Abstract
Consequences of cardiac myocyte-specific ablation of KATP channels in transgenic mice expressing dominant negative Kir6 subunits. Am J Physiol Heart Circ Physiol 291: H543–H551, 2006. First published February 24, 2006; doi:10.1152/ajpheart.00051.2006.—Cardiac ATP-sensitive K+ (KATP) channels are formed by Kir6.2 and SUR2A subunits. We produced transgenic mice that express dominant negative Kir6.x pore-forming subunits (Kir6.1-AAA or Kir6.2-AAA) in cardiac myocytes by driving their expression with the α-myosin heavy chain promoter. Weight gain and development after birth of these mice were similar to nontransgenic mice, but an increased mortality was noted after the age of 4–5 mo. Transgenic mice lacked cardiac KATP channel activity as assessed with patch clamp techniques. Consistent with a decreased current density observed at positive voltages, the action potential duration was increased in these mice. Some myocytes developed EADs after isoproterenol treatment. Hemodynamic measurements revealed no significant effects on ventricular function (apart from a slightly elevated heart rate), whereas in vivo electrophysiological recordings revealed a prolonged ventricular effective refractory period in transgenic mice. The transgenic mice tolerated stress less well as evident from treadmill stress tests. The proarrhythmogenic features and lack of adaptation to a stress response in transgenic mice suggest that these features are intrinsic to the myocardium and that KATP channels in the myocardium have an important role in protecting the heart from lethal arrhythmias and adaptation to stress situations.
Keywords: potassium channels, ATP-sensitive K+ channel, heart, ventricle, stress responses
Atp-Sensitive (KATP) channels are prominently expressed in the heart. They were first described in ventricular myocytes by use of the patch clamping technique (22, 36). Under pathophysio-logical states, a clear role for KATP channels has been identified, including their participation in K+ loss from the ischemic heart, arrhythmogenesis, and the protection afforded by ischemic preconditioning (7, 23, 28). Ventricular KATP channels are expressed at a high density (21). Despite the abundant expression of KATP channels in ventricular myocytes, the physiological role that these channels may have in ventricular myocytes remains poorly elucidated at best.
At the molecular level, KATP channels are understood to be multisubunit protein complexes. Transmembrane Kir6 pore-forming subunits allow K+ ions to permeate the channel complex, whereas SUR accessory subunits serve to act as receptors for a variety of pharmacological compounds that promote or inhibit KATP channel opening (28). The Kir6.x subunit subfamily consists of two members, Kir6.1 and Kir6.2. Similarly, two SUR subunits exist (SUR1 and SUR2) (28). Alternative splicing of SUR2 gives rise to at least two functionally relevant isoforms (SUR2A and SUR2B) with distinct pharmacological profiles (28). Because KATP channels are hetero-octameric complexes consisting of four Kir6.x and four SURx subunits (30), in principle a large degree of freedom for subunit assembly is possible. This heterogeneous assembly of KATP channels may explain, in part, the large diversity of native KATP channels found in various tissue types (1). The consensus is that ventricular KATP channels consist of Kir6.2/ SUR2A heteromultimers and that KNDP channels in vascular smooth muscle consist of Kir6.1 and SUR2B subunits (28). Gene targeting approaches confirm an important role for these subunits in the respective tissues because Kir6.2(−/−) mice lack functional sarcolemmal KATP channels and both Kir6.1(−/−) and SUR2(−/−) mice develop coronary abnormalities (2, 17). However, these results do not explain the presence of Kir6.1 or SUR1 in cardiac myocytes (17, 19, 32) or the observation that anti-SUR1 antisense oligonucleotides inhibit KATP channels of ventricular myocytes (41). Thus the molecular composition of KATP channels and the functional roles of the individual subunits may therefore not be as clear-cut as previously thought. Gene targeting experiments demonstrated that (as expected) Kir6.2-deficient mice do not have functional KATP channels in ventricular myocytes (33). Although the initial report suggested no apparent cardiac abnormalities, subsequent studies demonstrated that Kir6.2(−/−) mice fail to adapt to sympathetic stress. They develop lethal cardiac arrhythmias and sudden death with sympathetic challenge (42). Because Kir6.2 subunits are lost in all tissues (central and peripheral nervous tissue, heart, and other tissues), it is not possible from these experiments to conclude whether these defects originate primarily from the absence of Kir6.2 in the heart or whether other systemic malfunctions may be involved (the sympathetic nervous system, for example). We developed transgenic mouse lines to specifically target KATP channels in the ventricular myocyte by driving Kir6.x dominant negative subunit expression by the α-myosin heavy chain promoter. We show that these mice have many of the hallmarks of the Kir6.2(−/−) mice (including intolerance to exercise stress). We conclude that the functional roles of KATP channels in the intact mouse (particularly under stress conditions) originate at least in part from the role of these channels within the cardiac myocyte.
MATERIALS AND METHODS
For a full description of the methods, please see the online supplement (http://ajpheart.physiology.org/cgi/content/full/00051.2006/DC1).
Production of transgenic constructs
Amino acid residues in the rat Kir6.1 and mouse Kir6.2 subunit pore regions (Gly-Phe-Gly) were mutated to Ala-Ala-Ala by site-directed mutagenesis (QuickChange, Stratagene). The Kir6.1-AAA construct was COOH-terminal tagged with enhanced green fluorescent protein (eGFP) and the Kir6.2-AAA had a FLAG epitope. The constructs were subcloned into the pBS-αMHC vector (Dr. J. Robbins, University of Cincinnati, Cincinnati, OH).
Generation of transgenic mice
The transgenic cassettes were excised, purified, and microinjected into the pronuclei of fertilized FVB/N zygotes. The injections were performed at the Transgenic/ES cell Core Facility (Skirball Institute, New York University School of Medicine). Surviving embryos were reimplanted into pseudopregnant FVB/N foster mothers. Genotyping was performed by PCR.
RNA extraction and semi-quantitative RT-PCR
RNA was prepared from mouse hearts. After reverse transcription (Promega), PCR was performed using the following primers: Kir6.2: (1065F: 5′-TTCACCATGTCCTTCCTGTG-3′) and (1008R: 5′-ACCAATGGTCACCTGGACCTC-3′, product: 173 bp); Kir6.2AAA-FLAG: (1077F and 1272R, product: 350 bp).
Western blotting
Western blotting was performed using crude membrane fractions obtained from mouse heart and brain using previously described procedures (18). Equal amount of proteins were separated on 12% SDS-PAGE, transferred to polyvinylidene difluoride membranes, and immunoblotted with anti-Kir6.1 NAF-1 (1: 1,500) or anti-Kir6.2 G16 antibody (1: 500, Santa Cruz Biotechnology). The secondary antibodies were horseradish peroxidase conjugated, and detection was by chemiluminescence.
Histology
Hearts were rapidly excised from mice after death with CO2, rinsed in Dulbecco's PBS, and fixed in 10% formalin. Sections of 5-μm thickness were cut from paraffin-embedded tissue and stained with hematoxylin and eosin.
