Abstract
The synthetic triterpenoid, CDDO-Me, has potent antiproliferative and antioxidant properties. However, its immunomodulatory effects in the context of LPS challenge are incompletely defined. Pretreatment with oral CDDO-Me significantly improved survival following lethal-dose LPS challenge in mice. To define this protection further, we measured effects of CDDO-Me pretreatment on splenocyte populations and cytokine production following LPS challenge, using low-level LPS pretreatment as an in vivo control for reducing cytokine production. Despite similar decreases in levels of LPS-inducible, circulating proinflammatory cytokines (IL-12p70, IFN-γ, IL-6, IL-17, and IL-23) and increases in heme oxygenase 1 (HO-1) protein expression, low-dose LPS and CDDO-Me pretreatments markedly differed in their overall response profiles. Splenocytes from LPS-pretreated mice contained reduced numbers of dendritic cells, increased percentages of Th17 and T-regulatory cells, lower levels of TLR-inducible IL-6, and higher levels of TLR-inducible IL-10. In contrast, CDDO-Me protection against LPS challenge had no impact on absolute numbers or distribution of splenocyte subsets, despite attenuating in vivo induction of proinflammatory cytokines in an IL-10-independent manner. Together, these results suggest that CDDO-Me pretreatment uniquely confers protection against LPS challenge by modulating the in vivo immune response to LPS. Thus, CDDO-Me potentially represents a novel oral agent for use in LPS-mediated inflammatory diseases.
Introduction
The synthetic triterpenoids, derivatives of naturally occurring oleanolic acids, have established anti-inflammatory, antiproliferative, and antioxidant properties (Suh and others 1999; Dinkova-Kostova and others 2005). In particular, the synthetic triterpenoid, 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO), and its imidazole (CDDO-Im) and methyl ester (CDDO-Me) derivatives induce heme oxygenase 1 (HO-1) protein expression at nanomolar concentrations (Dinkova-Kostova and others 2005; Liby and others 2005). In addition, the synthetic triterpenoids directly suppress NF-κB activation (Ahmad and others 2006; Shishodia and others 2006; Yore and others 2006) and STAT phosphorylation in malignant cells (Liby and others 2006) and inhibit transcription of TNF-α- and IFN-γ-inducible COX-2 and nitric oxide synthetase (iNOS) in murine macrophages (Honda and others 1998; Suh and others 1998). These compounds have also been shown to induce TGF-β-signaling cascades in murine epithelial cells (Suh and others 2003). However, less is known about the anti-inflammatory effects of these agents in the context of in vivo LPS challenge (Thimmulappa and others 2006b).
The immune response to LPS requires activation of Tolllike receptor 4 (TLR4), which leads to both MyD88-dependent, early phase NF-κB transcription of proinflammatory cytokines (IL-1β, TNF-α, and IL-6) and MyD88-independent, late phase NF-κB transcription of IFN-β (Akira and Takeda 2004). Through innate immune cell activation, LPS–TLR4 ligation bridges innate and adaptive proinflammatory responses (Akira and others 2001). LPS further promotes inflammation by inducing production of reactive oxygen species, an effect balanced by antioxidant enzymes like HO-1 (Asehnoune and others 2004; Bhattacharyya and others 2004), which subsequently modulates innate (Chauveau and others 2005) and adaptive (Xia and others 2006) immune cell function. The link between LPS-induced inflammation and oxidation–reduction reactions (Wiesel and others 2000; Kapturczak and others 2004; Zhang and others 2005) predicts that agents with combined anti-inflammatory and antioxidant properties may be advantageous in attenuating or even preventing endotoxemia.
Regulation of LPS-mediated immune response is vital to ensure that acute inflammation does not progress to chronic inflammatory disease and that the host does not succumb to an overwhelming inflammatory demise (Pinsky 2004). Anti-inflammatory cytokines like IL-10 (Pestka and others 2004) and TGF-β (Li and others 2006) induced by low-level LPS exposure prior to LPS challenge directly modulate immune cell function (West and Heagy 2002), through effects on TLR4-signaling cascades (Nomura and others 2000; Escoll and others 2003) and NF-κB activation (West and others 2000; Fan and Cook 2004).
We hypothesized that pretreatment with CDDO-Me would decrease the proinflammatory cytokine response to LPS challenge through its induction of anti-inflammatory soluble factors and HO-1. We used a model of LPS pretreatment as an established in vivo control for attenuating LPS-inducible cytokine production (Balkhy and Heinzel 1999). Like low-dose LPS pretreatment, CDDO-Me significantly reduced the proinflammatory response to LPS challenge and induced HO-1. However, CDDO-Me pretreatment did not alter splenocyte immune cell subtypes or TLR-inducible cytokine profiles. Together, these results suggest CDDO-Me pretreatment modulates the in vivo LPS response and that the anti-inflammatory effects of CDDO-Me may be clinically useful in preventing or in treating endotoxin-mediated diseases.
Materials and Methods
Mice
Female C57BL/6J (Ly5.1, CD45.2) mice aged 12–16 weeks were purchased from Jackson Laboratory (Bar Harbor, ME) and the National Cancer Institute (C57BL/6NCr; Frederick, MD). The Institutional Animal Care and Use Committee at Case Western Reserve University approved all animal experiments.
Reagents
Pathogen-associated molecular pattern (PAMP) agonists included phosphorothioated CpG-containing oligodeoxynucleotide 1826 (5′-TCCATGACGTTCCTGACGTT, Oligos Etc., Westerville, OR); Resiquimod (R848; PharmaTech, China), and Salmonella enteritidis lipopolysaccharide (LPS; Sigma-Aldrich, St. Louis, MO). The LPS reagent is TLR4-dependent; as it has been shown to induce endotoxin tolerance in C3H/HeN and TLR2-KO C57BL/6 mice, but not in TLR4-deficient C3H/HeJ mice (Greene and others 2006).
Preparation of the synthetic triterpenoid, CDDO-Me
Synthesis of the methyl ester derivative of CDDO, CDDO-Me, has been previously published (Honda and others 1998, 2000). CDDO-Me was dissolved in vehicle (DMSO) prior to administration as single intraperitoneal (Sallusto and others 1999) injection. Nanomole (10, 100, and 500 nmol) and micromole (1 μmol) doses of CDDO-Me were used to optimize the anti-inflammatory effect, at which the 100 nmol dose was established.
In vivo injections
Intraperitoneal injections were administered as 50 or 100 μL volume using a 1-mL syringe with a 27-gauge needle. LPS-pretreated mice were given 25–50 μg (~1–2 mg/kg) IP daily for 2 consecutive days (Days -3 and -2) (Balkhy and Heinzel 1999). Vehicle control animals received DMSO alone (100 μL) as a single IP injection on Day -2. CDDO-Mepretreated animals received a single IP injection (100 μL) or gavage (200 μL) containing the desired dose of CDDO-Me (Day -2). All LPS-challenged mice (LPS-, DMSO-, and CDDO-Me-pretreated) received 300 μg IP LPS 48 h after the last dose of LPS, vehicle, or triterpenoid, respectively (Day 0). For mortality experiments, CDDO-Me was given via gavage using sesame oil as the vehicle (200 μL). The LD50 dose for LPS (850 μg, ~40 mg/kg) was administered as a single IP injection on Day 0.
