Abstract
Immune suppression is a major cause of morbidity and mortality in the septic patient. Apoptotic loss of immune effector cells such as CD4 T and B cells is a key component in the loss immune competence in sepsis. Inhibition of lymphocyte apoptosis has led to improved survival in animal models of sepsis. Using qRT-PCR of isolated splenic CD4 T and B cells, we determined that Bim and PUMA, two key cell death proteins, are markedly up-regulated during sepsis. Lymphocytes have been notoriously difficult to transfect with siRNA. Consequently a novel, cyclodextrin polymer-based, transferrin receptor-targeted, delivery vehicle was employed to co-administer siRNA to Bim and PUMA to mice immediately after cecal ligation and puncture. Anti-apoptotic siRNA based therapy markedly decreased lymphocyte apoptosis and prevented the loss of splenic CD4 T and B cells. Flow cytometry confirmed in vivo delivery of siRNA to CD4 T and B cells and also demonstrated decreases in intracellular Bim and PUMA protein. In conclusion, Bim and PUMA are two critical mediators of immune cell death in sepsis. Use of a novel cyclodextrin polymer-based, transferrin receptor-targeted siRNA delivery vehicle enables effective administration of anti-apoptotic siRNAs to lymphocytes and reverses the immune cell depletion that is a hallmark of this highly lethal disorder.
Keywords: lymphocyte, transferrin, cyclodextrin, Bim, PUMA, apoptosis
Introduction
Sepsis continues to be the leading cause of mortality in patients in the intensive care unit.(1) Although death in sepsis can be due to a “cytokine storm”-mediated, hyperinflammatory response, the majority of deaths occur late in the course of sepsis and are often due to either the inability of the host to clear the primary infection, or the development of hospital-acquired infections.(2–6) Septic patients are now known to enter a period of immune suppression, termed “immunoparalysis”, and their ability to survive sepsis is related, in part, to their propensity to restore immune competence.(6)
One of the major pathological features of sepsis-induced immune suppression is loss of immune effector cells, specifically, lymphocytes and dendritic cells by apoptosis.(7–11) Three independent autopsy studies of adult, pediatric, and neonatal septic patients noted marked sepsis-induced apoptotic depletion of CD4+ T cells and B cells.(7, 12, 13) The loss of large numbers of lymphocytes and dendritic cells hampers the ability of the host to sustain an effective immune response, thereby resulting in the inability to clear primary infections and susceptibility to nosocomial infections.(6) The fact that prevention of apoptosis in clinically-relevant animal models of sepsis results in improved survival supports the hypothesis that apoptosis is a critical pathogenic mechanism in sepsis.(14–16)
Mammalian apoptotic cell death in sepsis proceeds through two distinct pathways, i.e., the death receptor pathway and the mitochondrial pathway, that converge to activate caspases (cell death proteases) resulting in cellular demolition.(17–19) The extrinsic, or death receptor, pathway is triggered by signaling through tumor necrosis factor (TNF) receptor family members, such as, TNF, Trail, and Fas.(20, 21) The mitochondrial (intrinsic) death pathway responds to various stress stimuli, e.g., oxidative stress, radiation, and cytokine withdrawal that cause activation of the pro-apoptotic BH3-only proteins, including Bim, Noxa, and PUMA.(17) Previous work from a number of groups, including our own, has shown that both the death receptor and mitochondrial pathways are activated in sepsis, implying that sepsis induces multiple independent triggers of cell death.(17–19, 22) The activation of pro-apoptotic BH3-only proteins makes them natural targets for therapeutics intended to decrease apoptosis.(19) In this regard, we noted that both Bim-null and PUMA-null mice have a highly significant decrease in apoptotic death of immune effector cells in sepsis.(19) Hence, methods to recapitulate the genetic deletion of Bim and PUMA such as RNA interference might provide similar beneficial effects.
After the discovery of RNA interference and small interfering RNA (siRNA) molecules that mediate it, translational applications of siRNA based therapy were immediately realized and this discovery has become an effective tool to “knockdown” genes of interest in a multitude of research models.(23) Recently, researchers have demonstrated the efficacy of siRNA in animal models of infectious disease, including sepsis.(24–27)
A critical challenge in the development of siRNA therapeutics has been the various obstacles to delivery in vivo. Effective in vivo delivery of siRNA has been extremely difficult both in terms of achieving intracellular delivery and release of the siRNA to permit efficacy and minimizing delivery vehicle-related toxicity.(23) Naked siRNA is highly vulnerable to circulating nucleases which render it ineffective. Liposomal delivery agents have shown some successes in delivery to the liver but may carry with them unfavorable toxicity profiles.(28) A siRNA delivery vehicle that appears to overcome these limitations is a targeted, cyclodextrin polymer-based (RONDEL™) system developed by Calando Pharmaceuticals.(29) The three delivery components condense the siRNA within nanocomplexes (which protect the siRNA from nuclease degradation), stabilize them, and target them to transferrin receptors on target cells, respectively. This delivery system is part of the first siRNA-containing drug candidate for oncology to reach the clinic, CALAA-01, for which dosing initiated in 2008.
The purpose of the present study was to identify transcriptionally-regulated proapoptotic genes in sepsis and to determine whether RNA interference targeting those genes individually or in combination could prevent sepsis-induced lymphocyte apoptosis. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed in splenic B and CD4 T cells obtained from septic and sham mice to determine activation of death genes and to reveal potential targets for siRNA-based therapy. Based upon the qRT-PCR findings and previous results, selected anti-apoptotic siRNAs (alone and in combination) were tested for their ability to prevent sepsis-induced death of immune cells when administered after the onset of sepsis. A novel, targeted, cyclodextrin-containing polymer-based delivery agent was used for intracellular delivery of the siRNAs.
Material and Methods
Cecal ligation and puncture (CLP) model of sepsis
6–8 week old male CD1 mice (22–28 g body weight) were purchased from Charles River Laboratories and underwent CLP according to the protocol approved by the Washington University Animal Studies Committee and as previously described.(30–32) Mice were anesthetized with isofluorane and a midline abdominal incision was made. The cecum was mobilized, ligated below the ileocecal valve, and punctured twice with a 25 gauge needle resulting in a polymicrobial peritonitis. The abdominal wall was closed in two layers and the mice received 1 mL of 0.9% saline s.q. for volume resuscitation. Each sham-operated mouse had its cecum externalized but not ligated or punctured. All mice were allowed free access to food and water throughout the study.
Tissue sampling for Spleen CD4+ T and B Cells
After excision, spleens were placed in 1 mL of culture medium (900 mL 1640-RPMI (GIBCO, Carlsbad, CA), 50 mL fetal bovine serum, 10 mL HEPES (Invitrogen, Carlsbad, CA), 10 mL non-essential amino acid (NEAA; Invitrogen), 10 mL Antibiotic/antimyotics (Invitrogen), 10 mL glutamax (Invitrogen), and 10 mL 2ME (360mL/L PBS; Sigma, St. Louis, MO)). Splenocytes were disaggregated over nylon mesh filter and the splenocyte pellet was resuspended in MACS buffer (PBS (phosphate buffered saline) pH 7.2, 0.4 % BSA (bovine serum albumin), and 1mM EDTA). The cell suspension underwent ficoll gradient separation. The WBC layer was diluted with MACS buffer to 10 mL at 4°C and cells were counted using a Beckman Coulter Vicell A cell counter (Fullerton, CA) using trypan blue to determine the percentage of live cells. CD4+ T and B cells were isolated as the flow-through of a magnetic column using positive selection (MACS LS Columns #130-042-401, CD4+ T-cell and B-cell Isolation Kit; #130-049–201 and #130-049-501, Miltenyi Biotec) and washed. Immunomagnetic depletion-enriched CD4+ T and B cells were used for quantitative real time-PCR (qRT-PCR) experiments. The purity of isolated resting CD4+ T and B cells was generally >90%, as measured by fluorescence activated cell sorter (FACS) analysis.