In vivo electrocardiographic and electrophysiological studies
Electrocardiographic and electrophysiological parameters were examined as described previously (10). Programmed electrical stimulation was performed consisting of a train of eight beats followed by a single extrastimulus for the determination of ventricular effective refractory period and double extrastimuli to test for inducible arrhythmias. Arrhythmias are defined as described previously (10, 37). To examine the electrophysiological response to β-adrenergic stimulation (42), we repeated measurements of electrocardiographic and electrophysiological parameters after administration of dobutamine (100 μg/kg ip).
Hemodynamic studies
Mice were anesthetized with Avertin, intubated endotracheally, and mechanically ventilated. A 1.4F Mikro-Tip catheter pressure transducer (Millar, Houston, TX) was placed into the right carotid and advanced retrograde into the left ventricle.
Epifluorescence measurements of epicardial electrical activity
Isolated hearts were perfused in the Langendorff mode and loaded with di-4-ANEPPS (Molecular Probes). High-resolution optical mapping of voltage-dependent fluorescence during normal sinus rhythm and pacing was acquired using a charge-coupled device camera (Dalsa). Conduction velocity was measured during epicardial pacing.
Treadmill exercise test
A two-track treadmill (Columbus Instruments, Columbus, OH) was used. The exercise-stress protocol consisted of stepwise increases in either incline or velocity at 3-min intervals. A shock grid at the end of the treadmill delivered a minimal painful stimulus to enforce running. Ten days before exercise stress, mice were acclimated daily. Animal protocols were approved by the Institutional Animal Care and Use Committee at the New York University.
Electrophysiological recordings in isolated ventricular myocytes
Whole-cell macroscopic currents were recorded at room temperature. The pipette solution consisted of (in mmol/l) 115 l-aspartate, 10 KCl, 5 EGTA, 5.92 MgCl2, 5 Na2ATP, and 10 HEPES, pH adjusted to 7.2 with KOH. The bath solution consisted of (in mmol/l) 143 NaCl, 5.4 KCl, 1.8 CaCl2, 0.5 MgCl2, 0.33 NaH2PO4, 5 glucose, and 5 HEPES, pH adjusted to 7.4 with NaOH. KATP channel currents were evoked by bath application of 100 μmol/l dinitrophenol (DNP; Sigma) and were recorded from a holding potential of −60 mV using a ramp (100 mV/s) pulse protocol from −120 to +40 mV, repeated at 1 Hz. Action potentials were recorded in current-clamp mode using pipettes filled with (in mmol/l) KCl 120, MgCl2 1, Na2ATP 5, HEPES 10, EGTA 0.5, and CaCl2 0.01.
Immunofluorescence microscopy
Immunocytochemistry was performed on isolated mouse cardiomyocytes as described (19). Primary antibodies used were rabbit anti-Kir6.1 (NAF1, 1:200) (19). The secondary antibodies used were Cy3-conjugated donkey anti-rabbit IgG (Jackson Imunoresearch). Some cells were loaded with Mito-Tracker red CMXRos (50 nmol/l; Molecular Probes) by incubating live cells for 20 min at room temperature.
Data analysis
Data are expressed as means ± SE. Significance between groups was determined by paired or unpaired t-tests, ANOVA, or the χ2 test. P < 0.05 was considered statistically significant.
RESULTS
Generation of transgenic mice lacking cardiac KATP channels
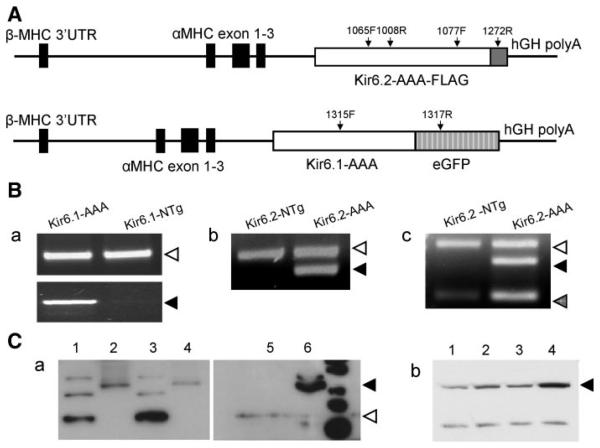
We utilized a dominant negative strategy to transgenically target KATP channels in ventricular myocytes. The pore regions of Kir6.1 or Kir6.2 subunits were mutated by replacing the Gly-Phe-Gly residues to Ala-Ala-Ala, which act to suppress wild-type Kir6 currents in a dominant negative manner (27). Expression of these subunits was driven by the cardiac-myocyte-specific α-myosin heavy chain (α-MHC) promoter (Fig. 1). α-MHC-Kir6.1-AAA-eGFP (hereafter referred as Kir6.1-AAA) transgenic mice were produced in a FVB/N background, whereas α-MHC-Kir6.2-AAA-FLAG (referred as Kir6.2-AAA) mice were in a C57BL/6 background. Both of these mice expressed the respective transgene specifically in the heart, as verified by RT-PCR and Western blotting (Fig. 1, B and C). Kir6.2 protein levels were higher in Kir6.2-AAA transgenic mice compared with their control littermates. However, Kir6.2 expression levels were similar in heart membrane fractions of Kir6.1-AAA transgenic mice and their control littermates. Similarly, by Northern blot we determined that native Kir6.1 mRNA expression was not affected in Kir6.2-AAA transgenic mouse hearts compared with their control littermates (data not shown). We produced several lines of each transgenic construct. None of them exhibited adverse morphological or functional phenotypes at an early age. The results shown here are from one line of each transgenic model.
Fig. 1.
Kir6.x transgenic construct, gene, and protein expression levels. A: transgenic cassettes. Top: α-myosin heavy chain (α-MHC)-Kir6.2AAA-FLAG construct. Boxes represent exons; solid boxes are α-MHC exons, open box represents the Kir6.2-AAA coding region, and shaded box represents the FLAG epitope. Arrows depict positions of the PCR primers used. Below is the α-MHC-Kir6.1AAA-eGFP construct (same shading as before, except that the shaded box represents the eGFP coding region). B.a: PCR-based genotyping of Kir6.1-AAA mice using tail genomic DNA as the template. As a control, primers were used to amplify GAPDH (452 bp). B.b: PCR-based genotyping using tail genomic DNA from Kir6.2-AAA mice as template. B.c: RT-PCR using as a template cDNA obtained from the hearts of Kir6.2-AAA mice and their control littermates. The 3 bands correspond to Kir6.2(168 bp), Kir6.2AAA-FLAG (350 bp), and GAPDH (452 bp). C.a: Western blot of membrane fractions obtained from the hearts or brains of Kir6.1-AAA mice and their control littermates. The lanes are heart membrane fractions from transgenic animals (lane 2, 10 μg protein; lane 4, 50 μg protein) or from nontransgenic animals (lane 3, 50 μg protein) as well as membrane fractions obtained from transgenic mouse brains (lane 1, 10 μg protein). Native Kir6.1 has apparent molecular size of 44 kDa, whereas Kir6.1AAA-eGFP migrates around 70 kDa. C.b: Western blot to illustrate Kir6.2 protein expression levels in heart membrane fractions from Kir6.1-AAA mice (lane 2), Kir6.2-AAA mice (lane 4), from their respective control littermates (lanes 1 and 3, respectively). The primary antibody used was anti-Kir6.2-G16 (1:500).
