Abstract
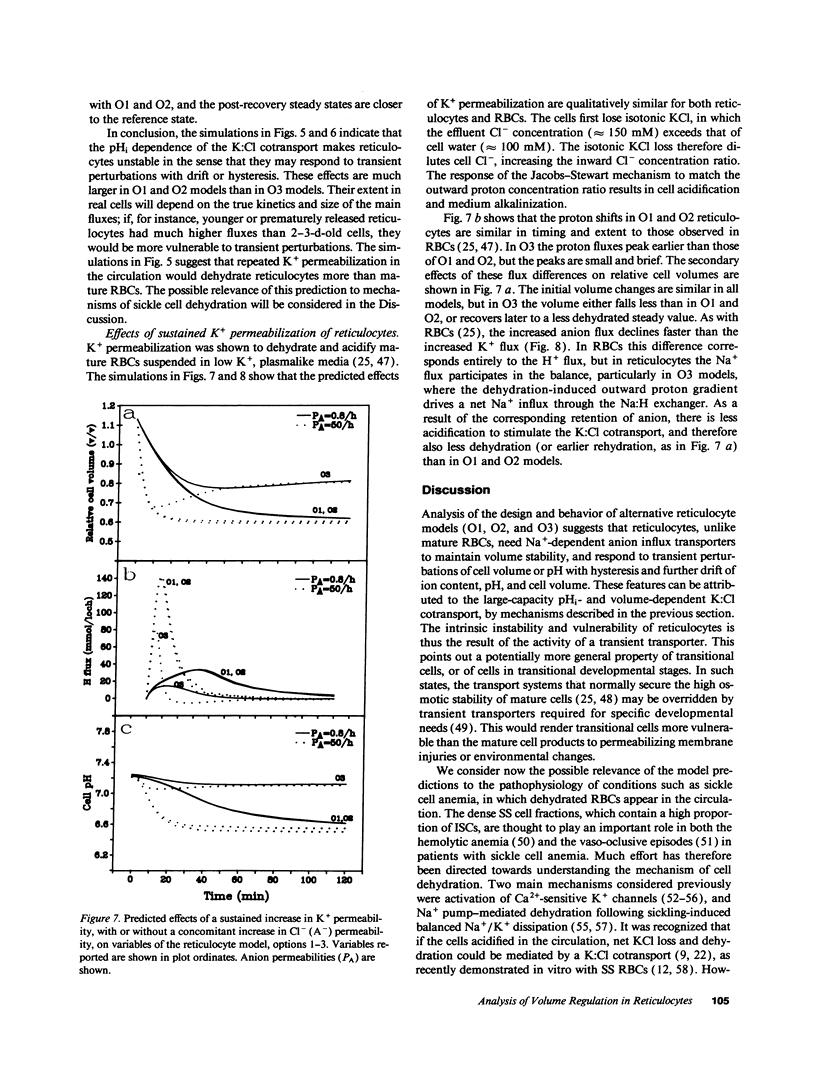
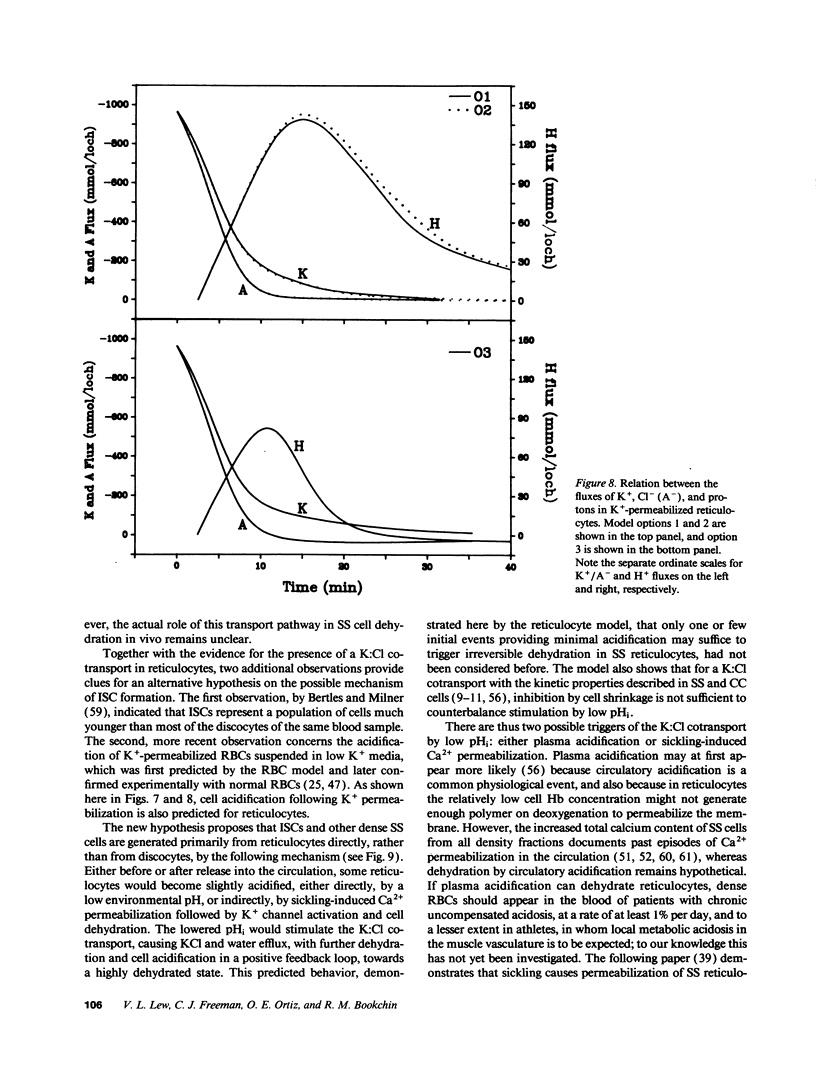
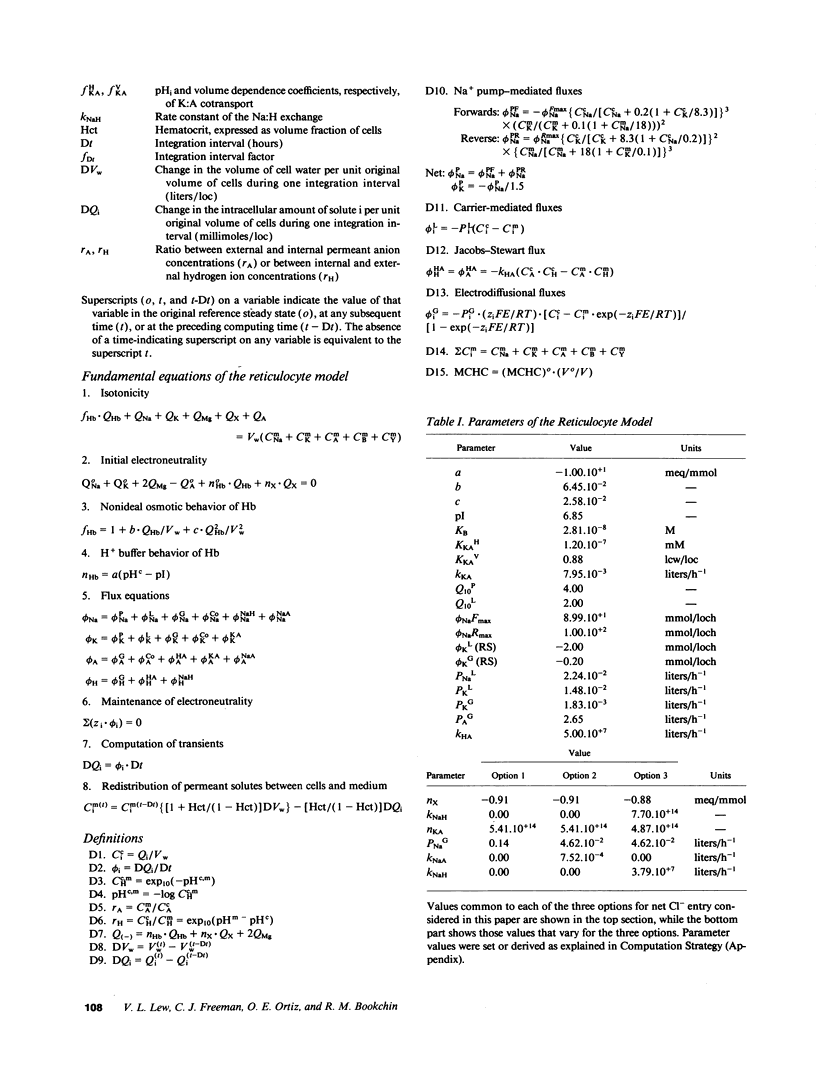
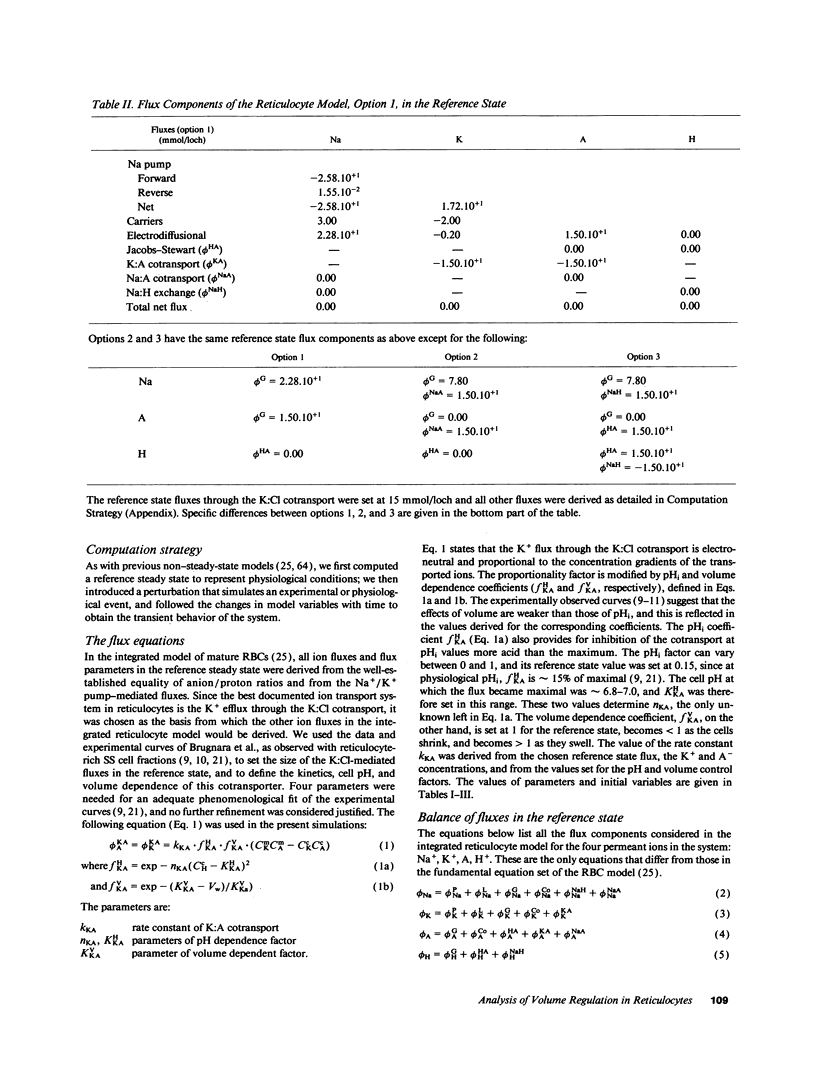
We developed a mathematical model of the reticulocyte, seeking to explain how a cell with similar volume but much higher ionic traffic than the mature red cell (RBC) regulates its volume, pH, and ion content in physiological and abnormal conditions. Analysis of the fluxbalance required by reticulocytes to conserve volume and composition predicted the existence of previously unsuspected Na(+)-dependent Cl- entry mechanisms. Unlike mature RBCs, reticulocytes did not tend to return to their original state after brief perturbations. The model predicted hysteresis and drift in cell pH, volume, and ion contents after transient alterations in membrane permeability or medium composition; irreversible cell dehydration could thus occur by brief K+ permeabilization, transient medium acidification, or the replacement of external Na+ with an impermeant cation. Both the hysteresis and drift after perturbations were shown to depend on the pHi dependence of the K:Cl cotransport, a major reticulocyte transporter. This behavior suggested a novel mechanism for the generation of irreversibly sickled cells directly from reticulocytes, rather than in a stepwise, progressive manner from discocytes. Experimental tests of the model's predictions and the hypothesis are described in the following paper.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson P. S. Kinetic properties of the plasma membrane Na+-H+ exchanger. Annu Rev Physiol. 1985;47:545–560. doi: 10.1146/annurev.ph.47.030185.002553. [DOI] [PubMed] [Google Scholar]
- Aronson P. S., Nee J., Suhm M. A. Modifier role of internal H+ in activating the Na+-H+ exchanger in renal microvillus membrane vesicles. Nature. 1982 Sep 9;299(5879):161–163. doi: 10.1038/299161a0. [DOI] [PubMed] [Google Scholar]
