Abstract
Chronic kidney disease (CKD) is associated with a high risk of death from coronary artery disease and may modify the response to standard cardiovascular therapies. Treatment of subjects with CKD should ideally be based on evidence from randomized, clinical trials, but how often subjects with CKD have been excluded from these trials is uncertain. We undertook this study in order to quantify how often subjects with moderate to advanced CKD were excluded from large cardiovascular trials. MEDLINE and the reference list of selected articles were searched in order to identify large, randomized, controlled trials of five different coronary artery disease therapies published between 1998 and 2005. Exclusion criteria and reported clinical characteristics of subjects were abstracted. Rates of exclusion and reporting of baseline characteristics of study participants were compared for CKD, diabetes, history of smoking, and hypertension. Eighty-six trials randomizing 411 653 patients were identified. More than 80% of trials excluded subjects with end-stage renal disease and 75.0% excluded patients with CKD. Subjects with diabetes, hypertension, or a history of smoking were excluded less than 4% of the time. Baseline renal function of study participant was reported in only 7% of trials. Patients with CKD are frequently excluded from coronary artery disease trials and renal function of randomized subjects is rarely reported. These findings reinforce the notion that available data on the treatment of coronary artery disease in subjects with CKD have significant limitations and should be generalized to the treatment of subjects with CKD cautiously.
Keywords: chronic kidney disease, coronary artery disease, cardiovascular disease
Roughly 11% of the US population has chronic kidney disease (CKD),1 and by 2030 there will be more than two million people with end-stage renal disease (ESRD) and many times that number with moderate impairment of kidney function.2 Both ESRD and moderate CKD are associated with high risks of death from coronary artery disease.3,4 This risk is not fully explained by traditional cardiovascular risk factors, and the usual relationship of these risk factors to cardiovascular outcomes is significantly altered in the setting of renal failure.3–10
Given the unique features of coronary artery disease in CKD it is possible that established cardiovascular therapies, or at least those tested solely in populations with normal renal function, will prove to be less effective in patients with CKD than in those with normal renal function. Alternatively, the high baseline risk in patients with CKD could magnify the absolute benefit of treatment with standard cardiovascular therapies. Indeed, the importance of specifically testing established cardiovascular therapies in subjects with CKD was highlighted by the negative findings of two recent trials that randomized patients with advanced kidney disease to treatment with statins vs placebo.11,12 Thus, both theoretical considerations and the available randomized evidence suggest that uncertainty about the appropriate role for standard therapies in subjects with CKD is warranted when those therapies have not been broadly tested on subjects with impaired kidney function.
How often renal function has been used to exclude participation in clinical trials and whether renal function is used to exclude participation in clinical trials more frequently than other high-risk conditions have not been systematically studied or quantified. We undertook the current study in order to systematically determine whether subjects with moderate CKD or dialysis-dependent ESRD are excluded from enrolment in large, coronary artery disease trials more frequently than subjects with other high-risk conditions such as diabetes, hypertension, or smoking and to compare the extent to which those trials report on the presence of these conditions at baseline.
RESULTS
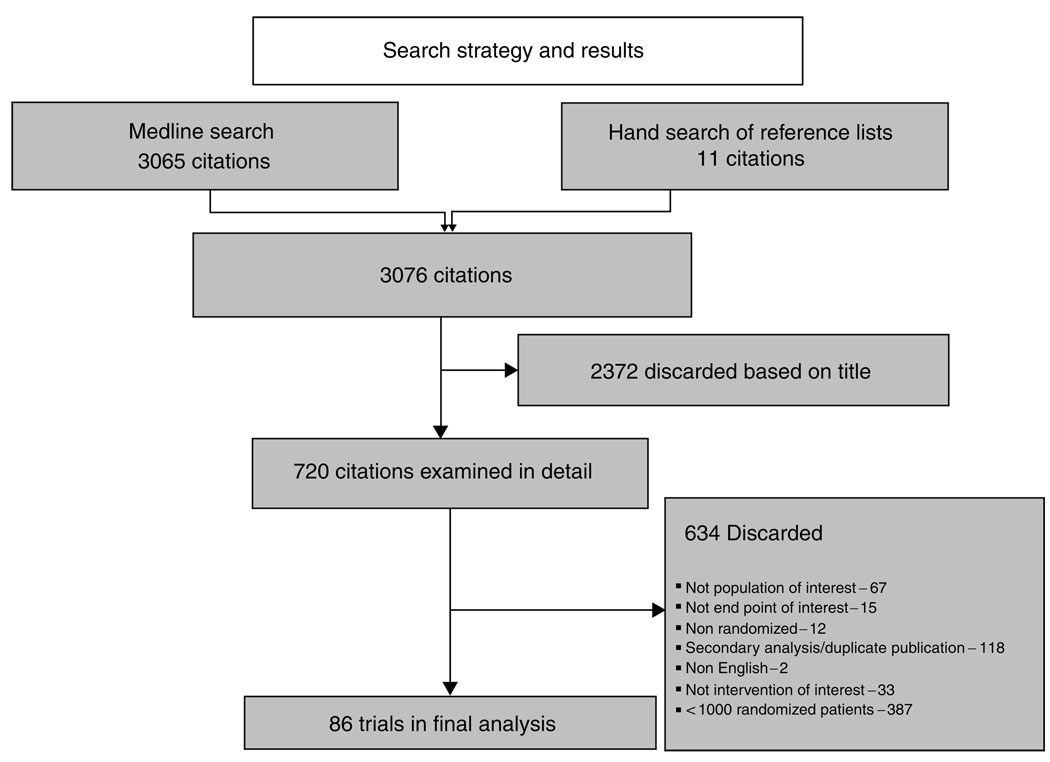
A total of 3076 articles were identified via electronic and hand searches. As shown in Figure 1, 86 trials randomizing 411 653 subjects met the inclusion criteria and were selected for further analysis. Twenty-one trials randomized patients to GPIIb–IIIa inhibitors, 15 to oral anti-platelet agents, 30 to statins, seven to beta blockers, and 18 to percutaneous coronary intervention or devices. Five trials randomized patients to more than one of these interventions (Table 1).
Figure 1.
Search strategy and identification of trials for inclusion in analysis.
Table 1.
Exclusion of patients with renal disease according to trial intervention
| Intervention | Number of trials |
Number excluding subjects with ESRD (%) |
Number excluding subjects with renal insufficiency (%) |
|---|---|---|---|
| Anti-platelet agents | 15 | 9 (60.0) | 6 (46.6) |
| Beta blocker | 7 | 4 (57.1) | 4 (57.1) |
| GIIb/IIIa inhibitor | 21 | 18 (85.7) | 18 (85.7) |
| Percutaneous interventions |
18 | 14 (77.7) | 11 (61.1) |
| Statin | 30 | 27 (90.0) | 27 (90.0) |
| Totala | 86 | 69 (80.2) | 64 (74.4) |
Five trials randomized subjects to more than one of these therapies leaving 86 unique trials.
Subjects with chronic renal insufficiency or ESRD were most likely to be excluded from trials of statins or GIIb/IIIa inhibitors –90% (95% confidence interval (CI): 73.5–97.9) of statin trials and 85.7% (95% CI: 63.7–97.0) GIIb/IIIa trials excluded subjects with either condition. Trials of oral anti-platelet agents included subjects with CKD more frequently, but approximately half used CKD as an exclusion criteria – 60% (95% CI: 32.3–83.7) excluded subjects with ESRD and 46.6% (95% CI: 16.3–67.7) excluded subjects with earlier stages of CKD.
Exclusion of patients with CKD was typically based on the serum creatinine. In only five trials was the threshold based on estimated GFR or creatinine clearance. A threshold creatinine clearance of 30 cm3/min or less was used in five trials. Two trials used a threshold creatinine below 1.5 mg/dl, 35 trials used a threshold between 1.5 and 2.0 mg/dl, and 18 trials used a threshold creatinine above 2.0 mg/dl.
All five types of trials were unlikely to report the baseline renal function or frequency of renal impairment in trial participants. This information was reported most frequently in trials of beta blocking agents, but was still available only 28.6% (95% CI: 3.7–70.9) of the time in this setting. Results from the individual trials are summarized in Tables 2–6.
Table 2.