Organ and peripheral blood collections
After euthanasia, mice underwent jugular vein ligation and dissection for organ harvests (spleen, lung, and liver). Organs and sera from LPS-challenged groups were collected 5 h post-LPS challenge on Day 0, and those from pretreated and control groups were collected 48 h after the last respective pretreatment (Day 0 as well) unless otherwise indicated. All individual serum samples were placed immediately on ice after collection and were centrifuged at 12,000g before plasma was obtained and was frozen at -20°C.
For Western blots and FACS analyses for T-regulatory (Treg) and Th17 cells measurements, individual organs were separately collected and analyzed. For all other FACS analyses and for cell-culture stimulations, spleens within each experimental group were harvested and were pooled (n = 4) in order to ensure adequate numbers for indicated analyses.
Cell-culture stimulations
Pooled, unselected splenocytes were cultured at 1 × 107 cells/mL in TCM/1% Nutridoma medium consisting of RPMI 1640 and PMEM in 1:1 ratio and supplemented with Hepes, l-glutamine, l-arginine, nonessential amino acids, and penicillin/streptomycin. Cells were stimulated with medium alone or with medium containing LPS (1 μg/mL), CpG oligodeoxynucleotide 1826 (10 μg/mL), a TLR9 agonist, or Resiquimod (R848, 1 μg/mL), a TLR 7/8 agonist. Conditions were performed in triplicate on sterile 96-well, flat-bottomed polypropylene culture plates and incubated at 37°C for 24 h. After the culture period, plates were centrifuged and supernatants were collected and were frozen at -20°C. In preliminary studies, higher levels of inducible cytokines (IL-6 and IL-10) were measured from splenocyte cultures stimulated with R848 and CpG 1826 than with LPS stimulation (data not shown). Therefore, cross-tolerant R848 and CpG 1826 stimulation was used to measure cytokine induction profiles in splenocyte cultures (Sato and others 2002; Dalpke and others 2005).
Flow cytometry
Techniques used for flow cytometry were as previously published (Auletta and others 2004). In brief, lymphoid phenotypes were defined by their respective stains: APC anti-CD3, PE anti-CD49b/Pan-NK (DX5), and streptavidin-PerCP/biotin anti-CD19, IgM, or CD45R/B220. Polymorphonuclear (PMN) cells were distinguished from macrophages using MAb Gr-1 and CD11b and forward and side scatter characteristics, with PMNs defined as CD11b+Gr-1+ and macrophages as CD11b+Gr-1-. Dendritic cells (DCs) were identified by APC-MHC II (I-A/I-E), PE-CD11b, and streptavidin-PerCP/biotin anti-CD11c stains. Specifically, myeloid (CD11c+CD11bhigh/intermediateCD8α-), lymphoid (CD11c+CD11blowCD8α+), and plasmacytoid (CD11c+B220+Ly6C+) DCs were separately identified. Total DC content was quantified using combination CD11c+CD11b+ staining (Pulendran and others 1997). Activation markers for CD4+ and CD8+ T cells included CD25 and CD69 staining, and those for DCs included MHC-II and CD86. At least 20,000 events were analyzed per conjugated MAb condition. Absolute numbers of defined cell populations were calculated via multiplying total number of splenocytes per experimental group with the percentages generated as above.
FoxP3 and IL-17 expression were determined by intracellular staining. In brief, for the FoxP3 expression, splenocytes were extracellular stained with anti-CD4, anti-CD8, and anti-CD25 (BD Biosciences, Mountain View, CA), fixed and permeabilized, and then intracellularly stained with anti-FoxP3 (eBioscience, San Diego, CA). For intracellular IL-17 cytokine staining, cells were extracellular stained with anti-CD4, fixed and permealized, and then intracellularly stained with anti-IL-17. Flow cytometric analysis was performed using a FACSCalibur flow cytometer (Becton Dickinson). After initially gating on CD4+ cells, intracellular staining for FoxP3 and IL-17 was used to define Treg (CD4+CD25+FoxP3+) and Th17 (CD4+IL-17+) splenocyte phenotypes, respectively.
ELISA
Cell-culture supernatants were assayed for murine IL-6 and IFN-γ using double-sandwich ELISA techniques as previously described (Auletta and others 2004). Murine IL-12p70 (Quantikine M Mouse IL-12p70; R&D Systems, Minneapolis, MN), TGF-β1 (Human TGF-β1 DuoSet; R&D Systems), IL-10 (DuoSet Mouse IL-10; R&D Systems), IL-17 (DuoSet Mouse IL-17; R&D Systems), and IL-23 (Mouse IL-23 (p19/p40) ELISA; eBioscience, San Diego, CA) were measured according to manufacturer's kit instructions.
Western blots
Splenocytes were washed twice with cold PBS, and then lysed in RIPA buffer containing Complete Mini Protease Inhibitor Cocktail Tablet (Roche, Indianapolis, IN). Protein was quantified using the bicinchoninic acid assay (Pierce, Rockford, IL). Lysates were clarified by centrifugation at 14,000g for 15 min and aliquots of the cell extracts containing equal amounts of protein were analyzed. Total cell lysates (50 μg) were separated by SDS-PAGE (4%–20% Tris-glycine gel) and transferred to a nitrocellulose membrane (Invitrogen, Carlsbad, CA). Membranes were immunostained with HO-1 (Santa Cruz Biotechnology, Santa Cruz, CA) and were visualized by incubation with appropriate HRP-conjugated secondary antibody (Southern Biotech, Birmingham, AL) followed by SuperSignal West Pico Chemiluminescent Substrate detection (Pierce, Rockford, IL). Membranes were stripped and re-probed with antibodies for β-actin (Cell Signaling Technology, Danvers, MA).
Statistics
The 2-tailed, unpaired t-tests were used to test for significant differences between individual means and the one-way ANOVA was used to measure for significant differences across means. Statistical significance was defined as a P value <0.05.
Results
CDDO-Me attenuates induction of inflammatory cytokines following in vivo lipopolysaccharide challenge
Low-dose LPS pretreatment reduces the proinflammatory in vivo cytokine response following LPS challenge (West and Heagy 2002). To test the degree to which the synthetic triterpenoid CDDO-Me would reduce LPS-inducible inflammatory cytokines, we pretreated C57BL/6 mice with either low-dose LPS (25–50 μg for 2 consecutive days), vehicle (DMSO), or CDDO-Me (single 100 nmol dose) and then challenged them with LPS (300 μg IP) 48 h after the last dose of LPS, DMSO, or CDDO-Me. Age-matched, untreated control mice received only LPS challenge, and sera were collected from all groups 5 h post-LPS challenge.