Phenotypic analysis of transgenic mice
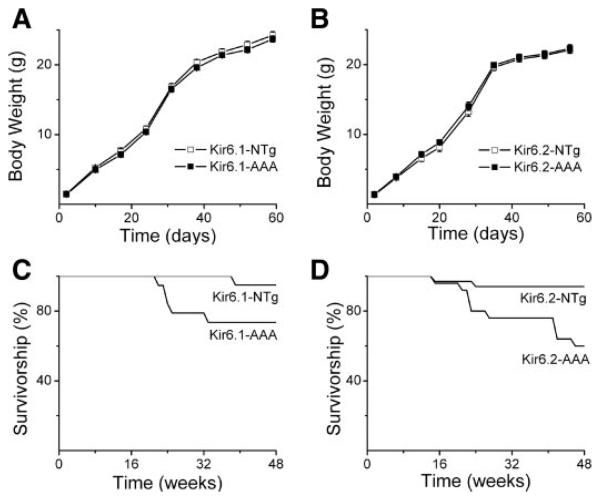
The Kir6 pore-mutant transgenic mice had a birth ratio as expected from Mendelian principles, suggesting that mice developed normally in utero (data not shown). The male-to-female ratio was close to 1:1. Mice developed normally after birth, as evident from weight-gain charts constructed during the first 2 mo after birth (Fig. 2, A and B). Body length (measured from nose to tip of tail) was also not different between transgenic groups and their control littermate groups (data not shown). At the end of 8 wk, animals were killed and the weights of several tissues were obtained. There was no significant difference between transgenics and control littermates in the weights of the heart, liver, kidney, or brain (data not shown).
Fig. 2.
Growth curves and survivorship of Kir6.x transgenic mice and their control littermates. A and B: body weight recorded over the first 60 days after birth for Kir6.1-AAA mice, Kir6.2-AAA mice, transgenic mice, and their respective control (nontransgenic) littermates. C and D: survivorship, defined as the number of living mice expressed as a percentage of the number of mice within a preselected group of mice, over the first 48 wk after birth. Shown are survival rates of Kir6.1-AAA mice (C; n = 41) and their control littermates (n = 34) as well as Kir6.2-AAA mice (D; n = 34) and their control littermates (n = 30).
Up to about 16 wk of age, survival rate was identical in the transgenic groups and control littermates. Thereafter, both Kir6.x transgenic mice exhibited an increased mortality compared with their control littermates (Fig. 2, C and D). To avoid the effect of age-related mortality, younger mice (3–4 mo old) were used for the most of studies. Although not studied in detail, there was no evidence of the vasospasm and early death observed in Kir6.1 (−) mice (17). Thus there are clearly distinct roles for cardiac KATP channels and those in the coronary vascular smooth muscle and/or endothelium.
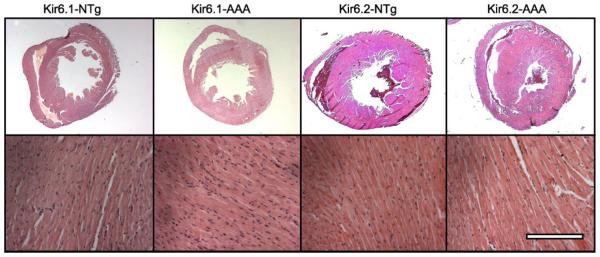
Histologically, there were no obvious differences between the hearts of transgenic and control littermates (Fig. 3).
Fig. 3.
Gross anatomy (top) and histology (bottom) of Kir6.1-AAA and Kir6.2-AAA mice as well as their control littermates. Scale bar is 2.5 mm for top images and 250 μm for bottom.
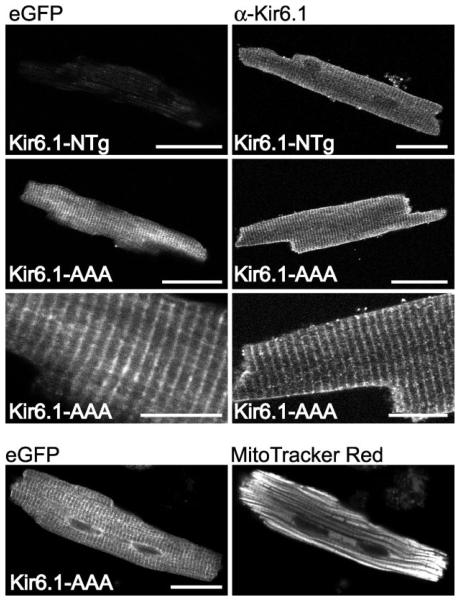
Subcellular expression of Kir6.1-AAA-eGFP in cardiac myocytes
The COOH-terminal flag failed to be recognized in tissues from transgenic animals (not shown). This may be due to masking of the epitope or possibly cleavage of the FLAG epitope. The Kir6.1 pore mutant was tagged with eGFP, which remained intact in vivo as demonstrated by a band of ~70 kDa in SDS-PAGE of heart lysates (Fig. 1D). The Kir6.1-AAA-eGFP subunits were distributed in a sarcomeric pattern (Fig. 4A), which is a subcellular distribution similar to that obtained for native Kir6.1 subunits (e.g., Fig. 4B1; see also Ref. 19). The expression pattern observed either by eGFP fluorescence or anti-Kir6.1 staining was not similar to mitochondrial staining (Fig. 4C).
Fig. 4.
Subcellular localization of Kir6.1 and Kir6.1-AAA-eGFP subunits in isolated cardiac myocytes from the hearts of Kir6.1-AAA mice. Left: eGFP fluorescence. Right: immunocytochemistry performed with an anti-Kir6.1 antibody (NAF-1). Bottom 2 panels depict eGFP fluorescence and MitoTracker red fluorescence recorded simultaneously from a myocyte of a transgenic animal. Scale bar is 20 μm for all panels, except the higher magnifications (3rd row from the top), where the scale bar is 10 μm.
