Abstract
Vascular tissue engineering should provide more biocompatible and functional conduits than synthetic vascular grafts. Understanding the cell-scaffold interactions and developing an efficient delivery system for growth factors and other biomolecules to control the signaling between the cells and the scaffold are fundamental issues in a wide range of tissue engineering research fields. Type I collagen is a natural scaffold extensively used in vascular tissue engineering and is a widely used vehicle in biomolecule delivery. It this paper, we will discuss type I collagen as a vascular tissue engineering scaffold, describe strategies for elucidating the interaction between cells and type I collagen scaffolds using various imaging techniques and summarize our work on the development of a chimeric collagen binding growth factor-based local delivery system.
Keywords: type I collage, growth factor, confocal microscopy, local delivery, scaffold
In the United States, cardiovascular disease has been the leading cause of death for many years; nearly 2300 Americans died of cardiovascular disease each day and coronary heart disease caused approximately 1 of every 6 deaths in the United States in 2006.[1] Bypasses are widely used to treat vascular diseases and in 2006, 448 000 inpatient bypass procedures were performed in the United States.[1] However, there are still a large number of patients who don’t have suitable vessels for the surgery[2] and thus require synthetic vessels as alternatives.[3, 4] Because of the drawbacks of the synthetic vessels, efforts have been made in vascular tissue engineering to develop biocompatible functional conduits.[5, 6] Cells, scaffolds and biomolecular signals are the three principle components of tissue engineering.[7] An ideal scaffold should mimic the biochemical and biomechanical properties of the extra cellular matrix of the tissue to be engineered and should support vascular cell viability and optimal phenotypic characterization. An ideal tissue engineering system also requires better control of the biomolecular signals between cells and scaffold. An efficient way to regulate and target the signal is to deliver desired growth factors locally to the cells. In this paper, we will focus on type I collagen - one of the most widely used natural scaffolds. We will cover the application of type I collagen as a vascular tissue engineering scaffold, describe strategies for elucidating the interaction between cells and type I collagen-based 3-D scaffolds and discuss a local growth factor delivery strategy based on a chimeric collagen binding growth factor using type 1 collagen as the delivery vehicle.
Application of type I collagen as scaffold for vascular tissue engineering
Both synthetic and natural materials have been used as scaffolds for vascular tissue engineering. Even though the chemical properties of synthetic materials are controllable and reproducible, the biocompatibility, biomechanical stability, immunoreactivity and and infectability and inflammation remain as unmet challenges. Natural scaffold are commonly large molecule components of the extracellular matrix (ECM) in vivo, or have similar macromolecular properties to natural ECM, and include collagen,[8, 9] fibrin,[10] hyaluronic acid,[11] alginate,[12] chitosan.[13] Collagens comprise 25% of the total protein mass of most mammals and have minimal cross-species immunological reactivity.[14] Most hydrogel-based scaffolds do not naturally adhere cells nor promote cell function. Collagen is a rare exception because it is rich of integrin-binding domains which enhance cell attachment and growth. At least 19 different types of collagen have been confirmed. The collagen molecule is a three-stranded structure consisting of three polypeptide chains twined around one another; and each chain has an individual twist in opposite directions. [14] The strands are held together primarily by hydrogen bonds and also by covalent bonds providing supplementary binding. The molecular weight of the basic collagen molecule is about 300 kDa and the length and width of a rod-shaped collagen fiber is 300 and 1.5 nanometers respectively.[14] Among all the types of collagen, type I collagen is the most favorable for vascular tissue engineering because it is a major ECM protein within the wall of a blood vessel, and SMCs and fibroblasts naturally reside in collagen-rich matrices. It is also relatively easily shaped into tubular configurations.[15, 16] Therefore it has been extensively used in vascular tissue engineering from the first tissue engineered blood vessel[17] until today.
Elucidation of cell-type 1 collagen scaffold interactions
In 1986, Weinberg and Bell[17] established a model for vascular tissue engineering which used smooth muscle cell-populated collagen to construct the media layer of blood vessel. It was a milestone in tissue engineering history. However, they failed to show the requisite mechanical strength. In 1993, L’Heureux et. al. modified this model using cells from different sources, but encountered the same problem.[18] Recently, scientists began to understand that the biomechanical strength of a hydrogel-based tissue engineered blood vessel (TEBV) is dependent largely upon hydrogel compaction by smooth muscle cells (SMCs) or fibroblasts within the scaffolds. Various methods have been employed to increase the compaction by SMCs and hthereby increase the mechanical strength of the engineered vessel, including in vitro mechanical preconditioning or biochemical stimulation of SMCs. SMCs contribute most of the contractility of an artery and the three dimensional extracellular matrix, where SMCs reside, is composed primarily of type I collagen. It is therefore critical to understand the mechanisms by which cells interact with the matrix in order to facilitate the engineering of a better TEBV.