- BERNSTEIN R. E. Alterations in metabolic energetics and cation transport during aging of red cells. J Clin Invest. 1959 Sep;38:1572–1586. doi: 10.1172/JCI103936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORSOOK H., LINGREL J. B., SCARO J. L., MILLETTE R. L. Synthesis of haemoglobin in relation to the maturation of erythroid cells. Nature. 1962 Oct 27;196:347–350. doi: 10.1038/196347a0. [DOI] [PubMed] [Google Scholar]
- BRECHER G., STOHLMAN F., Jr Reticulocyte size and erythropoietic stimulation. Proc Soc Exp Biol Med. 1961 Aug-Sep;107:887–891. doi: 10.3181/00379727-107-26785. [DOI] [PubMed] [Google Scholar]
- Berkowitz L. R., Orringer E. P. Cell volume regulation in hemoglobin CC and AA erythrocytes. Am J Physiol. 1987 Mar;252(3 Pt 1):C300–C306. doi: 10.1152/ajpcell.1987.252.3.C300. [DOI] [PubMed] [Google Scholar]
- Bertles J. F., Milner P. F. Irreversibly sickled erythrocytes: a consequence of the heterogeneous distribution of hemoglobin types in sickle-cell anemia. J Clin Invest. 1968 Aug;47(8):1731–1741. doi: 10.1172/JCI105863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookchin R. M., Ortiz O. E., Lew V. L. Activation of calcium-dependent potassium channels in deoxygenated sickled red cells. Prog Clin Biol Res. 1987;240:193–200. [PubMed] [Google Scholar]
- Bookchin R. M., Ortiz O. E., Somlyo A. V., Somlyo A. P., Sepulveda M. I., Hockaday A., Lew V. L. Calcium-accumulating inside-out vesicles in sickle cell anemia red cells. Trans Assoc Am Physicians. 1985;98:10–20. [PubMed] [Google Scholar]
- Brugnara C., Bunn H. F., Tosteson D. C. Regulation of erythrocyte cation and water content in sickle cell anemia. Science. 1986 Apr 18;232(4748):388–390. doi: 10.1126/science.3961486. [DOI] [PubMed] [Google Scholar]
- Brugnara C., Kopin A. S., Bunn H. F., Tosteson D. C. Regulation of cation content and cell volume in hemoglobin erythrocytes from patients with homozygous hemoglobin C disease. J Clin Invest. 1985 May;75(5):1608–1617. doi: 10.1172/JCI111867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugnara C., Tosteson D. C. Cell volume, K transport, and cell density in human erythrocytes. Am J Physiol. 1987 Mar;252(3 Pt 1):C269–C276. doi: 10.1152/ajpcell.1987.252.3.C269. [DOI] [PubMed] [Google Scholar]
