Abstract
BACKGROUND:
Deep vein thrombosis (DVT) is the formation of a blood clot within the deep veins. During periods of sitting, blood flow is decreased and this contributes to an increased risk of DVT. Trials have shown that 5% to 10% of passengers undertaking long-haul flights develop asymptomatic calf DVT.
AIM:
To investigate the safety and efficacy of a novel neuromuscular device that augments peripheral blood flow.
METHODS:
Thirty healthy volunteers were assessed while seated. Each subject had one leg connected to the stimulator and the other leg immobile acting as control. Fifteen sequential electrical stimulations were applied for 5 min each followed by a 10 min recovery phase. The following noninvasive measurements were performed before, during and after the stimulation programs: photoplethysmography, strain gauge plethysmography, laser Doppler fluxmetry, transcutaneous oxygen tension, pulse oximetry, superficial femoral vein blood flow and vessel diameter (ultrasound); discomfort questionnaires were also administered.
RESULTS:
During neuromuscular stimulation, significant increases in blood volume flow and velocity and skin capillary blood flow were found; transdermal skin oxygen levels were maintained. No changes were observed in heart rate, blood pressure, oxygen saturation or femoral vein vessel diameter.
CONCLUSIONS:
Using a newly developed device, electrical nerve stimulation of the lower leg significantly increased blood flow; the device in the present study is, therefore, a promising tool for the development of a novel DVT prevention device. Because this method of electrical nerve stimulation is virtually pain free, the present study has significant implications for the prevention of DVT in hospitals, outpatient settings and community care settings, as well as in preventing travel-related thrombosis.
Keywords: Blood volume and flow, Electrical stimulation, Stenosis, Thrombosis, Veins
Deep vein thrombosis (DVT) is the formation of a blood clot within the deep veins. Clot formation has been attributed to three main factors – the so-called Virchow’s triad. These factors are endothelial injury, blood stasis and hypercoagulability (1). It has been shown that the majority of thrombi that occur after surgery originate in the soleal veins and venous valve pockets (1–3). The annual incidence of DVT after surgery in the United States and Europe is estimated to be approximately 160 per 100,000 patients; the incidence of fatal pulmonary embolism is estimated to be 50 per 100,000 patients (4).
The common factors that are associated with a greater than average risk of DVT are age older than 40 years, cancer, trauma, previous DVT or pulmonary embolism, recent surgery, obesity and estrogen therapy in women. In addition, surgery of the lower limb, in particular to the hip or knee, carries a specific risk of DVT ranging between 40% and 84% (5,6). Pharmacological methods for the prevention of DVT reduce blood coagulability (7), but are intrinsically associated with significant risk of bleeding, necessitating clinical supervision (8), and are, therefore, contraindicated for some categories of patients. Mechanical methods include graduated compression stockings (9–11), in which a pressure of 18 mmHg at the ankle produces venous compression and increased venous blood flow velocity (12). However, compression stockings can be uncomfortable to wear and individual patient compliance is highly variable. Intermittent pneumatic compression (IPC) devices (13–15) consist of an inflatable garment for the arm, leg or foot. The garment is intermittently inflated and deflated. If IPC is applied to the foot, it increases leg blood flow by activating the foot pump (foot impulse technology) (16). Most compression devices consist of plastic sleeves, which enclose the whole leg and, therefore, have been proven to be uncomfortable to wear and can cause sweating beneath the plastic sleeve. Furthermore, the size, weight and external power source requirements contribute to poor compliance, which limits the efficacy of IPC devices (17). The combination of techniques such as compression stockings and heparin, or compression stockings and IPC devices, has been shown to have an incremental protective effect (18–20). In relation to the prevention of travel-related DVT, the methods currently available include clinical compression stockings and in-flight foot exercisers, such as the Airogym Exerciser (Airogym Ltd, UK). However, stockings often prove to be uncomfortable to wear, applied pressures are highly variable and travellers generally fail to maintain use of the foot exercise devices throughout the period of travel.
Direct electrical stimulation of the lower limb muscles has been shown to be effective in improving blood flow (21–24). However, these studies of direct electrical muscle stimulation have not led to the development of effective and easy-to-use devices for blood flow enhancement and, therefore, the potential prevention of DVT. The failure to widely adopt direct muscle stimulators may be explained by the elevated discomfort experienced at high intensities of direct electrical stimulation to the muscle. The limited number of available devices that use this technique are mostly only used under general anesthesia.
The main objective of the present study was to evaluate the efficacy of a novel transdermal neuromuscular device applied to the common peroneal nerve on blood flow in the lower limb.