Ventricular myocytes from transgenic mice lack sarcolemmal KATP channel currents
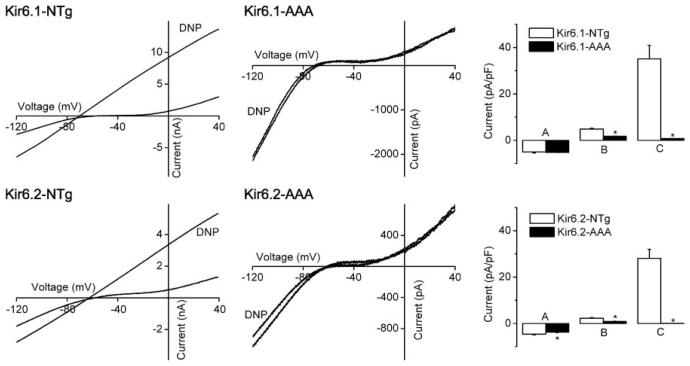
Whole-cell patch clamping was performed. There were no differences in the membrane capacitances or reversal potentials of the current-voltage relationships between controls and transgenics (not shown). The current density at −90 mV was similar between the Kir6.1-AAA transgenics and their control littermates (a small difference was observed for Kir6.2-AAA transgenics), but current density at 0 mV was smaller in myocytes from transgenic mice (Fig. 5). KATP channel currents were activated by metabolic inhibition in control myocytes, which were partly reversed by glibenclamide (1 μmol/l, data not shown), but DNP (100 μmol/l) failed to elicit KATP channel currents in transgenic mice. For example, in Kir6.1 control littermates, the maximal effect of DNP was within 5.7 ± 1.6 min, whereas no DNP-activated current was observed even after 11 ± 1.1 min of DNP application in Kir6.1-AAA cardiomyocytes (at which time hypercontracture occurred; not shown). Similar data were seen with the Kir6.2-AAA transgenic mice (Fig. 5). Similarly, pinacidil or bimakalim failed to activate KATP channels in transgenic myocytes (not shown). Thus both transgenic mice lack sarcolemmal KATP channels.
Fig. 5.
Lack of sarcolemmal ATP-sensitive K+ (KATP) channels in cardiac myocytes from Kir6.1-AAA and Kir6.2-AAA mice. Whole cell patch clamping was performed using enzymatically isolated ventricular myocytes. Ramp voltage-clamp pulses were applied from a holding potential of −60 mV. KATP channels were activated by metabolic inhibition (100 μmol/l dinitrophenol) until maximum KATP channel activation occurred; usually within a few minutes. If no KATP channel current was activated, perfusion was stopped when cell hypercontracture occurred. The genotypes are indicated in the individual panels. In the bar graphs, A refers to the current densities at −90 mV in Tyrode's solution, B to the current densities at 0 mV in Tyrode's solution, and C to the current densities at 0 mV in the presence of dinitrophenol (DNP). Data are expressed as means ± SE of 6–10 experiments from 3–4 animals.
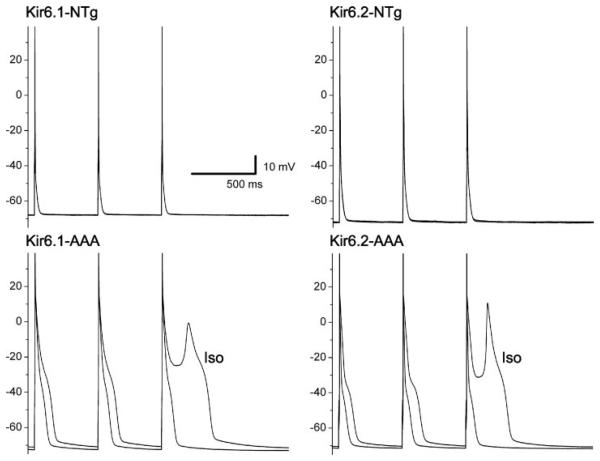
Prolonged action potentials in Kir6.x transgenic mice
Action potentials were recorded under current-clamp conditions. There were no differences in the resting membrane potentials (Table 1). However, the action potential duration was prolonged in both transgenic mice. Isoproterenol (1 μmol/l) led to an action potential prolongation (Table 1) and some of the transgenic (but not control) myocytes developed early afterdepolarizations (EADs; 2 of 9 cells in the Kir6.1-AAA group and 1 of 9 cells in the Kir6.2-AAA group; Fig. 6).
Table 1.
Action potential duration in Kir6.x pore mutant mice with and without isoproterenol
| Kir6.1 control (n = 10) |
Kir6.1-AAA (n = 9) |
Kir6.2 control (n = 10) |
Kir6.2-AAA (n = 9) |
|
|---|---|---|---|---|
| Vm (mV) | −67.5±0.8 | −69.5±0.6 | −69.9±0.9 | −68.3 ±0.9 |
| 2 Hz in Tyrode's | ||||
| APD50, ms | 8.4±0.6 | 19.7±3.9* | 7.4± 1.1 | 50.1±17.8* |
| APD90, ms | 32.3±5.0 | 86.9±9.2* | 30.5±4.4 | 118.9±21.7* |
| 2 Hz in isoproterenol | ||||
| APD50, ms | 9.9±0.9† | 43.1±19.2 | 9.4± 1.9 | 55.5±19.5* |
| APD90, ms | 35.2±4.9 | 120.0±27.8* | 31.2±4.8 | 127.9±26.7* |
| 4 Hz in Tyrode's | ||||
| APD50, ms | 8.2±0.6 | 25.1±6.2* | 7.7± 1.3 | 50.8±16.2* |
| APD90, ms | 35.1±5.6 | 97.3±11.4* | 32.0±5.1 | 116.2±17.0* |
| 4 Hz in isoproterenol | ||||
| APD50, ms | 11.3±1.3† | 57.2±25.6 | 11.8±3.4† | 56.8±16.6* |
| APD90, ms | 45.1±8.2† | 138.7±27.9† | 37.4±7.8 | 132.2±19.4* |
Data are means ± SE. APD50, action potential duration at 50% repolarization; APD90, action potential duration at 90% repolarization.
P < 0.05 compared with their control littermates;
P < 0.05 compared with the data in Tyrode's solution.
Fig. 6.
Action potential recordings obtained under current-clamping conditions. The mouse genotype is illustrated above each panel. Each panel contains 2 action potential traces (super-imposed in the top 2 panels): 1 recorded during control conditions and another recorded within a few minutes after addition of isoproterenol (Iso). The pacing rate was 2 Hz. Note the prolonged action potential duration in the transgenic myocytes, which was further prolonged by isoproterenol.
Ventricular function is unchanged in Kir6.x transgenic mice
We next examined hemodynamic parameters of anesthetized transgenic mice. At baseline, Kir6.1-AAA transgenic mice had a modestly but significantly increased heart rates compared with age-matched controls (Table 2); the heart rate was not different between Kir6.2-AAA and their control littermates. Other hemodynamic parameters, including left ventricular systolic pressure, left ventricular end-diastolic pressure, +dP/dt (where P is pressure and t is time), −dP/dt, and the relaxation constant Tau, were unchanged, suggesting that ventricular function was largely unchanged in the transgenic mice.
Table 2.
