Abstract
Background
During the menopausal transition, total testosterone remains unchanged while estrogen decreases markedly creating a state of relative androgen excess. We hypothesized that change in the testosterone/estradiol (T/E) ratio during the menopausal transition would be associated with incident metabolic syndrome.
Methods and Results
The association between incident metabolic syndrome and total estradiol, total testosterone, sex hormone binding globulin, the free androgen index (FAI), baseline ratio of total testosterone over total estradiol and the change of this ratio over time was evaluated in a multiethnic cohort of 1,862 pre- and perimenopausal women without diabetes enrolled in the Study of Women’s Health Across the Nation (SWAN). New cases (n=257) of the metabolic syndrome were identified in the cohort during 6296 woman-years of follow-up. The age adjusted total T/E ratio increased 10.1% per year during the 5 years of follow-up. Neither baseline nor change in estradiol were associated with incident metabolic syndrome. Low sex hormone biding globulin, free androgen index and high total testosterone at baseline all increased the risk of metabolic syndrome but their change over time did not. Both baseline total T/E ratio (1.41; 95% CI=1.17-1.1.69; P 0.001) and its rate of change (1.24; 95% CI=1.01-1.52; P 0.04) were associated with increased incident metabolic syndrome independent of ethnicity.
Conclusions
The interaction between testosterone and estradiol during the menopausal transition, rather than the individual change of each over time, is a factor in determination of risk for developing the metabolic syndrome during the menopausal transition. This relationship was independent of ethnicity and other factors associated with prevalent metabolic syndrome prior to the onset of the menopausal transition.
Keywords: Menopause, Testosterone, Estrogen, Metabolic, Syndrome
Introduction
It has long been the belief that the estrogen deficiency during the menopausal transition was responsible for the increased risk of cardiovascular disease seen in postmenopausal women. However, prospective randomized clinical trails have not demonstrated cardiovascular disease protection from estrogen therapyi,ii,iii, calling this long standing hypothesis into question. Less attention has been paid to role of endogenous androgens on cardiovascular risk factors in women despite studies suggesting that androgens may play an important role. High testosterone levels predict insulin resistance and incident type 2 diabetes in postmenopausal womeniv. Increased testosterone and low sex hormone biding globulin (SHBG) levels have also been individually linked to the some of the components of the metabolic syndrome and diabetes in both pre and postmenopausal womenv,vi,vii,viii,ix,x,xi. Low SHBG has been demonstrated to be independently associated with MS in postmenopausal women, after adjustment for BMI, in a recent sample from the Women’s Health Studyxii.
During the menopausal transition testosterone levels remain unchanged while estradiol levels decrease. Thus, the testosterone to estradiol ratio at baseline increases over time creating a state of “relative androgen excess”xiii,xiv,xv. The “relative androgen excess” created during the menopausal transition, may help explain the increasing risk of cardiovascular disease in postmenopausal women.
The metabolic syndrome generates a greater sensitivity for the detection of cardiovascular disease than its individual constituentsxvi,xvii,xviii,xix. We hypothesized that the relative excess of androgens created during the menopausal transition would be associated with an increased risk of developing the metabolic syndrome. We used the prospective data for the first five years of follow-up of the 3,302 premenopausal and early perimenopausal women enrolled in the Study of Women’s Health Across the Nation (SWAN), a multicenter, multiethnic, community-based, longitudinal study of perimenopausal women, to test this hypothesis.
Methods
Study Population
The design, sampling strategy, cohort recruitment and enrollment of SWAN have been described in detail elsewherexx. In brief, participants were enrolled at seven clinical sites. Recruitment techniques were designed to generate a community-based sample of women at each of the seven sites. All seven sites enrolled Caucasian women, and each site also enrolled women belonging to one pre-specified minority ethnic group. African-American women were enrolled in Boston, Chicago, the Detroit area, and Pittsburgh. Japanese, Chinese, and Hispanic women were enrolled in Los Angeles, Oakland California, and Hudson County New Jersey, respectively. Eligibility criteria for entry into the SWAN longitudinal cohort were as follows: age between 42–52; the presence of a uterus and at least one ovary; no current use of estrogens or other medications known to affect ovarian function; at least one menstrual period in the 3 months before screening; and self-identification as a member of one of the five eligible ethnic groups. The Institutional Review Board at each SWAN center approved the study. All women provided written informed consent prior to participation in the study.
A total of 3,302 women were enrolled. At baseline, the cohort consisted of 1550 Caucasians, 935 African-Americans, 281 Japanese, 250 Chinese, and 286 Hispanics at baseline. Women were followed yearly. The data reported here represent the analysis of the first 5 years of follow-up. From the full SWAN cohort a total of 1,862 women were used for these analyses and 1,440 women were excluded from the analysis for the presence of the following confounders: presence of the metabolic syndrome or diabetes at entry (N=844), abnormal TSH (0.1 – 9.99 uIU/ml; N=65) or abnormal testosterone (> 100 ng/ml; N=73) at any single visit, or use of hormone therapy or surgical menopause (N=239). Remaining exclusions were for missing or incomplete follow-up data (n=219).
Questionnaires and Physical Measurements
Demographic information, history of hypertension or diabetes, smoking status, perceived stress, physical activity, race/origin, and menopausal status were obtained using standardized questionnaires. Primary race/ethnicity was self-defined as one of the five groups noted above. Nutrient intake for total fat consumption, percent of total calories consumed from fat per day were assessed using the NCI (Block) food frequency questionnaire (FFQ), which was enhanced to accommodate the ethnic diversity in SWANxxi. Information on alcohol consumption was derived from the FFQ and categorized based on amount of alcohol consumptionxxii. Standardized protocols were used to measure height, weight, waist circumference, and blood pressure.