METHODS
Volunteers
Thirty healthy volunteers were recruited by advertisement to staff and students of Queen Mary University of London (London, UK), Barts and The London NHS (London) and to the general community, as approved by the Central Office for Research Ethics Committees (reference 05/Q0604/11). Volunteers were provided with information sheets, and written informed consent was obtained. Volunteers were initially invited to participate in a screening evaluation, which included a medical history, physical examination and colour flow duplex ultrasound of the lower legs to exclude bilateral DVT. The specific inclusion and exclusion criteria are presented in Tables 1 and 2. The rationale for these criteria was to reduce confounding variables and provide a basis for matching future study groups.
TABLE 1.
Inclusion criteria
| Health | Good general health/fitness |
| Age | Between 18 and 65 years |
| Medical history | No abnormal findings; absence of deep vein thrombosis and hematological disorders or indications |
| Body mass index | Between 18 kg/m2 and 34 kg/m2 |
| Drugs | No history of drug abuse (including alcohol) |
| Medication | No medication during the 30 days preceding the study and no medication during the course of the study |
TABLE 2.
Exclusion criteria
| Health | Organ dysfunction, any clinically significant deviation from normal in the physical determinations |
| Age | <18 or >65 years |
| Medical history | Hematological disorders, previous DVT/PE, peripheral arterial disease (ankle-brachial pressure index <0.9), varicose veins or lower limb ulceration, musculoskeletal disorders, recent surgery and recent trauma to lower limb; and history of gastrointestinal, hepatic, renal, cardiovascular, endocrine, neurological, dermatological, rheumatological, metabolic (including diabetes), psychiatric, hematological (especially in relation to clotting or coagulation) or systemic disease judged to be significant |
| Body mass index | Chronic obesity (body mass index >34 kg/m2) |
| Pregnancy | Positive pregnancy test |
| Medication | Any medication in the previous 30 days |
| Tobacco consumption | Smoker |
| Pulse rate | <50 beats/min |
| Blood pressure | Systolic blood pressure >160 mmHg or <80 mmHg, or diastolic pressure of >90 mmHg or <60 mmHg |
| Blood donation | Donation of blood within 8 weeks of the screening period or during the investigation |
| Participation in other clinical study | Participation in any clinical study during the 8 weeks preceding the active period of the study |
DVT Deep vein thrombosis; PE Pulmonary embolism
Stimulator and electrodes
A modified electrical stimulator offering a range of preset stimulation currents and pulse frequencies was used, employing custom stimulation protocols and electrodes. These devices are commonly used under the supervision of a physiotherapist as well as by members of the public for therapeutic purposes such as exercising or toning muscles. The waveform was specifically designed for motor nerve stimulation as opposed to direct muscle stimulation. Pulse amplitudes ranged from 1 mA to 40 mA, with frequencies ranging from 1 Hz to 5 Hz (Table 3), which were significantly lower than those used in physiotherapy and transcutaneous electrical nerve stimulation protocols (25–27). In all cases, the pulse consisted of a charge-balanced pulse of 200 μs in width. Two electrodes were connected to the stimulator box and placed over the common peroneal nerve, as shown in Figure 1. This nerve is related to the reaction of the venous muscle pumps of the lower leg (foot and calf pump).
TABLE 3.
The stimulation program sequence
| Program number | Amplitude, mA | Frequency, Hz |
|---|---|---|
| 1 | 1 | 1 |
| 2 | 1 | 3 |
| 3 | 1 | 5 |
| 4 | 5 | 1 |
| 5 | 5 | 3 |
| 6 | 5 | 5 |
| 7 | 10 | 1 |
| 8 | 10 | 3 |
| 9 | 10 | 5 |
| 10 | 20 | 1 |
| 11 | 20 | 3 |
| 12 | 20 | 5 |
| 13 | 40 | 1 |
| 14 | 40 | 3 |
| 15 | 40 | 5 |
Program current settings were defined as follows: on the bench (not in contact with a human body), peak voltage was measured (using an oscilloscope) between terminals across a fixed 2000 ohm resistor. The equivalent current was then calculated by Ohm’s law (voltage = current × resistance). In vivo, variations in skin resistance, tissue resistance and quality of contact will give varying values of both current and voltage (because the device has a substantial internal resistance); therefore, the values given serve only to identify the setting and do not necessarily represent the actual value of current delivered to the subject
Figure 1).
