Abstract
Many features of the suprachiasmatic nucleus (SCN) are the same in diurnal and nocturnal animals, suggesting that differences in phase preference are determined by mechanisms downstream from the SCN. Here, we examined this hypothesis by characterizing rhythmic expression of PER1 and PER2 in several extra-SCN areas in the brains of a diurnal murid rodent, Arvicanthis niloticus (grass rats). In the shell of the nucleus accumbens, dorsal striatum, piriform cortex, and CA1 of the hippocampus, both PER1 and PER2 were rhythmic, with peak expression occurring at ZT10. PER1 in the dentate gyrus also peaked at ZT10, but PER2 was arrhythmic in this region. In general, these patterns are 180° out of phase with those reported for nocturnal species. In a second study, we examined inter-individual differences in the multioscillator system of grass rats. Here, we housed grass rats in cages with running wheels, under which conditions some individuals spontaneously adopt a day active (DA) and others a night active (NA) phase preference. In the majority of the extra-SCN regions sampled, the patterns of PER1 and PER2 expression of NA grass rats resembled those of nocturnal species, while those of DA grass rats were similar to the ones seen in grass without access to running wheels. In contrast, the rhythmic expression of both PER proteins was identical in the SCN and ventral subparaventricular zone (vSPZ) of DA and NA animals. Differences in the phase of oscillators downstream from the SCN, and perhaps the vSPZ, appear to determine the phase preference of particular species, as well as that of members of a diurnal species that show voluntary phase reversals. The latter observation has important implications for the understanding of health problems associated with human shift work.
Keywords: suprachiasmatic nucleus, Extra-SCN oscillators, Arvicanthis niloticus, night active, day active, human shift work
1. Introduction
Circadian rhythms in physiological, metabolic, and behavioral functions are endogenous, and when entrained to the light-dark cycle, they enable organisms to anticipate daily environmental challenges. In mammals, the suprachiasmatic nucleus (SCN) of the hypothalamus is the primary circadian pacemaker, which is necessary for the maintenance of a multitude of rhythms, as well as for their entrainment to the day-night cycle (Moore and Eichler, 1972, Stephan and Zucker, 1972, Ralph et al., 1990, Klein et al., 1991, Dibner et al., 2010). At the cellular level, the SCN generates rhythms using molecular mechanisms of transcriptional and translational feedback loops involving sets of clock genes (Welsh et al., 1995, Reppert and Weaver, 2001, Ko and Takahashi, 2006, Welsh et al., 2010). Most of what we know about the functioning of the mammalian SCN comes from studies using nocturnal laboratory species, but consistent with the pioneer work of Schwartz and colleagues (Schwartz et al., 1983), there is a growing literature showing that some fundamental features of the SCN are common to species with widely varying activity patterns (see Smale et al., 2008 for a review). Most importantly, the coupling of rhythms in expression of circadian clock genes in the SCN to the light-dark cycle are very similar in day- and night-active species [reviewed in (Smale et al., 2008)], as are rhythms in expression of genes that are part of SCN output pathways (Dardente et al., 2004, Lambert et al., 2005, Mahoney et al., 2009). These observations have lead to the suggestion that differences in the circadian systems of diurnal and nocturnal species reside downstream from the SCN circadian pacemaker (Kalsbeek et al., 2008, Smale et al., 2008).
The molecular clock machinery first described in the SCN is also present in several brain regions and peripheral tissues (Mendoza and Challet, 2009, Dibner et al., 2010). These extra-SCN oscillators may regulate rhythms in region specific functions (Hastings et al., 2008, Mendoza and Challet, 2009). Studies with nocturnal laboratory rodents have reported rhythmic expression of the clock genes Per1, Per2, Bmal1, Clock, and Cry, and their protein products in extra-SCN brain regions (Abe et al., 2002, Shieh, 2003, Guilding and Piggins, 2007, Feillet et al., 2008, Amir and Stewart, 2009b). These extra-SCN oscillators need circadian signals from the SCN to entrain to the light/dark cycle (Yamazaki et al., 2000, Abe et al., 2002, Amir et al., 2004, Lamont et al., 2005, Guilding et al., 2009). Very little information is available on clock gene expression in the extra-SCN oscillators of diurnal animals (Mrosovsky et al., 2001, Vosko et al., 2009). We recently reported that there are PER1 and PER2 rhythms in the amygdala and the oval nucleus of the bed nucleus of the stria terminalis (BNST-ov) of the diurnal grass rat (Arvicanthis niloticus) (Ramanathan et al., 2008b, Ramanathan et al., 2010). In those regions, PER1 and 2 expression peaks during the light phase of the light-dark cycle, approximately 180° out of phase with the peaks reported for the same brain regions in nocturnal species (Amir et al., 2004, Lamont et al., 2005, Angeles-Castellanos et al., 2007, Feillet et al., 2008). These results are consistent with the hypothesis that a phase reversal of extra-SCN oscillators contributes to differences in phase preference of nocturnal and diurnal species ( see also Lambert and Weaver, 2006).
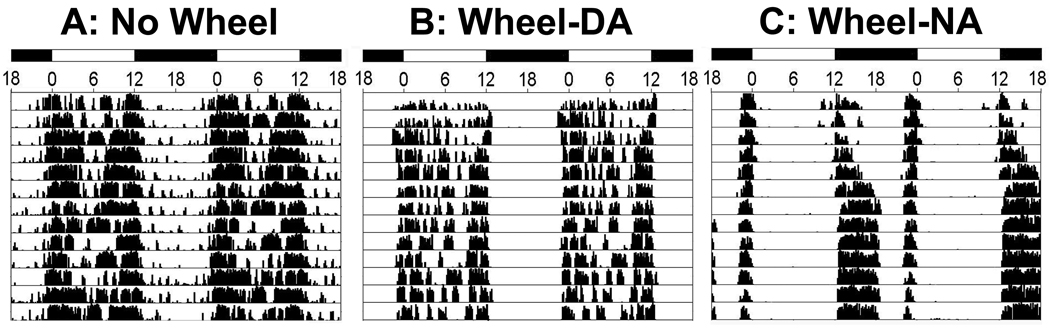
In addition to light, the phase of several extra-SCN oscillators can be modulated by a variety of non-photic cues that alter activity patterns of animals. These include, for example, meal schedules (Wakamatsu et al., 2001, Angeles-Castellanos et al., 2007, Feillet et al., 2008, Verwey and Amir, 2009), chronic exposure of methamphetamine in the drinking water (Masubuchi et al., 2000, Iijima et al., 2002), cocaine sensitization (Abarca et al., 2002, Uz et al., 2002, Manev and Uz, 2006), and hormonal rhythms (von Gall et al., 2002, Amir et al., 2004, Lamont et al., 2005, Amir and Stewart, 2009a). One non-photic cue that has a major impact on the activity rhythms of grass rats is access to a running wheel. Although these animals are fundamentally diurnal in the laboratory and in the field (Katona and Smale, 1997, McElhinny et al., 1997, Blanchong and Smale, 2000), when given free access to running wheels, a subset of them switches to a predominantly night active (NA) pattern, while the rest continues to be day active (DA) (Blanchong et al., 1999; See Figure 1). This switch takes place without any apparent changes in the SCN, or in the region just above the nucleus, the ventral subparaventricular zone (vSPZ); e.g. patterns of Fos expression in the SCN and vSPZ are unaffected as the grass rats become predominantly nocturnal (Rose et al., 1999, Mahoney et al., 2000, Mahoney et al., 2001, Smale et al., 2001, Schwartz and Smale, 2005). This does not appear to be the case for rhythms in other brain regions. For example, rhythms in Fos expression in orexin-producing neurons of the perifornical lateral hypothalamus are completely reversed in DA and NA grass rats (Nixon and Smale, 2004). Thus, engaging in activity during the normal resting period could change the phase of extra-SCN brain oscillators, without affecting those of the SCN or vSPZ, which could disrupt the temporal organization of the organism. Such changes in the internal temporal program may contribute to the many health problems associated with human shift work. Animals that voluntarily shift their activity to their resting phase provide an excellent model to study the physiological and behavioral consequences of individual differences in phase preference for the display of activity in humans (e.g., Gau et al., 2007).
Figure 1.
Double-plotted actograms depicting the activity patterns of grass rats from our colony when they are housed without access to running wheels (panel A), or with access to running wheels and showing either a day-active (DA) chronotype (panel B) or a night-active (NA) chronotype (panel C). The dark bars at the top represent the 12-hour dark periods and the numbers below the bars refer to Zeitgeber times.
Here we further document that species can be quite different with respect to the phase of extra-SCN oscillators (Experiment 1), and we evaluate the hypothesis that the phases of many extra-SCN oscillators relative to the LD cycle are more malleable than are those of the SCN and the vSPZ (Experiment 2). In Experiment 1 we monitored rhythms of PER1 and PER2 expression in the dorsal striatum (DSt), piriform cortex (PC), shell of the nucleus accumbens (Acc-Shell) dentate gyrus (DG) and area CA1 of the hippocampus and compared them to those reported for nocturnal rodents. In Experiment 2,, we looked at whether voluntary changes in phase preference for the display of activity are accompanied by changes in phase in oscillators outside the SCN and vSPZ. Thus, Experiment 2 tested the hypothesis that features that accompany phase differences in the display of activity between species, also accompany chronotype differences within a species.
2. Experimental procedures
2.1 Animals and Housing
Adult male grass rats from our breeding colony at Michigan State University, East Lansing, MI, USA, were housed individually in Plexiglas cages (34 × 28 × 17 cm), under a 12:12 h light-dark (LD) cycle, with a red light (< 5 lx) on all the time, and with free access to food (PMI Nutrition Prolab RMH 2000, Brentwood, MO, USA) and water. For Experiment 2 (see below), the animals were housed in the same Plexiglas cages, but now equipped with running wheels (26 cm diameter; 8 cm width). The colony room was entered at irregular intervals to check the health of the animals and to replenish food and water. All experiments were performed in compliance with guidelines established by the Michigan State University All University Committee on Animal Use and Care, and the National Institute of Health guide for the Care and Use of Laboratory Animals.
2.2 Perfusion and Tissue Preparation
Animals were perfused at ZTs 2, 6, 10, 14, 18, and 22. At the prescribed ZT, the animals were deeply anesthetized with sodium pentobarbital and perfused transcardially with 0.01M phosphate buffered saline (PBS), pH 7.2, followed by 4% paraformaldehyde (Sigma, St. Louis, MO USA) in 0.1 M phosphate buffer. An aluminum hood covered the heads of the animals perfused during the dark period to prevent exposure to light. Brains were removed and post-fixed for 4 h in 4% paraformaldehyde and transferred to 20% sucrose solution overnight, then stored in cryoprotectant at −20° C until sectioning. Brains were sectioned coronally at 30 µm using a freezing microtome. The sections were stored in cryoprotectant at −20° C until processing.
