Abstract
Background
In countries with a high seroprevalence of human immunodeficiency virus type 1 (HIV-1), HIV infection contributes significantly to infant mortality. We investigated antiretroviral-treatment strategies in the Children with HIV Early Antiretroviral Therapy (CHER) trial.
Methods
HIV-infected infants 6 to 12 weeks of age with a CD4 lymphocyte percentage (the CD4 percentage) of 25% or more were randomly assigned to receive antiretroviral therapy (lopinavir–ritonavir, zidovudine, and lamivudine) when the CD4 percentage decreased to less than 20% (or 25% if the child was younger than 1 year) or clinical criteria were met (the deferred antiretroviral-therapy group) or to immediate initiation of limited antiretroviral therapy until 1 year of age or 2 years of age (the early antiretroviral-therapy groups). We report the early outcomes for infants who received deferred antiretroviral therapy as compared with early antiretroviral therapy.
Results
At a median age of 7.4 weeks (interquartile range, 6.6 to 8.9) and a CD4 percentage of 35.2% (interquartile range, 29.1 to 41.2), 125 infants were randomly assigned to receive deferred therapy, and 252 infants were randomly assigned to receive early therapy. After a median follow-up of 40 weeks (interquartile range, 24 to 58), antiretroviral therapy was initiated in 66% of infants in the deferred-therapy group. Twenty infants in the deferred-therapy group (16%) died versus 10 infants in the early-therapy groups (4%) (hazard ratio for death, 0.24; 95% confidence interval [CI], 0.11 to 0.51; P<0.001). In 32 infants in the deferred-therapy group (26%) versus 16 infants in the early-therapy groups (6%), disease progressed to Centers for Disease Control and Prevention stage C or severe stage B (hazard ratio for disease progression, 0.25; 95% CI, 0.15 to 0.41; P<0.001). Stavudine was substituted for zidovudine in four infants in the early-therapy groups because of neutropenia in three infants and anemia in one infant; no drugs were permanently discontinued. After a review by the data and safety monitoring board, the deferred-therapy group was modified, and infants in this group were all reassessed for initiation of antiretroviral therapy.
Conclusions
Early HIV diagnosis and early antiretroviral therapy reduced early infant mortality by 76% and HIV progression by 75%. (ClinicalTrials.gov number, NCT00102960.)
Infants with human immunodeficiency virus type 1 (HIV-1) infection have higher rates of disease progression and mortality than older children,1-3 even with a high percentage of CD4 lymphocytes (CD4 percentage).4 Whereas early initiation of antiretroviral therapy may be appropriate for infants, continuing treatment for life is problematic, given the limitations of the available drugs, the long-term toxicity of antiretroviral therapy, adherence issues, the risk of resistance to antiretroviral therapy, and limited resources. This trial addresses the optimal time of initiation and duration of antiretroviral therapy in infants with in utero or intrapartum HIV-1 infection. We hypothesized that early initiation of limited antiretroviral therapy soon after primary infection, when the immune system is most immature, would be beneficial and would delay the time to initiation of continuous antiretroviral therapy. In accordance with the recommendation, in June 2007, of the the data and safety monitoring board, we report the early outcomes for infants who were randomly assigned to receive deferred therapy as compared with those assigned to receive early antiretroviral therapy.
Methods
Study Design
The Children with HIV Early Antiretroviral Therapy (CHER) trial is a phase 3, randomized, open-label trial conducted by the Comprehensive International Program for Research in AIDS — South Africa in collaboration with the Medical Research Council Clinical Trials Unit, United Kingdom, and the Division of AIDS (DAIDS) of the National Institutes of Health (NIH). The study is being conducted in two centers in South Africa: the Perinatal HIV Research Unit, Chris Hani Baragwanath Hospital, in Soweto, and the Children's Infectious Diseases Clinical Research Unit, Tygerberg Children's Hospital, in Cape Town.
We enrolled infants 6 to 12 weeks of age who had HIV infection (defined by a positive polymerase-chain-reaction [PCR] test for HIV-1 DNA and a plasma HIV-1 RNA level on PCR of >1000 copies per milliliter) and a CD4 percentage of 25% or more. Exclusion criteria are listed in the Supplementary Appendix, available with the full text of this article at www.nejm.org. Infants were randomly assigned to receive one of three treatments: early limited antiretroviral therapy for 96 weeks, early limited antiretroviral therapy for 40 weeks, or deferred therapy. Immunologic criteria for initiating antiretroviral therapy in the deferred-therapy group or reinitiating antiretroviral therapy in the early-therapy groups were a CD4 percentage of less than 20%5 or, in the case of children younger than 12 months, a CD4 percentage of less than 25% or a CD4 count of less than 1000 cells per cubic millimeter, according to World Health Organization (WHO) guidelines updated in 2006.6 Clinical criteria for initiating or reinitiating antiretroviral therapy7 were Centers for Disease Control and Prevention (CDC) stage C or investigator-selected (severe) stage B events (see the Supplementary Appendix), including symptomatic lymphoid interstitial pneumonitis, bronchiectasis, nephropathy, cardiomyopathy, and failure to thrive. GlaxoSmithKline provided lamivudine and zidovudine, and the South African Department of Health provided lopinavir–ritonavir. Written informed consent was obtained from the parents or legal guardians of all the infants. The authors vouch for the completeness and accuracy of the data.
Study Treatment
First-line antiretroviral therapy consisted of zidovudine at a dose of 240 mg per square meter of body-surface area twice daily and lamivudine at a dose of 4 mg per kilogram of body weight twice daily, with lopinavir–ritonavir given at a dose of 300 mg of lopinavir plus 75 mg of ritonavir per square meter twice daily until 6 months of age,8 then at a dose of 230 mg of lopinavir plus 57.5 mg of ritonavir per square meter twice daily. The second-line regimen was predefined and consisted of didanosine, abacavir, and nevirapine (or efavirenz instead of nevirapine if the child was older than 3 years of age). The criteria for switching to second-line therapy are listed in the Supplementary Appendix.