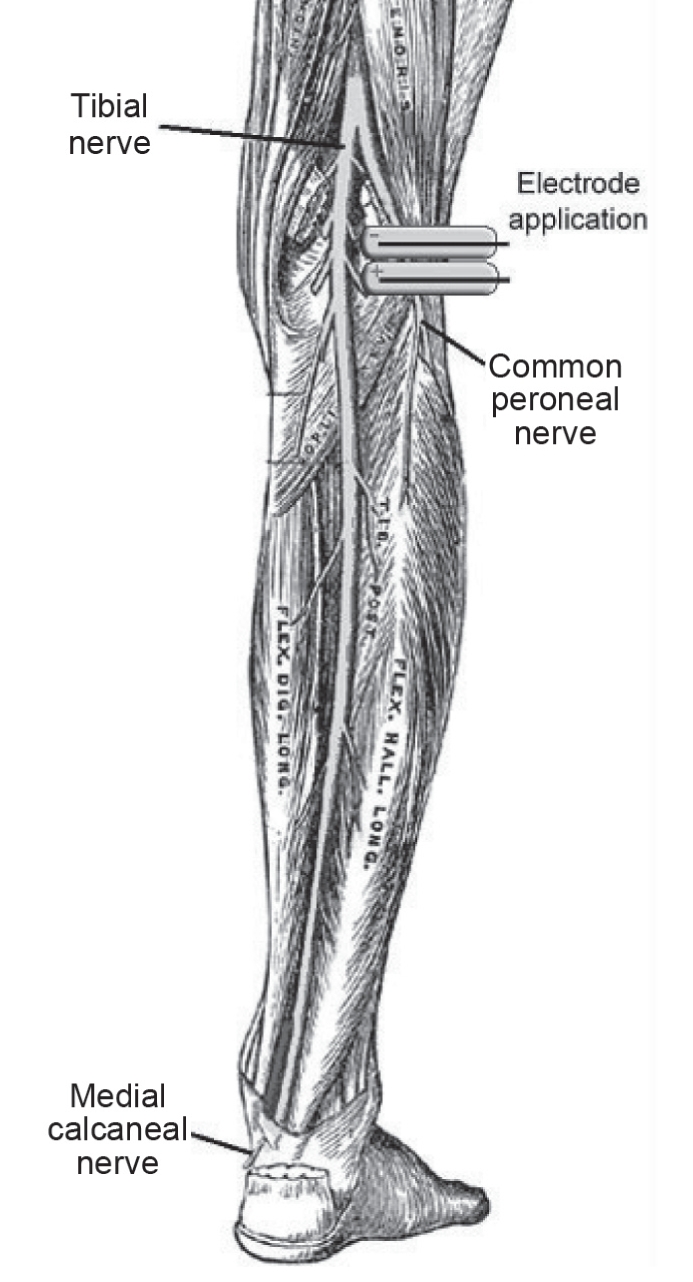
Electrode application on the common peroneal nerve. Illustration of the nerves of the right lower extremity in posterior view adjusted from reference 37
Study methodology
The present study was performed in a temperature- and humidity-controlled environment (temperature 24±1°C, relative humidity 30% to 40%) using an air-conditioning unit (Airedale International Air Conditioning Ltd, UK); environmental data were recorded with a hygrometer (TH105; ABB Kent-Taylor Inc, USA). The effects of electrical stimulation on lower limb blood flow were investigated in 30 healthy volunteers during a 4 h period of sitting in an economy airline seat. The leg clearance distance was set at 86 cm by positioning a toe bar. Each subject was positioned in the seat by a safety belt to maintain close uniformity of posture and was actively encouraged to remain as passive as could be tolerated. A succession of 15 different stimulation programs was applied to each subject according to a two-dimensional matrix of amplitude and frequency, as shown in Table 3. A randomly selected leg was stimulated while the contralateral leg remained immobile as a control for the duration of the study. Each stimulation program was 5 min long, followed by 5 min of response recording (stimulator off) and a 5 min recovery phase to allow vascular re-equilibration before the next sequence. Blood pressure was measured before stimulation and at every hour until the end of the study period (4 h) using a digital blood pressure monitor (UA-767PC; A&D Instruments Ltd, UK). During the 4 h, changes in blood flow and volume, microcirculatory flux and physiological measures related to the heart were recorded bilaterally using standard noninvasive techniques, including photoplethysmography (PPG) (MedSonics Ltd, USA), strain gauge plethysmography (SPG) (Hokanson EC4; DE Hokanson Inc, USA; and MedaSonic Ltd, USA), laser Doppler fluxmetry (Laser Doppler Perfusion & Temperature Monitor DRT4; Moor Instruments Ltd, UK), transcutaneous oxygen tension (TCM4 Tina; Radiometer Ltd, UK), colour flow duplex ultrasound (Philips IU22; Philips Healthcare, USA) and pulse oximetry (Datex-Ohmeda Ltd, UK). Changes in these parameters were compared with baseline values (at rest) and those determined by voluntary muscle action; ie, subjects were asked to perform 10 dorsiflexions with the heel on the ground. This represented the theoretical maximum physiological response that can be obtained in the sitting position. Furthermore, subjects were asked to evaluate acceptance and tolerability of the electrical stimulation sequences by the use of a questionnaire, which included a verbal rating scale (VRS) and a visual analogue scale. At the end of the assessments, the subject’s deep veins were re-examined with duplex ultrasound to exclude the development of thrombi. During the second visit (within two weeks), the stimulation sequence was reversed (starting at stimulation program 15 and proceeding to stimulation program 1) for each subject.