2.3 Experiment 1: PER1/2 expression in extra-SCN oscillators of grass rats without access to wheels
2.3.1 Immunocytochemical Procedures
Adjacent sections from the brains of 36 animals (n =6/sampling ZT) were subjected to ICC procedures for detecting either PER1 or PER 2 expression as previously described (Ramanathan et al., 2006). Briefly, Free-floating sections were rinsed 3 × 10 min in 0.01M PBS before the first as well as all subsequent incubations. All sections were incubated in 5% Normal Donkey serum (NDS; Jackson) in 0.01M PBS with 0.3% Triton X-100 for 1 h at room temperature. The sections were incubated in the primary antibody (mPER1# 1177, made in rabbit, 1:20,000, a gift from Dr. D. R. Weaver, University of Massachusetts, MA, USA) on a rotator for 48 h at 4° C. On third day, they were incubated in biotinylated secondary antibody (Donkey anti Rabbit; 1:200, Jackson), followed by Avidin-Biotin peroxidase complex (ABC Vectastain Kit; Vector Laboratories, Burlingame, Ca, USA). Protein was visualized by reacting with diaminobenzidine (0.5mg/ml Sigma) in sodium acetate buffer (pH 7.2) and 3% hydrogen peroxide. Nickel sulfate was added to yield a purple reaction product. For detecting PER2 immunoreactivity, the same procedure was followed except that for PER2, the primary antibody (made in rabbit, and generously provided by Dr. D. R. Weaver, University of Massachusetts, MA, USA) was diluted to 1:10,000. All tissue was mounted onto gelatin-coated slides, dehydrated and cover slipped. To control for non-specific staining resulting from the procedure, all the steps of the protocol were replicated excluding the incubation with the primary antibody. This control procedure produced no staining in any of the anatomical areas examined in the two experiments.
2.3.2 Data Collection and Analysis
We selected single sections from several extra-SCN regions using the rat brain atlas of (Paxinos and Watson, 2007). For each region sampled, the approximate level of the section is given in reference to bregma from that atlas. To quantify PER1 and PER2 in extra-SCN brain regions, immunoreactive nuclear staining was counted bilaterally in the shell of the nucleus accumbens (Acc-Shell; Bregma= 0.70mm), piriform cortex (PC; Bregma= 0.70mm), dorsal striatum (DSt; Bregma=0.70mm), hippocampus CA1; Bregma =−2.80mm) and dentate gyrus (DG; Bregma =−2.80mm). Pictures were taken from each region with a digital camera (MBF Bioscience Inc, 2007) attached to a Zeiss light microscope (Carl Zeiss, Gottingen, Germany). Pictures were arranged in Photoshop. For every region, labeled cells of either PER1 or PER2 were counted within a region defined by a counting box with the following dimensions: Acc-Shell (800×500 µm), PC (700×100 µm), DSt (800×1000 µm), CA1 (500×100µm), DG (500×100 µm). Bilateral cell counts were done using NIH Image J software (National Institute of Health, Bethesda, MD). Immunoreactive labeled cells were distinguished from background staining using circularity and contrast intensity thresholds, which were determined separately for each area counted. All counts were performed by an investigator who was not aware of the time of perfusion of each animal. Total bilateral counts of each area were used for statistical analysis. One-way analyses of variance (ANOVA) were used to assess the effect of ZT on PER expression in each region and for each protein. Significant F ratios were followed by comparisons between ZTs using Fisher’s Least Significant Difference (LSD) tests. We used SPSS version 17 for all statistical tests for both Experiment 1 and 2, and for both experiments significant differences had p values equal to or less than .05.
2.4 Experiment 2: PER1/2 expression in the SCN and extra-SCN oscillators of DA and NA grass rats with access to running wheels
2.4.1 Chronotype Determination
Wheel running data were continuously collected using a DSI Dataquest 3 system (MiniMitter, Sunriver, OR, USA) and actograms were generated using the Vital View program (MiniMitter, Sunriver, OR, USA). After obtaining a stable wheel running rhythm for two weeks, animals were classified as DA or NA based on their activity profiles. An animal was classified as DA if daily activity ceased within 2 h after lights-out and classified as NA if activity continued for more than four hours after lights-out (Blanchong et al., 1999; see Figure 1). Animals exhibiting intermediate activity patterns were discarded from the study. This approach resulted in 42 DA and 48 NA animals (7 and 8/sampling ZT, respectively).
2.4.2 Data Collection and Analysis
The ICC procedures for detecting PER1and PER2 were identical to those described for Experiment 1. In addition to the extra-SCN regions sampled in Experiment 1, we included the BNST-ov (Bregma=−0.26 mm) and the central and basolateral amygdala (CEA and BLA; Bregma=−2.12 mm) in the data collection for Experiment 2. Similar to Experiment 1, a single section from each area was selected for counting and cell counts were done as previously described in Experiment 1, except that for these three regions, rather than using a sampling box, we counted all cells expressing PER1 or PER2 bilaterally in each region.
To quantify PER1 and PER2 labeled cells in the SCN and the ventral subparaventricular zone (vSPZ), sections were selected through the rostral, middle and caudal of the SCN. Pictures were taken using a light microscope and were formatted for figures using Adobe Photoshop 7. Bilateral cell counts were done on one section per level in the SCN and the vSPZ using Image J software. To quantify labeled PER1 and PER2 cells in the vSPZ, a rectangular box (215µm×160µm) was placed dorsal to the SCN close to the third ventricle and labeled cells within this sampling box were counted. For the SCN counts, all labeled cells within the boundaries of the SCN were counted. The boundaries of the SCN were determined using archival sections stained for vasopressin. For the SCN and vSPZ, preliminary ANOVAs for the effect of level of section failed to detect significant differences and the average of the three sections was used for subsequent analyses.
Individual two-way (ZT x Chronotype) ANOVAs were conducted for each brain region and each PER protein, using total bilateral counts (or averages for the SCN and vSPZ) of labeled cells as the dependent variable. Significant interactions were followed by analyses of simple main effects of ZT for each chronotype, and when appropriate, post hoc comparisons of individual ZTs used Fisher’s LSD tests. In addition, when interaction effects were observed, independent samples t-tests were used to analyze the simple main effects of Chronotype within each ZT. In the CEA, one animal was excluded from the analysis as an outlier, because the values for PER2 expression were four standard deviations above the mean.
3. Results
3.1 Experiment 1: PER1 and PER2 expression in extra-SCN oscillators of grass rats without access to wheels
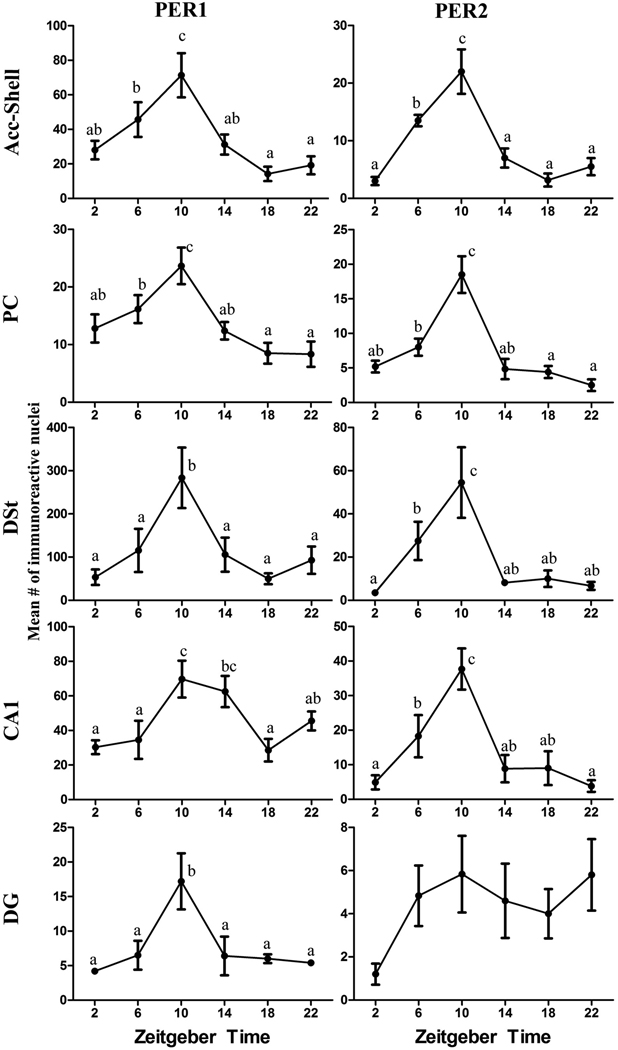
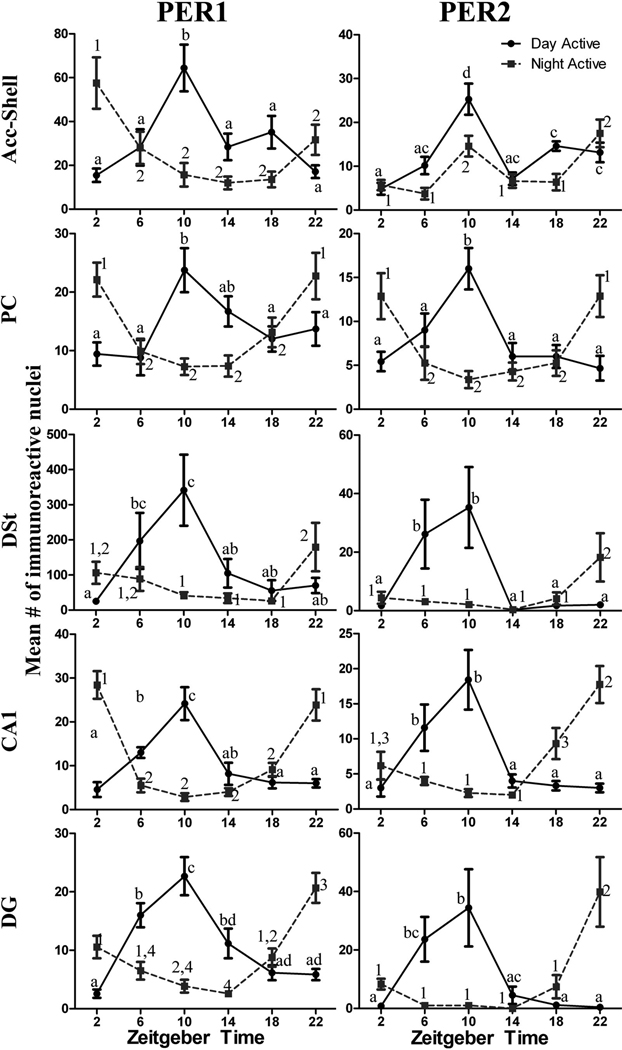
The statistical results from the one-way ANOVAs for each brain regions and PER protein are shown in Table 1, and the differences across ZTs for each brain region and PER protein are shown in Figure 2. Figure 3 shows the locations of the sampling boxes and representative examples of PER1 and PER2 staining in the five brain regions. For PER1 there was a significant effect of ZT for all the brain regions. For PER2 a significant effect of ZT was evident in all regions except in the DG, where PER2 expression was relatively low and arrhythmic. For all areas with significant rhythmic expression of PER1 and/or PER2, peak values were seen at ZT10.