Total RNA extraction and reverse transcription
Total RNA from spleen cells was isolated from the prepared tissues as described above using a commercially-available kit (QIAshredder™ and RNeasy Mini Kit, Qiagen GmbH., Hilden, Germany) following the manufacturer’s instructions. To avoid amplification of contaminating genomic DNA, all samples were treated with ribonuclease-free deoxyribonuclease (Qiagen) for 15 min. The quantity and purity of extracted RNA was measured with a spectrophotometer (Spectronic BioMate 3 UV-Vis, Thermo Electron Co., Madison, WI). The quality of the extracted mRNA was verified on an Agilent 2100 Bioanalyzer (Santa Clara, CA). Purified total RNA was reverse-transcribed into cDNA using random primers.
Quantitative Real-time polymerase chain reaction (qRT-PCR)
qRT-PCR was performed at early (8 h) and late (18 h) time points after sepsis or sham surgery to identify transcriptionally-regulated candidate genes involved in mediating apoptosis. The reaction was performed in 96-well microtiter plates with an ABI Prism 7500 Sequence Detector System. Primers and reagents were purchased from Applied Biosystems (Foster City, CA). TaqMan glyceraldehyde-3-phosphate dehydrogenase (GAPDH) endogenous control reagent was used as internal control for normalization. Cell threshold values for each gene were determined and fold-induction in comparison to GAPDH was calculated. qRT-PCR results were recorded for each gene as fold-change vs. sham. PCR products were evaluated by dissociation curves to confirm single amplicons and the absence of significant primer-dimer contamination.
Determination of Cellular Transferrin Receptor Expression
The cyclodextrin polymer-containing siRNA delivery vehicle employed in the present study contains the human transferrin protein as a ligand to enhance cellular uptake. In order to determine which classes of immune cells express the transferrin receptor (CD71), and thus are more likely to have increased siRNA uptake, splenocytes from sham-operated (n=5) and septic mice (n=6) were isolated ~24 h after surgery and CD71 expression on specific subsets was measured using an anti-CD71 antibody (BD Biosciences, San Diego, CA). Fluorescence was detected using BD FACScan.
Determining delivery of siRNAs to splenic immune cells via knockdown of green fluorescence protein (GFP)
To confirm delivery of siRNA to splenic immune cells, mice transgenic for GFP in all nucleated cells (stock# 004353) were obtained from Jackson Laboratories and injected via tail vein with siRNA to GFP (100 µg per animal, Sigma-Aldrich, St. Louis, MO) 24 h and 48 h before tissue harvesting. The siRNA was diluted in D5W to a concentration of 4 µg/µL, then an equal volume of a solution containing delivery components (also in D5W) was added to formulate it, yielding a final siRNA concentration of 2 µg/µL. This formulated siRNA solution was then further diluted in D5W (1:1 vol:vol) to give a final injection volume of 100 µL. The siRNA was delivered using a transferrin receptor-mediated, cyclodextrin-based polymer provided by Calando Pharmaceuticals (Pasadena, CA). Adamantane is conjugated to one terminus of linear PEG and the adamantine-PEG interacts with cyclodextrin non-covalently via inclusion complex formation. This delivery agent has been tested in non-human primates and has been shown to have minimal systemic toxicity.(29) Knockdown of GFP in various tissues splenic subsets was determined by flow cytometry and compared to splenocytes harvested from GFP mice receiving diluent only or a formulated inactive (non-coding) siRNA.
siRNA sequences and determination of siRNA knockdown in vitro
HeLa (human) and C2C12 (murine) cells were used to evaluate the potency of three different MISSION® pre-designed siRNA constructs (Sigma-Aldrich) to Bim or to PUMA. After 24 h of treatment with siRNAs, total RNA was extracted with the Gen Elute Mammalian Total RNA kit (Sigma-Aldrich, St Louis, MO). Gene expression was determined by qRT-PCR. First strands were synthesized using M-MLV reverse transcriptase (Sigma-Aldrich). Human or mouse cyclophilin was used as the normalization control in subsequent quantitative analysis for the HeLa and C2C12 cells, respectively. Primers and FAM labeled TaqMan probes were used for target transcript analyses (Applied Biosystems). The PCR was performed on a MX3000 instrument (Stratagene, La Jolla, CA). The program consisted of an initial reverse transcription at 42°C for 15 min, denaturation at 94°C for 3 min, followed by 40 cycles of amplification (denaturation at 94°C for 15 s, annealing and extension at 60°C for 1 min).
Quantitation of intracellular pro-apoptotic proteins via flow cytometry
To verify that the anti-apoptotic siRNAs were acting by direct effects on target proteins, intracellular Bim and PUMA were measured using flow cytometry. Harvested splenocytes were fixed in1% paraformaldehyde and washed with Perm/Wash solution (BD Bioscience). Primary rabbit anti-mouse antibody staining for Bim and PUMA (Cell Signaling) was done overnight at 4°C. Cells were then washed and stained with the donkey anti-rabbit secondary antibody and cell surface antibodies (CD3 for T cells, CD20 for B cells). CD3 was used as a cell surface marker to identify more than one class of T cells that are important in sepsis, e.g., CD4+, CD8+, and gamma/delta T cells. Staining for CD3 allowed us to obtain a general picture of the effect of siRNA therapy to all classes of T cells. Cells were analyzed for mean fluorescence intensity via flow cytometry.
Treatment of septic mice with siRNA to BH3 family members
Mice received 100µL of diluent (sham and CLP) or siRNA (50µg per mouse) treatment at the time of surgery via retro-orbital venous plexus injections. Treatment groups included sham (n=6), CLP alone (n=9), siRNA to PUMA (n=9), siRNA to BIM (n=9), siRNA to both PUMA and BIM (n=6), or a non-coding inactive siRNA (n=6). Mice receiving siRNA to both Puma and Bim received 50µg of siRNA to each. All siRNA was formulated as described above.
Determination of efficacy of siRNA via quantification of absolute cell counts
In order to determine if anti-apoptotic siRNAs were effective in preventing sepsis-induced cell death, absolute cell counts for various splenic subsets were quantitated. Approximately 24 hours post-surgery, mice were sacrificed and spleens were harvested for analysis. Splenocytes were dissociated by gently pressing through a 70-µm filter and then washed. The total number of viable cells (based upon exclusion of trypan blue) was determined using a Beckman-Coulter cell counter. Splenocytes were also stained with fluorochrome-conjugated antibodies to cell subset-specific surface markers (CD4 and CD8 for T cells, B220 for B cells) and the percentage of the different cell subset was determined by flow cytometric analysis (BD FACScan).