Data acquisition and analysis
A CED 1401 computer interface system (Cambridge Electronic Design Ltd, UK) and Spike 2 software (Cambridge Electronic Design Ltd) allowed simultaneous data acquisition of PPG, SPG and laser Doppler fluxmetry. Transcutaneous oxygen tension values, measured by colour flow duplex ultrasound and pulse oximetry, were hand recorded. All parameters were measured at baseline (at rest), at voluntary muscle action (dorsiflexion), during the 5 min stimulation period and/or during the 5 min recovery phase. ANOVA with adjusted sum of squares was conducted for each dependent parameter against stimulation settings of frequency and current. Statistical analysis for the comparison of data obtained at each stimulus and baseline or dorsiflexion was performed using Minitab software (Minitab Ltd, UK); P≤0.05 was considered to be significant. Data shown in the Results section represent the mean of data obtained from 30 volunteers and error bars show the standard error of the difference.
RESULTS
The effect of electrical stimulation on blood flow
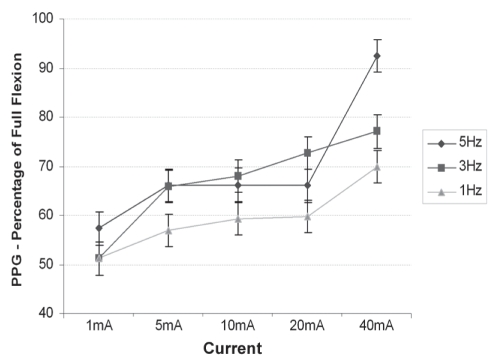
PPG placed on one of the dorsal foot veins measured relative changes in optical reflectance resulting from blood emptying from the vein. Figure 2 shows the emptying response at each stimulation as a percentage of that measured during 10 sequential full dorsiflexions. All PPG values were at least 50% of full dorsiflexion; higher current settings produced significant venous emptying (P=0.0004, R2=0.56).
Figure 2).
Photoplethysmography (PPG) measurements showing venous emptying response versus stimulation current, by frequency, compared with full flexion. Data presented as mean ± standard error of the difference
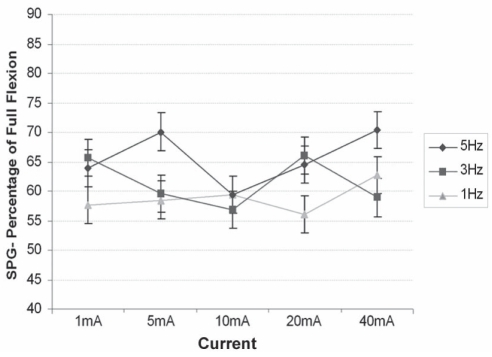
SPG placed bilaterally on the mid-calf measured the amplitude of the cyclic change in calf circumference during the stimulation cycle. Figure 3 shows the percentage of calf circumference change in each stimulation program versus dorsiflexion.
Figure 3).
Strain gauge plethysmography (SPG) measurements showing percentage of calf circumference change at 15 different stimulation programs versus foot flexion. Data presented as mean ± standard error of the difference
Stimulation frequency had a highly significant positive effect on SPG amplitude (overall P<0.001, R2=0.84). The values for SPG were between 55% and 70% of full dorsiflexion.
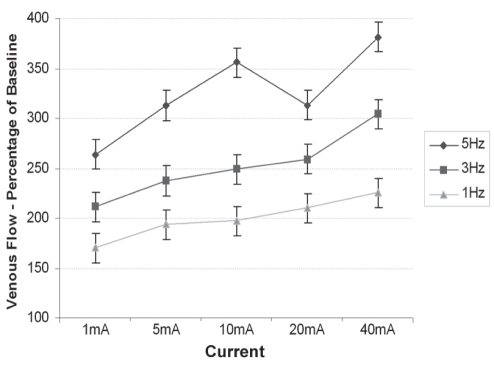
Venous volume flow and peak velocity were measured in the superficial femoral vein by pulsed Doppler using duplex ultrasound. Figure 4 shows the mean venous volume flow at each stimulation versus baseline. All stimulations showed a significant increase (P<0.01) in venous volume flow compared with baseline, with both amplitude (R2=0.55) and frequency (R2=0.82) showing a positive correlation. Mean vessel diameter was measured by imaging ultrasound, and no significant change in mean vessel diameter was found throughout the study (data not shown).
Figure 4).
Ultrasonography measurements showing venous volume flow at 15 different stimulation programs versus baseline. Data presented as mean ± standard error of the difference
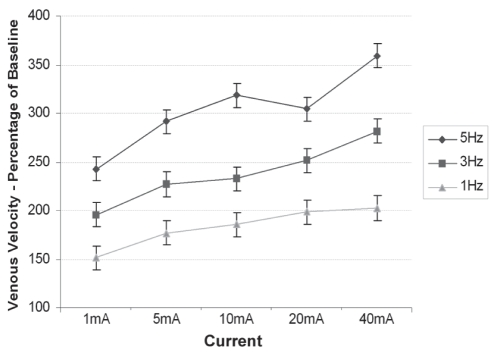
Figure 5 shows the mean peak venous velocity at each stimulation program versus baseline. There was a significant increase (P<0.01) in blood flow velocity at all stimulations compared with baseline, again with both frequency (R2=0.72) and current (R2=0.74) having strong positive correlations.
