Abstract
Breast cancer progression is driven by altered gene expression. We show that the RIN1 gene, which encodes a RAS effector regulating epithelial cell properties, is silenced in breast tumor cell lines compared to cultured human mammary epithelial cells. We also report that RIN1 is often reduced in human breast tumor cells compared to morphologically normal breast glandular cells. At least two silencing mechanisms appear to be involved. Overexpression of the transcription repressor SNAI1 (Snail) was observed in ZR75-1 cells, and SNAI1 knockdown restored RIN1 expression. In addition, DNA methylation within the RIN1 promoter and the first exon in KPL-1 cells suggested that epigenetic modifications may contribute to silencing, and demethylation was shown to restore RIN1 expression. Re-expression of RIN1 was shown to inhibit anchorage independent growth in soft agar. In addition, RIN1 expression inhibited both the initiation and progression of tumorigenesis for two breast tumor cell lines in a mouse model, consistent with a tumor suppressor function. We also show that RIN1 acts as a negative regulator of tumor cell invasive growth and that this requires the ABL kinase signaling function of RIN1, suggesting a mechanism through which RIN1 silencing may contribute to breast cancer progression.
Keywords: RAS, RIN1, Snail, breast cancer, tumor suppressor, invasive growth
Introduction
Among the most common loss-of-function events reported for breast cancers are mutations in TP53 and PTEN (1), with less frequent mutations reported in several other tumor suppressor genes (reviewed in (2)). In addition to these inactivation mutations, there appears to be a common role for transcriptional silencing of tumor suppressor genes during breast tumor progression and metastasis. Several mechanisms of gene silencing during tumorigenesis have been described. Covalent modification of DNA (e.g. CpG methylation) and/or histones (e.g. methylation, deacetylation) can greatly reduce the rate of transcription initiation for some target genes. Target genes may also be silenced by a reduction in transcription activator proteins or by overexpression of transcription repressor proteins. CDH1 (E-cadherin), which encodes a component of adherens junctions and promotes epithelial over mesenchymal functions during normal development and transformation (reviewed in (3)), is subject to mutations as well as silencing by epigenetic mechanisms during tumor progression (4). In addition, transcriptional repressors such as SNAI1 (Snail), which silence CDH1 and other pro-epithelial genes, are induced during developmental EMT and overexpressed in some metastatic tumor cells ((5) and reviewed in (6)). These observations suggest that the regulated expression of pro-epithelial cell genes, which evolved to orchestrate developmental transitions, can be exploited by tumor cells during expansion and metastasis.
Among the most common events in human tumors are activating mutations in RAS genes (HRAS, KRAS and NRAS). And while only a small fraction of breast tumors have such mutations, these tumors often have elevated RAS signaling due to overexpression of upstream receptor tyrosine kinases such as ERBB2, EGFR, MET and RON (7–10). Signaling pathways downstream of RAS are mediated by at least a half dozen direct RAS effectors (reviewed in (11)). Among these, BRAF and PIK3CA (a.k.a. PI3K), which stimulate mitosis and block apoptosis, respectively, are mutationally activated in a variety of tumors and tumor cells including some breast cancer cell lines (reviewed in (12); Cancer Genome Project). Given the diversity of signals from RAS, and the established role of RAS in development, the contribution of tumor suppressor pathways downstream of RAS also needs to be considered.
The RAS effector RIN1 activates ABL tyrosine kinases and RAB5 GTPases to regulate cytoskeletal remodeling and endocytic pathways that promote normal epithelial functions (13). Silencing of RIN1 leads to increased motility of epithelial cells (14). Here we report that RIN1 expression is silenced in a large proportion of breast tumor cell lines as well as in tumor tissue samples. Further, we identify multiple mechanisms for RIN1 silencing. We directly confirm the breast tumor suppressor function of RIN1 and provide evidence supporting a role for RIN1 in restraining invasive growth of epithelial cells.
Materials and Methods
Cell lines and culture conditions
The breast cancer cell lines MDA-MB-231, BT-549, KPL-1, ZR75-1, T47D, Hs578t, BT20 and BT549 were grown in DMEM with 10% FBS. MCF10A was cultured in DMEM/F12 with hEGF (20ng/ml), hydrocortisone (500 ng/ml), insulin (10 μg/ml), cholera toxin (100 ng/ml) and 5% equine serum. Normal human mammary epithelial cells (HMECs) were obtained from Clonetics and were cultured in MEGM (Clonetics). Lentivirus stock production and viral transduction of cells was performed as previously described (13). Blasticidin (Invitrogen) was used at 20 μg/ml for selection of transduced cells. Zebularine (obtained from the National Cancer Institute) was used at 150 ng/ml.
For soft agar growth assays, 1 x 104 breast tumor cells were seeded into a 10-cm culture dish containing 0.35% low-melting agarose over a 0.7% agarose layer, both in culture medium, and incubated for 3–5 weeks at 37°C. Colonies were then stained with MTT (250 μg/ml) and counted.
Invasive growth assays were performed using modified Boyden chambers coated with Matrigel (BD Biosciences). The upper chamber was seeded with 2 x 105 cells and these were allowed to migrate at 37°C for 18 hours toward a lower reservoir of DMEM with HGF (10 ng/ml) before fixing (2% paraformaldehyde for 20 min), staining (crystal violet for 1 hr), and counting cells that had passed through the membrane separating the chambers.
RNA preparations and RT-PCR
Total RNA was extracted from breast tumor and normal breast cell lines using TRIzol® reagent (Invitrogen, Life Technologies) following the manufacturer’s protocol. Isolated RNA was then used to synthesize cDNA using an iScript cDNA synthesis kit (Biorad). Real time PCR was performed using the iCycler™ PCR platform (Biorad). Thermal cycling conditions were as follows: an initial incubation at 95 °C for 10 min followed by 40 cycles of 95 °C for 30 s, 55 °C for 1 min and 72° C for 30 s. Followed by a final cycle of 95°C for 1 min, 55 °C for 30 sec and 95°C for 30 s. IQ SYBR Green Supermix (Biorad) was used in accordance with the manufacturer's instructions. The primers used were: RIN1 5’-GGCAGCAGAGGAGTAGCTTGA and 5’-GCTTGCTGGCGCTAAAAGG; SNAI1 5’-GAAAGGCCTTCAACTGCAAA and 5’-TGACATCTGAGTGGGTCTGG; SNAI2 5' ATGAGGAATCTGGCTGCTGT 3' and 5' CAGGAGAAAATGCCTTTGGA 3'; β-actin (ACTB) 5’-CATTGCCGACAGGATGCA and 5’-CGCTCAGGAGGAGCAATGAT.