Collagen remodeling has been extensively studied with some traditional techniques, performed on a fixed sample with an electron microscope or at a macroscopic level by measuring the changes of length, width and height of the collagen-cell constructions.[19, 20] Both methods have provided limited information. Cells remodel scaffolds by continuously rearranging of the microstructure of the scaffold as cells remodel the surrounding matrix either in vivo or in vitro. Therefore, it is important to understand their interaction dynamically. Moreover, in growth factor-incorporated systems, cells will alter the release of growth factors dynamically. Growth factors are in the namometer range and, even though they are commonly clustered, the aggregated growth factor microspheres are still at the micrometer level. Therefore it is also important to study the these dynamics with high resolution. We have developed a new microscopic approach to better understand their temporal and spatial interaction dynamics in six dimensions: three spacial dimensions (X, Y, and Z), the time dimension, a spectral dimension (multichannel of fluorophores) and a multi-position dimension (mosaic imaging).[9] First live time lapse phase imaging enables long term dynamic study of collagen remodeling, and the locomotion characteristics of the cells were studied with computer assisted cell tracking. Fluorescent and reflection confocal microscopy were used to acquire images and quantification of microscale changes of both smooth muscle cells and collagen fibers during remodeling. Even though the time lapses z-stack images were acquired, visualizing and presenting the 3-D structure dynamically is still challenging. In our lab, we developed two image processing techniques to accomplish real 3-D visualization. First, 3-D volume rendered images were generated at each time point, and a 3-D video was generated using the 3-D images as frames. This 3-D presentation is the first report in the literature as far as we know. Another image processing technique which we developed for dynamic visualization is the multi-window dynamics view which enables viewing the multiple focal planes at each time point.
It is well known that cell contractility, which is mediated by the cytoskeletal system, plays a major in role in matrix remodeling, cell-matrix interaction and growth factor release. However, little has been reported on direct simultaneous viewing of cell cytoskeleton component changes and related scaffold changes at the microscale level. In our lab, we used our confocal micrscopic techniques to directly visualize the α-smooth muscle actin (α-SMA) fibers and collagen fibers. The directional relationship between actin fibers and collagen fibers was analyzed by Fast Fourier Transform (FFT) and the results provided quantitative information showing that actin fibers play an important role in collagen scaffold remodeling. Reflection confocal microscopy provides non-label and noninvasive means to visualize collagen fiber reorganization at high resolution. However, one limitation is that under the high magnification objective and within one visual field, very few cells, often only one, can be viewed. Data based on few cells is too limited and arbitrary judgments may occur. To balance the resolution and field of view requirements, mosaic imaging is a good choice. Some two dimensional mosaic techniques have been successfully used in studies of the ocean-floor[21] and of X-ray[22] images. Applying mosaic techniques to 3-D collagen matrix and cells is challenging because of the complex structure. Moreover, most of previous mosaic imaging studies were based on motor-controlled stages at low magnification. Image stitching using a motor-controlled stage may generate mismatched images because of the limitation of the accuracy of the stage as well as the hydrogel sample which may independently move as the stage moves. These errors are tolerable in low magnification images but not in high magnification images. The solution for this 3-D hydrogel mosaic imaging is to use software image registration to stitch the images. In other words, the software finds the overlay parts of two adjacent images and corrects image position including tilt. The speed and accuracy were also improved by generating a position file from the cell channels, which have simpler structure compared with the collagen channel. The position information in the file was then used to adjust the images in the collagen channel. Stack mosaic imaging was simply achieved by using the same position file in the other focal planes. Thus, we created a simple and cost effective way to generate multi-channel, multi-focal plane mosaic imaging without using an expensive motor controlled stage while simultaneously eliminating the mechanical errors.