- Brugnara C., Tosteson D. C. Inhibition of K transport by divalent cations in sickle erythrocytes. Blood. 1987 Dec;70(6):1810–1815. [PubMed] [Google Scholar]
- Brugnara C., Van Ha T., Tosteson D. C. Acid pH induces formation of dense cells in sickle erythrocytes. Blood. 1989 Jul;74(1):487–495. [PubMed] [Google Scholar]
- Brugnara C., Van Ha T., Tosteson D. C. Properties of K+ transport in resealed human erythrocyte ghosts. Am J Physiol. 1988 Sep;255(3 Pt 1):C346–C356. doi: 10.1152/ajpcell.1988.255.3.C346. [DOI] [PubMed] [Google Scholar]
- Bureau M., Banerjee R. Structure-volume relationships in hemoglobin. A densitometric and dilatometric study of the oxy leads to deoxy transformation. Biochimie. 1976;58(4):403–407. doi: 10.1016/s0300-9084(76)80249-0. [DOI] [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. The nature of the membrane sites controlling anion permeability of human red blood cells as determined by studies with disulfonic stilbene derivatives. J Membr Biol. 1972 Dec 29;10(3):311–330. doi: 10.1007/BF01867863. [DOI] [PubMed] [Google Scholar]
- Canessa M., Fabry M. E., Blumenfeld N., Nagel R. L. Volume-stimulated, Cl(-)-dependent K+ efflux is highly expressed in young human red cells containing normal hemoglobin or HbS. J Membr Biol. 1987;97(2):97–105. doi: 10.1007/BF01869416. [DOI] [PubMed] [Google Scholar]
- Canessa M., Fabry M. E., Nagel R. L. Deoxygenation inhibits the volume-stimulated, Cl(-)-dependent K+ efflux in SS and young AA cells: a cytosolic Mg2+ modulation. Blood. 1987 Dec;70(6):1861–1866. [PubMed] [Google Scholar]
- Canessa M., Spalvins A., Nagel R. L. Volume-dependent and NEM-stimulated K+,Cl- transport is elevated in oxygenated SS, SC and CC human red cells. FEBS Lett. 1986 May 5;200(1):197–202. doi: 10.1016/0014-5793(86)80538-5. [DOI] [PubMed] [Google Scholar]
- Cass A., Dalmark M. Equilibrium dialysis of ions in nystatin-treated red cells. Nat New Biol. 1973 Jul 11;244(132):47–49. doi: 10.1038/newbio244047a0. [DOI] [PubMed] [Google Scholar]