Screening, Randomization, and Follow-up
Infants who were exposed to HIV were identified from programs for the prevention of mother-to-child transmission of HIV-1 in the Western Cape and Gauteng provinces. PCR for HIV-1 DNA was performed from 4 weeks of age when cotrimoxazole prophylaxis commenced. In Gauteng, the regimen for the prevention of mother-to-child transmission of HIV-1 was single-dose nevirapine administered to both the mother and the neonate; in the Western Cape, the regimen was zidovudine administered to mothers from 34 weeks' gestation and to neonates for 7 days and single-dose nevirapine administered to both.
The randomization schedule was prepared centrally by the trial statistician and faxed to the study sites. Randomization was stratified according to clinical center, and blocks, varying randomly in size, were used to ensure balance in the number of infants assigned to each group according to center.
After randomization, the infants were seen every 4 weeks until week 24, then every 8 weeks until week 48, and every 12 weeks thereafter. At each scheduled visit, the evaluation included documentation of any HIV-related clinical events, a complete blood count with a differential count, measurements of aminotransferase levels, and a CD4 cell count with calculation of the CD4 percentage. The pharmacist at each site measured adherence to the assigned medication by comparing the amount of drug dispensed with that returned at the subsequent visit. Toxicity was graded according to the criteria of the DAIDS9 of the National Institute of Allergy and Infectious Diseases (NIAID), at the NIH. The study was also conducted under a Food and Drug Administration investigational-new-drug application (number 71,494).
Outcomes
The primary outcome was the time to death or failure of the first-line antiretroviral therapy. The latter was defined as any of the following: failure to reach a CD4 percentage of 20% or more by week 24 of therapy or a decrease in the CD4 percentage to less than 20% after the first 24 weeks of antiretroviral therapy (immunologic failure), severe CDC stage B or stage C events (clinical failure), or toxicity requiring more than one drug substitution within the same class or a switch to a new class or requiring permanent discontinuation of treatment (i.e., regimen-limiting toxicity failure). An independent end-point review committee reviewed all deaths and CDC stage C and severe stage B events without knowledge of CD4 values, status of antiretroviral therapy, or randomized treatment assignments. Investigators at each of the two centers remained unaware of the primary and secondary outcomes at the other center.
Review and Monitoring
The trial was approved by the ethics committees of both participating institutions. According to the protocol, the study is reviewed at least annually by the independent DAIDS international data and safety monitoring board of Africa; this board can recommend termination or modification of the study because of safety or efficacy concerns. The guiding statistical criterion for “proof beyond reasonable doubt” is based on a difference of at least 3 SD in the log relative hazard (or nominal P<0.001) in any interim analysis (according to the Haybittle-Peto rule). Two such annual reviews have been conducted. In addition, a subcommittee of the data and safety monitoring board reviews all deaths according to randomized treatment assignments and can call for an unscheduled review by the full data and safety monitoring board if there are any safety concerns; the subcommittee has performed three reviews (approximately every 3 months).
At the second annual review of the data and safety monitoring board, in June 2007, by which time accrual had been completed, strong evidence of a difference in mortality emerged between infants who had been randomly assigned to receive early antiretroviral therapy and those assigned to the deferred-therapy regimen. The data and safety monitoring board recommended dissemination of these early findings and urgent evaluation of children in the deferred-therapy group who were not receiving antiretroviral therapy for possible initiation of antiretroviral therapy. The board also recommended that all three groups be continued, with modification in the deferred-therapy group as mentioned above.
Statistical Analysis
The sample size was estimated with the use of the methods previously developed for sample size and estimation of statistical power in complex clinical trials.10,11 We assumed that the cumulative probability of death or regimen failure in the deferred-therapy group would be 0.06, 0.17, 0.28, 0.39, and 0.49 by years 1, 2, 3, 4, and 5, respectively. We assumed that for the first 5 years of follow-up, the annual hazard ratio for disease progression or death would be 0.51, 0.57, 0.78, 0.85, and 0.88 in the group that received early limited antiretroviral therapy for 40 weeks and 0.51, 0.27, 0.43, 0.67, and 0.74 in the group that received early limited antiretroviral therapy for 96 weeks, as compared with the deferred-therapy group. These assumptions were based on estimates of death rates, time to initiation of antiretroviral therapy, and time to failure of antiretroviral therapy in other pediatric cohorts.4,12 Under these assumptions, the planned sample size of 375 children (125 per group), enrolled over a period of 18 months and followed for a minimum of 3.5 years, would provide 80% power to reject the null hypothesis of no difference among the three groups in the time to the primary outcome, on the basis of a global log-rank test with a two-sided alpha level of 0.05.
The planned primary analysis was to first test the null hypothesis of no difference among the three groups in the time to death or regimen failure by means of a global log-rank test with two degrees of freedom (with stratification according to site). If the null hypothesis was rejected, then each of the early-therapy groups was to be compared with the deferred-therapy group in terms of the average hazard during the follow-up period with the use of a Cox proportional-hazards model, also stratified according to site. Although pairwise comparisons of all groups were performed, we report the comparison of the deferred-therapy group with the combined early-therapy groups, preserving blinding between the early-therapy groups, as recommended by the data and safety monitoring board. All analyses are based on complete data as of June 20, 2007.
We used the intention-to-treat approach to compare the early-therapy groups combined with the deferred-therapy group. Time-to-event methods (i.e., Kaplan-Meier plots and the log-rank test stratified according to site) were used to compare the two groups for the time to the primary end point and survival. Cox proportional-hazards modeling was used to estimate a summary hazard ratio for death or treatment failure for the early-therapy groups combined as compared with the deferred-therapy group. The frequency of grade 3 or 4 adverse events in the two groups was compared with the use of a chi-square test. The time to initiation of continuous antiretroviral therapy in the deferred-therapy group was estimated with the use of Kaplan-Meier methods. Changes in the CD4 percentage and the CD4 count over time were summarized with the use of point estimates of mean changes from baseline documented at each visit. All reported P values are two-sided and have not been adjusted for multiple testing.