Quantification of the degree of scaffold remodeling is another step we took to study the cell-scaffold interaction. A traditional way of quantifying the spatial changes of collagen scaffolds is measuring the dimension changes of the collagen gel globally, an approach which limited the study to the macroscale level. Low accuracy and limited information generated from macroscale studies have led to an incomplete understanding of the remodeling process. In our lab, perform the quantification at the microscale level by analyzing the digitized confocal images at micrometer level, enabling a better understanding of the mechanism of remodeling. We quantify the compaction in the remodeling process, but also, by FFT, the alignment, which cannot be accomplished in more traditional techniques. After the transform, total orientation of both cells and collagen fibers are clearly shown and the Alignment Index is generated from the spectral plot, which provides quantitative information.
Application of a growth factor delivery strategy using type I collagen as the delivery vehicle
Natural polymers and their derivatives in the form of gels and sponges have been used extensively as delivery vehicles. Collagen in particular is a readily available ECM component that allows cell infiltration and remodeling, making it highly suitable for biomolecule delivery. To the best of our knowledge, the first description of using collagen as a delivery vehicle was reported in 1973.[23] Since then various collagen based delivery systems have been used (table 1).
Table 1.
Different forms of collagen used to delivery various biomolecules
| Biomolecules delivered | Collagen form | Representative ref. |
|---|---|---|
| Pil | Collagen gel | 23 |
| INS, GH | collagen gel | 24 |
| RA | collagen sponge | 25 |
| 5-FU | collagen sponge | 26 |
| GM, VCM | collagen gel | 27 |
| TGF-beta2 | Injectable collagen gel | 28 |
| NGF beta | collagen/HA composite | 29 |
| TGF-beta1 | collagen-HA/TCP microspheres | 30 |
| bBMP | collagen/HA composite | 31 |
| rhBMP-2 | collagen sponge | 32 |
| VEGF | collagen vitrigel membrane | 33 |
| R136K-CBD | collagen gel | 8 |
Abbreviations: Pil: pilocarpine; INS: insulin; GH: growth hormone; RA: retinoic acid; 5-FU: 5-fluorouracil; GM: gentamicin; VCM: vancomycin; TGF: transforming growth factor; NGF: Nerve growth factor; BMP: bone morphogenetic proteins; VEGF: vascular endothelial growth factor; R136K: relatively thrombin-resistant mutant derivative of FGF-1; CBD: collagen binding domain.
Most of the collagen based delivery system just trap the biomolecules inside the collagen fibril networks and release largely depends on micro dimension of the collagen fibers and growth factors. A major drawback to this delivery strategy is that the time course is not well controlled. There is typically a burst release within the first few hours and total release time is comparatively short. This is partly due to the large pore size of the scaffold material compared with the relatively small size of most growth factors. For example, the pore size of type I collagen scaffolds commonly varies from 66.8 μm (at 1.67mg/ml concentration) to 37.5 μm (at 2.5mg/ml concentration),[24] while the size of most growth factors are at the nanometer level or lower. Decreasing the pore size of collagen by increasing the gel concentration may extend the retention of the growth factor inside the collagen hydrogel. However, this reduces the nutrient transport and limits cell growth and viability. In order to overcome these problems, we constructed a chimeric collagen binding fusion growth factor - R136K-CBD. It consists of R136K, which is a relatively thrombin-resistant mutant derivative of FGF-1, and a collagen binding domain (CBD). [25] We demonstrated that R136K-CBD has a significantly higher binding affinity to a 3-D collagen scaffold than FGF-1 and R136K. Furthermore, R136K-CBD showed still greater binding affinity compared with FGF-1 and R136K in a cell populated collagen gel. We then fluorescently labeled R136K-CBD and by confocal microscopic imaging and analysis, we found that R136K-CBD shows uniform distribution throughout collagen gel, which suggests that incorporated R136K-CBD would likely have an essentially uniform biologic effect on SMCs. Growth factor internalization was directly visualized by our microcopic techniques. Both R136K-CBD and the SMCs were fluorescently labeled (different labels) and incubated within the collagen hydrogel. The SMCs were then isolated from the collagen and the internalized R136-CBD was found to be localized to cytoplasm and the perinuclear region. To better understand the delivery mechanism, the internalization was also studied in 3-D in an “on-site” visualization mode without isolating SMCs from the collagen hydrogel. R136K-CBD binding to collagen fibers was visualized by reflection and fluorescent confocal microscopy. By image volume rendering of SMCs and collagen fibers, their spatial relationships were clearly visualized. More importantly, R136K-CBD elicited a greater mitogenic effect on SMCs in the 3-D collagen scaffold. Up to 7 days, R136K-CBD-stimulated groups showed continuous increasing cell proliferation whereas the proliferation rate dropped in R136K- and FGF-1-stimulated groups. At Day 7, R136K-CBD demonstrated a 2.0 and 2.1 fold greater mitogenic effect than R136K and FGF-1, respectively.