Table 1.
Experiment 1: PER1 and PER2 in extra-SCN oscillators of the grass rats without access to wheels
| Region | PER1 | PER2 |
|---|---|---|
| Acc-Shell | F= 7.11, df =5, P<0.001 | F= 15.94, df =5, P<0.001 |
| PC | F= 6.18, df =5, P=0.001 | F= 16.02, df =5, P<0.001 |
| DSt | F= 4.12, df =5, P=0.006 | F= 6.13, df =5, P=0.001 |
| CA1 | F= 5.32, df =5, P=0.001 | F= 8.00, df =5, P<0.001 |
| DG | F= 4.52, df =5, P=0.004 | F= 1.314, df=5, p=.289 |
Figure 2.
Line graphs showing rhythms of PER1 and PER2 expression in the Acc-Shell, PC, DSt, CA1, and DG of male grass rats. Animals were kept in a 12-h LD cycle and sacrificed at 4-hour intervals starting at ZT 2. The data are presented as means [± standard error of the mean (SEM)] for each ZT. Significant differences (P<0.05) are noted by different letters.
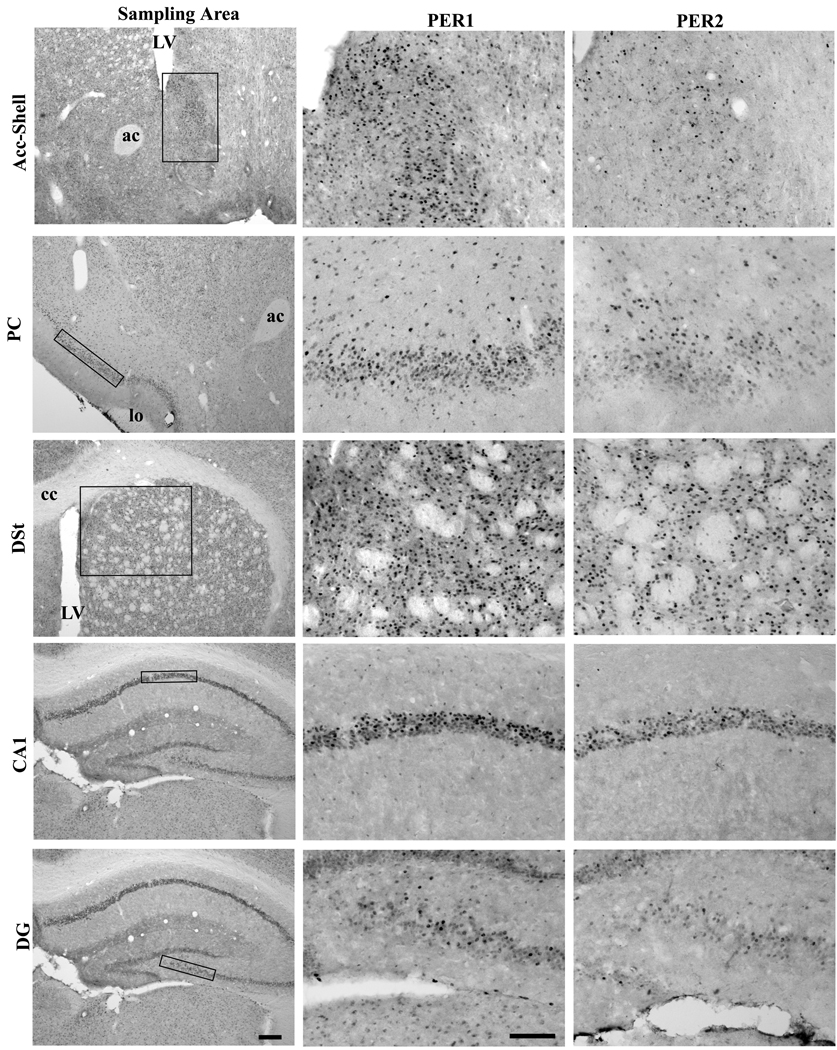
Figure 3.
Photomicrographs depicting the PER1 and PER2 expression in the Acc-Shell, the PC, the DSt, the CA1, and the DG. The first column represents the sampling areas of the five regions. The second and third columns display representative PER1 and PER2 staining, respectively. The representative animals were perfused at the ZT10. LV-lateral ventricle, ac-anterior commissure, lo-lateral olfactory track. cc-corpus callosum, Scale bars of the first and second columns are 200um and 100 um, respectively.
3.2 Experiment 2: PER1 and PER2 expression in the SCN, the vSPZ and other extra-SCN oscillators of DA and NA grass rats with access to running wheels
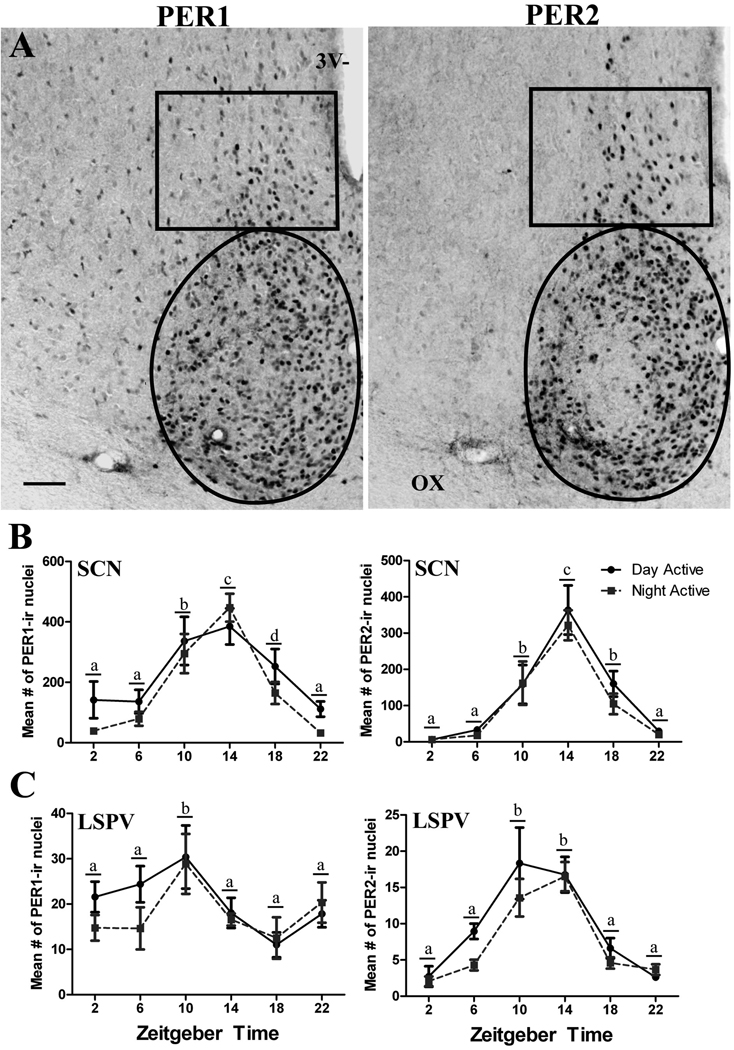
Figure 4A illustrates the sampling areas and PER1/2 labeling in the SCN and the vSPZ. In the SCN, the two-way ANOVA revealed a significant effect of ZT on PER1 (F = 17.31, df = 5, P< 0.001) and PER2 (F = 25.08, df = 5, P< 0.001) expression, with no chronotype differences (P=0.065 for PER1; P=0.352 for PER2) or significant interaction (P=0.555 for PER1; P=0.958 for PER2). Figure 4B shows the results of the post-hoc comparison across ZTs; for both PER proteins and for both chronotypes peak values were seen at ZT14. In the vSPZ both PER1 and PER2 expression were significantly affected by ZT (F = 3.67, df = 5, P=0.005 for PER1 and F = 19.82, df = 5, P< 0.001 for PER2) but we found no chronotype differences (P=0.300 for PER1; P=0.112 for PER2) and no significant interaction effect (P=0.691 for PER1; P=0.618 for PER2). The results of the multiple post-hoc comparisons for PER1 revealed that ZT10 was significantly different from all other ZTs and for PER2 that ZT10 and 14 were significantly different from all other ZTs (see Figure 4C). The spatial distribution of labeled cells in the SCN and the vSPVZ was indistinguishable across chronotypes. For PER2, the SCN of both chronotypes featured a central region that was relatively free of labeled neurons (Figure 4A, right panel) and that corresponds to the area where calbindin positive cells are abundant (Mahoney et al., 2000).
Figure 4.
(A) Photomicrographs of coronal sections through the SCN and vSPZ of representative NA animals sampled at ZT14. Scale bar 100um. 3V-third ventricle, OX-optic chiasms. (B) Line graphs showing the mean (±SEM) number of PER1 and PER2 expressing cells for each ZT in the SCN and the vSPZ of DA and the NA male grass rats. Animals were kept in a 12-h LD cycle and sacrificed at 4-h intervals starting at ZT2. Solid black lines represent DA and dotted grey lines represent NA grass rats. Significant differences (P<0.05) are noted by different letters.