Biochemical Measurements and Calculation of Relative Androgen Excess
Biochemical analyses were done on the samples obtained at baseline and years 1, 3, 5 of follow-up. Blood was drawn on days 2-5 of the follicular phase after an 8 hour fast. Lipids and glucose were assayed at the Medical Research Laboratories, (Lexington, Kentucky, USA) which is certified by the National Heart Lung and Blood Institute, Centers for Disease Control Part III program. Lipid and lipoprotein fractions were analyzed on EDTA treated plasma. Total cholesterol and triglycerides were analyzed by enzymatic methods. HDL-C was isolated using heparin-2M manganese chloride. Serum insulin was measured using the RIA (DPC Coat-a-count, Los Angeles, CA) procedure and monitored monthly as part of the quality assurance program by the Diabetes Diagnostic Laboratory at the University of Missouri. Glucose was measured using a hexokinase-coupled reaction (Boehringer Mannheim Diagnostics, Indianapolis, IN).
Hormone assays were conducted at the University of Michigan SWAN Endocrine Laboratory using the ACS-180 automated analyzer (Bayer Diagnostics Corp, Norwood, MA). Sex hormone binding globulin (SHBG) was measured using a newly developed, two-site chemiluminescent assay with a lower limit of detection was 2 Nm; intra and interassay CV’s were 9.9% and 6.1%, respectively. The estradiol (E2) assay modifies the rabbit anti-E2-6 ACS-180 immunoassay to increase sensitivity, with a lower limit of detection (LLD) of 1.0 pg/mL; intra and interassay CV’s were 10.6% and 6.4%, respectively. The testosterone (T) assay modifies the rabbit polyclonal anti-T ACS-180 immunoassay, with a LLD of 2.19 ng/dL; intra and interassay CV’s were 10.5% and 8.5%, respectively.
Calculation of Free Androgen Index, T/E2 molar ratio and Relative Androgen Excess
The free androgen index (FAI) was used to estimate of the amount of free T, as it correlates well with the free T level as measured by equilibrium dialysis (FAI = 100*T(ng/ml)/28.84*SHBG(nM))xxiii. The Free Estrogen Index (FEI) was calculated as 100*E2(pg/ml)/272.11*SHBG(nM). To quantify the amount of T relative to E2, the molar ratio of log(FAI/FEI) was used. The log(FAI/FEI) is equivalent to the molar ratio of log(T/E2) as SHBG is represented in both the numerator and denominator and thus cancels out of the equation. Relative androgen excess is defined as the difference between the baseline T/E2 and each follow-up visit.
Definition of the Metabolic Syndrome
Currently there is no uniform well-accepted definition for the metabolic syndrome. We used the American Heart Assocation/National Heart Blood and Lung Institutes definition for the metabolic syndrome because the ethnic diversity of our study population included Asians; a group at increased risk of diabetes hypertension and dyslipidemia at lower overall adiposityxxiv,xxv,xxvi,xxvii. Any subject with any 3 of the 5 criteria was considered to have the metabolic syndrome: a) waist circumference ≥ 88 ( ≥ 80 for Asians), b) triglycerides ≥ 150 md/dl, c) HDL < 50 mg/dl, d) elevated blood pressure ≥ 130 mmHg systolic or ≥ 85 mm Hg diastolic or on antihypertensive drug treatment, e) fasting blood glucose ≥ 100 mg/dl.
Statistical methods
Ethnic differences in baseline characteristics were tested using standard Chi-squared or one-way ANOVA’s. Where necessary, variables were log-transformed. In order to asses how hormones changed over time, the unadjusted means of T, E2, T/E2 and RAE were plotted against time. In addition, change over time was estimated using mixed models, with each hormone modeled as a simple function of time, adjusting for baseline age. MS incidence rates were calculated as the total number of cases divided by the total number of person-years of follow-up. A univariate complimentary log-log model was used to test for ethnic differences in MS incidence.
The association between incident MS and hormone levels was modeled using a discrete-time proportional hazards model, i.e., a complementary log-log modelxxviii,xxix. A discrete-time model instead of a Cox analysis has been used since our identification of incident MS cases occurs only at several discrete times (i.e. the 01, 03 and 05 visits), which is known as grouped or interval-censored data. In each model, incident MS was modeled as a function of baseline (log-transformed) hormone and average annual change in hormone levels between baseline and each follow-up visit (on a log scale). All models were adjusted for ethnicity, clinical site, age at baseline, a time interval indicator, and ‘phlebotomy in window’ (whether phlebotomy at each visit was in days 2-5 of the menstrual cycle).
The ‘residual BMI’, a term designed to capture departure from expected change in BMI, was added into the models to correct for difference in weight over time as waist circumference is included in the definition of the metabolic syndrome. This latter variable was calculated as the error term from a simple model of each follow-up BMI as a function of baseline BMI. All other covariates, including physical activity, level of education, alcohol consumption, smoking, total fat consumption, percent of total calories consumed from fat per day, income and perceived stress, were taken from the baseline visit and included in final models only where statistical significance was achieved. Analysis was carried out using SAS Version 8. Model fit was verified using the Hosmer and Lemeshow goodness-of-fit testxxx.
The primary reason for participant drop-out was the initiation of hormone therapy. The validity of all models was assessed by investigating the impact of hormone therapy users on model results.