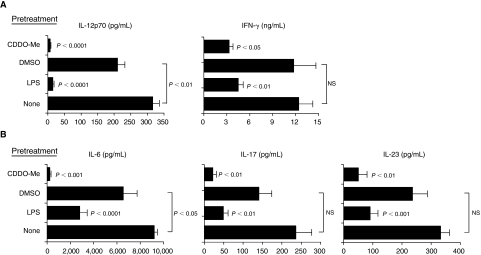
Mice pretreated with a single 100 nmol dose of CDDO-Me had significantly reduced levels of circulating proinflammatory cytokines following LPS challenge relative to vehicle-pretreated mice (Fig. 1). Levels of circulating IL-12p70 and IFN-γ (Fig. 1A) as well as IL-6, IL-17, and IL-23 in CDDO-Me-pretreated mice were significantly decreased versus vehicle-pretreated mice (Fig. 1B). In general, cytokine reduction in CDDO-Me-pretreated, LPS-challenged mice was as profound as reduction in LPS-pretreated, LPS-challenged mice.
FIG. 1.
Synthetic triterpenoid, CDDO-Me potently reduces levels of circulating IL-12p70 and IFN-γ (A) and levels of IL-6, IL-17, and IL-23 (B) in response to in vivo lipopolysaccharide (LPS) challenge. Cytokine levels were measured from plasma collected 5 h after mice (n = 6 mice per group) were challenged with LPS (300 μg IP). Mice were pretreated with low-dose LPS (25–50 μg intraperitoneal injection for 2 consecutive days prior to LPS challenge, Days -3 and -2), with vehicle alone (DMSO 100 μL, Day -2) or with the synthetic triterpenoid, CDDO-Me (100 nmol IP, Day -2). Mice did not receive any pretreatment on Day -1 (rest). All LPS challenges occurred 48 h (Day 0) after the last pretreatment (LPS, DMSO, or CDDO-Me) was administered. Untreated, age-matched control mice (none) were similarly challenged with LPS on Day 0. Cytokine levels (mean ± SEM) for each experimental group from 1 of 3 independent experiments are shown. Indicated P values (unpaired, 2-tailed t-tests) are for mean comparisons between groups pretreated with either low-dose LPS (LPS) or synthetic triterpenoid (CDDO-Me) and their respective control group (none or DMSO, respectively) unless otherwise indicated. NS = not statistically significant (P > 0.05).
Pretreatment with CDDO-Me has no effect on circulating IL-10 following LPS challenge
We next evaluated the ability of CDDO-Me to induce anti-inflammatory cytokines. We hypothesized that levels of the anti-inflammatory cytokines IL-10 and TGF-β1 would increase in mice pretreated with either low-dose LPS or CDDO-Me. Plasma samples were collected from mice 48 h after pretreatment with low-dose LPS, DMSO (vehicle), or CDDO-Me. Similar groups of mice were challenged with LPS and their plasma was collected 5 h post-LPS challenge. Circulating cytokines in these experimental groups were then compared with levels from untreated, age-matched control mice.
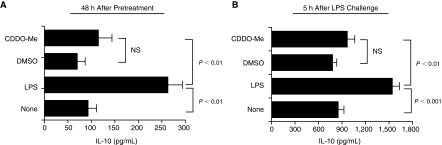
Levels of circulating IL-10 were higher in plasma from LPS-pretreated mice relative to CDDO-Me-pretreated and control animals (Fig. 2A). Following LPS challenge, increased IL-10 levels were also measured in plasma from LPS-pretreated mice relative to plasma levels in control and CDDO-Me-pretreated mice (Fig. 2B). Analysis of total TGF-β1 showed no significant difference in plasma levels between experimental groups and controls (data not shown). Together, these results show that LPS pretreatment associates with IL-10 induction and that high-level IL-10 induction persists in these mice following subsequent LPS challenge. In contrast, the anti-inflammatory effects of CDDO-Me are independent of IL-10 induction.
FIG. 2.
Pretreatment with synthetic triterpenoid, CDDO-Me has no effect on circulating IL-10 following lipopolysaccharide (LPS) challenge. (A) IL-10 levels in pretreated mice. Systemic IL-10 induction was measured from individual plasma samples (n = 6 mice per group) 48 h following pretreatment with low-dose LPS (LPS), vehicle (DMSO), and synthetic triterpenoid (CDDO-Me). (B) IL-10 levels in LPS-challenged mice. Systemic IL-10 was measured in plasma samples from the indicated experimental groups 5 h after LPS challenge. Cytokine levels (mean ± SEM) for each experimental group from 1 of 3 independent experiments are shown. Indicated P values (unpaired, 2-tailed t-tests) are mean comparisons between groups pretreated with either low-dose LPS (LPS) or synthetic triterpenoid (CDDO-Me) and between LPS-pretreated and control groups unless otherwise indicated. NS = not statistically significant (P > 0.05).
CDDO-Me pretreatment preserves splenocyte immune cell populations
In mice pretreated with low-dose LPS, decreases in TNF-α-inducible IL-12 and IL-12-dependent IFN-γ correlate with DC and natural killer (NK) cell depletion, respectively (Balkhy and Heinzel 1999). Therefore, we measured splenocyte immune cell populations using flow cytometry to define the effects of CDDO-Me pretreatment on levels of immune cells in spleen. Spleens were harvested 48 h following the last administration of low-dose LPS, DMSO (vehicle), and CDDO-Me. Spleens from age-matched, untreated mice served as controls. Spleens were pooled from each experimental group (n = 4 spleens per group), and innate and adaptive immune cell populations were defined in unselected splenocytes by flow cytometry.
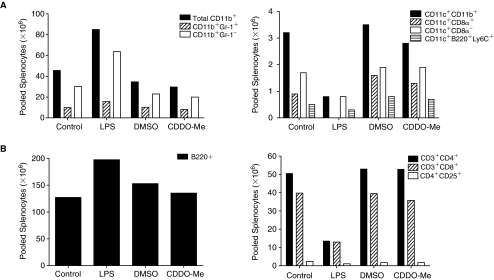
LPS-pretreated mice had marked cachexia and splenomegaly relative to untreated control and CDDO-Me-pretreated animals (Fig. 3). Profiles in splenocyte immune cells differed between mice given low-dose LPS and CDDO-Me (Fig. 4). In comparison with untreated control mice, LPS-pretreated mice had 2-fold increase in splenocyte myeloid (CD11b+) populations, especially macrophages (CD11b+Gr-1-), but 3-fold decrease in splenocyte DC populations, with undetectable levels of lymphoid DCs (CD11c+CD8α+) (Fig. 4A). Decreases in splenocyte NK cells (DX5+) were also measured from LPS-pretreated mice relative to control mice (data not shown). In contrast, mice pretreated with CDDO-Me had similar profiles in myeloid cells, DCs, and NK cells as control animals. With respect to adaptive immune cells, LPS-pretreated mice had increased splenocyte B cells, but markedly reduced T cells in comparison with control mice and mice pretreated with CDDO-Me (Fig. 4B).
FIG. 3.
Lipopolysaccharide (LPS) pretreatment produces changes in whole body (A) and whole spleen (B) weights. Six CD57BL/6 mice comprised each of 4 experimental groups including control (none), LPS-pretreated (LPS), vehicle-pretreated (DMSO), and synthetic triterpenoid-pretreated (CDDO-Me) mice. Forty-eight hours after the last pretreatment dose, mice were individually weighed and also had their respective spleens weighed. Weight data (mean ± SEM) from 1 of 2 independent experiments are shown. Indicated P values (unpaired, 2-tailed t-tests) are mean comparisons between experimental groups.