Invasive hemodynamic parameters in control and Kir6.x transgenic mice
| Kir6.1 Control | Kir6.1-AAA | Kir6.2 Control | Kir6.2-AAA | |
|---|---|---|---|---|
| Hemodynamic parameters | ||||
| LV systolic pressure, mmHg | 81.6±1.3 | 79.6±2.2 | 79.4±4.2 | 74.4±8.9 |
| LVEDP, mmHg | 3.7±1.1 | 4.4±1.0 | 1.9 ±1.1 | 2.5±2.1 |
| Heart rate, beats/min | 416.0±2.2 | 429.7±3.0* | 442.0±6.0 | 442.0±8.0 |
| +dP/dt mmHg/s | 8,646±528 | 8,442±775 | 6,490 ±1006 | 4,986 ±889 |
| −dP/dt mmHg/s | −6855±636 | −6337±800 | −5745±477 | −4925±759 |
| Tau, ms | 8.0±2.1 | 10.2±2.5 | 6.3 ±1.5 | 9.2±3.3 |
Data are group means ± SE; LV, left ventricular; LVEDP, left ventricular end-diastolic pressure; dP/dt change in pressure over time; Tau, relaxation constant. n = 5 controls and 6 Kir6.1-AAA; n = 5 controls and n = 5 Kir6.2-AAA.
P < 0.05 compared with matched controls.
Kir6.x transgenic mice have an increased refractory period
In vivo electrophysiological parameters were similar in anesthetized transgenic mice and their control littermates. The basal heart rate was slightly elevated in Kir6.1-AAA transgenic mice (decreased R-R interval, Table 3). This was not observed with Kir6.2-AAA transgenic mice. Other electrocardiographic parameters were unchanged. Interestingly, the QRS amplitude was significantly decreased in Kir6.2-AAA (but not Kir6.1-AAA) mice.
Table 3.
Comparison of electrocardiographic and electrophysiological parameters in nontransgenic and Kir6.x-AAA transgenic mice
| Baseline |
Postdobutamine |
Baseline |
Postdobutamine |
|||||
|---|---|---|---|---|---|---|---|---|
| Ntg | Kir6.1AAA | Ntg | Kir6.1AAA | Ntg | Kir6.2AAA | Ntg | Kir6.2AAA | |
| P-wave duration, ms | 23.0±0.4 | 22.0±0.6 | 22.4±1.0 | 22.8±0.3 | 17.8±1.1 | 24.3±2.7 | 21.5±1.3† | 24.3±1.8 |
| P-R interval, ms | 42.4±2.4 | 36.8±1.2 | 41.0±3.6 | 35.3±1.0 | 38.0±1.9 | 42.0±3.3 | 38.3±0.9 | 44.0±2.7 |
| QRS duration, ms | 9.0±0.4 | 9.5±0.3 | 8.6±0.5 | 9.3±0.5 | 8.5±0.9 | 9.5±0.7 | 7.8±0.8 | 10.8±0.6* |
| R-R interval, ms | 140.8±10.8 | 112.5±3.2* | 119.0±15.5 | 108.7±3.4 | 139.5±8.5 | 137.8±6.8 | 124.0±4.5 | 131.8±3.9 |
| QRS amplitude, μV | 111.9 ± 18.7 | 132.4±9.0 | 107.4±15.6 | 113.7±7.5 | 132.1 ±8.8 | 44.7±3.8* | 112.7±14.3 | 40.4±6.7* |
| QTc, ms | 170.3±5.8 | 158.0±6.7 | 150.5±5.6 | 164.5±9.8 | 174.9±7.0 | 158.3 ± 11.7 | 169.1 ±1.1 | 159.9±6.8 |
| VERP100, ms | 28.2±2.2 | 43.2±2.0† | 29.0±2.3 | 43.6±1.2† | 26.3 ±1.5 | 59.0±6.8* | 28.3 ±2.6 | 60.0±7.7* |
| VERP80, ms | 29.6±2.2 | 45.5±2.2† | 30.8±2.5 | 49.0±3.7* | 28.5±2.3 | 61.5±6.6* | 29.0±2.7 | No 1:1 capture in 2 of 4 |
Values are means ± SE; n = 5 controls and 6 Kir6.1-AAA mice; n = 4 controls and 5 Kir6.2-AAA mice. Ntg, respective nontransgenic littermate controls. QTc, corrected QT interval; VERP80 and VERP100, ventricular effective refractory periods, respectively, at pacing lengths of 80 ms or 100 ms.
P < 0.05 compared with matched Ntg controls;
P < 0.05 compared with baseline.
The ventricular effective refractory period was determined by using programmed electrical stimulation (10) and was prolonged in both transgenic mice. Overall, the incidence of nonsustained ventricular tachycardia was very low and not different between groups. None of the mice tested exhibited sustained ventricular tachycardia.
We also investigated the response to β-adrenoceptor activation. Dobutamine had essentially similar effects in the transgenic animals as observed in their nontransgenic littermates. In this regard, the transgenic animals differ from the Kir6.2(−/−) mice, which have a compromised response to isoproterenol (14).
No conduction abnormalities in Kir6.x transgenic mice
Sinus rhythm activation patterns of activation were similar in transgenics and controls (not shown). Pacing experiments revealed no differences in conduction velocities of either the left or right ventricles (not shown).
Kir6.x transgenic mice exhibit a compromised exercise capacity
Kir6.2(−/−) mice are known to have a reduced exercise capacity (42). To examine the effect of cardiac-specific loss of KATP channels, we performed a treadmill test using one of the transgenic models. Kir6.1-AAA transgenic mice (mean age of 18 wk) performed less well on this test, both in terms of the time on the treadmill and the workload performed. The running time on treadmill was 29.6 ± 3.4 min (vs. 41.1 ± 3.8 min for control littermates, P < 0.05) and the workload performed was 10.4 ± 2.9 J (vs. 19.9 ± 4.3 J in control, P < 0.05).
DISCUSSION
We generated two transgenic mouse models that specifically lack cardiac myocyte KATP channels. Young mice did not have an overt phenotype, whereas older mice exhibited increased mortality. Hemodynamics and ECG parameters were relatively normal, but with programmed electrical stimulation the transgenic mice have a prolonged refractory period. Action potentials were prolonged in myocytes from transgenic mice, and early afterpotentials could be provoked with β-receptor stimulation. Transgenic mice performed less well in an exercise stress test.
Both Kir6.x transgenic mice lack KATP channels
A clear role for Kir6.2 subunits has been identified in forming cardiac sarcolemmal KATP channels (28). However, Kir6.1 subunits are also expressed in ventricular cells (19) (also see Fig. 4) and atrial KATP channels may have a Kir6.1 component (38), but the functional role of this subunit is far from clear. We found transgenic expression of either Kir6.1 or Kir6.2 dominant negative subunits to abolish sarcolemmal KATP channels. The mechanism by which this occurred is not entirely clear, especially because Kir6.1 and Kir6.2 subunits may not have an identical subcellular localization (19, 31). The most reasonable explanation is that these mutant subunits competed with native Kir6.2 subunits for the available SUR2A pool, thus preventing the latter from trafficking normally and forming functional KATP channels. This explanation is consistent with the recent observations that transgenic overexpression of SUR1 and Kir6.2 constructs also demonstrate marked dominant-negative phenotypes (6, 13). Regardless of the mechanism, transgenic expression of mutant Kir6.x subunits resulted in hearts lacking KATP channels, as assessed with patch-clamp methods. Having two independent models of mice lacking KATP channels with essentially identical features strongly rules against integrational nonspecificity of the transgenes.