The results of the two-way ANOVAS for each PER protein and for each of the five brain regions that were also included in Experiment 1 are summarized in Table 2. For PER1 expression, there was a significant ZT x Chronotype interaction for the Acc-Shell, PC, DSt, CA1 and DG. Follow-up analyses of the simple main effects revealed significant effects of ZT for both Chronotypes. The post-hoc comparisons across ZTs for PER1 expression in these brain areas (see Figure 5) showed that peak values occurred at ZT10 in DA animals and between ZT22 and ZT2 in the NA group. For PER2 expression, there was a significant ZT x Chronotype interaction for the same five regions (see Table 2), and there were significant simple main effects of ZT in all these brain regions. Figure 5 shows the results of post-hoc comparisons across ZTs. Peak expression of PER2 occurred at ZT10 for DA animals and between ZT22 and ZT2 for NA animals; however in the Acc-Shell, there was also an elevation in PER2 production at ZT10.
Table 2.
Experiment 2: PER1 and PER2 expression in extra-SCN oscillators of DA and NA grass rats with access to running wheels
| PER1 | PER2 | |||
|---|---|---|---|---|
| Region | 2-way ANOVA | 1-way ANOVA | 2-way ANOVA | 1-way ANOVA |
| ZT×Chronotype Interaction |
Simple Main Effect of Time | ZT×Chronotype Interaction | Simple Main Effect of Time | |
| Acc-Shell | F= 9.811, df =5, P<0.001 | DA (F= 6.686, df =5,P<0.001) | F= 3.120, df =5, P=0.013 | DA (F= 9.26, df =5, P<0.001) |
| NA (F= 5.81, df =5, P<0.001) | NA (F= 7.48, df =5, P<0.001) | |||
| PC | F= 8.42, df =5, P<0.001 | DA (F= 3.94, df =5, P=0.006) | F= 9.307, df =5, P<0.001 | DA (F= 6.75, df =5, P<0.001) |
| NA (F= 7.34, df =5, P<0.001) | NA (F= 5.64, df =5, P<0.001) | |||
| DSt | F= 5.184, df =5, P<0.001 | DA (F= 4.52, df =5, P=0.003) | F= 5.331, df =5, P<0.001 | DA (F= 4.43, df =5, P=0.003) |
| NA (F= 2.55, df =5, P=0.042) | NA (F= 2.73, df =5, P=0.032) | |||
| CA1 | F= 26.63, df =5, P<0.001 | DA (F= 12.50, df =5, P<0.001) | F= 14.69, df =5, P<0.001 | DA (F= 7.26, df =5, P<0.001) |
| NA (F= 19.69, df =5, P<0.001) | NA (F= 13.37, df =5, P<0.001) | |||
| DG | F= 22.46, df =5, P<0.001 | DA (F= 15.16, df =5, P<0.001) | F= 8.77, df =5, P<0.001 | DA (F= 4.59, df =5, P=0.003) |
| NA (F= 13.31, df =5, P<0.001) | NA (F= 7.44, df =5, P<0.001) | |||
Figure 5.
Line graphs showing the mean (± SEM) number of PER1 and PER2 expressing cells for each ZT in the Acc-Shell, PC, DSt, CA1, and DG of DA and NA male grass rat. Animals were kept in a 12-h LD cycle and sacrificed at 4-hour intervals starting at ZT2. Solid black lines represent DA and dotted grey lines represent NA grass rats. Significant differences (P<0.05) are noted by different letters (DA) or different numbers (NA).
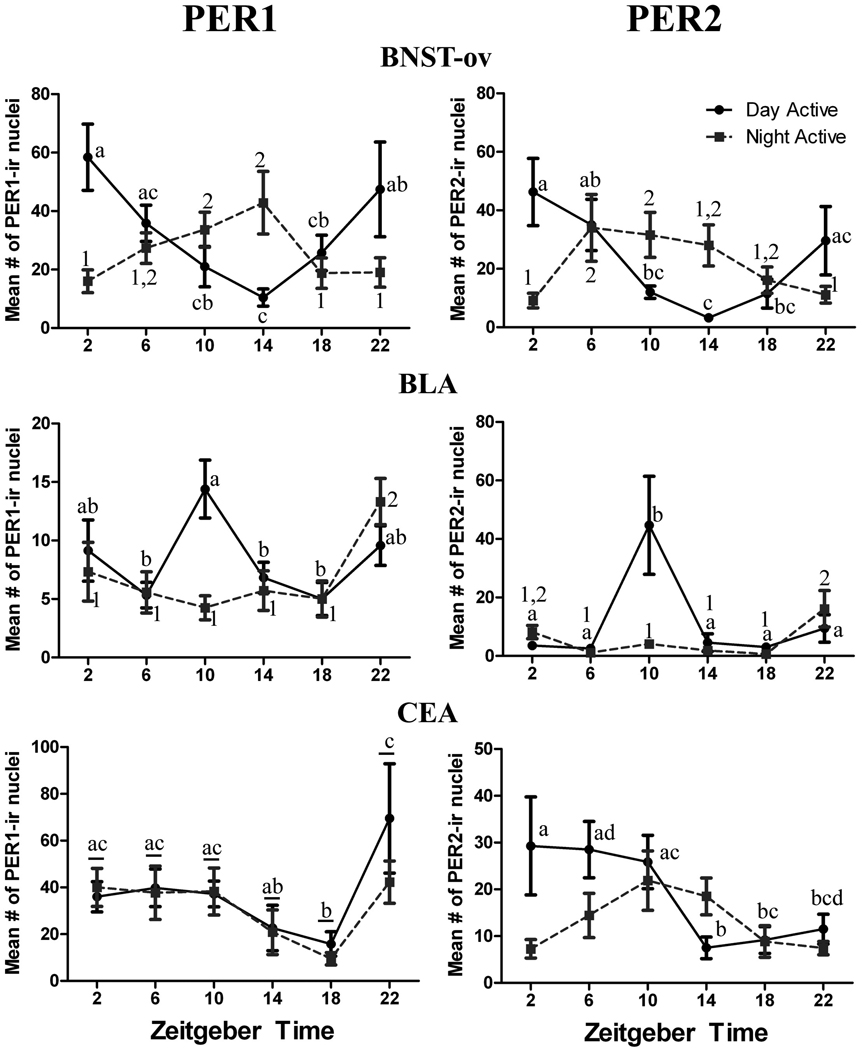
The results of the two-way ANOVAs for the BNST-ov and the two regions of the amygdala are summarized for each PER protein in Table 3, and Figure 6 shows the results of post-hoc comparisons across ZTs. Figure 7 shows the sampling area and representative labeling for each of the three brain regions. For PER1 in both the BNST-ov and the BLA there was a significant ZT x Chronotype interaction effect, and the simple main effects of ZT were also significant for both chronotypes. In the BNST-ov peak expression of PER1 was seen at ZT2 and at ZT14 in DA and NA animals, respectively. The results for PER2 in the BNST-ov were very similar (see Table 3 and Figure 6 right panel) to those for PER1, except that peak values occurred earlier (ZT6 – ZT14) in the case of NA animals. In the BLA, peak expression of both PER1 and PER2 was evident at ZT10 in DA animals and for PER1 at ZT22 in NA animals. PER2 expression in the BLA of NA animals was relatively low for most of the 24 period, but showed a significant elevation at ZT22. In the CEA the analysis of PER1 expression did not reveal a significant interaction effect, but there was a significant main effect of ZT for both chronotypes, and the pattern of PER1 expression featured sustained levels between ZT22 and ZT10 in both groups. For PER2 expression in the CEA, there was a significant interaction effect, and a significant simple main effect of ZT only for the DA animals. In that group PER2 expression was sustained between ZT2 and ZT10, with a drop at ZT14.
Table 3.
Experiment 2: PER1 and PER2 expression in extra-SCN oscillators of DA and NA grass rats with access to running wheels
| PER1 | PER2 | ||||
|---|---|---|---|---|---|
| Region | 2-way ANOVA | 1-way ANOVA | 2-way ANOVA | 1-way ANOVA | |
| Time×Chronotype Interaction |
Main Effect of Time |
Simple Main Effect of Time |
Time×Chronotype Interaction |
Simple Main Effect of Time |
|
| BNST-OV | F= 5.979, df =5, p<0.001 | - | DA (F= 3.575, df=5, p=.010) | F= 4.73, df =5, p=0.001 | DA (F= 3.39, df =5, p=0.018) |
| NA (F= 2.791, df=5, p=.030) | NA (F= 2.72, df =5, p=0.034) | ||||
| BLA | F= 3.14, df =5, p=0.013 | - | DA (F= 3.06, df =5, p=0.023) | F= 5.63, df =5, p<0.001 | DA (F= 5.32, df =5, p=0.001) |
| NA (F= 4.10, df =5, p=0.004) | NA (F= 3.48, df =5, p=.011) | ||||
| CEA | - | F=4.47, df=5, p=.001 | - | F=2.48, df =5, p=0.040 | DA (F=2.735, df=5, p=.036) |
| NA (F=2.289. df=5, p=.065) | |||||
Figure 6.
Line graphs showing the mean (± SEM) number of PER1 and PER2 expressing cells for each ZT in the BNST-ov, BLA, and CEA of DA and NA male grass rat. Animals were kept in a 12-h LD cycle and sacrificed at 4-hour intervals starting at ZT2. Other details as in Figure 5, except that in the CEA for the PER1 data (lower left panel) significant differences between ZTs are indicated by different letters for both the NA and DA groups.