Trials of GIIb/IIIa inhibitors
| Trials | Intervention | ESRD excluded |
Exclusion creatinine (mg/dl) |
Other conditions excluded |
Renal function reported |
All other conditions reported |
|---|---|---|---|---|---|---|
| ASSENT-326 | Tenecteplase+enoxaparin, heparin or, abciximab+heparin |
Y | >2.5(male)>2.0 (female) | N | N | Y |
| BRAVO27 | Lotrafiban vs placebo | Y | CrCl<30 | N | N | Y |
| CADILLAC28 | Abciximab vs placebo | Y | >2.0 | N | N | Y |
| EPISTENT29 | Stent vs angioplasty abciximab vs placebo | N | NA | N | N | Y |
| ESPRIT30 | Eptifibatide vs placebo | Y | >4.0 | N | N | Y |
| EXCITE31 | Xemilofiban vs placebo | Y | >1.5 | N | N | S, H |
| GUSTO IV-ACS32 | Abciximab vs placebo | Y | >1.5 | N | N | Y |
| GUSTO V33 | Reteplase vs reteplase+abciximab | N | NA | N | N | Y |
| ISAR-REACT34 | Abciximab vs placebo | N | NA | N | N | Y |
| OPUS-TIMI 1635 | Orbofiban vs placebo | Y | >1.6 | N | Y | H |
| PARAGON36 | Lamifiban vs placebo | Y | >2.0 | N | N | Y |
| PARAGON -B37 | ASA vs coumadin vs placebo | Y | CrCl<30 | N | N | Y |
| PRISM38 | Tirofiban vs heparin | Y | >2.5 | N | N | Y |
| PRISM-PLUS39 | Tirofiban vs tirofiban+heparin vs heparin | Y | >2.5 | N | N | Y |
| PURSUIT40 | Eptifibatide vs placebo | Y | >2.0 | N | N | Y |
| REPLACE-241 | Heparin+GIIb/IIIa inhibitor vs bivalirudin | Y | >4.0 | N | N | Y |
| Second SYMPHONY42 | Sirafiban+aspirin vs sirafiban vs aspirin | Y | >1.5 | N | Y | Y |
| SYMPHONY43 | ASA vs sibrafiban | Y | >1.5 | N | N | Y |
| TARGET44 | Abciximab vs tirofiban | Y | >2.5 | N | N | Y |
| TETAMI45 | Enoxaparin vs enoxaparin+tirofiban vs heparin+tirofiban vs heparin |
Y | CrCl<30 | N | N | Y |
ASA, aspirin; CrCl, creatinine clearance; D, diabetic patients excluded or percent of patients with diabetes not reported; ESRD, end-stage renal disease; H, hypertension excluded or percent of patients with hypertension not reported; N, no; NA, not applicable; S, smokers excluded or percent of smokers not reported; Y, yes.
Note: To convert serum creatinine in mg/dl to µmol/l multiply by 88.4.
Table 6.
Non-GIIb/IIIa oral anti-platelet therapy
| Trial | Intervention | Excluded | Creatinine (mg/dl) |
Conditions Excluded |
Function Reported |
Other Conditions Reported |
|---|---|---|---|---|---|---|
| CLARITY-TIMI 2898 | Clopidogrel vs placebo | Y | >2.5 | N | N | Y |
| CLASSICS99 | 3 regimens of plavix+ASA | Y | >2.0 | N | N | Y |
| CREDO100 | Clopidogrel vs placebo | Y | >3.0 | N | N | Y |
| CURE101 | Clopidogrel vs placebo | N | NA | N | N | Y |
| HOT102 | ASA vs Placebo/vitamin E. vs Placebo | N | N | N | Y | Y |
| Primary Prevention Project103 | ASA vs Placebo/vitamin E. vs Placebo | N | NA | N | N | Y |
| Second SYMPHONY42 | ASA vs sibrafiban+ASA vs sibrafiban | Y | >1.5 | N | Y | Y |
| STAMI104 | Ticlopidine vs ASA | Y | >1.5 × normal | N | N | Y |
| STARS105 | ASA vs ASA+coumadin vs ASA+ticlopidine | N | NA | N | N | Y |
| SYMPHONY43 | ASA vs sibrafiban | Y | >1.5 | N | N | Y |
| Taniuchi et al.106 | Ticlopidine vs clopidogrel | N | NA | N | S | |
| TIM107 | Triflusal vs ASA | Y | Investigator discretion | N | N | Y |
| TPT108 | ASA vs coumadin vs placebo | Y | Investigator discretion | N | N | D |
| WARIS II109 | ASA vs coumadin vs coumadin+ASA | N | NA | N | N | Y |
| Women’s Health Study110 | ASA vs placebo | N | NA | N | N | Y |
ASA, aspirin; CrCl, creatinine clearance; D, diabetic patients excluded or percent of patients with diabetes not reported; H, hypertension excluded or percent of patients with hypertension not reported; N, no; NA, not applicable; S, smokers excluded or percent of smokers not reported; Y, yes.
Note: To convert serum creatinine in mg/dl to µmol/l multiply by 88.4.
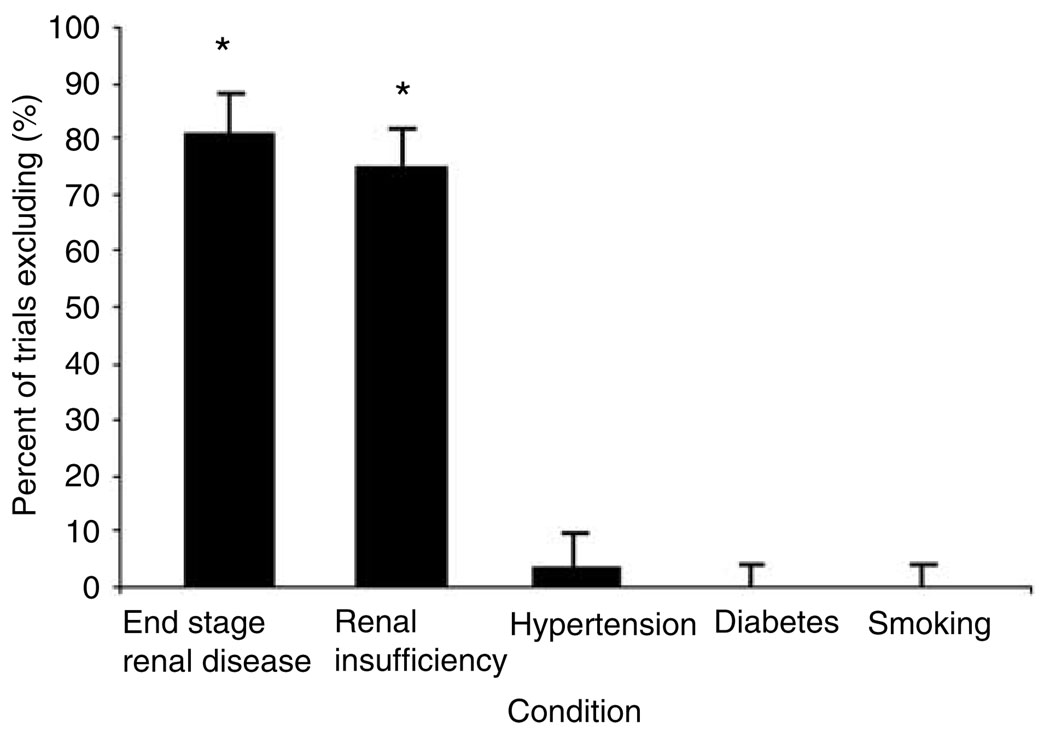
Out of 86 trials, 69 (80.6%, 95% CI: 70.2–88.0) excluded patients with ESRD and 64 (74.4, 95% CI: 63.9–88.2) excluded patients with moderate to severe renal insufficiency. Patients with diabetes, hypertension, or a history of smoking were rarely excluded from participation in trials. Only three studies (3.5%, 95% CI: 0.7–9.8) excluded patients with diabetes and no study excluded patients with hypertension or a history of smoking (P < 0.0001 for all comparisons, Table 1 and Figure 2).
Figure 2.
Percent of cardiovascular trials excluding subjects with ESRD, CKD, hypertension, diabetes, or smoking from participation. *P < 0.0001 for comparisons with diabetes, hypertension, and smoking.