For tissue-based expression analysis, primary tumor epithelial cells and matching normal epithelial cells were isolated by laser capture microdissection (LCM) from sectioned breast tissue obtained from the UCLA Tissue Procurement Core Laboratory. Mammary epithelial cells were isolated onto LCM transfer film (Arcturus, Mountain View, CA), as described (15) using an LCM microscope (Pixcell IIe, Arcturus). RNA was then purified using the PicoPure RNA isolation kit (Arcturus),
Immunoprecipitations, Immunoblotting and Immunohistochemistry
For semi-quantitative analysis, RIN1 was first immunoprecipitated from breast tumor cell line lysates using polyclonal anti-RIN1 (BD Biosciences) and immunoblotted using monoclonal anti-RIN1 (BD Biosciences). Immunohistochemistry was performed using established protocols (16) with monoclonal anti-RIN1 (1:250 dilution) or polyclonal (rabbit)-anti-human RIN1 (1:400 dilution). Monoclonal anti-CDH1 (E-cadherin) (BD Biosciences) antibodies were used at 1:400 dilution. Non-immune antibody (mouse IgG1) was used as a negative control. Biotinylated Universal Antibody (Vectastain) was used as the secondary stain. Tissue samples were counterstained with hematoxylin and eosin.
Viral constructs, gene silencing and methylation analysis
The self-inactivating lentivirus vector M4 has been previously described (13). M4-blast is a modified version of this vector that includes an SV40 promoter-driven blasticidin resistance cassette derived from pcDNA6 (Invitrogen Life Technologies). The RIN1-directed shRNA lentivirus construct M4-shRIN1-blast was created by first converting the M4 unique BamHI site to a BstBI site and then removing the CMV promoter between the flanking BstBI sites. Oligonucleotide primers, one encoding a RIN1 shRNA, were used to PCR amplify U6 promoter sequence and the resulting BamHI – EcoRI fragment was cloned into pKS (Stratagene) then moved as an XbaI – EcoRI fragment to M4 cut with NheI and EcoRI. A Blasticidin resistance gene cassette from pcDNA6 (Invitrogen) was then inserted into the EcoRI site of the modified M4 vector. The upstream U6 promoter primer (5’– 3’) was GCTGGGATCCCAAGGTCGGGCAGGAAGAGGG -3’ (BamHI underlined). The RIN1-directed shRNAs primers (5’ – 3’) were #753: GCTTCGGCAGCACATATACTACTGGAGGTCTCTGTGGACACGCGCACTTTTTGGA AGGTGTGCGTGTCCACAGAGATCTCCAGTTTTTGAATTCTG (EcoRI) and #2264: GCTTCGGCAGCACATATACTATTCAGCAGTTGTCTCAGACTGTTCCTTGGGGAAT AGTCTGAGACAGCTGCTGAATTTTTGAATTCTG (EcoRI).
SNAI1-directed siRNA (Ambion, cat#AM16708) and scrambled sequence control siRNA (Ambion, cat#4615) were transfected into ZR75-1 cells using siPORT (Ambion).
Methylation sites were identified by sequence analysis of bisulfite-treated genomic DNA and was carried out by Seqwright using the primers:
5’-TGTAAAACGACGGCCAGTTGTGTGGAGGTGGTATTTTTTA –3’ and
5’-CAGGAAACAGCTATGACCCATCCCCAATAAATAACACTTC – 3’.
In Vivo Tumor Formation
In vivo tumor assays were carried out using established protocol (17) with the following modifications. Tumor cells were washed twice with PBS and then incubated in serum-free DMEM for 2 hrs at 37°C. Cells were subsequently incubated with versene: trypsin (4:1 mixture) for 5 minutes. Cells were then collected using serum free DMEM and soybean trypsin inhibitor (Sigma-Aldrich).
Tumor cells (2 x 106 cells transduced with M4-blast or M4-RIN1-blast) were injected into the mammary fat pads of 4–6 week old female nude mice (Charles River Laboratories). Tumor growth was monitored at 3–4 day intervals. Upon completion of the assay, tumors were removed and sectioned. Sectioned tumor tissue was immuno-stained for RIN1 protein using polyclonal anti-RIN1 as described above.
Results
RIN1 is silenced in breast tumor cells
Based on its expression in mammary epithelial cells (13) and its function as a RAS effector, we examined whether RIN1 expression is altered in breast tumors. We initially examined five breast tumor cell lines, all derived from invasive ductal carcinomas, and observed a significant reduction in RIN1 protein levels compared to normal human mammary epithelial cells (Fig. 1A).
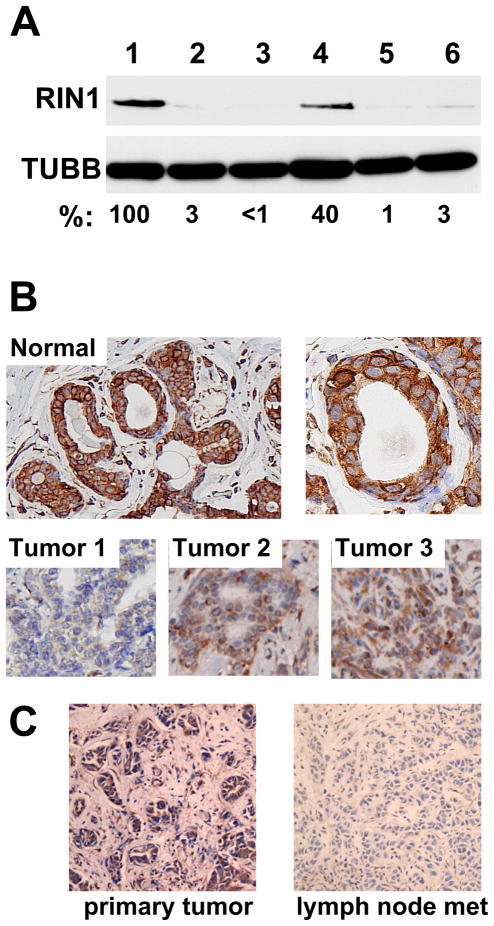
Figure 1.