Results
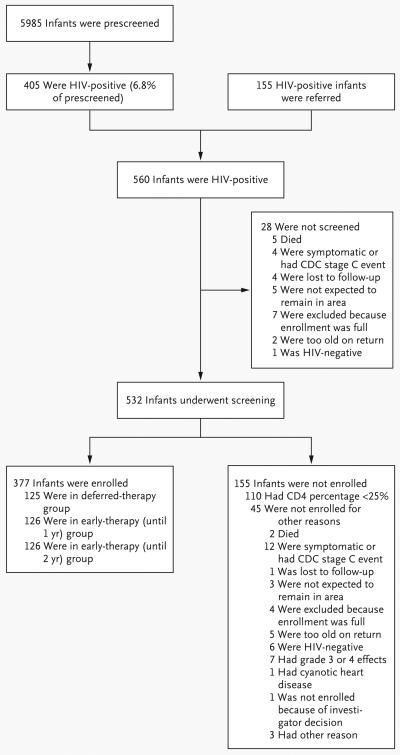
Of 5985 infants born to mothers in programs for the prevention of mother-to-child transmission of HIV-1, 405 were HIV-positive (6.8%). The transmission rate was higher in Soweto (8.4%) than in Cape Town (5.6%), probably because of different regimens for the prevention of mother-to-child transmission of HIV-1. An additional 155 HIV-infected infants were referred from other infant diagnosis programs. Among 560 HIV-infected infants considered for the trial, 110 were ineligible because the CD4 percentage was less than 25%, and 45 were ineligible for other reasons (Fig. 1). Between August 2005 and February 2007, a total of 377 infants were enrolled; 125 infants were randomly assigned to the deferred-therapy group, and 252 infants were randomly assigned to the early-therapy groups. Two pairs of twins were enrolled: the first pair underwent randomization, and the second pair of twins were nonrandomly assigned to the same group.
Figure 1.
Screening and Enrollment.
The median follow-up as of June 20, 2007, was 40 weeks (interquartile range, 24 to 58). Baseline characteristics were well matched in the study groups (Table 1). A total of 14% of the infants were breast-fed. Most mothers (62%) and infants (51%) received single-dose nevirapine for the prevention of mother-to-child transmission of HIV-1; 20% of the mothers and 27% of the infants received zidovudine and nevirapine. A smaller proportion (11% of the mothers and 16% of the infants) received no prophylaxis for the prevention of mother-to-child transmission of HIV-1.
Table 1.
Baseline Characteristics of Infants Enrolled in the Trial.*
| Variable | Early Antiretroviral Therapy (N = 252) |
Deferred Antiretroviral Therapy (N = 125) |
Total (N = 377) |
|---|---|---|---|
| Female sex — no. (%) | 147 (58.3) | 74 (59.2) | 221 (58.6) |
| Age — wk | |||
| Median | 7.4 | 7.1 | 7.4 |
| Interquartile range | 6.6 to 8.9 | 6.4 to 8.9 | 6.6 to 8.9 |
| Maternal antiretroviral therapy for prevention of mother-to-child transmission of HIV-1 — no. (%) | |||
| No therapy | 26 (10.3) | 15 (12.0) | 41 (10.9) |
| Nevirapine | 162 (64.3) | 72 (57.6) | 234 (62.1) |
| Zidovudine | 8 (3.2) | 5 (4.0) | 13 (3.4) |
| Zidovudine plus nevirapine | 51 (20.2) | 26 (20.8) | 77 (20.4) |
| Maternal therapy with highly active antiretroviral therapy — no. (%) | 2 (0.8) | 5 (4.0) | 7 (1.9) |
| Infant antiretroviral therapy for prevention of mother-to-child transmission of HIV-1 — no. (%) | |||
| No therapy | 40 (15.9) | 20 (16.0) | 60 (15.9) |
| Nevirapine | 124 (49.2) | 68 (54.4) | 192 (50.9) |
| Zidovudine | 5 (2.0) | 1 (0.8) | 6 (1.6) |
| Zidovudine plus nevirapine | 69 (27.4) | 34 (27.2) | 103 (27.3) |
| Breast-fed infants — no. (%) | 35 (13.9) | 19 (15.2) | 54 (14.3) |
| Weight — kg | |||
| Median | 4.4 | 4.5 | 4.4 |
| Interquartile range | 4.0 to 4.9 | 4.0 to 5.0 | 4.0 to 4.9 |
| Weight for age — z score | |||
| Median | −0.8 | −0.5 | −0.7 |
| Interquartile range | −1.5 to 0.0 | −1.4 to 0.2 | −1.5 to 0.0 |
| Weight for length — z score | |||
| Median | 0.3 | 0.3 | 0.3 |
| Interquartile range | 0.2 to 0.3 | 0.2 to 0.3 | 0.2 to 0.3 |
| Head circumference for age — z score | |||
| Median | −0.1 | 0.0 | −0.0 |
| Interquartile range | −0.9 to 0.6 | −0.7 to 0.6 | −0.8 to 0.6 |
| CDC class — no. (%)† | |||
| N and A | 237 (94.0) | 121 (96.8) | 358 (95.0) |
| B | 11 (4.4) | 3 (2.4) | 14 (3.7) |
| CD4 percentage | |||
| Median | 35.1 | 35.6 | 35.2 |
| Interquartile range | 29.1 to 40.8 | 29.1 to 43.8 | 29.1 to 41.2 |
| CD4 cell count — per mm3 | |||
| Median | 2035 | 2044 | 2039 |
| Interquartile range | 1519 to 2754 | 1585 to 2960 | 1541 to 2789 |
| Hemoglobin — g/dl | |||
| Median | 10.1 | 10.2 | 10.1 |
| Interquartile range | 9.3 to 10.7 | 9.4 to 11 | 9.4 to 10.8 |
| Total lymphocyte count — per mm3 | |||
| Median | 6065 | 6250 | 6110 |
| Interquartile range | 4510 to 7735 | 4450 to 7500 | 4490 to 7690 |
| Plasma HIV-1 RNA — log10 copies/ml | |||
| Median | 5.9 | 5.9 | 5.9 |
| Interquartile range | 5.6 to 5.9 | 5.6 to 5.9 | 5.6 to 5.9 |
Information was missing for four infants in the early-therapy group and one infant in the deferred-therapy group.