Results
Baseline characteristics of the final analytic sample are shown in Table 1. The distributions of all variables differed very significantly across ethnic groups (p<0.01 to p<0.001) except for marginally significant between ethnic differences for SHBG (p=0.04) and FAI (p=0.06). The T/E2 molar ratio did not differ significantly by ethnic group. Overall, Hispanics were considerably more likely to be less well educated. Chinese and Japanese women were more likely to be pre-menopausal at baseline. Chinese women were also considerably less likely to smoke, drink alcohol and consumed lower % of KCAL from fat while Caucasians were more likely to be physically active. The components of the metabolic syndrome, E2, T differed across ethnic groups. No differences were seen in SHBG, FAI and T/E2 molar ratio across ethnic groups.
Table 1.
Baseline characteristics of the study sample by ethnicity
| Variable | Full Sample N=1862 |
Caucasian N=911 |
Afr. American N=450 |
Hispanic N=113 |
Chinese N=186 |
Japanese N=202 |
P-value for Ethnic Differences |
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Age * | 46.0 (4.13) | 45.7 (4.07) | 45.8 (4.09) | 46.3 (3.53) | 46.5 (3.88) | 46.9 (4.19) | 0.009 |
| Education | <0.001 | ||||||
| ≤ High School | 21% | 12% | 25% | 71% | 27% | 18% | |
| > High School | 32% | 31% | 40% | 21% | 21% | 34% | |
| ≥College | 47% | 57% | 35% | 8% | 52% | 48% | |
| Menopause Status | 0.008 | ||||||
| Early perimenopause | 43% | 45% | 49% | 39% | 34% | 35% | |
| Premenopause | 57% | 55% | 51% | 61% | 66% | 65% | |
| Tobacco use | <0.001 | ||||||
| Never | 61% | 55% | 57% | 70% | 95% | 64% | |
| Previous | 25% | 33% | 21% | 18% | 4% | 23% | |
| Current | 14% | 12% | 22% | 13% | 1% | 13% | |
| Alcohol consumption | <0.001 | ||||||
| None | 48% | 36% | 56% | 45% | 81% | 55% | |
| < median (31.2 Kcal) | 26% | 29% | 23% | 39% | 14% | 21% | |
| > median | 26% | 35% | 21% | 16% | 5% | 24% | |
| Physical activity score * | 7.75 (2.45) | 8.30 (2.40) | 7.35 (2.20) | 6.70 (1.93) | 7.25 (2.40) | 7.75 (2.05) | <0.001 |
| Perceived stress * | 8.00 (4.00) | 8.00 (4.00) | 8.00 (5.00) | 10.0 (5.00) | 8.00 (4.00) | 9.00 (3.00) | <0.001 |
| Dietary Variables | |||||||
| % KCAL from fat /day * | 32.5 (9.74) | 33.0 (9.73) | 34.0 (9.25) | 33.0 (9.95) | 28.7 (9.71) | 29.9 (9.01) | <0.001 |
| Dietary fat, grams/day * | 58.6 (36.3) | 58.4 (34.3) | 65.4 (48.2) | 57.3 (32.5) | 52.4 (25.6) | 57.8 (29.9) | <0.001 |
| Physical Measurements | |||||||
|
Waist circumference
(cm) * |
78.0 (15.2) | 78.0 (15.2) | 84.0 (17.7) | 82.0 (13.0) | 74.3 (8.5) | 70.5 (9.3) | <0.001 |
|
Systolic blood pressure
(mmHg) * |
111.0 (17.0) | 110.0(16.0) | 118.0 (22.0) | 119.0(14.0) | 108.0 (19.0) | 109.0(17.0) | <0.001 |
|
Diastolic blood pressure
(mmHg) * |
73.0 (12.8) | 72.0 (12.0) | 74.0 (15.0) | 80.0 (3.0) | 71.0 (14.0) | 73.0 (11.0) | <0.001 |
| Biochemical Measurements | |||||||
| Blood drawn | <.0001 | ||||||
| In cycle day 2-5 | 81% | 83% | 78% | 82% | 74% | 86% | |
| Not in 2-5/unknown | 19% | 17% | 22% | 18% | 26% | 14% | |
| Triglyceride (mg/dL) * | 81.0 (43.0) | 82.0 (42.0) | 74.0 (37.0) | 92.0 (50.0) | 85.0 (44.0) | 83.0 (48.0) | <0.001 |
| HDL (mg/dL) * | 58.0 (16.0) | 58.0 (16.0) | 57.0 (16.0) | 52.5 (12.0) | 61.0 (17.0) | 62.5 (16.5) | <0.001 |
| Glucose (mg/dL) * | 89.0 (9.0) | 88.0 (9.0) | 90.0 (10.0) | 87.0 (11.0) | 91.0 (8.0) | 90.0 (8.0) | <0.001 |
| Estradiol (pg/mL) * | 59.7 (57.2) | 60.2 (54.6) | 61.7 (60.3) | 66.5 (79.9) | 53.6 (53.8) | 54.9 (53.5) | 0.009 |