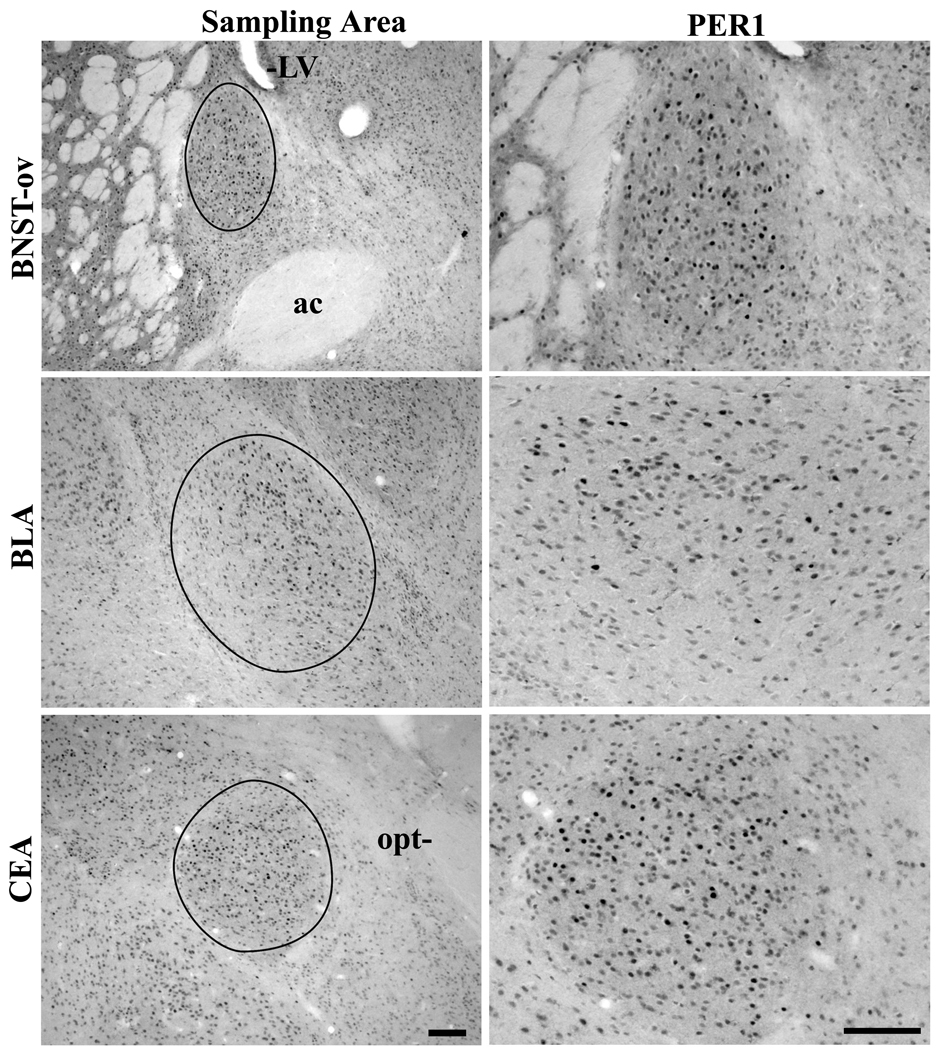
Figure 7.
Photomicrographs depicting the PER1 expression in the BNST-ov, BLA and CEA. The first column of photomicrographs represents the sampling areas of the three regions. The second column of photomicrographs displays representative PER1 staining. The representative DA animals were perfused at peak PER1 expression for each region (ZT2, 10, and 22, respectively). LV-lateral ventricle, ac-anterior commissure, opt-optic track. Scale bars of the first and second columns are 100um.
The results of the analysis of the simple main effects of Chronotype for all areas (except for the CEA), and for both proteins are presented in Table 4. Because of the differences in patterns (i.e., in most cases an antiphase relationship) of protein expression between NA and DA grass rats, there was a complex profile of significant differences between the two groups of animals, without a consistent trend for differences in amount of PER1 and PER2 expression between chronotypes.
Table 4.
Experiment 2: T-tests for simple main effects of Chronotype within each ZT
| Region |
ZT2 |
ZT6 |
ZT10 |
ZT14 |
ZT18 |
ZT22 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PER1 | PER2 | PER1 | PER2 | PER1 | PER2 | PER1 | PER2 | PER1 | PER2 | PER1 | PER2 | |
| Acc-Shell | * | ns | ns | * | * | ns | * | ns | * | * | ns | ns |
| PC | * | * | ns | ns | * | * | * | ns | ns | ns | ns | * |
| DSt | * | ns | ns | ns | * | * | ns | ns | ns | ns | ns | ns |
| CA1 | * | ns | ns | ns | * | * | ns | ns | ns | * | * | * |
| DG | * | * | * | * | * | * | * | ns | ns | ns | * | * |
| BLA | ns | ns | ns | ns | * | * | ns | ns | ns | * | ns | ns |
| BNST-ov | * | * | ns | ns | ns | ns | * | * | ns | ns | ns | ns |
| CEA | ns | ns | ns | ns | ns | ns | ns | * | ns | ns | ns | ns |
significant difference, ns- not significant
4. Discussion
Experiment 1: One important finding of Experiment 1 and of previous studies (Ramanathan et al., 2008b, Ramanathan et al., 2010) is that the phases of PER1 and PER2 rhythms outside of the SCN with respect to the light-dark cycle are remarkably different between diurnal grass rats and nocturnal laboratory rodents (Angeles-Castellanos et al., 2007, Feillet et al., 2008, Amir and Stewart, 2009b) kept in standard laboratory cages, with no access to wheels. These differences in phase are evident in several areas of the brain involved in learning and memory, fear and anxiety, motivation and autonomic regulation (Amir et al., 2004, Lamont et al., 2005, Angeles-Castellanos et al., 2007, Feillet et al., 2008), and rhythms in many of these functions are likely to be phase reversed between diurnal and nocturnal species. Since our previous and current work has used a single endpoint (i.e., PER protein production) it is important for follow up work to include the expression of other clock genes and clock-controlled genes in these extra-SCN regions, and to monitor neural activity in these areas.
Specific comparisons between the PER1 and PER2 rhythms of grass rats and those of nocturnal species are complicated by the fact that in some brain structures nocturnal species show rhythmic expression of only one of the two proteins (Feillet et al., 2008), whereas both are rhythmically expressed in almost all extra-SCN oscillators of grass rats (Ramanathan et al., 2008b, Ramanathan et al., 2010), and present data); lack of rhythmic expression of PER2 in the DG of grass rats being the exception (present data). Although there may be specific roles for PER1 and PER2 in different brain regions (Zheng et al., 2001), one way to facilitate comparisons across species is to focus on the rhythmic expression of PER, combining the patterns of PER1 and PER2 expression for each structure. When this is done for the Acc-Shell, PC, DSt, CA1, and DG the general pattern that emerges is one in which PER expression is elevated in the late morning in grass rats and in the late night in nocturnal rodents (Amir et al., 2006, Angeles-Castellanos et al., 2007, Feillet et al., 2008). A similar comparison for the BNST-ov and the CEA shows peak levels early in the day for grass rats without wheels (Ramanathan et al., 2008b, Ramanathan et al., 2010) and early in the night for nocturnal rodents (Amir et al., 2004, Lamont et al., 2005, Angeles-Castellanos et al., 2007, Feillet et al., 2008). Finally in the BLA of grass rats PER expression is high from ZT2 – ZT10, but it does not peak until late night in nocturnal rodents (Lamont et al., 2005, Feillet et al., 2008, Ramanathan et al., 2008b, Ramanathan et al., 2010). In contrast to these species differences in extra-SCN oscillators, the phase of PER1 and PER2 rhythms in the SCN is virtually identical across species, regardless of phase preference for the display of activity (Ramanathan et al., 2006, Smale et al., 2008). Taken together, these observations support the claim that differences between diurnal and nocturnal mammals depend upon mechanisms that reside downstream from the SCN (Smale et al., 2003, Smale et al., 2008), and appear to involve phase shifts in the rhythmic expression of PER1 and PER2 in extra-SCN oscillators.
Extra-SCN oscillators within the brain, as a rule, depend upon SCN inputs to sustain rhythmicity [with the exceptions of the olfactory bulb (Granados-Fuentes et al., 2004) and retina (Tosini et al., 2008)] and to be entrained to the light-dark cycle (Abe et al., 2002, Amir et al., 2004). However, the phase and robustness of clock gene rhythms in many extra-SCN oscillators are determined in part by factors, such as hormones and meal patterns that do not affect the clock-gene rhythms of the SCN (Angeles-Castellanos et al., 2007, Feillet et al., 2008, Amir and Stewart, 2009b, Verwey and Amir, 2009). Thus, although the SCN is necessary for sustaining their rhythms, extra-SCN oscillators show functional plasticity, and their phase is heavily influenced by inputs from other sources.. The phase reversal of several extra-SCN oscillators in nocturnal and diurnal species, suggests that the phase of rhythms of clock-controlled genes in several extra-SCN regions is likely to differ in diurnal and nocturnal brains. Documenting those postulated differences and how they affect neural activity in these brain regions are important directions for future research on the neural determinants of phase preference across mammalian species.
Experiment 2: The results of Experiment 2 show that when grass rats switch to a predominantly nocturnal pattern of activity, the rhythms in PER1 and PER2 in most extra-SCN oscillators adopt a phase relationship with respect to the light-dark cycle that resembles that of nocturnal species. Thus the behavioral plasticity displayed by a subset of wheel running grass rats is matched by functional plasticity in extra-SCN oscillators. This was clearly the case for both PER proteins in the PC, DSt, CA1, DG, BNST-ov and the BLA and for PER1 in the Acc-Shell. In these regions, the peak in protein production seen at ZT10 in DA grass rats was absent in NA individuals, which in turn showed peak protein production 12 hours later; the only exceptions being the pattern of PER1 expression in the CEA, which showed a peak about 10 hours later than the one reported for nocturnal mice (Feillet et al., 2008), and the presence of a peak at ZT10 for PER2 expression in the Acc-Shell of NA grass rats. The shifts in phase in extra-SCN oscillators of NA grass rats contrast sharply with the lack of any difference in the phase of the SCN PER1 and PER2 rhythms between the two chronotypes. Thus, individual phase preference for the display of activity in grass rats is not predictable from the phase of the SCN oscillator, as it appears to be the case for predicting phase preferences across mammalian species (Smale et al. 2008).
The causal direction for the shift in NA grass rats with respect to the display of activity and the phase of extra-SCN oscillators is not known. Thus, the animals may become night active first, with the nocturnal activity then setting the phase of the extra-SCN oscillators or, alternatively, a shift in the phase of the oscillators, triggered by wheel running, may result in the phase reversal in the display of activity. Experiments that assess the phase of extra-SCN oscillators in grass rats immediately after a chronotype shift may shed light on the question of causality.
Separate from questions about the directional of causality of the phase shifts, are questions related to the mechanisms that could mediate the shift in the phases of extra-SCN oscillators. One possibility is that these extra-SCN oscillators phase shift, not because they become functionally independent of the SCN, but because of a change in the coupling parameters between the oscillator of the SCN and those that reside in extra-SCN regions. This situation would be analogous to the splitting phenomenon seen in hamsters exposed to constant light, in which the right and left SCN become stably coupled 180° out of phase with each other (de la Iglesia et al., 2000). Although this coupling pattern has been well documented for the interactions between the self-sustained oscillators of each SCN, to our knowledge it has never been observed between the SCN and damp oscillators, thus this possible explanation remains speculative. Alternatively, the switch may take place in a self-sustained oscillator, other than the SCN, with that switch affecting the phase of extra-SCN oscillators that do not display self-sustained cycles. The self-sustained oscillator of the main olfactory bulb is a possible candidate for this role. The main olfactory bulb provides direct and indirect input to many of the extra-SCN areas that show PER1/2 rhythms (Shipley et al., 2004), particularly to the PIR, where inputs from the olfactory bulb control a rhythm in olfactory sensitivity (Granados-Fuentes et al., 2006). Experiments determining the phase of the olfactory bulb oscillator in NA and DA grass rats as well as the use of olfactory bulbectomies are needed to test this hypothesis. Finally, for at least some extra-SCN oscillators, the phase shift may be mediated by a change in the phase of rhythms of adrenal glucocorticoid production (see Amir and Steward, 2009 for a review). Studies combining assessment of adrenal rhythms with adrenalectomies and timed hormone replacement paradigms are needed to test that hypothesis.