Future directions
Our current microscopic studies on dynamic interactions between cells and collagen are on samples without mechanical stimulation. Since mechanical stimulation has been shown to increase the mechanical strength of collagen-cell constructs, we are developing techniques to dynamically image the sample while under mechanical stimulation. For collagen binding domain-based delivery systems, more complex delivery strategies are being developed to accomplish both short term and long term delivery of multiple peptides, including the encapsulation of the CBD-growth factor in biodegradable microspheres allowing its binding to the collagen following its release from the microspheres and eventually internalization by the cells.
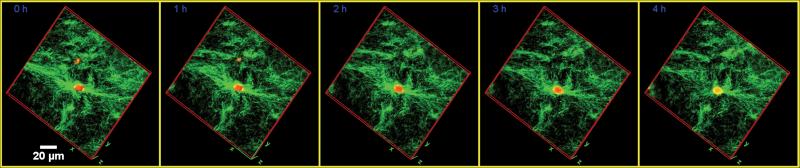
Figure 1.
Time lapse images of SMCs interacting with type I collagen scaffold. SMCs labeled with PKH26 and collagen fibers are visualized by florescent and reflection confocal microcopy respectively. 3-D volume rendered images are made at each time point.
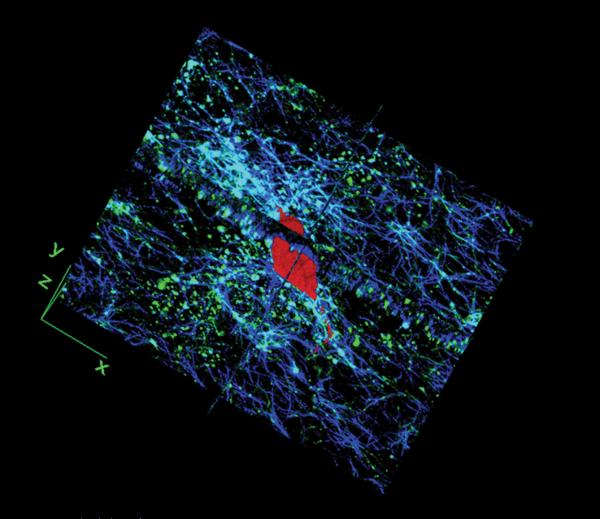
Figure 2.
The SMC (red) is inside collagen scaffold (blue) and R136K-CBD (green) binds to collagen fibers. R136K-CBD and collagen fibers are shown in orthogonal view.
Acknowledgments
This project was supported by grants from the NIH R01-HL41272 (HPG), Department of Veteran’s Affairs (HPG) and Chicago Association for Research and Education in Science(HPG).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lloyd-Jones D, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Campbell JH, Efendy JL, Campbell GR. Novel vascular graft grown within recipient’s own peritoneal cavity. Circ Res. 1999;85(12):1173–8. doi: 10.1161/01.res.85.12.1173. [DOI] [PubMed] [Google Scholar]
- 3.Bader A, et al. Engineering of human vascular aortic tissue based on a xenogeneic starter matrix. Transplantation. 2000;70(1):7–14. [PubMed] [Google Scholar]
- 4.Xue L, Greisler HP. Biomaterials in the development and future of vascular grafts. J Vasc Surg. 2003;37(2):472–80. doi: 10.1067/mva.2003.88. [DOI] [PubMed] [Google Scholar]
- 5.Stegemann JP, Kaszuba SN, Rowe SL. Review: advances in vascular tissue engineering using protein-based biomaterials. Tissue Eng. 2007;13(11):2601–13. doi: 10.1089/ten.2007.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas AC, Campbell GR, Campbell JH. Advances in vascular tissue engineering. Cardiovasc Pathol. 2003;12(5):271–6. doi: 10.1016/s1054-8807(03)00086-3. [DOI] [PubMed] [Google Scholar]
- 7.Griffith LG, Naughton G. Tissue engineering--current challenges and expanding opportunities. Science. 2002;295(5557):1009–14. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 8.Pang Y, et al. Local delivery of a collagen-binding FGF-1 chimera to smooth muscle cells in collagen scaffolds for vascular tissue engineering. Biomaterials. 31(5):878–85. doi: 10.1016/j.biomaterials.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pang Y, et al. The temporal and spatial dynamics of microscale collagen scaffold remodeling by smooth muscle cells. Biomaterials. 2009;30(11):2023–31. doi: 10.1016/j.biomaterials.2008.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xue L, Greisler HP. Angiogenic effect of fibroblast growth factor-1 and vascular endothelial growth factor and their synergism in a novel in vitro quantitative fibrin-based 3-dimensional angiogenesis system. Surgery. 2002;132(2):259–67. doi: 10.1067/msy.2002.125720. [DOI] [PubMed] [Google Scholar]