- Chipperfield A. R. An effect of chloride on (Na+K) co-transport in human red blood cells. Nature. 1980 Jul 17;286(5770):281–282. doi: 10.1038/286281a0. [DOI] [PubMed] [Google Scholar]
- Clark M. R., Guatelli J. C., White A. T., Shohet S. B. Study on the dehydrating effect of the red cell Na+/K+-pump in nystatin-treated cells with varying Na+ and water contents. Biochim Biophys Acta. 1981 Sep 7;646(3):422–432. doi: 10.1016/0005-2736(81)90311-4. [DOI] [PubMed] [Google Scholar]
- Clarkson D. R., Moore E. M. Reticulocyte size in nutritional anemias. Blood. 1976 Nov;48(5):669–677. [PubMed] [Google Scholar]
- Dalmark M. Chloride and water distribution in human red cells. J Physiol. 1975 Aug;250(1):65–84. doi: 10.1113/jphysiol.1975.sp011043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhm J. Furosemide-sensitive K+ (Rb+) transport in human erythrocytes: modes of operation, dependence on extracellular and intracellular Na+, kinetics, pH dependency and the effect of cell volume and N-ethylmaleimide. J Membr Biol. 1987;98(1):15–32. doi: 10.1007/BF01871042. [DOI] [PubMed] [Google Scholar]
- Eaton J. W., Skelton T. D., Swofford H. S., Kolpin C. E., Jacob H. S. Elevated erythrocyte calcium in sickle cell disease. Nature. 1973 Nov 9;246(5428):105–106. doi: 10.1038/246105a0. [DOI] [PubMed] [Google Scholar]
- Escobales N., Canessa M. Amiloride-sensitive Na+ transport in human red cells: evidence for a Na/H exchange system. J Membr Biol. 1986;90(1):21–28. doi: 10.1007/BF01869682. [DOI] [PubMed] [Google Scholar]
- Escobales N., Canessa M. Ca2+-activated Na+ fluxes in human red cells. Amiloride sensitivity. J Biol Chem. 1985 Oct 5;260(22):11914–11923. [PubMed] [Google Scholar]
- Fabry M. E., Benjamin L., Lawrence C., Nagel R. L. An objective sign in painful crisis in sickle cell anemia: the concomitant reduction of high density red cells. Blood. 1984 Aug;64(2):559–563. [PubMed] [Google Scholar]