Determination of efficacy of siRNA therapy via quantitation of apoptosis
To verify that the anti-apoptotic siRNAs were acting mechanistically to block sepsis-induced cell death, apoptosis was quantified. Two independent methods, detection of active caspase-3 and TUNEL staining, were utilized as complementary methods. Cells were fixed in 1% paraformaldehyde for 30 min at room temperature, washed, and permeabilized with 90% methanol on ice for 30 min. Active caspase-3 was quantitated per the manufacturer’s recommendations using antibodies to the cleaved fragment of caspase-3 (Cell Signaling, catalog #9661, Danvers, MA). A secondary PE-labeled donkey anti-rabbit IgG antibody was used to detect the primary antibody. Apoptosis was quantitated by the TUNEL method using a commercially-available Apo-BrdU Kit (Phoenix Flow Systems, Inc., San Diego, CA) per the manufacturer’s instructions. A secondary anti-biotin PE-labeled antibody was used for detecting BrdU-labeled strand breaks. T cells were identified using CD3 cell surface marker, and B cells were identified using CD20.
Quantitation of plasma cytokine levels
Due to the similarity of the structure of siRNA to viral RNA, siRNA treatment may elicit an inflammatory response by activation of Toll-like receptors. To determine the effect of siRNA on systemic inflammation, plasma cytokines were quantitated in CLP-induced septic and sham-operated mice that were treated with siRNA to Bim (n=5 and n=3, respectively) or with vehicle alone (n=5 and n=3, respectively). These were compared to septic and sham-operated mice that were given diluent (n=5 and n=3, 12 respectively). Blood was obtained by cardiac puncture in heparinized syringes at approximately 24 h post-treatment. Plasma was obtained by centrifugation and stored at −80°C. Levels of cytokines in plasma were quantitated using BD FACS Array and the Inflammation Kit per the manufacturer’s recommendations. Samples were run in duplicate. The cytokines examined and their respective lower limits of detection were IL-10 (17.5 pg/mL), IL-6 (5 pg/mL), MCP-1 (22.7 pg/mL), TNF-α (7.3 pg/mL), IL-12p70 (10.7 pg/mL), and IFN-γ (2.5 pg/mL).
Statistical analysis
Data are reported as mean ± SEM. Data were analyzed with the statistical software program PRISM (GraphPad, San Diego, CA, USA). One-way ANOVA with Tukey’s multiple comparison post-test was used to compare three or more groups.
Results
Sepsis induces time-dependent expression of BH3 cell death genes
Mice subjected to CLP or sham surgery were sacrificed at 8 and 18 hours post-surgery and CD4 T cells and B cells were isolated (n=3 per group). Then, using quantitative real-time PCR, expression of selected genes involved in regulation of cell survival was determined. At 8 hours post surgery, pro-apoptotic BIM was up-regulated in septic animals compared to sham-operated in B cells (average relative quantification 2.07 ± 0.2 vs. 0.94 ± 0.05, p≤0.01 [Fig. 1a]). Bim expression returned to baseline by 18 hours post-surgery (Fig. 1b). PUMA expression in B cells of septic animals was not significantly different compared to controls, however.
Figure 1. A and B. Quantitation of messenger RNA in B cells 8 and 18 hours after sepsis.
Each gene shown is quantified relative to levels of sham operated mice. We show here that Bim expression is increased early when compared to sham (* p≤0.01) and then returns to sham levels by 18 hours (0.92 ± 0.1). PUMA expression in B cells was not affected by sepsis. Notably, expression of Pdcd-1 is markedly up-regulated early in sepsis (**p≤0.05).
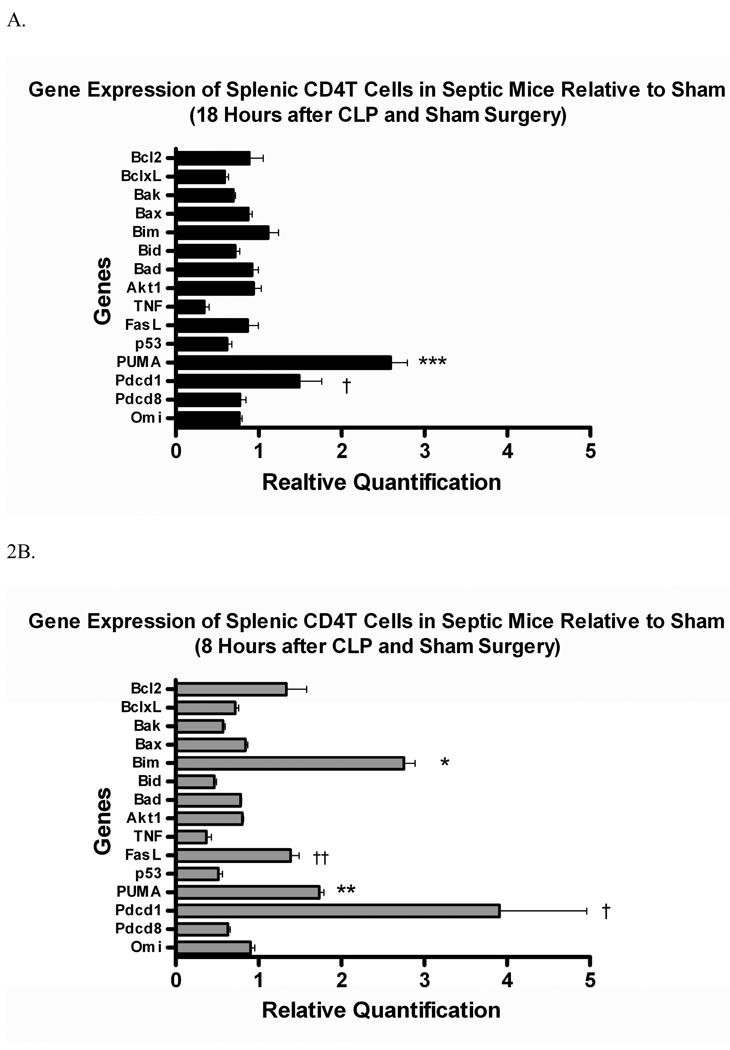
In CD4 T cells, Bim expression was also increased after 8 hours in septic vs. control animals, and this also returned to baseline by 18 hours (3.02 ± 0.15 vs. 1.18 ± 0.31 at 8 hours, p≤0.01 [Fig. 2a]). In contrast, PUMA expression was increased early (1.65 ± 0.05 vs. 0.96 ± 0.08, p≤0.01) in septic animals and remained elevated at 18 hours (3.44 ± 0.26 vs. 1.36 ± 0.18, p≤0.01) when compared to sham-operated animals (Fig. 2). Notably, the gene for Programmed cell death receptor - 1 (Pdcd-1) was up-regulated in both B and T cells early in sepsis. The role of Pdcd-1 in sepsis is still unclear and further study is needed (see discussion).
Figure 2. Quantitation of messenger RNA in CD4 T cells at 8 and 18hrs after sepsis.
Each gene shown here is quantified relative to levels in sham operated mice. Bim expression is increased early in sepsis (3.02 ± 0.15 vs. 1.18 ± 0.31, * p≤0.01) but decreases by 18 hrs. PUMA is upregulated in T cells during sepsis at both 8 (**p≤0.01) and 18 hours (***p≤0.01) during sepsis. Interestingly, Pdcd-1 is upregulated in CD4 T cells at 8 and 18 hours after sepsis (†p≤0.01). Fas-L is also upregulated early in sepsis (†† p≤0.05) but by 18 hours has returned to baseline levels.