The Centers for Disease Control and Prevention (CDC) classification system is for HIV infection in children younger than 13 years of age.
Follow-up
Treatment was not completed in 14 infants in the early-therapy groups (6%) and in 4 infants in the deferred-therapy group (3%). The reasons were withdrawal of consent, for three infants in the early-therapy groups (1.2%) and for one infant in the deferred-therapy group (0.8%), and loss to follow-up, for the other 14 infants.
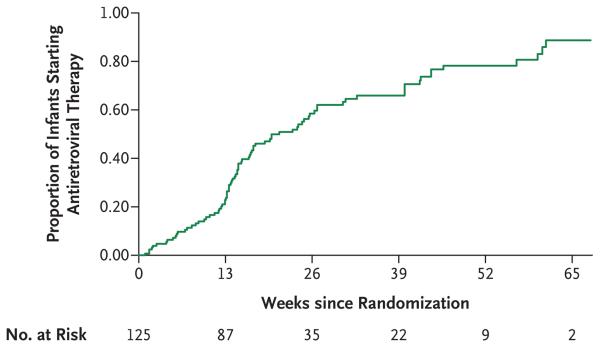
In the early-therapy groups, infants received antiretroviral therapy for 99% of the follow-up period, and in the deferred-therapy group, infants received antiretroviral therapy for 35% of the follow-up period. Eighty-three children in the deferred-therapy group (66%) started antiretroviral therapy according to the protocol criteria (63% met immunologic criteria and 36% met clinical criteria); 51 of these children (41%) were younger than 26 weeks of age. The estimated median time to the initiation of antiretroviral therapy in the deferred-therapy group was 21.1 weeks (Fig. 2). The adherence rate, defined as the percentage of drug received, was 87.8% for zidovudine, 90.2% for lamivudine, and 92.1% for lopinavir–ritonavir.
Figure 2.
Time to Initiation of Antiretroviral Therapy in the Deferred-Therapy Group.
Mortality and Failure of the First Regimen
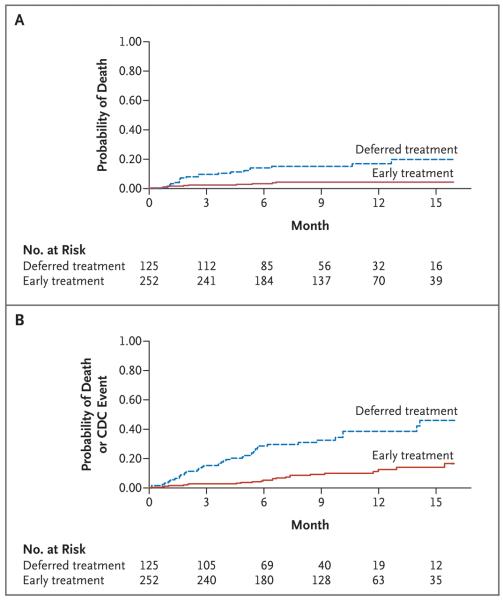
Thirty-two infants reached the primary end point of death or failure of the first regimen: 11 of 252 infants in the early-therapy groups combined and 21 of 125 in the deferred-therapy group (hazard ratio for early therapy as compared with deferred therapy, 0.25; 95% confidence interval [CI], 0.12 to 0.51). A total of 30 of 32 primary end points were deaths: 10 of 252 infants in the early-therapy groups (4%) died and 20 of 125 infants in the deferred-therapy group (16%) died (hazard ratio, 0.24; 95% CI, 0.11 to 0.51; P<0.001) (Fig. 3A and Table 2). Average rates of death were 5 per 100 person-years in the early-therapy groups and 21 per 100 person-years in the deferred-therapy group (Table 2). The death rate was much higher in the first 26 weeks after randomization and declined thereafter in both groups (Table 2); 20 of 30 infants died before 26 weeks of age. Fifteen infants in the deferred-therapy group died before receiving antiretroviral therapy; the other 5 infants received antiretroviral therapy, but death occurred within 1 month after initiation of antiretroviral therapy in four of five infants.
Figure 3. Probability of Death or a First Event, According to Treatment Group.
Panel A shows the probability of death. Panel B shows the probability of death or the onset of a CDC stage C or severe stage B event.
Table 2.
Mortality Rates.
| Variable | Early Antiretroviral Therapy (N = 252) |
Deferred Antiretroviral Therapy (N = 125) |
Total (N = 377) |
Hazard Ratio (95% CI)* |
P Value |
|---|---|---|---|---|---|
| Deaths — no. (%) | 10 (4) | 20 (16) | 30 (8) | 0.24 (0.11–0.51) | <0.001 |
| Person-years of follow-up — no. | 205 | 94 | 299 | ||
| Overall death rate per 100 person-years — % (95% CI) | 4.9 (2.3–9.0) | 21.2 (13.0–32.7) | 10.0 (6.8–14.3) | ||
| Rate of death during follow-up — % (95% CI) | |||||
| 0–13 wk | 9.8 (3.6–21.4) | 40.6 (21.0–71.0) | |||
| >13–26 wk | 3.7 (0.4–13.2) | 19.7 (6.4–45.9) | |||
| >26–52 wk | 3.1 (0.4–11.2) | 6.8 (0.8–24.7) |
Hazard ratios for death are for the comparison of the early-therapy groups with the deferred-treatment group.