- Flatman P. W. Sodium and potassium transport in ferret red cells. J Physiol. 1983 Aug;341:545–557. doi: 10.1113/jphysiol.1983.sp014823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman J. C., Hoffman J. F. Ionic and osmotic equilibria of human red blood cells treated with nystatin. J Gen Physiol. 1979 Aug;74(2):157–185. doi: 10.1085/jgp.74.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman C. J., Bookchin R. M., Ortiz O. E., Lew V. L. K-permeabilized human red cells lose an alkaline, hypertonic fluid containing excess K over diffusible anions. J Membr Biol. 1987;96(3):235–241. doi: 10.1007/BF01869305. [DOI] [PubMed] [Google Scholar]
- Fröhlich O. Relative contributions of the slippage and tunneling mechanisms to anion net efflux from human erythrocytes. J Gen Physiol. 1984 Dec;84(6):877–893. doi: 10.1085/jgp.84.6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDOS G. The function of calcium in the potassium permeability of human erythrocytes. Biochim Biophys Acta. 1958 Dec;30(3):653–654. doi: 10.1016/0006-3002(58)90124-0. [DOI] [PubMed] [Google Scholar]
- Glader B. E., Nathan D. G. Cation permeability alterations during sickling: relationship to cation composition and cellular hydration of irreversibly sickled cells. Blood. 1978 May;51(5):983–989. [PubMed] [Google Scholar]
- Grinstein S., Rothstein A. Mechanisms of regulation of the Na+/H+ exchanger. J Membr Biol. 1986;90(1):1–12. doi: 10.1007/BF01869680. [DOI] [PubMed] [Google Scholar]
- Gunn R. B., Dalmark M., Tosteson D. C., Wieth J. O. Characteristics of chloride transport in human red blood cells. J Gen Physiol. 1973 Feb;61(2):185–206. doi: 10.1085/jgp.61.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M., Forbush B., 3rd [3H]bumetanide binding to duck red cells. Correlation with inhibition of (Na + K + 2Cl) co-transport. J Biol Chem. 1986 Jun 25;261(18):8434–8441. [PubMed] [Google Scholar]