Transferrin receptor (CD71) is broadly expressed on many immune effector cells
Flow cytometry showed that CD71 was expressed to a variable degree on all immune cell subsets examined (Fig. 3). The highest expression of CD71 was on B cell and dendritic cells, in which ~75–80% of cells were positive. Approximately 35% of monocytes/macrophages from sham-operated mice expressed CD71 and this increased to ~60% with sepsis. NK, CD4, and CD8 T cells had relatively lower levels of expression compared to other immune effector cells; CD71 expression approached 25% of these populations.
Figure 3. Tranferrin Receptor (CD71) Expression on Immune Effector Cells during Sepsis.
Transferrin Receptor expression was measured on splenocyte subsets after sham (n=5) and CLP (n=6). The proportion of cells positive for transferrin receptors remains, for the most part, constant between different cell types despite the onset of sepsis.
siRNA to GFP decreases fluorescence intensity in splenic immune cells
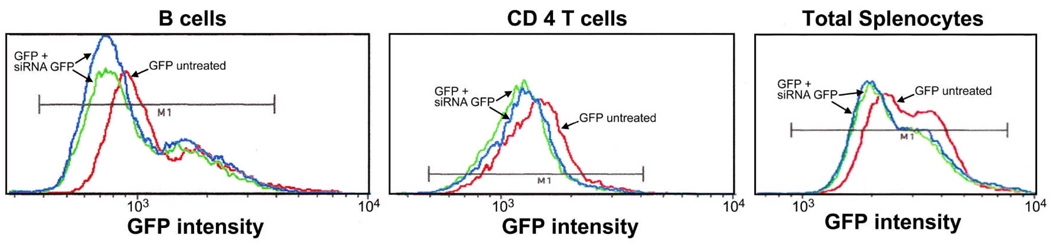
CD4 T and B cells from transgenic GFP mice that were treated with siRNA to GFP showed decreased GFP fluorescence as evidenced by the decrease in mean fluorescence intensity (MFI) compared to mice treated with saline or non-coding siRNA in splenic immune effector cells (Fig. 4). Note that the decrease in GFP fluorescence was greater in B vs. T cells, possibly reflecting the fact that a higher % of B cells express the CD71 receptor thereby being more likely to take up the formulated siRNA.
Figure 4. Knockdown of GFP in Splenic Immune Cells.
B (B220+) cells and CD4 T cells of GFP transgenic mice treated with siRNA to GFP show decreased mean fluorescence intensity when compared to untreated GFP mice. Each curve represents one mouse. The data are representative of n=3 studies.
In vitro knockdown of Bim and Puma
Mouse C2C12 cells were treated with 3 different constructs of siRNA to either Bim or PUMA to evaluate the relative potency of the individual siRNA constructs. Gene expression in cells treated with the inactive non-coding siRNA (MOCK) was set at 100%. qRT-PCR shows a significant decrease in proapoptotic gene expression in cells treated with the corresponding siRNAs (data not shown). siRNAs used for the in vivo work (the 1st construct of 3 different designs, for both Bim and Puma) were effective in decreasing target gene expression by > 80% in vitro.
siRNA to PUMA and Bim decrease cell protein expression in vivo during sepsis
Splenic T cells of septic mice treated with siRNA to PUMA had significantly less intracellular PUMA compared to septic controls (Fig 5). Splenic T cells of septic mice treated with siRNA to Bim also exhibited less intracellular Bim staining when compared to septic controls (Fig. 6). Septic animals treated with siRNA to both PUMA and Bim demonstrated lower levels of both proteins in splenic T cells vs. non-treated septic mice (Figs. 5 and 6). The protein changes in B cells of siRNA treated septic mice mirrored those seen in T cells (supplemental data). The effect of the siRNA to decrease intracellular protein was also specific to its target, i.e., siRNA to PUMA decreased PUMA but not Bim. Similarly, siRNA to Bim decreased Bim, but not PUMA (data not shown).
Figure 5. A and B siRNA to PUMA decreases intra-cellular PUMA expression in Splenic T cells.
Splenic T cells of septic mice treated with siRNA to PUMA or PUMA and Bim in combination have decreased intracellular levels of PUMA when compared to controls (mean fluorescence intensity 9.80 ± 0.13 and 11.7 ± 0.7 vs. 25.7 ± 0.9, n=3 in each group *p<.001). P+B are data from mice treated with both siRNA to PUMA and Bim. NC, mice treated with non-coding siRNA.
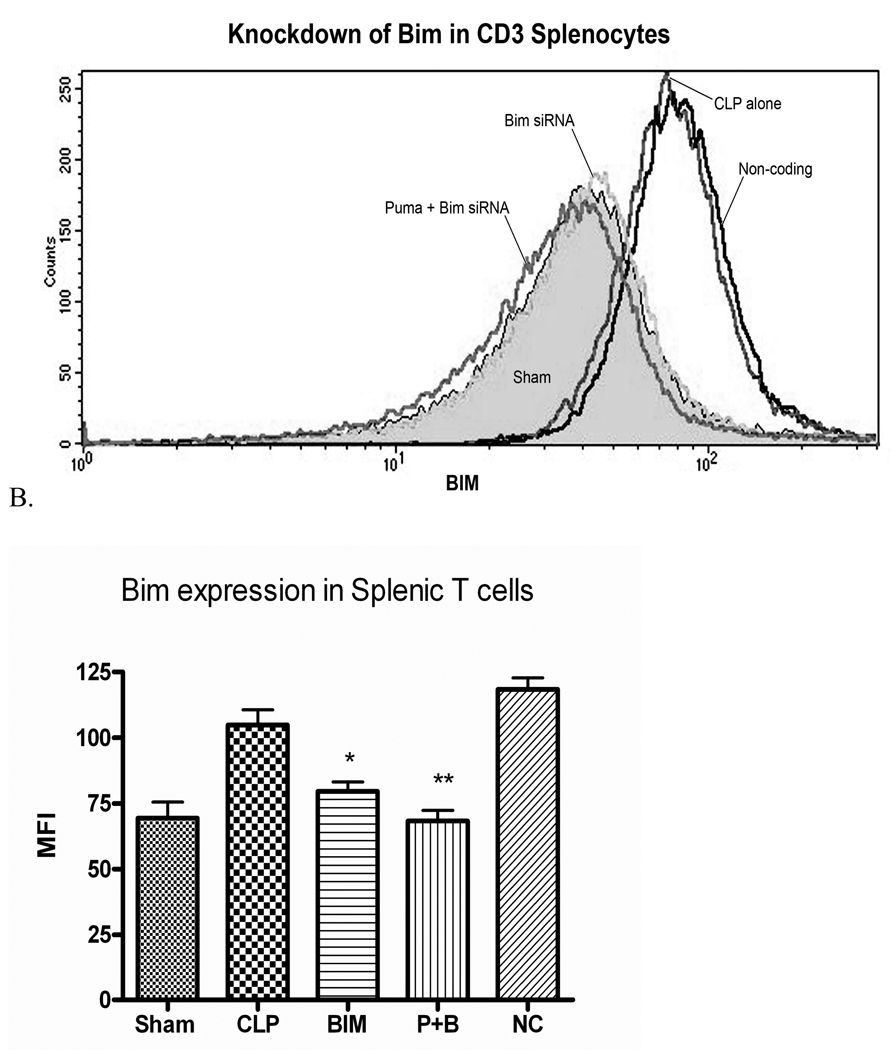
Figure 6. A and B siRNA to Bim decreases intracellular Bim expression in T (CD3+) cells after Sepsis.