Sarcolemmal vs. mitochondrial KATP channels
The transgenic mice lacked sarcolemmal KATP channels. However, mitochondria also contain KATP channels (26). We saw no obvious colocalization of the Kir6.1-AAA subunits (tagged with eGFP), with the mitochondrial-specific dye MitoTracker red (similar experiments could not be performed to localize Kir6.2-AAA subunits because the FLAG epitope was not detected in transgenic myocytes). Thus our data suggest that sarcolemmal, but not mitochondrial, KATP channels were lost in our transgenic mouse models. However, we cannot rule out the possibility of disruption of the mitochondria KATP channel activity without further experimentation.
Action potential duration lengthening in transgenic myocytes
Similar to the Kir6.2(−/−) mice (33), the shape of the current-voltage relation was similar (but not identical) in control and transgenic myocytes under voltage-clamp conditions. The current densities at −90 mV were similar between the transgenic and control groups. However, the current density at 0 mV was smaller in transgenics (Fig. 5), which is consistent with the longer action potentials in transgenics. One interpretation of these data is that there may be a small but significant activation of KATP channels at near-physiological pacing rates in the nontransgenic mouse, which is not present in the transgenic mouse. Such an interpretation would be consistent with reports that KATP channel blockade with glibenclamide increases the rat ventricular action potential duration under nonischemic conditions in some studies (3, 40) and that glibenclamide caused QT prolongation in a clinical trial of patients with type II diabetes (20). However, a contribution of KATP channels to the action potential is not always observed (16, 33), and the relative contribution of KATP channels to the action potential may depend on unidentified factors (such as the cellular condition, pacing rate, stress, etc.). Our data do not directly address the issues of whether KATP channels are open under basal conditions or the possibility of metabolic compromise of the isolated myocytes. It is also possible that a degree of remodeling may have occurred in some of the other repolarizing membrane currents, such as the alterations in the Ca2+ current, as observed in another transgenic mouse model over-expressing a constituently active KATP channel subunit (5).
KATP channels and arrhythmogenesis
The effective refractory period was prolonged in transgenic mice lacking cardiac KATP channels. Experimentally, incessant ventricular tachycardia can be partly explained by an increase of functional conduction block and/or decrease of the wavelength of excitation (conduction velocity × refractory period) as actions favoring reentry. A shortened refractory period may therefore be proarrhythmic (29). Thus, although at first sight a prolonged effective refractory period may be considered antiarrhythmic, many drugs (including those with class III action) are in fact proarrhythmic by prolonging the action potential duration, which in turn increases the effective refractory period. The link between KATP channel activity and refractoriness has been noted by others. For example, blockade of KATP channels with glibenclamide increases the effective refractory period in an isolated rabbit heart preparation (40). Furthermore, the progressive shortening of the effective refractory period characteristic of atrial fibrillation has been argued to be due to activation of the KATP channels during rapid atrial rates (39). Conversely, KATP channel opening by pinacidil or cromakalim has been described to be associated with a reduction in effective refractory periods in experimental models (4, 35). Taken together, the lengthened effective refractory period and possible dispersion of refractoriness caused by the absence of KATP channel may be responsible for an increased susceptibility to arrhythmias. Conversely, the argument can be made that KATP channels, by shortening the action potential and effective refractory period, may have a protective role against the onset of ventricular arrhythmias. We found mechanistic support for possible arrhythmic features of myocytes lacking KATP channels at the cellular level. First, the action potential was prolonged in myocytes from transgenic mice. Second, EADs appeared after isoproterenol treatment in some transgenic, but not in control littermates, myocytes. A similar observation has been made for Kir6.2(−/−) mice, in which catecholamine challenge predisposes the myocardium to afterpotentials (14). Furthermore, KATP channel blockade with glibenclamide also predisposes to afterpotentials (25). Prolonged action potentials increase the susceptibility of EAD occurrence, which is a possible explanation for this arrhythmia in our transgenic mouse model. However, there may also be a role for a poorly understood link between KATP channels and intracellular Ca2+ cycling, which has been most clearly demonstrated in transgenic mice expressing cardiac ATP-insensitive mutant Kir6.2 subunits (5). This collection of in vivo and in vitro data underscores the role that KATP channels may have in repolarization (particularly at high pacing rates and with catecholamine challenge) and the role that KATP channels may have in protecting the heart against potentially lethal arrhythmias. A caveat to note is that we failed to observe any serious arrhythmias in the in vivo electrophysiology study with the β-agonist dobutamine.
A role of cardiac KATP channels in cardiovascular function under conditions of stress
Evidence is compiling to show that KATP channels contribute to excitability under conditions of stress. In addition to the numerous studies published to demonstrate a role for KATP channels during hypoxia and ischemic events (8, 34), as well as participation in protection of the heart during ischemic preconditioning (7, 23), recent studies additionally point to a role for KATP channels in more “physiological” stress conditions, such as exercise and rapid heart rates. For example, in an exercise-stress test, Kir6.2 knockout mice perform at a significantly reduced level compared with ageand gender-matched nontransgenic controls (42). When subjected to chronic exercise, these mice also exhibited less augmentation in exercise capacity and lacked metabolic improvement in body fat composition and glycemic handling (11). The fact that our transgenic mice are also intolerant of stress further underscores the important role of KATP channels in protection against a stress response.
Cardiac intrinsic vs. extrinsic cardiovascular effects of KATP channel deletion
Although they are powerful tools, gene-knockout approaches can overemphasize certain important aspects of gene function and may overlook more subtle effects of protein function. Furthermore, gene knockout results in lack of the target protein in all somatic cells and it may be difficult to dissociate the role of a protein in a particular organ or biological system from other somatic effects. The creation of Kir6.2(−/−) and Sur2(−/−) mice has been invaluable in understanding the role of KATP channels (28). However, interpretation of phenotypes can be complicated by loss of KATP channels in multiple tissues. For example, the deficit in a stress response of Kir6.2(−/−) mice could result from absence of KATP channels in the nervous system (centrally or peripherally), in skeletal muscle, or in the heart itself. Furthermore, even though isolated hearts from Kir6.2(−/−) mice were used in some studies to delineate the role of KATP channels in ischemia-reperfusion and preconditioning (9, 34), the contribution of KATP channels to norepinephrine release from sympathetic nerve endings (24) and the associated effects of neurotransmitters on ischemia-related events (12) cannot be excluded. Using a transgenic approach to ablate KATP channels specifically in cardiac muscle, we were able to recapitulate the impaired stress response, demonstrating that this response is intrinsic (at least in part) to the myocardium. Our transgenic mice also shared other features with Kir6.2(−/−) mice, including a predisposition of the myocardium to EADs (and thus a high risk for induction of triggered activity and ventricular arrhythmias), suggesting that KATP channel-mediated protection against arrhythmias is also a myocardial-intrinsic feature. The prolonged effective refractory period in our transgenic mice can be interpreted along the same lines (because invasive electrophysiology studies have not been performed in Kir6.2 knockout mice, these data cannot be directly compared with that mouse model).