| Testosterone (ng/dL) * | 39.5 (22.9) | 40.9 (23.6) | 39.0 (21.1) | 35.2 (25.0) | 38.9 (21.3) | 37.5 (22.5) | 0.005 |
| SHBG (nM) * | 44.3 (29.8) | 43.8 (29.6) | 44.9 (27.9) | 46.3 (27.4) | 41.9 (26.9) | 47.7 (38.6) | 0.04 |
| Free Androgen Index * | 3.13 (3.06) | 3.19 (3.12) | 3.03 (2.75) | 3.13 (3.49) | 3.33 (3.16) | 2.88 (2.92) | 0.06 |
|
Testosterone/Estradiol
Molar Ratio * |
6.08 (7.49) | 6.11 (7.28) | 5.65 (7.80) | 5.62 (9.17) | 6.62 (7.45) | 6.23 (7.52) | 0.25 |
- Median (IQRange)
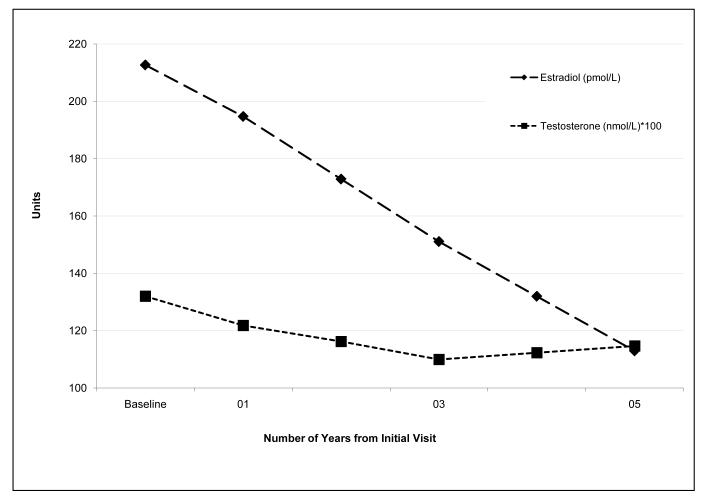
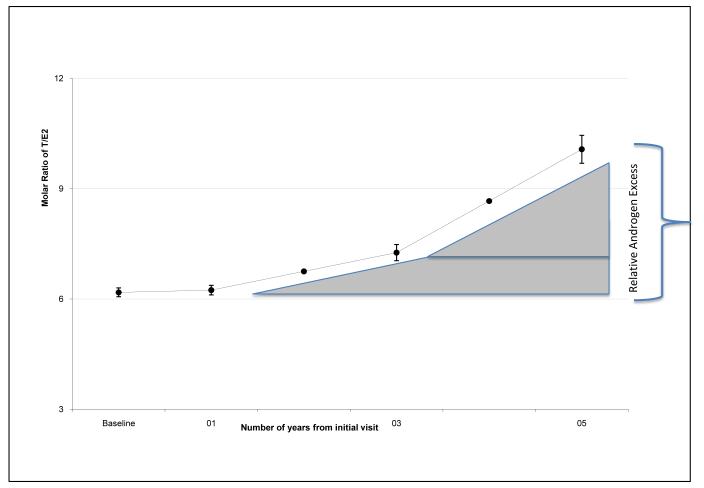
During the follow-up period, the age-adjusted estradiol levels decreased by an estimated 12.0% each year (P<0.0001), while the average annual age-adjusted decrease in total testosterone was much less (3.1% per year, P<0.0001, Figure 1). At baseline, the unadjusted median T/E2 molar ratio ranged from 5.62 in Hispanics to 6.62 in Chinese women (table 1). The age adjusted T/E2 molar ratio increased 10.1% per year during the 5 years of follow-up (P<0.001) creating a state of androgen excess relative to the baseline T/E2 values (Figure 2).
Figure 1.
The changing relationship of estradiol to testosterone as the menopausal transition progresses. Estradiol is shown in pmol/L and testosterone in pmol/L × 100 with units on the Y axis depicting hormone concentration. The X axis depicts time since initial baseline visit in years. Note the large decline in estradiol and the modest decline in testosterone over time.
Figure 2.
Change in the molar ratio of testosterone to estradiol as the menopausal transition progresses. The Yaxis shows the Ratio, and the X axis is identical to Figure 1. The shaded triangle indicates the increase in the testosterone to estradiol ratio, or the relative androgen excess, over the indicated period of time.
There were 257 new cases of the metabolic syndrome during 6296 person-years of follow-up. Hispanic had a greater incidence rate than Caucasians (8.81 vs. 4.12 per 100 person years) while Chinese women had lower rates than Caucasians (2.34 vs. 4.12 per 100 person years) (Table 2).
Table 2.
Incident metabolic syndrome reported as number of cases per 100 person years
| Race/Ethnicity | Total Observations in Person years |
# Cases | % Incidence Rate (95% CI) |
|---|---|---|---|
| Caucasian | 2989 | 123 | 4.12 (3.40 - 4.83) |
| African American | 1482 | 67 | 4.52 (3.46 - 5.58) |
| Hispanic | 329 | 29 | 8.81 (5.75 - 11.88) |
| Chinese | 728 | 17 | 2.34 (1.24 - 3.43) |
| Japanese | 768 | 21 | 2.73 (1.58 - 3.89) |
|
| |||
| Full Sample | 6296 | 257 | 4.08 (3.59 - 4.57) |
Note: The incidence rate is significantly different across ethnic groups, P<0.001. Chinese and Hispanic women are significantly different from Caucasian women at α=0.05 level.
Individual models were created to asses the association of E2 (model 1), T (model 2), SHBG (model 3), FAI (model 4) and the T/E2 molar ratio, RAE (model 5) with the incidence of the metabolic syndrome (Table 3). Neither the baseline E2 or its rate of change were associated with incidence (model 1). Although higher baseline T, FAI, and lower SHBG levels were associated with higher incidence, the models for rates of change over time were not statistically significantly (models 2-4). Unlike FAI and SHBG, both a higher baseline T/E2 molar ratio and change over time in the RAE were associated with increased risk of developing MS (model 5).
Table 3.
