Abstract
γδ T lymphocytes are a numerically small subset of T cells with potent cytotoxic activity against a variety of tumor cells. Human γδ T cells expressing the Vγ9Vδ2 T cell antigen receptor recognize endogenous pyrophosphate molecules that are overproduced in transformed cells. Moreover, the intracellular accumulation of such pyrophosphates is strongly enhanced by aminobisphosphonates used in the treatment of osteoporosis and bone metastasis in certain cancer patients. A new concept of cancer immunotherapy is based on the endogenous activation of γδ T cells with aminobisphosphonates plus low-dose interleukin-2.
Introduction and context
Approximately 1-5% of peripheral blood T cells express the γδ T-cell receptor instead of the conventional αβ T-cell receptor [1]. The αβ versus γδ T-cell lineage commitment during intrathymic T-cell development seems to be controlled by the signal strength provided to the γδ T-cell receptor [2]. In healthy donors, most blood γδ T cells carry a specific T-cell receptor composed of Vγ9 and Vδ2 elements. In addition to effector functions shared with αβ T cells, the Vγ9Vδ2 T cells can acquire professional antigen-presenting capacity characteristic of dendritic cells [3]. In contrast to αβ T cells, Vγ9Vδ2 T cells do not see processed antigenic peptides presented by major histocompatibility complex molecules, but rather recognize small phosphorylated non-peptide molecules (‘phosphoantigens’) produced by many microorganisms but also by transformed eukaryotic cells [4,5]. While microbial phosphoantigens are active at pico- to nanomolar concentrations, micromolar concentrations of the eukaryotic phosphoantigen isopentenyl pyrophosphate (IPP) are required for γδ T-cell activation. Such high concentrations are not achieved in the mevalonate pathway of isoprenoid synthesis used in non-transformed cells. Interestingly, human Vγ9Vδ2 T cells can kill a broad variety of epithelial tumor and leukemia/lymphoma cells [6,7]. The sensitivity of tumor cells to γδ T-cell-mediated killing is increased upon treatment of tumor cells with aminobisphosphonates (N-BPs), drugs that are used in clinical practice for the treatment of osteoporosis and bone metastasis in cancer patients [8]. N-BPs inhibit the IPP-processing enzyme farnesyl diphosphate synthase (FPPS), thereby leading to an accumulation of IPP, which is then sensed by the γδ T cells [9]. γδ T cells are poor producers of interleukin-2 (IL-2), which is required for expansion of γδ T cells. Therefore, attempts to activate tumor-reactive γδ T cells endogenously by treating patients with N-BPs must take into consideration an appropriate supply of IL-2. Alternative strategies consider the adoptive transfer of in vitro expanded tumor-reactive γδ T cells [10-14].
Recent advances
The critical role of FPPS in the control of intracellular IPP levels, and thus of the sensitivity of tumor cells toward γδ T-cell killing, has been recently demonstrated using short hairpin RNA-mediated knock-down of FPPS [15]. Knock-down of FPPS caused tumor cells, which otherwise were not recognized by γδ T cells, to be susceptible to γδ T-cell killing [15]. Therefore, Vγ9Vδ2 T cells recognize and kill tumor cells on the basis of the unbalanced isoprenoid metabolic pathway in transformed cells, a pathway that is stable in non-malignant cells.
The discovery that N-BPs activate γδ T cells by inhibiting FPPS, thereby leading to accumulation of IPP, has paved the way for proof-of-principle studies to activate γδ T cells in patients with advanced cancer. In a phase I clinical trial, Dieli and colleagues [16] treated patients with hormone-refractory prostate cancer with a standard application of the N-BP zoledronate (4 mg intravenous infusion every 21 days) either with or without additional low-dose (6 × 105 IU) subcutaneous application of IL-2. Various parameters, including subset analysis of γδ T cells, and serum levels of prostate-specific antigen and cytokines, were monitored over time. Although the two cohorts comprised only a few patients, statistically significant effects of zoledronate plus IL-2 on the mobilization and effector cell maturation of γδ T cells were recorded. Very importantly, the two cohorts showed distinct clinical outcomes, with clinical responses seen in six of nine patients treated with zoledronate plus IL-2 but only in one of nine patients treated with zoledronate alone. Interestingly, a correlation between favorable outcome at 12 months and γδ T-cell numbers or functional status (or both) was observed [16]. Similarly, Wilhelm and colleagues had previously shown that the combined application of N-BP plus low-dose IL-2 can induce objective tumor responses in patients with lymphoid malignancies [17]. Together, these studies support the view that application of N-BPs plus IL-2 is safe, induces in vivo activation/maturation of γδ T cells, and may have beneficial effects in advanced cancer (Figure 1a).