Reduced levels of RIN1 protein in breast tumor cell lines and tissues. A. Upper panel: RIN1 protein levels determined by immunoprecipitation (polyclonal anti-RIN1) and immunoblot (monoclonal anti-RIN1). Extracts were normalized based on total protein and confirmed by anti-tubulin immunoblot. Cells examined were 1 = HMEC; 2 = T47D; 3 = SKBR3; 4 = MBA-MD-231; 5 = BT20; 6 = MCF7. Protein levels, as a percent of HMEC control, are indicated. B. Immunohistochemical staining of RIN1 in normal human mammary tissue (top) and three breast tumor samples (bottom). The same staining protocol applied to embedded MCF10A and KPL-1 cells was performed to validate specificity (Fig. S1A) C. Immunohistochemical staining of RIN1 in a primary ductal carcinoma (left) and in one of sixteen involved lymph nodes from the same patient (right). Both images obtained with 200X magnification.
In normal mammary tissue, RIN1 protein is enriched in ductal epithelial cells with localization to the cytoplasm and plasma membrane (Fig. 1B). Consistent with the tumor cell line analysis, we observed reduced levels of RIN1 in primary breast tumor cells, although the degree and extent of silencing was not uniform (Fig. 1B). The same technique applied to cell lines confirmed staining specificity (Fig. S1A). These findings indicate that reduced RIN1 protein levels occur in breast tumor tissue as well as cell lines derived from such tumors. In addition, lymph node metastatic tumors had RIN1 levels that were low and generally weaker than primary tumors from the same patients (Fig. 1C).
We next examined whether lower RIN1 protein levels in tumor cells reflected a decrease in RIN1 mRNA. RIN1 transcript levels from 9 breast tumor cell lines and normal human mammary epithelial cells were quantified by real time PCR. The normalized results indicated consistent silencing of RIN1 message in these established breast tumor cell lines (Fig. 2A).
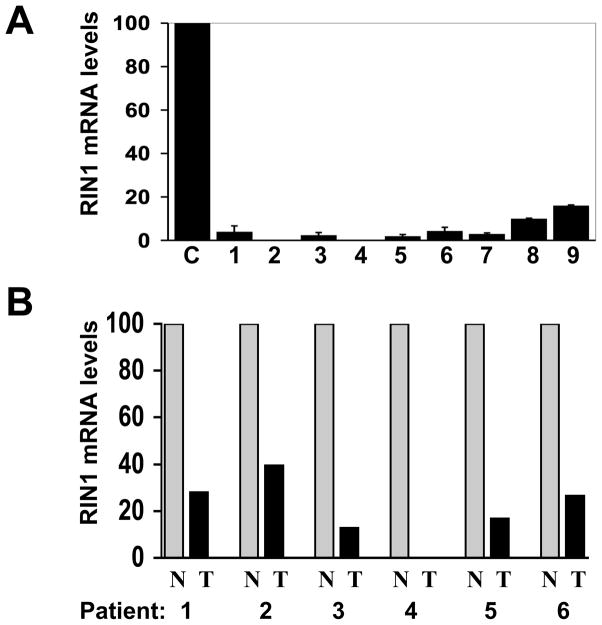
Figure 2.
RIN1 expression is reduced in breast tumor cell lines and tissues. A. Real Time PCR quantification of RIN1 message levels. Cell examined were C = HMEC; 1 = T47D; 2 = Hs578t; 3 = SKBR3; 4 = BT549; 5 = ZR75-1; 6 = BT20; 7 = KPL-1; 8 = MCF7 and 9 = MBA-MD-231. Standard error was calculated from two independent evaluations. B. RIN1 message levels in laser microdissected normal (N) and tumor (T) breast tissue from invasive ductal carcinomas removed from six patients.
We also tested whether RIN1 message levels were reduced in primary human breast tumor samples compared to glandular epithelial cells from normal human breast tissue. Mammary epithelial cells collected using LCM from tumor and surrounding normal tissue were analyzed by real time PCR. All six of the patient samples that were tested had reduced RIN1 transcript levels in tumor compared with surrounding normal mammary epithelial tissue (Fig. 2B), indicating that RIN1 silencing occurs at a high frequency in human breast malignancies. Immunohistochemical analysis of the same patient samples showed RIN1 protein levels commensurate with the observed reduction in transcripts (data not shown).
RIN1 Silencing Mechanisms in Breast Tumor Cells
The human RIN1 promoter includes 20 binding sites for SNAI1 (a.k.a. Snail), a transcriptional repressor of epithelial genes such as E-cadherin (18), and 3 of these SNAI1 sites are well conserved in mammals (Fig. S3). In addition, overexpression of SNAI1 is seen in multiple types of epithelial-derived tumors (5), and correlates with breast tumor recurrence (19). We therefore examined breast tumor cell lines with low RIN1 levels and found that ZR75-1 cells have nearly four fold higher levels of SNAI1 than the normal mammary epithelial cell line MCF10A (Fig. 3A). The closely related transcription repressor SNAI2 (a.k.a. Slug) showed unchanged or reduced expression in the same set of tumor lines (Fig. 3B). To determine whether elevated levels of SNAI1 contribute to the silencing of RIN1 in this cell line, we used a SNAI1-targeted siRNA to reduce levels of SNAI1 in ZR75-1 cells. This treatment reduced SNAI1 mRNA levels and at the same time increased the mRNA levels of RIN1 (Fig. 3C), consistent with SNAI1 acting as a repressor of RIN1 expression.