In summary, disruption of KATP channels specifically in the myocardium leads to some of the same phenotypes observed in Kir6.2 knockout mice. These phenotypes, which include increased mortality, electrophysiological alterations, and lack of adaptation to a stress response, suggest that these features are intrinsic to the myocardium and that cardiac KATP channels have an important role in protecting the heart from lethal arrhythmias and adaptation to stress situations.
Supplementary Material
Acknowledgments
GRANTS
These studies were supported by the American Heart Association (EIA to W. A. Coetzee), by the National Heart, Lung, and Blood Institute Grant 1 RO1 HL-64838 (W. A. Coetzee), and in part by the Seventh Masonic District Association.
REFERENCES
- 1.Ashcroft FM. Adenosine 5′-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- 2.Chutkow WA, Pu J, Wheeler MT, Wada T, Makielski JC, Burant CF, McNally EM. Episodic coronary artery vasospasm and hypertension develop in the absence of Sur2 KATP channels. J Clin Invest. 2002;110:203–208. doi: 10.1172/JCI15672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faivre JF, Findlay I. Effects of tolbutamide, glibenclamide and diazoxide upon action potentials recorded from rat ventricular muscle. Biochim Biophys Acta. 1989;984:1–5. doi: 10.1016/0005-2736(89)90334-9. [DOI] [PubMed] [Google Scholar]
- 4.Ferrier GR, Howlett SE. Pretreatment with pinacidil promotes arrhythmias in an isolated tissue model of cardiac ischemia and reperfusion. J Pharmacol Exp Ther. 2005;313:823–830. doi: 10.1124/jpet.104.081349. [DOI] [PubMed] [Google Scholar]
- 5.Flagg TP, Charpentier F, Manning-Fox J, Remedi MS, Enkvetchakul D, Lopatin A, Koster J, Nichols C. Remodeling of excitation-contraction coupling in transgenic mice expressing ATP-insensitive sarcolemmal KATP channels. Am J Physiol Heart Circ Physiol. 2004;286:H1361–H1369. doi: 10.1152/ajpheart.00676.2003. [DOI] [PubMed] [Google Scholar]
- 6.Flagg TP, Remedi MS, Masia R, Gomes J, McLerie M, Lopatin AN, Nichols CG. Transgenic overexpression of SUR1 in the heart suppresses sarcolemmal KATP. J Mol Cell Cardiol. 2005;39:647–656. doi: 10.1016/j.yjmcc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Gross GJ, Fryer RM. Sarcolemmal versus mitochondrial ATP-sensitive K+ channels and myocardial preconditioning. Circ Res. 1999;84:973–979. doi: 10.1161/01.res.84.9.973. [DOI] [PubMed] [Google Scholar]
- 8.Grover GJ. Pharmacology of ATP-sensitive potassium channel (KATP) openers in models of myocardial ischemia and reperfusion. Can J Physiol Pharmacol. 1997;75:309–315. doi: 10.1139/cjpp-75-4-309. [DOI] [PubMed] [Google Scholar]
- 9.Gumina RJ, Pucar D, Bast P, Hodgson DM, Kurtz CE, Dzeja PP, Miki T, Seino S, Terzic A. Knockout of Kir6.2 negates ischemic preconditioning-induced protection of myocardial energetics. Am J Physiol Heart Circ Physiol. 2003;284:H2106–H2113. doi: 10.1152/ajpheart.00057.2003. [DOI] [PubMed] [Google Scholar]
- 10.Gutstein DE, Danik SB, Sereysky JB, Morley GE, Fishman GI. Subdiaphragmatic murine electrophysiological studies: sequential determination of ventricular refractoriness and arrhythmia induction. Am J Physiol Heart Circ Physiol. 2003;285:H1091–H1096. doi: 10.1152/ajpheart.00100.2003. [DOI] [PubMed] [Google Scholar]
- 11.Kane GC, Behfar A, Yamada S, Perez-Terzic C, O'Cochlain F, Reyes S, Dzeja PP, Miki T, Seino S, Terzic A. ATP-sensitive K+ channel knockout compromises the metabolic benefit of exercise training, resulting in cardiac deficits. Diabetes. 2004;53(Suppl 3):S169–S175. doi: 10.2337/diabetes.53.suppl_3.s169. [DOI] [PubMed] [Google Scholar]
- 12.Kent KM, Epstein SE. Neural basis for the genesis and control of arrhythmias associated with myocardial infarction. Cardiology. 1976;61:61–74. doi: 10.1159/000169748. [DOI] [PubMed] [Google Scholar]
- 13.Koster JC, Knopp A, Flagg TP, Markova KP, Sha Q, Enkvetchakul D, Betsuyaku T, Yamada KA, Nichols CG. Tolerance for ATP-insensitive KATP channels in transgenic mice. Circ Res. 2001;89:1022–1029. doi: 10.1161/hh2301.100342. [DOI] [PubMed] [Google Scholar]
- 14.Liu XK, Yamada S, Kane GC, Alekseev AE, Hodgson DM, O'Cochlain F, Jahangir A, Miki T, Seino S, Terzic A. Genetic disruption of Kir6.2, the pore-forming subunit of ATP-sensitive K+ channel, predisposes to catecholamine-induced ventricular dysrhythmia. Diabetes. 2004;53(Suppl 3):S165–S168. doi: 10.2337/diabetes.53.suppl_3.s165. [DOI] [PubMed] [Google Scholar]
- 16.MacKenzie I, Saville VL, Waterfall JF. Differential class III and glibenclamide effects on action potential duration in guinea-pig papillary muscle during normoxia and hypoxia/ischaemia. Br J Pharmacol. 1993;110:531–538. doi: 10.1111/j.1476-5381.1993.tb13843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miki T, Suzuki M, Shibasaki T, Uemura H, Sato T, Yamaguchi K, Koseki H, Iwanaga T, Nakaya H, Seino S. Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nat Med. 2002;8:466–472. doi: 10.1038/nm0502-466. [DOI] [PubMed] [Google Scholar]
- 18.Morrissey A, Parachuru L, Leung M, Lopez G, Nakamura TY, Tong X, Yoshida H, Srivastava S, Dhar Chowdhury P, Artman M, Coetzee WA. Expression of KATP channel subunits during perinatal maturation in the mouse heart. Pediatr Res. 2005;58:185–192. doi: 10.1203/01.PDR.0000169967.83576.CB. [DOI] [PubMed] [Google Scholar]
- 19.Morrissey A, Rosner E, Lanning J, Parachuru L, Dhar Chowdhury P, Han S, Lopez G, Tong X, Yoshida H, Nakamura TY, Artman M, Giblin JP, Tinker A, Coetzee WA. Immunolocalization of KATP channel subunits in mouse and rat cardiac myocytes and the coronary vasculature. BMC Physiol. 2005;5:1–18. doi: 10.1186/1472-6793-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Najeed SA, Khan IA, Molnar J, Somberg JC. Differential effect of glyburide (glibenclamide) and metformin on QT dispersion: a potential adenosine triphosphate sensitive K+ channel effect. Am J Cardiol. 2002;90:1103–1106. doi: 10.1016/s0002-9149(02)02776-5. [DOI] [PubMed] [Google Scholar]