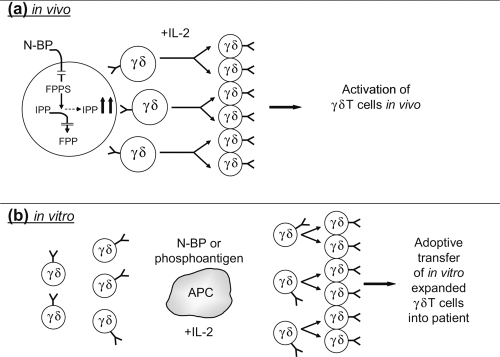
Figure 1. Approaches to immunotherapy with γδ T cells.
(a) N-BPs inhibit farnesyl diphosphate synthase (FPPS), thus preventing processing of isopentenyl pyrophosphate (IPP) to farnesyl diphosphate (FPP). This leads to accumulation of IPP which then activates Vγ9Vδ2 T cells. Vγ9Vδ2 T cells require exogenous interleukin (IL)-2 for cellular expansion. The combined application of N-BP plus IL-2 leads to in vivo activation of γδ T cells. (b) Alternatively, γδ T cells can be activated in vitro with N-BP or synthetic phosphoantigens in the presence of antigen-presenting cells (APC) and can be subsequently expanded to large cell numbers by an exogenous supply of IL-2 for subsequent adoptive transfer into cancer patients. The cell preparation can be performed under GMP (Good Manufacturing Practice) conditions.
In a case study reported by Laggner et al. [18], regression of lung and bone metastases was observed in a patient with advanced stage melanoma upon systemic treatment with zoledronate and localized radiotherapy. Although γδ T-cell subsets were analyzed, it is difficult to ascertain a substantial role of γδ T-cell activation in the resolution of metastases in this single case, particularly since IL-2 was omitted in the treatment of this patient [18].
In addition to their γδ T-cell activating properties, N-BPs also exhibit direct anti-tumor activities by both inhibiting proliferation and inducing apoptosis in tumor cells [19]. While zoledronate seems to be the most potent γδ T-cell-activating substance among the N-BPs licensed for clinical application [9], derivatives of zoledronate with further improved γδ T-cell-stimulating capacity and enhanced direct anti-tumor activity are under development [20]. Such modified N-BPs might also exert improved in vivo activation of γδ T cells when given to patients together with IL-2.
An alternative and not mutually exclusive γδ T-cell-based immunotherapeutic strategy is the adoptive transfer of in vitro expanded Vγ9Vδ2 T cells from tumor patients (Figure 1b). Recently, efficient protocols for the large-scale in vitro expansion of Vγ9Vδ2 T cells based on stimulation with synthetic phosphoantigens [11,13,14] or zoledronate [21] have been established. First results indicate that the repetitive adoptive transfer of in vitro expanded γδ T cells is well tolerated and may induce anti-tumor responses in patients with solid tumors, including renal cell carcinoma [13,14] and myeloma [12].
Implications for clinical practice
The protocol developed by Dieli et al. [16] for the in vivo activation of γδ T cells based on zoledronate plus low-dose IL-2 application is ready to be explored in larger clinical trials and in other tumor entities with poor prognosis, for example, pancreatic ductal adenocarcinoma where it might be combined with standard regimens such as gemcitabine. It is conceivable that this protocol can be further improved, for instance, by combination with tumor-targeting monoclonal antibodies. Along this line, it has been shown that B-cell lymphoma or breast tumor cell killing by Fcγ receptor-expressing γδ T cells is enhanced in the presence of targeting antibodies rituximab (CD20) or trastuzumab (HER2/neu), respectively [22]. Moreover, a γδ T-cell-stimulating synthetic phosphoantigen was found to enhance the depletion of CD20+ B cells by rituximab in a non-human primate model in vivo, pointing to the possible use of phosphoantigen plus anti-CD20 antibodies in the treatment of CD20+ leukemias and lymphomas [23]. Furthermore, cytokines promoting homeostatic proliferation and survival of T cells, such as IL-15 [24], or cytokines potentiating the cytolytic activity and pro-inflammatory response, such as IL-21 [25], might be combined with IL-2 or used instead of IL-2. This could be considered both for in vivo application together with N-BPs and for optimization of in vitro expansion of γδ T cells. In addition, future study protocols might include the combination of in vivo activation of γδ T cells (by N-BP or phosphoantigen plus IL-2) followed by the adoptive transfer of in vitro expanded γδ T cells, Finally, it should be stressed that γδ T-cell-based immunotherapy is not expected to replace established therapeutic protocols. Rather, it might offer additional benefit to the patient, for instance, in combination with conventional chemotherapy [26].