Results from multivariate models. Each row represents a separate multivariable model.†
| Models | Baseline hormone | Change in hormone from baseline | ||
|---|---|---|---|---|
| Risk Ratio (95% CI) | P Value | Risk Ratio (95% CI) | P Value | |
| Model 1: E2 | 0.85 (0.69 - 1.05) | 0.13 | 0.90 (0.73 - 1.11) | 0.32 |
| Model 2: T | 2.04 (1.45 - 2.86) | <0.001 | 1.28 (0.81 - 2.02) | 0.29 |
| Model 3: SHBG | 0.58 (0.48 - 0.70) | <0.001 | 0.80 (0.58 - 1.10) | 0.20 |
| Model4: FAI | 1.77 (1.50 - 2.09) | <0.001 | 1.22 (0.93 - 1.60) | 0.16 |
| Model 5: RAE | 1.41 (1.17 - 1.69) ‡ | 0.001 | 1.24 (1.01 - 1.52) | 0.04 |
All models were adjusted for ethnicity, age at baseline, residual BMI, ‘phlebotomy in window’, site, physical activity, level of education.
Relative Androgen Excess baseline value is the T/E2 molar ratio at study entry.
The associations between risk of MS and either baseline T/E2 molar ratio and RAE did not vary significantly by ethnicity, with coefficients similar in magnitude and direction across all ethnic groups. Interaction terms between ethnicity and baseline hormone levels, ethnicity and change in hormone levels, and baseline hormone and change in hormone were not found to be significant.
To estimate for potential changes in risk associated with hormone therapy use, each model was rerun including hormone therapy users and adjusted for hormone therapy use via an indicator variable. The model coefficients for each hormone and its change were unchanged in direction and significance level when the indicator variable was placed in the model. The indicator variable for hormone therapy use was not significant, suggesting that hormone therapy use was not associated with risk of MS.
Discussion
Higher baseline testosterone/estradiol ratio and change over time in the relative androgen excess were associated with increased risk of developing the metabolic syndrome during the menopausal transition. Neither baseline estradiol nor its decline over time was associated with increased overall risk. While the baseline testosterone, FAI, and SHBG all appeared to be associated with an increased risk of developing metabolic syndrome, their change over time was not. These findings suggest that there is a complex interaction between testosterone and estradiol, rather than simply changes in testosterone, free androgen index and SHBG, determines a woman’s risk for developing the metabolic syndrome during the menopausal transition.
Our results highlight that estrogen deficiency by itself is not as important as the relationship between testosterone and estradiol during the menopausal transition. This finding is supported by primary and secondary prevention randomized, clinical trials of postmenopausal women that do not demonstrate a benefit of exogenous estradiol1-3. Observational cohort studies have demonstrated that increased endogenous estradiol in the postmenopause is not associated with reduced cardiovascular events4.
Endogenous and exogenous testosterone influence a woman’s cardiovascular risk factors. Unbound circulating testosterone best reflects a woman’s overall androgen statusxxxi. High free androgen index levels have been associated with a more atherogenic lipid profile independent of metabolic factors and health behaviors5. High free androgen index has been associated with the metabolic syndrome in postmenopausal women6. Administration of testosterone to normal females and female transsexuals leads to insulin resistance as measured by an insulin clampxxxii,xxxiii. Such doses are typically supraphysiological for women, and at lower doses of testosterone, carbohydrate metabolism has been reported to be unaffected in two short term studies of over 1,000 women, although specific measures were not shown and long term data are not available.xxxiv,xxxv We have previously demonstrated that high free androgen index and low SHBG were strongly and consistently related to adverse cardiovascular risk factors during cross sectional analysis of the SWAN cohort. Even after adjusting for body mass index, free androgen index was most strongly associated with PAI-1, tPA, triglycerides and glucose while SHBG was most strongly associated with PAI-1, triglycerides and HDLxxxvi,xxxvii. The present findings extend the logic of examining androgens in women in a longitudinal fashion, as they appear to be strongly associated with adverse intermediate outcomes such as metabolic syndrome.
The Prospective Cardiovascular Munster Program was used to estimate the risk of an acute coronary events in the Melbourne Women’s Midlife Health Project, a cohort of 438 Australian midline women were followed through the menopausal transitionxxxviii. In this study, lower than average estradiol levels, estradiol levels that decreased, and high free testosterone levels were associated with increased risk of a coronary event. While our study supports the increased risk seen with free androgen index, neither our study nor other previous studies support their findings as it pertains to decreasing estrogen levels4-6.
During our prospective analysis, both the free androgen index and SHBG at baseline were associated with an increased risk for developing the metabolic syndrome, but their change over time was not. This suggests that the baseline values in free androgen index and SHBG predict a woman’s overall risk independent of the change over time in these variables. This is in agreement with previously published data from SWAN, in which baseline characteristics of the women were associated with the presence of the metabolic syndrome. In this study, SHBG and FAI remained independently associated with metabolic syndrome at baseline after adjusting for other factors such as age and waist circumference. Thus, although circulating free androgens and SHBG may predict a womans’s risk before she enters the menopausal transition, the change in these two factors over time does not explain why a woman’s risk of developing the metabolic syndrome increases during the menopausal transition. In our cohort, the testosterone/estradiol molar ratio increased over time creating a state of relative androgen excess during the menopausal transition (Figure 2). Both the baseline testosterone/estradiol molar ratio and the change over time in the relative androgen excess were associated with increased risk of developing the metabolic syndrome after correcting for multiple confounding variables. The associated risk seen with relative androgen excess was independent of ethnicity notwithstanding the ethnic differences in the baseline values of E, T and incidence of MS across the ethnic groups suggesting that ethnicity may predict an individual woman’s overall riskxxxix, but not the manner in which her risk changes during the transition.
Thus, not only does the baseline testosterone/estradiol molar ratio and its change over time suggests that the complex interplay between both of testosterone and estradiol during the menopausal transition may be responsible for increasing the risk of MS. Physiological interplay between androgens and estrogens in various endocrine targets is well established typically having opposing actions38. Androgen and estrogen responses occur at the promoter regions of selected genes independently through distinct response elements. This androgen estrogen interplay may reflect the combined effects of sex steroid specific regulated gene expression, each hormone may affect the expression of the other’s receptor, or each receptor may interact with each other39-42.