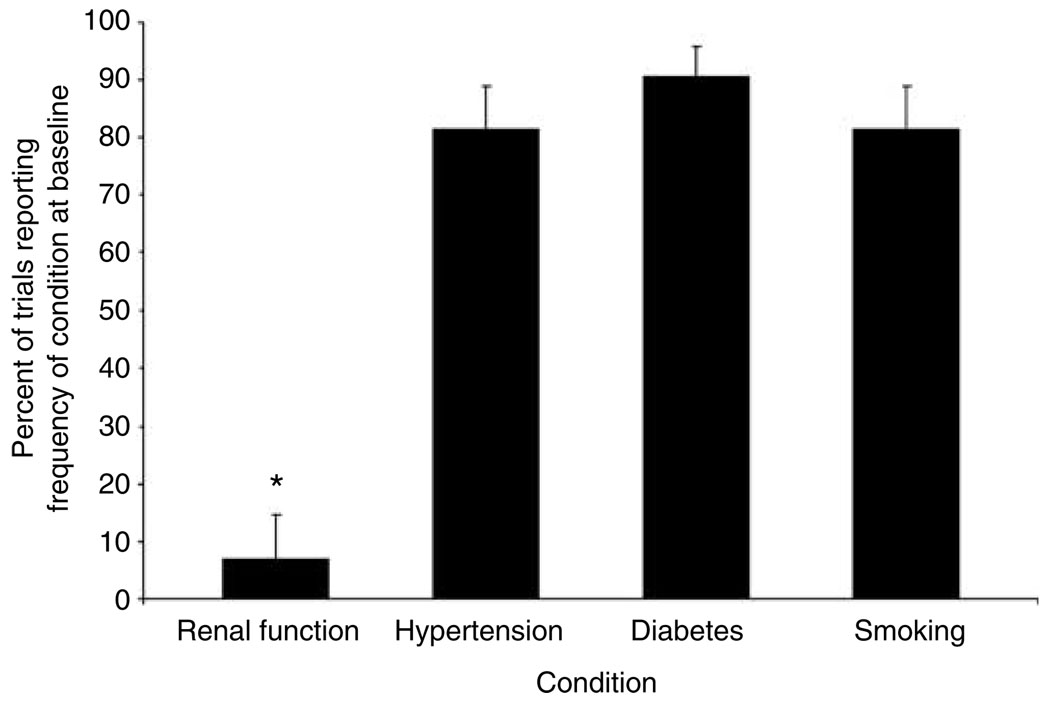
The percentage of patients with impaired renal function or the mean baseline serum creatinine of randomized patients was reported by only six of 86 studies (7.0, 95% CI: 2.6–14.6). The percentage of patients with hypertension (or mean baseline blood pressure), diabetes, or a history of smoking was all reported significantly more frequently (P < 0.0001 for all comparisons). Baseline blood pressure and smoking history were each recorded in 70 of 86 (81.4, 95% CI: 71.6–89.0) studies. The baseline percentage of subjects with diabetes was reported in 75 out of 83 studies (90.4%, 95% CI: 81.9–95.7) that included diabetic patients, Figure 3.
Figure 3.
Percent of cardiovascular trials reporting on the presence of CKD, hypertension, diabetes, or smoking at baseline. *P < 0.0001 for comparisons with diabetes, hypertension, and smoking.
DISCUSSION
We systematically reviewed the reports of large, randomized coronary artery disease trials published between 1998 and 2005 to assess whether these trials exclude patients with moderate or dialysis-dependent CKD more frequently than they exclude subjects with other cardiovascular risk-factors. We found that more than 80% of trials exclude patients with ESRD and nearly 75% exclude patients with moderate renal insufficiency whereas subjects with other common risk factors for cardiovascular disease are excluded only rarely. Further, we found that fewer than 10% of trials provide information on baseline serum creatinine. Data on the estimated glomerular filtration rate or creatinine clearance of randomized subjects, which are better markers of renal function than serum creatinine alone, are provided even less frequently. Our results thus demonstrate that among common cardiovascular risk factors, there is a unique failure of large cardiovascular trials to produce data on the treatment of cardiovascular disease in the setting of moderate or advanced CKD.
This failure to test coronary artery disease therapies in patients with advanced renal insufficiency has significant implications when considered in light of elevated cardiovascular mortality rates in subjects with CKD,4 the fact that as many as 50% of subjects admitted with a myocardial infarction have stage 3 or worse CKD13 and the common failure to administer standard cardiovascular therapies to patients with CKD even when they are diagnosed with myocardial infarction.14–16 Because there is insufficient randomized evidence on the effectiveness of typical therapies in subjects with CKD, it is difficult to know whether this low use represents appropriate concern about the use of unproven therapies, appropriate response to comorbidity in patients with CKD,17 or whether it partially explains the high rates of cardiovascular morbidity and mortality in the CKD population.5–8,18
More importantly, there is a growing body of literature suggesting that standard treatment strategies may act differently in patients with and without CKD 14,15,19 – a concept reinforced by two trials of statins that were conducted in populations with CKD.11,12 Available evidence, including the present study, thus suggests that the general standard of care for coronary artery disease should be extrapolated to subjects with CKD cautiously, except in those rare cases where subjects with CKD have been randomized in significant numbers.
This failure of large coronary artery disease trials to include patients with CKD is particularly concerning when one considers that the number of randomized controlled trail published in nephrology is low and compounded by poor quality and reporting20 and thus unlikely to provide definitive answers on cardiovascular care in this population. Our findings, highlight and quantify the limitations of current evidence on the treatment of cardiovascular disease in subjects with CKD and provide a strong rationale for including greater numbers of subjects with CKD in future trials or for specifically targeting subjects with CKD as the population for future trials of standard and emerging therapies of coronary artery disease. Finally, the reliance of serum creatinine as the measure of renal function in the majority of studies we analyzed highlights the need to educate cardiovascular trialists on the availability of better estimates of renal function for use during randomization and follow-up of patients.
Our findings should be interpreted within the context of our methodology. Our search was limited to peer-reviewed trials randomizing at least 1000 patients to five different therapies. We cannot rule out the possibility that small trials or trials of other therapies are more likely to include patients with kidney disease. However, our results were consistent across a broad array of both medical and interventional therapies. Furthermore, large trials with power to provide definitive answers to clinical questions provide important data that profoundly influences evidenced-based medical practice. The clinical importance of our findings would be only slightly diminished in the event that small or unpublished trials are more likely to enrol subjects with kidney disease than the trials we studied.
We did not directly assess whether separate, smaller trials are being conducted in patients with renal insufficiency. Nevertheless, our search strategy incidentally identified only a single, randomized trial published between 1998 and March 2005 that tested whether a drug or device prevents myocardial infarction or cardiovascular death in patients with renal insufficiency.21
Finally, the low rate of reporting on the baseline renal function of trial participants that we found does not account for the possibility of subsequent publication of subgroup analyses of the treatment effect in subjects with vs without renal insufficiency. Such publications can partially mitigate the import of an initial failure to report the percent of subjects with renal disease, but that initial failure nevertheless reflects a markedly different approach by clinical trialists towards CKD as a coronary disease risk factor than they have for other common conditions such as hypertension, diabetes, and smoking. Given the high risk of cardiovascular events in subjects with CKD and high frequency of this condition in the general population, we find this practice puzzling. Additionally, subgroup analyses, particularly if not pre-specified, are susceptible to false-positive and false-negative findings.22 Further, their publication is frequently delayed by several years compared to the initial publication of the overall results. For both reasons, having enough information to draw conclusions about a trial’s relevance to subjects with CKD at the time of initial publication would be preferable to current practices.
An important question raised by our findings is whether there are sufficiently compelling reasons to exclude patients with CKD despite the clear need for better data about this group. Inclusion of a new subgroup of patients in cardiovascular trials might skew the overall results or the power to detect a therapeutic effect by altering the rate of cardiovascular events, the case-fatality rate, or the frequency of non-cardiovascular death in the trial cohort, although stratified randomization or limiting the numbers of randomized patients with a particularly high-risk condition can be used to mitigate the influence of that condition on trial results. Moreover, the risk of cardiovascular disease among patients with moderate renal impairment is actually similar to the risk among patients with diabetes, smoking, or hypertension,5–9,18,23 and randomized trials are unlikely to randomize substantially more patients with moderate or advanced renal insufficiency than they currently randomize with diabetes or hypertension. The risk profile is significantly different in patients with ESRD where the mortality rate is significantly higher than in the general population.24 However, roughly half of deaths in patients with ESRD are due to cardiovascular disease,25 and any loss of power owing to an increased rate of non-cardiovascular deaths might well be neutralized by a concurrent increase in the rate of cardiovascular morbidity.