Acknowledgments
Cited work from the author’s lab has been continuously supported by the Deutsche Forschungsgemeinschaft (German Research Foundation).
Abbreviations
- FPPS
farnesyl diphosphate synthase
- IL
interleukin
- IPP
isopentenyl pyrophosphate
- N-BP
aminobisphosphonate
Competing Interests
The author declares that he has no competing interests.
The electronic version of this article is the complete one and can be found at: http://f1000.com/reports/m/2/45
References
- 1.Hayday A. γδ T cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 2.Hayes SM, Love PE. TCR signal strength influences αβ/γδ lineage fate. Immunity. 2005;22:583–93. doi: 10.1016/j.immuni.2005.03.014. [DOI] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by Harald von Boehmer 02 Jun 2005
- 3.Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human γδ T cells. Science. 2005;309:264–8. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]; F1000 Factor 8.4 ExceptionalEvaluated by Pedro Romero 29 Jun 2005, Dan Conrad 21 Jul 2005, Stephen Jameson 09 Aug 2005, Peter Van Endert 16 Aug 2005
- 4.Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunologic memory of human Vγ2Vδ2 T cells: discriminating friend from foe through the recognition of prenylpyrophosphate antigens. Immunol Rev. 2007;215:59–76. doi: 10.1111/j.1600-065X.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 5.Kabelitz D. Small molecules for the activation of human γδ T cell responses against infection. Recent Pat Anti-Infect Drug Discov. 2008;3:1–9. doi: 10.2174/157489108783413218. [DOI] [PubMed] [Google Scholar]
- 6.Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of γδ T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96:384–92. [PubMed] [Google Scholar]
- 7.Wrobel P, Shojaei H, Schittek B, Gieseler F, Wollenberg B, Kalthoff H, Kabelitz D, Wesch D. Lysis of a broad range of epithelial tumour cells by human γδ T cells: Involvement of NKG2D ligands and T-cell receptor- versus NKG2D-dependent recognition. Scand J Immunol. 2007;66:320–8. doi: 10.1111/j.1365-3083.2007.01963.x. [DOI] [PubMed] [Google Scholar]
- 8.Gober HJ, Kistowska M, Angman L, Jenö P, Mori L, de Libero G. Human T cell receptor γδ T cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–8. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson K, Roelofs AJ, Jauhiainen M, Mönkkönen H, Mönkkönen J, Rogers MJ. Activation of γδ T cells by bisphosphonates. Adv Exp Med Biol. 2010;658:11–20. doi: 10.1007/978-1-4419-1050-9_2. [DOI] [PubMed] [Google Scholar]
- 10.Kabelitz D, Wesch D, He W. Perspectives of γδ T cells in tumor immunology. Cancer Res. 2007;67:5–8. doi: 10.1158/0008-5472.CAN-06-3069. [DOI] [PubMed] [Google Scholar]
- 11.Salot S, Bercegeay S, Dreno B, Saiagh S, Scaglione V, Bonnafous C, Sicard H. Large scale expansion of Vγ9Vδ2 T lymphocytes from human peripheral blood mononuclear cells after a positive selection using MACS ‘TCR γ/δ+ T cell isolation kit’. J Immunol Methods. 2009;347:12–8. doi: 10.1016/j.jim.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Abe Y, Muto M, Nieda M, Nakagawa Y, Nicol A, Kaneko T, Goto S, Yokokawa K, Suzuki K. Clinical and immunological evaluation of zoledronate-activated Vγ9γδ T-cell-based immunotherapy for patients with multiple myeloma. Exp Hematol. 2009;37:956–68. doi: 10.1016/j.exphem.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi H, Tanaka Y, Yagi J, Osaka Y, Nakazawa H, Uchiyama T, Minato N, Toma H. Safety profile and anti-tumor effects of adoptive immunotherapy using gamma-delta T cells against advanced renal cell carcinoma: a pilot study. Cancer Immunol Immunother. 2007;56:469–76. doi: 10.1007/s00262-006-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennouna J, Bompas E, Neidhardt EM, Rolland F, Philip I, Galéa C, Salot S, Saiagh S, Audrain M, Rimbert M, Lafaye-de Micheaux S, Tiollier J, Négrier S. Phase-I study of Innacell γδ™, an autologous cell-therapy product highly enriched in γ9δ2 T lymphocytes, in combination with IL-2, in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother. 2008;57:1599–609. doi: 10.1007/s00262-008-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Herold MJ, Kimmerl B, Müller I, Rincon-Orozco B, Kunzmann V, Herrmann T. Reduced expression of the mevalonate pathway enzyme farnesyl pyrophosphate synthase unveils recognition of tumor cells by Vγ9Vδ2 T cells. J Immunol. 2009;182:8118–24. doi: 10.4049/jimmunol.0900101. [DOI] [PubMed] [Google Scholar]