A total of 12 children (40%) died at home: 8 in the deferred-therapy group (6.4%) versus 4 in the early-therapy groups (1.6%); most deaths were unexpected and rapid. The cause of death was determined in two patients: one had gastroenteritis and one had disseminated tuberculosis. The cause of death was not determined in the remaining 10 infants. Hospital deaths were due to gastroenteritis (in five infants in the deferred-therapy group [4%] vs. four infants in the early-therapy groups [1.6%]), pneumonia (in four infants in the deferred-therapy group), Pneumocystis jiroveci pneumonia (in two infants in the deferred-therapy group), cytomegalovirus (CMV) (in one infant in the deferred-therapy group), liver failure (granulomatous steatohepatitis detected on postmortem examination in one infant in an early-therapy group), and the sudden infant death syndrome (in one infant in an early-therapy group).
HIV Disease Progression
Disease progression occurred in 16 infants in the early-therapy groups (6.3%), as compared with 32 infants in the deferred-therapy group (25.6%) (hazard ratio, 0.25; 95% CI, 0.15 to 0.41; P<0.001) (Table 3). P. jiroveci pneumonia, CMV disease, and esophageal candidiasis occurred only in the deferred-therapy group. Four children had P. jiroveci pneumonia (two cases were definitive and two were presumptive). Failure to thrive was the most common event in both the deferred-therapy group and the early-therapy groups. The time to death or of the first CDC stage C or severe stage B event is shown in Figure 3B. Forty-one infants in the early-therapy groups were hospitalized (16.3%), as compared with 46 infants in the deferred-therapy group (36.8%) (Table 3).
Table 3.
Adverse Events.
| Adverse Event | Early Antiretroviral Therapy (N = 252) |
Deferred Antiretroviral Therapy (N = 125) |
Total (N = 377) |
|---|---|---|---|
| All adverse events — no. of events | 1356 | 1063 | 2419 |
| Clinical adverse event | |||
| Stage C event — no. of events | |||
| HIV wasting | 3 | 3 | 6 |
| Pneumocystis jiroveci pneumonia | 0 | 4 | 4 |
| Recurrent bacterial infections | 0 | 2 | 2 |
| HIV encephalopathy | 1 | 8 | 9 |
| CMV pneumonitis | 0 | 2 | 2 |
| Disseminated CMV infection | 0 | 1 | 1 |
| Esophageal candidiasis | 0 | 1 | 1 |
| Extrapulmonary tuberculosis | 1 | 1 | 2 |
| Severe stage B event — no. | |||
| Failure to thrive* | 12 | 12 | 24 |
| Lymphoid interstitial pneumonitis | 0 | 1 | 1 |
| Chronic lung disease | 1 | 0 | 1 |
| Stage C or severe stage B event | |||
| No. of events | 18 | 35 | 53 |
| No. of participants with event† | 16 | 32 | |
| Most frequently reported infections — no. of infections (no./100 person-years) | |||
| Grade 3 or 4 gastroenteritis | 29 (14.1) | 36 (38.2) | 65 (21.7) |
| Grade 1 or 2 gastroenteritis | 180 (87.5) | 123 (130.5) | 303 (101.0) |
| Grade 3 or 4 pneumonia | 25 (12.2) | 25 (26.5) | 50 (16.7) |
| Grade 1 or 2 pneumonia | 66 (32.1) | 56 (59.4) | 122 (40.7) |
| Meningitis | 1 (0.5) | 6 (6.4) | 7 (2.3) |
| Tuberculosis | 17 (8.3) | 19 (20.2) | 36 (12.0) |
| Laboratory grade 3 or 4 adverse event — total no. of events (no. of drug-related events)‡ | |||
| Alanine or aspartate aminotransferase elevation | 6 (2) | 1 (0) | 7 (2) |
| Anemia | 5 (3) | 2 (1) | 7 (4) |
| Neutropenia | 19 (10) | 3 (3) | 22 (13) |
| Thrombocytopenia | 3 (1) | 1 (0) | 4 (1) |
| Elevated γ-glutamyltransferase§ | 0 (0) | 2 (0) | 2 (0) |
| Hypernatremia | 6 (0) | 5 (0) | 11 (0) |
| Hyponatremia | 1 (0) | 1 (0) | 2 (0) |
| Hyperkalemia | 1 (0) | 0 (0) | 1 (0) |
| Hypokalemia | 1 (0) | 1 (0) | 2 (0) |
| Total events | 42 (16) | 16 (4) | 58 (20) |
| Serious adverse event — no. (%) | |||
| Participants with ≥1 event¶ | 55 (21.8) | 55 (44.0) | 110 (29.2) |
| Death | 10 (4.0) | 20 (16.0) | 30 (8.0) |
| Life-threatening event | 9 (3.6) | 4 (3.2) | 13 (3.4) |
| New or prolonged hospitalization | 41 (16.3) | 46 (36.8) | 87 (23.1) |
| Persistent or significant disability or incapacity | 0 | 1 (0.8) | 1 (0.3) |
Failure to thrive was defined as the documented loss of body weight or body weight crossing two major percentile lines (50th, 25th, 10th, or 5th) and failure to gain weight within 4 weeks with standard management, with no other cause identified during investigation, or for patients already below the 5th percentile, failure of weight gain to parallel the 5th percentile (in the absence of chronic diarrhea and fever for >30 days).13 CMV denotes cytomegalovirus.
Some of these participants may have had more than one event.
This category includes all adverse events deemed to be possibly, probably, or definitely drug-related.
An elevation was considered to be present if the assayed value was 10 or more times the upper limit of the normal age-adjusted range.