A reduction in the glomerular filtration rate can alter the safety profile of some drugs and devices, and might be an appropriate reason to exclude patients from participation in trials, especially phase I and phase II trials. However, none of the therapies we studied is contraindicated in patients with renal insufficiency. Indeed, a high proportion of trials of beta blockers, statins, and anti-platelet therapies excluded patients with renal insufficiency despite the widespread clinical use of these agents in studies on patients with ESRD.14
Finally, if the altered epidemiology and pathophysiology of cardiovascular disease in patients with renal disease10 confers resistance to standard therapies, then the inclusion of patients with renal insufficiency in trials will decrease the observed treatment effect and bias towards the null hypothesis. Belief in this theory may partially explain why patients with renal disease are so frequently excluded from cardiovascular trials, but it remains an unproven hypothesis that at a minimum deserves to be tested in clinical trials.
A significant and growing minority of people in this country has CKD. They suffer from an increased risk of developing and dying from coronary artery disease and whether they respond differently to cardiovascular therapies is uncertain. It is therefore crucial that we advance our knowledge of how to treat and prevent cardiovascular disease in this population. Unfortunately, large, contemporary trials have largely ignored these patients despite compelling reasons to increase our understanding of the unique features of their disease. Government and industry-funded trials must enrol greater numbers of patients with impaired kidney function in trials or should specifically target trials towards the CKD population so that we can provide the best possible cardiovascular care for this crucial population.
MATERIALS AND METHODS
Definitions
Our objective in this study was to understand how contemporary clinical research applies to patients with renal disease. Because small trials or trials published only in abstract form are less likely to influence clinical practice or lead to Food and Drug Administration approval of new drugs and devices than larger trials that have been published in peer-reviewed journals, we restricted our analysis to published trials that randomized at least 1000 subjects. We looked at five different interventions selected because as a group they represent the standard therapies now used for the treatment of coronary artery disease. Trials randomizing subjects to beta-blockers, GIIb/IIIa inhibitors, non-GIIb/IIIa oral anti-platelet agents, HMG-CoA reductase inhibitors, or a percutaneous coronary intervention or coronary stents were included in this study. In order to examine contemporary practices, we restricted our analysis to trials whose principal results were published in peer-reviewed journals between January 1998 and March 2005.
Trials that studied the aforementioned agents for purposes other than the treatment of coronary artery disease were excluded from further analysis. Thus, trials were included when at least one of the following conditions was met: (a) ischemic coronary artery disease was a prerequisite for randomization; (b) the primary end point assessed the occurrence of new or recurrent coronary artery disease that included myocardial infarction, coronary revascularization, and cardiovascular death; and (c) the primary end point assessed the effect of a medication on the low-density lipoprotein cholesterol concentration. The last criterion was chosen because low-density lipoprotein cholesterol lowering has been widely accepted as a valid surrogate end point for statin therapy, is widely used to guide the use of statins, and has been used to justify the approval of new statin medications.
We chose not to study trials of angiotensin-converting enzyme inhibitors) and angiotensin receptor blockers. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers have recently emerged as important agents in the treatment and prevention of cardiovascular disease. However, in contrast to the other therapies we studied, the use of angiotensin-converting enzyme inhibitors and/or angiotensin receptor blockers forms the standard of care in the therapy of CKD. These agents are routinely prescribed to reduce proteinuria and slow progression of renal disease in patients with CKD regardless of their role in the treatment of cardiovascular disease. Thus, whereas it would have been interesting to study trials of these medications, we felt that the clear nephrologic indications for their use mitigated the clinical impact of exclusion of patients with CKD from cardiovascular trials of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers.
Search strategy
MEDLINE searches using the PubMed interface were conducted for the period between January 1998 and March 2005. Search terms included the following MeSH headings: beta blocker, platelet glycoprotein GPIIb–IIIa complex, HMG-CoA reductase inhibitors, angioplasty, stent, aspirin, and ticlopidine. Additional search terms included the generic names of individual beta blockers, GIIb/IIIa inhibitors, statins, and anti-platelet agents. The following limits were used: English-language; randomized, controlled trial; and adults greater than 19 years old. The reference lists of selected trials were also manually reviewed in order to identify additional studies. Eligibility for inclusion was determined after review of titles, abstracts or, where necessary, the full manuscript.
Data abstraction and analysis
For each trial, the exclusion criteria were abstracted from the published reports. Exclusion of subjects with CKD was defined as the use of ‘renal insufficiency’ or a threshold of serum creatinine, blood urea nitrogen, or creatinine clearance to exclude subjects from randomization. Authors were contacted for clarification of the exclusion criteria when the published reports were unclear or when individual investigators were allowed to exclude patients based on the presence of conditions they believed would compromise life expectancy or a subject’s ability to comply with the assigned treatment. In the event that the authors could not to be reached for clarification, trials were considered to have included patients with CKD. The proportion of studies excluding patients with moderate CKD or dialysis-dependent CKD was calculated from these data.
For comparative purposes, we also assessed how frequently subjects with a clinical history of diabetes, smoking, or hypertension – other common risk factors for cardiovascular disease – were excluded from trial participation. In addition, we abstracted whether studies reported the baseline percentage of patients with diabetes, a history of smoking, CKD, or hypertension. Reporting on the mean baseline serum creatinine was considered equivalent to reporting on the number of subjects with CKD. Reporting mean baseline blood pressure was considered equivalent to reporting on the percentage of subjects with diagnosed hypertension.
Proportions were compared using an exact binomial test. P < 0.05 was considered significant. All calculations were performed in Stata version 8.0 (Stata Corporation, College Station, TX, USA).
Table 3.
Interventional trials
| Trial | Intervention | ESRD excluded |
Exclusion creatinine (mg/dl) |
Other conditions excluded |
Renal function reported |
All other conditions reported |
|---|---|---|---|---|---|---|
| ARTS46 | Stent vs CABG | Y | >1.8 | N | N | Y |
| ASCENT47 | Stent vs stent | N | NA | N | N | H |
| CADILLAC28 | Stent vs angioplasty | Y | >2.0 | N | N | Y |
| CONSERVE48 | Low pressure vs conventional stent | N | NA | N | N | Y |
| DANAMI 249 | Angioplasty vs thrombolysis | Y | >2.8 | N | N | Y |
| DELIVER50 | Paclitaxel-coated vs bare metal stent | Y | >2.5 | N | N | Y |
| EPISTENT29 | Stent vs angioplasty | N | NA | N | N | Y |
| FRISC II51 | Invasive vs conservative | Y | >1.8 | N | N | Y |
| Kastrati et al.52 | Stent vs stent | N | NA | N | N | Y |
| Mauri et al.53 | Cutting baloon vs stent | Y | >2.0 | |||
| RITA 154 | PTCA vs CABG | Y | NA | N | N | S, H |
| RITA 255 | PTCA vs CABG | Y | NA | N | N | S, H |
| RITA 356 | Early vs symptom-driven angiography | Y | NA | N | N | S |
| SCORES57 | Self expanding vs balloon expanding stents | Y | >1.4 | N | N | Y |
| SIRIUS58 | Sirolimus-coated vs bare metal stent | Y | >3.0 | N | N | Y |
| Stent-PAMI59 | Stent vs angioplasty | Y | >2.5 | N | N | S, H |
| TACTICS-TIMI-1860 | Invasive vs conservative | Y | >2.5 | N | N | S, H |
| Taxus-IV61 | Paclitaxel-coated vs bare metal stent | Y | >2.0 | N | N | Y |
CABG, coronary artery bypass grafting; CrCl, creatinine clearance; D, diabetic patients excluded or percent of patients with diabetes not reported; ESRD, end-stage renal disease; H, hypertension excluded or percent of patients with hypertension not reported; N, no; NA, not applicable; PCTA, percutaneous trans coronary angioplasty; S, smokers excluded or percent of smokers not reported; Y, yes.
Note: To convert serum creatinine in mg/dl to µmol/l multiply by 88.4.
Table 4.