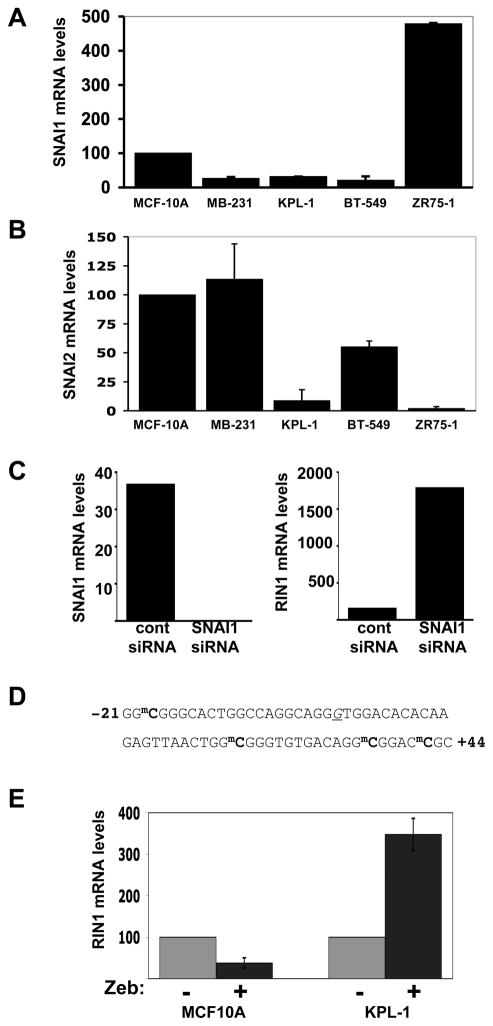
Figure 3.
SNAI1 overexpression and DNA methylation contribute to RIN1 silencing in breast tumor cells. A. SNAI1 (Snail) expression levels in MCF10A (normalized) and the indicated breast tumor cell lines, quantified by real time PCR. B. SNAI2 (Slug) expression levels in MCF10A (normalized) and the indicated breast tumor cell lines, quantified by real time PCR. A & B Standard error was calculated from two independent evaluations. C. ZR75-1 cells transfected with control or SNAI1-direced siRNA were analyzed for SNAI1 expression (left) and RIN1 expression (right). Greater than 99% SNAI1 silencing was achieved with this reagent. D. RIN1 gene sequence from KPL-1 cells, showing positions of methylated cytosines (mC). Nucleotides are numbered relative to the transcription start (underlined). E. Normalized RIN1 message levels (real time PCR) in MCF10A and KPL-1 cells following treatment with the de-methylating agent Zebularine. Standard error was calculated from two independent evaluations.
We next considered whether RIN1 silencing might sometimes occur through DNA methylation, a common gene silencing mechanism in tumor cells. DNA methylation typically occurs at CpG dinucleotides within the promoter and first exon sequence of a gene, and subsequently leads to the recruitment of chromatin remodeling complexes (20). We used methylation-specific sequence analysis to check for cytosine methylation in the RIN1 promoter region. Several methylated cytosines were found in the promoter and first exon sequences of the RIN1 gene in KPL-1 cells (Fig. 3D). However, no methylation was detected in the same region of the mammary epithelial cell line MCF10A cells or the breast tumor lines MDA-MB-231, ZR75-1 and BT549. To determine if RIN1 promoter methylation correlated with reduced expression, we next tested for reactivation of RIN1 expression following treatment with the non-nucleoside demethylating agent zebularine (21). Treatment over the course of 3 cell doublings increased RIN1 expression in KPL-1 cells (Fig. 3E), suggesting that DNA methylation was involved in silencing of RIN1 in these cells. In contrast, Zebularine treatment of MCF10A cells resulted in a slight reduction of RIN1 expression (MCF10A cells expresses RIN1 at levels comparable to immortalized HMECs (Fig. S1B)). These results indicate that the recovery of RIN1 expression in KPL-1 cells was not a generalized non-specific activating effect of zebularine. The region of the RIN1 promoter methylated in KPL-1 cells does not conform to the standard criteria for a “CpG island”, the name given to sequences with a high-density of CpG dinucleotides that are frequently targeted for DNA methylation (ccnt.hsc.usc.edu/cpgislands2). This raises the possibility that targeting factors may be involved in directing methylation to the RIN1 promoter.
Restoration of RIN1 Expression Inhibits Tumor Phenotypes
To directly evaluate the tumor suppressor properties of RIN1, we engineered ectopic expression through stable transduction of the breast tumor lines KPL-1 and MDA-MB-231 with a RIN1 expression vector and expression levels were assessed by immunoblot (Fig. S3A). Expression of RIN1 suppressed the growth of KPL-1 cells in soft agar (Fig. 4A), a measure of anchorage independence and a common phenotype of tumor cells. In the well-studied and aggressively metastatic tumor cell line MDA-MB-231, restored RIN1 expression did not noticeably affect the number or size of colonies formed (data not shown). One possible explanation for this is that additional alterations in this cell line, including the KRASG13D allele, have rendered it less sensitive to the suppressing effects of RIN1 in this assay. In addition, levels of ectopic RIN1 expression were not as high in MDA-MB-231 cells as they were in KPL-1 cells.