A potential mechanism that could explain our findings are the effects of androgens on inulin resistance. Exogenous administration of androgens to women has been demonstrated to increase insulin resistance31,32. As SHBG levels are suppressed by hyperinsulinemia, the relative androgen excess during the menopausal transition could lead to an insulin resistant state that subsequently further suppresses levels of SHBG causing higher levels of free testosterone. As low SHBG levels have been previously associated with an increased risk of a variety of cardiovascular risk factors and incident cardiovascular disease in post-menopausal women independent of insulin, obesity and dyslipidemia, further study is needed to assess if this proposed mechanism is valid43,44.
Our study has limitations. A small number of women in our cohort may have developed polycystic ovarian syndrome (PCOS) during follow-up. However, in the SWAN cohort, women with PCOS were largely excluded at entry as 87% of SWAN participants reported an intermenstrual interval of 21-35 days at baseline and the eligibility requirement of at least one menstrual period within the past 3 months. We also excluded from the analyses women with the metabolic syndrome and diabetes at entry, further decreasing the presence of women who may have had PCOS in this analysis. It must also be kept in mind that our blood sampling is restricted to the early follicular phase of the cycle. During the early stages of the menopausal transition, estradiol and, to a lesser extent, testosterone, are secreted in varying quantities over the menstrual cycle and thus this single assessment may not be reflective of the entire hormone exposure.
Our results imply that the complex interplay between testosterone and estradiol has been a relatively overlooked factor as a cause of morbidity in women at mid-life. The current lack of evidence that exogenous estrogen decreases cardiovascular morbidity and mortality in postmenopausal women argues that there is not a simple, single hormone that influences cardiovascular disease risk in peri and postmenopausal women. An understanding of how changes in the testosterone over estradiol molar ratio influence the ontogeny of the metabolic syndrome and insulin resistance may be important for the development of new cardiovascular disease prevention strategies. Moreover, the recent enthusiasm for the use of testosterone as a form of hormonal replacement for postmenopausal women30,45 will need to take into account the possible introduction of an adverse metabolic profile in at least some women, prior to its widespread initiation.
Acknowledgments
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health, DHHS, through the National Institute on Aging, the National Institute of Nursing Research and the NIH Office of Research on Women’s Health (Grants NR004061, AG017104, AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495).
Clinical Centers: University of Michigan, Ann Arbor - MaryFran Sowers, PI; Massachusetts General Hospital, Boston, MA - Robert Neer, PI 1994 - 1999; Joel Finkelstein, PI 1999-present; Rush University, Rush University Medical Center, Chicago, IL - Lynda Powell, PI; University of California, Davis/Kaiser - Ellen Gold, PI; University of California, Los Angeles - Gail Greendale, PI; University of Medicine and Dentistry - New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; Nanette Santoro, PI 2004 – present; and the University of Pittsburgh, Pittsburgh, PA - Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD - Marcia Ory 1994 – 2001; Sherry Sherman 1994 – present; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor - Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001; University of Pittsburgh, Pittsburgh, PA – Kim Sutton-Tyrrell, PI 2001 – present.
We thank the study staff at each site and all the women who participated in SWAN.
J.I.T. was supported by Grant HD-01457 as a BIRCWH Scholar, NS was supported by HD041978.
References
- i.Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, Hsia J, Hulley S, Herd A, Khan S, Newby LK, Waters D, Vittinghoff E, Wenger N, HERS Research Group Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) JAMA. 2002;288:49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]; JAMA. 2002;288:1064. Erratum in. [Google Scholar]
- ii.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J, Writing Group for the Women’s Health Initiative Investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- iii.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O’Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S, Women’s Health Initiative Steering Committee Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- iv.Oh JY, Barrett-Connor E, Wedick NM, Wingard DL, Rancho Bernardo Study Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo study. Diabetes Care. 2002;25:55–60. doi: 10.2337/diacare.25.1.55. [DOI] [PubMed] [Google Scholar]
- v.Haffner SM, Valdez RA. Endogenous sex hormones: impact on lipids, lipoproteins, and insulin. American Journal of Medicine. 1995;98:40S–47S. doi: 10.1016/s0002-9343(99)80058-8. [DOI] [PubMed] [Google Scholar]
- vi.Pugeat M, Moulin P, Cousin P, et al. Interrelations between sex hormone-binding globulin (SHBG), plasma lipoproteins and cardiovascular risk. Journal of Steroid Biochemistry & Molecular Biology. 1995;53:567–572. doi: 10.1016/0960-0760(95)00102-6. [DOI] [PubMed] [Google Scholar]