Figure 5).
Mean peak venous velocity at 15 different stimulation programs versus baseline. Data presented as mean ± standard error of the difference
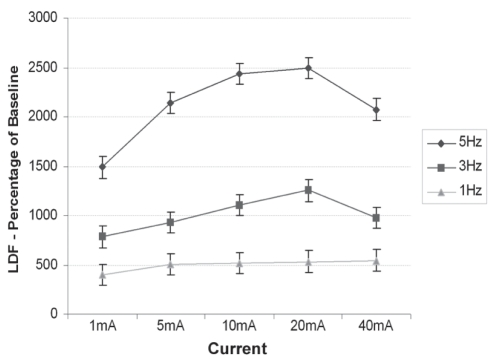
Figure 6 shows a substantial increase of the microcirculatory flux compared with baseline for all stimulation settings. Both frequency and current had significant effects (P<0.01), with a strongly positive frequency correlation (R2=0.86). There was also a significant increase in the temperature of the skin surface comparing the stimulated with the unstimulated leg (P=0.04). Skin temperatures in both the stimulated and unstimulated leg decreased by approximately 1°C compared with the individual baseline, which was expected because subjects sat in a restricted position without any physiological movement apart from the contraction of the stimulated leg (data not shown).
Figure 6).
Laser Doppler fluxmetry (LDF) measurements of micro-circulatory flux on the dorsum of the foot bilaterally for each stimulation setting as a percentage of baseline. Data presented as mean ± standard error of the difference
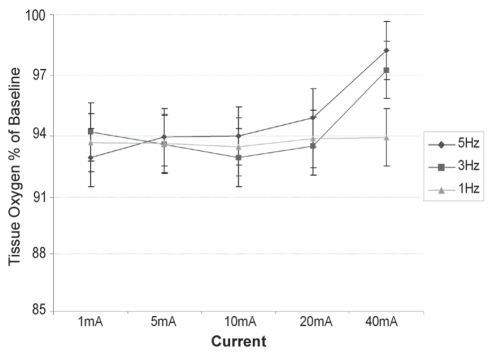
Figure 7 shows that, in all cases, there was a bilateral decrease in tissue oxygen during the course of the experiment. Values obtained from the stimulated leg (Figure 7) were significantly higher (P=0.03) than the unstimulated values for all settings above 1 mA. The data for the stimulated leg indicate that, although tissue oxygen levels still fell during the session, higher currents had a beneficial effect in maintaining these levels.
Figure 7).
Transcutaneous oxygen tension on the dorsum of the foot versus stimulation program as a percentage of baseline. Data presented as mean ± standard error of the difference
There were no significant changes to oxygen saturation (measured by pulse oximetry) or heart rate during any stimulation settings. This supports the safety of the device because no changes to important physiological parameters were observed (data not shown).
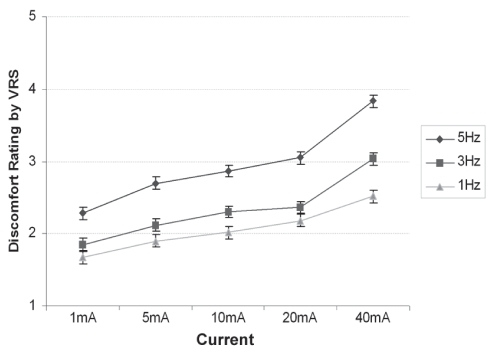
The majority of stimulation programs were rated by the subjects as minimal sensations; only stimulation program 15 (the highest amplitude and the highest frequency) reached a moderate discomfort level (Figure 8). (Discomfort rating by VRS: 1 = no sensation, 2 = minimal sensation, 3 = mild discomfort, 4 = moderate discomfort, 5 = severe discomfort.) The intrasubject variation between successive visits was generally small, and this supports the validity of the measurement methods. The VRS showed a correlation with both frequency and current.
Figure 8).
Verbal rating scale (VRS) scores for discomfort, by stimulation settings. Data presented as mean ± standard error of the difference
DISCUSSION
The present study tested a novel method for the prevention of DVT by enhancing blood flow via electrical nerve stimulation. The technology used in the study is novel and potentially advantageous compared with similar existing muscle electrostimulation (MEST) methods – first, due to an achieved blood flow increase via indolent nerve stimulation instead of painful direct muscle stimulation, and second, due to its small size, resulting in a wide range of application possibilities. Furthermore, by stimulating the nerve proximal to the posterior/anterior bifurcation, there is simultaneous activation of the tibialis, peroneus longus and lateral gastrocnemius muscles. Together, their contraction provides a near-isometric compression of the venous valve system within the lower leg, possibly evacuating blood more effectively than by contracting the gastrocnemius alone.