FIG. 4.
Synthetic triterpenoid, CDDO-Me pretreatment preserves levels of innate and adaptive immune cell populations in spleen, while lipopolysaccharide (LPS) pretreatment reduces splenocyte immune cell populations. (A) Innate immune cell populations. Spleens were harvested from individual mice in each group and pooled (n = 4 spleens per group) prior to being stained with conjugated monoclonal antibodies (MAb) and analyzed using flow cytometry. Data are absolute numbers (× 106) of indicated innate immune cells measured from pooled splenocytes as described in the Materials and Methods. Total myeloid (CD11b+) cells were further classified as macrophages (CD11b+Gr-1-) and polymorphonuclear cells (CD11b+Gr-1+) (Left figure). Total dendritic cells (CD11c+CD11b+) were further classified as myeloid (CD11c+CD8-), lymphoid (CD11c+CD8+), and plasmacytoid (CD11c+B220+Ly6C+) (Right figure). (B) Adaptive immune cell populations. Absolute numbers (× 106) of B cells (B220+) (Left figure) and T-cell subtypes (CD3+CD4+; CD3+CD8+; and CD25 expression on gated CD3+CD4+cells, CD4+CD25+) (Right figure) are shown. Representative results are from 1 of 3 independent experiments.
Together, these results suggest that CDDO-Me pretreatment attenuates the inflammatory cytokine response to LPS challenge without inducing systemic (cachexia) and hematopoietic (splenomegaly with B cell and CD11b+ cell hyperplasia) stress responses. Furthermore, CDDO-Me pretreatment preserves lymphoid immune cell content and overall host integrity.
An increase in splenocyte Th17 and T-regulatory cells follows LPS pretreatment but not pretreatment with CDDO-Me
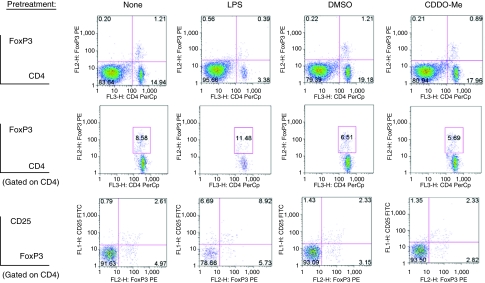
Th17 (CD4+IL-17+) and Treg (CD4+CD25+FoxP3+) cells have critical, opposing roles in inflammatory-mediated immune responses (Iwakura and Ishigame 2006; Weaver and others 2006). We measured differences in splenocyte CD4+ content between LPS- and CDDO-Me-pretreated mice compared with age-matched control mice and mice pretreated with vehicle alone. Specifically, we used IL-17 and FoxP3 intracellular staining to define Th17 and Treg populations, respectively. Increased percentages of Th17 (Fig. 5) and Treg cells (Fig. 6) were observed in spleens of LPS-pretreated mice, but not in mice pretreated with CDDO-Me. Differences in splenocyte Th17 and Treg cells between LPS- and CDDO-Me-pretreated groups persisted following LPS challenge (data not shown). These results further support the distinction in lymphoid response between LPS pretreatment and CDDO-Me pretreatment, particularly with respect to CD4+ effector cells; as CDDO-Me pretreatment had no effect on inducing or reducing Th17 and Treg cells.
FIG. 5.
Splenocyte CD4+IL-17+ (Th17) cells are increased following lipopolysaccharide (LPS) pretreatment, but are not increased following synthetic triterpenoid, CDDO-Me pretreatment. Whole spleens from individual mice (n = 1 spleen per indicated pretreatment group) were used to define splenocyte Th17 cell content. Indicated percentages are percentages of total cells as determined by analytical software. Relevant isotype controls were used to set the illustrated region gates (data not shown). Axes to histograms for each row are indicated to the left of the histograms. Representative results are from 1 of 3 independent experiments.
FIG. 6.
Splenocyte CD4+CD25+FoxP3+ (Treg) cells are increased in lipopolysaccharide (LPS)-pretreated mice, but not in synthetic triterpenoid, CDDO-Me-pretreated mice. Whole individual spleens (n = 1 spleen per group) from each of the indicated pretreatment groups were used to measure Treg cells. Indicated percentages are percentages of total cells as determined by analytical software. Relevant isotype controls were used to set the illustrated region gates (data not shown). Axes to histograms for each row are indicated to the left of the histograms. Representative results are from 1 of 3 independent experiments.
CDDO-Me pretreatment in vivo does not modulate TLR-inducible cytokine production in vitro
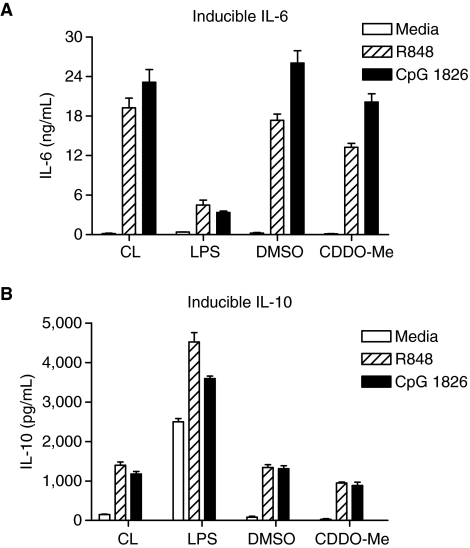
To determine how observed differences in splenocyte immune cell populations influenced cytokine expression, we next examined cytokine induction profiles from splenocyte cultures of LPS- and CDDO-Me-pretreated mice. We compared TLR-inducible cytokines from cell cultures containing unselected splenocytes from control, vehicle-, LPS-, and CDDO-Me-pretreated mice. We used TLR7/8 agonist, R848, and TLR9 agonist, CpG ODN 1826, as cross-tolerant cell-culture stimulants as described in the Materials and Methods (Sato and others 2002; Dalpke and others 2005). We hypothesized that splenocyte cultures from either LPS- or CDDO-Me-pretreated mice would have diminished production of TLR-inducible proinflammatory cytokines (IL-6) and an increase in inducible anti-inflammatory cytokines (IL-10) when compared with stimulated control splenocyte cultures.
In splenocyte cultures from LPS-pretreated mice, TLR-inducible IL-6 levels were lower than levels measured from control splenocyte cultures (Fig. 7A). However, IL-10 levels in LPS-pretreated splenocyte cultures were higher than levels in TLR-stimulated control splenocyte cultures (Fig. 7B), correlating with higher circulating IL-10 levels in these mice (Fig. 2). Surprisingly, levels of inducible IL-6 and IL-10 measured in supernatants of TLR-stimulated splenocyte cultures from CDDO-Me-pretreated mice were similar to control levels.
FIG. 7.