Splenic T cells of septic mice treated with siRNA to Bim or the combination of PUMA and Bim show a decrease in intracellular staining for Bim (mean fluorescence intensity 79.6 ± 3.5 and 68.3 ± 3.9 vs. 104.8 ± 5.9, n=3 in each group, * p≤0.05, **p≤.01). P+B are data from mice treated with siRNA to PUMA and Bim. NC, mice treated with non-coding siRNA.
siRNA to PUMA, Bim, or both in combination prevents splenic cell loss after sepsis
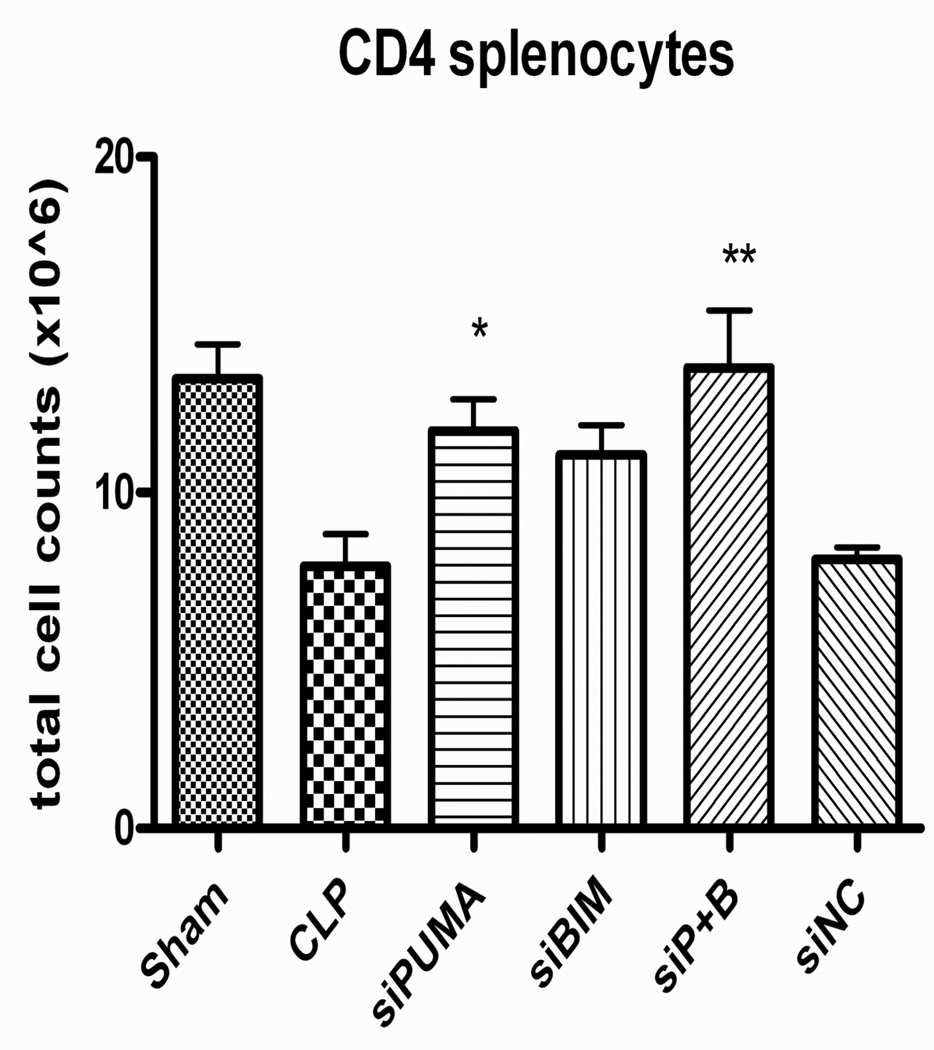
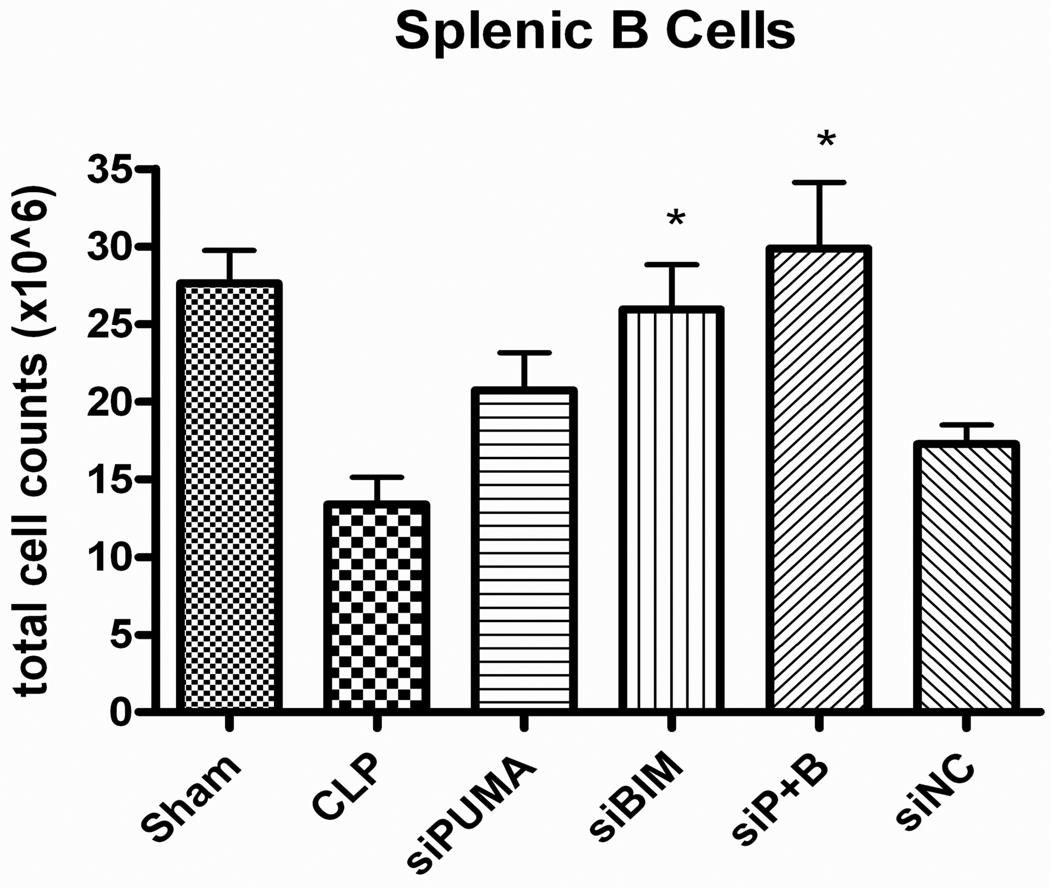
Initial studies comparing 50 µg and 100 µg doses of siRNA to pro-apoptotic BH3 proteins did not show a dose dependence to their anti-apoptotic effect (data not shown). Therefore, a dose of 50 µg of siRNA was selected for subsequent studies. Mice that received siRNA to PUMA after CLP demonstrated significantly higher numbers of splenic CD4 T cells 24 h after surgery than mice that received diluent, (11.9 ± 0.9 × 106 vs. 7.8 ± 1.0 × 106, p≤0.05 [Fig. 7]). Mice that received siRNAs to both PUMA and Bim also had significantly higher numbers of surviving CD4 T (13.7 ± 1.7 × 106, p≤0.01 [Fig. 7]) and B cells (29.9 ± 4.3 × 106, p≤0.01 [Fig. 8]) than septic controls. Mice treated with Bim siRNA had significantly higher number of B cells survive after sepsis when compared to CLP alone (25.9 ± 2.9 × 106 vs. 13.4 ± 1.75 × 106, p<0.01 [Fig. 8]). In other subtypes of splenocytes, there was a trend toward a larger number of surviving cells but the data do not reach statistically significant levels (data not shown).