A participant may have had an event that was included in more than one category of serious adverse events.
Change in the CD4 Percentage during Follow-up
The mean changes from baseline in the CD4 percentage were as follows: at 12 weeks, +4.8% in the early-therapy groups versus −7.5% in the deferred-therapy group (absolute difference between the early-therapy groups and the deferred-therapy group, 12.3%; P<0.001); at 24 weeks, +5.9% versus −5.6% (absolute difference, 11.5%, P<0.001); at 32 weeks, +4.5% versus −4.8% (absolute difference, 9.3%; P<0.001); by week 40, when antiretroviral therapy had been initiated in more infants in the deferred-therapy group, the between-group difference had decreased to 6.7%.
Grade 3 and 4 Drug-Related Events
Of 58 grade 3 or 4 laboratory abnormalities, 20 were related to antiretroviral therapy and occurred in 19 children (Table 3). Fifteen of these children were in the early-therapy groups, and four were in the deferred-therapy group. In the early-therapy groups, neutropenia occurred in 10 children, anemia in 3, and elevated aminotransferase levels in 2. In the deferred-therapy group, three infants had neutropenia and one had elevated aminotransferase levels. Four children, all in the early-therapy groups, switched from zidovudine to stavudine because of neutropenia (in three children) or anemia and neutropenia (in one). No other drugs were discontinued.
Discussion
These data show that antiretroviral therapy initiated at a median age of 7 weeks reduced early mortality from 16% to 4% as compared with antiretroviral therapy initiated according to a threshold CD4 percentage or clinical progression of HIV disease; this is a relative reduction of 76%. A rapid decrease in CD4 values, rapid disease progression, and sudden death were all evident among infants in the deferred-therapy group, despite very close follow-up and regular CD4 monitoring. Although 66% of infants in this group received antiretroviral therapy, mainly because of a decreasing CD4 percentage, excess deaths could not be prevented. Another, smaller South African trial showed similarly rapid decreases in the CD4 percentage, with 85% of infants meeting the criteria for initiation of antiretroviral therapy (i.e., CD4 percentage <20%) by 6 months of age.14
More than one third of deaths in our study occurred at home, before caregivers recognized the need for medical attention. Mortality was consistently higher among infants in the deferred-therapy group than among those in the early-therapy groups throughout the first year of life, although differences were greatest among the youngest infants. Despite the randomization of only asymptomatic infants with high CD4 percentages, the majority of deaths occurred within 6 months after randomization. In 27 of the 30 deaths, there were no antecedent CDC stage C or severe stage B events, and the infants died rapidly from the first significant clinical event, most commonly gastroenteritis or pneumonia. Deaths due to gastroenteritis occurred twice as frequently in the deferred-therapy group as in the early-therapy groups, suggesting that early antiretroviral therapy has a protective effect in infants in whom replacement feeding is common. Whether breast-feeding would have an additional beneficial effect remains to be investigated. Safe infant-feeding practices were promoted, irrespective of the feeding choice. Failure to thrive was twice as frequent in the deferred-therapy group as in the early-therapy groups; however, the occurrence of failure to thrive in infants receiving antiretroviral therapy underscores the importance of nutritional, caregiving, and social factors in these patients.
Although the logistics of HIV testing in early infancy were considerable, the low mother-to-child transmission rate (6.8%) in field conditions highlights the success of programs for the prevention of this type of transmission in the areas where the study was conducted. The timing of HIV infection — in utero or during the perinatal period — is unknown, since the first PCR test was performed at 4 weeks of age. The fact that the majority of the children became infected despite the use of regimens for the prevention of mother-to-child transmission suggests that many of the infections were acquired in utero and that such infections may have contributed to the high rates of rapid progression.15 More rapid disease progression has also been reported among infants infected with HIV despite the use of regimens to prevent mother-to-child transmission of HIV-1 in countries with sufficient resources.16 Our sample size limits comparisons of HIV disease progression among infants in the deferred-therapy group according to whether or not they had received a regimen for the prevention of mother-to-child transmission of HIV-1.
Luzuriaga et al. found better HIV suppression in infants who began to receive antiretroviral therapy before 3 months of age than in infants who began to receive this therapy later.17 A number of U.S. and European cohort studies have reported better outcomes in infants receiving early antiretroviral therapy. However, interpretation of the data may be biased by the lack of randomization.18-21 The HIV Paediatric Prognostic Markers Collaborative Study (HPPMCS),4 a meta-analysis of data from untreated HIV-infected children in the United States and Europe, showed a risk of death that was increased by a factor of six for a 1-year-old child with high CD4 values as compared with a child 5 years of age with high CD4 values. The Cross Continents Collaboration for Kids study of untreated HIV-infected African children showed a poorer predictive value of CD4 values in young children after infancy and much higher mortality rates than those reported in the HPPMCS.22 Current guidelines recommend the initiation of antiretroviral therapy on the basis of a low CD4 percentage or count, a high viral load, or the presence of clinical symptoms, whereas the treatment of asymptomatic infants with high CD4 values is not mandated. Results from the CHER trial provide support for early antiretroviral therapy to reduce mortality among infants who acquire HIV infection despite regimens for the prevention of mother-to-child transmission of HIV-1.6,23-25
In the CHER trial, infants received a protease inhibitor-based regimen as first-line antiretroviral therapy, according to South-African guidelines. A nevirapine-based regimen may not be advisable for early treatment in infants exposed to single-dose nevirapine.26 Since guidelines for most resource-limited countries recommend starting antiretroviral therapy with nevirapine, the implementation of early antiretroviral therapy may pose considerable challenges. The estimated probability of death in the deferred-therapy group (17%) is lower than the 35% probability reported in African birth cohorts before the introduction of antiretroviral therapy or widespread cotrimoxazole prophylaxis.3 By comparison, early natural-history studies in the United States and Europe showed mortality that was lower but rates of disease progression that were similar to the rates among infants in the deferred-therapy group in our study.2,27
These data provide strong support for the initiation of antiretroviral therapy from an early age, regardless of the CD4 percentage or count. A good program for the prevention of mother-to-child transmission of HIV-1, encompassing early diagnosis in infants, is fundamental to the success of any early antiretroviral-therapy strategy.