Levels of TLR-inducible IL-6 (A) and IL-10 (B) differ between lipopolysaccharide (LPS)-pretreated mice and mice pretreated with synthetic triterpenoid, CDDO-Me. Levels in inducible IL-6 and IL-10 (mean ± SD) were measured from supernatants of splenocyte cultures from control (CL), LPS (LPS)-, vehicle (DMSO)-, and CDDO-Me pretreated mice. Each stimulation condition was performed in triplicate. Unselected splenocytes (1 × 107 cells/mL) were stimulated with media alone (TCM/1%NDM) or media containing R848 (Resiquimod) (1 μg/ml) or CpG ODN 1826 (10 μg/ml). Twenty-four hour supernatants were collected for ELISA. Mean inducible levels of IL-6 and IL-10 among experimental groups and across stimulation conditions were significantly different (P < 0.0001, 2-way ANOVA).
Both LPS and CDDO-Me pretreatments induce HO-1
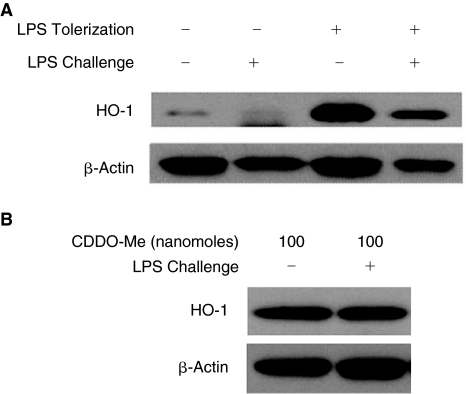
The synthetic triterpenoids potently induce HO-1 expression (Dinkova-Kostova and others 2005). In addition, the anti-inflammatory properties of the synthetic triterpenoids are intrinsically linked to their ability to induce HO-1 (Liby and others 2005). Therefore, we measured HO-1 expression from splenocyte lysates from experimental groups in order to correlate the observed attenuation in inflammatory cytokine levels with HO-1 induction. We also compared HO-1 expression between groups pretreated with low-dose LPS and CDDO-Me and their respective LPS-challenged counterparts to determine if HO-1 induction was preserved following LPS challenge.
HO-1 expression was increased in lysates from LPS-pretreated mice and this increase persisted in mice subsequently challenged with LPS (Fig. 8A). As predicted, pretreatment with CDDO-Me induced HO-1 expression (Fig. 8B). The increase in HO-1 observed in lysates from mice pretreated with CDDO-Me also persisted following a subsequent challenge with LPS. Together, these data are consistent with the hypothesis that HO-1 induction assumes an important role in modulating the immune response to LPS challenge following LPS and CDDO-Me pretreatments.
FIG. 8.
Heme oxygenase 1 (HO-1) protein expression is increased in both lipopolysaccharide (LPS) (A)- and synthetic triterpenoid, CDDO-Me-pretreated (B) mice. Spleen lysates of indicated experimental groups were prepared and analyzed by Western blots as described in the Materials and Methods. Whole spleens (n = 1 spleen per indicated pretreatment group) were individually collected and processed from LPS-pretreated and from CDDO-Me-pretreated mice 48 h after the last pretreatment as well as from LPS- and CDDO-Me-pretreated mice 5 h following LPS challenge. Relative band densities for HO-1 were calculated using band densitometry software (ImageJ Software) and used to standardize HO-1 expression relative to protein loading to confirm HO-1 expression (data not shown).
Pretreatment with CDDO-Me protects mice from lethal-dose LPS challenge
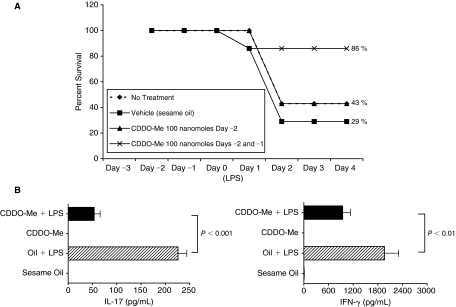
Pretreatment with CDDO-Me significantly attenuated inflammatory cytokine induction following LPS challenge (Fig. 1). Therefore, we hypothesized that CDDO-Me would prevent endotoxic death in mice challenged with lethal-dose LPS (LD50 = 850 μg, ~40 mg/kg). For survival studies, CDDO-Me was suspended in sesame oil and given by oral gavage at doses up to 200 nmol, a dose at which plasma levels of CDDO-Me are known to be similar to those achieved with a single IP dose of 100 nmol; as the drug is ~60% orally bioavailable.
We compared survival in mice receiving 2 consecutive daily doses of 100 nmol CDDO-Me prior to challenge with lethal-dose LPS to survival in mice giving either lethal-dose LPS alone, vehicle alone followed by LPS challenge, or one daily 100 nmol dose of CDDO-Me prior to lethal-dose LPS challenge. As shown in Figure 9A, mice pretreated with 200 nmol CDDO-Me had a 86% survival advantage compared with mice pretreated with vehicle alone (29%) or mice pretreated with 100 nmol CDDO-Me (43%). In addition, protection conferred by 200 nmol gavage dose of CDDO-Me (Fig. 9B) correlated with an ability to repress LPS-induced cytokines in a manner equivalent to that observed with a 100 nmol IP dose of CDDO-Me (Fig. 1).
FIG. 9.
(A) Synthetic triterpenoid, CDDO-Me protects mice against lethal-dose lipopolysaccharide (LPS) challenge. Mice were either pretreated with 100 nmol CDDO-Me on Day -2 or with 100 nmol CDDO-Me on Days -2 and -1 (total pretreatment dose = 200 nmol) prior to lethal-dose LPS challenge (LD50 = 850 μg single IP injection, 20 mg/kg) on Day 0. Untreated control mice and vehicle-only mice (sesame oil, 200 μL gavages on Days -2 and -1) also received lethal-dose LPS on Day 0. Percent survival was determined for 4 days following LPS challenge, and mice surviving on Day 4 were euthanized according to protocol. Each experimental group contained 7 mice at the start of the experiment. Representative results are from 1 of 2 independent experiments. (B) Survival advantage following 200 nmol pretreatment associates with reduction in circulating IL-17 and IFN-γ following LPS challenge. IL-17 and IFN-γ levels were measured from plasma of mice pretreated with vehicle (sesame oil) or CDDO-Me (200 nmol) alone and from vehicle- and CDDO-Me-pretreated mice 5 h after LPS challenge (300 μg). Cytokine levels from mice pretreated with vehicle or CDDO-Me alone were below level of detection. Cytokine levels (mean ± SEM) from 1 experiment (n = 6 mice per experimental group) are shown. Indicated P values (unpaired, 2-tailed t-tests) are mean comparisons between experimental groups.
Collectively, these results suggest that pretreatment with CDDO-Me likely protects against endotoxemia through mechanisms involving induction in HO-1 and reduction in inflammatory cytokine production.