The food entrainable oscillator (Stephan, 2002) is another extra-SCN self-sustained oscillator that may be involved in the phase shifts of NA grass rats. There is evidence that feeding schedules can affect the phase of PER1/2 rhythms in some extra-SCN brain regions (Angeles-Castellanos et al., 2007, Feillet et al., 2008, Verwey and Amir, 2009) at least in nocturnal species, thus possible changes in meal patterns associated with the switch to nocturnal activity may contribute to the phase reversal of extra-SCN oscillators in NA grass rats. This explanation appears unlikely for two reasons. First, in animals fed standard laboratory chow, as it was the case here, protracted daily periods of food deprivation are needed to shift extra-SCN oscillators (Angeles-Castellanos et al., 2007, Feillet et al., 2008, Verwey and Amir, 2009); our animals had ad libitum access to food. Second, shifts induced by scheduled feeding are typically seen in only a sub-set of these extra-SCN oscillators (Angeles-Castellanos et al., 2007, Feillet et al., 2008), in sharp contrast with the almost universal shift displayed by our NA grass rats. However, since all the observations about food entrainment come from studies with nocturnal rodents, data on how the extra-SCN oscillators of grass rats respond to feeding schedules are needed before abandoning the hypothesis that voluntary changes in meal patterns contribute to the phase adopted by these oscillators in NA individuals.
As was the case for the SCN, a switch to nocturnal activity did not affect the phase of the PER1 and PER2 rhythms in the vSPZ. This is also the case for rhythms in Fos expression in this area, which are the same in DA and NA grass rats (Schwartz and Smale 2005). Although the vSPZ of grass rats is clearly functionally and anatomically distinct from their SCN (Nunez et al., 1999, Mahoney et al., 2000, Mahoney et al., 2001, Schwartz et al., 2004, Schwartz, 2006, Schwartz et al., 2009), the vSPZ of the grass rats may share some functional features with the SCN in circadian regulation (Schwartz et al., 2004, Schwartz, 2006, Schwartz et al., 2009) and may be fundamentally different from other extra-SCN oscillators. There is a remarkable overlap in the terminal fields of SCN and vSPZ projections (Watts et al., 1987, Schwartz, 2006), and in grass rats the phase of extra-SCN oscillators may be influenced by signals generated by both the SCN and the vSPZ (Schwartz et al. 2009).
The PER1 and PER2 rhythms of most extra-SCN oscillators of NA grass rats resemble the patterns of nocturnal species (Amir et al., 2004, Lamont et al., 2005, Angeles-Castellanos et al., 2007, Feillet et al., 2008), but we do not know if this shift is reflected in all rhythmic functions of the individual brain regions. Therefore, it is possible that in NA grass rats, while some rhythms in clock controlled genes adopt the new phase of the local oscillators, other rhythmic functions may continue to obey the phase setting signals of the SCN and/or the vSPZ. That situation could result in the decoupling of rhythms that are normally in phase with each other, and thus, in the disruption of the temporal organization of neural systems responsible for behavioral and regulatory functions. In addition to their phase reversals, NA grass rats lacked rhythmic expression of PER2 in the CEA, and showed a pattern of PER2 expression in the ACC-Shell that appears to have features common to both diurnal (elevation at ZT10) and nocturnal (peak at ZT22) species, which could represent an additional challenges for the orchestration of circadian functions in these animals.
The normal nocturnal elevation of pineal melatonin production is evident in NA grass rats (Martin-Fairey et al., personal communication). This is not surprising given the constant phase of the SCN and its tight control of the pineal melatonin rhythm in rodents (Klein and Moore, 1979, Kalsbeek et al., 2000, Challet, 2007). Even though there is no consensus about the specific role of melatonin in the regulation of sleep or other behavioral rhythms, melatonin is likely to have very different effects in diurnal and nocturnal species, (Dollins et al., 1994, Lavie, 1997, Huber et al., 1998, Mailliet et al., 2001, Pandi-Perumal et al., 2008) and in the case of NA grass rats, melatonin peaks when activity levels are high. One interesting observation is that despite their night-active profile, NA grass rats show an abrupt increase in the display of sleep in the late night (i.e., at ZT20, see Schwartz and Smale, 2005 and the actogram of Figure 1C), which may be due to the putative sleep promoting actions of melatonin in diurnal species (Lavie, 1997). This mismatch between melatonin production and activity may represent another form of circadian disruption in NA grass rats. Human night-shift workers are at risk for many health and mental health problems (Scott, 2000). The NA grass rats provides a model to study how voluntary phase reversals in the display of activity, which accompany night-shift work, affects the phase of multiple oscillators (present results) and the quality of sleep (Schwartz and Smale, 2005) independently of changes in exposure to light.
For most brain regions the pattern of expression of PER1 and PER2 was identical for DA grass rats and grass rats housed without wheels. This was the case for rhythms of both proteins in the Acc-Shell, PC, DSt, CA1, (present results) and BNST-ov (present results and (Ramanathan et al., 2008b, Ramanathan et al., 2010), and with the exception of a drop in PER1 expression at ZT6 in the DA animals, also true for the BLA (present results and (Ramanathan et al., 2008b, Ramanathan et al., 2010). Thus, the overall profile of most extra-SCN oscillators is not affected by wheel running in animals that do not switch to a nocturnal display of activity. There were, however, exceptions. DA grass rats showed a peak at ZT22 in the expression of PER1 in the CEA, whereas in grass rats without wheels the peak was at ZT2; (Ramanathan et al., 2008b). Peak PER1 expression in NA grass rats was also at ZT22, which is different from the time of peak expression in nocturnal mice (i.e., ZT12; (Feillet et al., 2008). In the case of PER1 rhythms in the CEA, wheel running appears to phase advance the rhythm by about 4 hours regardless of the effect of wheel running on the animals’ phase preference for the display of activity. Similar effects of wheel running, independent of chronotype, have been reported for the expression of Fos in some components of the reward system of the brain of grass rats (Castillo-Ruiz et al., 2010). The change in patterns of PER1 expression in the CEA of both DA and NA grass rats resulted in a deviation from the phase lock that has been universally reported for PER rhythms in the BNST-ov and in the CEA (Ramanathan et al., 2008b, Amir and Stewart, 2009a, Amir and Stewart, 2009b, Ramanathan et al., 2010). This was particularly salient in the case of the NA grass rats; for that group the phase difference between the CEA and the BNST-ov was between 10 and 12 hours. Although many consider the CEA and the BNST-ov to be a single functional and anatomical unit (Alheid, 2003, Amir and Stewart, 2009a, Amir and Stewart, 2009b), there is evidence of subtle functional differences between these two components of the extended amygdala (Walker et al., 2003). Our observations raise the possibility that engaging in exercise decouples rhythms in functions associated with the BNST-ov and the CEA, particularly in the case of NA grass rats, and further research with these animals should be instrumental in determining the function of local oscillators in these two areas of the extended amygdala.
Another interesting difference between both DA grass rats and animals without access to wheels was the emergence of a rhythm of PER2 expression in the DG in animals with wheel access. This rhythmic expression of PER2 in the DG appears to be a reaction to exercise, since it was also evident in NA grass rats. For both chronotypes, the novel PER2 rhythm was in phase with that of PER1 and completely reversed between DA and NA animals. The DG is the most active region of the adult mammalian brain with respect to neurogenesis, and wheel running has been shown to enhance neurogenesis in the DG of adult rodents (van Praag et al., 1999, Holmes et al., 2004, Tamai et al., 2008). Since PER2 may play a role in modulating neurogenesis (Borgs et al., 2009) it is now important to determine if the enhanced and rhythmic production of PER2 increases the amplitude of rhythms in DG neurogenesis of grass rats, and if such rhythms are reversed between DA and NA grass rats.
5. Conclusions
In summary, our results indicate that many extra-SCN oscillators are 180° out of phase between diurnal grass rats and nocturnal species (Experiment 1). This confirms and extends previous work using this animal model to study the patterns of PER1and PER2 expression in the brain (Ramanathan et al., 2008a, Ramanathan et al., 2008b, Ramanathan et al., 2010) as well as in the liver (Lambert and Weaver, 2006). These differences in phase stand in contrast to the cross-species consistency of the phase of the oscillator within the SCN (Challet, 2007, Smale et al., 2008). Taken together these observations add support to the hypothesis that the evolution of differences between the brains of diurnal and nocturnal species involved changes in coupling between oscillators within the SCN and those in other regions of the brain or major organs of the body. The results of Experiment 2 suggest that the same principle applies to differences within a species. That is, phase reversals in the activity rhythms of grass rats were associated with phase reversals of rhythms in almost all extra-SCN regions, but not of rhythms within the SCN. Our results also indicate that in contrast to other extra-SCN oscillators, that of the vSPZ remains locked to the light-dark cycle regardless of the phase of the overt activity rhythm. This observation supports the view that the vSPZ may work with the SCN to mediate light-entrainable rhythms, but that it may do this differently in nocturnal and diurnal species (Smale et al., 2003, Smale et al., 2008).
The current data raise two general questions relating to intra- and inter-specific differences in the multi-oscillator system that coordinates circadian rhythms. First: what causes the differences in coupling between oscillators within the SCN/vSPZ region and those elsewhere in the brain? The patterns suggest that while the mechanisms coupling the SCN to the LD cycle are invariant, those coupling oscillators in the SCN to oscillators in other brain regions have changed across evolutionary time and can change within the lifespan of individuals. The reversals seen in these oscillators in NA grass rats suggest that the influence of the SCN on their phase may be extremely weak, and they raise the question of what the non-photic mechanisms are that can so dramatically modify the phase of these oscillators. The second major question raised by our observations is: what are the consequences of phase reversals of extra-SCN oscillators? Again, the question is relevant to both the inter- and the intra-specific differences revealed here. Further studies of NA grass rats are needed to determine if the shifts in the local oscillators disrupt the phase relations of region-specific rhythmic functions. It is possible that the reversals in the phase of PER rhythms displayed by NA grass rats create conflicts between circadian signals from the SCN and vSPZ and those emanating from local oscillators. Such disruptions are likely to affect the temporal organization of many important functions, and may be related to the multiple pathologies associated with human shift work (Scott, 2000, Mahoney, 2010).