- Haas M., McManus T. J. Effect of norepinephrine on swelling-induced potassium transport in duck red cells. Evidence against a volume-regulatory decrease under physiological conditions. J Gen Physiol. 1985 May;85(5):649–667. doi: 10.1085/jgp.85.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. C., Ellory J. C. Evidence for the presence of volume-sensitive KCl transport in 'young' human red cells. Biochim Biophys Acta. 1986 Jun 26;858(2):317–320. doi: 10.1016/0005-2736(86)90338-x. [DOI] [PubMed] [Google Scholar]
- Hunter M. J. Human erythrocyte anion permeabilities measured under conditions of net charge transfer. J Physiol. 1977 Jun;268(1):35–49. doi: 10.1113/jphysiol.1977.sp011845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M. H., Stewart D. R. THE ROLE OF CARBONIC ANHYDRASE IN CERTAIN IONIC EXCHANGES INVOLVING THE ERYTHROCYTE. J Gen Physiol. 1942 Mar 20;25(4):539–552. doi: 10.1085/jgp.25.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings M. L. Kinetics and mechanism of anion transport in red blood cells. Annu Rev Physiol. 1985;47:519–533. doi: 10.1146/annurev.ph.47.030185.002511. [DOI] [PubMed] [Google Scholar]
- KILLMANN S. A. ON THE SIZE OF NORMAL HUMAN RETICULOCYTES. Acta Med Scand. 1964 Nov;176:529–533. doi: 10.1111/j.0954-6820.1964.tb00654.x. [DOI] [PubMed] [Google Scholar]
- Kaji D. Volume-sensitive K transport in human erythrocytes. J Gen Physiol. 1986 Dec;88(6):719–738. doi: 10.1085/jgp.88.6.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf P. A., Fuhrmann G. F., Rothstein S., Rothstein A. The relationship between anion exchange and net anion flow across the human red blood cell membrane. J Gen Physiol. 1977 Mar;69(3):363–386. doi: 10.1085/jgp.69.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauf P. K., Adragna N. C., Garay R. P. Activation by N-ethylmaleimide of a latent K+-Cl- flux in human red blood cells. Am J Physiol. 1984 May;246(5 Pt 1):C385–C390. doi: 10.1152/ajpcell.1984.246.5.C385. [DOI] [PubMed] [Google Scholar]
- Lauf P. K., Theg B. E. A chloride dependent K+ flux induced by N-ethylmaleimide in genetically low K+ sheep and goat erythrocytes. Biochem Biophys Res Commun. 1980 Feb 27;92(4):1422–1428. doi: 10.1016/0006-291x(80)90445-3. [DOI] [PubMed] [Google Scholar]