Discussion
Therapies modulating immune cell function may offer protection against overwhelming sepsis following exposure to endotoxin. The synthetic triterpenoids are novel antiproliferative and anti-inflammatory agents that inhibit NF-κB activation, regulate JAK/STAT signaling, and induce HO-1 expression. The data presented here show that pretreatment with the synthetic triterpenoid, CDDO-Me, significantly reduces the in vivo inflammatory cytokine response following LPS challenge, induces HO-1 protein expression in the spleen, and protects mice against lethal-dose LPS. Notably, CDDO-Me pretreatment protects against a lethal LPS challenge without altering immune cell subsets numbers or distribution, yet maintains inducible cytokine profiles similar to non-LPS-exposed control animals, effects that contrast to low-dose LPS pretreatment. Together, these results suggest that CDDO-Me confers protection against endotoxemia by modulating the immune response to LPS in a way that differs from the complex biological phenotype of low-dose LPS pretreatment (ie, endotoxin tolerance) (West and Heagy 2002).
Levels in systemic JAK/STAT-dependent cytokines, including IFN-γ (JAK1/STAT1), IL-6 (JAK3/STAT3), and IL-12 (Tyk2/STAT4), were significantly reduced in CDDO-Me-pretreated mice and subsequently challenged with LPS. Our results show for the first time that CDDO-Me reduces systemic IL-17 (STAT3) (Chen and others 2007) and IL-23 (Tyk2/STAT3) production following LPS challenge. One potential explanation for decreased induction in JAK/STAT-dependent cytokines may involve up-regulation of STAT inhibitors like SOCS1 (Nakagawa and others 2002). For example, CDDO-Im induces SOCS1 and SHP-1 in human melanoma and lung cancer cells, which correlate with dose-dependent suppression in constitutive and IL-6-inducible STAT3 and STAT5 phosphorylation and inhibition of the JAK kinase, Tyk2 (Liby and others 2006). An additional mechanism for suppression in proinflammatory induction and preservation in levels of immune cells may be HO-1 induction, as demonstrated in splenocyte lysates in mice pretreated with CDDO-Me. HO-1 has established anti-inflammatory and antioxidant roles (Otterbein and Choi 2000; Ryter and others 2006) and also can modulate immune cell function, including LPS-inducible DC maturation and proinflammatory cytokine production (Chauveau and others 2005) and potentially regulatory cell function (Brusko and others 2005).
Induction of HO-1 requires the transcription factor, nuclear factor erythroid 2-related factor 2 (Nrf2). Once released from its cytoplasmic negative regulator, Keap1, Nrf2 translocates to the nucleus, heterodimerizes with other proteins like Mafs, and then binds to antioxidant response elements (ARE) (Otterbein and others 2000) to induce HO-1 expression (Zhang and Gordon 2004). HO-1 induction by CDDO-Me is dependent upon the Keap1-Nrf2-ARE-signaling pathway (Liby and others 2005). HO-1 plays a critical role in protecting against LPS-induced end organ damage and mortality (Wiesel and others 2000). For example, HO-1-deficient splenocytes produce 20- to 40-fold higher levels of proinflammatory cytokines after LPS stimulation in culture (Kapturczak and others 2004). Furthermore, Nrf2-deficient mice have increased susceptibility to endotoxin and cecal ligation and puncture-induced sepsis due to augmented expression of NF-κB-dependent proinflammatory gene expression (Thimmulappa and others 2006a).
Despite the established role of HO-1 in conferring protection against LPS challenge and the proven ability of the synthetic triterpenoids to induce HO-1, published experience using these novel compounds in the context of LPS challenge is limited. In one study, CDDO-Im increases Nrf2 activity in murine neutrophils, reduces production in both LPS-inducible ROS and inflammatory cytokines and chemokines, and protects against lethality (Thimmulappa and others 2006b). The data presented here extend this experience by demonstrating that CDDO-Me pretreatment induces HO-1 expression in lymphoid organs, that HO-1 induction correlates with reduction in systemic levels of proinflammatory cytokines, and that enteral administration of CDDO-Me can protect mice against lethal LPS challenge.
CDDO-Me pretreatment associates with a disparity in levels of systemic and splenocyte IL-6 induction following in vivo LPS challenge and in vitro TLR stimulation, respectively, which contrasts with similar attenuation in levels of systemic and splenocyte IL-6 induction following low-dose LPS pretreatment. However, LPS pretreatment leads to a reduction in splenocyte populations like DCs (Wysocka and others 2001) and such effects on cell depletion or function likely contribute to decreased IL-6 induction in this setting (West and Heagy 2002).
We have previously noted dissociation between hematopoietic (splenocyte culture) and systemic (plasma) IL-6 levels following in vitro and in vivo TLR challenge in the context of murine syngeneic bone marrow transplantation (Auletta and others 2004). In these experiments, wild-type (WT) transplant recipient mice receiving IL-6 KO bone marrow (KO BM into WT mice) had near absent IL-6 induction from splenocyte cultures following CpG 1826 stimulation, but retained non-transplant, control-level plasma IL-6 induction following in vivo LPS challenge. In contrast, IL-6 KO recipients receiving WT BM (WT BM into KO mice) had measurable CpG-inducible IL-6 induction from splenocyte cultures, but near absent plasma IL-6 levels following LPS challenge. These data indicate that non-hematopoietic tissues are a significant source for systemic IL-6, which can be modulated directly by CDDO-Me. In this regard, CDDO has recently been shown to decrease NF-κB activation and increase Nrf2 activation in pulmonary epithelium, resulting in decreased levels of IL-1β, IL-6, and TNF-α measured in bronchoalveolar lavages from mice challenged with LPS (Nichols and others 2009).
In conclusion, the synthetic triterpenoid, CDDO-Me, targets inflammatory cytokine production through negative regulation of cytokine-signaling cascades, including inhibition of NF-κB-dependent gene transcription, and the induction of HO-1 in both hematopoietic and non-hematopoietic cells. Furthermore, in preserving immune cell function by not inducing a generalized state of immune deactivation (Yadavalli and others 2001) or immune suppression (Lionakis and Kontoyiannis 2003; Mueller 2008), CDDO-Me could potentially assume a therapeutically advantageous role in modulating LPS-mediated disease without compromising host defense.
Acknowledgments
The authors wish to acknowledge Dr. Michael Sporn (Dartmouth College) for supplying the synthetic triterpenoid for early experiments, Fong Xu and Janet Robinson for technical support, and the Cytometry Core Facility of the Case Comprehensive Cancer Center for its supportive resources (P30 CA 43703). Research support was provided by the National Institutes of Health (AI57801 J.J.A.), by a sponsored research agreement between Case Western Reserve University and Reata Pharmaceuticals, Inc. (J.J.A.), and by the Center for Stem Cell and Regenerative Medicine at Case Western Reserve University (J.J.A.).
Author Disclosure Statement
C.J.M. is a paid employee and share option holder of Reata Pharmaceuticals, Inc. All other authors have no competing financial interests.