Beta blockers
| Trial | Intervention | ESRD excluded |
Exclusion creatinine (mg/dl) |
Other conditions excluded |
Renal function reported |
All Other conditions reported |
|---|---|---|---|---|---|---|
| CAPPP62 | Captopril vs diuretic or beta blocker | Y | >1.7 | N | Y | Y |
| CONVINCE63 | Verapamil vs atenolol or hydrochlorothiazide | Y | >2.0 | N | N | Y |
| CAPRICORN64 | Carvedilol vs placebo | N | NA | N | N | Y |
| LIFE65 | Losartan vs atenolol | Y | >1.8 | N | N | Y |
| JBCMI66 | Beta blocker vs calcium channel blocker | Y | ‘Severe renal insufficiency’ |
N | N | Y |
| NORDIL67 | Diltiazem vs beta blockers or diuretics | N | N | N | Y | Y |
| STOP-Hypertension 268 | ACE inhibitor or calcium channel blocker vs diuretic or beta blocker |
N | N | N | N | Y |
ACE, angiotensin-converting enzyme; CrCl, creatinine clearance; D, diabetic patients excluded or percent of patients with diabetes not reported; ESRD, end-stage renal disease; H, hypertension excluded or percent of patients with hypertension not reported; N, no; NA, not applicable; S, smokers excluded or percent of smokers not reported; Y, yes;
Note: To convert serum creatinine in mg/dl to mmol/l multiply by 88.4.
Table 5.
Statin trials
| Trial | Intervention | ESRD excluded |
Exclusion creatinine (mg/dl) |
Other conditions excluded |
Renal function reported |
All other conditions reported |
|---|---|---|---|---|---|---|
| ALERT11 | Fluvastatin vs placebo | Y | CrCl<20 | N | N | Y |
| ALLHAT-LLT69 | Pravastatin vs placebo | Y | >2.0 | N | N | Y |
| ALLIANCE70 | Atorvastatin vs usual care | Investigator discretion |
Investigator discretion | N | N | y |
| AFCAPS/TexCAPS71 | Lovastatin vs placebo | Y | Investigator discretion | N | N | Y |
| ASCOT-LLA72 | Atorvastatin vs placebo | Y | >2.2 | N | Y | Y |
| Atorvastatin Comparitive Cholesterol Efficacy73 |
Atorvastatin vs fluvastatin vs lovastatin vs pravastatin |
Y | >2.0 | N | N | S, D, H |
| A to Z74 | Aggressive vs conservative simvastatin | Y | >2.0 | N | N | Y |
| Bays, et al.75 | Vytorin vs ezetimibe or simvastatin | N | NA | N | N | S, D, H |
| Bruckert, et al.76 | Fluvastatin vs placebo | Y | CrCl<30 | N | N | Y |
| CARDS77 | Atorvastatin vs placebo | Y | >1.6 | N | Y | Y |
| Cerivastatin Pivotal Trial78 | High dose cerivasatin vs low dose vs placebo |
Y | Significant abnormality | D | N | Da |
| CHALLENGE79 | Atovastatin vs simvastatin | Y | >2.0 | N | N | H |
| Dujovne, et al.80 | Cerivastatin vs pravastatin | Y | >2.0 | D | N | Da, H |
| Discovery81 | Atorvastatin vs rosuvastatin | Y | >2.5 | N | N | Y |
| FLARE82 | Fluvastatin vs placebo | N | N | N | N | Y |
| GREACE83 | Atovastatin vs usual care | Y | >1.3 | N | Y | S |
| Heart Protection Study84 | Simvastatin vs placebo | Y | >2 × ULN | N | N | S |
| Kyushu Lipid Intervention85 | Pravastatin vs placebo | Y | >2.0 | N | N | Y |
| Lipid Intervention in Kyoto86 | Pravastatin vs conventional Lipid-lowering drugs |
Y | ‘Severe’ | N | N | D, H |
| LIPID87 | Pravastatin vs placebo | Y | >1.8 | N | N | Y |
| LIPS88 | Fluvastatin vs placebo | Y | >1.8 | N | N | Y |
| MIRACL89 | Atorvastatin vs placebo | Y | N | N | N | Y |
| MERCURY I90 | Rosuvastatin vs atorvastatin vs simvastatin vs pravastatin |
Y | >2.5 | N | N | S, D, H |
| Olsson, et al.91 | Extended release fluvastatin vs immediate elease |
Y | >1.5 | D | N | S, Da, H |
| PACT92 | Pravastatin vs placebo | Y | ‘Severe renal disease’ | N | N | H |
| PaCT93 | Pravastatin vs cholestyramine | N | N | N | N | Y |
| PROSPER94 | Pravastatin vs placebo | Y | >2.3 | N | N | Y |
| STELLAR95 | Rosuvastatin vs atorvastatin vs simvastatin vs pravastatin |
Y | >2.0 | N | N | H, S |
| TARGET TANGIBLE96 | Atorvastatin vs simvastatin | Y | >1.8 | N | N | Y |
| TIMI-2297 | Pravastatin vs atorvastatin | Y | >2.0 | N | N | Y |
CrCl, creatinine clearance; D, diabetic patients excluded or percent of patients with diabetes not reported; ESRD, end-stage renal disease; H, hypertension excluded or percent of patients with hypertension not reported; N, no; NA, not applicable; S, smokers excluded or percent of smokers not reported; ULN, upper limit of normal; Y, yes.
Note: To convert serum creatinine in mg/dl to µmol/l multiply by 88.4.
Patients with diabetes excluded from participation.
ACKNOWLEDGMENTS
This work was supported by NIH Grant T32 DK007527-22. Dr Kuntz is currently employed at Medtronic Inc., a maker of cardiovascular devices.
REFERENCES
- 1.Coresh J, Astor BC, Greene T, et al. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 2.Gilbertson DT, Craig AS, Jay LX, Collins AJ. Projecting the US ESRD population to 2030. J Am Soc Nephrol. 2003;14:F-PO881. [Google Scholar]
- 3.Anavekar NS, McMurray JJ, Velazquez EJ, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 5.Muntner P, He J, Hamm L, et al. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol. 2002;13:745–753. doi: 10.1681/ASN.V133745. [DOI] [PubMed] [Google Scholar]
- 6.Wannamethee SG, Shaper AG, Perry IJ. Serum creatinine concentration and risk of cardiovascular disease: a possible marker for increased risk of stroke. Stroke. 1997;28:557–563. doi: 10.1161/01.str.28.3.557. [DOI] [PubMed] [Google Scholar]
- 7.Mann JF, Gerstein HC, Pogue J, et al. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Intern Med. 2001;134:629–636. doi: 10.7326/0003-4819-134-8-200104170-00007. [DOI] [PubMed] [Google Scholar]
- 8.Fried LF, Shlipak MG, Crump C, et al. Renal insufficiency as a predictor of cardiovascular outcomes and mortality in elderly individuals. J Am Coll Cardiol. 2003;41:1364–1372. doi: 10.1016/s0735-1097(03)00163-3. [DOI] [PubMed] [Google Scholar]
- 9.Salahudeen AK, Fleischmann E, Ahmed A, Bower JD. Anemia and iron target realization in 1998: clinical management of anemia in 1,639 patients on hemodialysis. ASAIO J. 2001;47:511–515. doi: 10.1097/00002480-200109000-00023. [DOI] [PubMed] [Google Scholar]
- 10.Baigent C, Burbury K, Wheeler D. Premature cardiovascular disease in chronic renal failure. Lancet. 2000;356:147–152. doi: 10.1016/S0140-6736(00)02456-9. [DOI] [PubMed] [Google Scholar]
- 11.Holdaas H, Fellstrom B, Jardine AG, et al. Effect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicentre, randomised, placebo-controlled trial. Lancet. 2003;361:2024–2031. doi: 10.1016/S0140-6736(03)13638-0. [DOI] [PubMed] [Google Scholar]
- 12.Wanner C, Krane V, Marz W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 13.Sosnov J, Lessard D, Goldberg RJ, et al. Differential symptoms of acute myocardial infarction in patients with kidney disease: a community-wide perspective. Am J Kidney Dis. 2006;47:378–384. doi: 10.1053/j.ajkd.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Trespalacios FC, Taylor AJ, Agodoa LY, Abbott KC. Incident acute coronary syndromes in chronic dialysis patients in the United States. Kidney Int. 2002;62:1799–1805. doi: 10.1046/j.1523-1755.2002.00638.x. [DOI] [PubMed] [Google Scholar]
- 15.Wright RS, Reeder GS, Herzog CA, et al. Acute myocardial infarction and renal dysfunction: a high-risk combination. Ann Intern Med. 2002;137:563–570. doi: 10.7326/0003-4819-137-7-200210010-00007. [DOI] [PubMed] [Google Scholar]
- 16.Berger AK, Duval S, Krumholz HM. Aspirin, beta-blocker, and angiotensin-converting enzyme inhibitor therapy in patients with end-stage renal disease and an acute myocardial infarction. J Am Coll Cardiol. 2003;42:201–208. doi: 10.1016/s0735-1097(03)00572-2. [DOI] [PubMed] [Google Scholar]