- Lew V. L., Bookchin R. M. Volume, pH, and ion-content regulation in human red cells: analysis of transient behavior with an integrated model. J Membr Biol. 1986;92(1):57–74. doi: 10.1007/BF01869016. [DOI] [PubMed] [Google Scholar]
- Lew V. L., Ferreira H. G., Moura T. The behaviour of transporting epithelial cells. I. Computer analysis of a basic model. Proc R Soc Lond B Biol Sci. 1979 Nov 30;206(1162):53–83. doi: 10.1098/rspb.1979.0091. [DOI] [PubMed] [Google Scholar]
- Lew V. L., Hockaday A., Sepulveda M. I., Somlyo A. P., Somlyo A. V., Ortiz O. E., Bookchin R. M. Compartmentalization of sickle-cell calcium in endocytic inside-out vesicles. Nature. 1985 Jun 13;315(6020):586–589. doi: 10.1038/315586a0. [DOI] [PubMed] [Google Scholar]
- McManus T. J., Haas M., Starke L. C., Lytle C. Y. The duck red cell model of volume-sensitive chloride-dependent cation transport. Ann N Y Acad Sci. 1985;456:183–186. doi: 10.1111/j.1749-6632.1985.tb14863.x. [DOI] [PubMed] [Google Scholar]
- Panet R., Atlan H. Characterization of a potassium carrier in rabbit reticulocyte cell membrane. J Membr Biol. 1980;52(3):273–280. doi: 10.1007/BF01869195. [DOI] [PubMed] [Google Scholar]
- Panuska J. R., Cirino N. M., Midulla F., Despot J. E., McFadden E. R., Jr, Huang Y. T. Productive infection of isolated human alveolar macrophages by respiratory syncytial virus. J Clin Invest. 1990 Jul;86(1):113–119. doi: 10.1172/JCI114672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs J. R. Volume-sensitive K influx in human red cell ghosts. J Gen Physiol. 1988 Nov;92(5):685–711. doi: 10.1085/jgp.92.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serjeant G. R., Serjeant B. E., Milner P. F. The irreversibly sickled cell; a determinant of haemolysis in sickle cell anaemia. Br J Haematol. 1969 Dec;17(6):527–533. doi: 10.1111/j.1365-2141.1969.tb01403.x. [DOI] [PubMed] [Google Scholar]
- Wiley J. S., Shaller C. C. Selective loss of calcium permeability on maturation of reticulocytes. J Clin Invest. 1977 Jun;59(6):1113–1119. doi: 10.1172/JCI108735. [DOI] [PMC free article] [PubMed] [Google Scholar]