Acknowledgements
The authors wish to thank Anthony Francis Yuhas, Jessica Schrader, Alexandra Castillo-Ruiz, Carmel Martin-Fairey, Dorela Shuboni, Shannon Cramm, Daniel Star and Steve Dressler. Dr. Lily Yan provided technical advice and made many insightful suggestions that improved the quality of this paper. This work was supported by the National Institute of Mental Health RO1 MH53433 to LS.
A comprehensive list of abbreviations
- Acc-Shell
Accumbens Shell
- BNST-ov
Oval nucleus of the bed nucleus of stria terminalis
- BLA
Basolateral amygdale
- CEA
Central amygdale
- DSt
Dorsal striatum
- DG
Dentate gyrus
- DA
Day active
- LD
Light-dark cycle
- vSPZ
ventral subparaventricular zone
- NA
Night active
- PC
Piriform cortex
- SCN
Suprachiasmatic nucleus
- ZT
Zeitgeber time
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc Natl Acad Sci U S A. 2002;99:9026–9030. doi: 10.1073/pnas.142039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Circadian rhythms in isolated brain regions. The Journal of Neuroscience. 2002;22:350–356. doi: 10.1523/JNEUROSCI.22-01-00350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alheid GF. Extended amygdala and basal forebrain. Ann N Y Acad Sci. 2003;985:185–205. doi: 10.1111/j.1749-6632.2003.tb07082.x. [DOI] [PubMed] [Google Scholar]
- Amir S, Harbour VL, Robinson B. Pinealectomy does not affect diurnal PER2 expression in the rat limbic forebrain. Neurosci Lett. 2006;399:147–150. doi: 10.1016/j.neulet.2006.01.041. [DOI] [PubMed] [Google Scholar]
- Amir S, Lamont EW, Robinson B, Stewart J. A circadian rhythm in the expression of PERIOD2 protein reveals a novel SCN-controlled oscillator in the oval nucleus of the bed nucleus of the stria terminalis. J Neurosci. 2004;24:781–790. doi: 10.1523/JNEUROSCI.4488-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir S, Stewart J. Behavioral and hormonal regulation of expression of the clock protein, PER2, in the central extended amygdala. Prog Neuropsychopharmacol Biol Psychiatry. 2009a;33:1321–1328. doi: 10.1016/j.pnpbp.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Amir S, Stewart J. Motivational Modulation of Rhythms of the Expression of the Clock Protein PER2 in the Limbic Forebrain. Biol Psychiatry. 2009b;65:829–834. doi: 10.1016/j.biopsych.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Angeles-Castellanos M, Mendoza J, Escobar C. Restricted feeding schedules phase shift daily rhythms of c-Fos and protein Per1 immunoreactivity in corticolimbic regions in rats. Neuroscience. 2007;144:344–355. doi: 10.1016/j.neuroscience.2006.08.064. [DOI] [PubMed] [Google Scholar]
- Blanchong JA, McElhinny TL, Mahoney MM, Smale L. Nocturnal and diurnal rhythms in the unstriped Nile rat, Arvicanthis niloticus. J Biol Rhythms. 1999;14:364–377. doi: 10.1177/074873099129000777. [DOI] [PubMed] [Google Scholar]
- Blanchong JA, Smale L. Temporal patterns of activity of the unstriped Nile rat, Arvicanthis niloticus. Journal of Mammalogy. 2000;81:595–599. [Google Scholar]
- Borgs L, Beukelaers P, Vandenbosch R, Nguyen L, Moonen G, Maquet P, Albrecht U, Belachew S, Malgrange B. Period 2 regulates neural stem/progenitor cell proliferation in the adult hippocampus. BMC Neurosci. 2009;10:30. doi: 10.1186/1471-2202-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Ruiz A, Nixon JP, Smale L, Nunez AA. Neural activation in arousal and reward areas of the brain in day-active and night-active grass rats. Neuroscience. 2010;165:337–349. doi: 10.1016/j.neuroscience.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challet E. Minireview: Entrainment of the suprachiasmatic clockwork in diurnal and nocturnal mammals. Endocrinology. 2007;148:5648–5655. doi: 10.1210/en.2007-0804. [DOI] [PubMed] [Google Scholar]
- de la Iglesia HO, Meyer J, Caprino A, Jr, Schwartz WJ. Antiphase oscillation of the left and right suprachiasmatic nuclei. Science. 2000;290:799–801. doi: 10.1126/science.290.5492.799. [DOI] [PubMed] [Google Scholar]
- Dardente H, Menet JS, Challet E, Tournier BB, Pevet P, Masson-Pevet M. Daily and circadian expression of neuropeptides in the suprachiasmatic nuclei of nocturnal and diurnal rodents. Brain Res Mol Brain Res. 2004;124:143–151. doi: 10.1016/j.molbrainres.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- Dollins AB, Zhdanova IV, Wurtman RJ, Lynch HJ, Deng MH. Effect of inducing nocturnal serum melatonin concentrations in daytime on sleep, mood, body temperature, and performance. Proc Natl Acad Sci U S A. 1994;91:1824–1828. doi: 10.1073/pnas.91.5.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feillet CA, Mendoza J, Albrecht U, Pevet P, Challet E. Forebrain oscillators ticking with different clock hands. Mol Cell Neurosci. 2008;37:209–221. doi: 10.1016/j.mcn.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Gau SS-F, Shang C-Y, Merikangas KR, Chiu Y-N, Soong W-T, Cheng T-A. Association between morningness-eviningness and behavioral/emotional problems among adolescents. Journal of Biological Rhythms. 2007;22:268–274. doi: 10.1177/0748730406298447. [DOI] [PubMed] [Google Scholar]
- Granados-Fuentes D, Prolo LM, Abraham U, Herzog ED. The suprachiasmatic nucleus entrains, but does not sustain, circadian rhythmicity in the olfactory bulb. J Neurosci. 2004;24:615–619. doi: 10.1523/JNEUROSCI.4002-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granados-Fuentes D, Tseng A, Herzog ED. A circadian clock in the olfactory bulb controls olfactory responsivity. The Journal of Neuroscience. 2006;26:12219–12225. doi: 10.1523/JNEUROSCI.3445-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilding C, Hughes AT, Brown TM, Namvar S, Piggins HD. A riot of rhythms: neuronal and glial circadian oscillators in the mediobasal hypothalamus. Mol Brain. 2009;2:28. doi: 10.1186/1756-6606-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilding C, Piggins HD. Challenging the omnipotence of the suprachiasmatic timekeeper: are circadian oscillators present throughout the mammalian brain? Eur J Neurosci. 2007;25:3195–3216. doi: 10.1111/j.1460-9568.2007.05581.x. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Maywood ES, Reddy AB. Two decades of circadian time. J Neuroendocrinol. 2008;20:812–819. doi: 10.1111/j.1365-2826.2008.01715.x. [DOI] [PubMed] [Google Scholar]
- Holmes MM, Galea LA, Mistlberger RE, Kempermann G. Adult hippocampal neurogenesis and voluntary running activity: circadian and dose-dependent effects. J Neurosci Res. 2004;76:216–222. doi: 10.1002/jnr.20039. [DOI] [PubMed] [Google Scholar]
- Huber R, Deboer T, Schwierin B, Tobler I. Effect of melatonin on sleep and brain temperature in the Djungarian hamster and the rat. Physiol Behav. 1998;65:77–82. doi: 10.1016/s0031-9384(98)00125-5. [DOI] [PubMed] [Google Scholar]
- Iijima M, Nikaido T, Akiyama M, Moriya T, Shibata S. Methamphetamine-induced, suprachiasmatic nucleus-independent circadian rhythms of activity and mPer gene expression in the striatum of the mouse. European Journal of Neuroscience. 2002;16:921–929. doi: 10.1046/j.1460-9568.2002.02140.x. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Garidou ML, Palm IF, Van Der Vliet J, Simonneaux V, Pevet P, Buijs RM. Melatonin sees the light: blocking GABA-ergic transmission in the paraventricular nucleus induces daytime secretion of melatonin. Eur J Neurosci. 2000;12:3146–3154. doi: 10.1046/j.1460-9568.2000.00202.x. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Verhagen LA, Schalij I, Foppen E, Saboureau M, Bothorel B, Buijs RM, Pevet P. Opposite actions of hypothalamic vasopressin on circadian corticosterone rhythm in nocturnal versus diurnal species. Eur J Neurosci. 2008;27:818–827. doi: 10.1111/j.1460-9568.2008.06057.x. [DOI] [PubMed] [Google Scholar]
- Klein DC, Moore RY. Pineal N-acetyltransferase and hydroxyindole-O-methyltransferase: control by the retinohypothalamic tract and the suprachiasmatic nucleus. Brain Res. 1979;174:245–262. doi: 10.1016/0006-8993(79)90848-5. [DOI] [PubMed] [Google Scholar]
- Klein DC, Moore RY, Reppert SM. Suprachiasmatic Nucleus: The Mind’s Clock. Vol. 19. New York: Oxford University Press; 1991. pp. 388–399. [Google Scholar]
- Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15(Spec No 2):R271–R277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- Lambert CM, Machida KK, Smale L, Nunez AA, Weaver DR. Analysis of the Prokineticin 2 System in a Diurnal Rodent, the Unstriped Nile Grass Rat (Arvicanthis niloticus) J Biol Rhythms. 2005;20:206–218. doi: 10.1177/0748730405275135. [DOI] [PubMed] [Google Scholar]
- Lambert CM, Weaver DR. Peripheral gene expression rhythms in a diurnal rodent. J Biol Rhythms. 2006;21:77–79. doi: 10.1177/0748730405281843. [DOI] [PubMed] [Google Scholar]
- Lamont EW, Robinson B, Stewart J, Amir S. The central and basolateral nuclei of the amygdala exhibit opposite diurnal rhythms of expression of the clock protein Period2. Proc Natl Acad Sci U S A. 2005;102:4180–4184. doi: 10.1073/pnas.0500901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie P. Melatonin: role in gating nocturnal rise in sleep propensity. J Biol Rhythms. 1997;12:657–665. doi: 10.1177/074873049701200622. [DOI] [PubMed] [Google Scholar]
- Mahoney M, Bult A, Smale L. Phase response curve and light-induced fos expression in the suprachiasmatic nucleus and adjacent hypothalamus of Arvicanthis niloticus. J Biol Rhythms. 2001;16:149–162. doi: 10.1177/074873001129001854. [DOI] [PubMed] [Google Scholar]