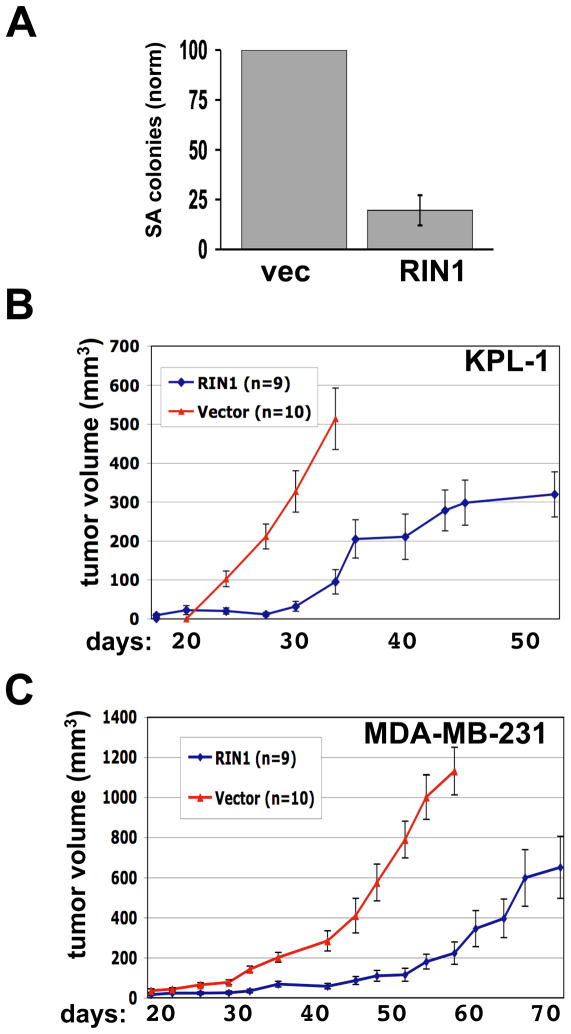
Figure 4.
A. RIN1 suppresses anchorage independent growth of breast tumor cells. KPL-1 cells transduced with a blasticidin resistance vector or a RIN1 expression construct were grown in soft agar suspension medium, and visible colonies were quantified. Standard error was calculated from two independent evaluations. B. RIN1 suppresses tumorigenicity of breast tumor cells in mice. Transduced KPL-1 cells (Blast or RIN1) were injected into mammary fat pads of nude mice (n = 10 for Blast vector cells; n = 9 for RIN1 transduced cells) and tumor volumes calculated at the indicated days. C. Same experiment as in “B” but using MDA-MB-231 cells.
Restoration of RIN1 expression also inhibited the ability of KPL-1 cells to form tumors following injection into the mammary fat pads of nude mice. Compared to cells transduced with empty vector, KPL-1 cells transduced with RIN1 showed approximately a ten-day delayed onset of detectable tumors (Fig. 4B). In addition, cells transduced with RIN1 showed reduced kinetics of tumor growth (15 ± 3 mm3/day) compared with vector-transduced cells (42 ± 5 mm3/day). To eliminate the possibility that the tumors that eventually arose from RIN1 transduced cells might have resulted from silencing of ectopic RIN1 expression, we stained tissue from tumors collected at the termination of the assay. We found that elevated RIN1 expression was retained in the tumors that arose from RIN1-transduced breast tumor cells (Fig. S3B), consistent with a role for RIN1 in suppressing, but not completely blocking, tumor formation.
We next examined whether ectopic RIN1 levels would reduce tumor formation by MDA-MB-231 cells. These cells retain more endogenous RIN1 than KPL-1 cells, but less than normal mammary epithelial cells (Fig. 1A and Fig. S4A). RIN1 expression caused a substantial delay in the formation of palpable tumors from these cells as well, compared to vector transduced cells (Fig. 4C). Again, a marked reduction in tumor growth kinetics (23 ± 4 mm3 per day), compared with control cells (30 ± 2 mm3 per day), was observed. These results from breast tumor cell lines that differ in their degree of silencing suggest that RIN1 suppresses an early step in tumor formation as well as a function required for tumor progression.
RIN1 is a Negative Regulator of Tumor Cell Invasive Growth
Previous analysis has demonstrated that RIN1 is an inhibitor of mammary epithelial cell migration (13, 14), raising the possibility that silencing of RIN1 might promote the invasive growth associated with tumor spread. Indeed, MDA-MB-231 cells stably transduced with RIN1 had a reduced capacity for invasive growth through a Matrigel substrate, compared to vector control MDA-MB-231 cells (Fig. 5A).
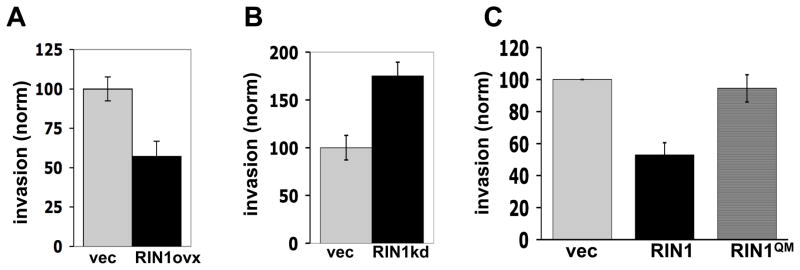
Figure 5.
RIN1 suppresses invasive growth of breast tumor cells. A. MDA-MB-231 cells, transduced with vector or a RIN1 construct (RIN1ovx), were allowed to invade through a Matrigel-coated filter. Cells passing to the lower chamber were fixed and counted. B. MDA-MB-231 cells transduced with a control or a RIN1-directed shRNA construct (RIN1kd) tested for invasive growth as in panel “A”. There were no differences in cell proliferation rates among vector, RIN1ovx and RIN1kd cells over a 48 hour period (data not shown) indicating that cell expansion was not a factor in this assay. Immunoblots demonstrating RIN1 expression increase and decrease are shown in figures S4A & S4B. C. MDA-MB-231 cells were transduced with a control vector, wild type RIN1 or RIN1QM (expression shown in Fig. S4A) and tested for invasive growth as in panel “A”. Standard errors were calculated from two independent evaluations.
Because these cells retain a significant level of RIN1 expression (Figs. 1A and 2A), we asked whether a further reduction of RIN1 might lead to an additional increase in cell invasion potential. Using shRNA to stably silence RIN1 expression in MDA-MB-231 cells we observed enhanced invasive growth (Fig. 5B) compared to control cells. This is consistent with a role for RIN1 as an invasive growth inhibitor in mammary epithelial cells.