- vii.Haffner SM, Valdez RA, Morales PA, et al. Decreased sex hormone-binding globulin predicts noninsulin-dependent diabetes mellitus in women but not in men. Journal of Clinical Endocrinology & Metabolism. 1993;77:56–60. doi: 10.1210/jcem.77.1.8325960. [DOI] [PubMed] [Google Scholar]
- viii.Goodman-Gruen D, Barrett-Connor E. Sex hormone-binding globulin and glucose tolerance in postmenopausal women. The Rancho Bernardo Study. Diabetes Care. 1997;20:645–649. doi: 10.2337/diacare.20.4.645. [DOI] [PubMed] [Google Scholar]
- ix.Mudali S, Dobs AS, Ding J, Cauley JA, Szklo M, Golden SH. Endogenous postmenopausal hormones and serum lipids: the atherosclerosis risk in communities study. J Clin Endocrinol Metab. 2005;90:1202–9. doi: 10.1210/jc.2004-0744. [DOI] [PubMed] [Google Scholar]
- x.Golden SH, Ding J, Szklo M, Schmidt MI, Duncan BB, Dobs A. Glucose and insulin components of the metabolic syndrome are associated with hyperandrogenism in postmenopausal women: the atherosclerosis risk in communities study. Am J Epidemiol. 2004;160:540–8. doi: 10.1093/aje/kwh250. [DOI] [PubMed] [Google Scholar]
- xi.Rexrode KM, Manson JE, Lee IM, Ridker PM, Sluss PM, Cook NR, Buring JE. Sex hormone levels and risk of cardiovascular events in postmenopausal women. Circulation. 2003;108:1688–93. doi: 10.1161/01.CIR.0000091114.36254.F3. [DOI] [PubMed] [Google Scholar]
- xii.Weinberg ME, Manson JE, Buring JE, Cook NR, Seely EW, Ridker PM, Rexrode KM. Low sex-hormone binding globulin is associated with the metabolic syndrome in postmenopausal women. Metabolism. 2006;55:1473–80. doi: 10.1016/j.metabol.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xiii.Burger HG, Dudley EC, Cui J, Dennerstein L, Hopper JL. A prospective longitudinal study of serum testosterone, dehydroepiandrosterone sulfate, and sex hormone-binding globulin levels through the menopause transition. J Clin Endocrinol Metab. 2000;85:2832–8. doi: 10.1210/jcem.85.8.6740. [DOI] [PubMed] [Google Scholar]
- xiv.Longcope C, Franz C, Morello C, Baker R, Johnston CC., Jr Steroid and gonadotropin levels in women during the peri-menopausal years. Maturitas. 1986;8:189–196. doi: 10.1016/0378-5122(86)90025-3. [DOI] [PubMed] [Google Scholar]
- xv.Rannevik G, Jeppsson S, Johnell O, Bjerre B, Laurell-Borulf Y, Svanberg L. A longitudinal study of the perimenopausal transition: altered profiles of steroid and pituitary hormones, SHBG and bone mineral density. Maturitas. 1995;2:103–113. doi: 10.1016/0378-5122(94)00869-9. [DOI] [PubMed] [Google Scholar]
- xvi.Malik S, Wong ND, Franklin SS, Kamath TV, L’Italien GJ, Pio JR, Williams GR. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245–50. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- xvii.Lempiainen P, Mykkanen L, Pyorala K, Laakso M, Kuusisto J. Insulin resistance syndrome predicts coronary heart disease events in elderly nondiabetic men. Circulation. 1999;100:123–8. doi: 10.1161/01.cir.100.2.123. [DOI] [PubMed] [Google Scholar]
- xviii.Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, Taskinen MR, Groop L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–9. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- xix.Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Bonadonna RC, Muggeo M, Bruneck study Carotid atherosclerosis and coronary heart disease in the metabolic syndrome: prospective data from the Bruneck study. Diabetes Care. 2003;26:1251–7. doi: 10.2337/diacare.26.4.1251. [DOI] [PubMed] [Google Scholar]
- xx.Sowers MF, Crawford SL, Sternfeld B, Morgenstein D, Gold EB, Greendale GA, Evans D, Neer R, Matthews K, Sherman S, Lo A, Weiss G, Kelsey J. SWAN: a multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, editors. Menopause: biology and pathobiology. Academic Press; San Diego: 2000. pp. 175–188. [Google Scholar]
- xxi.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–69. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- xxii.Huang MH, Harrison GG, Mohamed MM, Gornbein JA, Henning SM, Go VL, Greendale GA. Assessing the accuracy of a food frequency questionnaire for estimating usual intake of phytoestrogens. Nutr Cancer. 2000;37:145–54. doi: 10.1207/S15327914NC372_5. [DOI] [PubMed] [Google Scholar]
- xxiii.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–72. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- xxiv.WHO, International Obesity Task Force, International Association for the Study of Obesity . The Asian-Pacific perspective: redefining obesity and its treatment. Health Communications; Australia: 2000. [Google Scholar]
- xxv.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F, American Heart Association. National Heart, Lung, and Blood Institute Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005 Oct 25;112(17):2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- xxvi.Deurenberg-Yap M, Yan TB, Kai CS, Deurenberg P, Van Staveren WA. Manifestation of cardiovascular risk factors at low levels of body mass index and waist-to-hip ratio in Singaporean Chinese. Asia Pacific Journal of Clinical Nutrition. 1999;80:323–330. doi: 10.1046/j.1440-6047.1999.00091.x. [DOI] [PubMed] [Google Scholar]
