuno M, et al. Suppression of rat lower extremity postoperative reperfusion injury with edaravone. Int J Angiol. 2006;15:34–6. doi: 10.1055/s-0031-1278238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamamura M, Miyamoto Y, Mitsuno M, et al. Edaravone suppresses postoperative reperfusion injury in rat lower extremity: An immunohistological study. Int J Angiol. 2007;16:17–9. doi: 10.1055/s-0031-1278238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamura M, Miyamoto Y, Mitsuno M, et al. Suppression of postoperative intimal hyperplasia of vein graft with edaravone in a rat model. Int J Angiol. 2005;14:151–4. doi: 10.1055/s-0031-1278269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamamoto Y, Kuwahara T, Watanabe K, Watanabe K. Antioxidant activity of 3-methyl-1-phenyl-2-pyrazolon-5-one. Redox Report. 1996;2:333–8. doi: 10.1080/13510002.1996.11747069. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe K, Watanabe K, Hayase T. Radical scavenging mechanism of MCI-186. Jpn Pharmacol Ther. 1997;25(Suppl):S1699–S1707. [Google Scholar]
- 8.Watanabe T, Yuki S, Egawa M, Nishi H. Protective effects of MCI-186 on cerebral ischemia: Possible involvement of free radical scavenging and antioxidant actions. J Pharmacol Exp Ther. 1994;268:1597–604. [PubMed] [Google Scholar]