Figure 7. Absolute splenic T cell counts in septic mice after siRNA treatment.
Mice receiving siRNA to PUMA and siRNAs to both PUMA and Bim had significantly higher number of CD4+ splenocytes when compared to mice undergoing CLP alone (*p<0.05, **p<0.01).
Figure 8. Absolute Splenic B cell counts in septic mice after siRNA treatment.
Mice receiving siRNA to Bim and to PUMA and Bim in combination had a significantly higher number of surviving B cells after CLP induced sepsis when compared to septic controls (*p<.01).
siRNA to PUMA and Bim in combination decreases splenic lymphocyte apoptosis
Splenic T and B cells of septic mice that were administered siRNAs to PUMA and Bim had significantly lower percentages of cells that were positive for active caspase-3 and TUNEL when compared to controls (Figs. 9 & 10). Mice that received siRNA to Bim had a lower percentage of B cells positive for active caspase-3 and TUNEL compared to controls (Figs. 10). Similarly, mice that received siRNA to Bim had a decreased percentage of TUNEL positive T cells after sepsis (Fig. 9). Treatment with PUMA siRNA alone did not significantly decrease apoptosis in either splenic T or B cells (Figs. 9 & 10).
Figure 9. A and B Quantitation of Apoptosis in Splenic T cells.
Mice receiving siRNA to a combination of PUMA and Bim had less T (CD3) cells that were positive for active caspase 3 and TUNEL than septic controls (Caspase 3 = 7.07 ± 0.6 vs. 11.37 ± 1.08, * p≤0.05; TUNEL = 11.89 ± 2.2 vs. 19.54 ± 1.7, * p≤0.05). Mice treated with siRNA to Bim had significantly less CD3+ T cells that were positive for TUNEL than septic controls ( 11.63 ± 0.93, ** p≤0.01).
Figure 10. A and B Quantitation of Apoptosis in Splenic B cells.
Mice treated with siRNA to Bim have a decreased percentage of B (CD20) cells that are positive for active Caspase 3 and TUNEL when compared to septic controls (Caspase 3 = 8.41 ± 0.73 vs. 12.55 ± 0.62, * p≤0.05; TUNEL = 11.37 ± 1.2 vs. 18.93 ± 1.8, **p≤0.01). Mice treated with siRNA to the combination of PUMA and Bim had similar results when compared to septic controls (Caspase 3 = 7.84 ± 0.7, *p≤0.05; TUNEL = 10.43 ± 1.8, **p≤0.01).
siRNA did not affect pro-inflammatory cytokine production in septic animals
Mice undergoing CLP have increased levels of pro-inflammatory cytokines TNF-α, IL-6, and MCP-1, as noted previously.(32) These levels are not significantly different from mice treated with siRNA or with the delivery agent alone. Neither siRNA, nor vehicle alone, affected cytokine levels in animals undergoing sham operation (Fig. 11).
Figure 11. Systemic cytokine profiles of mice treated with siRNA.
Mice treated with siRNA to Bim do not show significant differences in the levels of proinflammatory cytokines such as IL-6, TNF-α, and MCP-1. Treatment with the delivery vehicle alone does not alter the cytokine profile in either septic or sham-operated animals.
Discussion
The present results strongly support the potential powerful role of siRNA-based therapy in infectious disease. Few studies have examined the ability of siRNA-based therapy to ameliorate the pathogenic response that occurs during infection. Geisbert et al., found that siRNA to the polymerase gene of Ebola virus was able to protect guinea pigs from a lethal dose of the virus.(25) Sorensen and colleagues protected mice from a lethal dose of LPS by treating with siRNA to TNF-alpha.(26) Two reports using siRNA in sepsis have been published. Wesche-Soldato et. al., reported that the hydrodynamic delivery of naked siRNA to Fas or caspase-8 administered after the onset CLP decreased apoptosis in hepatocytes and splenocytes and improved survival.(27) Our laboratory reported that pre-treatment of mice with siRNA to Bim delivered via a liposomal delivery agent protected against sepsis-induced lymphocyte apoptosis and improved survival.(24) The current work extends findings from previous studies by demonstrating that simultaneous targeting of two critical mediators of sepsis-induced apoptosis is feasible. Inhibition of Bim and PUMA conjointly had a greater effect than inhibiting either one alone as evidenced by the lower % of splenocytes that were positive for active caspase-3 and TUNEL when compared to either siRNA to Bim of siRNA to PUMA individually.
The current results are also of interest because they show that different cell death triggers may be activated in different classes of immune cells during cells. For example, Bim was upregulated in both T and B cells while PUMA was upregulated only in T cells. This finding of differential up-regulation of cell death genes may be reflected in the fact that siRNA to Bim but not siRNA to PUMA decreased splenic B cell apoptosis as determined by active caspase-3 and TUNEL. Other notable findings from the qRT-PCR studies were the dramatic rapid increase in the gene for Programmed cell death receptor - 1 (Pdcd-1) in splenic T cells. Pdcd-1 is a relatively recently discovered negative costimulatory cell surface receptor expressed on T cells.(34) Activation of the Pdcd-1 pathway induces anergic non-functional T cells and dampens the immune response. Interestingly, microarray studies on CD4 T cells from patients with trauma also demonstrated a marked increase in Pdcd-1, and the investigators postulated that increased Pdcd-1 expression could be a mechanism for the immune dysfunction that characterizes these patients.(35) The present data in sepsis are consistent with the results in trauma and support the potential role of Pdcd-1 in the immune suppression that is a hallmark of sepsis. We did not elect to use anti-Pdcd-1 siRNA based therapy in the present study because Pdcd-1 is a cell surface receptor and therefore it is more easily targeted using an antibody-based approach; such studies are currently underway.