- 17.Abbott KC, Bohen EM, Yuan CM, et al. Use of beta-blockers and aspirin after myocardial infarction by patient renal function in the Department of Defense health care system. Am J Kidney Dis. 2006;47:593–603. doi: 10.1053/j.ajkd.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Garg AX, Clark WF, Haynes RB, House AA. Moderate renal insufficiency and the risk of cardiovascular mortality: results from the NHANES I. Kidney Int. 2002;61:1486–1494. doi: 10.1046/j.1523-1755.2002.00270.x. [DOI] [PubMed] [Google Scholar]
- 19.Azar RR, Prpic R, Ho KK, et al. Impact of end-stage renal disease on clinical and angiographic outcomes after coronary stenting. Am J Cardiol. 2000;86:485–489. doi: 10.1016/s0002-9149(00)00998-x. [DOI] [PubMed] [Google Scholar]
- 20.Strippoli GF, Craig JC, Schena FP. The number, quality, and coverage of randomized controlled trials in nephrology. J Am Soc Nephrol. 2004;15:411–419. doi: 10.1097/01.asn.0000100125.21491.46. [DOI] [PubMed] [Google Scholar]
- 21.Cice G, Ferrara L, D’Andrea A, et al. Carvedilol increases two-year survivalin dialysis patients with dilated cardiomyopathy: a prospective, placebo-controlled trial. J Am Coll Cardiol. 2003;41:1438–1444. doi: 10.1016/s0735-1097(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 22.Brookes ST, Whitley E, Peters TJ, et al. Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives. Health Technol Assess. 2001;5:1–56. doi: 10.3310/hta5330. [DOI] [PubMed] [Google Scholar]
- 23.Wilson PW, D’Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 24.United States Renal Data System. USRDS 2004 annual data report: atlas of end-stage renal disease in the United States. Bethesda: 2004
- 25.NIH. USRDS 2003 annual data report: atlas of end-stage renal disease in the United States. 2003 [PubMed]
- 26.Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT-3 randomised trial in acute myocardial infarction. Lancet. 2001;358:605–613. doi: 10.1016/S0140-6736(01)05775-0. [DOI] [PubMed] [Google Scholar]
- 27.Topol EJ, Easton D, Harrington RA, et al. Randomized, double-blind, placebo-controlled, international trial of the oral IIb/IIIa antagonist lotrafiban in coronary and cerebrovascular disease. Circulation. 2003;108:399–406. doi: 10.1161/01.CIR.0000084501.48570.F6. [DOI] [PubMed] [Google Scholar]
- 28.Stone GW, Grines CL, Cox DA, et al. Comparison of angioplasty with stenting, with or without abciximab, in acute myocardial infarction. N Engl J Med. 2002;346:957–966. doi: 10.1056/NEJMoa013404. [DOI] [PubMed] [Google Scholar]
- 29.Randomised placebo-controlled and balloon-angioplasty-controlled trial to assess safety of coronary stenting with use of platelet glycoprotein-IIb/IIIa blockade. The EPISTENT Investigators. Evaluation of Platelet IIb/IIIa Inhibitor for Stenting. Lancet. 1998;352:87–92. doi: 10.1016/s0140-6736(98)06113-3. [DOI] [PubMed] [Google Scholar]
- 30.ESPRIT Investigators. Novel dosing regimen of eptifibatide in planned coronary stent implantation (ESPRIT): a randomised, placebo-controlled trial. Lancet. 2000;356:2037–2044. doi: 10.1016/S0140-6736(00)03400-0. [DOI] [PubMed] [Google Scholar]
- 31.O’Neill WW, Serruys P, Knudtson M, et al. Long-term treatment with a platelet glycoprotein-receptor antagonist after percutaneous coronary revascularization. EXCITE Trial Investigators. Evaluation of Oral Xemilofiban in Controlling Thrombotic Events. N Engl J Med. 2000;342:1316–1324. doi: 10.1056/NEJM200005043421803. [DOI] [PubMed] [Google Scholar]
- 32.Simoons ML. Effect of glycoprotein IIb/IIIa receptor blocker abciximab on outcome in patients with acute coronary syndromes without early coronary revascularisation: the GUSTO IV-ACS randomised trial. Lancet. 2001;357:1915–1924. doi: 10.1016/s0140-6736(00)05060-1. [DOI] [PubMed] [Google Scholar]
- 33.Topol EJ. Reperfusion therapy for acute myocardial infarction with fibrinolytic therapy or combination reduced fibrinolytic therapy and platelet glycoprotein IIb/IIIa inhibition: the GUSTO V randomised trial. Lancet. 2001;357:1905–1914. doi: 10.1016/s0140-6736(00)05059-5. [DOI] [PubMed] [Google Scholar]
- 34.Kastrati A, Mehilli J, Schuhlen H, et al. A clinical trial of abciximab in elective percutaneous coronary intervention after pretreatment with clopidogrel. N Engl J Med. 2004;350:232–238. doi: 10.1056/NEJMoa031859. [DOI] [PubMed] [Google Scholar]
- 35.Cannon CP, McCabe CH, Wilcox RG, et al. Oral glycoprotein IIb/IIIa inhibition with orbofiban in patients with unstable coronary syndromes (OPUS-TIMI 16) trial. Circulation. 2000;102:149–156. doi: 10.1161/01.cir.102.2.149. [DOI] [PubMed] [Google Scholar]
- 36.International, randomized, controlled trial of lamifiban (a platelet glycoprotein IIb/IIIa inhibitor), heparin, or both in unstable angina. The PARAGON Investigators. Platelet IIb/IIIa Antagonism for the Reduction of Acute coronary syndrome events in a Global Organization Network. Circulation. 1998;97:2386–2395. doi: 10.1161/01.cir.97.24.2386. [DOI] [PubMed] [Google Scholar]
- 37.Global Organization Network (PARAGON)-B Investigators. Randomized, placebo-controlled trial of titrated intravenous lamifiban for acute coronary syndromes. Circulation. 2002;105:316–321. doi: 10.1161/hc0302.102573. [DOI] [PubMed] [Google Scholar]
- 38.A comparison of aspirin plus tirofiban with aspirin plus heparin for unstable angina. Platelet Receptor Inhibition in Ischemic Syndrome Management (PRISM) Study Investigators. N Engl J Med. 1998;338:1498–1505. doi: 10.1056/NEJM199805213382103. [DOI] [PubMed] [Google Scholar]
- 39.Inhibition of the platelet glycoprotein IIb/IIIa receptor with tirofiban in unstable angina and non-Q-wave myocardial infarction. Platelet Receptor Inhibition in Ischemic Syndrome Management in Patients Limited by Unstable Signs and Symptoms (PRISM-PLUS) Study Investigators. N Engl J Med. 1998;338:1488–1497. doi: 10.1056/NEJM199805213382102. [DOI] [PubMed] [Google Scholar]
- 40.Inhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromes. The PURSUIT Trial Investigators. Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy. N Engl J Med. 1998;339:436–443. doi: 10.1056/NEJM199808133390704. [DOI] [PubMed] [Google Scholar]
- 41.Lincoff AM, Bittl JA, Harrington RA, et al. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA. 2003;289:853–863. doi: 10.1001/jama.289.7.853. [DOI] [PubMed] [Google Scholar]
- 42.Second Symphony Investigators. Randomized trial of aspirin, sibrafiban, or both for secondary prevention after acute coronary syndromes. Circulation. 2001;103:1727–1733. doi: 10.1161/01.cir.103.13.1727. [DOI] [PubMed] [Google Scholar]
- 43.Comparison of sibrafiban with aspirin for prevention of cardiovascular events after acute coronary syndromes: a randomised trial. The SYMPHONY Investigators. Sibrafiban versus Aspirin to Yield Maximum Protection from Ischemic Heart Events Post-acute Coronary Syndromes. Lancet. 2000;355:337–345. [PubMed] [Google Scholar]
- 44.Topol EJ, Moliterno DJ, Herrmann HC, et al. Comparison of two platelet glycoprotein IIb/IIIa inhibitors, tirofiban and abciximab, for the prevention of ischemic events with percutaneous coronary revascularization. N Engl J Med. 2001;344:1888–1894. doi: 10.1056/NEJM200106213442502. [DOI] [PubMed] [Google Scholar]
- 45.Cohen M, Gensini GF, Maritz F, et al. The safety and efficacy of subcutaneous enoxaparin versus intravenous unfractionated heparin and tirofiban versus placebo in the treatment of acute ST-segment elevation myocardial infarction patients ineligible for reperfusion (TETAMI): a randomized trial. J Am Coll Cardiol. 2003;42:1348–1356. doi: 10.1016/s0735-1097(03)01040-4. [DOI] [PubMed] [Google Scholar]