- xxvii.Ko GT, Chan JC, Cockram CS, Woo J. Prediction of hypertension, diabetes, dyslipidaemia or albuminuria using simple anthropometric indexes in Hong Kong Chinese. Int J Obes Relat Metab Disord. 1999;23:1136–1142. doi: 10.1038/sj.ijo.0801043. [DOI] [PubMed] [Google Scholar]
- xxviii.Prentice RL, Gloeckler A. Regression analysis of grouped survival data with application to breast cancer data. Biometrics. 1978;34:57–67. [PubMed] [Google Scholar]
- xxix.Paul D. Allison “Survival Analysis using the SAS System: A practical guide”. SAS Institute Inc.; Cary, NC: 1995. [Google Scholar]
- xxx.Hosmer DW, Lemeshow S. Logistic Regression. John Wiley & Sons, Inc.; New York: 1989. Applied. [Google Scholar]
- xxxi.Braunstein GD. Androgen insufficiency in women: summary of critical issues. Fertil Steril. 2002;77(Suppl 4):S94–9. doi: 10.1016/s0015-0282(02)02962-x. [DOI] [PubMed] [Google Scholar]
- xxxii.Polderman KH, Gooren LJ, Asscheman H, Bakker A, Heine RJ. Induction of insulin resistance by androgens and estrogens. J Clin Endocrinol Metab. 1994;79:265–71. doi: 10.1210/jcem.79.1.8027240. [DOI] [PubMed] [Google Scholar]
- xxxiii.Diamond MP, Grainger D, Diamond MC, Sherwin RS, Defronzo RA. Effects of methyltestosterone on insulin secretion and sensitivity in women. J Clin Endocrinol Metab. 1998;83:4420–5. doi: 10.1210/jcem.83.12.5333. [DOI] [PubMed] [Google Scholar]
- xxxiv.Buster JE, Kingsberg SA, Aguiree O, Brown C, Breaux JG, Buch A, Rodenberg CA, Wekselman K, Casson P. Testosterone patch for low sexual desire in surgically menopausal women: a randomized trial. Obstet Gynecol. 2005;105:944–52. doi: 10.1097/01.AOG.0000158103.27672.0d. [DOI] [PubMed] [Google Scholar]
- xxxv.Simon J, Braunstein G, Nachtigall L, Utian W, Katz M, Miller S, Waldbaum A, Couchard C, Derzko C, Buch A, Rodenberg C, Lucas J, Davis S. Testosterone patch increases sexual activity and desire in surgically menopausal women with hypoactive sexual desire disorder. J Clin Endocrinol Metab. 2005;90:5226–5233. doi: 10.1210/jc.2004-1747. [DOI] [PubMed] [Google Scholar]
- xxxvi.Sutton-Tyrrell K, Wildman RP, Matthews KA, Chae C, Lasley BL, Brockwell S, Pasternak RC, Lloyd-Jones D, Sowers MF, Torréns JI for the SWAN Investigators Sex Hormone Binding Globulin and the Free Androgen Index are Related to CV Risk Factors in Multi-Ethnic Pre and Peri-Menopausal Women Enrolled in The Study of Women’s Health Across the Nation (SWAN) Circulation. 2005;111:1242–9. doi: 10.1161/01.CIR.0000157697.54255.CE. [DOI] [PubMed] [Google Scholar]
- xxxvii.Sowers M, Derby C, Jannausch ML, Torrens JI, Pasternak R. Insulin resistance, hemostatic factors, and hormone interactions in pre- and perimenopausal women: SWAN. J Clin Endocrinol Metab. 2003;88:4904–10. doi: 10.1210/jc.2003-030350. [DOI] [PubMed] [Google Scholar]
- xxxviii.Guthrie JR, Taffe JR, Lehert P, Burger HG, Dennerstein L. Association between hormonal changes at menopause and the risk of a coronary event: a longitudinal study. Menopause. 2004;11:315–22. doi: 10.1097/01.gme.0000094208.15096.62. [DOI] [PubMed] [Google Scholar]
- xxxix.Bild DE, Detrano R, Peterson D, Guerci A, Liu K, Shalar E, Ouyang P, Jackson S, Saad MF. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2005;111:1313–20. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 38.Krietmann B, Bayard F. Androgen interaction with oestrogen receptor in human tissues. J. Steroid Biochem. 1979;11:1589–1595. doi: 10.1016/0022-4731(79)90354-6. [DOI] [PubMed] [Google Scholar]
- 39.Poulin R, Simard J, Labrie C, Petitclerc L, Dumont M, Lagace L, Labrie F. Down-regulation of estrogen receptors by androgens in the ZR-75-1 human breast cancer cell line. Endocrinology. 1989;125:392–399. doi: 10.1210/endo-125-1-392. [DOI] [PubMed] [Google Scholar]
- 40.Tetsuka M, Hillier SG. Androgen receptor gene expression in rat granulosa cells: the role of follicle-stimulating hormone and steroid hormones. Endocrinology. 1996;137:4392–4397. doi: 10.1210/endo.137.10.8828500. [DOI] [PubMed] [Google Scholar]
- 41.Adesanya-Famuyiwa OO, Zhou J, Wu G, Bondy C. Localization and sex steroid regulation of androgen receptor gene expression in rhesus monkey uterus. Obstet. Gynecol. 1999;93:265–270. doi: 10.1016/s0029-7844(98)00384-6. [DOI] [PubMed] [Google Scholar]
- 42.Panet-Raymond V, Gottlieb B, Beitel LK, Pinsky L, Trifiro MA. Interactions between androgen and estrogen receptors and the effects on their transactivational properties. Mol Cell Endocrinol. 2000 Sep 25;167(1-2):139–50. doi: 10.1016/s0303-7207(00)00279-3. [DOI] [PubMed] [Google Scholar]
- 43.Lapidus L, Lindstedt G, Lundberg PA, Bengtsson C, Gredmark T. Concentrations of sex-hormone binding globulin and corticosteroid binding globulin in serum in relation to cardiovascular risk factors and to 12-year incidence of cardiovascular disease and overall mortality in postmenopausal women. Clin Chem. 1986;32:146–52. [PubMed] [Google Scholar]
- 44.Reinecke H, Bogdanski J, Woltering A, Breithardt G, Assmann G, Kerber S, von Eckardstein A. Relation of serum levels of sex hormone binding globulin to coronary heart disease in postmenopausal women. Am J Cardiol. 2002;90:364–8. doi: 10.1016/s0002-9149(02)02490-6. [DOI] [PubMed] [Google Scholar]
- 45.Cameron DR, Braunstein GD. Androgen replacement therapy in women. Fertil Steril. 2004;82:273–89. doi: 10.1016/j.fertnstert.2003.11.062. [DOI] [PubMed] [Google Scholar]