Supplementary Material
Acknowledgments
Supported by a grant from the National Institute of Allergy and Infectious Diseases of the National Institutes for Health through the Comprehensive International Program of Research on AIDS network (U19 AI53217); the Departments of Health of the Western Cape and Gauteng, South Africa; and GlaxoSmith-Kline.
Drs. Violari and Cotton report receiving lecture fees from Abbott Laboratories; Dr. Babiker, research support from Abbott Laboratories; and Dr. Gibb, research support from the Meningitis Research Foundation, GlaxoSmithKline, and Gilead. No other potential conflict of interest relevant to this article was reported.
The views expressed in this report do not necessarily reflect the views or policies of the National Institute of Allergy and Infectious Diseases, nor does mention of trade names, commercial projects, or organizations imply endorsement by the U.S. government.
We thank the families and children who participated in the trial.
Appendix
The following investigators, clinical centers, and committees participated in the Children with HIV Early Antiretroviral Therapy (CHER) trial: South Africa — A. Violari, J. McIntyre, W. Pelser, J. Steyn, S. Madhi, A. Naeem-Sheik, M. Budge, M. Saleh, S. Cassimjee, E. Lazarus, S. Mashinini, S. Dlamini, V. Kemese, J. Bolton (Perinatal HIV Research Unit, University of the Witwatersrand, Johannesburg); M.F. Cotton, H. Rabie, A. Janse van Rensburg, E. Dobbels, G. Fourie, M. Bester, W. Orange, R. Arendze, C. Andrea, M. Smuts, K. Smith, T. Louw, A. Abrahams, K. Kelly, A. Bohle, I. Mong, J. Howard, T. Cyster, G. Solomon, G. Benjamin, J. Mkalipi, E. Barnes (Children's Infectious Diseases Clinical Research Unit, Stellenbosch University, Tygerberg); I. Sanne, G. Gray, R. Panchia, C. Davies, M. Cornell (Comprehensive International Program for Research in AIDS — South Africa [CIPRA-SA]); W. Stevens, D. Glencross (CIPRA-SA Laboratory Core); S. Spector, C. van der Horst (CIPRA-SA Scientific Advisory Committee); United Kingdom — A.G. Babiker, D.M. Gibb, (Medical Research Council, Clinical Trials Unit [MRC CTU], London) J.-M. Steens, W.X. Snowden, N. Thoofer, E. Loeliger (Glaxo-SmithKline); United States — E. Handelsman, K. Reese, P. Jean-Phillipe, J. Nadler (Division of Acquired Immunodeficiency Syndrome, National Institute of Allergy and Infectious Diseases [NIAID], National Institutes of Health [NIH]), J. McNamara (Division of Allergy, Immunology, and Transplantation, NIAID, NIH), R. Hoff (Regional Emerging Diseases Intervention Center), S. Lehrman (Merck), C. Oster (Walter Reed Army Institute of Research). End Point and Clinical Events Review Committee: T. Peto (chair) (John Radcliffe Hospital, University of Oxford, Oxford, United Kingdom); L. Levin (Right to Care, Johannesburg); D. Gibb (MRC CTU, London). Data Safety and Monitoring Board: S. Ellenberg (chair) (University of Pennsylvania, Philadelphia); R. DerSimonian (executive secretary); A. Dodoo (University of Ghana, Accra); D. Harrington (Harvard School of Public Health, Boston); A. Kamali (MRC Uganda, Entebbe); E. Katabira (Makerere University Uganda, Kampala); C. Lombard (MRC South Africa, Cape Town); C. Luo (UNICEF, New York); M.F. Marshall (University of Minnesota, Minneapolis); L. Mokgatlhe (University of Botswana, Gaborone); A. Mwinga (Centers for Disease Control Zambia, Lusaka); A. Nunn (MRC CTU, London); H. Saloojee (University of the Witwatersrand, Johannesburg); M. Sande (University of Washington, Seattle); J. Schoeman (Stellenbosch University, Tygerberg, South Africa); J. Singh (Nelson Mandela School of Medicine, Durban); R. Boss-Victoria (Bowie State University, Bowie, MD); T. Chipato (University of Zimbabwe, Harare); S. Emerson (University of Washington, Seattle); J. Mfutso-Bengo (University of Malawi, Blantyre); and P. Mwaba (University of Zambia, Lusaka).