- Mahoney MM. Shift work, jet lag, and female reproduction. Int J Endocrinol. 2010:813764. doi: 10.1155/2010/813764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney MM, Nunez AA, Smale L. Calbindin and Fos within the suprachiasmatic nucleus and the adjacent hypothalamus of Arvicanthis niloticus and Rattus norvegicus. Neuroscience. 2000;99:565–575. doi: 10.1016/s0306-4522(00)00212-8. [DOI] [PubMed] [Google Scholar]
- Mahoney MM, Ramanathan C, Hagenauer MH, Thompson RC, Smale L, Lee T. Daily rhythms and sex differences in vasoactive intestinal polypeptide, VIPR2 receptor and arginine vasopressin mRNA in the suprachiasmatic nucleus of a diurnal rodent, Arvicanthis niloticus. Eur J Neurosci. 2009;30:1537–1543. doi: 10.1111/j.1460-9568.2009.06936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailliet F, Galloux P, Poisson D. Comparative effects of melatonin, zolpidem and diazepam on sleep, body temperature, blood pressure and heart rate measured by radiotelemetry in Wistar rats. Psychopharmacology (Berl) 2001;156:417–426. doi: 10.1007/s002130100769. [DOI] [PubMed] [Google Scholar]
- Manev H, Uz T. Clock genes: influencing and being influenced by psychoactive drugs. Trends Pharmacol Sci. 2006;27:186–189. doi: 10.1016/j.tips.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Masubuchi S, Honma S, Abe H, Ishizaki K, Namihira M, Ikeda M, Honma K. Clock genes outside the suprachiasmatic nucleus involved in manifestation of locomotor activity rhythm in rats. Eur J Neurosci. 2000;12:4206–4214. [PubMed] [Google Scholar]
- McElhinny TL, Smale L, Holekamp KE. Patterns of body temperature, activity, and reproductive behavior in a tropical murid rodent, Arvicanthis niloticus. Physiol Behav. 1997;62:91–96. doi: 10.1016/s0031-9384(97)00146-7. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Challet E. Brain clocks: from the suprachiasmatic nuclei to a cerebral network. Neuroscientist. 2009;15:477–488. doi: 10.1177/1073858408327808. [DOI] [PubMed] [Google Scholar]
- Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N, Edelstein K, Hastings MH, Maywood ES. Cycle of period gene expression in a diurnal mammal (Spermophilus tridecemlineatus): implications for nonphotic phase shifting. J Biol Rhythms. 2001;16:471–478. doi: 10.1177/074873001129002141. [DOI] [PubMed] [Google Scholar]
- Nixon JP, Smale L. Individual differences in wheel-running rhythms are related to temporal and spatial patterns of activation of orexin A and B cells in a diurnal rodent (Arvicanthis niloticus) Neuroscience. 2004;127:25–34. doi: 10.1016/j.neuroscience.2004.04.052. [DOI] [PubMed] [Google Scholar]
- Nunez AA, Bult A, McElhinny TL, Smale L. Daily rhythms of Fos expression in hypothalamic targets of the suprachiasmatic nucleus in diurnal and nocturnal rodents. J Biol Rhythms. 1999;14:300–306. doi: 10.1177/074873099129000713. [DOI] [PubMed] [Google Scholar]
- Pandi-Perumal SR, Trakht I, Srinivasan V, Spence DW, Maestroni GJ, Zisapel N, Cardinali DP. Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog Neurobiol. 2008;85:335–353. doi: 10.1016/j.pneurobio.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3rd ed. San Diego, CA: Acadamic Press; 2007. [DOI] [PubMed] [Google Scholar]
- Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Ramanathan C, Nunez AA, Martinez GS, Schwartz MD, Smale L. Temporal and spatial distribution of immunoreactive PER1 and PER2 proteins in the suprachiasmatic nucleus and peri-suprachiasmatic region of the diurnal grass rat (Arvicanthis niloticus) Brain Res. 2006;1073–1074:348–358. doi: 10.1016/j.brainres.2005.11.082. [DOI] [PubMed] [Google Scholar]
- Ramanathan C, Nunez AA, Smale L. Daily rhythms in PER1 within and beyond the suprachiasmatic nucleus of female grass rats (Arvicanthis niloticus) Neuroscience. 2008a;156:48–58. doi: 10.1016/j.neuroscience.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan C, Smale L, Nunez AA. Rhythms in expression of PER1 protein in the amygdala and bed nucleus of the stria terminalis of the diurnal grass rat (Arvicanthis niloticus) Neurosci Lett. 2008b;441:86–89. doi: 10.1016/j.neulet.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan C, Stowie A, Smale L, Nunez A. PER2 rhythms in the amygdala and bed nucleus of the stria terminalis of the diurnal grass rat (Arvicanthis niloticus) Neurosci Lett. 2010;473:220–223. doi: 10.1016/j.neulet.2010.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annual Review of Physiology. 2001;63:647–676. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- Rose S, Novak CM, Mahoney MM, Nunez AA, Smale L. Fos expression within vasopressin-containing neurons in the suprachiasmatic nucleus of diurnal rodents compared to nocturnal rodents. J Biol Rhythms. 1999;14:37–46. doi: 10.1177/074873099129000425. [DOI] [PubMed] [Google Scholar]
- Schwartz MD. Arvicanthis niloticus. East Lansing: Michigan State University; 2006. Neural substrates of diurnality in the Nile grass rat. [Google Scholar]
- Schwartz MD, Nunez AA, Smale L. Differences in the suprachiasmatic nucleus and lower subparaventricular zone of diurnal and nocturnal rodents. Neuroscience. 2004;127:13–23. doi: 10.1016/j.neuroscience.2004.04.049. [DOI] [PubMed] [Google Scholar]
- Schwartz MD, Nunez AA, Smale L. Rhythmic cFos expression in the ventral subparaventricular zone influences general activity rhythms in the Nile grass rat, Arvicanthis niloticus. Chronobiol Int. 2009;26:1290–1306. doi: 10.3109/07420520903415742. [DOI] [PubMed] [Google Scholar]
- Schwartz MD, Smale L. Individual differences in rhythms of behavioral sleep and its neural substrates in Nile grass rats. J Biol Rhythms. 2005;20:526–537. doi: 10.1177/0748730405280924. [DOI] [PubMed] [Google Scholar]
- Schwartz WJ, Reppert SM, Eagan SM, Moore-Ede MC. In vivo metabolic activity of the suprachiasmatic nuclei: a comparative study. Brain Research. 1983;274:184–187. doi: 10.1016/0006-8993(83)90538-3. [DOI] [PubMed] [Google Scholar]
- Scott AJ. Shift work and health. Prim Care. 2000;27:1057–1079. doi: 10.1016/s0095-4543(05)70189-5. [DOI] [PubMed] [Google Scholar]
- Shieh KR. Distribution of the rhythm-related genes rPERIOD1, rPERIOD2, and rCLOCK, in the rat brain. Neuroscience. 2003;118:831–843. doi: 10.1016/s0306-4522(03)00004-6. [DOI] [PubMed] [Google Scholar]
- Shipley MT, Ennis M, Puche AC. Olfactory System. In: Paxinos G, editor. The rat nervous system. London: Elsevier; 2004. pp. 923–964. [Google Scholar]
- Smale L, Castleberry C, Nunez AA. Fos rhythms in the hypothalamus of Rattus and Arvicanthis that exhibit nocturnal and diurnal patterns of rhythmicity. Brain Res. 2001;899:101–105. doi: 10.1016/s0006-8993(01)02205-3. [DOI] [PubMed] [Google Scholar]
- Smale L, Lee T, Nunez AA. Mammalian diurnality: some facts and gaps. J Biol Rhythms. 2003;18:356–366. doi: 10.1177/0748730403256651. [DOI] [PubMed] [Google Scholar]
- Smale L, Nunez AA, Schwartz MD. Rhythms in a diurnal brain. Biological Rhythm Research. 2008;39:305–318. [Google Scholar]
- Stephan FK. The "other" circadian system: food as a Zeitgeber. J Biol Rhythms. 2002;17:284–292. doi: 10.1177/074873040201700402. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai S, Sanada K, Fukada Y. Time-of-day-dependent enhancement of adult neurogenesis in the hippocampus. PLoS One. 2008;3:e3835. doi: 10.1371/journal.pone.0003835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosini G, Pozdeyev N, Sakamoto K, Iuvone PM. The circadian clock system in the mammalian retina. Bioessays. 2008;30:624–633. doi: 10.1002/bies.20777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uz T, Javaid JI, Manev H. Circadian differences in behavioral sensitization to cocaine: putative role of arylalkylamine N-acetyltransferase. Life Sci. 2002;70:3069–3075. doi: 10.1016/s0024-3205(02)01559-x. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Verwey M, Amir S. Food-entrainable circadian oscillators in the brain. Eur J Neurosci. 2009;30:1650–1657. doi: 10.1111/j.1460-9568.2009.06960.x. [DOI] [PubMed] [Google Scholar]
- von Gall C, Garabette ML, Kell CA, Frenzel S, Dehghani F, Schumm-Draeger PM, Weaver DR, Korf HW, Hastings MH, Stehle JH. Rhythmic gene expression in pituitary depends on heterologous sensitization by the neurohormone melatonin. Nat Neurosci. 2002;5:234–238. doi: 10.1038/nn806. [DOI] [PubMed] [Google Scholar]
- Vosko AM, Hagenauer MH, Hummer DL, Lee TM. Period gene expression in the diurnal degu (Octodon degus) differs from the nocturnal laboratory rat (Rattus norvegicus) Am J Physiol Regul Integr Comp Physiol. 2009;296:R353–R361. doi: 10.1152/ajpregu.90392.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakamatsu H, Yoshinobu Y, Aida R, Moriya T, Akiyama M, Shibata S. Restricted-feeding-induced anticipatory activity rhythm is associated with a phase-shift of the expression of mPer1 and mPer2 mRNA in the cerebral cortex and hippocampus but not in the suprachiasmatic nucleus of mice. Eur J Neurosci. 2001;13:1190–1196. doi: 10.1046/j.0953-816x.2001.01483.x. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Watts AG, Swanson LW, Sanchez-Watts G. Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. J Comp Neurol. 1987;258:204–229. doi: 10.1002/cne.902580204. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, Li Q, Sun ZS, Eichele G, Bradley A, Lee CC. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell. 2001;105:683–694. doi: 10.1016/s0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]