- 21.Nichols CG, Lederer WJ. The regulation of ATP-sensitive K+ channel activity in intact and permeabilized rat ventricular myocytes. J Physiol. 1990;423:91–110. doi: 10.1113/jphysiol.1990.sp018013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 23.O'Rourke B. Myocardial KATP channels in preconditioning. Circ Res. 2000;87:845–855. doi: 10.1161/01.res.87.10.845. [DOI] [PubMed] [Google Scholar]
- 24.Oe K, Sperlagh B, Santha E, Matko I, Nagashima H, Foldes FF, Vizi ES. Modulation of norepinephrine release by ATP-dependent K+-channel activators and inhibitors in guinea-pig and human isolated right atrium. Cardiovasc Res. 1999;43:125–134. doi: 10.1016/s0008-6363(99)00052-8. [DOI] [PubMed] [Google Scholar]
- 25.Pasnani JS, Ferrier GR. Differential effects of glyburide on premature beats and ventricular tachycardia in an isolated tissue model of ischemia and reperfusion. J Pharmacol Exp Ther. 1992;262:1076–1084. [PubMed] [Google Scholar]
- 26.Paucek P, Mironova G, Mahdi F, Beavis AD, Woldegiorgis G, Garlid KD. Reconstitution and partial purification of the glibenclamide-sensitive, ATP-dependent K+-channel from rat liver and beef heart mitochondria. J Biol Chem. 1992;267:26062–26069. [PubMed] [Google Scholar]
- 27.Pountney DJ, Sun ZQ, Porter LM, Nitabach MN, Nakamura TY, Holmes D, Rosner E, Kaneko M, Manaris T, Holmes TC, Coetzee WA. Is the molecular composition of KATP channels more complex than originally thought? Biochemical and electrophysiological evidence for heteromultimeric assembly of the KATP channel subunits Kir6.1 and Kir6.2. J Mol Cell Cardiol. 2001;33:1541–1546. doi: 10.1006/jmcc.2001.1407. [DOI] [PubMed] [Google Scholar]
- 28.Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol. 2003;81:133–176. doi: 10.1016/s0079-6107(02)00053-6. [DOI] [PubMed] [Google Scholar]
- 29.Sheridan DJ, Culling W. Electrophysiological effects of alpha-adrenoceptor stimulation in normal and ischemic myocardium. J Cardiovasc Pharmacol. 1985;7(Suppl 5):S55–S60. doi: 10.1097/00005344-198500075-00012. [DOI] [PubMed] [Google Scholar]
- 30.Shyng S, Nichols CG. Octameric stoichiometry of the KATP channel complex. J Gen Physiol. 1997;110:655–664. doi: 10.1085/jgp.110.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh H, Hudman D, Lawrence CL, Rainbow RD, Lodwick D, Norman RI. Distribution of Kir6.0 and SUR2 ATP-sensitive potassium channel subunits in isolated ventricular myocytes. J Mol Cell Cardiol. 2003;35:445–459. doi: 10.1016/s0022-2828(03)00041-5. [DOI] [PubMed] [Google Scholar]
- 32.Sun X, Cao K, Yang G, Huang Y, Hanna ST, Wang R. Selective expression of Kir6.1 protein in different vascular and non-vascular tissues. Biochem Pharmacol. 2004;67:147–156. doi: 10.1016/j.bcp.2003.08.041. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki M, Li RA, Miki T, Uemura H, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Ogura T, Seino S, Marban E, Nakaya H. Functional roles of cardiac and vascular ATP-sensitive potassium channels clarified by Kir6.2-knockout mice. Circ Res. 2001;88:570–577. doi: 10.1161/01.res.88.6.570. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki M, Sasaki N, Miki T, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Seino S, Marban E, Nakaya H. Role of sarcolemmal KATP channels in cardioprotection against ischemia/reperfusion injury in mice. J Clin Invest. 2002;109:509–516. doi: 10.1172/JCI14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szilvassy Z, Koltai M, Ferdinandy P, Jakab I, Lonovics J, Tarrade T, Allard M, Braquet PG. Cromakalim and cicletanine against pacing-induced myocardial ischemia in conscious rabbits. Life Sci. 1994;54:L125–L130. doi: 10.1016/0024-3205(94)00870-1. [DOI] [PubMed] [Google Scholar]
- 36.Trube G, Hescheler J. Inward-rectifying channels in isolated patches of the heart cell membrane: ATP-dependence and comparison with cell-attached patches. Pflügers Arch. 1984;401:178–184. doi: 10.1007/BF00583879. [DOI] [PubMed] [Google Scholar]
- 37.Vaidya D, Morley GE, Samie FH, Jalife J. Reentry and fibrillation in the mouse heart. A challenge to the critical mass hypothesis. Circ Res. 1999;85:174–181. doi: 10.1161/01.res.85.2.174. [DOI] [PubMed] [Google Scholar]
- 38.Van Bever L, Poitry S, Faure C, Norman RI, Roatti A, Baertschi AJ. Pore loop-mutated rat Kir6.1 and Kir6.2 suppress KATP current in rat cardiomyocytes. Am J Physiol Heart Circ Physiol. 2004;287:H850–H859. doi: 10.1152/ajpheart.00054.2004. [DOI] [PubMed] [Google Scholar]
- 39.Vereckei A, Gogelein H, Wirth KJ, Zipes DP. Effect of the cardioselective, sarcolemmal KATP channel blocker HMR 1098 on atrial electrical remodeling during pacing-induced atrial fibrillation in dogs. Cardiovasc Drugs Ther. 2004;18:23–30. doi: 10.1023/B:CARD.0000025752.63337.b2. [DOI] [PubMed] [Google Scholar]
- 40.Xiao XH, Holley LK. Reducing electrical defibrillation thresholds with glibenclamide in an isolated rabbit heart preparation. J Cardiovasc Pharmacol. 1997;30:576–582. doi: 10.1097/00005344-199711000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Yokoshiki H, Sunagawa M, Seki T, Sperelakis N. Antisense oligodeoxynucleotides of sulfonylurea receptors inhibit ATP-sensitive K+ channels in cultured neonatal rat ventricular cells. Pflügers Arch. 1999;437:400–408. doi: 10.1007/s004240050794. [DOI] [PubMed] [Google Scholar]
- 42.Zingman LV, Hodgson DM, Bast PH, Kane GC, Perez-Terzic C, Gumina RJ, Pucar D, Bienengraeber M, Dzeja PP, Miki T, Seino S, Alekseev AE, Terzic A. Kir6.2 is required for adaptation to stress. Proc Natl Acad Sci USA. 2002;99:13278–13283. doi: 10.1073/pnas.212315199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.