The present study highlights the fact that during sepsis multiple triggers of cell death, e.g., Bim and PUMA, are induced. Bim is induced by a number of stimuli including cytokine withdrawal and oxidative stress.(36, 37) PUMA is a key activator of p53-mediated apoptosis and is induced by nuclear injury, such as occurs during radiation, and may occur during sepsis.(38, 39) We have previously shown that siRNA to Bim decreased apoptosis in polymicrobial sepsis.(24) In the present study we demonstrated that siRNA to PUMA also prevented sepsis-induced death of immune effector cells. Furthermore, results suggest that there is an advantage to targeting both PUMA and Bim in that the combination of both was able to decrease apoptosis in both T and B cells by two different detection methods. We have shown that Bim knockout mice have extensive protection against apoptosis (17), and others have shown that lymphocytes that lack Bim are resistant to chemotherapeutics and cytokine deprivation.(36, 37) Bim has been shown to be crucial for prevention of autoimmunity by removal of superfluous hematopoietic cells (37), whereas loss of PUMA does not lead to the same buildup of cells during hematopoiesis.(39) PUMA knockout does render T cells resistant to apoptotic stimuli such as γ-irradiation and cytokine deprivation.(38, 39) The above findings may explain why knockdown of Bim more effectively blocks apoptosis in sepsis compared to PUMA. Targeting both Bim and PUMA allows for coverage of the two most potent BH3-only proteins.
Another important finding was the ability of the cyclodextrin polymer-based, transferrin-mediated delivery system to effectively deliver siRNAs to immune effector cells. The results showing decreased GFP fluorescence in total splenocytes including CD4 T cells and B cells (Fig. 4) following in vivo administration of siRNA to GFP are particularly impressive given the well-recognized resistance of lymphocytes to siRNA uptake.(40–43) The decrease in intracellular concentrations of PUMA and Bim protein using their respective siRNAs (Figs. 5 & 6) strongly supports the contention that this delivery system is effective. Furthermore, previous studies have shown that this vehicle has minimal toxicity after repeated doses in non-human primates.(29) The present results demonstrate that siRNA can be delivered in sepsis using this delivery system without noticeable off-target effects - there was no significant difference in pro-inflammatory cytokines between mice receiving either siRNA or the vehicle alone versus septic controls (Fig. 11). Currently, work is being done in our lab using phage display to identify targets on CD4 T cells and dendritic cells that would be more specific to those classes of cells in order to enhance delivery.
The ability to deliver siRNAs to selected classes of immune cells may represent a major advance in infectious disease. Work from numerous groups has shown that cells of the innate and adaptive immune system rapidly die by apoptotic cell death during infection due to myriad of diverse pathogens, including, Gram-negative and Gram-positive bacteria, fungae, and viruses.(3) The death of the cells severely compromises the ability of the host to mount an effective immune response. For example, particular micro-organisms have an early propensity to target host phagocytic cells for death thereby preventing the host from eliminating the invading pathogens.(3) Thus, delivery of anti-apoptotic siRNAs that maintain immune cell viability could result in improved survival and several groups have studies consistent with this hypothesis.(24, 27)
One potential limitation of anti-apoptotic based therapy in sepsis should be noted. Recent evidence indicates that cell death is more complex than originally appreciated. Multiple pathways of cell death may be activated in single dying cells and cross talk between the cell death pathways exists. Thus, blocking apoptosis may in some instances lead to an alternative form of cell death such as necrosis or autophagy.(44) Another potential limitation to siRNA therapy is the duration of response. Before any clinical applications are attempted, the duration of the response to treatment with siRNA will also have to be determined. In vitro, inactive non-dividing cells siRNA treatment can have an effect that lasts up to two weeks, but in rapidly dividing cells this effect is limited by how often the cell divides and may be as short as 24–48 hours in duration.(23)
In summary, the present results demonstrate that sepsis induces multiple triggers of apoptosis that are specific to different classes of immune cells. The safe and effective simultaneous delivery of anti-apoptotic siRNAs to selected classes of immune cells including CD4 T and B cells is possible using a cyclodextrin polymer-based, transferrin targeted delivery system. siRNA-based anti-apoptotic therapy offers great promise in modulating the survival of host immune cells that are critical to the elimination of selected pathogens.
Acknowledgements
This work was supported by NIH grants GM44118, GM55195, and T32 GM008795-08, the Defense Threat Reduction Agency, and by the Alan A. and Edith L. Wolff Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Zeni F, Freeman B, Natanson C. Anti-inflammatory therapies to treat sepsis and septic shock: a reassessment. Crit Care Med. 1997;25(7):1095–1100. doi: 10.1097/00003246-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348(6):138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 4.Meakins JL, Pietsch JB, Bubenick O, Kelly R, Rode H, Gordon J, MacLean LD. Delayed hypersensitivity: indicator of acquired failure of host defenses in sepsis and trauma. Ann Surg. 1977;186(3):241–250. doi: 10.1097/00000658-197709000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lederer JA, Rodrick ML, Mannick JA. The effects of injury on the adaptive immune response. Shock. 1999;11(3):153–159. doi: 10.1097/00024382-199903000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Frazier WJ, Hall MW. Immunoparalysis and adverse outcomes from critical illness. Pediatr Clin North Am. 2008;55(3):647–668. doi: 10.1016/j.pcl.2008.02.009. xi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27(7):1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Hotchkiss RS, Tinsley KW, Swanson PE, Grayson MH, Osborne DF, Wagner TH, Cobb JP, Coopersmith C, Karl IE. Depletion of dendritic cells, but not macrophages, in patients with sepsis. J Immunol. 2002;168(5):2493–2500. doi: 10.4049/jimmunol.168.5.2493. [DOI] [PubMed] [Google Scholar]
- 9.Chung CS, Yang S, Song GY, Lomas J, Wang P, Simms HH, Chaudry IH, Ayala A. Inhibition of Fas signaling prevents hepatic injury and improves organ blood flow during sepsis. Surgery. 2001;130(2):339–345. doi: 10.1067/msy.2001.116540. [DOI] [PubMed] [Google Scholar]
- 10.Chung CS, Song GY, Lomas J, Simms HH, Chaudry IH, Ayala A. Inhibition of Fas/Fas ligand signaling improves septic survival: differential effects on macrophage apoptotic and functional capacity. J Leukoc Biol. 2003;74(2003):344–351. doi: 10.1189/jlb.0102006. [DOI] [PubMed] [Google Scholar]