- 16.Dieli F, Vermijlen D, Fulfaro F, Caccamo N, Meraviglia S, Cicero G, Roberts A, Buccheri S, D’Asaro M, Gebbia N, Salerno A, Eberl M, Hayday A. Targeting human γδ T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007;67:7450–7. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by Dietrich Kabelitz 12 May 2010
- 17.Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F, Ruediger T, Tony HP. γδ T cells for immune therapy of patients with lymphoid malignancy. Blood. 2003;102:200–6. doi: 10.1182/blood-2002-12-3665. [DOI] [PubMed] [Google Scholar]
- 18.Laggner U, Lopez JS, Perera G, Warbey VS, Sita-Lumsden A, O’Doherty MJ, Hayday A, Harries M, Nestle FO. Regression of melanoma metastases following treatment with the n-bisphosphonate zoledronate and localized radiotherapy. Clin Immunol. 2009;131:367–73. doi: 10.1016/j.clim.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Clezardin P, Ebertino FH, Fournier PG. Bisphosphonates and cancer-induced bone disease: beyond their anti-resorptive activity. Cancer Res. 2005;65:4971–4. doi: 10.1158/0008-5472.CAN-05-0264. [DOI] [PubMed] [Google Scholar]
- 20.Simoni D, Gebbia N, Invidiata FP, Eleopra M, Marchetti P, Rondanin R, Baruchello R, Provera S, Marchioro C, Tolomeo M, Marinelli L, Limongelli V, Novellino E, Kwaasi A, Dunford J, Buccheri S, Caccamo N, Dieli F. Design, synthesis, and biological evaluation of novel aminobisphosphonates possessing an in vivo antitumor activity through a γδ-T lymphocytes-mediated activation mechanism. J Med Chem. 2008;51:6800–7. doi: 10.1021/jm801003y. [DOI] [PubMed] [Google Scholar]
- 21.Kondo M, Sakuta K, Noguchi A, Ariyoshi N, Sato K, Sato S, Sao K, Hosoi A, Nakajima J, Yoshida Y, Shiraishi K, Nakagawa K, Kakimi K. Zoledronate facilitates large-scale ex vivo expansion of functional γδ T cells from cancer patients for use in adoptive immunotherapy. Cytotherapy. 2008;10:842–56. doi: 10.1080/14653240802419328. [DOI] [PubMed] [Google Scholar]
- 22.Tokuyama H, Hagi T, Mattarollo SR, Morley J, Wang Q, Fai-So H, Moriyasu F, Nieda M, Nicol AJ. Vγ9Vδ2 T cell cytotoxicity against tumor cells is enhanced by monoclonal antibody drugs - rituximab and trastuzumab. Int J Cancer. 2008;122:2526–34. doi: 10.1002/ijc.23365. [DOI] [PubMed] [Google Scholar]
- 23.Gertner-Dardenne J, Bonnafous C, Bezombes C, Capietto AH, Scaglione V, Ingoure S, Cendron D, Gross E, Lepage JF, Quillet-Mary A, Ysebaert L, Laurent G, Sicard H, Fournié JJ. Bromohydrin pyrophosphate enhances antibody-dependent cell-mediated cytotoxicity induced by therapeutic antibodies. Blood. 2009;113:4875–84. doi: 10.1182/blood-2008-08-172296. [DOI] [PubMed] [Google Scholar]
- 24.Boyman O, Létourneau S, Krieg C, Sprent J. Homeostatic proliferation and survival of naïve and memory T cells. Eur J Immunol. 2009;39:2088–94. doi: 10.1002/eji.200939444. [DOI] [PubMed] [Google Scholar]
- 25.Thedrez A, Harly C, Morice A, Salot S, Bonneville M, Scotet S. IL-21-mediated potentiation of antitumor cytolytic and proinflammatory responses of human Vγ9Vδ2 T cells for adoptive immunotherapy. J Immunol. 2009;182:3423–31. doi: 10.4049/jimmunol.0803068. [DOI] [PubMed] [Google Scholar]
- 26.Mattarollo SR, Kenna T, Nieda M, Nicol AJ. Chemotherapy and zoledronate sensitize solid tumour cells to Vγ9Vδ2 T cell cytotoxicity. Cancer Immunol Immunother. 2007;56:1285–97. doi: 10.1007/s00262-007-0279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]