RIN1 is a binding partner and activator of ABL tyrosine kinases (13), which in turn regulate actin remodeling and cell motility. We tested the contribution of this signaling pathway to invasive growth blockade using a RIN1 mutant. RIN1QM carries tyrosine to phenylalanine substitutions at positions 36, 121, 148 and 295. This mutant shows reduced levels of tyrosine phosphorylation and ABL binding (14), severely compromising its ABL activation function (Fig. S4C). RIN1QM was unable to block cell invasion (Fig. 5C), strongly implicating ABL stimulation as a required pathway for RIN1 mediated tumor suppression.
Discussion
We have shown that the RAS effector RIN1 has the properties of a breast tumor suppressor. As with a growing number of tumor suppressor genes, RIN1 appears to be transcriptionally silenced, rather than deleted, during tumor progression. We identify two potential mechanisms causing reduced expression. These include DNA methylation of the RIN1 promoter and transcriptional repression through elevated levels of SNAI1, an established repressor of genes defining epithelial cell properties (22). Consistent with this observation, RIN1 expression was significantly reduced in mammary epithelial cells following treatment with TGFβ (14), a factor that confers mesenchymal phenotypes and promotes tumor metastasis in part through SNAI1.
In addition to transcription repressor-based silencing, DNA methylation of the RIN1 promoter was observed in a breast tumor cell line, suggesting that alterations in chromatin structure may contribute to silencing. Other breast tumor cell lines with low RIN1 expression showed no indication of either mechanism, implying that additional means, such as miRNAs, might be employed to silence this locus in a reversible manner. The involvement of alternate transcription silencing mechanisms, including SNAI1 overexpression and promoter methylation, has also been reported for the breast tumor suppressor CDH1 (5, 23). These observations highlight the limitation of therapeutic approaches that target only specific silencing mechanisms.
The most intensely studied RAS effectors (RAF and PI3K) enhance transformation and are themselves mutationally activated in many tumors (reviewed in (12)). There is, however, precedence for a RAS effector with tumor suppressor properties. RASSF1 is frequently silenced in tumors and shows the hallmarks of a tumor suppressor (24). Other members of the RASSF family, which share a RAS association domain, are potential tumor suppressors as well (25–27). RASSF proteins appear to enhance apoptosis (28) and silencing of RASSF genes likely promotes tumor cell survival.
RIN1 functions through two downstream pathways involved in maintenance of epithelial properties. By activating ABL tyrosine kinases, RIN1 blocks the cytoskeletal rearrangements associated with cell dissociation and migration (13). We present evidence that signaling through ABL tyrosine kinases is required for RIN1-mediated blockade of invasive growth. This is consistent with a proposed inhibitory role for ABL in breast cancer cell tumorigenicity (29), although other studies (30, 31) suggest a more complex role for ABL signaling in breast cancer. RIN1 also signals downstream through RAB5 proteins to promote endocytosis (32, 33). This down regulates growth factor receptors required for directed migration and enhances TGFβ signaling (14). RAB5 signaling might also contribute to the initiation and/or progression of primary tumors, but there is as yet no direct evidence for this.
E-cadherin is another protein that contributes to epithelial character in normal cells while its loss facilitates cell motility and is characteristic of tumor cells (3). Like RIN1, CDH1 is silenced by promoter hypermethylation as well as SNAI1-mediated repression in breast tumors (34). Also like RIN1, loss of CDH1 and other epithelial markers does not strictly correlate with tumor grade or metastatic potential in vivo (35). These observations lend support to a model of tumor progression in which gene expression is dynamically regulated. In early stages, matrix invasion and intravasation of primary tumor cells through endothelial cell barriers should select for the silencing of genes that inhibit mesenchymal phenotypes or are associated with tumor metastasis. Such genes might include CDH1 (36), BRMS1 (37), NDRG1 (a.k.a. Drg-1 and CAP43) (38), NME1 (a.k.a. nm23-H1) (39), CD82 (a.k.a. KAI1) (40), SERPINB5 (a.k.a. maspin) (41), MKK4 (42), RRM1 (43), PEBP1 (a.k.a. RKIP) (44) and RIN1 (this work). Resistance to silencing may partly explain why a relatively moderate increase in RIN1 expression from a viral promoter had such a strong tumor suppressor effect in MDA-MB-231 cells. Subsequently, establishment of secondary tumors may remove, or even reverse, this selective pressure for silencing and lead to restored expression of invasion suppressor genes. Finally, it should be considered that culture conditions may exert their own selective pressure for tumor cell lines to display mesenchymal properties, making them more representative of particular tumor stages.
The loss of epithelial cell characteristics together with a gain of mesenchymal phenotypes, as seen in many cancer cells, has prompted a comparison between tumor progression and the cell transitions that occur during development. Indeed, during organ formation, epithelial cells appear to have a natural plasticity that allows them to adopt mesenchymal characteristics as needed. A striking example is the multiple waves of Snail gene expression that occur during vertebrate morphogenesis (45). And, just as epithelial cells adopt mesenchymal phenotypes only temporarily during development, the metastatic spread of tumor cells may also require forward and reverse transitions between epithelial and mesenchymal characteristics.
It is worth noting that the RIN1 gene is located less than 1 kb downstream of, and in the same orientation as, the breast cancer metastasis suppressor gene BRMS1 (chr 11: 65,856,118–65,869,158). The relative position of these genes is highly conserved in mammals. A reduction in BRMS1 expression is associated with the metastatic potential of breast tumor cells, and restored expression blocks metastasis (37). The BRMS1 gene product functions as a transcriptional co-repressor (46, 47) and normally enhances apoptosis of non-adherent cells. This raises the intriguing possibility that the RIN1 and BRMS1 gene products work in concert to promote stable mammary epithelial cell structures. Combined repression of both the RIN1 and BRMS1 genes may collaboratively facilitate cell migration during development, as well as the spread of tumor cells during metastasis. We are currently exploring this hypothesis.