References
- Ahmad R. Raina D. Meyer C. Kharbanda S. Kufe D. Triterpenoid CDDO-Me blocks the NF-kappaB pathway by direct inhibition of IKKbeta on Cys-179. J Biol Chem. 2006;281(47):35764–35769. doi: 10.1074/jbc.M607160200. [DOI] [PubMed] [Google Scholar]
- Akira S. Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Akira S. Takeda K. Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2(8):675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- Asehnoune K. Strassheim D. Mitra S. Kim JY. Abraham E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J Immunol. 2004;172(4):2522–2529. doi: 10.4049/jimmunol.172.4.2522. [DOI] [PubMed] [Google Scholar]
- Auletta JJ. Devecchio JL. Ferrara JL. Heinzel FP. Distinct phases in recovery of reconstituted innate cellular-mediated immunity after murine syngeneic bone marrow transplantation. Biol Blood Marrow Transplant. 2004;10(12):834–847. doi: 10.1016/j.bbmt.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Balkhy HH. Heinzel FP. Endotoxin fails to induce IFN-gamma in endotoxin-tolerant mice: deficiencies in both IL-12 heterodimer production and IL-12 responsiveness. J Immunol. 1999;162(6):3633–3638. [PubMed] [Google Scholar]
- Bhattacharyya J. Biswas S. Datta AG. Mode of action of endotoxin: role of free radicals and antioxidants. Curr Med Chem. 2004;11(3):359–368. doi: 10.2174/0929867043456098. [DOI] [PubMed] [Google Scholar]
- Brusko TM. Wasserfall CH. Agarwal A. Kapturczak MH. Atkinson MA. An integral role for heme oxygenase-1 and carbon monoxide in maintaining peripheral tolerance by CD4+CD25+ regulatory T cells. J Immunol. 2005;174(9):5181–5186. doi: 10.4049/jimmunol.174.9.5181. [DOI] [PubMed] [Google Scholar]
- Chauveau C. Rémy S. Royer PJ. Hill M. Tanguy-Royer S. Hubert FX. Tesson L. Brion R. Beriou G. Gregoire M. Josien R. Cuturi MC. Anegon I. Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL-10 expression. Blood. 2005;106(5):1694–1702. doi: 10.1182/blood-2005-02-0494. [DOI] [PubMed] [Google Scholar]
- Chen Z. Laurence A. O'Shea JJ. Signal transduction pathways and transcriptional regulation in the control of Th17 differentiation. Semin Immunol. 2007;19(6):400–408. doi: 10.1016/j.smim.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalpke AH. Lehner MD. Hartung T. Heeg K. Differential effects of CpG-DNA in Toll-like receptor-2/-4/-9 tolerance and cross-tolerance. Immunology. 2005;116(2):203–212. doi: 10.1111/j.1365-2567.2005.02211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkova-Kostova AT. Liby KT. Stephenson KK. Holtzclaw WD. Gao X. Suh N. Williams C. Risingsong R. Honda T. Gribble GW. Sporn MB. Talalay P. Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc Natl Acad Sci USA. 2005;102(12):4584–4589. doi: 10.1073/pnas.0500815102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escoll P. del Fresno C. García L. Vallés G. Lendínez MJ. Arnalich F. López-Collazo E. Rapid up-regulation of IRAK-M expression following a second endotoxin challenge in human monocytes and in monocytes isolated from septic patients. Biochem Biophys Res Commun. 2003;311(2):465–472. doi: 10.1016/j.bbrc.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Fan H. Cook JA. Molecular mechanisms of endotoxin tolerance. J Endotoxin Res. 2004;10(2):71–84. doi: 10.1179/096805104225003997. [DOI] [PubMed] [Google Scholar]
- Greene JA. DeVecchio JL. Gould MP. Auletta JJ. Heinzel FP. In vivo and in vitro regulation of type I IFN synthesis by synergistic effects of CD40 and type II IFN. J Immunol. 2006;176(10):5995–6003. doi: 10.4049/jimmunol.176.10.5995. [DOI] [PubMed] [Google Scholar]
- Honda T. Rounds BV. Bore L. Finlay HJ. Favaloro FG., Jr Suh N. Wang Y. Sporn MB. Gribble GW. Synthetic oleanane and ursane triterpenoids with modified rings A and C: a series of highly active inhibitors of nitric oxide production in mouse macrophages. J Med Chem. 2000;43(22):4233–4246. doi: 10.1021/jm0002230. [DOI] [PubMed] [Google Scholar]
- Honda T. Rounds BV. Gribble GW. Suh N. Wang Y. Sporn MB. Design and synthesis of 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, a novel and highly active inhibitor of nitric oxide production in mouse macrophages. Bioorg Med Chem Lett. 1998;8(19):2711–2714. doi: 10.1016/s0960-894x(98)00479-x. [DOI] [PubMed] [Google Scholar]
- Iwakura Y. Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116(5):1218–1222. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapturczak MH. Wasserfall C. Brusko T. Campbell-Thompson M. Ellis TM. Atkinson MA. Agarwal A. Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse. Am J Pathol. 2004;165(3):1045–1053. doi: 10.1016/S0002-9440(10)63365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MO. Wan YY. Sanjabi S. Robertson AK. Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- Liby K. Hock T. Yore MM. Suh N. Place AE. Risingsong R. Williams CR. Royce DB. Honda T. Honda Y. Gribble GW. Hill-Kapturczak N. Agarwal A. Sporn MB. The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling. Cancer Res. 2005;65(11):4789–4798. doi: 10.1158/0008-5472.CAN-04-4539. [DOI] [PubMed] [Google Scholar]
- Liby K. Voong N. Williams CR. Risingsong R. Royce DB. Honda T. Gribble GW. Sporn MB. Letterio JJ. The synthetic triterpenoid CDDO-Imidazolide suppresses STAT phosphorylation and induces apoptosis in myeloma and lung cancer cells. Clin Cancer Res. 2006;12(14 Pt 1):4288–4293. doi: 10.1158/1078-0432.CCR-06-0215. [DOI] [PubMed] [Google Scholar]
- Lionakis MS. Kontoyiannis DP. Glucocorticoids and invasive fungal infections. Lancet. 2003;362(9398):1828–1838. doi: 10.1016/S0140-6736(03)14904-5. [DOI] [PubMed] [Google Scholar]
- Mueller NJ. New immunosuppressive strategies and the risk of infection. Transpl Infect Dis. 2008;10(6):379–384. doi: 10.1111/j.1399-3062.2008.00346.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa R. Naka T. Tsutsui H. Fujimoto M. Kimura A. Abe T. Seki E. Sato S. Takeuchi O. Takeda K. Akira S. Yamanishi K. Kawase I. Nakanishi K. Kishimoto T. SOCS-1 participates in negative regulation of LPS responses. Immunity. 2002;17(5):677–687. doi: 10.1016/s1074-7613(02)00449-1. [DOI] [PubMed] [Google Scholar]
- Nichols DP. Ziady AG. Shank SL. Eastman JF. Davis PB. The triterpenoid CDDO limits inflammation in preclinical models of cystic fibrosis lung disease. Am J Physiol Lung Cell Mol Physiol. 2009;297(5):L828–L836. doi: 10.1152/ajplung.00171.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura F. Akashi S. Sakao Y. Sato S. Kawai T. Matsumoto M. Nakanishi K. Kimoto M. Miyake K. Takeda K. Akira S. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface toll-like receptor 4 expression. J Immunol. 2000;164(7):3476–3479. doi: 10.4049/jimmunol.164.7.3476. [DOI] [PubMed] [Google Scholar]