- 46.Serruys PW, Unger F, Sousa JE, et al. Comparison of coronary-artery bypass surgery and stenting for the treatment of multivessel disease. N Engl J Med. 2001;344:1117–1124. doi: 10.1056/NEJM200104123441502. [DOI] [PubMed] [Google Scholar]
- 47.Baim DS, Cutlip DE, Midei M, et al. Final results of a randomized trial comparing the MULTI-LINK stent with the Palmaz-Schatz stent for narrowings in native coronary arteries. Am J Cardiol. 2001;87:157–162. doi: 10.1016/s0002-9149(00)01308-4. [DOI] [PubMed] [Google Scholar]
- 48.Brener SJ, Midei MG, Nukta D, et al. A randomized multicenter trial comparing a new, low-pressure versus a conventional coronary stent: primary results from the CONSERVE trial. J Invasive Cardiol. 2003;15:128–132. [PubMed] [Google Scholar]
- 49.Andersen HR, Nielsen TT, Rasmussen K, et al. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Engl J Med. 2003;349:733–742. doi: 10.1056/NEJMoa025142. [DOI] [PubMed] [Google Scholar]
- 50.Lansky AJ, Costa RA, Mintz GS, et al. Non-polymer-based paclitaxel-coated coronary stents for the treatment of patients with de novo coronary lesions: angiographic follow-up of the DELIVER clinical trial. Circulation. 2004;109:1948–1954. doi: 10.1161/01.CIR.0000127129.94129.6F. [DOI] [PubMed] [Google Scholar]
- 51.Wallentin L, Lagerqvist B, Husted S, et al. Outcome at 1 year after an invasive compared with a non-invasive strategy in unstable coronary-artery disease: the FRISC II invasive randomised trial. FRISC II Investigators. Fast Revascularisation during Instability in Coronary artery disease. Lancet. 2000;356:9–16. doi: 10.1016/s0140-6736(00)02427-2. [DOI] [PubMed] [Google Scholar]
- 52.Kastrati A, Dirschinger J, Boekstegers P, et al. Influence of stent design on 1-year outcome after coronary stent placement: a randomized comparison of five stent types in 1147 unselected patients. Catheter Cardiovasc Interv. 2000;50:290–297. doi: 10.1002/1522-726x(200007)50:3<290::aid-ccd5>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 53.Mauri L, Bonan R, Weiner BH, et al. Cutting balloon angioplasty for the prevention of restenosis: results of the Cutting Balloon Global Randomized Trial. Am J Cardiol. 2002;90:1079–1083. doi: 10.1016/s0002-9149(02)02773-x. [DOI] [PubMed] [Google Scholar]
- 54.Henderson RA, Pocock SJ, Sharp SJ, et al. Long-term results of RITA-1 trial: clinical and cost comparisons of coronary angioplasty and coronary-artery bypass grafting. Randomised Intervention Treatment of Angina. Lancet. 1998;352:1419–1425. doi: 10.1016/s0140-6736(98)03358-3. [DOI] [PubMed] [Google Scholar]
- 55.Henderson RA, Pocock SJ, Clayton TC, et al. Seven-year outcome in the RITA-2 trial: coronary angioplasty versus medical therapy. J Am Coll Cardiol. 2003;42:1161–1170. doi: 10.1016/s0735-1097(03)00951-3. [DOI] [PubMed] [Google Scholar]
- 56.Fox KA, Poole-Wilson PA, Henderson RA, et al. Interventional versus conservative treatment for patients with unstable angina or non-ST-elevation myocardial infarction: the British Heart Foundation RITA 3 randomised trial. Randomized Intervention Trial of unstable Angina. Lancet. 2002;360:743–751. doi: 10.1016/s0140-6736(02)09894-x. [DOI] [PubMed] [Google Scholar]
- 57.Han RO, Schwartz RS, Kobayashi Y, et al. Comparison of self-expanding and balloon-expandable stents for the reduction of restenosis. Am J Cardiol. 2001;88:253–259. doi: 10.1016/s0002-9149(01)01636-8. [DOI] [PubMed] [Google Scholar]
- 58.Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–1323. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 59.Grines CL, Cox DA, Stone GW, et al. Coronary angioplasty with or without stent implantation for acute myocardial infarction. Stent Primary Angioplasty in Myocardial Infarction Study Group. N Engl J Med. 1999;341:1949–1956. doi: 10.1056/NEJM199912233412601. [DOI] [PubMed] [Google Scholar]
- 60.Cannon CP, Weintraub WS, Demopoulos LA, et al. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med. 2001;344:1879–1887. doi: 10.1056/NEJM200106213442501. [DOI] [PubMed] [Google Scholar]
- 61.Stone GW, Ellis SG, Cox DA, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350:221–231. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]
- 62.Hansson L, Lindholm LH, Niskanen L, et al. Effect of angiotensin-converting-enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project (CAPPP) randomised trial. Lancet. 1999;353:611–616. doi: 10.1016/s0140-6736(98)05012-0. [DOI] [PubMed] [Google Scholar]
- 63.Black HR, Elliott WJ, Grandits G, et al. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trial. JAMA. 2003;289:2073–2082. doi: 10.1001/jama.289.16.2073. [DOI] [PubMed] [Google Scholar]
- 64.Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001;357:1385–1390. doi: 10.1016/s0140-6736(00)04560-8. [DOI] [PubMed] [Google Scholar]
- 65.Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 66.Japanese beta-Blockers and Calcium Antagonists Myocardial Infriction (JBCMI) Investigators. Comparison of the effects of beta blockers and calcium antagonists on cardiovascular events after acute myocardial infarction in Japanese subjects. Am J Cardiol. 2004;93:969–973. doi: 10.1016/j.amjcard.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 67.Hansson L, Hedner T, Lund-Johansen P, et al. Randomised trial of effects of calcium antagonists compared with diuretics and beta-blockers on cardiovascular morbidity and mortality in hypertension: the Nordic Diltiazem (NORDIL) study. Lancet. 2000;356:359–365. doi: 10.1016/s0140-6736(00)02526-5. [DOI] [PubMed] [Google Scholar]
- 68.Hansson L, Lindholm LH, Ekbom T, et al. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension-2 study. Lancet. 1999;354:1751–1756. doi: 10.1016/s0140-6736(99)10327-1. [DOI] [PubMed] [Google Scholar]
- 69.ALLHAT Officers and Coordinators for the ALLHAT Collabarative Research Group. The Anti-hypertensive and Lipid-Lowering Treatment to prevent Heart Attack Trial. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT) JAMA. 2002;288:2998–3007. doi: 10.1001/jama.288.23.2998. [DOI] [PubMed] [Google Scholar]
- 70.Koren MJ, Hunninghake DB. Clinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics: the alliance study. J Am Coll Cardiol. 2004;44:1772–1779. doi: 10.1016/j.jacc.2004.07.053. [DOI] [PubMed] [Google Scholar]
- 71.Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 72.Sever PS, Dahlof B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial - Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 73.Andrews TC, Ballantyne CM, Hsia JA, Kramer JH. Achieving and maintaining National Cholesterol Education Program low-density lipoprotein cholesterol goals with five statins. Am J Med. 2001;111:185–191. doi: 10.1016/s0002-9343(01)00799-9. [DOI] [PubMed] [Google Scholar]
- 74.de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316. doi: 10.1001/jama.292.11.1307. [DOI] [PubMed] [Google Scholar]
- 75.Bays HE, Ose L, Fraser N, et al. A multicenter, randomized, double-blind, placebo-controlled, factorial design study to evaluate the lipid-altering efficacy and safety profile of the ezetimibe/simvastatin tablet compared with ezetimibe and simvastatin monotherapy in patients with primary hypercholesterolemia. Clin Ther. 2004;26:1758–1773. doi: 10.1016/j.clinthera.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 76.Bruckert E, Lievre M, Giral P, et al. Short-term efficacy and safety of extended-release fluvastatin in a large cohort of elderly patients. Am J Geriatr Cardiol. 2003;12:225–231. doi: 10.1111/j.1076-7460.2003.02000.x. [DOI] [PubMed] [Google Scholar]