Supplementary Material
References
- 1.Kurose K, Gilley K, Matsumoto S, Watson PH, Zhou XP, Eng C. Frequent somatic mutations in PTEN and TP53 are mutually exclusive in the stroma of breast carcinomas. Nat Genet. 2002;32:355–7. doi: 10.1038/ng1013. [DOI] [PubMed] [Google Scholar]
- 2.Baselga J, Norton L. Focus on breast cancer. Cancer Cell. 2002;1:319–22. doi: 10.1016/s1535-6108(02)00066-1. [DOI] [PubMed] [Google Scholar]
- 3.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–58. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Lombaerts M, van Wezel T, Philippo K, et al. E-cadherin transcriptional downregulation by promoter methylation but not mutation is related to epithelial-to-mesenchymal transition in breast cancer cell lines. Br J Cancer. 2006;94:661–71. doi: 10.1038/sj.bjc.6602996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batlle E, Sancho E, Franci C, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nature cell biology. 2000;2:84–9. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 6.Vernon AE, LaBonne C. Tumor metastasis: a new twist on epithelial-mesenchymal transitions. Curr Biol. 2004;14:R719–21. doi: 10.1016/j.cub.2004.08.048. [DOI] [PubMed] [Google Scholar]
- 7.Kang JY, Dolled-Filhart M, Ocal IT, et al. Tissue microarray analysis of hepatocyte growth factor/Met pathway components reveals a role for Met, matriptase, and hepatocyte growth factor activator inhibitor 1 in the progression of node-negative breast cancer. Cancer research. 2003;63:1101–5. [PubMed] [Google Scholar]
- 8.Maggiora P, Marchio S, Stella MC, et al. Overexpression of the RON gene in human breast carcinoma. Oncogene. 1998;16:2927–33. doi: 10.1038/sj.onc.1201812. [DOI] [PubMed] [Google Scholar]
- 9.Ro J, North SM, Gallick GE, Hortobagyi GN, Gutterman JU, Blick M. Amplified and overexpressed epidermal growth factor receptor gene in uncultured primary human breast carcinoma. Cancer research. 1988;48:161–4. [PubMed] [Google Scholar]
- 10.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–12. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 11.Colicelli J. Human RAS superfamily proteins and related GTPases. Sci STKE. 2004;250:RE13. doi: 10.1126/stke.2502004re13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez–Viciana P, Tetsu O, Oda K, Okada J, Rauen K, McCormick F. Cancer targets in the Ras pathway. Cold Spring Harb Symp Quant Biol. 2005;70:461–7. doi: 10.1101/sqb.2005.70.044. [DOI] [PubMed] [Google Scholar]
- 13.Hu H, Bliss JM, Wang Y, Colicelli J. RIN1 is an ABL tyrosine kinase activator and a regulator of epithelial-cell adhesion and migration. Curr Biol. 2005;15:815–23. doi: 10.1016/j.cub.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 14.Hu H, Milstein M, Bliss JM, Thai M, Malhotra G, Colicelli J. Integration of TGFbeta and RTK signaling through a RAB5 GEF regulates directed cell migration. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wadehra M, Braun J, Goodglick L. One-step RT-PCR for screening microdissected tissue samples. Biotechniques. 2002;32:242, 4, 6, 8. doi: 10.2144/02322bm02. [DOI] [PubMed] [Google Scholar]
- 16.Dohadwala M, Yang SC, Luo J, et al. Cyclooxygenase-2-dependent regulation of E-cadherin: prostaglandin E(2) induces transcriptional repressors ZEB1 and snail in non-small cell lung cancer. Cancer research. 2006;66:5338–45. doi: 10.1158/0008-5472.CAN-05-3635. [DOI] [PubMed] [Google Scholar]
- 17.Sharp JA, Waltham M, Williams ED, Henderson MA, Thompson EW. Transfection of MDA-MB-231 human breast carcinoma cells with bone sialoprotein (BSP) stimulates migration and invasion in vitro and growth of primary and secondary tumors in nude mice. Clinical & experimental metastasis. 2004;21:19–29. doi: 10.1023/b:clin.0000017167.17065.61. [DOI] [PubMed] [Google Scholar]
- 18.Cano A, Perez-Moreno MA, Rodrigo I, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nature cell biology. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 19.Moody SE, Perez D, Pan TC, et al. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005;8:197–209. doi: 10.1016/j.ccr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Baylin SB, Ohm JE. Epigenetic gene silencing in cancer - a mechanism for early oncogenic pathway addiction? Nat Rev Cancer. 2006;6:107–16. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- 21.Cheng JC, Weisenberger DJ, Gonzales FA, et al. Continuous zebularine treatment effectively sustains demethylation in human bladder cancer cells. Mol Cell Biol. 2004;24:1270–8. doi: 10.1128/MCB.24.3.1270-1278.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–61. doi: 10.1242/dev.01907. [DOI] [PubMed] [Google Scholar]
- 23.Yoshiura K, Kanai Y, Ochiai A, Shimoyama Y, Sugimura T, Hirohashi S. Silencing of the E-cadherin invasion-suppressor gene by CpG methylation in human carcinomas. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:7416–9. doi: 10.1073/pnas.92.16.7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burbee DG, Forgacs E, Zochbauer-Muller S, et al. Epigenetic inactivation of RASSF1A in lung and breast cancers and malignant phenotype suppression. Journal of the National Cancer Institute. 2001;93:691–9. doi: 10.1093/jnci/93.9.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vos MD, Martinez A, Ellis CA, Vallecorsa T, Clark GJ. The pro-apoptotic Ras effector Nore1 may serve as a Ras-regulated tumor suppressor in the lung. J Biol Chem. 2003;278:21938–43. doi: 10.1074/jbc.M211019200. [DOI] [PubMed] [Google Scholar]