- 11.Tan H, et al. Thermosensitive injectable hyaluronic acid hydrogel for adipose tissue engineering. Biomaterials. 2009;30(36):6844–53. doi: 10.1016/j.biomaterials.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stevens MM, et al. A rapid-curing alginate gel system: utility in periosteum-derived cartilage tissue engineering. Biomaterials. 2004;25(5):887–94. doi: 10.1016/j.biomaterials.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Jiang T, et al. Biologically active chitosan systems for tissue engineering and regenerative medicine. Curr Top Med Chem. 2008;8(4):354–64. doi: 10.2174/156802608783790974. [DOI] [PubMed] [Google Scholar]
- 14.Lee CH, Singla A, Lee Y. Biomedical applications of collagen. Int J Pharm. 2001;221(1-2):1–22. doi: 10.1016/s0378-5173(01)00691-3. [DOI] [PubMed] [Google Scholar]
- 15.Stegemann JP, Nerem RM. Altered response of vascular smooth muscle cells to exogenous biochemical stimulation in two- and three-dimensional culture. Exp Cell Res. 2003;283(2):146–55. doi: 10.1016/s0014-4827(02)00041-1. [DOI] [PubMed] [Google Scholar]
- 16.Miller EJ, Rhodes RK. Preparation and characterization of the different types of collagen. Methods Enzymol. 1982;82(Pt A):33–64. doi: 10.1016/0076-6879(82)82059-4. [DOI] [PubMed] [Google Scholar]
- 17.Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231(4736):397–400. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- 18.L’Heureux N, et al. In vitro construction of a human blood vessel from cultured vascular cells: a morphologic study. J Vasc Surg. 1993;17(3):499–509. doi: 10.1067/mva.1993.38251. [DOI] [PubMed] [Google Scholar]
- 19.Li S, et al. Signal transduction in matrix contraction and the migration of vascular smooth muscle cells in three-dimensional matrix. J Vasc Res. 2003;40(4):378–88. doi: 10.1159/000072702. [DOI] [PubMed] [Google Scholar]
- 20.Franco C, et al. Doxycycline alters vascular smooth muscle cell adhesion, migration, and reorganization of fibrillar collagen matrices. Am J Pathol. 2006;168(5):1697–709. doi: 10.2353/ajpath.2006.050613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marks RL, Rock SM, Lee MJ. Real-time video mosaicking of the ocean floor. Oceanic Engineering, IEEE Journal of. 1995;20(3):229–241. [Google Scholar]
- 22.Loo BW, Jr., Meyer-Ilse W, Rothman SS. Automatic image acquisition, calibration and montage assembly for biological X-ray microscopy. J Microsc. 2000;197(Pt 2):185–201. doi: 10.1046/j.1365-2818.2000.00644.x. [DOI] [PubMed] [Google Scholar]
- 23.Rubin AL, et al. Collagen as a vehicle for drug delivery. Preliminary report. J Clin Pharmacol. 1973;13(8):309–12. doi: 10.1002/j.1552-4604.1973.tb00217.x. [DOI] [PubMed] [Google Scholar]
- 24.Friedl P, et al. Migration of highly aggressive MV3 melanoma cells in 3-dimensional collagen lattices results in local matrix reorganization and shedding of alpha2 and beta1 integrins and CD44. Cancer Res. 1997;57(10):2061–70. [PubMed] [Google Scholar]
- 25.Brewster LP, et al. Construction and characterization of a thrombin-resistant designer FGF-based collagen binding domain angiogen. Biomaterials. 2008;29(3):327–36. doi: 10.1016/j.biomaterials.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]