- 77.Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 78.Insull W, Jr, Isaacsohn J, Kwiterovich P, et al. Efficacy and safety of cerivastatin 0.8mg in patients with hypercholesterolaemia: the pivotal placebo-controlled clinical trial. Cerivastatin Study Group. J Int Med Res. 2000;28:47–68. doi: 10.1177/147323000002800201. [DOI] [PubMed] [Google Scholar]
- 79.Karalis DG, Ross AM, Vacari RM, et al. Comparison of efficacy and safety of atorvastatin and simvastatin in patients with dyslipidemia with and without coronary heart disease. Am J Cardiol. 2002;89:667–671. doi: 10.1016/s0002-9149(01)02337-2. [DOI] [PubMed] [Google Scholar]
- 80.Dujovne CA, Knopp R, Kwiterovich P, et al. Randomized comparison of the efficacy and safety of cerivastatin and pravastatin in 1,030 hypercholesterolemic patients. The Cerivastatin Study Group. Mayo Clin Proc. 2000;75:1124–1132. doi: 10.4065/75.11.1124. [DOI] [PubMed] [Google Scholar]
- 81.Strandberg TE, Feely J, Sigurdsson EL. Twelve-week, multicenter, randomized, open-label comparison of the effects of rosuvastatin 10 mg/d and atorvastatin 10 mg/d in high-risk adults: a DISCOVERY study. Clin Ther. 2004;26:1821–1833. doi: 10.1016/j.clinthera.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 82.Serruys PW, Foley DP, Jackson G, et al. A randomized placebo-controlled trial of fluvastatin for prevention of restenosis after successful coronary balloon angioplasty; final results of the fluvastatin angiographic restenosis (FLARE) trial. Eur Heart J. 1999;20:58–69. doi: 10.1053/euhj.1998.1150. [DOI] [PubMed] [Google Scholar]
- 83.Athyros VG, Papageorgiou AA, Mercouris BR, et al. Treatment with atorvastatin to the National Cholesterol Educational Program goal versus ‘usual’ care in secondary coronary heart disease prevention. The GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) study. Curr Med Res Opin. 2002;18:220–228. doi: 10.1185/030079902125000787. [DOI] [PubMed] [Google Scholar]
- 84.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 85.Pravastatin use and risk of coronary events and cerebral infarction in Japanese men with moderate hypercholesterolemia: the Kyushu Lipid Intervention Study. J Atheroscler Thromb. 2000;7:110–121. doi: 10.5551/jat1994.7.110. [DOI] [PubMed] [Google Scholar]
- 86.Sasaki S, Nakagawa M, Nakata T, et al. Effects of pravastatin on exercise electrocardiography test performance and cardiovascular mortality and morbidity in patients with hypercholesterolemia: Lipid Intervention Study in Kyoto. Circ J. 2002;66:47–52. doi: 10.1253/circj.66.47. [DOI] [PubMed] [Google Scholar]
- 87.Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med. 1998;339:1349–1357. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 88.Serruys PW, de Feyter P, Macaya C, et al. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;287:3215–3222. doi: 10.1001/jama.287.24.3215. [DOI] [PubMed] [Google Scholar]
- 89.Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA. 2001;285:1711–1718. doi: 10.1001/jama.285.13.1711. [DOI] [PubMed] [Google Scholar]
- 90.Schuster H, Barter PJ, Stender S, et al. Effects of switching statins on achievement of lipid goals: Measuring Effective Reductions in Cholesterol Using Rosuvastatin Therapy (MERCURY I) study. Am Heart J. 2004;147:705–713. doi: 10.1016/j.ahj.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 91.Olsson AG, Pauciullo P, Soska V, et al. Comparison of the efficacy and tolerability of fluvastatin extended-release and immediate-release formulations in the treatment of primary hypercholesterolemia: a randomized trial. Clin Ther. 2001;23:45–61. doi: 10.1016/s0149-2918(01)80029-1. [DOI] [PubMed] [Google Scholar]
- 92.Thompson PL, Meredith I, Amerena J, et al. Effect of pravastatin compared with placebo initiated within 24 h of onset of acute myocardial infarction or unstable angina: the Pravastatin in Acute Coronary Treatment (PACT) trial. Am Heart J. 2004;148:e2. doi: 10.1016/j.ahj.2003.10.052. [DOI] [PubMed] [Google Scholar]
- 93.Eriksson M, Hadell K, Holme I, et al. Compliance with and efficacy of treatment with pravastatin and cholestyramine: a randomized study on lipid-lowering in primary care. J Intern Med. 1998;243:373–380. doi: 10.1046/j.1365-2796.1998.00294.x. [DOI] [PubMed] [Google Scholar]
- 94.Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 95.Jones PH, Davidson MH, Stein EA, et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial) Am J Cardiol. 2003;92:152–160. doi: 10.1016/s0002-9149(03)00530-7. [DOI] [PubMed] [Google Scholar]
- 96.Marz W, Wollschlager H, Klein G, et al. Safety of low-density lipoprotein cholestrol reduction with atorvastatin versus simvastatin in a coronary heart disease population (the TARGET TANGIBLE trial) Am J Cardiol. 1999;84:7–13. doi: 10.1016/s0002-9149(99)00183-6. [DOI] [PubMed] [Google Scholar]
- 97.Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 98.Sabatine MS, Cannon CP, Gibson CM, et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med. 2005;352:1179–1189. doi: 10.1056/NEJMoa050522. [DOI] [PubMed] [Google Scholar]
- 99.Bertrand ME, Rupprecht HJ, Urban P, et al. Double-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting: the clopidogrel aspirin stent international cooperative study (CLASSICS) Circulation. 2000;102:624–629. doi: 10.1161/01.cir.102.6.624. [DOI] [PubMed] [Google Scholar]
- 100.Steinhubl SR, Berger PB, Mann JT, III, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288:2411–2420. doi: 10.1001/jama.288.19.2411. [DOI] [PubMed] [Google Scholar]
- 101.Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 102.Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755–1762. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 103.de Gaetano G. Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Collaborative Group of the Primary Prevention Project. Lancet. 2001;357:89–95. doi: 10.1016/s0140-6736(00)03539-x. [DOI] [PubMed] [Google Scholar]
- 104.Scrutinio D, Cimminiello C, Marubini E, et al. Ticlopidine versus aspirin after myocardial infarction (STAMI) trial. J Am Coll Cardiol. 2001;37:1259–1265. doi: 10.1016/s0735-1097(01)01164-0. [DOI] [PubMed] [Google Scholar]
- 105.Leon MB, Baim DS, Popma JJ, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med. 1998;339:1665–1671. doi: 10.1056/NEJM199812033392303. [DOI] [PubMed] [Google Scholar]
- 106.Taniuchi M, Kurz HI, Lasala JM. Randomized comparison of ticlopidine and clopidogrel after intracoronary stent implantation in a broad patient population. Circulation. 2001;104:539–543. doi: 10.1161/hc3001.093435. [DOI] [PubMed] [Google Scholar]
- 107.Cruz-Fernandez JM, Lopez-Bescos L, Garcia-Dorado D, et al. Randomized comparative trial of triflusal and aspirin following acute myocardial infarction. Eur Heart J. 2000;21:457–465. doi: 10.1053/euhj.1999.1874. [DOI] [PubMed] [Google Scholar]
- 108.Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. The Medical Research Council's General Practice Research Framework. Lancet. 1998;351:233–241. [PubMed] [Google Scholar]
- 109.Hurlen M, Abdelnoor M, Smith P, et al. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2002;347:969–974. doi: 10.1056/NEJMoa020496. [DOI] [PubMed] [Google Scholar]
- 110.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]