- 26.Eckfeld K, Hesson L, Vos MD, Bieche I, Latif F, Clark GJ. RASSF4/AD037 is a potential ras effector/tumor suppressor of the RASSF family. Cancer research. 2004;64:8688–93. doi: 10.1158/0008-5472.CAN-04-2065. [DOI] [PubMed] [Google Scholar]
- 27.Hesson LB, Wilson R, Morton D, et al. CpG island promoter hypermethylation of a novel Ras-effector gene RASSF2A is an early event in colon carcinogenesis and correlates inversely with K-ras mutations. Oncogene. 2005;24:3987–94. doi: 10.1038/sj.onc.1208566. [DOI] [PubMed] [Google Scholar]
- 28.Vos MD, Dallol A, Eckfeld K, et al. The RASSF1A tumor suppressor activates Bax via MOAP-1. J Biol Chem. 2006;281:4557–63. doi: 10.1074/jbc.M512128200. [DOI] [PubMed] [Google Scholar]
- 29.Noren NK, Foos G, Hauser CA, Pasquale EB. The EphB4 receptor suppresses breast cancer cell tumorigenicity through an Abl-Crk pathway. Nature cell biology. 2006;8:815–25. doi: 10.1038/ncb1438. [DOI] [PubMed] [Google Scholar]
- 30.Srinivasan D, Plattner R. Activation of Abl tyrosine kinases promotes invasion of aggressive breast cancer cells. Cancer research. 2006;66:5648–55. doi: 10.1158/0008-5472.CAN-06-0734. [DOI] [PubMed] [Google Scholar]
- 31.Srinivasan D, Sims JT, Plattner R. Aggressive breast cancer cells are dependent on activated Abl kinases for proliferation, anchorage-independent growth and survival. Oncogene. 2007 doi: 10.1038/sj.onc.1210714. [DOI] [PubMed] [Google Scholar]
- 32.Barbieri MA, Kong C, Chen PI, Horazdovsky BF, Stahl PD. The SRC homology 2 domain of Rin1 mediates its binding to the epidermal growth factor receptor and regulates receptor endocytosis. J Biol Chem. 2003;278:32027–36. doi: 10.1074/jbc.M304324200. [DOI] [PubMed] [Google Scholar]
- 33.Tall GG, Barbieri MA, Stahl PD, Horazdovsky BF. Ras-activated endocytosis is mediated by the Rab5 guanine nucleotide exchange activity of RIN1. Dev Cell. 2001;1:73–82. doi: 10.1016/s1534-5807(01)00008-9. [DOI] [PubMed] [Google Scholar]
- 34.Cheng CW, Wu PE, Yu JC, et al. Mechanisms of inactivation of E-cadherin in breast carcinoma: modification of the two-hit hypothesis of tumor suppressor gene. Oncogene. 2001;20:3814–23. doi: 10.1038/sj.onc.1204505. [DOI] [PubMed] [Google Scholar]
- 35.Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer research. 2006;66:8319–26. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- 36.Liu YN, Lee WW, Wang CY, Chao TH, Chen Y, Chen JH. Regulatory mechanisms controlling human E-cadherin gene expression. Oncogene. 2005;24:8277–90. doi: 10.1038/sj.onc.1208991. [DOI] [PubMed] [Google Scholar]
- 37.Seraj MJ, Samant RS, Verderame MF, Welch DR. Functional evidence for a novel human breast carcinoma metastasis suppressor, BRMS1, encoded at chromosome 11q13. Cancer research. 2000;60:2764–9. [PubMed] [Google Scholar]
- 38.Bandyopadhyay S, Wang Y, Zhan R, et al. The tumor metastasis suppressor gene Drg-1 down-regulates the expression of activating transcription factor 3 in prostate cancer. Cancer research. 2006;66:11983–90. doi: 10.1158/0008-5472.CAN-06-0943. [DOI] [PubMed] [Google Scholar]
- 39.Steeg PS, Bevilacqua G, Kopper L, et al. Evidence for a novel gene associated with low tumor metastatic potential. Journal of the National Cancer Institute. 1988;80:200–4. doi: 10.1093/jnci/80.3.200. [DOI] [PubMed] [Google Scholar]
- 40.Dong JT, Lamb PW, Rinker-Schaeffer CW, et al. KAI1, a metastasis suppressor gene for prostate cancer on human chromosome 11p11.2. Science. 1995;268:884–6. doi: 10.1126/science.7754374. [DOI] [PubMed] [Google Scholar]
- 41.Zou Z, Anisowicz A, Hendrix MJ, et al. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994;263:526–9. doi: 10.1126/science.8290962. [DOI] [PubMed] [Google Scholar]
- 42.Kim HL, Vander Griend DJ, Yang X, et al. Mitogen-activated protein kinase kinase 4 metastasis suppressor gene expression is inversely related to histological pattern in advancing human prostatic cancers. Cancer research. 2001;61:2833–7. [PubMed] [Google Scholar]
- 43.Gautam A, Li ZR, Bepler G. RRM1-induced metastasis suppression through PTEN-regulated pathways. Oncogene. 2003;22:2135–42. doi: 10.1038/sj.onc.1206232. [DOI] [PubMed] [Google Scholar]
- 44.Fu Z, Smith PC, Zhang L, et al. Effects of raf kinase inhibitor protein expression on suppression of prostate cancer metastasis. Journal of the National Cancer Institute. 2003;95:878–89. doi: 10.1093/jnci/95.12.878. [DOI] [PubMed] [Google Scholar]
- 45.Dale JK, Malapert P, Chal J, et al. Oscillations of the snail genes in the presomitic mesoderm coordinate segmental patterning and morphogenesis in vertebrate somitogenesis. Dev Cell. 2006;10:355–66. doi: 10.1016/j.devcel.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 46.Meehan WJ, Samant RS, Hopper JE, et al. Breast cancer metastasis suppressor 1 (BRMS1) forms complexes with retinoblastoma-binding protein 1 (RBP1) and the mSin3 histone deacetylase complex and represses transcription. J Biol Chem. 2004;279:1562–9. doi: 10.1074/jbc.M307969200. [DOI] [PubMed] [Google Scholar]
- 47.Liu Y, Smith PW, Jones DR. Breast cancer metastasis suppressor 1 functions as a corepressor by enhancing HDAC1-mediated deacetylation of RelA/p65 and promoting apoptosis. Mol Cell Biol. 2006;26:8683–96. doi: 10.1128/MCB.00940-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.