Based on the analysis of all data acquired from the 30 healthy volunteers, it can be concluded that electrical stimulation of the common peroneal nerve significantly enhances both venous volume and venous velocity in the lower limb, compared with baseline. PPG measured in dorsal foot veins showed significant venous emptying at all stimulation programs, indicating the device’s potential to produce prolonged improved dorsal foot vein emptying compared with a restricted short-time effect obtained by natural dorsiflexion. Laser Doppler fluxmetry measurements were increased up to 25-fold in the stimulated leg compared with baseline and the unstimulated leg. Skin temperature was increased at all stimulation programs in the stimulated leg compared with the unstimulated leg. Because metabolism is not altered during the stimulation programs, the increase in skin temperature is an indicator of increased blood flow, even in the superficial layers of the skin. Ultrasound measurements confirmed an increase in venous volume and venous velocity in the stimulated leg at all stimulations compared with baseline. Venous blood flow increases in the superficial femoral vein were exceptionally high (up to 100%) in the present study, compared with MEST results of foot or calf muscle electrical stimulation (28), which showed an increase in the femoral venous blood flow of only up to 25%. It is well known that an increase in venous volume and velocity causes venous emptying and facilitates clearance within the soleal sinuses and valve cusps, which are the most common sites of DVT formation (3,29,30).
Parameters were included to ensure appropriate safety testing of the device. Pulse oximetry measurements ensured constant monitoring of heart rate and saturated oxygen levels throughout the study and confirmed that the mean oxygen saturation was stable and always above 97% in all the subjects. There were no differences in mean oxygen saturation and heart rate between baseline values and values at each stimulation program. Furthermore, blood pressure measurements showed no differences before, during or after electrical stimulation. This is a strong indicator of the safety of the device because there were no significant physiological changes of measures related to the heart. Duplex ultrasound measurements of the blood vessel diameter (superficial femoral vein) showed no significant change in the mean vessel diameter throughout the stimulation programs, which additionally supports the safety of the device.
Pain due to high currents, as described in MEST studies, constitutes one of the main problems for the acceptance of electrical muscle stimulation in conscious patients. Within the present study, the subjects were asked to complete a discomfort questionnaire to assess the optimal electrical stimulation setting for development of an out-of-hospital DVT prevention device. Discomfort assessment by VRS showed that the majority of stimulations were rated as minimal sensations, and only the highest amplitude and frequency – stimulation program 15 – reached the moderate discomfort level. Efficient venous blood flow and microcirculatory flux increases were demonstrated at lower stimulation levels.
SPG measurements showed that all stimulations produced values between 55% and 70% of dorsiflexion, indicating that increases in microcirculatory flux are possible without substantive distortion of the calf. This is an additional measure supporting the potential ease of use of the device, and suggests the potential for improved compliance. Additionally, it has been shown that muscular contractions at 30% of the maximum possible muscle contraction (equivalent to dorsiflexion) result in significant blood flow increases (31). Finally, tissue oxygen was maintained at 92% or greater at all stimulation programs in the stimulated leg compared with baseline. Tissue oxygen values were also significantly higher in the stimulated leg compared with the unstimulated leg at most stimulation programs.
The present study demonstrates that electrical nerve stimulation using this novel technique is safe, and significantly increases blood flow and velocity by magnitudes that have not been shown before. Additionally, the device was well accepted and tolerated by healthy volunteers and, therefore, ideal for the development of a DVT prevention device for use in and out of the hospital. The small size of the device will also encourage future developments of commercially available DVT prevention devices for air travel because there are currently no comparable electrical devices available. The only current clinically recommended solution for DVT prevention on long-haul flights is graduated compression stockings, which are cumbersome and uncomfortable.
Furthermore, the increases in capillary blood flow in the skin (laser Doppler fluxmetry data), venous blood flow (ultrasound data) and preserved tissue oxygen are strong indicators for potential use of the device in wound care. It has been shown that electrical stimulation affects the inflammation and proliferation phases by increasing blood flow, enhancing tissue oxygenation, promoting phagocytosis (32), and stimulating fibroblast (33) and epithelial cells (28). Hence, it has been widely used as an additional therapy in wound care management (eg, for pressure, venous and ischemic ulcers) (34–36).
The enhancement of venous return from the lower limbs and the substantive increases of microcirculatory flux will reduce peripheral vascular resistance. These findings may also be relevant to the management of heart failure. To evaluate a physical device for out-of-hospital DVT prevention, the following aspects should be addressed: safety, effectiveness, portability, size, ease of use and cost. To our knowledge, there are no studies on electrical nerve stimulation of the lower limb to evaluate the efficacy of potential out-of-hospital DVT prevention devices. The present study provides a detailed analysis of blood flow changes during the application of electrical nerve stimulation of the common peroneal nerve of the lower limb. Due to the highly significant data shown in the present study, we believe that the tested device has great potential for the development of a novel, easy-to-use, pain-free DVT prophylaxis device, with a potential for use in an outpatient setting.