- Otterbein LE. Bach FH. Alam J. Soares M. Tao Lu H. Wysk M. Davis RJ. Flavell RA. Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6(4):422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- Otterbein LE. Choi AM. Heme oxygenase: colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol. 2000;279(6):L1029–L1037. doi: 10.1152/ajplung.2000.279.6.L1029. [DOI] [PubMed] [Google Scholar]
- Pestka S. Krause CD. Sarkar D. Walter MR. Shi Y. Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–979. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- Pinsky MR. Dysregulation of the immune response in severe sepsis. Am J Med Sci. 2004;328(4):220–229. doi: 10.1097/00000441-200410000-00005. [DOI] [PubMed] [Google Scholar]
- Pulendran B. Lingappa J. Kennedy MK. Smith J. Teepe M. Rudensky A. Maliszewski CR. Maraskovsky E. Developmental pathways of dendritic cells in vivo: distinct function, phenotype, and localization of dendritic cell subsets in FLT3 ligand-treated mice. J Immunol. 1997;159(5):2222–2231. [PubMed] [Google Scholar]
- Ryter SW. Alam J. Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86(2):583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- Sallusto F. Palermo B. Lenig D. Miettinen M. Matikainen S. Julkunen I. Forster R. Burgstahler R. Lipp M. Lanzavecchia A. Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur J Immunol. 1999;29(5):1617–1625. doi: 10.1002/(SICI)1521-4141(199905)29:05<1617::AID-IMMU1617>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Sato S. Takeuchi O. Fujita T. Tomizawa H. Takeda K. Akira S. A variety of microbial components induce tolerance to lipopolysaccharide by differentially affecting MyD88-dependent and -independent pathways. Int Immunol. 2002;14(7):783–791. doi: 10.1093/intimm/dxf046. [DOI] [PubMed] [Google Scholar]
- Shishodia S. Sethi G. Konopleva M. Andreeff M. Aggarwal BB. A synthetic triterpenoid, CDDO-Me, inhibits IkappaBalpha kinase and enhances apoptosis induced by TNF and chemotherapeutic agents through down-regulation of expression of nuclear factor kappaB-regulated gene products in human leukemic cells. Clin Cancer Res. 2006;12(6):1828–1838. doi: 10.1158/1078-0432.CCR-05-2044. [DOI] [PubMed] [Google Scholar]
- Suh N. Honda T. Finlay HJ. Barchowsky A. Williams C. Benoit NE. Xie QW. Nathan C. Gribble GW. Sporn MB. Novel triterpenoids suppress inducible nitric oxide synthase (iNOS) and inducible cyclooxygenase (COX-2) in mouse macrophages. Cancer Res. 1998;58(4):717–723. [PubMed] [Google Scholar]
- Suh N. Roberts AB. Birkey Reffey S. Miyazono K. Itoh S. ten Dijke P. Heiss EH. Place AE. Risingsong R. Williams CR. Honda T. Gribble GW. Sporn MB. Synthetic triterpenoids enhance transforming growth factor beta/Smad signaling. Cancer Res. 2003;63(6):1371–1376. [PubMed] [Google Scholar]
- Suh N. Wang Y. Honda T. Gribble GW. Dmitrovsky E. Hickey WF. Maue RA. Place AE. Porter DM. Spinella MJ. Williams CR. Wu G. Dannenberg AJ. Flanders KC. Letterio JJ. Mangelsdorf DJ. Nathan CF. Nguyen L. Porter WW. Ren RF. Roberts AB. Roche NS. Subbaramaiah K. Sporn MB. A novel synthetic oleanane triterpenoid, 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, with potent differentiating, antiproliferative, and anti-inflammatory activity. Cancer Res. 1999;59(2):336–341. [PubMed] [Google Scholar]
- Thimmulappa RK. Lee H. Rangasamy T. Reddy SP. Yamamoto M. Kensler TW. Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest. 2006a;116(4):984–995. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmulappa RK. Scollick C. Traore K. Yates M. Trush MA. Liby KT. Sporn MB. Yamamoto M. Kensler TW. Biswal S. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochem Biophys Res Commun. 2006b;351(4):883–889. doi: 10.1016/j.bbrc.2006.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver CT. Harrington LE. Mangan PR. Gavrieli M. Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24(6):677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- West MA. Clair L. Kraatz J. Rodriguez JL. Endotoxin tolerance from lipopolysaccharide pretreatment induces nuclear factor-kappaB alterations not present in C3H/HeJ mice. J Trauma. 2000;49(2):298–305. doi: 10.1097/00005373-200008000-00018. [DOI] [PubMed] [Google Scholar]
- West MA. Heagy W. Endotoxin tolerance: A review. Crit Care Med. 2002;30(1 Supp):S64–S73. [PubMed] [Google Scholar]
- Wiesel P. Patel AP. DiFonzo N. Marria PB. Sim CU. Pellacani A. Maemura K. LeBlanc BW. Marino K. Doerschuk CM. Yet SF. Lee ME. Perrella MA. Endotoxin-induced mortality is related to increased oxidative stress and end-organ dysfunction, not refractory hypotension, in heme oxygenase-1-deficient mice. Circulation. 2000;102(24):3015–3022. doi: 10.1161/01.cir.102.24.3015. [DOI] [PubMed] [Google Scholar]
- Wysocka M. Robertson S. Riemann H. Caamano J. Hunter C. Mackiewicz A. Montaner LJ. Trinchieri G. Karp CL. IL-12 suppression during experimental endotoxin tolerance: dendritic cell loss and macrophage hyporesponsiveness. J Immunol. 2001;166(12):7504–7513. doi: 10.4049/jimmunol.166.12.7504. [DOI] [PubMed] [Google Scholar]
- Xia ZW. Zhong WW. Xu LQ. Sun JL. Shen QX. Wang JG. Shao J. Li YZ. Yu SC. Heme oxygenase-1-mediated CD4+CD25high regulatory T cells suppress allergic airway inflammation. J Immunol. 2006;177(9):5936–5945. doi: 10.4049/jimmunol.177.9.5936. [DOI] [PubMed] [Google Scholar]
- Yadavalli GK. Auletta JJ. Gould MP. Salata RA. Lee JH. Heinzel FP. Deactivation of the innate cellular immune response following endotoxic and surgical injury. Exp Mol Pathol. 2001;71(3):209–221. doi: 10.1006/exmp.2001.2387. [DOI] [PubMed] [Google Scholar]
- Yore MM. Liby KT. Honda T. Gribble GW. Sporn MB. The synthetic triterpenoid 1-[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole blocks nuclear factor-kappaB activation through direct inhibition of IkappaB kinase beta. Mol Cancer Ther. 2006;5(12):3232–3239. doi: 10.1158/1535-7163.MCT-06-0444. [DOI] [PubMed] [Google Scholar]
- Zhang X. Shan P. Qureshi S. Homer R. Medzhitov R. Noble PW. Lee PJ. Cutting edge: TLR4 deficiency confers susceptibility to lethal oxidant lung injury. J Immunol. 2005;175(8):4834–4838. doi: 10.4049/jimmunol.175.8.4834. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Gordon GB. A strategy for cancer prevention: stimulation of the Nrf2-ARE signaling pathway. Mol Cancer Ther. 2004;3(7):885–893. [PubMed] [Google Scholar]