REFERENCES
- 1.Diaz C, Hanson C, Cooper ER, et al. Disease progression in a cohort of infants with vertically acquired HIV infection observed from birth: the Women and Infants Transmission Study (WITS) J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18:221–8. doi: 10.1097/00042560-199807010-00004. [DOI] [PubMed] [Google Scholar]
- 2.Tovo PA, de Martino M, Gabiano C, et al. Prognostic factors and survival in children with perinatal HIV-1 infection: the Italian register for HIV infections in children. Lancet. 1992;339:1249–53. doi: 10.1016/0140-6736(92)91592-v. [DOI] [PubMed] [Google Scholar]
- 3.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–43. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 4.Dunn D. Short-term risk of disease progression in HIV-1-infected children receiving no antiretroviral therapy or zidovudine monotherapy: a meta-analysis. Lancet. 2003;362:1605–11. doi: 10.1016/s0140-6736(03)14793-9. [DOI] [PubMed] [Google Scholar]
- 5.Scaling up antiretroviral therapy in resource-limited settings: guidelines for a public health approach. World Health Organization; Geneva: 2002. [PubMed] [Google Scholar]
- 6.Antiretroviral therapy for HIV infection in infants and children: towards universal access — recommendations for a public health approach. World Health Organization; Geneva: 2006. [PubMed] [Google Scholar]
- 7.Revised classification system for human immunodeficiency virus infection in children less than 13 years of age. MMWR Morb Mortal Wkly Rep. 1994;43:1–10. [PubMed] [Google Scholar]
- 8.Chadwick EG, Capparelli EV, Yogev R, et al. Pharmacokinetics, safety and efficacy of lopinavir/ritonavir in infants less than 6 months of age: 24 week results. AIDS. 2008;22:249–55. doi: 10.1097/QAD.0b013e3282f2be1d. [DOI] [PubMed] [Google Scholar]
- 9.National Institute of Allergy and Infectious Diseases, Division of AIDS Table for grading the severity of adult and pediatric adverse events. 2004 December; [Google Scholar]
- 10.Barthel FM, Babiker A, Royston P, Parmar MK. Evaluation of sample size and power for multi-arm survival trials allowing for non-uniform accrual, non-proportional hazards, loss to follow-up and cross-over. Stat Med. 2006;25:2521–42. doi: 10.1002/sim.2517. [DOI] [PubMed] [Google Scholar]
- 11.Royston P, Babiker A. Complex sample size calculation in randomised controlled trials with a survival or binary outcome. Stata J. 2002;2:151–63. [Google Scholar]
- 12.Paediatric European Network for Treatment of AIDS (PENTA) Comparison of dual nucleoside-analogue reverse-transcriptase inhibitor regimens with and without nelfinavir in children with HIV-1 who have not previously been treated: the PENTA 5 randomised trial. Lancet. 2002;359:733–40. doi: 10.1016/S0140-6736(02)07874-1. [DOI] [PubMed] [Google Scholar]
- 13.WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. World Health Organization; Geneva: 2007. p. 20. [Google Scholar]
- 14.Mphatswe W, Blanckenberg N, Tudor-Williams G, et al. High frequency of rapid immunological progression in African infants infected in the era of perinatal HIV prophylaxis. AIDS. 2007;21:1253–61. doi: 10.1097/QAD.0b013e3281a3bec2. [DOI] [PubMed] [Google Scholar]
- 15.Mayaux MJ, Burgard M, Teglas JP, et al. Neonatal characteristics in rapidly progressive perinatally acquired HIV-1 disease. JAMA. 1996;275:606–10. [PubMed] [Google Scholar]
- 16.Kuhn L, Abrams EJ, Weedon J, et al. Disease progression and early viral dynamics in human immunodeficiency virus-infected children exposed to zidovudine during prenatal and perinatal periods. J Infect Dis. 2000;182:104–11. doi: 10.1086/315678. [DOI] [PubMed] [Google Scholar]
- 17.Luzuriaga K, McManus M, Mofenson L, Britto P, Graham B, Sullivan JL. A trial of three antiretroviral regimens in HIV-1–infected children. N Engl J Med. 2004;350:2471–80. doi: 10.1056/NEJMoa032706. [DOI] [PubMed] [Google Scholar]
- 18.Berk DR, Falkovitz-Halpern MS, Hill DW, et al. Temporal trends in early clinical manifestations of perinatal HIV infection in a population-based cohort. JAMA. 2005;293:2221–31. doi: 10.1001/jama.293.18.2221. [DOI] [PubMed] [Google Scholar]
- 19.Chiappini E, Galli L, Tovo PA, et al. Virologic, immunologic, and clinical benefits from early combined antiretroviral therapy in infants with perinatal HIV-1 infection. AIDS. 2006;20:207–15. doi: 10.1097/01.aids.0000200529.64113.3e. Erratum, AIDS 2006;20:1789. [DOI] [PubMed] [Google Scholar]
- 20.Faye A, Le Chenadec J, Dollfus C, et al. Early versus deferred antiretroviral multi-drug therapy in infants infected with HIV type 1. Clin Infect Dis. 2004;39:1692–8. doi: 10.1086/425739. [DOI] [PubMed] [Google Scholar]
- 21.Goetghebuer T, Haelterman E, Chenadec J, et al. Early versus deferred highly active antiretroviral therapy in HIV infected infants: a European collaborative cohort study; IV Dominique DORMONT International Conference; Paris. December 13-15, 2007. [Google Scholar]
- 22.Cross Continents Collaboration for Kids (3Cs4Kids) Analysis and Writing Committee Markers for predicting mortality in untreated HIV-infected children in resource-limited settings: a meta-analysis. AIDS. 2008;22:97–105. doi: 10.1097/01.aids.0000302262.51286.a5. [DOI] [PubMed] [Google Scholar]
- 23.Sharland M, Blanche S, Castelli G, Ramos J, Gibb DM. PENTA guidelines for the use of antiretroviral therapy, 2004. HIV Med. 2004;5(Suppl 2):61–86. doi: 10.1111/j.1468-1293.2004.00227.x. [DOI] [PubMed] [Google Scholar]
- 24.Working Group on Antiretroviral Therapy and Medical Management of HIV-Infected Children . Guidelines for the use of antiretroviral agents in pediatric HIV infection. Department of Health and Human Services; Bethesda, MD: Oct 26, 2006. Accessed October 26, 2008, at http://aidsinfo.nih.gov. [Google Scholar]
- 25.National Department of Health . National antiretroviral treatment guidelines. Jacana Publishers; Pinetown, South Africa: 2004. [Google Scholar]
- 26.Lockman S, Shapiro RL, Smeaton LM, et al. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N Engl J Med. 2007;356:135–47. doi: 10.1056/NEJMoa062876. [DOI] [PubMed] [Google Scholar]
- 27.Abrams EJ, Wiener J, Carter R, et al. Maternal health factors and early pediatric antiretroviral therapy influence the rate of perinatal HIV-1 disease progression in children. AIDS. 2003;17:867–77. doi: 10.1097/00002030-200304110-00012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.