Footnotes
FUNDING SOURCE: Sky Medical Technology, UK.
CONFLICT OF INTEREST: Drs Arthur T Tucker and Duncan S Bain are named inventors of the nerve stimulation technology described in this study, on behalf of Sky Medical Technology.
REFERENCES
- 1.Malone PC, Agutter PS. The aetiology of deep venous thrombosis. QJM. 2006;99:581–93. doi: 10.1093/qjmed/hcl070. [DOI] [PubMed] [Google Scholar]
- 2.Sevitt S. Pathology and pathogenesis of deep vein thrombosis. In: Poller L, editor. Recent Advances in Thrombosis. Edinburgh: Churchill Livingstone; 1973. pp. 17–38. [Google Scholar]
- 3.Nicolaides AN, Kakkar VV, Field ES, Renney JT. The origin of deep vein thrombosis: A venographic study. Br J Radiol. 1971;44:653–63. doi: 10.1259/0007-1285-44-525-653. [DOI] [PubMed] [Google Scholar]
- 4.Nicolaides AN, Breddin HK, Fareed J, et al. Prevention of venous thromboembolism. International Consensus Statement. Guidelines compiled in accordance with the scientific evidence. Int Angiol. 2001;20:1–37. [PubMed] [Google Scholar]
- 5.Kim YH. The incidence of deep vein thrombosis after cementless and cemented knee replacement. J Bone Joint Surg Br. 1990;72:779–83. doi: 10.1302/0301-620X.72B5.2211755. [DOI] [PubMed] [Google Scholar]
- 6.Clarke MT, Green JS, Harper WM, Gregg PJ. Screening for deep-venous thrombosis after hip and knee replacement without prophylaxis. J Bone Joint Surg Br. 1997;79:787–91. doi: 10.1302/0301-620x.79b5.7627. [DOI] [PubMed] [Google Scholar]
- 7.Kujath P, Spannagel U, Habscheid W. Incidence and prophylaxis of deep venous thrombosis in outpatients with injury of the lower limb. Haemostasis. 1993;23(Suppl 1):20–6. doi: 10.1159/000216905. [DOI] [PubMed] [Google Scholar]
- 8.Bounameaux H, Righini M, Perrier A. Venous thromboembolism: Contemporary diagnostic and therapeutic aspects. Vasa. 2008;37:211–26. doi: 10.1024/0301-1526.37.3.211. [DOI] [PubMed] [Google Scholar]
- 9.Holford CP. Graded compression for preventing deep venous thrombosis. Br Med J. 1976;2:969–70. doi: 10.1136/bmj.2.6042.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scurr JH, Ibrahim SZ, Faber RG, Le Quesne LP. The efficacy of graduated compression stockings in the prevention of deep vein thrombosis. Br J Surg. 1977;64:371–3. doi: 10.1002/bjs.1800640521. [DOI] [PubMed] [Google Scholar]
- 11.Wells PS, Lensing AW, Hirsh J. Graduated compression stockings in the prevention of postoperative venous thromboembolism. A meta-analysis. Arch Intern Med. 1994;154:67–72. [PubMed] [Google Scholar]
- 12.Sigel B, Edelstein AL, Felix WR, Jr, Memhardt CR. Compression of the deep venous system of the lower leg during inactive recumbency. Arch Surg. 1973;106:38–43. doi: 10.1001/archsurg.1973.01350130040009. [DOI] [PubMed] [Google Scholar]
- 13.Sabri S, Roberts VC, Cotton LT. Prevention of early postoperative deep vein thrombosis by intermittent compression of the leg during surgery. Br Med J. 1971;4:394–6. doi: 10.1136/bmj.4.5784.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicolaides AN, Fernandes e Fernandes J, Pollock AV. Intermittent sequential pneumatic compression of the legs in the prevention of venous stasis and postoperative deep venous thrombosis. Surgery. 1980;87:69–76. [PubMed] [Google Scholar]
- 15.Hull RD, Raskob GE, Gent M, et al. Effectiveness of intermittent pneumatic leg compression for preventing deep vein thrombosis after total hip replacement. JAMA. 1990;263:2313–7. [PubMed] [Google Scholar]
- 16.Fordyce MJ, Ling RS. A venous foot pump reduces thrombosis after total hip replacement. J Bone Joint Surg Br. 1992;74:45–9. doi: 10.1302/0301-620X.74B1.1732264. [DOI] [PubMed] [Google Scholar]
- 17.Comerota AJ, Katz ML, White JV. Why does prophylaxis with external pneumatic compression for deep vein thrombosis fail? Am J Surg. 1992;164:265–8. doi: 10.1016/s0002-9610(05)81083-9. [DOI] [PubMed] [Google Scholar]