- 11.Le Tulzo Y, Pangault C, Gacouin A, Guilloux V, Tribut O, Amiot L, Tattevin P, Thomas R, Fauchet R, Drenou B. Early circulating lymphocyte apoptosis in human septic shock is associated with poor outcome. Shock. 2002;18(6):487–494. doi: 10.1097/00024382-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Felmet KA, Hall MW, Clark RS, Jaffe R, Carcillo JA. Prolonged lymphopenia, lymphoid depletion, and hypoprolactinemia in children with nosocomial sepsis and multiple organ failure. J Immunol. 2005;174(6):3765–3772. doi: 10.4049/jimmunol.174.6.3765. [DOI] [PubMed] [Google Scholar]
- 13.Toti P, De Felice C, Occhini R, Schuerfeld K, Stumpo M, Epistolato MC, Vatti R, Buonocore G. Spleen depletion in neonatal sepsis and chorioamnionitis. Am J Clin Pathol. 2004;122(5):765–771. doi: 10.1309/RV6E-9BMC-9954-A2WU. [DOI] [PubMed] [Google Scholar]
- 14.Hotchkiss RS, Chang KC, Swanson PE, Tinsley KW, Hui JJ, Klender P, Xanthoudakis S, Roy S, Black C, Grimm E, Aspiotis R, Han Y, Nicholson DW, Karl IE. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat Immunol. 2000;1(6):496–501. doi: 10.1038/82741. [DOI] [PubMed] [Google Scholar]
- 15.Hotchkiss RS, Swanson PE, Knudson CM, Chang KC, Cobb JP, Osborne DF, Zollner KM, Buchman TG, Korsmeyer SJ, Karl IE. Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J Immunol. 1999;162(7):4148–4156. [PubMed] [Google Scholar]
- 16.Braun JS, Novak R, Herzog KH, Bodner SM, Cleveland JL, Tuomanen EI. Neuroprotection by a caspase inhibitor in acute bacterial meningitis. Nat Med. 1999;5(3):298–302. doi: 10.1038/6514. [DOI] [PubMed] [Google Scholar]
- 17.Chang KC, Unsinger J, Davis CG, Schwulst SJ, Muenzer JT, Strasser A, Hotchkiss RS. Multiple triggers of cell death in sepsis: death receptor and mitochondrial-mediated apoptosis. FASEB J. 2007;21(3):708–719. doi: 10.1096/fj.06-6805com. [DOI] [PubMed] [Google Scholar]
- 18.Roy S, Nicholson DW. Cross-talk in cell death signaling. J Exp Med. 2000;192(8):F21–F25. [PMC free article] [PubMed] [Google Scholar]
- 19.Strasser A. The role of BH3-only proteins in the immune system. Nat Rev Immunol. 2005;5(3):189–200. doi: 10.1038/nri1568. [DOI] [PubMed] [Google Scholar]
- 20.Lavrik I, Golks A, Krammer PH. Death receptor signaling. J Cell Sci. 2005;118(pt 2):265–267. doi: 10.1242/jcs.01610. [DOI] [PubMed] [Google Scholar]
- 21.Thorburn A. Death receptor-induced cell killing. Cell Signal. 2004;16(2):139–144. doi: 10.1016/j.cellsig.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Hotchkiss RS, Osmon SB, Chang KC, Wagner TH, Coopersmith CM, Karl IE. Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. J Immunol. 2005;174(8):5110–5118. doi: 10.4049/jimmunol.174.8.5110. [DOI] [PubMed] [Google Scholar]
- 23.de Fougerolles A, Vornlocher HP, Maraganore J, Lieberman J. Interfering with disease: a progress report on siRNA-based therapeutics. Nat Rev Drug Discov. 2007;6(6):443–453. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwulst SJ, Muenzer JT, Peck-Palmer OM, Chang KC, Davis CG, McDonough JS, Osborne DF, Walton AH, Unsinger J, McDunn JE, Hotchkiss RS. BIM siRNA DECREASES LYMPHOCYTE APOPTOSIS AND IMPROVES SURVIVAL IN SEPSIS. Shock. 2008;30(2):127–134. doi: 10.1097/shk.0b013e318162cf17. [DOI] [PubMed] [Google Scholar]
- 25.Geisbert TW, Hensley LE, Kagan E, Yu EZ, Geisbert JB, Daddario-DiCaprio K, Fritz EA, Jahrling PB, McClintock K, Phelps JR, Lee AC, Judge A, Jeffs LB, MacLachlan I. Postexposure protection of guinea pigs against a lethal ebola virus challenge is conferred by RNA interference. J Infect Dis. 2006;193(12):1650–1657. doi: 10.1086/504267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sorensen DR, Leirdal M, Sioud M. Gene silencing by systemic delivery of synthetic siRNAs in adult mice. J Mol Biol. 2003;327(4):761–766. doi: 10.1016/s0022-2836(03)00181-5. [DOI] [PubMed] [Google Scholar]
- 27.Wesche-Soldato DE, Chung CS, Lomas-Neira J, Doughty LA, Gregory SH, Ayala A. In vivo delivery of caspase-8 or Fas siRNA improves the survival of septic mice. Blood. 2005;106(7):2295–2301. doi: 10.1182/blood-2004-10-4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bumcrot D, Manoharan M, Koteliansky V, Sah DW. RNAi therapeutics: a potential new class of pharmaceutical drugs. Nat Chem Biol. 2006;2(12):711–719. doi: 10.1038/nchembio839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heidel JD, Yu Z, Liu JY, Rele SM, Liang Y, Zeidan RK, Kornbrust DJ, Davis ME. Administration in non-human primates of escalating intravenous doses of targeted nanoparticles containing ribonucleotide reductase subunit M2 siRNA. Proc Natl Acad Sci U S A. 2007;104(14):5715–5721. doi: 10.1073/pnas.0701458104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wichterman KA, Baue AE, Chaudry IH. Sepsis and Septic Shock - a review of laboratory models and a proposal. J Surg Res. 1980;29:189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 31.Wang P, Chaudry IH. A single hit model of sepsis: cecal ligation and puncture. Sepsis. 1988;2:227–233. [Google Scholar]
- 32.Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW, 3rd, Bland KI, Choudry IH. Cecal ligation and puncture. Shock. 2005 Suppl 1:52–57. doi: 10.1097/01.shk.0000191414.94461.7e. [DOI] [PubMed] [Google Scholar]
- 33.Osuchowski MF, Welch K, Siddiqui J, Remick DG. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol. 2006;177(3):1967–1974. doi: 10.4049/jimmunol.177.3.1967. [DOI] [PubMed] [Google Scholar]
- 34.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bandyopadhyay G, De A, Laudanski K, Li F, Lentz C, Bankey P, Miller-Graziano C. Negative signaling contributes to T-cell anergy in trauma patients. Crit Care Med. 2007;35(3):794–801. doi: 10.1097/01.CCM.0000256847.61085.A5. [DOI] [PubMed] [Google Scholar]
- 36.Willis SN, Adams JM. Life in the balance: how BH3-only proteins induce apoptosis. Curr Opin Cell Biol. 2005;17(6):617–625. doi: 10.1016/j.ceb.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, Adams JM, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286(5445):1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 38.Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ, Adams JM, Strasser A. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302(5647):1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 39.Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J, MacLean KH, Han J, Chittenden T, Ihle JN, McKinnon PJ, Cleveland JL, Zambetti GP. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 2003;4(4):321–328. doi: 10.1016/s1535-6108(03)00244-7. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Lu H, LiWang P, Sili U, Templeton NS. Optimization of gene expression in nonactivated circulating lymphocytes. Mol Ther. 2003;8(4):629–636. doi: 10.1016/s1525-0016(03)00231-4. [DOI] [PubMed] [Google Scholar]
- 41.Marodon G, Mouly E, Blair EJ, Frisen C, Lemoine FM, Klatzmann D. Specific transgene expression in human and mouse CD4+ cells using lentiviral vectors with regulatory sequences from the CD4 gene. Blood. 2003;101(9):3416–3423. doi: 10.1182/blood-2002-02-0578. [DOI] [PubMed] [Google Scholar]
- 42.Goffinet C, Keppler OT. Efficient nonviral gene delivery into primary lymphocytes from rats and mice. FASEB J. 2006;20(3):500–502. doi: 10.1096/fj.05-4651fje. [DOI] [PubMed] [Google Scholar]
- 43.Peer D, Zhu P, Carman CV, Lieberman J, Shimaoka M. Selective gene silencing in activated leukocytes by targeting siRNAs to the integrin lymphocyte function-associated antigen-1. Proc Natl Acad Sci U S A. 2007;104(10):4095–4100. doi: 10.1073/pnas.0608491104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73(4):1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]