- 18.Torngren S. Low dose heparin and compression stockings in the prevention of postoperative deep venous thrombosis. Br J Surg. 1980;67:482–4. doi: 10.1002/bjs.1800670710. [DOI] [PubMed] [Google Scholar]
- 19.Wille-Jorgensen P. Prophylaxis of postoperative thromboembolism with combined methods. Semin Thromb Hemost. 1991;17(Suppl 3):272–9. [PubMed] [Google Scholar]
- 20.Scurr JH, Coleridge-Smith PD, Hasty JH. Regimen for improved effectiveness of intermittent pneumatic compression in deep venous thrombosis prophylaxis. Surgery. 1987;102:816–20. [PubMed] [Google Scholar]
- 21.Currier DP, Petrilli CR, Threlkeld AJ. Effect of graded electrical stimulation on blood flow to healthy muscle. Phys Ther. 1986;66:937–43. doi: 10.1093/ptj/66.6.937. [DOI] [PubMed] [Google Scholar]
- 22.Liu HI, Currier DP, Threlkeld AJ. Circulatory response of digital arteries associated with electrical stimulation of calf muscle in healthy subjects. Phys Ther. 1987;67:340–5. doi: 10.1093/ptj/67.3.340. [DOI] [PubMed] [Google Scholar]
- 23.Faghri PD, Van Meerdervort HF, Glaser RM, Figoni SF. Electrical stimulation-induced contraction to reduce blood stasis during arthroplasty. IEEE Trans Rehabil Eng. 1997;5:62–9. doi: 10.1109/86.559350. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan RE, Czyrny JJ, Fung TS, Unsworth JD, Hirsh J. Electrical foot stimulation and implications for the prevention of venous thromboembolic disease. Thromb Haemost. 2002;88:200–4. [PubMed] [Google Scholar]
- 25.Sindrup JH, Avnstorp C, Steenfos HH, Kristensen JK. Transcutaneous PO2 and laser Doppler blood flow measurements in 40 patients with venous leg ulcers. Acta Derm Venereol. 1987;67:160–3. [PubMed] [Google Scholar]
- 26.Bickel CS, Slade JM, Warren GL, Dudley GA. Fatigability and variable-frequency train stimulation of human skeletal muscles. Phys Ther. 2003;83:366–73. [PubMed] [Google Scholar]
- 27.Bonifer N, Anderson KM. Application of constraint-induced movement therapy for an individual with severe chronic upper-extremity hemiplegia. Phys Ther. 2003;83:384–98. [PubMed] [Google Scholar]
- 28.Zhao M, Bai H, Wang E, Forrester JV, McCaig CD. Electrical stimulation directly induces pre-angiogenic responses in vascular endothelial cells by signaling through VEGF receptors. J Cell Sci. 2004;117:397–405. doi: 10.1242/jcs.00868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicolaides AN, Kakkar VV, Renney JT. Soleal sinuses and stasis. Br J Surg. 1971;58:307. [PubMed] [Google Scholar]
- 30.Flanc C, Kakkar VV, Clarke MB. The detection of venous thrombosis of the legs using 125-I-labelled fibrinogen. Br J Surg. 1968;55:742–7. doi: 10.1002/bjs.1800551007. [DOI] [PubMed] [Google Scholar]
- 31.Barcroft H, Millen JL. The blood flow through muscle during sustained contraction. J Physiol. 1939;97:17–31. doi: 10.1113/jphysiol.1939.sp003789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chakravarti B, Chakravarti DN. Phagocytosis: An overview. Pathol Immunopathol Res. 1987;6:316–42. doi: 10.1159/000157062. [DOI] [PubMed] [Google Scholar]
- 33.Kamrin BB. Induced collagenolytic activity by electrical stimulation of embryonic fibroblasts in tissue culture. J Dent Res. 1974;53:1475–83. doi: 10.1177/00220345740530063001. [DOI] [PubMed] [Google Scholar]
- 34.Kloth LC, Feedar JA. Acceleration of wound healing with high voltage, monophasic, pulsed current. Phys Ther. 1988;68:503–8. doi: 10.1093/ptj/68.4.503. [DOI] [PubMed] [Google Scholar]
- 35.Mulder GD. Treatment of open-skin wounds with electric stimulation. Arch Phys Med Rehabil. 1991;72:375–7. [PubMed] [Google Scholar]
- 36.Feedar JA, Kloth LC, Gentzkow GD. Chronic dermal ulcer healing enhanced with monophasic pulsed electrical stimulation. Phys Ther. 1991;71:639–49. doi: 10.1093/ptj/71.9.639. [DOI] [PubMed] [Google Scholar]
- 37.Gray H. In: Anatomy of the human body. 20th edn. Lewis WH, editor. Philadelphia: Lea & Febiger; 1918. [Google Scholar]